Abstract
Objective
Several studies report a polygenic component of risk for Alzheimer’s disease. Understanding whether this polygenic signal is associated with educational, cognitive and behavioural outcomes in children could provide an earlier window for intervention.
Methods
We examined whether polygenic risk scores (PRS) at varying P-value thresholds in children from the Avon Longitudinal Study of Parents and Children were associated with academic achievement, cognitive and behavioural measures in childhood and adolescence.
Results
We did not detect any evidence that the genome-wide significant PRS (5x10-8) were associated with these outcomes. PRS at the highest P-value threshold examined (P ≤ 5x10-1) were associated with lower academic achievement in adolescents (Key Stage 3; β: -0.03; 95% confidence interval: -0.05, -0.003) but the effect was attenuated when single nucleotide polymorphisms (SNPs) associated with educational attainment were removed. These PRS were associated with lower IQ (β: -0.04; 95% CI: -0.07, -0.02) at age 8 years with the effect remaining after removing SNPs associated with educational attainment.
Conclusions
SNPs mediating the biological effects of Alzheimer’s disease are unlikely to operate early in life. The evidence of association between PRS for Alzheimer’s disease at liberal thresholds and cognitive measures suggest shared genetic pathways between Alzheimer’s disease, academic achievement and cognition.
Keywords: ALSPAC, Alzheimer’s disease, behavioural, cognitive, polygenic risk scores
Key Messages
This is the first time that the effect of genetic variants for Alzheimer’s disease on academic achievement, cognitive and behavioural measures are being investigated in a large sample of children.
Genetic variants most strongly associated with Alzheimer’s disease are not associated with any of examined outcomes.
Alzheimer’s disease may share genetic pathways with cognition and academic achievement, as indicated by findings at liberal P-value thresholds.
Individuals with substantially increased risk of Alzheimer’s disease later in life have similar academic achievement to other individuals in the population.
Our study suggests that the preclinical effects for Alzheimer’s disease are unlikely to operate in childhood.
Introduction
Alzheimer’s disease is a heritable neurodegenerative disease which, in addition to other dementia forms, affects 47 million individuals worldwide.1 The long prodromal phase is characterized by cognitive decline and behavioural disturbances.2,3 As Alzheimer’s disease exerts a heavy socioeconomic burden,4 identifying modifiable factors earlier in life is important for preventing or delaying the onset of the disease.
Genome-wide association studies (GWAS)5 have identified several single nucleotide polymorphisms (SNPs) associated with late-onset Alzheimer’s disease, all exerting low to modest effects [except for the ε4 allele in the apolipoprotein E (ApoE) gene)].6,7 The effects of common genetic risk variants for complex diseases, including Alzheimer’s disease, can be considered en masse to calculate a polygenic risk score (PRS) for disease.8–10 These scores can be used as an indicator of genetic risk for Alzheimer’s disease (irrespective of whether an individual will develop Alzheimer’s disease) to investigate the genetic overlap between Alzheimer’s disease and other diseases or traits. The overall SNP heritability of Alzheimer’s disease (24–35%) identified in GWAS11 is higher when SNPs of small effect size are also considered, indicating that there are many SNPs below the genome-wide level of significance contributing to increasing genetic risk for Alzheimer’s disease.8
Pathophysiological changes resulting in gradual cognitive and functional decline can occur more than two decades before the onset of clinical symptoms.12,13 This presents a challenge in the development of effective treatments and highlights the need for intervention preceding the initiation of the disease process. There is an established association between PRS for Alzheimer’s disease, cognitive outcomes and educational attainment in adults.14–16 However, the association between academic achievement, as well as cognitive outcomes, and behavioural difficulties in young ages is understudied.
In our study, we investigated whether a PRS for Alzheimer’s disease is associated with academic achievement at Key Stages 3, 4 and 5, childhood IQ at 8 and 15 years and behavioural difficulties at 9 and 12 years using a large population sample.
Methods
Participants
The Avon Longitudinal Study of Parents and Children (ALSPAC) is a prospective birth cohort study which recruited pregnant women residing in the former Avon Health Authority area with expected delivery dates between April 1991 and December 1992; 14 541 pregnant women were initially enrolled, with 14 062 children born. A detailed description of the cohort has been published previously.17,18 Detailed information on health and development of children and their parents was collected from regular clinic visits and completion of questionnaires. The study website contains details of all the data that are available through a fully searchable data dictionary [http://www.bris.ac.uk/alspac/researchers/data-access/data-dictionary/]. Ethical approval was obtained from the ALSPAC Law and Ethics Committee and the local ethics committees.
ALSPAC genetic data
A total of 9912 ALSPAC children were genotyped on the Illumina HumanHap550-quad SNP genotyping platform. After quality control (QC) assessment published elsewhere,19,20 imputation and restriction to one child per family, genetic data were available for 7977 individuals (QC procedures in Supplementary material, available as Supplementary data at IJE online).
