Abstract
Background
Cardiac myocyte hypertrophy results from clinical conditions that include hypertension and valvular heart disease, and can result in heart failure. This study aimed to investigate the expression and role of the long noncoding RNA FTX (lnc-FTX), an X-inactive-specific transcript (XIST) regulator transcribed from the X chromosome, in hypertrophy of neonatal mouse cardiac myocytes induced by angiotensin II (Ang II) in vitro.
Material/Method
Cardiac myocytes were isolated from neonatal mice and cultured with and without Ang II. Immunofluorescence, with localization of an antibody to alpha-smooth muscle actin (α-SMA), was used to identify the neonatal mouse cardiac myocytes. Quantitative real-time polymerase chain reaction (qRT-PCR) measured gene expression levels. The cell counting kit-8 (CCK-8) assay was used to determine cell viability, and Western blot measured protein expression. StarBase v2.0 bioinformatics software was used for target gene prediction and was confirmed with the luciferase reporter assay.
Results
The expression of lnc-FTX was reduced in mouse cardiac myocytes treated with Ang II. Overexpression of lnc-FTX reduced cell apoptosis, cardiomyocyte contractility, and the expression of c-Jun, A-type natriuretic peptide (ANP), and B-type natriuretic peptide (BNP) induced by Ang II. The target of lnc-FTX was micro-RNA 22 (miRNA-22). The mechanism of action of lnc-FTX in neonatal mouse cardiac myocytes was through suppression of the PI3K/Akt signaling pathway by promoting the release of PTEN by sponging miRNA-22.
Conclusions
The expression of lnc-FTX was associated with reduced hypertrophy of neonatal mouse cardiac myocytes and regulated the PTEN/PI3K/Akt signaling pathway by sponging miRNA-22.
MeSH Keywords: Heart Diseases; MicroRNAs; RNA, Long Noncoding
Background
Clinically, cardiac hypertrophy can be identified on imaging studies that include chest X-radiograph or computed tomography (CT), supported by studies of cardiac function [1]. Cardiac hypertrophy results from clinical conditions that include hypertension and valvular heart disease but is also associated with dilated and hypertrophic cardiomyopathy [2–4]. Cardiac hypertrophy is an adaptive response to maintain cardiac output and cardiac contractility and results from hypertrophy of cardiac myocytes. Cardiac hypertrophy may be physiological as a consequence of physical exercise, or pathological, which results in irreversible myocardial thickening and can result in heart failure [5]. Heart failure has a high mortality rate [6]. Therefore, early intervention and control of hypertrophy of cardiac myocytes are required to prevent heart failure. Currently, treatment for patients with heart failure is symptomatic and aimed at reducing disease progression, and new approaches to treatment are needed.
Long noncoding RNAs (lncRNAs) are more than 200 nucleotides in length and are without the ability to encode protein, and were initially believed to represent transcriptional ‘noise’ [7]. Currently, lncRNAs have been shown to regulate gene expression in physiological and pathological processes, including glucose metabolism, diabetes, cancer, myocardial hypertrophy, and atherosclerosis [8–12]. Studies have identified pivotal roles for lncRNA in the regulation of gene expression in cardiac hypertrophy. For example, lnc-MAGI1-IT1 has been identified as a negative regulator of cardiac hypertrophy by inactivating the Wnt/beta-catenin pathway [13].
The long noncoding RNA FTX (lnc-FTX), an X-inactive-specific transcript (XIST) regulator transcribed from the X chromosome, was reported to possess protective effects in cardiomyocyte hypertrophy by sponging miR-330-3p [14]. Lnc-FTX has been investigated in gliomas, in the development of drug resistance in acute myeloid leukemia (AML) and in hepatocellular carcinoma (HCC) [15–17]. Recently, lnc-FTX has been shown to participate in the apoptosis cascade in cardiomyocytes, but the effects of lnc-FTX in cardiac hypertrophy remain unknown. An in vitro animal model of angiotensin II (Ang II)-induced cardiac myocyte hypertrophy has been previously reported [18–22].
Therefore, this study aimed to investigate the expression and role of lnc-FTX in hypertrophy of neonatal mouse cardiac myocytes induced by angiotensin II (Ang II) in vitro.
