Abstract
Background
A retrospective study of data from a prospective clinical registry was conducted to evaluate the prognostic role of serum calprotectin in patients with diabetes who underwent percutaneous coronary intervention (PCI) for acute coronary syndrome (ACS).
Material/Methods
Data were retrieved for 273 patients with diabetes mellitus who underwent PCI for primary ACS in a single center. Serum calprotectin levels were measured before PCI. Baseline clinical data included the Global Registry of Acute Coronary Events (GRACE) risk score for ACS. All patients underwent regular follow-up for major adverse cardiovascular events (MACE) during 12 months after PCI, including target vessel revascularization (TVR), defined as the need for an unplanned repeat PCI or coronary artery procedure. The predictive value of serum calprotectin for MACE was analyzed by using univariate and multivariate analysis and receiver operating characteristic (ROC) curve analysis.
Results
At the final follow-up, 47 of the 273 patients studies experienced MACE. Optimal cutoff values for serum calprotectin levels predictive for MACE stratified patients into a high calprotectin group and a low calprotectin group. The incidence of MACE and TVR in the high calprotectin group was significantly greater than in the low calprotectin group (21.9% vs. 11.5%; P=0.02). Multivariate analysis, adjusted for confounders, showed that the serum level of calprotectin was an independent risk factor for MACE (HR, 1.56; 95% CI, 1.08–4.62; P=0.01).
Conclusions
In patients with diabetes and the co-morbidity of ACS, a high serum level of calprotectin was associated with a significantly increased risk for MACE following PCI.
MeSH Keywords: Acute Coronary Syndrome, Diabetes Mellitus, Leukocyte L1 Antigen Complex, Percutaneous Coronary Intervention
Background
Worldwide, coronary artery disease (CAD) is a major cause of morbidity and mortality, with acute coronary syndrome (ACS) due to reduced blood flow in the coronary arteries having a main role [1,2]. The most effective treatment for patients with ACS is immediate cardiac reperfusion using percutaneous coronary intervention (PCI) [3]. However, the long-term clinical outcome for patients with ACS following PCI remains unsatisfactory [4,5]. Diabetes mellitus is a significant risk factor for patients with ACS [6]. Patients with diabetes and ACS are more likely to have occlusive disease of small caliber coronary vessels and are also at an increased risk of in-stent restenosis or thrombosis following PCI, with worse outcome [7,8]. Therefore, identification of indicators that can improve risk prediction and risk stratification is crucial for improving the clinical efficacy of PCI in patients with diabetes who have ACS.
Diabetes mellitus is also significantly associated with hypertension, hyperlipidemia, and atherosclerosis, which are risk factors for ACS [9,10]. Also, because ACS is due to the complications of atherosclerosis, the syndrome is associated with the expression of inflammatory mediators [11]. Calprotectin, also known as S100A8/A9, is constitutively expressed in human neutrophils, monocytes, and macrophage, which are cells that promote atherosclerosis [12]. Increased serum levels of calprotectin have previously been shown to be predictive for microvascular changes in patients with diabetes [13]. Also, significantly increased levels of serum calprotectin are found in patients with ACS compared with patients with stable coronary artery disease (CAD) [14,15]. However, the predictive value of serum levels of calprotectin for outcome following PCI in patients with diabetes who have ACS Remains to be determined.
Therefore, a retrospective study of data from a prospective clinical registry was conducted to evaluate the prognostic role of serum calprotectin in patients with diabetes who underwent PCI for ACS.
Material and Methods
Patients
A retrospective analysis of data from a prospective registry at a single center was conducted according to the declaration of Helsinki and ethical guidelines and was approved by the Institutional Review Board (IRB) of the Affiliated Hospital of Jining Medical University (No. Clinic 2019021). All enrolled patients provided signed and written informed consent. Data were retrospectively reviewed from 273 consecutive patients with diabetes with acute coronary syndrome (ACS) who underwent percutaneous coronary intervention (PCI) between May 2016 and May 2018 in the Fifth Department of Cardiology of the Affiliated Hospital of Jining Medical University.