Polygenic risk scores
PRS were computed according to the method described by the International Schizophrenia Consortium10 based on the summary statistics from the GWAS of Alzheimer’s disease by the IGAP consortium.5 Details about IGAP and PRS are in the Supplementary material, available as Supplementary data at IJE online. Our main analysis focused on P-value thresholds P ≤ 5x10-8, 5x10-2 and 5x10-1, although more thresholds were tested (Supplementary material, available as Supplementary data at IJE online). The number of SNPs in the PRS at each P-value threshold is provided in Supplementary Table 1, available as Supplementary data at IJE online. A list of 19 SNPs used to generate the PRS at the genome-wide significant threshold is provided in Table 1.
Table 1.
SNPs included in PRS with a genome-wide significance threshold as reported in IGAP stage 1
| Marker | Chromosome | Position | Nearest gene | A1 | A2 | OR |
|---|---|---|---|---|---|---|
| rs6656401 | 1 | 207692049 | CR1 | A | G | 1.17 |
| rs6733839 | 2 | 127892810 | BIN1 | T | C | 1.21 |
| rs35349669 | 2 | 234068476 | INPP5D | T | C | 1.07 |
| rs190982 | 5 | 88223420 | MEF2C | G | A | 0.92 |
| rs10948363 | 6 | 47487762 | CD2AP | G | A | 1.10 |
| rs2718058 | 7 | 37841534 | NME8 | G | A | 0.93 |
| rs1476679 | 7 | 100004446 | ZCWPW1 | C | T | 0.92 |
| rs11771145 | 7 | 143110762 | EPHA1 | A | G | 0.90 |
| rs28834970 | 8 | 27195121 | PTK2B | C | T | 1.10 |
| rs10838725 | 11 | 47557871 | CELF1 | C | T | 1.08 |
| rs983392 | 11 | 59923508 | MS4A6A | G | A | 0.90 |
| rs10792832 | 11 | 85867875 | PICALM | A | G | 0.88 |
| rs11218343 | 11 | 121435587 | SORL1 | C | T | 0.76 |
| rs17125944 | 14 | 53400629 | FERMT2 | C | T | 1.13 |
| rs10498633 | 14 | 92926952 | SLC24A4 | T | G | 0.90 |
| rs4147929 | 19 | 1063443 | ABCA7 | A | G | 1.14 |
| rs429358/rs7412 | 19 | 45411941/45412079 | APOE | ε4 | ε2/3 | 3.86/1.47 |
| rs7274581 | 20 | 55018260 | CASS4 | C | T | 0.87 |
Additional information provided: chromosomal and base pair position, nearest gene, minor allele (A1) and major allele (A2), odds ratio (OR).
Measures
Academic achievement
Academic achievement measures were attained through linkage to compulsory UK educational assessments from the National Pupil Database21 and were evaluated at three time points during the pupils’ education; Key Stages 3, 4 and 5. Key Stage 3 national tests are taken when children are 14 years old and include English, Mathematics and Science assessments. For Key Stage 4, General Certificate of Secondary Education (GCSE) examinations determine transition into post-compulsory education. Students study up to 12 subjects (eight on average) of which some are compulsory (e.g. English and Mathematics). The Key Stage 4 scores were analysed as the total point score, which is capped at the student’s eight best GCSE (and equivalent) qualifications.22 For Key Stage 5, examinations are taken when the students are 18 years old (A levels) and range from A* to E. Test scores at all Key Stages were provided as a scaled total points score. A binary variable was generated to investigate whether PRS was associated with progression to Key Stage 5 tests for children with available GCSE results.
Intelligence quotient
Total IQ scores were collected when children were 8 years old, using the computerized version of the Wechsler Intelligence Scale for Children (WISC-III).23 Verbal and performance subtests were administered, their scores were scaled to age, and the total IQ scores derived. At 15 years, children were administered the Vocabulary and Matrix Reasoning subcategories of the Wechsler Abbreviated Scale of Intelligence (WASI).24 The verbal, performance and total IQ scores are normative IQs, with a mean of 100 and a standard deviation of 15.24 Mean IQ differed from age 8 (IQ = 105.0) to age 15 (IQ = 92.4). A thorough investigation was carried out by ALSPAC, and it was concluded that there were no systematic errors in the way the WASI (or the WISC) was scored. These tests are designed for different age ranges and cannot be interpreted interchangeably or to reflect change over time.25
Behavioural problems
Behavioural problems in childhood were measured by the Strengths & Difficulties Questionnaire (SDQ),26 completed by mothers when the children were 9 and 12 years old. More details on SDQ can be found in Supplementary material, available as Supplementary data at IJE online. Due to the skewed nature of the SDQ variables (with most children demonstrating no problems), the five SDQ subscales were dichotomized as in previous studies.27,28
Examining the association between the PRS and the outcomes
Linear and ordinary logistic regression models were used for continuous and categorical outcomes, respectively. For the cognitive outcomes (IQ, academic achievement), z-scores were calculated to enable comparison of the magnitude of regression coefficients across outcomes and time points. Models were adjusted for age, sex and the first three ancestry-informative principal components (Model 1). Analyses were performed in Stata 14.29 As the outcome measures are highly interdependent, we used principal component analysis (PCA) to extract factors, using a cut-off threshold of an eigenvalue of ≥1 (Kaiser rule).30 Our results were interpreted according to the American Statistical Association guidance,31,32 by presenting raw summary statistics and using PCA to assist with interpreting our findings.