Material and Methods
Isolation, culture, and treatment of mouse cardiac myocytes
Ten-week-old C57BL/6 mice were obtained from Soochow University. All animal experiments were conducted according to the ethical guidelines of Soochow University. The isolation of mouse cardiac myocytes was performed in aseptic conditions, as previously described [23]. The isolated cells were incubated in 96-well plates in Dulbecco’s modified Eagle’s medium (DMEM) at 37°C at a cell density of 3×104. The cells were treated with 10−8 mol/L of angiotensin II (Ang II) for 24 h to develop the in vitro cell model of cardiac myocyte hypertrophy. After treatment with Ang II, the cells were transfected with long noncoding RNA FTX (lnc-FTX) mimic or the pcDNA expression vector. The cell model treated with Ang II, and cells without Ang II treatment represented the control groups. The mouse cardiac myocytes were divided into four study groups: the control group; the Ang II treated group; the pcDNA + Ang II treated group; and the lnc-FTX mimic+Ang II treated group.
Immunofluorescence
An immunofluorescent staining assay was performed to identify the isolated cardiac myocytes. Polychlorinated formaldehyde (4%) was used to fix the cells for 30 min. The cells were incubated with a primary antibody to alpha-smooth muscle actin (α-SMA) (1: 100), followed by a secondary fluorescein-conjugated antibody. After washing the cells, the cell nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI). The immunofluorescence-stained mouse cardiac myocytes were observed by using fluorescence microscopy.
Cell Counting Kit-8 (CCK-8) assay
The density of cells seeded in 96-well plates was 1×104, and the cells were incubated for 24 h, 48 h, and 72 h respectively. After Ang II treatment or transfection with the lnc-FTX mimic, 10 μL of CCK-8 solution was added to each well and incubated with the cells for 2 h. The absorbance at 450 nm was measured using a Multiskan FC microplate reader (Thermo Fisher Scientific, Waltham, MA, USA).
The surface area of cardiac myocytes
The cells in the control group, the Ang II treated group, the pcDNA+Ang II treated group, and the lnc-FTX mimic+Ang II-treated group were stained by using crystal violet. Then, 100 random cardiomyocytes from the four study groups were selected for cell surface area assessment by using Image-pro Plus version 7.0 image analysis (Olympus, Tokyo, Japan).
Quantitative real-time polymerase chain reaction (qRT-PCR)
The total RNA from the mouse cardiac myocytes from the four study groups were separated by using an RNA isolation kit (Thermo Fisher Scientific, Waltham, MA, USA). Then, qRT-PCR was performed under the following cycle condition: 95°C for 10 seconds; 40 cycles at 95°C for 10 seconds; and 60 cycles at 72°C for 20 seconds. The 2−DDCt method was used to calculate the expression of the target.
Western blot
Western blot was performed to evaluate the protein expression levels in the mouse cardiac myocytes in the four study groups. The total proteins were separated with 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were transferred to the nitrocellulose membranes after blocking with bovine serum albumin (BSA) in phosphate-buffered saline (PBS). After washing, the membranes were incubated with the primary antibodies for 1h. The amount of primary antibody was used according to the area of the membranes (0.1 mL/m2). After washing three times with 0.1% Tween-PBST, the membranes were incubated with the secondary antibody. The E-Gel imaging system (Thermo Fisher Scientific, Waltham, MA, USA) was used to obtain protein bands.
Luciferase reporter assay
StarBase v2.0 biological information software was used to predict the target gene. For further confirmation of the target gene, the luciferase reporter assay was used. The wild-type or mutant 3′UTR binding site were inserted into a pmirGLO dual-luciferase miRNA target expression vector (Promega, Madison, WI, USA) to construct the luciferase reporter plasmid and were co-transfected with or without the miRNA-22 mimic into the cells. The miRNA-22 NC co-transfected with luciferase reporter plasmid (wild-type or mutant type) were included as controls. After incubation for 48 h, the luciferase reporter assay system (Promega, Madison, WI, USA) was applied to determine relative luciferase activity.
Statistical analysis
Data were expressed as the mean±standard deviation (SD). Data were analyzed using SPSS version 20.0 software (SPSS Inc., Chicago, IL, USA). One-way or two-way analysis of variance (ANOVA) was used to determine significant differences between the study groups. A P-value <0.05 was considered to be statistically significant.