All patients enrolled in the study underwent PCI for primary ACS, which was diagnosed according to the 2015 guidelines from the European Society of Cardiology [16]. Patients with ACS had ST-segment elevation myocardial infarction (STEMI), none-ST-segment elevation myocardial infarction (NSTEMI), and unstable angina pectoris [16]. Diabetes mellitus was diagnosed according to the current clinical criteria recommended in the 2016 Standards of Medical Care in Diabetes in 2016 [17].
The exclusion criteria included prior myocardial infarction (MI) or coronary artery bypass graft (CABG), a history of organic heart disease, or co-morbidities that included infection or inflammatory disease, liver or kidney disease, and long-term antiplatelet and anticoagulant treatments. PCI was performed by using a drug-eluting stent according to the standard approach. The success criteria for PCI included the Thrombolysis in Myocardial Infarction (TIMI) flow grade 3 and residual stenosis <10%. Appropriate standard treatment was given in the perioperative period following PCI according to the discretion of the attending cardiologist. Data from all patients were collected from the hospital information system by one author and checked by another author. The Global Registry of Acute Coronary Events (GRACE) risk score for ACS was calculated for each patient according to the current guidelines [18,19].
Laboratory analysis
Peripheral venous blood samples were collected before PCI to avoid contamination with contrast media. Blood samples were centrifuged at 4000 rpm for 10 min to separate the serum. Then, the separated serum was aliquoted into Eppendorf tubes and stored at −80°C for further analysis. Serum calprotectin levels were measured using a commercial calprotectin enzyme-linked immunosorbent assay (ELISA) kit (Hycult Biotech Inc., Wayne, PA, USA), according to the manufacturer’s instructions. All routine laboratory tests were performed using the standardized clinical laboratory methods used at the Affiliated Hospital of Jining Medical University. Left ventricular ejection fraction (LVEF) and left atrial diameter (LAD) were evaluated by echocardiography on hospital admission.
Follow-up and clinical evaluation
All 273 patients were regularly followed-up each month after discharge by telephone communication or face-to-face clinic visits. At 12-month follow-up, the primary composite endpoint of major adverse cardiovascular events (MACE) was evaluated that included non-fatal MI, cardiovascular death, and the need for revascularization procedures due to target vessel revascularization (TVR), which was defined by the need for an unplanned repeat PCI or coronary artery procedure, such as bypass.
Statistical analysis
Statistical analysis was performed using SPSS version 20.0 software (IBM, Chicago, IL, USA). Data with a normal distribution were evaluated using the Kolmogorov-Smirnov test. Continuous variables were expressed as the mean±standard deviation (SD), and categorical variables as frequencies and percentages. Student’s t-test was used to compare continuous variables. Comparisons of categorical variables were conducted using the chi-squared (χ2) test or Fisher’s exact test. A receiver operating curve (ROC) was used to analyze the optimal cutoff value of calprotectin for predicting MACE at 12 months following PCI in patients with diabetes and ACS. The relationship of clinical variables with MACE was examined by univariate analysis and the multivariate Cox proportional regression model. The event-free survival curve was created based on the Kaplan-Meier method. A P-value <0.05 was considered to be statistically significant.
Results
Baseline characteristics
Baseline characteristics of the 273 enrolled patients with diabetes who underwent percutaneous coronary intervention (PCI) for acute coronary syndrome (ACS) were evaluated. Serum values for calprotectin allowed the patients in the study to be divided into two groups (low and high serum calprotectin) based on the serum levels of calprotectin, <4.1 μg/mL (n=122) (44.7%) and ≥4.1 μg/mL (n=151) (55.3%), as shown Table 1. During the study period, the mean age of all enrolled patients was 63.4±8.5 years, and 62.4% were male. The median serum level of calprotectin was 4.1 μg/mL (range, 0.8–11.3 μg/mL). The mean value of the Global Registry of Acute Coronary Events (GRACE) risk score for ACS was 136.78±38.83. Patients with a high calprotectin level had significantly increased levels of fasting plasma glucose, and GRACE scores, and significantly lower high-density lipoprotein cholesterol (HDL-C) levels than patients with a low calprotectin level (all P<0.05) (Table 1).