Sensitivity analysis
Results from Model 1 were compared with those based on scores that excluded SNPs within the ApoE gene (Chr. 19: 44 400–46 500 kb) (Model 2) due to the large effect sizes within that region, which may have been driving any observed associations. They were also compared with scores including the two SNPs tagging the ApoE gene (rs7412 and rs429358) (Supplementary material, available as Supplementary data at IJE online) (Model 3). To exclude the possibility that the effect of the PRS on academic achievement and cognitive outcomes is driven by SNPs associated with educational attainment, we repeated the analysis with a PRS excluding SNPs associated with educational attainment. at P ≤ 5x10-8 and at P ≤ 5x10-2. The number of SNPs removed at each PRS P-value threshold is provided in Supplementary Table 2, available as Supplementary data at IJE online. We also compared indicators of socioeconomic status (SES) for participants with and without genetic data (Supplementary Table 3, available as Supplementary data at IJE online).
Results
Sample description
The number of children with genetic data and total IQ scores was 5300 at 8 years and 3724 at 15 years. The number of children with educational outcomes was 3990 at 14 years (Key Stage 3) and 6535 at 15 years (Key Stage 4). Among the 6535 children who sat GCSE examinations, 3977 (61%) children also sat Key Stage 5 examinations. IQ scores and academic achievement variables were highly correlated, as described in Supplementary Table 4, available as Supplementary data at IJE online. A total of 5525 children had genetic data and a SDQ score. Detailed descriptive statistics are provided in Tables 2 and 3. We found four components to have an eigenvalue above 1 (adjusted_ P-value = 1.25x10-2). In total, these principal components explained 66% of variation in all the outcomes examined.
Table 2.
Characteristics of ALSPAC children with available genetic and IQ, or education data
| IQ/education measure | N | % male | Mean age in years (SD) | Mean score (SD) |
|---|---|---|---|---|
| IQ at age 8 | 5300 | 49.7% | 8.6 (0.3) | 105.0 (16.4) |
| IQ at age 15 | 3724 | 48.0% | 15.4 (0.3) | 92.4 (13.1) |
| Key Stage 3 | 6029 | 50.8% | 14.1 (0.3) | 106.5 (24.3) |
| Key Stage 4 | 6535 | 50.5% | 15.0 (0.04) | 133.8 (3.8) |
| Key Stage 5 | 3990 | 45.9% | 16.3 (0.6) | 764.3 (252.7) |
ALSPAC, Avon Longitudinal Study of Parents and Children; IQ, intelligence quotient; SD, standard deviation.
Table 3.
Descriptive statistics for SDQ at age 9
| Outcome | Range | Cut-off indicating problems | N | N (%) with behavioural problems |
|---|---|---|---|---|
| Total difficulties | 0 to 40 | ≥17 | 5525 | 246 (4.45%) |
| Hyperactivity | 0 to 10 | ≥7 | 5547 | 434 (7.82%) |
| Emotional symptoms | 0 to 10 | ≥5 | 5532 | 349 (6.31%) |
| Conduct problems | 0 to 10 | ≥4 | 5546 | 378 (6.82%) |
| Peer problems | 0 to 10 | ≥4 | 5541 | 441 (7.96%) |
| Prosocial behaviours | 0 to 10 | ≤6 | 5553 | 817 (14.7%) |
SDQ, strengths and difficulties questionnaire.
Academic achievement
At a SNP inclusion threshold of P ≤ 5 x 10-1, there was evidence that a PRS was associated with lower total points at Key Stage 3 [β: -0.03; 95% confidence interval (CI): -0.05, -0.003, P = 0.03, Table 4] and lower total points at Key Stage 4 (β: -0.03; 95% CI: -0.05, -0.003, P = 0.03, Table 4). The direction of effect sizes was similar for SNP inclusion threshold of P ≥ 5x10-2 (Table 4). The evidence of association when considering only genome-wide significant SNPs (P ≤ 5x10-8) was weak (Table 4). We could not detect any evidence that a PRS at the examined thresholds was associated with increased odds of individuals with GCSE results sitting Key Stage 5 examinations (Supplementary Table 6, available as Supplementary data at IJE online).
Table 4.