Results
Expression of long noncoding RNA FTX (lnc-FTX) in mouse cardiac myocytes
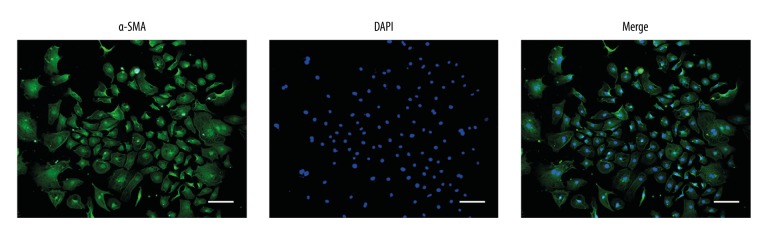
As shown in Figure 1, 95% of the isolated mouse cells were positively stained using immunofluorescence for alpha-smooth muscle actin (α-SMA), confirming their identity as cardiac myocytes. The cells showed a variety of cell morphology, including triangular shapes, star, and spindle shapes. The expression of long noncoding RNA FTX (lnc-FTX) was higher in the control groups than in the groups treated with angiotensin II (Ang II), indicating that lnc-FTX was involved in mouse cardiac myocyte hypertrophy that occurred in vitro.
Figure 1.
Immunofluorescence with localization of an antibody to alpha-smooth muscle actin (α-SMA) was used to identify the neonatal mouse cardiac myocytes.
Cell viability of cardiac myocytes following overexpression of lnc-FTX
The mouse cardiac myocytes included four study groups: the control group; the Ang II treated group; the pcDNA+Ang II treated group; and the lnc-FTX mimic+Ang II treated group. The overexpression of lnc-FTX was achieved by transfection with the lnc-FTX mimic into the cells, as shown in Figure 2A. Compared with the control, cell viability was decreased in the group with Ang II treatment (Figure 2B). Decreased cell viability induced by Ang II was increased by lnc-FTX overexpression, indicating that lnc-FTX overexpression reduced apoptosis of cardiac myocytes induced by Ang II.
Figure 2.
Long noncoding RNA FTX (lnc-FTX) expression, cell viability, myocardial beat frequency, and cell surface area in the study groups. (A) Expression of long noncoding RNA FTX (lnc-FTX) in the study groups. *** P<0.001 vs. the control group. ### P<0.001 vs. the miRNA-NC group. (B) The cell viability in the study groups at 24 h, 48 h, and 72 h. * P<0.05, *** P<0.001 vs. the control group. # P<0.05, ### P<0.001 vs. pcDNA+the angiotensin II (Ang II) group. (C) The myocardial beat frequency in the study groups. ** P<0.01 vs. control group; ## P<0.01 pcDNA (72 h)+the Ang II group (24 h). (D) Cell surface area in the study groups. *** P<0.001 vs. the control (72 h) group; ### P<0.001 vs. pcDNA (72 h)+the Ang II group (24 h).
lnc-FTX reduced mouse cardiac myocyte hypertrophy induced by Ang II
As shown in Figure 2C, the cardiac myocyte beat frequency was increased by Ang II treatment, in contrast to the normal cells. The increased cardiac myocyte beat frequency was reduced by lnc-FTX overexpression, confirming the protective effect of lnc-FTX on cardiac myocyte hypertrophy induced by Ang II. This finding was supported by the results of the assessment of the cardiac myocyte cell surface area (Figure 2D). The cell surface area was increased in the group treated with Ang II compared with the control group. The larger cell surface area induced by Ang II was reduced by lnc-FTX overexpression, indicating the protective effects of lnc-FTX on mouse cardiac myocyte hypertrophy.
