Abstract
Objective
To evaluate the association between adherence to the Dietary Approaches to Stop Hypertension (DASH) diet and overall and cause-specific mortality in the Golestan Cohort Study (GCS).
Methods
A total of 50 045 participants aged 40 years or older were recruited from Golestan Province, Iran, from 2004 to 2008 and followed for a mean of 10.64 years. The DASH diet score was calculated for each individual based on food groups. The primary outcome measure was death from any cause.
Results
During 517 326 person-years of follow-up, 6763 deaths were reported. After adjustment for potential confounders, DASH diet score was inversely associated with risk of death from all causes and cancers [hazard ratio (HR): 0.86; 95% confidence interval (CI): 0.75, 0.98; and HR: 0.65; 95% CI: 0.47, 0.90, respectively]. A higher DASH diet score was associated with lower risk of gastrointestinal cancer mortality in men (HR: 0.55; 95% CI: 0.30, 0.99). A greater adherence to DASH diet was also associated with lower other-cancer mortality in women (HR: 0.50; 95% CI: 0.24, 0.99). No association between DASH diet score and cardiovascular disease mortality was observed, except that those dying of cardiovascular disease were younger than 50 years of age and smokers.
Conclusions
Our findings suggest that maintaining a diet similar to the DASH diet is independently associated with reducing the risk of total death, cancers, and especially gastrointestinal cancers in men.
Keywords: Diet, dietary approaches to stop hypertension, mortality, cancer, cardiovascular disease, DASH
Key Messages
Higher adherence to the Dietary Approaches to Stop Hypertension (DASH) diet has been shown to be beneficial for the metabolic disorders, such as hypertension, cardiovascular disease and type 2 diabetes.
It has been suggested that adherence to a DASH-style diet may be related to reduced risk of mortality.
An inverse association between adherence to a DASH-style diet and risk of death, particularly from chronic diseases, was observed in a developing country.
Conformity to the DASH diet and the association with risk of some specific causes of mortality were considerably different between the sexes.
A higher conformity with the DASH diet was associated with lower risk of overall mortality and mortality from cardiovascular disease among current smokers.
This study suggests that people with selected risk factors may benefit more than others from adherence to a DASH dietary pattern.
Introduction
Evaluation of dietary patterns, including combinations of specific foods and dietary components, provides us with a more accurate and comprehensive snapshot of dietary exposures than assessing foods or nutrients separately, which ignores probable interaction or association among numerous nutrients.1 One of the established dietary patterns is the Dietary Approaches to Stop Hypertension (DASH) diet, which is rich in fruits, vegetables, whole grains, low-fat dairy products and legumes/nuts but low in sodium, added sugars and processed/red meat. The DASH-style diet was initially designed and evaluated for reducing blood pressure2; however, it has since been considered to be beneficial for the other metabolic disorders, such as cardiovascular disease (CVD),3–6 type 2 diabetes4,7 and some cancers.8,9 Thus, it seems that adherence to a DASH-style diet may be related to reduced risk of mortality. Although the beneficial impact of DASH diet components on survival has been shown previously,10–14 the association between the total dietary pattern and mortality has been less likely to be evaluated. As some causes of mortality, such as CVD and cancers, could have been influenced by exposures during the early decades of life, and it is not feasible to conduct a long-term randomized clinical trial to see the impact of adherence to a DASH-style diet on mortality as an endpoint, data from cohort studies with participants following a diet similar to the DASH pattern may be helpful in elucidating the long-term effectiveness of the DASH diet on health and survival. The aim of this study was to evaluate the association between DASH diet adherence and overall and cause-specific mortality in the Golestan Cohort Study (GCS) .
Methods
Study population
The design of the GCS has been reported previously.15 The follow-up flow chart is presented as Supplementary Figure 1, available as Supplementary data at IJE online. This cohort was launched in 2004 in Golestan Province, in northeastern Iran, by recruiting 50 045 adults, aged between 40 and 87 years, from Gonbad city and 326 rural villages (a 20% urban, 80% rural cohort). After excluding those participants with extremely low or high energy intakes (<500 or >5000 kcal/day), prevalent cancers at baseline, missing or incomplete information on the food frequency questionnaire (FFQ) and/or the general lifestyle questionnaire (including questions on demographics, education, socio-economic status, history of diabetes, smoking, alcohol and opium use, and anthropometric measurements), and those with an unreasonable body mass index (BMI) (<15 or >50 kg/m2), 48 633 individuals were available for this analysis (27 975 women and 20 658 men). The study was approved by the Institutional Review Boards of the Digestive Disease Research Center (DDRC) of Tehran University of Medical Sciences, the US National Cancer Institute (NCI) and the World Health Organization International Agency for Research on Cancer (IARC). All participants provided written informed consent before enrollment.
Dietary assessment
A valid and reliable FFQ that was designed for the GCS was used to collect the dietary intakes in this study.16 Information on typical portion size, consumption frequency and servings consumed each time was collected for each food item at enrollment. Consumption frequency of each food item was questioned on a daily, weekly or monthly basis and converted into daily intakes; portion sizes were then converted into grams using household measures.17,18 The collected data were analysed using Nutritionist V (First Databank, Hearst Corp, San Bruno, CA, USA). A DASH diet score was calculated for each FFQ. The DASH diet score was constructed based on the foods and nutrients emphasized or minimized in the DASH diet, focusing on eight components: high intake of fruits, vegetables, low-fat dairy products, whole grains, and nuts/legumes; and low intake of soft drinks and sweets, red/processed meats and sodium. For calculation of DASH score, the amount of each dietary food group was converted to its equal serving size number. Owing to the lack of accurate data on consumed salt, only data with respect to sodium in foods were used to estimate sodium intake (i.e. without considering the salt used during cooking and/or added at the table). For each of the eight components, intake (in serving) was ranked to quintiles.3 For each food group, a maximum score of 5 points could be achieved when the intake met the recommendation, whereas lower intakes were scored proportionately. For the five groups of fruits, vegetables, dairy, whole grains and beans/nuts, a lowest quintile intake received a score of 1 point and a highest quintile intake received a score of 5 points. For the remaining components (red/processed meat, soft drinks/sweets and sodium), low intake was desirable. Therefore, the lowest quintile received 5 points and the highest was given 1 point. The resulting eight component scores were summed to create the overall DASH diet score, which could range from 8 to 40. DASH diet score was categorized into the following four groups: DASH score (DS) 1: 9–20, DS2: 21–25, DS3: 26–30, and DS4: 31–39.
Assessment of potential confounders
All participants underwent interviews conducted by trained physicians and/or technicians, and information on demographics and baseline lifestyle behaviour was collected using a structured lifestyle questionnaire. Anthropometric indices were measured after the interviews.15 Weight, height, body mass index (BMI) and waist-to-hip ratio (WHR) were measured according to WHO recommendations.19 Data on the intensity, duration and frequency of physical activity were obtained.20 Physical activity was categorized, based on the metabolic equivalent of task per minute per week, into tertiles. Other potential confounders assessed in this cohort study included age, sex, smoking status, opium and alcohol consumption, wealth score, diabetes and hypertension.21 Participants were regarded as smokers if they had smoked at least once a week for a period of 6 months or more. Lifetime pack-years of cigarette smoking were estimated by multiplying frequency of use per day by the duration of use in years. Pack-years of cigarette smoking were categorized into 0, 0.1–5, 5–10, 10–20 and >20. Opium users or alcohol drinkers were described as those who used these substances at least once a week, for a minimum of 6 months. Information on wealth score was a surrogate of socio-economic status (SES)22 and calculated from appliance ownership. These scores were categorized in quartiles.
Follow-up and cause of death ascertainment
Details of the follow-up procedures of this cohort study have been described previously.15 Briefly, all participants were followed annually. The GCS team completed a case review questionnaire during each phone call or home visit and recorded the vital status of the participants. During a mean follow-up of 10.64 years, only 454 of 48 633 participants (0.9%) were lost to follow-up. The primary endpoint was death from any cause. Any reported death was confirmed by a physician visit and a completed validated verbal autopsy questionnaire.23 Furthermore, two internists independently reviewed all the verbal autopsy information and medical records and ascertained the cause of death. In case of conflict between the two internists, all data were reviewed by a third, more skilled, internist and final diagnosis was made.15 For this analysis, the most prevalent causes of death among the participants were assessed, including mortality from CVD, cancer (all; gastrointestinal, including alimentary tract, liver and pancreas; lung; and other cancers), respiratory diseases, infectious disorders, and other causes. Injury deaths were included as controls. Analysis was done only on individuals with confirmed causes of death.