Associations between PRS for Alzheimer’s disease and educational, cognitive and behavioural measures
|
P-value threshold for SNP inclusion |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome | P = 5 x 10-8 (19 SNPs) | P = 5 x 10-2 (45 040) | P = 5 x 10-1 (240 803 SNPs) | ||||||
| Educational | β (95% CI) | P | R2 | β (95% CI) | P | R2 | β (95% CI) | P | R2 |
| Key Stage 3 points | 0.01 (-0.01, 0.04) | 0.33 | 2.2 x 10-3 | −0.002 (-0.03, 0.02) | 0.87 | 2.1 x 10-3 | −0.03 (-0.05, -0.003) | 0.03 | 2.9 x 10-3 |
| Key Stage 4 points | 0.001 (-0.02, 0.02) | 0.96 | 3.6 x 10-3 | −0.01 (-0.04, 0.01) | 0.31 | 3.7 x 10-3 | −0.03 (-0.05, -0.003) | 0.03 | 4.3 x 10-3 |
| Key Stage 5 points | 0.01 (-0.02, 0.04) | 0.55 | 1.6 x 10-3 | −0.01 (-0.04, 0.02) | 0.46 | 1.6 x 10-3 | −0.03 (-0.06, 0.003) | 0.08 | 2.2 x 10-3 |
| Cognitive | |||||||||
| Total IQ, age 8 | 0.0002 (-0.03, 0.03) | 0.99 | 2.7 x 10-4 | −0.03 (-0.06, -0.01) | 0.01 | 1.5 x 10-3 | −0.04 (-0.07, -0.02) | 0.002 | 2.1 x 10-3 |
| Total IQ, age 15 | −0.01 (-0.04, 0.02) | 0.44 | 1.3 x 10-3 | −0.01 (-0.05, 0.02) | 0.38 | 1.3 x 10-3 | −0.02 (-0.05, 0.01) | 0.20 | 1.5 x 10-3 |
| OR (95% CI) | P | R2 | OR (95% CI) | P | Pseudo-R2 | OR (95% CI) | P | Pseudo-R2 | |
| Behavioural | |||||||||
| Total difficulties, age 9 | 1.00 (0.89, 1.14) | 0.95 | 5.3 x 10-4 | 1.02 (0.90, 1.16) | 0.78 | 5.6 x 10-4 | 1.05 (0.92, 1.19) | 0.46 | 7.9 x 10-4 |
| Prosocial, age 9 | 0.97 (0.90, 1.04) | 0.36 | 1.4 x 10-3 | 0.98 (0.91, 1.06) | 0.58 | 1.3 x 10-3 | 1.00 (0.93, 1.07) | 0.95 | 1.2 x 10-3 |
| Hyperactivity, age 9 | 0.99 (0.90, 1.09) | 0.86 | 1.3 x 10-3 | 1.02 (0.92, 1.12) | 0.73 | 1.3 x 10-3 | 1.05 (0.96. 1.16) | 0.29 | 1.6 x 10-3 |
| Emotional symptoms, age 9 | 0.94 (0.84, 1.05) | 0.25 | 2.9 x 10-3 | 1.00 (0.90, 1.11) | 0.96 | 2.3 x 10-3 | 1.07 (0.96, 1.19) | 0.23 | 2.9 x 10-3 |
| Conduct problems, age 9 | 0.96 (0.86, 1.07) | 0.43 | 9.5 x 10-4 | 0.99 (0.89, 1.10) | 0.88 | 7.3 x 10-4 | 1.01 (0.91, 1.13) | 0.79 | 7.5 x 10-4 |
| Peer problems, age 9 | 1.01 (0.91, 1.11) | 0.91 | 4.5 x 10-4 | 0.93 (0.85, 1.03) | 0.16 | 1.1 x 10-3 | 0.97 (0.88, 1.07) | 0.53 | 5.8 x 10-4 |
| Total difficulties, age 12 | 1.01 (0.90, 1.14) | 0.85 | 1.7 x 10-3 | 1.05 (0.93, 1.17) | 0.46 | 2.0 x 10-3 | 1.04 (0.92, 1.16) | 0.56 | 1.9 x 10-3 |
| Prosocial, age 12 | 0.93 (0.86, 1.00) | 0.05 | 2.0 x 10-3 | 1.04 (0.97, 1.13) | 0.28 | 1.4 x 10-3 | 1.10 (1.02, 1.18) | 0.02 | 2.4 x 10-3 |
| Hyperactivity, age 12 | 0.97 (0.87, 1.08) | 0.56 | 4.9 x 10-4 | 1.06 (0.95, 1.18) | 0.30 | 7.7 x 10-4 | 1.07 (0.96, 1.20) | 0.20 | 1.0 x 10-3 |
| Emotional symptoms, age 12 | 1.07 (0.96, 1.20) | 0.19 | 1.4 x 10-3 | 1.12 (1.00, 1.25) | 0.05 | 2.3 x 10-3 | 1.16 (1.04, 1.26) | 0.01 | 3.5 x 10-3 |
| Conduct problems, age 12 | 0.99 (0.89, 1.10) | 0.81 | 1.6 x 10-3 | 1.04 (0.94, 1.16) | 0.46 | 1.8 x 10-3 | 1.05 (0.94, 1.16) | 0.42 | 1.8 x 10-3 |
| Peer problems, age 12 | 0.96 (0.87, 1.06) | 0.43 | 3.0 x 10-4 | 0.96 (0.87, 1.06) | 0.40 | 3.3 x 10-4 | 1.00 (0.91, 1.11) | 0.92 | 9.4 x 10-5 |
PRS, polygenic risk scores; SNPs, single nucleotide polymorphisms.