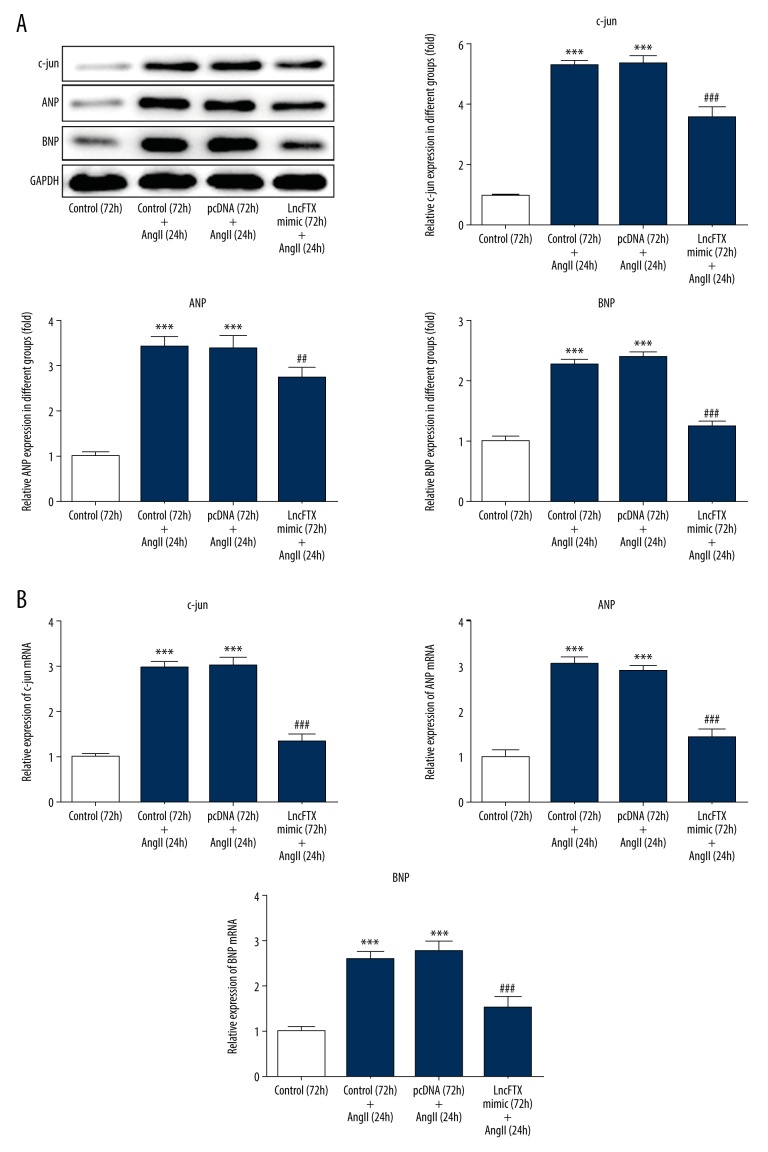
Lnc-FTX reduced the expression of c-Jun, A-type natriuretic peptide (ANP), and B-type natriuretic peptide (BNP)
Indicators of cardiac myocyte hypertrophy that were investigated included c-Jun, ANP, and BNP. The expression of c-Jun, ANP, and BNP was significantly increased by Ang II when compared with the control group (Figure 3A, 3B). The increased expression of c-Jun, ANP, and BNP were reversed by lnc-FTX overexpression, indicating that lnc-FTX overexpression had a protective effect on hypertrophy of mouse cardiac myocytes induced by Ang II through downregulation of c-Jun, ANP, and BNP.
Figure 3.
The levels of c-Jun, A-type natriuretic peptide (ANP), and B-type natriuretic peptide (BNP) evaluated by Western blot and quantitative real-time polymerase chain reaction (qRT-PCR). (A) The levels of c-Jun, ANP, and BNP evaluated by western blot in the different study groups. (B) The levels of c-Jun, ANP, and BNP evaluated by qRT-PCR in the different study groups ** P<0.01 and *** P<0.001 vs. the control (72 h) group; ## P<0.01 and ### P<0.001 vs. pcDNA (72 h)+the angiotensin II (Ang II) (24 hr) group.
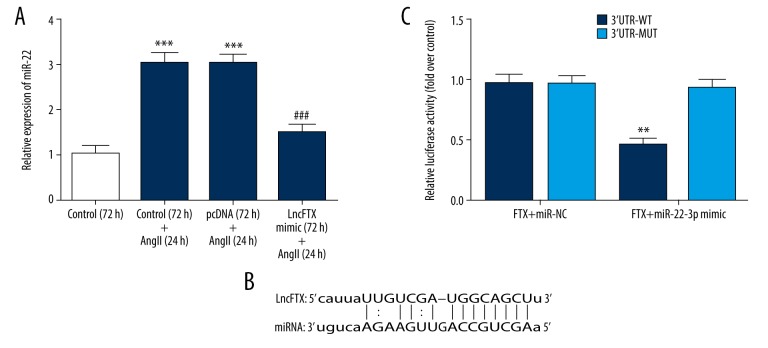
Identification of micro-RNA 22 (miRNA-22) as the target of long noncoding RNA FTX (lnc-FTX)
Also, miR-22 was predicted as the target gene of lnc-FTX, as shown using StarBase v2.0 software (Figure 4B). The expression of miRNA-22 was increased in the Ang II treatment group compared with the control group. The high miRNA-22 expression in the Ang II treatment group was reduced by lnc-FTX overexpression, which indicated that miRNA-22 was the possible target of lnc-FTX in hypertrophy of mouse cardiac myocytes induced by Ang II (Figure 4A). The luciferase reporter assay was performed to confirm these findings. As shown in Figure 4C, the relative luciferase activity was lowest in the FTX WT+ miRNA-22 mimic group, for all the groups, which further supported that miRNA-22 was the target for lnc-FTX.