Primary and secondary outcomes
The primary outcome measure was a significant association between DASH score and total mortality. Secondary outcome measures were the association between DASH score and causes of death across the strata of the known risk factors for death.
Statistical analysis
Participants were divided into four groups according to their DASH score, and characteristics of participants were presented according to DASH score. Participants’ characteristics were compared between groups of DASH score using an analysis of covariance (ANCOVA) test for continuous variables and the chi-squared (χ2) test for categorical variables. To test for associations between DASH score and risk of overall and cause-specific mortality, Cox proportional hazards regression models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs). In the Cox models, age and energy-adjusted and multivariate-adjusted HRs were calculated for each covariate. In the multivariate model, the HRs were adjusted for potential confounders, including age in years (continuous), energy intake (continuous), sex (male, female), BMI (continuous), pack-years of cigarette smoking (0, 0.1–5, 5–10, 10–20, >20), opium user (non-user, opium user), wealth score (quartiles 1, 2, 3 and 4), physical activity (tertiles 1, 2 and 3), history of diabetes (yes, no) and history of hypertension (yes, no). Alcohol consumption was also included in the list of confounders; however, since the results were not changed considerably, it was omitted. Follow-up time was considered for each participant from the date they were recruited to the study until the date of death, failure to follow-up or the end of follow-up date (30 July 2018), whichever occurred first. We also stratified the analysis by major risk factors at baseline to evaluate potential interactions between these factors and the DASH score in relation to risk of overall and some common specific causes of mortality. Interactions were tested using the likelihood ratio test (LRT). All the statistical analyses were done in SPSS (version 19; SPSS Inc, Chicago, IL, USA) and P ≤ 0.05 was considered significant.
Results
The mean age [standard deviation (SD)] of participants at baseline was 52.03 (8.9) years. Overall, 57.5% were women and 79.7% lived in rural areas. During 517 326 person-years of follow-up, we documented 6 763 deaths (3003 women, 3760 men), including 2753 cardiovascular deaths, 1319 cancer deaths (681 gastrointestinal cancer deaths, 91 lung cancer deaths, 547 other cancer deaths), 382 respiratory disease deaths, 252 infectious disease deaths, 325 injury deaths, 621 deaths from other causes and 1111 deaths from unconfirmed causes. Baseline characteristics of the participants are shown in Table 1. Participants with higher DASH scores were older and more likely to have a higher BMI, WHR, wealth score and be alcohol drinkers compared with those with the lowest score. Furthermore, participants in the top group of the DASH score were less likely to smoke or use opium, were more likely to report a history of diabetes and hypertension and were less active. Calculated DASH scores for all FFQs ranged from 9 to 39, and the mean DASH score (SD) of all participants was 24.00 (3.71). Participants with higher DASH scores tended to consume more energy, fibre, calcium, magnesium and potassium (Table 1).
Table 1.
Characteristics of participants according to the DASH score categoriesa
| DASH score ranges |
||||
|---|---|---|---|---|
| DS1 (9–20) | DS2 (21–25) | DS3 (26–30) | DS4 (31–39) | |
| n (%) | 8698 (17.9) | 23 237 (47.8) | 14 666 (30.2) | 2032 (4.2) |
| Men % | 3738 (43.0) | 9783 (42.1) | 6275 (42.8) | 862 (42.4) |
| Age (y)b | 52.75±9.16 | 51.93±8.87 | 51.60±8.67 | 53.26±9.32 |
| BMI (kg/m2)b | 25.47±5.27 | 26.41 ±5.35 | 27.59 ±5.37 | 28.39 ±5.06 |
| Waist-to-hipb | 0.95 ± 0.08 | 0.95 ± 0.08 | 0.96 ± 0.08 | 0.97 ± 0.07 |
| Smoker ever, %b | 1659 (18.1) | 4037(17.4) | 2424 (16.5) | 286 (14.1) |
| Pack-years of smoking, %b | ||||
| 0 | 7039 (80.9) | 19 200 (82.6) | 12 242 (83.5) | 1 746 (85.9) |
| 0.1–5 | 474 (5.4) | 1302 (5.6) | 821 (5.6) | 103 (5.1) |
| 5.1–10 | 230 (2.6) | 604 (2.6) | 372 (2.5) | 40 (2.0) |
| 10.1–20 | 368 (4.2) | 826 (3.6) | 523 (3.6) | 52 (2.6) |
| 20< | 587 (6.7) | 1305 (5.6) | 708 (4.8) | 91 (4.5) |
| Alcohol ever used, %b | 214 (2.5) | 707 (3.0) | 649 (4.4) | 111 (5.5) |
| Opium ever used, %b | 1770 (20.3) | 4083 (17.6) | 2144 (14.6) | 222 (10.9) |
| History of diabetes, %b | 209 (2.4) | 1127 (4.9) | 1499 (10.2) | 536 (26.4) |
| History of hypertension, %b | 1590 (18.3) | 4286 (18.4) | 3152 (21.5) | 575 (28.3) |
| Wealth score, %b | ||||
| 1st quartile | 3181 (36.6) | 6951 (29.9) | 2900 (19.8) | 253 (12.5) |
| 2nd quartile | 2393 (27.5) | 5669 (24.4) | 2960 (20.2) | 338 (16.6) |
| 3rd quartile | 1957 (22.5) | 5661 (24.4) | 3703 (25.2) | 512 (25.2) |
| 4th quartile | 1167 (13.4) | 4956 (21.3) | 5103 (34.8) | 929 (45.7) |
| Physical activity, %b | ||||
| 1st tertile | 3091 (35.6) | 8042 (34.7) | 5263 (36.0) | 758 (37.4) |
| 2nd tertile | 2678 (30.9) | 7438 (32.1) | 4549 (31.1) | 678 (33.4) |
| 3rd tertile | 2908 (33.5) | 7710 (33.2) | 4816 (32.9) | 592 (29.2) |
| Total energy intake (kcal)b | 1, 978.95±592.33 | 2, 129.59±587.80 | 2306.85±579.10 | 2302.45±524.90 |
| Total dietary fibre (g/d)b,c | 20.78±0.04 | 22.27±0.02 | 23.92±0.03 | 26.40±0.08 |
| Dietary calcium (mg/d)b,c | 624.09±1.96 | 683.96±1.19 | 762.91±1.51 | 855.31±4.01 |
| Dietary magnesium (mg/d)b,c | 433.03±0.74 | 447.64±0.45 | 458.54±0.45 | 488.02±1.51 |
| Dietary potassium (mg/d)b,c | 2654.72±4.31 | 2764.10±2.61 | 2967.08±3.32 | 3241.59±8.85 |
| Saturated fatty acids (g/d)b,c | 40.74±0.13 | 40.59±0.08 | 39.41±0.10 | 34.75±0.27 |
| Monounsaturated fatty acids (g/d)b,c | 18.83±0.04 | 19.27±0.03 | 19.83±0.03 | 19.87±0.09 |
| Polyunsaturated fatty acids (g/d)b,c | 9.09 ±0.06 | 9.71±0.04 | 10.10±0.05 | 12.54±0.12 |
| Trans-fatty acids (g/d)b,c | 0.41±0.002 | 0.38±0.001 | 0.33±0.001 | 0.25±0.004 |
| Components of the DASH score | ||||
| Fruit (servings/d)b | 0.81±0.66 | 1.29±1.09 | 2.10±1.51 | 2.94±1.92 |
| Vegetables (servings/d)b | 0.74±0.39 | 1.10±0.65 | 1.64±0.90 | 2.18±1.15 |
| Whole grains (servings/d)b | 0.92±0.51 | 1.10±0.50 | 1.16±0.50 | 1.21±0.49 |
| Nuts and legumes (servings/d)b | 0.18±0.18 | 0.34±0.31 | 0.55±0.45 | 0.71±0.68 |
| Low-fat dairy (servings/d)b | 0.33±0.27 | 0.50±0.36 | 0.75±0.46 | 0.97±0.51 |
| Red and processed meats (servings/d)b | 0.55 ±0.55 | 0.53±0.60 | 0.52±0.62 | 0.37±0.60 |
| Sweetened beverages and sweets (servings/d)b | 3.85±2.48 | 3.15±2.37 | 2.56±2.08 | 1.40±1.19 |
| Sodium (mg/d)b | 4723.53±1761.98 | 4718.41±1614.99 | 4832.72±1574.12 | 4558.35±1492.07 |
DS, DASH score; BMI, Body mass index.