Adjusted P-value threshold = 0.0125
Cognitive measures
At 8 years and using an SNP inclusion threshold of P≤5x10-1, there was strong evidence of a PRS being associated with lower total IQ (β: -0.04; 95% CI: -0.07, -0.02, P = 0.002, Table 4) as well as lower verbal (β: -0.04; 95% CI: -0.07, -0.01, P = 0.003) and performance IQ (β: -0.03; 95% CI: -0.06, -0.01, P = 0.01, Supplementary Table 7, available as Supplementary data at IJE online). The pattern of results was similar for a threshold of P≥5x10-2, whereas the evidence of association when considering only genome-wide significant SNPs (P≤5x10-8) was weak (Table 4). At 15 years, we could not detect any evidence of association between PRS for Alzheimer’s and total IQ or IQ domains, using either a genome-wide significant SNP threshold or more liberal SNP inclusion thresholds (Table 4; Supplementary Table 8, available as Supplementary data at IJE online). However, the direction of effects was the same as for age 8, with PRS suggesting lower total IQ (β: -0.02; 95% CI: -0.05, 0.01, P = 0.20 at P≤5x10-1, Table 4). Also, there was some evidence of association between PRS at SNP inclusion thresholds of P≥5x10-2 with matrix reasoning scores (Supplementary Table 8, available as Supplementary data at IJE online).
Behavioural difficulties
At all of the examined P-value thresholds, there was weak evidence that a PRS was associated with the SDQ components at 9 years (Supplementary Table 9, available as Supplementary data at IJE online). At 12 years and an SNP inclusion threshold P ≤5x10-1, there was some evidence that a PRS may be associated with the prosocial and emotional score (Table 4). At an SNP inclusion threshold of P≤5x10-2, there was evidence of association for a PRS with higher odds of having an abnormal emotional symptoms score (OR: 1.16; 95% CI: 1.04, 1.26, P = 0.01 at P≤5x10-1, Table 4). We could not detect any evidence for association between a PRS at the genome-wide significant threshold and the SDQ domains.
Sensitivity analysis
The size and direction of effect estimates for PRS excluding the SNPs within the ApoE gene were similar for most outcomes (Supplementary Tables 5–10, available as Supplementary data at IJE online). The direction of effects and variance explained by the two SNPs tagging the ApoE region was similar to that explained by the genome-wide significant PRS (with ApoE) for most outcomes (Supplementary Tables 5–10, available as Supplementary data at IJE online). Results for PRS excluding the SNPs associated with educational attainment at 5x10-8 were similar to results in the main analysis. When SNPs associated with educational attainment at P≤5x10-2 were removed, evidence of association was attenuated (Supplementary Tables 11–18, available as Supplementary data at IJE online). In our comparison of participants with and without genetic data in terms of SES, availability of genetic data was associated with indicators of SES (Supplementary Table 3, available as Supplementary data at IJE online).
Discussion
This is one of the first studies investigating whether common genetic variants predisposing to a higher risk of Alzheimer’s disease are associated with educational, cognitive and behavioural measures in children from a general population cohort study. Our findings indicate little evidence for association of the PRS at the genome-wide significant threshold with cognitive measures, academic achievement and behavioural difficulties. We found that PRS at liberal P-value thresholds were associated with lower academic achievement at 14 and 15 years, as well as lower total IQ and IQ domain scores at 8 years.
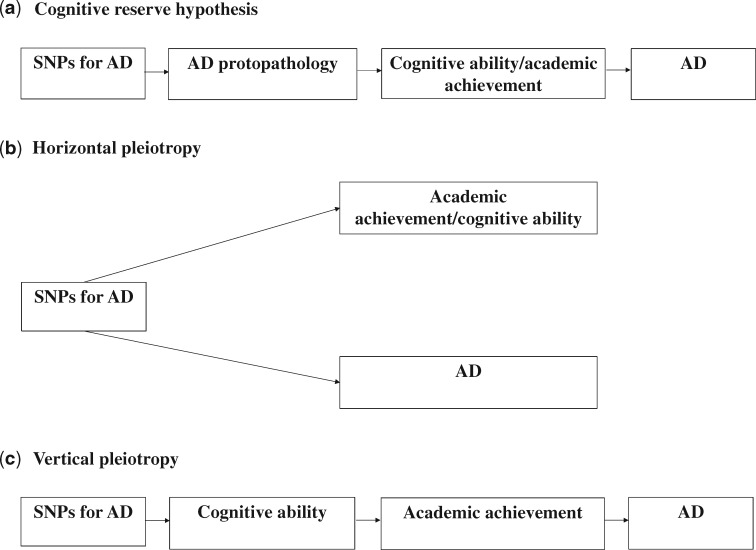
The use of PRS with P-value thresholds of varying strengths, as well as accounting for genetic variants associated with educational attainment, allowed for the testing of three possible mechanisms by which genetic variants for Alzheimer’s disease may affect the examined outcomes. The first possibility is that risk variants for Alzheimer’s are associated with disease protopathology in early life which manifests as lower IQ and poorer academic achievement in early life. Lower IQ and poorer academic achievement at young ages may reduce brain and cognitive reserve, which could result in reduced ability to tolerate and compensate for Alzheimer’s disease pathology through structural differences in the brain and/or pre-existing cognitive processing approaches/activation of compensatory mechanisms, respectively known as the brain/cognitive reserve hypothesis33 (Figure 1a). This is not supported by our findings, since genetic variants most strongly associated with Alzheimer’s disease (i.e. 5 x 10-8) should also have been associated with cognitive and educational measures in childhood to support this hypothesis. The second possibility is the presence of horizontal pleiotropy.34,35 SNPs associated with Alzheimer’s disease could be associated with lower academic achievement and lower cognitive ability through biological pathways that are completely unrelated to the disease (Figure 1b). This could be the case at least for cognitive ability, since evidence of association between the PRS at liberal thresholds and cognitive ability remained after the removal of SNPs associated with educational attainment.