Figure 4.
Identification of micro-RNA 22 (miRNA-22) as the target of long noncoding RNA FTX (lnc-FTX). (A) The levels of micro-RNA 22 (miRNA-22) in the study groups. *** P<0.001 vs. control (72 h) group; ### P<0.001 vs. pcDNA (72 h)+the angiotensin II (Ang II) (24 h) group. (B) The target gene predicted by StarBase v2.0. (C) Luciferase Reporter Assay (** P<0.01 vs. FTX+ miR-NC group).
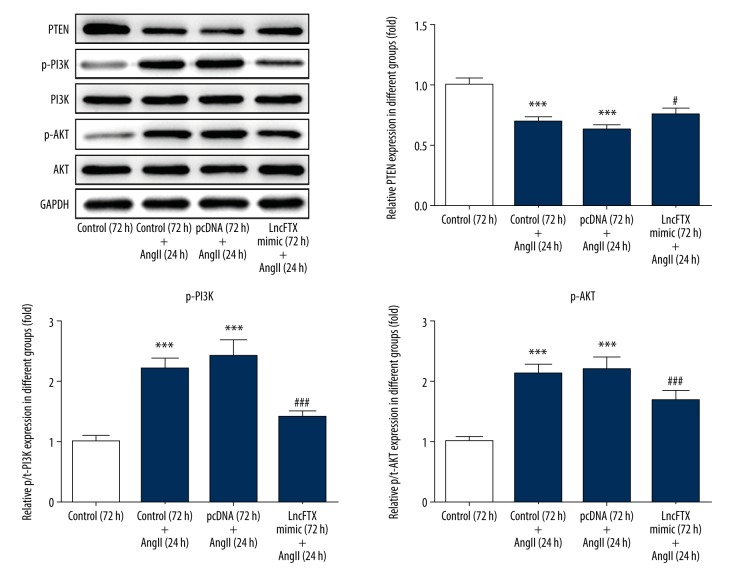
Mouse cardiac myocyte hypertrophy induced by Angi II promoted PTEN and suppressed PI3K/Akt signaling
The PI3K/Akt signaling pathway has previously been reported to be associated with hypertrophy of cardiac myocytes [24,25]. PTEN, as an antagonist of P13K, has also previously been reported to protect against hypertrophy of cardiac myocytes [26]. The expression of PTEN and the relevant proteins in the PI3K/Akt signaling pathway were evaluated in this study. The results showed that, when compared with the control group, PTEN expression was reduced by Ang II treatment. Also, the expression of PI3K and AKT were increased by Ang II treatment, which was consistent with previously published findings that showed PTEN was an antagonist of P13K [26]. The expression of PTEN, PI3K, and AKT induced by Ang II were partly reversed by lnc-FTX (Figure 5). PTEN expression induced by Ang II was increased by lnc-FTX, and the expression of PI3K and AKT induced by Ang II were reduced by lnc-FTX. These findings showed that lnc-FTX reduced mouse cardiac myocyte hypertrophy induced by Ang II by promoting the release of PTEN and inhibiting the PI3K/Akt signaling pathway.
Figure 5.
The expression of PTEN, PI3K, and AKT evaluated by Western blot in the study groups. *** P<0.001 vs. the control (72 h) group; # P<0.05, ## P<0.01 and ### P<0.001 vs. pcDNA (72 h)+the angiotensin II (Ang II) (24 h) group.