Values are means ± SDs for continuous variables and percentages for categorical variables.
These variables were statistically different across the DASH score range (P < 0.001), ANCOVA for quantitative variables and χ 2 test for qualitative variables.
Energy adjusted.
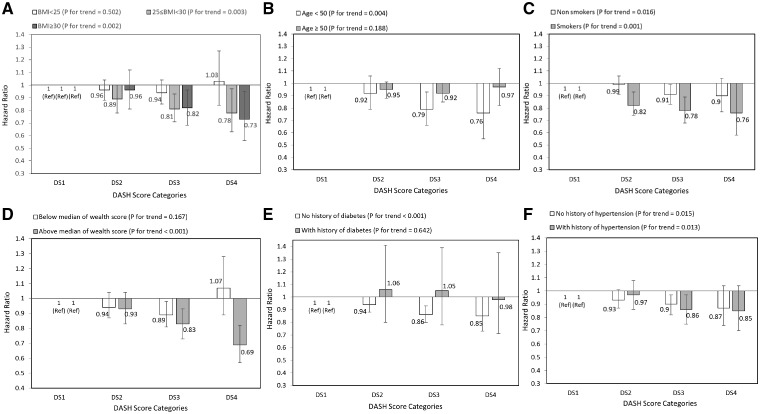
Table 2 indicates HRs for the association between DASH score quartiles and risk of mortality. The risk of mortality was lower in participants in the DS2, DS3 and DS4 groups in comparison with those in the first group (DS1). After adjustment for confounders, the results were the same in women and men when analysed separately. For further analysis, we stratified data by BMI, age, smoking, wealth score, history of diabetes and hypertension. The results showed that associations appeared stronger among overweight and obese participants than non-obese participants; for those below the age of 50 years rather than above the age of 50; for smokers than non-smokers; for participants who were above the median wealth score than below it; and for those who had no history of diabetes rather than a history of diabetes. The associations were similar among those with or without a history of hypertension (Figure 1) .
Table 2.
Hazard ratios for total mortality, according to the DASH score categoriesa
| DASH score range |
|||||
|---|---|---|---|---|---|
| DS1 (9–20) | DS2 (21–25) | DS3 (26–30) | DS4 (31–39) | P-value for trend | |
| Women | |||||
| No. of person-years | 4960 | 13454 | 8391 | 1170 | |
| No. of deaths | 579 | 1418 | 845 | 161 | |
| Model 1b | 1.00 | 0.94 (0.86, 1.04) | 0.91 (0.82, 1.02) | 1.08 (0.90, 1.28) | 0.712 |
| Model 2c | 1.00 | 0.98 (88, 1.08) | 1.00 (0.90, 1.12) | 1.27 (1.06, 1.52) | 0.078 |
| Model 3d | 1.00 | 0.92 (0.84, 1.02) | 0.86 (0.77, 0.97) | 0.90 (0.75, 0.99) | 0.034 |
| Men | |||||
| No. of person-years | 3 738 | 9 783 | 6 275 | 862 | |
| No. of deaths | 819 | 1, 795 | 990 | 156 | |
| Model 1b | 1.00 | 0.92 (0.85, 1.00) | 0.84 (0.76, 0.92) | 0.84 (0.70, 0.99) | <0.001 |
| Model 2c | 1.00 | 0.96 (88, 1.04) | 0.94 (0.86, 1.04) | 1.02 (0.86, 1.22) | 0.615 |
| Model 3d | 1.00 | 0.94 (0.86, 1.02) | 0.87 (0.79, 0.96) | 0.82 (0.68, 0.98) | 0.003 |
| Pooled | |||||
| Model 1b | 1.00 | 0.90 (0.85, 0.97) | 0.82 (0.76, 0.89) | 0.86 (0.75, 0.97) | <0.001 |
| Model 2c | 1.00 | 0.97 (0.91, 1.03) | 0.97 (0.90, 1.04) | 1.13 (1.00, 1.28) | 0.414 |
| Model 3d | 1.00 | 0.94 (0.88, 1.00) | 0.87 (0.81, 0.94) | 0.86 (0.75, 0.98) | <0.001 |
DS, DASH score.
Cox proportional hazards regression models for estimating HRs and 95% CIs.
Model 1: adjusted for age and energy intake.
Model 2: additionally adjusted for gender (except when stratified by gender), BMI, smoking, opium use, wealth score and physical activity.
Model 3: additionally adjusted for history of diabetes, history of hypertension.
Figure 1.
Multivariate hazard ratios of DASH score categories for total mortality according to risk factor status at baseline (Cox proportional hazards regression models for estimating HRs and 95% CIs, multivariable models were adjusted for age, energy intake, sex, BMI, smoking, opium use, wealth score, physical activity, history of diabetes and history of hypertension, except for the respective stratifying factor). Data are reported as HR (95% CI). DASH score categories: DS1: 9–20, DS2: 21–25, DS3: 26–30, and DS4: 31–39. A, BMI lower than 25 vs 25–30 and ≥30 (P = 0.187 for interaction); B, age <50 years vs ≥50 years (P = 0.260 for interaction); C, non-smokers vs smokers (P = 0.067 for interaction); D, wealth score below median vs above median (P = 0.024 for interaction); E, no diabetes vs diabetes (P = 0.179 for interaction); and F, no hypertension vs hypertension (P = 0.521 for interaction). Ref indicates reference group.
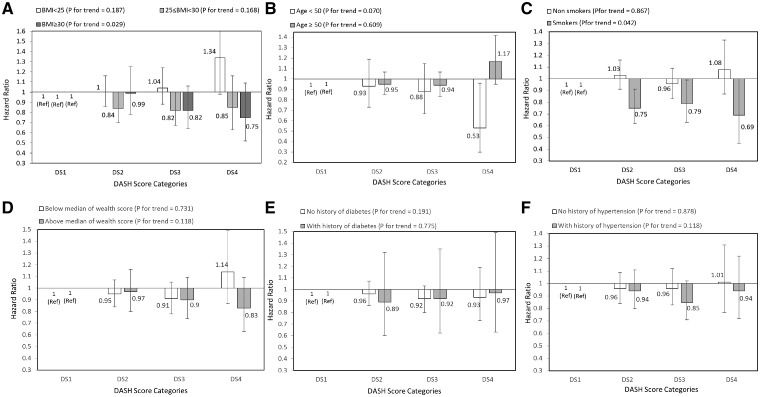
After multivariable adjustment, there was no significant association between adherence to the DASH diet and risk of death due to CVD (Table 3). However, after stratifying by main risk factors at baseline, reduced risks of CVD mortality tended to be significant among participants aged under 50 years compared with those above 50 years and those who had smoked compared with those who did not, albeit with wide CIs (Figure 2).
Table 3.