Figure 1.
Potential mechanisms of associations between PRS for Alzheimer’s disease and cognitive ability/academic achievement. Please note that these are not intended to be directed acyclic graphs. AD denotes Alzheimer’s disease. In panel (a), genetic variants for Alzheimer’s disease cause Alzheimer’s disease protopathology, which manifests as lower IQ and poorer academic achievement at young ages. This could result in reduced ability to tolerate and compensate for Alzheimer’s disease pathology. Panel (b) describes the situation where genetic variants that increase predisposition for Alzheimer’s disease affect academic achievement and/or cognitive ability through an independent pathway (horizontal pleiotropy). In panel (c), genetic variants used to instrument Alzheimer’s disease have their primary effect on Alzheimer’s disease through academic achievement and/or cognitive ability rather than vice versa.
Furthermore, a recent Mendelian randomization study examining the relationship between intelligence and Alzheimer’s disease identified some evidence of horizontal pleiotropy.36 For education, this explanation is not supported by our findings or by Mendelian randomization studies,36–38 which indicated a causal effect of education on Alzheimer’s disease without detecting the presence of pleiotropy.36,38 The third possibility is the presence of vertical pleiotropy34,35; genetic variants associated with Alzheimer’s disease are only associated with the disease because they reduce educational attainment and/or cognitive ability (Figure 1c). Our findings support this possibility, as the observed association between PRS for Alzheimer’s disease and academic achievement is fully attenuated when the genetic variants associated with educational attainment are removed.
We did not detect consistent associations between the PRS at liberal P-value thresholds across all time points tested. This could be due to either: (i) lower participation and consequently lower power to detect associations due to differences in sample size across ages; (ii) varying impact of environmental factors on behavioural changes across development; or (iii) difference in measures.
Comparison with other studies
In agreement with a previous study showing no effect of ApoE on cognitive outcomes in ALSPAC children, we did not find any evidence that the genome-wide significant PRS was associated with cognition.39 A recent study40 in children and adolescents from two independent cohorts in Brazil (N = 716) found that a PRS for Alzheimer’s disease was associated with lower scores in non-declarative memory exercises at P ≤ 0.01 and with reading and writing at more liberal P-value thresholds. They did not find strong evidence for cognitive tasks or brain structure in a comparable sample of adolescents (N = 1029, mean age 15 years). Our findings are also in agreement with those from adult populations for which there is an established association between a PRS for Alzheimer’s disease and adverse cognitive outcomes.15,16 In adults from the UK Biobank, there is an association between a higher PRS for Alzheimer’s disease with lower cognitive performance (P≥1x10-2) and educational attainment (P≥5x10-2).16 Furthermore, a phenome-wide analysis41 using linkage disequilibrium score regression showed negative genetic correlations between Alzheimer’s disease and cognitive outcomes. In line with our findings, this study did not detect associations between a PRS for Alzheimer’s disease at P ≤ 0.30 and different SDQ components.
Strengths and limitations
Our study benefits from using a large discovery GWAS of individuals with a clinical diagnosis of late-onset Alzheimer’s and a large sample of children representative of the population as a target sample. Our study differs from many earlier studies, in that we used academic achievement on a continuous scale, rather than educational attainment which is traditionally measured on a categorical scale. Thus, our results are far more precise and potentially very well powered to pick up effects. The use of the PRS in children allowed us to perform this investigation without the selection bias present in late life studies.
The inherent limitation of using PRS is the limited amount of phenotypic variance they explain, which is also true for this study (<1%). Genome-wide complex trait analysis has shown that variants achieving genome-wide significance for Alzheimer’s currently explain only 16.3% of phenotypic variance. When all SNPs in the dataset are included, the amount of phenotypic variance explained increases to 53.2%.42 We used a more liberal threshold for SNP inclusion than that of genome-wide significance because, although it might introduce noise,43 it increases power to detect individuals at highest/lowest risk.9 Another limitation of this study could be the differential attrition or non-participation by PRS, which has been shown to introduce collider bias into studies for psychiatric disorders. A recent study in addressing the issue of selection bias in genetic studies did not detect evidence for an association between PRS for Alzheimer’s disease and non-participation in ALSPAC.44
Our study is the first to examine the association between genetic variants for Alzheimer’s disease and both educational, cognitive and behavioural outcomes in childhood and adolescence in such a large sample. We show with great precision that individuals with substantially increased risk of Alzheimer’s disease later in life have similar educational attainment to other individuals in the population. This suggests that the risk factors for Alzheimer’s disease play a role after adolescence.