Discussion
Clinically, cardiac hypertrophy occurs in patients with hypertension, myocardial infarction, valvular heart disease, and hypertrophic cardiomyopathy, and persistent cardiac hypertrophy results in heart failure. Therefore, myocardial hypertrophy is an important pathophysiological process that requires further study for the prevention of heart failure. Previous studies have shown that long noncoding RNAs play essential roles in cardiac hypertrophy [27–29]. Long noncoding RNA FTX (lnc-FTX) was reported to be involved in cancer, but the effects of lnc-FTX in cardiac myocyte hypertrophy remain unknown. In this study, hypertrophy of mouse cardiac myocytes was induced by angiotensin II (Ang II). The expression of lnc-FTX was reduced in mouse cardiac myocyte hypertrophy induced by Ang II, and overexpression of lnc-FTX had a protective effect of reducing hypertrophy of mouse cardiac myocytes following treatment with Ang II.
The results of the present study showed that apoptosis of mouse cardiac myocytes, cell surface area, the expression of indicators of cardiac myocyte hypertrophy, c-Jun, A-type natriuretic peptide (ANP), and B-type natriuretic peptide (BNP), and myocardial beat frequency induced by Ang II were significantly increased when compared with the control group. These findings confirmed the successful construction of the murine cell model of cardiac myocyte hypertrophy. Also, the effects of Ang II were reduced by overexpression of lnc-FTX, which demonstrated the protective effects of lnc-FTX in mouse cardiac myocyte hypertrophy induced by Ang II.
The mechanism of the protective effects of lnc-FTX on mouse cardiac myocyte hypertrophy induced by Ang II was also investigated. As shown by the StarBase v2.0 target gene prediction software and the luciferase reporter assay, micro-RNA 22 (miRNA-22) was identified as the target of lnc-FTX. This finding was supported by a previous study, which showed that miRNA-22 promoted cardiac myocyte hypertrophy [30]. Therefore, the protective effects of lnc-FTX on hypertrophy of mouse cardiac myocytes was achieved by targeting miRNA-22, as lnc-FTX downregulated miRNA-22 by sponging miRNA-22. PTEN, which is expressed in myocardial cells, has previously been reported to control cardiac hypertrophy in vivo [26]. In this study, reduction of PTEN expression by Ang II treatment was reversed by lnc-FTX overexpression, which may be the protective mechanism of lnc-FTX. Also, miRNA-22 was previously reported to inhibit the expression of PTEN, which supports the findings of the present study [30]. In this study, overexpression of lnc-FTX resulted in downregulation of miRNA-22 and suppression of PTEN by reducing miRNA-22, resulting in upregulation of PTEN expression to protect mouse cardiac myocytes from hypertrophy.
The mechanisms of the relevant signaling pathway were also investigated in this study. Inhibition of the PI3K/Akt signaling pathway was previously reported to reduce cardiac myocyte hypertrophy [24,25]. In the present study, the expression of PI3K and Akt were increased by Ang II treatment, and this increased expression was reversed by lnc-FTX overexpression. Also, PTEN expression had the opposite effect to the expression of PI3K and Akt, which was consistent with the findings from a previously published study that showed that PTEN was a negative regulator of the PI3K/AKT signaling pathway [31]. The findings from the present study showed that lnc-FTX had a protective on hypertrophy of mouse cardiac myocytes induced by Ang II through inhibition of the PI3K/AKT signaling pathway by promoting the expression of PTEN and sponging miRNA-22.
Conclusions
The aim of this study was to investigate the expression and role of the long noncoding RNA FTX (lnc-FTX), an X-inactive-specific transcript (XIST) regulator transcribed from the X chromosome, in hypertrophy of neonatal mouse cardiac myocytes induced by angiotensin II (Ang II) in vitro. The expression of lnc-FTX was associated with reduced hypertrophy of neonatal mouse cardiac myocytes and regulated the PTEN/PI3K/Akt signaling pathway by sponging microRNA-22 (miRNA-22).
Acknowledgments
The authors would like to thank Fang Jia, Mengfei Wang, and Tianhong Yu for their helpful comments and suggestions.