Table 1.
Baseline characteristics and serum protectin levels of the patients with diabetes who underwent percutaneous coronary intervention (PCI) for acute coronary syndrome (ACS).
| Variables | Serum calprotectin level (μg/mL) | P-value | |
|---|---|---|---|
| <4.1 μg/mL (n=122) | ≥4.1 μg/mL (n=151) | ||
| Demographic characteristics | |||
| Age, years | 59.5±7.2 | 61.2±7.6 | 0.06 |
| Male | 74 (60.7%) | 95 (62.9%) | 0.70 |
| BMI, kg/m2 | 25.6±2.8 | 25.9±3.2 | 0.42 |
| Underlying diseases | |||
| Hypertension | 79 (64.8%) | 93 (61.6%) | 0.29 |
| Hyperlipidemia | 66 (54.1%) | 76 (50.3%) | 0.54 |
| Chronic kidney disease | 55 (45.1%) | 73 (48.3%) | 0.59 |
| Medication | |||
| OHA | 31 (25.4%) | 41 (27.2%) | 0.75 |
| Insulin | 56 (45.9%) | 80 (52.9%) | 0.24 |
| ACEIs/ARBs | 88 (72.1%) | 106 (70.2%) | 0.73 |
| Statins | 112 (92.6%) | 139 (92.1%) | 0.94 |
| Beta-blockers | 91 (75.2%) | 109 (72.2%) | 0.66 |
| Laboratory tests | |||
| LDL-C, mmol/l | 2.28±0.52 | 2.26±0.54 | 0.76 |
| HDL-C, mmol/l | 1.32±0.22 | 0.94±0.21 | <0.01 |
| TC, mmol/l | 4.33±1.07 | 4.36±1.02 | 0.81 |
| TG, mmol/l | 2.11±1.13 | 2.13±1.26 | 0.04 |
| FPG, mmol/l | 7.22±1.45 | 9.46±1.89 | <0.01 |
| HbA1c,% | 7.12±1.24 | 7.22±1.63 | 0.58 |
| Cr, μmol/l | 78.12±12.11 | 76.82±16.82 | 0.47 |
| eGFR, ml/min/1.73 m2 | 72.26±23.46 | 68.82±18.73 | 0.18 |
| hs-CRP (ng/ml) | 6.65±4.71 | 8.61±5.98 | <0.01 |
| PCI related data | |||
| Number of stents, per case | 1.71±1.12 | 1.73±1.14 | 0.88 |
| GRACE score | 132.16±37.22 | 143.52±35.68 | 0.01 |
| LVEF, % | 54.23±11.05 | 53.32±10.11 | 0.48 |
| LAD, mm | 31.42±2.62 | 32.12±4.62 | 0.14 |
DM – diabetes mellitus; BMI – body mass index; OHA – oral hypoglycemic agent; ACEIs – angiotensin-converting enzyme inhibitors; ARBs – angiotensin receptor blockers; LDL-C – low-density lipoprotein cholesterol; HDL-C – high-density lipoprotein cholesterol; FPG – fasting plasma glucose; TC – total cholesterol; TG – triglycerides; Cr – creatinine. LVEF – left ventricular ejection fraction; LAD – left atrial diameter; GRACE – Global Registry of Acute Coronary Events.
Incidence of major adverse cardiovascular events (MACE) during 12-month follow-up after PCI
Target vessel revascularization (TVR) was defined by the need for an unplanned repeat PCI or coronary artery procedure, such as bypass. During the 12-month follow-up, out of 273 patients included in this study, 47 (17.2%) patients experienced MACE, including 27 patients (9.9%) with TVR, 13 patients (4.8%) with non-fatal MI, and seven patients (2.6%) who died from a cardiac cause. Compared with patients with a low serum calprotectin level, the patients with a high serum calprotectin level had a significantly increased incidence of MACE (21.9% vs. 11.5%; P=0.02). No significant difference was observed between the two groups in terms of cardiac mortality (2.8% vs. 2.0%; P=0.92) and non-fatal MI (4.1% vs. 5.3%; P=0.21), but the TVR was significantly increased in patients with a low serum calprotectin level (4.9% vs. 13.9%; P=0.01).