Hazard ratiosa for cause-specific mortality, according to the DASH score range
| DASH score range |
|||||
|---|---|---|---|---|---|
| Cause of death | DS1 (9–20) (n = 8, 698) | DS2 (21–25) (n = 23, 237) | DS3 (26–30) (n = 14, 666) | DS4 (31–39) (n = 2, 032) | P-value for trend |
| Cardiovascular disease | |||||
| Women | |||||
| No. of deaths | 223 | 608 | 365 | 79 | |
| Model 1b | 1.00 | 1.07 (0.91, 1.24) | 1.06 (0.90, 1.25) | 1.40 (1.08, 1.82) | 0.064 |
| Model 2c | 1.00 | 1.10 (0.94, 1.28) | 1.15 (0.97, 1.37) | 1.64 (1.26, 2.1) | 0.002 |
| Model 3d | 1.00 | 1.02 (0.87, 1.19) | 0.93 (0.78, 1.11) | 1.05 (0.80, 1.38) | 0.722 |
| Men | |||||
| No. of deaths | 315 | 668 | 417 | 78 | |
| Model 1b | 1: 00 | 0.90 (0.79, 1.03) | 0.95 (0.82, 1.10) | 1.11 (0.87, 1.43) | 0.667 |
| Model 2c | 1: 00 | 0.91 (0.79, 1.04) | 0.98 (0.84, 1.14) | 1.19 (0.92, 1.53) | 0.359 |
| Model 3d | 1: 00 | 0.89 (0.78, 1.02) | 0.88 (0.76, 1.03) | 0.91 (0.70, 1.18) | 0.246 |
| Pooled | |||||
| Model 1b | 1: 00 | 0.95 (0.86, 1.05) | 0.96 (0.85, 1.07) | 1.18 (0.98, 1.41) | 0.416 |
| Model 2c | 1: 00 | 0.99 (0.90, 1.10) | 1.06 (0.94, 1.19) | 1.38 (1.15, 1.65) | 0.004 |
| Model 3d | 1: 00 | 0.95 (0.86, 1.05) | 0.91 (0.81, 1.05) | 0.97 (0.81, 1.17) | 0.99 |
| Cancer: | |||||
| All cancer | |||||
| Women | |||||
| No. of deaths | 134 | 257 | 152 | 20 | |
| Model 1b | 1.00 | 0.713 (0.58, 0.88) | 0.66 (0.52, 0.84) | 0.55 (0.34, 0.88) | 0.001 |
| Model 2c | 1.00 | 0.73 (0.59, 0.90) | 0.71 (0.56, 0.90) | 0.62 (0.38, 100) | 0.007 |
| Model 3d | 1.00 | 0.72 (0.58, 0.89) | 0.69 (0.54, 0.89) | 0.58 (0.36, 0.95) | 0.003 |
| Men | |||||
| No. of deaths | 176 | 392 | 163 | 25 | |
| Model 1b | 1.00 | 0.92 (0.77, 1.10) | 0.62 (0.50, 0.77) | 0.61 (0.40, 0.93) | 0.001 |
| Model 2c | 1.00 | 0.97 (0.81, 1.17) | 0.73 (0.58, 0.91) | 0.81 (0.52, 1.24) | 0.008 |
| Model 3d | 1.00 | 0.96 (0.80, 1.15) | 0.70 (0.56, 0.87) | 0.71 (0.45, 0.98) | <0.001 |
| Pooled | |||||
| Model 1b | 1.00 | 0.80 (0.70, 0.92) | 0.61 (0.52, 0.71) | 0.55 (0.40, 0.75) | <0.001 |
| Model 2c | 1.00 | 0.86 (0.75, 0.99) | 0.72 (0.61, 0.85) | 0.71 (0.52, 0.98) | <0.001 |
| Model 3d | 1.00 | 0.85 (0.74, 0.98) | 0.70 (0.59, 0.82) | 0.65 (0.47, 0.90) | <0.001 |
| GI cancer | |||||
| Women | |||||
| No. of deaths | 60 | 122 | 64 | 11 | |
| Model 1b | 1.00 | 0.77 (0.56, 1.05) | 0.64 (0.45, 0.92) | 0.67 (0.35, 1.28) | 0.026 |
| Model 2c | 1.00 | 0.80 (0.58, 1.09) | 0.72 (0.50, 1.04) | 0.82 (0.42, 1.59) | 0.156 |
| Model 3d | 1.00 | 0.79 (0.58, 1.08) | 0.70 (0.48, 1.01) | 0.76 (0.39, 1.49) | 0.104 |
| Men | |||||
| No. of deaths | 114 | 208 | 90 | 12 | |
| Model 1b | 1.00 | 0.76 (0.60, 0.95) | 0.53 (0.40, 0.70) | 0.45 (0.25, 0.82) | <0.001 |
| Model 2c | 1.00 | 0.79 (0.63, 0.99) | 0.61 (0.46, 0, 82) | 0.58 (0.32, 1.07) | 0.001 |
| Model 3d | 1.00 | 0.78 (0.62, 0.99) | 0.60 (0.45, 0.81) | 0.55 (0.30, 0.99) | <0.001 |
| Pooled | |||||
| Model 1b | 1.00 | 0.73 (0.61, 0.88) | 0.53 (0.43, 0.67) | 0.49 (0.32, 0.76) | <0.001 |
| Model 2c | 1.00 | 0.79 (0.66, 0.96) | 0.65 (0.52, 0.82) | 0.67 (0.43, 1.05) | <0.001 |
| Model 3d | 1.00 | 0.79 (0.65, 0.95) | 0.64 (0.51, 0.80) | 0.63 (0.40, 0.99) | <0.001 |
| Lung cancer | |||||
| Women | |||||
| No. of deaths | 5 | 6 | 8 | 0 | |
| Model 1b | 1.00 | 0.45 (0.14, 1.48) | 0.95 (0.30, 2.99) | 0 | 0.701 |
| Model 2c | 1.00 | 0.45 (0.14, 1.49) | 0.96 (0.30, 3.09) | 0 | 0.706 |
| Model 3d | 1.00 | 0.43(0.13, 1.44) | 0.85 (0.26, 2.81) | 0 | 0.536 |
| Men | |||||
| No. of deaths | 20 | 36 | 15 | 1 | |
| Model 1b | 1.00 | 0.75(0.43, 1.30) | 0.52 (0.26, 1.03) | 0.23 (0.30, 1.68) | 0.022 |
| Model 2c | 1.00 | 0.86(0.49, 1.51) | 0.67 (0.33, 1.35) | 0.34 (0.05, 2.65) | 0.157 |
| Model 3d | 1.00 | 0.86(0.49, 1.50) | 0.64 (0.32, 1.31) | 0.30 (0.04, 2.39) | 0.127 |
| Pooled | |||||
| Model 1b | 1.00 | 0.64(0.39, 1.05) | 0.54 (0.30, 0.96) | 0.15 (0.02, 1.10) | 0.008 |
| Model 2c | 1.00 | 0.77(0.46, 1.27) | 0.75 (0.41, 1.37) | 0.25 (0.03, 1.84) | 0.155 |
| Model 3d | 1.00 | 0.75(0.45, 1.25) | 0.71 (0.39, 1.30) | 0.20 (0.3, 1.57) | 0.101 |
| Other cancer | |||||
| Women | |||||
| No. of deaths | 69 | 129 | 80 | 9 | |
| Model 1b | 1.00 | 0.68 (0.51, 0.92) | 0.66 (0.47, 0.91) | 0.48 (0.24, 0.97) | 0.008 |
| Model 2c | 1.00 | 0.69 (0.52, 0.93) | 0.69 (0.49, 0.95) | 0.52 (0.26, 1.04) | 0.020 |
| Model 3d | 1.00 | 0.69 (51, 0.93) | 0.67 (0.48, 0.94) | 0.50 (0.24, 0.99) | 0.016 |
| Men | |||||
| No. of deaths | 42 | 148 | 58 | 12 | |
| Model 1b | 1.00 | 1.45 (1.03, 2.05) | 0.93 (0.62, 1.39) | 1.24 (0.65, 2.36) | 0.570 |
| Model 2c | 1.00 | 1.55 (1.10, 2.19) | 1.08 (0.72, 1.64) | 1.62 (0.84, 3.14) | 0.597 |
| Model 3d | 1.00 | 1.51 (1.07, 2.13) | 1.00 (0.66, 1.52) | 1.26 (0.64, 2.47) | 0.858 |
| Pooled | |||||
| Model 1b | 1.00 | 0.96 (0.77, 1.19) | 0.75 (0.58, 0.97) | 0.74 (0.46, 1.18) | 0.013 |
| Model 2c | 1.00 | 1.00 (0.80, 1.25) | 0.83 (0.64, 1.08) | 0.87 (0.54, 1.41) | 0.149 |
| Model 3d | 1.00 | 0.99 (0.79, 1.23) | 0.79 (0.61, 1.03) | 0.77 (0.47, 1.24) | 0.051 |
| Respiratory | |||||
| Women | |||||
| No. of deaths | 30 | 76 | 41 | 5 | |
| Model 1b | 1.00 | 1.02 (0.67, 1.56) | 0.94 (0.58, 1.52) | 0.70 (0.27, 1.82) | 0.502 |
| Model 2c | 1.00 | 1.06 (0.69, 1.62) | 1.12 (0.69, 1.80) | 1.07 (0.41, 2.81) | 0.672 |
| Model 3d | 1.00 | 1.03 (0.67, 1.58) | 1.03 (0.64, 1.68) | 0.81 (0.30, 2.17) | 0.902 |