Footnotes
Source of support: Departmental sources
References
- 1.Amin H, Siddiqui WJ. StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2019. Cardiomegaly. Available from [URL]: https://www.ncbi.nlm.nih.gov/books/NBK542296/ [Google Scholar]
- 2.Han Q, Liu Q, Zhang H, et al. Simvastatin improves cardiac hypertrophy in diabetic rats by attenuation of oxidative stress and inflammation induced by Calpain-1-mediated activation of nuclear factor-kappaB (NF-kappaB) Med Sci Monit. 2019;25:1232–41. doi: 10.12659/MSM.913244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kai H, Kudo H, Takayama N, et al. Molecular mechanism of aggravation of hypertensive organ damages by short-term blood pressure variability. Curr Hypertens Rev. 2014;10(3):125–33. doi: 10.2174/1573402111666141217112655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pagliaro P, Penna P. Hypertension, hypertrophy, and reperfusion injury. J Cardiovasc Med. 2017;18(3):131–35. doi: 10.2459/JCM.0000000000000435. [DOI] [PubMed] [Google Scholar]
- 5.Oka T, Akazawa H, Naito AT, Komuro I. Angiogenesis and cardiac hypertrophy: Maintenance of cardiac function and causative roles in heart failure. Circ Res. 2014;114(3):565–71. doi: 10.1161/CIRCRESAHA.114.300507. [DOI] [PubMed] [Google Scholar]
- 6.Mamas MA, Sperrin M, Watson MC, et al. Do patients have worse outcomes in heart failure than in cancer? A primary care-based cohort study with 10-year follow-up in Scotland. Eur J Heart Fail. 2017;19(9):1095–104. doi: 10.1002/ejhf.822. [DOI] [PubMed] [Google Scholar]
- 7.Comings DE. The structure and function of chromatin. Adv Hum Genet. 1972;3:237–431. doi: 10.1007/978-1-4757-4429-3_5. [DOI] [PubMed] [Google Scholar]
- 8.D’Angelo D, Mussnich P, Sepe R, et al. RPSAP52 lncRNA is overexpressed in pituitary tumors and promotes cell proliferation by acting as miRNA sponge for HMGA proteins. J Mol Med. 2019;97(7):1019–32. doi: 10.1007/s00109-019-01789-7. [DOI] [PubMed] [Google Scholar]
- 9.Zheng X, Han H, Liu GP, et al. LncRNA wires up Hippo and Hedgehog signaling to reprogramme glucose metabolism. EMBO J. 2017;36(22):3325–35. doi: 10.15252/embj.201797609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reichelt-Wurm S, Wirtz T, et al. Glomerular expression pattern of long non-coding RNAs in the type 2 diabetes mellitus BTBR mouse model. Sci Rep. 2019;9(1):9765. doi: 10.1038/s41598-019-46180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shao M, Chen G, Lv F, et al. LncRNA TINCR attenuates cardiac hypertrophy by epigenetically silencing CaMKII. Oncotarget. 2017;8(29):47565–73. doi: 10.18632/oncotarget.17735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holdt LM, Kohlmaier A, Teupser D. Long noncoding RNAs of the arterial wall as therapeutic agents and targets in atherosclerosis. Thromb Haemost. 2019;119(8):1222–36. doi: 10.1055/s-0039-1692680. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Q, Wang F, Wang F, Wu N. Long noncoding RNA MAGI1-IT1 regulates cardiac hypertrophy by modulating miR-302e/DKK1/Wnt/beta-catenin signaling pathway. J Cell Physiol. 2019 doi: 10.1002/jcp.28964. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Liu X, Chen L, et al. The long noncoding RNA XIST protects cardiomyocyte hypertrophy by targeting miR-330-3p. Biochem Biophys Res Commun. 2018;505(3):807–15. doi: 10.1016/j.bbrc.2018.09.135. [DOI] [PubMed] [Google Scholar]
- 15.Zhang W, Bi Y, Li J, et al. Long noncoding RNA FTX is upregulated in gliomas and promotes proliferation and invasion of glioma cells by negatively regulating miR-342-3p. Lab Invest. 2017;97(4):447–57. doi: 10.1038/labinvest.2016.152. [DOI] [PubMed] [Google Scholar]