Funding
This work was supported by a grant from the BRACE Alzheimer’s charity (BR16/028). The UK Medical Research Council and Wellcome (Grant ref: 102215/2/13/2) and the University of Bristol provide core support for ALSPAC. The collection of the WASI variable was supported by a grant from the Wellcome Trust (Grant ref: 076467/Z/05/Z). GWAS data were generated by Sample Logistics and Genotyping Facilities at Wellcome Sanger Institute and LabCorp (Laboratory Corporation of America) using support from 23andMe. L.D.H. and E.L.A. are supported by fellowships from the UK Medical Research Council (MR/M020894/1 and MR/P014437/1, respectively). All authors work in a unit that receives funding from the University of Bristol and the UK Medical Research Council (MC_UU_00011-1). This publication is the work of the authors, and E,S, will serve as a guarantor for the contents of this paper.
Supplementary Material
Acknowledgements
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses. We would also like to thank Neil Davies for his invaluable input. We also thank the International Genomics of Alzheimer's Project (IGAP) for providing summary results data for these analyses. The investigators within IGAP contributed to the design and implementation of IGAP and/or provided data but did not participate in analysis or writing of this report. IGAP was made possible by the generous participation of the control subjects, the patients and their families. The i–Select chips was funded by the French National Foundation on Alzheimer's disease and related disorders. EADI was supported by the LABEX (laboratory of excellence programme investment for the future) DISTALZ grant, Inserm, Institut Pasteur de Lille, Université de Lille 2 and the Lille University Hospital. GERAD was supported by the Medical Research Council (Grant ref: 503480), Alzheimer's Research UK (Grant ref: 503176), the Wellcome Trust (Grant ref: 082604/2/07/Z) and German Federal Ministry of Education and Research (BMBF): Competence Network Dementia (CND) (Grant ref: 01GI0102, 01GI0711, 01GI0420). CHARGE was partly supported by the NIH/NIA grant R01 AG033193 and the NIA AG081220 and AGES contract N01–AG–12100, the NHLBI grant R01 HL105756, the Icelandic Heart Association and the Erasmus Medical Center and Erasmus University. ADGC was supported by the NIH/NIA grants: U01 AG032984, U24 AG021886, U01 AG016976, and the Alzheimer's Association grant ADGC–10–196728.
Conflict of interest: None declared.
References
- 1.Alzheimer’s Association. 2015 Alzheimer’s disease facts and figures. Alzheimers Dement 2015;11:332–84. [DOI] [PubMed] [Google Scholar]
- 2. Geda YE, Knopman DS, Mrazek DA. et al. Depression, apolipoprotein E genotype, and the incidence of mild cognitive impairment: a prospective cohort study. Arch Neurol 2006;63:435–40. [DOI] [PubMed] [Google Scholar]
- 3. Gallagher D, Coen R, Kilroy D. et al. Anxiety and behavioural disturbance as markers of prodromal Alzheimer’s disease in patients with mild cognitive impairment. Int J Geriatr Psychiatry 2011;26:166–72. [DOI] [PubMed] [Google Scholar]
- 4. Mayeux R, Stern Y.. Epidemiology of Alzheimer disease. Cold Spring Harb Perspect Med 2012;2. doi: 10.1101/cshperspect.a006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lambert JC, Ibrahim-Verbaas CA, Harold D. et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet 2013;45:1452–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Genin E, Hannequin D, Wallon D. et al. APOE and Alzheimer disease: a major gene with semi-dominant inheritance. Mol Psychiatry 2011;16:903–07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Corder EH, Saunders A. M, Strittmatter WJ. et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 1993;261:921–23. [DOI] [PubMed] [Google Scholar]
- 8. Escott-Price V, Sims R, Bannister C. et al. Common polygenic variation enhances risk prediction for Alzheimer’s disease. Brain 2015;138:3673–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Escott-Price V, Shoai M, Pither R, Williams J, Hardy J.. Polygenic score prediction captures nearly all common genetic risk for Alzheimer’s disease. Neurobiol Aging 2017;49:214.e7–e11. [DOI] [PubMed] [Google Scholar]
- 10. Purcell SM, Wray NR, Stone JL. et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 2009;460:748–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee SH, Harold D, Nyholt DR. et al. Estimation and partitioning of polygenic variation captured by common SNPs for Alzheimer’s disease, multiple sclerosis and endometriosis. Hum Mol Genet 2013;22:832–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bateman RJ, Xiong C, Benzinger TLS. et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med 2012;367:795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nabers A, Perna L, Lange J. et al. Amyloid blood biomarker detects Alzheimer’s disease. EMBO Mol Med 2018;10:e8763.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Logue MW, Panizzon MS, Elman JA. et al. Use of an Alzheimer’s disease polygenic risk score to identify mild cognitive impairment in adults in their 50s. Mol Psychiatry 2019;24:421–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rawle M, Davis D, Bendayan R, Wong A, Kuh D, Richards M.. Apolipoprotein-E (ApoE) ϵ4 and cognitive decline over the adult life course. Transl Psychiatry 2018;8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hagenaars SP, Harris SE, Davies G. et al. Shared genetic aetiology between cognitive functions and physical and mental health in UK Biobank (N=112 151) and 24 GWAS consortia. Mol Psychiatry 2016;21:1624–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fraser A, Macdonald-Wallis C, Tilling K. et al. Cohort Profile: The Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int J Epidemiol 2013;42:97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Boyd A, Golding J, Macleod J. et al. Cohort Profile: The ‘children of the 90s—’—the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol 2013;42:111–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stergiakouli E, Martin J, Hamshere ML. et al. Shared genetic influences between attention-deficit/hyperactivity disorder (ADHD) traits in children and clinical ADHD. J Am Acad Child Adolesc Psychiatry 2015;54:322–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jones HJ, Stergiakouli E, Tansey KE. et al. Phenotypic manifestation of genetic risk for schizophrenia during adolescence in the general population. JAMA Psychiatry 2016;73:221–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Department for Education. National Pupil Database in England, 2002 2014. http://www.adls.ac.uk/department-for-education/dcsf-npd/? detail (5 February 2019, date last accessed).