| Men | |||||
| No. of deaths | 63 | 114 | 52 | 1 | |
| Model 1b | 1.00 | 0.81 (0.59, 1.10) | 0.66 (0.45, 0.96) | 0.08 (0.01, 0.55) | 0.001 |
| Model 2c | 1.00 | 0.87 (0.64, 1.19) | 0.83 (0.57, 1.21) | 0.12 (0.01, 0.93) | 0.056 |
| Model 3d | 1.00 | 0.88 (0.64, 1.20) | 0.84 (0.57, 1.22) | 0.13 (0.2, 0.94) | 0.062 |
| Pooled | |||||
| Model 1b | 1.00 | 0.84 (0.65, 1.07) | 0.69 (0.51, 0.92) | 0.27 (0.12, 0.61) | <0.001 |
| Model 2c | 1.00 | 0.94 (0.73, 1.20) | 0.92 (0.68, 1.23) | 0.47 (0.20, 1.08) | 0.190 |
| Model 3d | 1.00 | 0.92 (0.72, 1.19) | 0.89 (0.66, 1.19) | 0.41 (0.17, 0.95) | 0.104 |
| Infections | |||||
| Women | |||||
| No. of deaths | 22 | 51 | 35 | 6 | |
| Model 1b | 1.00 | 0.92 (0.56, 1.52) | 1.05 (0.61, 1.82) | 1.08 (0.44, 2.70) | 0.707 |
| Model 2c | 1.00 | 0.95 (0.57, 1.57) | 1.13 (0.65, 1.96) | 1.22 (0.48, 3.08) | 0.514 |
| Model 3d | 1.00 | 0.92 (0.56, 1.52) | 1.03 (0.59, 1.80) | 0.96 (0.37, 2.48) | 0.908 |
| Men | |||||
| No. of deaths | 36 | 61 | 37 | 4 | |
| Model 1b | 1.00 | 0.74 (0.49, 1.12) | 0.78 (0.49, 1.26) | 0.51 (0.18, 1.45) | 0.189 |
| Model 2c | 1.00 | 0.79 (0.52, 1.20) | 0.98 (0.61, 1.59) | 0.77 (0.27, 2.21) | 0.836 |
| Model 3d | 1.00 | 0.78 (0.51, 1.17) | 0.90 (0.55, 1.45) | 0.61 (0.21, 1.77) | 0.478 |
| Pooled | |||||
| Model 1b | 1.00 | 0.79 (0.57, 1.08) | 0.84 (0.59, 1.20) | 0.70 (0.36, 1.37) | 0.304 |
| Model 2c | 1.00 | 0.85 (0.62, 1.17) | 1.03 (0.72, 1.48) | 0.97 (0.49, 1.92) | 0.795 |
| Model 3d | 1.00 | 0.83 (0.60, 1.13) | 0.94 (0.65, 1.35) | 0.76(0.38, 1.53) | 0.629 |
| Injuries | |||||
| Women | |||||
| No. of deaths | 15 | 56 | 32 | 4 | |
| Model 1b | 1.00 | 1.42 (0.80, 2.51) | 1.31 (0.70, 2.44) | 1.13 (0.37, 3.43) | 0.688 |
| Model 2c | 1.00 | 1.43 (0.81, 2.54) | 1.34 (0.71, 2.53) | 1.18 (0.38, 3.61) | 0.617 |
| Model 3d | 1.00 | 1.45 (0.82, 2.58) | 1.39 (0.74, 2.63) | 1.32 (0.42, 4.1) | 0.470 |
| Men | |||||
| No. of deaths | 50 | 104 | 57 | 7 | |
| Model 1b | 1.00 | 0.80 (0.57, 1.12) | 0.68 (0.46, 1.00) | 0.59 (0.27, 1.31) | 0.061 |
| Model 2c | 1.00 | 0.84 (0.60, 1.18) | 0.78 (0.52, 1.16) | 0.74 (0.33, 1.65) | 0.220 |
| Model 3d | 1.00 | 0.83 (0.59, 1.16) | 0.75 (0.50, 1.11) | 0.64 (0.29, 1.47) | 0.131 |
| Pooled | |||||
| Model 1b | 1.00 | 0.90 (0.67, 1.20) | 0.76 (0.55, 1.05) | 0.65 (0.34, 1.24) | 0.068 |
| Model 2c | 1.00 | 0.98 (0.73, 1.30) | 0.91 (0.65, 1.27) | 0.84 (0.44, 1.60) | 0.463 |
| Model 3d | 1.00 | 0.97 (0.73, 1.30) | 0.90 (0.64, 1.25) | 0.81 (0.42, 1.56) | 0.401 |
| Other causes | |||||
| Women | |||||
| No. of deaths | 52 | 129 | 90 | 22 | |
| Model 1b | 1.00 | 0.96 (0.70, 1.33) | 1.10 (0.77, 1.55) | 1.62 (0.97, 2.67) | 0.086 |
| Model 2c | 1.00 | 1.00 (0.73, 1.38) | 1.20 (0.84, 1.72) | 1.97 (1.17, 3.23) | 0.016 |
| Model 3d | 1.00 | 0.91 (0.66, 1.26) | 0.94 (0.66, 1.34) | 1.11 (0.65, 1.89) | 0.834 |
| Men | |||||
| No. of deaths | 58 | 151 | 106 | 13 | |
| Model 1b | 1.00 | 1.11 (0.82, 1.50) | 1.31 (0.95, 1.82) | 1.00 (0.55, 1, 83) | 0.247 |
| Model 2c | 1.00 | 1.16 (0.85, 1.57) | 1.48 (1.05, 2.06) | 1.23 (0.67, 2, 28) | 0.042 |
| Model 3d | 1.00 | 1.09 (0.80, 1.48) | 1.23 (0.87, 1.72) | 0.75 (0.40, 1.41) | 0.819 |
| Pooled | |||||
| Model 1b | 1.00 | 1.02 (0.82, 1.27) | 1.16 (0.92, 1.47) | 1.27 (0.87, 1.86) | 0.086 |
| Model 2c | 1.00 | 1.80 (0.87, 1.34) | 1.33 (1.05, 1.70) | 1.58 (1.00, 2.34) | 0.002 |
| Model 3d | 1.00 | 1.00 (0.80, 1.25) | 1.07 (0.84, 1.38) | 0.93 (0.63, 1.34) | 0.826 |
DS, DASH score; GI, gastrointestinal.
Cox proportional hazards regression models for estimating HRs and 95% CIs.
Model 1: adjusted for age and energy intake.
Model 2: additionally adjusted for gender (except when stratified by gender), BMI, smoking, opium use, wealth score and physical activity.
Model 3: additionally adjusted for history of diabetes, history of hypertension.
Figure 2.
Multivariate HRs of DASH score categories for CVD according to risk factor status at baseline (Cox proportional hazards regression models for estimating HRs and 95% CIs, multivariable models were adjusted for age, energy intake, sex, BMI, smoking, opium use, wealth score, physical activity, history of diabetes and history of hypertension, except for the respective stratifying factor). Data are reported as HR (95% CI). DASH score categories: DS1: 9–20, DS2: 21–25, DS3: 26–30, and DS4: 31–39. A, BMI <25 vs 25–30 and ≥30 (P = 0.054 for interaction); B, age <50 years vs ≥50 years (P = 0.440 for interaction); C, non-smokers vs smokers (P = 0.029 for interaction); D, wealth score below median vs above median (P = 0.565 for interaction); E, no diabetes vs diabetes (P = 0.625 for interaction); and F, no hypertension vs hypertension (P = 0.234 for interaction). Ref indicates reference group.
Among deaths from cancer, after controlling for potential confounders, adherence to DASH score was associated with a diminished risk of all cancers (HR: 0.65; 95% CI: 0.47, 0.90; P for trend <0.001), gastrointestinal cancers (HR: 0.55; 95% CI: 0.30, 0.99 for men, and HR: 0.63; 95% CI: 0.40, 0.99 for pooled data; P for trend <0.001). Furthermore, women with the highest DASH score were 50% less likely to die from other cancers (HR: 0.50; 95% CI: 0.24, 0.99; P for trend =0.016). However, there was no significant association between DASH score and lung cancer and other cancer deaths.