- 16.Liu B, Ma X, Liu Q, et al. Aberrant mannosylation profile and FTX/miR-342/ALG3-axis contribute to development of drug resistance in acute myeloid leukemia. Cell Death Dis. 2018;9(6):688. doi: 10.1038/s41419-018-0706-7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Li X, Zhao Q, Qi J, et al. lncRNA Ftx promotes aerobic glycolysis and tumor progression through the PPARgamma pathway in hepatocellular carcinoma. Int J Oncol. 2018;53(2):551–66. doi: 10.3892/ijo.2018.4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu S, Cheng M, Guo X, et al. Down-regulation of miR-200c attenuates AngII-induced cardiac hypertrophy via targeting the MLCK-mediated pathway. J Cell Mol Med. 2019;23(4):2505–16. doi: 10.1111/jcmm.14135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bai Y, Sun X, Chu Q, et al. Caspase-1 regulate AngII-induced cardiomyocyte hypertrophy via upregulation of IL-1beta. Biosci Rep. 2018 doi: 10.1042/BSR20171438. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen F, Chen D, Zhang Y, et al. Interleukin-6 deficiency attenuates angiotensin II-induced cardiac pathogenesis with increased myocyte hypertrophy. Biochem Biophys Res Commun. 2017;494(3–4):534–41. doi: 10.1016/j.bbrc.2017.10.119. [DOI] [PubMed] [Google Scholar]
- 21.Dong ZX, Wan L, Wang RJ, et al. (−)-Epicatechin suppresses Angiotensin II-induced cardiac hypertrophy via the activation of the SP1/SIRT1 signaling pathway. Cell Physiol Biochem. 2017;41(5):2004–15. doi: 10.1159/000475396. [DOI] [PubMed] [Google Scholar]
- 22.Xiao YF, Zeng ZX, Guan XH, et al. FKBP12.6 protects heart from AngII-induced hypertrophy through inhibiting Ca(2+)/calmodulin-mediated signalling pathways in vivo and in vitro. J Cell Mol Med. 2018;22(7):3638–51. doi: 10.1111/jcmm.13645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Z-Z, Wang W, Jin H-Y, et al. Apelin is a negative regulator of Angiotensin II – mediated adverse myocardial remodeling and dysfunction. Hypertension. 2017;70(6):1165–75. doi: 10.1161/HYPERTENSIONAHA.117.10156. [DOI] [PubMed] [Google Scholar]
- 24.Qian W, Yu D, Zhang J, et al. Wogonin attenuates isoprenaline-induced myocardial hypertrophy in mice by suppressing the PI3K/Akt pathway. Front Pharmacol. 2018;9:896. doi: 10.3389/fphar.2018.00896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang X, Li X, Lin Q, Xu Q. Up-regulation of microRNA-203 inhibits myocardial fibrosis and oxidative stress in mice with diabetic cardiomyopathy through the inhibition of PI3K/Akt signaling pathway via PIK3CA. Gene. 2019;715:143995. doi: 10.1016/j.gene.2019.143995. [DOI] [PubMed] [Google Scholar]
- 26.Crackower MA, Oudit GY, Kozieradzki I, et al. Regulation of myocardial contractility and cell size by distinct PI3K-PTEN signaling pathways. Cell. 2002;110(6):737–49. doi: 10.1016/s0092-8674(02)00969-8. [DOI] [PubMed] [Google Scholar]
- 27.Yuan Y, Wang J, Chen Q, et al. Long non-coding RNA cytoskeleton regulator RNA (CYTOR) modulates pathological cardiac hypertrophy through miR-155-mediated IKKi signaling. Biochim Biophys Acta Mol Basis Dis. 2019;1865(6):1421–27. doi: 10.1016/j.bbadis.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J, Liang Y, Huang X, et al. STAT3-induced upregulation of lncRNA MEG3 regulates the growth of cardiac hypertrophy through miR-361-5p/HDAC9 axis. Sci Rep. 2019;9(1):460. doi: 10.1038/s41598-018-36369-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zou X, Wang J, Tang L, Wen Q. LncRNA TUG1 contributes to cardiac hypertrophy via regulating miR-29b-3p. In Vitro Cell Dev Biol Anim. 2019;55(7):482–490. doi: 10.1007/s11626-019-00368-x. [DOI] [PubMed] [Google Scholar]
- 30.Xu XD, Song XW, Li Q, et al. Attenuation of microRNA-22 derepressed PTEN to effectively protect rat cardiomyocytes from hypertrophy. J Cell Physiol. 2012;227(4):1391–98. doi: 10.1002/jcp.22852. [DOI] [PubMed] [Google Scholar]
- 31.Palabiyik O, Tastekin E, Doganlar ZB, et al. Alteration in cardiac PI3K/Akt/mTOR and ERK signaling pathways with the use of growth hormone and swimming, and the roles of miR21 and miR133. Biomed Rep. 2019;0(0):1–10. doi: 10.3892/br.2018.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]