- 22.The Department for Education. National Curriculum in England: Complete Framework for Key Stages 1 to 4 2014. https://www.gov.uk/government/publications/national-curriculum-in-england-framework-for-key-stages-1-to-4 (5 February 2019, date last accessed).
- 23. Wechsler D, Golombok S, Rust J.. WISC-III: Wechsler Intelligence Scale for Children—Third Edition UK Manual. Sidcup, UK: The Psychological Corporation, 1992. [Google Scholar]
- 24. Wechsler D. Manual for the Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Harcourt Assessment, 1999. [Google Scholar]
- 25. Strauss EH, Sherman EMS, Spreen O.. A Compendium of Neuropsychological Tests: Adminsitration, Norms, and Commentary.Oxford, UK: Oxford University Press, 2006. [Google Scholar]
- 26. Goodman R. The strengths and difficulties questionnaire: a research note. J Child Psychol Psychiatry 1997;38:581–86. [DOI] [PubMed] [Google Scholar]
- 27. Goodman R. Psychometric properties of the strengths and difficulties questionnaire. J Am Acad Child Adolesc Psychiatry 2001;40:1337–45. [DOI] [PubMed] [Google Scholar]
- 28. Stergiakouli E, Thapar A, Davey Smith G.. Association of acetaminophen use during pregnancy with behavioral problems in childhood: evidence against confounding. JAMA Pediatr 2016;170:964–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.StataCorp. Stata Statistical Software: Release 14. 2016. College Station, TX: Stata, 2016. [Google Scholar]
- 30. Kaiser HF. The application of electronic computers to factor analysis. Educ Psychol Meas 1960;20:141–51. [Google Scholar]
- 31. Sterne JAC, Smith Davey, Smith G.. Sifting the evidence—what’s wrong with significance tests? BMJ 2001;322:226.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wasserstein RL, Lazar NA.. The ASA’s statement on p-values: context, process, and purpose. Am Stat 2016;70:129–33. [Google Scholar]
- 33. Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol 2012;11:1006–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Davey Smith G, Hemani G.. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet 2014;23:R89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hemani G, Bowden J, Davey Smith G.. Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum Mol Genet 2018;27:R195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Anderson EL, Howe LD, Wade KH. et al. Education, intelligence and Alzheimer’s disease: evidence from a multivariable two-sample Mendelian randomization study. bioRxiv 2018. doi: https://doi.org/10.1101/401042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Larsson SC, Traylor M, Malik R, Dichgans M, Burgess S, Markus HS.. Modifiable pathways in Alzheimer’s disease: Mendelian randomisation analysis. BMJ 2017;359:j5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Anderson E, Wade KH, Hemani G. et al. The causal effect of educational attainment on Alzheimer’s disease: a two-sample Mendelian randomization study. bioRxiv 2017. doi: https://doi.org/10.1101/127993. [Google Scholar]
- 39. Taylor AE, Guthrie PAI, Davey Smith G. et al. IQ, educational attainment, memory and plasma lipids: associations with apolipoprotein e genotype in 5995 children. Biol Psychiatry 2011;70:152–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Axelrud LK, Santoro ML, Pine DS. et al. Polygenic risk score for Alzheimer’s disease: implications for memory performance and hippocampal volumes in early life. Am J Psychiatry 2018;175:555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Krapohl E, Euesden J, Zabaneh D. et al. Phenome-wide analysis of genome-wide polygenic scores. Mol Psychiatry 2016;21:1188–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ridge PG, Hoyt KB, Boehme K. et al. Assessment of the genetic variance of late-onset Alzheimer’s disease. Neurobiol Aging 2016;41:e13–200.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chatterjee N, Shi J, García-Closas M.. Developing and evaluating polygenic risk prediction models for stratified disease prevention. Nat Rev Genet 2016;17:392–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Taylor AE, Jones HJ, Sallis H. et al. Exploring the association of genetic factors with participation in the Avon Longitudinal Study of Parents and Children. Int J Epidemiol 2018;47:1207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.