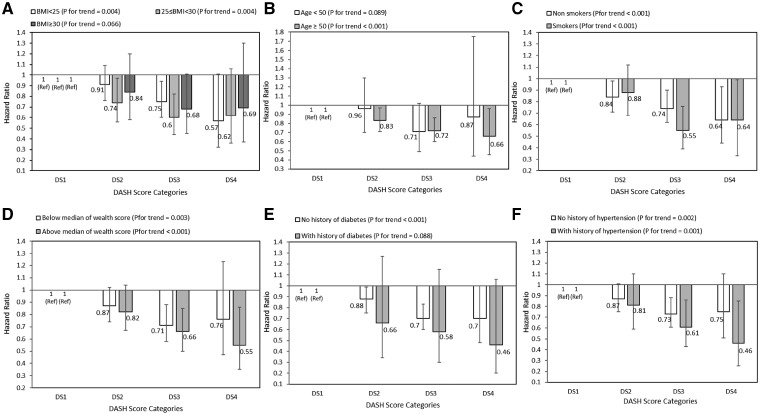
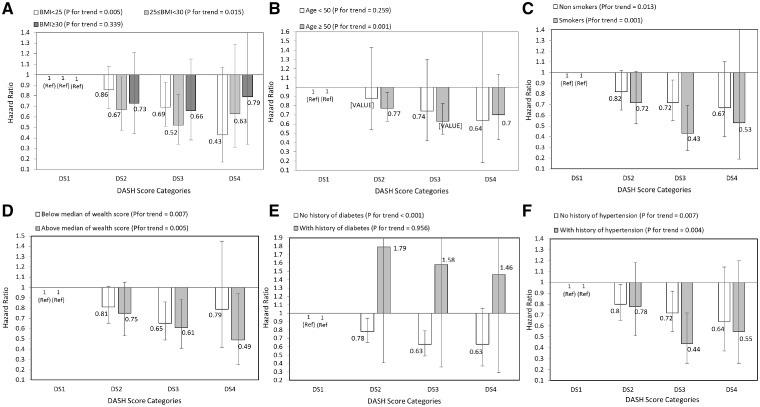
After stratifying analysis by selected risk factors at baseline, inverse significant association between DASH score and all cancer deaths was stronger among people aged above 50 years than those under 50 years, in those above the median wealth score than those below it, in participants without diabetes than those with diabetes, and in those with hypertension rather than normotensive participants (Figure 3). Additionally, adherence to DASH diet and gastrointestinal cancer mortality were similar across all strata, except across wealth score strata. A stronger inverse association was observed in individuals with an above-median wealth score compared with those with below-median wealth scores (Figure 4). The HRs and 95% CIs of these figures are provided in the Supplementary Tables 1–4, available as Supplementary data at IJE online.
Figure 3.
Multivariate hazard ratios of DASH score categories for all cancers according to risk factor status at baseline (Cox proportional hazards regression models for estimating HRs and 95% CIs, multivariable models were adjusted for age, energy intake, sex, BMI, smoking, opium use, wealth score, physical activity, history of diabetes and history of hypertension, except for the respective stratifying factor). Data are reported as HR (95% CI). DASH score categories: DS1: 9–20, DS2: 21–25, DS3: 26–30, and DS4: 31–39. A, BMI <25 vs 25–30 and ≥30 (P = 0.917 for interaction); B, age <50 years vs ≥50 years (P = 0.562 for interaction); C, non-smokers vs smokers (P = 0.172 for interaction); D, wealth score below median vs above median (P = 0.657 for interaction); E, no diabetes vs diabetes (P = 0.751 for interaction); and F, no hypertension vs hypertension (P = 0.918 for interaction). Ref indicates reference group.
Figure 4.
Multivariate hazard ratios of DASH score categories for all gastrointestinal cancers according to risk factor status at baseline (Cox proportional hazards regression models for estimating HRs and 95% CIs, multivariable models were adjusted for age, energy intake, sex, BMI, smoking, opium use, wealth score, physical activity, history of diabetes and history of hypertension, except for the respective stratifying factor). Data are reported as HR (95% CI). DASH score categories: DS1: 9–20, DS2: 21–25, DS3: 26–30, and DS4: 31–39. A, BMI <25 vs 25–30 and ≥30 (P = 0.801 for interaction); B, age <50 years vs ≥50 years (P = 0.970 for interaction); C, non-smokers vs smokers (P = 0.134 for interaction); D, wealth score below median vs above median (P = 0.637 for interaction); E, no diabetes vs diabetes (P = 0.527 for interaction); and F, no hypertension vs hypertension (P = 0.568 for interaction). Ref indicates reference group.
In respect of each component of the DASH score, the most noticeable finding was that vegetables, nuts and legumes and low-fat dairy intakes were associated with greater reduced risk of overall mortality and all cancer, gastrointestinal cancer and other cancer mortality (Supplementary Table 5, available as Supplementary data at IJE online). Association between DASH score and deaths from respiratory disease showed that the adjusted HR tended to be significant only in men (HR: 0.13; 95% CI: 0.2, 0.94; P for trend =0.062). After controlling for potential confounders, there was no significant association between adherence to DASH score and mortality from infections, injuries and other causes in either sex (Table 3).
Discussion
In this large prospective cohort study, we found greater adherence to a DASH diet pattern was associated with a 14–35% reduced risk of death overall and mortality due to all cancers. Furthermore, we found no association between the DASH diet pattern and risk of CVD mortality. A sex-specific analysis revealed that the highest versus lowest categories of DASH score were associated with a 45–87% reduced risk of gastrointestinal cancer mortality and respiratory disease deaths only in men, and with a 50% lower risk of mortality due to other cancers only in women.
To our knowledge, this is the first cohort evaluating the association between adherence to a DASH-style diet and mortality in the Middle East, with its special dietary properties. Our finding was consistent with previous investigations, in which greater accordance with the DASH-style diet was associated with decreased risk of overall and cancer mortality.24 However, we found no significant associations between DASH score and death from cardiovascular causes; such findings have also been reported in the Nurses’ Health Study and Women’s Health Initiative cohort study,5,25 in contrast to the previously reported results from the Multiethnic Cohort and the Singapore Chinese Heath Study, in which DASH score was more strongly associated with CVD than with cancer mortality.26,27 In our study, this association was observed in individuals younger than 50 years of age and smokers. This discrepancy may be due to variations in the baseline risk and dissimilarities in measurement of contributory factors in diverse populations. Furthermore, the limitation of FFQs for assessment of sodium intake, as a pivotal factor in the DASH score calculation, may have contributed to such a finding.
Conformity to the DASH diet and its association with risk of gastrointestinal cancer and other cancer (except lung cancer) mortality were considerably different between the sexes. Additionally, no association was revealed for lung cancer mortality in either men or women. To our knowledge, the association between DASH score and mortality from specific cancers has been assessed in few studies, indicating conflicting results.8,28–32 Discrepancy in the results might be explained by the fact that cancer is a heterogeneous endpoint, and nutritional factors may have played a more notable role in the cause of specific cancers. It is difficult to explain the sex-based differences in findings, but they might be related to heterogeneity of the baseline risk and dissimilarity of the hormone-dependent cancers between sexes.
Associations between some components of a DASH-style diet and mortality have also been previously shown.12,13,33–35 However, our analysis of individual food items of the DASH diet suggested that the aggregate of food items, instead of any specific food items, is associated with mortality risk. This might be because of nutritional factors interacting with each other. Several lines of evidence have revealed the role of dietary factors in respiratory diseases.36,37 However, studies evaluating the association between dietary patterns and respiratory death are limited. Our study is in agreement with Neelakantan et al.,26 who showed that higher adherence to the DASH diet pattern was associated with a noticeably decreased risk of respiratory disease mortality in men, although with a non-significant trend across categories of the DASH score. In spite of limited studies related to the role of diet in infectious diseases,38,39 we noted no association between adherence to the DASH diet and mortality due to infectious disease. Likewise, no association was observed between DASH diet pattern and mortality due to injury and other causes.
The DASH diet may exert its health benefits through oxidative stress and chronic inflammation modulation. Adherence to the DASH-style diet is linked to increased antioxidant capacity compared with a normal diet,40,41 implying that this dietary pattern might be attaining the mixed effect of dietary antioxidants, which may, partly, clarify the lower mortality risk. Additionally, other important dietary elements of the DASH diet, such as fibre and a low exposure to N-nitroso compounds, heterocyclic amines, polycyclic aromatic hydrocarbons and heme-iron caused by limited intake of red and processed meat, potentially inhibit initiation and progression of cancers.42–44 Furthermore, an association between low-grade inflammatory state and risk of CVD and neoplasms has been proposed by several studies.45,46 DASH diet consumption has also been indicated to beneficially affect systemic inflammation in individuals at risk of CVD or cancer.47–49
It seems that the inverse association we found between DASH score and mortality is less likely to be due to confounding by an overall healthy lifestyle, because it was observed despite the large differences in underlying confounding structure between the European/American studies and the current population. In our study, the people in the highest level of DASH score had higher BMI, alcohol consumption and a history of diabetes or hypertension. In contrast, in most previous reports from Western populations,8,50,51 people with higher adherence to the DASH diet were more likely to have healthier lifestyles. Even though opium use is known to be an important risk factor for death in this population,52 the inverse association between DASH score and mortality did not change after adjustment for opium use and other potential confounders, suggesting that this association was independent of other known confounders and risk factors.
Our results showed that higher conformity with the DASH diet is associated with lower risk of overall mortality and mortality from CVD among current smokers, who are regarded as individuals with high oxidative stress status.53 This would appear to indicate that people with selected risk factors may benefit more than others from adherence to a DASH dietary pattern. Likewise, previous studies reported that risk was lower among smokers with the highest adherence to the plant-based diets.54,55 This finding might be due to the ability of some of the main dietary recommendations (e.g. high consumption of vegetables and fruits) to detoxify and scavenge the reactive oxygen species induced by smoking.56,57 Furthermore, patterns of associations between the DASH score and mortality differed by age. In individuals younger than 50 years, in comparison to those aged 50 years or older, stronger associations with all-cause and CVD mortality and weaker association with cancer mortality were seen. Further studies are required to elucidate these varying patterns.
The findings of our study indicate that the inverse association between DASH score and mortality was stronger among participants who had a greater wealth score. It has been proposed that greater wealth might be accompanied by beneficial factors, such as better social environments and lower chronic stress, which may impact outcomes of health.58 Furthermore, distribution of the population across quartiles of DS diet was different: the lower number of participants were in the highest DS category, compared with lower DS categories. This might be due to low socio-economic status of the majority of our study participants. It has been shown that economic access to foods is an important factor for adherence to the DASH diet.59
The strengths of this study include its prospective nature, large sample size and high participation rate, the objective measurement of anthropometric data, the relatively long follow-up time and excellent (99.1 %) retention rate, and the central adjudication of deaths. Moreover, this study is the first to assess the association between adherence to a DASH diet and mortality in a general population in the Middle East region. Dietary intake in Middle-Eastern populations has its own unique properties, including large portion sizes and high intake of refined grains (white rice and bread). Furthermore, studies in these countries can provide unique opportunities to find associations between dietary patterns and illnesses because of the large socio-economic variation in the population.60
Limitations of this study include the potential weaknesses of FFQs, such as measurement error and recall bias. However, the FFQ is the most suitable tool for dietary assessment in cohort studies. Furthermore, sodium intake was not estimated accurately because an accurate estimation required the measurement of 24-h urine sodium excretion, and it is difficult to collect 24-h urine samples in such a large population. In addition, the nutrient database that has been used in the analysis for dietary intake data may be inappropriate for Iranian foods; however, we added our national dietary data for the analysis of our national food items. Moreover, due to the observational design of the study, we cannot conclude that the observed associations reflect cause and effect; however, the consistency of our results with previous studies, in the presence of different underlying patterns of confounding factors, may suggest causality. In conclusion, a higher adherence to a DASH-style dietary pattern was significantly associated with decreased mortality in Iran. This finding suggests that maintaining a diet similar to the DASH diet could help reduce the risk of death from chronic diseases in this and similar countries.
Funding
This work was supported financially by Digestive Disease Research Center (DDRC).
Supplementary Material
Acknowledgements
The authors wish to thank all the study participants and the local health workers (Behvarz) for their cooperation. We also would like to show our appreciation to all of the follow-up team. We received special support from the Social Security Organization of Iran, Golestan branch. This work was also supported by Tehran University of Medical Sciences, Cancer Research UK, the Intramural Research Program of the US National Cancer Institute at the NIH, and through various collaborative research agreements with the International Agency for Research on Cancer.
Conflict of interest: None declared.
References
- 1. Willett W. Nutritional Epidemiology. New York: Oxford University Press, 2012. [Google Scholar]
- 2. Vogt TM, Appel LJ, Obarzanek E. et al. Dietary approaches to stop hypertension: rationale, design, and methods. DASH Collaborative Research Group. J Am Diet Assoc 1999;99:S12–8. [DOI] [PubMed] [Google Scholar]
- 3. Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB.. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med 2008;168:713–20. [DOI] [PubMed] [Google Scholar]
- 4. Liese AD, Bortsov A, Gunther AL. et al. Association of DASH diet with cardiovascular risk factors in youth with diabetes mellitus: the SEARCH for Diabetes in Youth study. Circulation 2011;123:1410–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bertoia ML, Triche EW, Michaud DS. et al. Mediterranean and Dietary Approaches to Stop Hypertension dietary patterns and risk of sudden cardiac death in postmenopausal women. Am J Clin Nutr 2014;99:344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hekmatdoost A, Shamsipour A, Meibodi M, Gheibizadeh N, Eslamparast T, Poustchi H.. Adherence to the Dietary Approaches to Stop Hypertension (DASH) and risk of Nonalcoholic Fatty Liver Disease. Int J Food Sci Nutr 2016;67:1024–9. [DOI] [PubMed] [Google Scholar]
- 7. Liese AD, Nichols M, Sun X, D'Agostino RB Jr, Haffner SM.. Adherence to the DASH Diet is inversely associated with incidence of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes Care 2009;32:1434–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fung TT, Hu FB, Wu K, Chiuve SE, Fuchs CS, Giovannucci E.. The Mediterranean and Dietary Approaches to Stop Hypertension (DASH) diets and colorectal cancer. Am J Clin Nutr 2010;92:1429–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fung TT, Hu FB, Hankinson SE, Willett WC, Holmes MD.. Low-carbohydrate diets, dietary approaches to stop hypertension-style diets, and the risk of postmenopausal breast cancer. Am J Epidemiol 2011;174:652–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang X, Ouyang Y, Liu J. et al. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 2014;349:g4490.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hastert TA, Beresford SA, Sheppard L, White E.. Adherence to the WCRF/AICR cancer prevention recommendations and cancer-specific mortality: results from the Vitamins and Lifestyle (VITAL) study. Cancer Causes Control 2014;25:541–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eslamparast T, Sharafkhah M, Poustchi H. et al. Nut consumption and total and cause-specific mortality: results from the Golestan Cohort Study. Int J Epidemiol 2017;46:75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Golledge J, Moxon JV, Jones RE. et al. Reported amount of salt added to food is associated with increased all-cause and cancer-related mortality in older men in a prospective cohort study. J Nutr Health Aging 2015;19:805–11. [DOI] [PubMed] [Google Scholar]
- 14. Fuchs MA, Sato K, Niedzwiecki D. et al. Sugar-sweetened beverage intake and cancer recurrence and survival in CALGB 89803 (Alliance). PLoS One 2014;9:e99816.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pourshams A, Khademi H, Malekshah AF. et al. Cohort profile: The Golestan Cohort Study--a prospective study of oesophageal cancer in northern Iran. Int J Epidemiol 2010;39:52–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Malekshah AF, Kimiagar M, Saadatian-Elahi M. et al. Validity and reliability of a new food frequency questionnaire compared to 24 h recalls and biochemical measurements: pilot phase of Golestan cohort study of esophageal cancer. Eur J Clin Nutr 2006;60:971–7. [DOI] [PubMed] [Google Scholar]
- 17. Ghaffarpour M, Houshiar-Rad A, Kianfar H.. The manual for household measures, cooking yields factors and edible portion of foods. Tehran: Nashre Olume Keshavarzy. 1999;7:213. [Google Scholar]
- 18. Hashemian M, Poustchi H, Abnet CC. et al. Dietary intake of minerals and risk of esophageal squamous cell carcinoma: results from the Golestan Cohort Study. Am J Clin Nutr 2015;102:102–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Organization WH. STEPwise Approach to Surveillance (STEPS), Vol. 2016. Geneva, Switzerland: WHO, 2016. [Google Scholar]
- 20. Nalini M, Oranuba E, Poustchi H. et al. Causes of premature death and their associated risk factors in the Golestan Cohort Study, Iran. BMJ Open 2018;8:e021479.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Golozar A, Khademi H, Kamangar F. et al. Diabetes mellitus and its correlates in an Iranian adult population. PLoS One 2011;6:e26725.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Islami F, Kamangar F, Nasrollahzadeh D. et al. Socio-economic status and oesophageal cancer: results from a population-based case-control study in a high-risk area. Int J Epidemiol 2009;38:978–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Khademi H, Etemadi A, Kamangar F. et al. Verbal autopsy: reliability and validity estimates for causes of death in the Golestan Cohort Study in Iran. PLos One 2010;5:e11183.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schwingshackl L, Bogensberger B, Hoffmann G.. Diet quality as assessed by the healthy eating index, alternate healthy eating index, dietary approaches to stop hypertension score, and health outcomes: an updated systematic review and meta-analysis of cohort studies. J Acad Nutr Diet 2018;118:74–100. e111. [DOI] [PubMed] [Google Scholar]
- 25. Sotos-Prieto M, Bhupathiraju SN, Mattei J. et al. Association of changes in diet quality with total and cause-specific mortality. N Engl J Med 2017;377:143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Neelakantan N, Koh W-P, Yuan J-M, van Dam RM.. Diet-quality indexes are associated with a lower risk of cardiovascular, respiratory, and all-cause mortality among Chinese adults. J Nutr 2018;148:1323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Harmon BE, Boushey CJ, Shvetsov YB. et al. Associations of key diet-quality indexes with mortality in the Multiethnic Cohort: the Dietary Patterns Methods Project.Am J Clin Nutr 2015;101:587–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Izano MA, Fung TT, Chiuve SS, Hu FB, Holmes MD.. Are diet quality scores after breast cancer diagnosis associated with improved breast cancer survival? Nutr Cancer 2013;65:820–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schneider L, Su LJ, Arab L. et al. Dietary patterns based on the Mediterranean diet and DASH diet are inversely associated with high aggressive prostate cancer in PCaP. Ann Epidemiol 2019;29:16–22. [DOI] [PubMed] [Google Scholar]
- 30. Du M, Liu SH, Mitchell C, Fung TT.. Associations between diet quality scores and risk of postmenopausal estrogen receptor-negative breast cancer: A systematic review. J Nutr 2018;148:100–8. [DOI] [PubMed] [Google Scholar]
- 31. Dixon LB, Subar AF, Peters U. et al. Adherence to the USDA Food Guide, DASH Eating Plan, and Mediterranean dietary pattern reduces risk of colorectal adenoma. J Nutr 2007;137:2443–50. [DOI] [PubMed] [Google Scholar]
- 32. Vargas AJ, Neuhouser ML, George SM. et al. Diet quality and colorectal cancer risk in the Women's Health Initiative Observational Study. Am J Epidemiol 2016;184:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aune D, Giovannucci E, Boffetta P. et al. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality—a systematic review and dose-response meta-analysis of prospective studies. Int J Epidemiol 2017;46:1029–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Aune D, Keum N, Giovannucci E. et al. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: systematic review and dose-response meta-analysis of prospective studies. BMJ 2016;353:i2716.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nothlings U, Schulze MB, Weikert C. et al. Intake of vegetables, legumes, and fruit, and risk for all-cause, cardiovascular, and cancer mortality in a European diabetic population. J Nutr 2008;138:775–81. [DOI] [PubMed] [Google Scholar]
- 36. Zhai T, Li S, Hu W, Li D, Leng S.. Potential micronutrients and phytochemicals against the pathogenesis of chronic obstructive pulmonary disease and lung cancer. Nutrients 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Varraso R, Fung TT, Barr RG, Hu FB, Willett W, Camargo CA.. Prospective study of dietary patterns and chronic obstructive pulmonary disease among US women.Am J Clin Nutr 2007;86:488–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sinclair D, Abba K, Grobler L, Sudarsanam TD.. Nutritional supplements for people being treated for active tuberculosis. Cochrane Database Syst Rev 2011;11:CD006086. [DOI] [PubMed] [Google Scholar]
- 39. Gray MS, Wang HE, Martin KD. et al. Adherence to Mediterranean-style diet and risk of sepsis in the REasons for Geographic and Racial Differences in Stroke (REGARDS) cohort. Br J Nutr 2018;120:1415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lopes HF, Martin KL, Nashar K, Morrow JD, Goodfriend TL, Egan BM.. DASH diet lowers blood pressure and lipid-induced oxidative stress in obesity. Hypertension 2003;41:422–30. [DOI] [PubMed] [Google Scholar]
- 41. Hummel SL, Seymour EM, Brook RD. et al. Low-sodium dietary approaches to stop hypertension diet reduces blood pressure, arterial stiffness, and oxidative stress in hypertensive heart failure with preserved ejection fraction. Hypertension 2012;60:1200–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Abnet CC, Corley DA, Freedman ND, Kamangar F.. Diet and upper gastrointestinal malignancies. Gastroenterology 2015;148:1234–43. e1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ley SH, Sun Q, Willett WC. et al. Associations between red meat intake and biomarkers of inflammation and glucose metabolism in women. Am J Clin Nutr 2014;99:352–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cross AJ, Leitzmann MF, Gail MH, Hollenbeck AR, Schatzkin A, Sinha R.. A prospective study of red and processed meat intake in relation to cancer risk. PLoS Med 2007;4:e325.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pikarsky E, Porat RM, Stein I. et al. NF-κB functions as a tumour promoter in inflammation-associated cancer. Nature 2004;431:461.. [DOI] [PubMed] [Google Scholar]
- 46. Libby P. Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr 2006;83:456S–60S. [DOI] [PubMed] [Google Scholar]
- 47. Azadbakht L, Surkan PJ, Esmaillzadeh A, Willett WC.. The dietary approaches to stop hypertension eating plan affects C-reactive protein, coagulation abnormalities, and hepatic function tests among type 2 diabetic patients. J Nutr 2011;4141:1083–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hodson L, Harnden K, Roberts R, Dennis A, Frayn K.. Does the DASH diet lower blood pressure by altering peripheral vascular function? J Hum Hypertens 2010;24:312.. [DOI] [PubMed] [Google Scholar]
- 49. Asemi Z, Esmaillzadeh A.. DASH diet, insulin resistance, and serum hs-CRP in polycystic ovary syndrome: a randomized controlled clinical trial. Horm Metab Res 2015;47:232–8. [DOI] [PubMed] [Google Scholar]
- 50. Reedy J, Krebs-Smith SM, Miller PE. et al. Higher diet quality is associated with decreased risk of all-cause, cardiovascular disease, and cancer mortality among older adults. J Nutr 2014;144:881–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Miller PE, Cross A, Subar AF. et al. Comparison of 4 established DASH diet indexes: examining associations of index scores and colorectal cancer. The American Journal of Clinical Nutrition 2013;ajcn. 063602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Khademi H, Malekzadeh R, Pourshams A. et al. Opium use and mortality in Golestan Cohort Study: Prospective cohort study of 50 000 adults in Iran. BMJ 2012;344:e2502.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yanbaeva DG, Dentener MA, Creutzberg EC, Wesseling G, Wouters EF.. Systemic effects of smoking. Chest 2007;131:1557–66. [DOI] [PubMed] [Google Scholar]
- 54. Mitrou PN, Kipnis V, Thiébaut AC. et al. Mediterranean dietary pattern and prediction of all-cause mortality in a US population: results from the NIH-AARP Diet and Health Study. Arch Intern Med 2007;167:2461–8. [DOI] [PubMed] [Google Scholar]
- 55. Haveman-Nies A, de GL, Burema J, Cruz JAA, Osler M, van SW.. Dietary quality and lifestyle factors in relation to 10-year mortality in older Europeans: The SENECA study. Am J Epidemiol 2002;156:962–8. [DOI] [PubMed] [Google Scholar]
- 56. Struijk EA, May AM, Wezenbeek NL. et al. Adherence to dietary guidelines and cardiovascular disease risk in the EPIC-NL cohort. Int J Cardiol 2014;176:354–9. [DOI] [PubMed] [Google Scholar]
- 57. Grassi D, Desideri G, Ferri L, Aggio A, Tiberti S, Ferri C.. Oxidative stress and endothelial dysfunction: say NO to cigarette smoking! Curr Pharm Des 2010;16:2539–50. [DOI] [PubMed] [Google Scholar]
- 58. Hajat A, Kaufman JS, Rose KM, Siddiqi A, Thomas JC.. Long-term effects of wealth on mortality and self-rated health status. Am J Epidemiol 2011;173:192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mackenbach JD, Burgoine T, Lakerveld J. et al. Accessibility and affordability of supermarkets: Associations with the DASH diet. Am J Prev Med 2017;53:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Willett WC, Koplan JP, Nugent R, Dusenbury C, Puska P, Gaziano TA.. Prevention of chronic disease by means of diet and lifestyle changes In: Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, Jha P, Mills A, Musgrove P. (eds). Disease Control Priorities in Developing Countries. 2nd edn Washington (DC): World Bank, 2006. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.