Predictive value of serum calprotectin level for MACE
The results of receiver operating characteristic (ROC) analysis showed that area under the curve (AUC) of serum calprotectin level that was predictive for MACE was 0.79 (95% CI, 0.63–0.97; P<0.01), and optimal cutoff value was 3.8 μg/mL (Figure 1). The Kaplan-Meier curve for MACE-free survival according to the serum calprotectin level showed that there was a significant difference between the two groups (P=0.01) (Figure 2).
Figure 1.
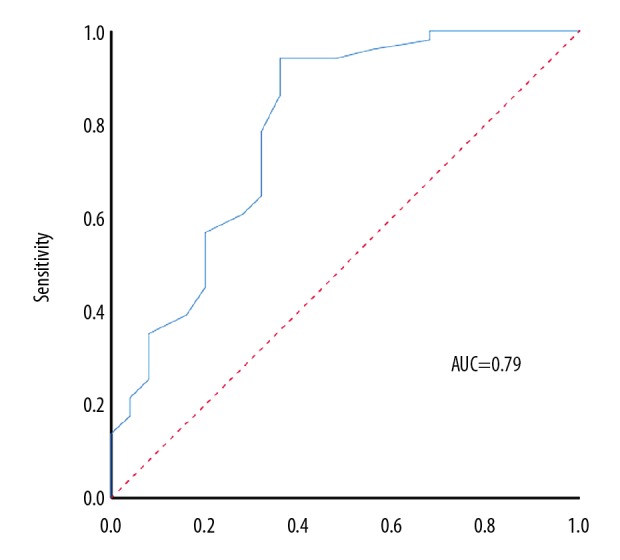
Receiver-operator characteristic (ROC) curve for the predictive level of serum calprotectin for major adverse cardiovascular events (MACE) in patients with diabetes who underwent percutaneous coronary intervention (PCI) for acute coronary syndrome (ACS). The area under the curve (AUC) is 0.79, with a 95% confidence interval (95% CI) of 0.63–0.97.
Figure 2.
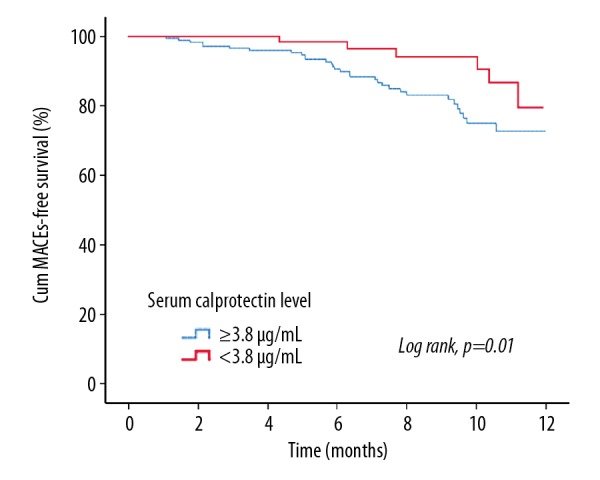
Kaplan-Meier event-free survival curves and serum calprotectin levels in patients with diabetes who underwent percutaneous coronary intervention (PCI) for acute coronary syndrome (ACS). Patients with lower serum calprotectin levels had a significantly improved 12-month major adverse cardiovascular event (MACE)-free survival compared with patients with higher serum calprotectin levels (P=0.01).
Univariate analysis showed that the serum calprotectin level was significantly associated with a risk for MACE following PCI (HR, 1.56; 95% CI, 1.08–4.62; P=0.01). Adjustment for potential confounding factors included age, body mass index (BMI), hypertension, hyperlipidemia, insulin therapy, the use of angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs), treatment with statins, the GRACE score, and the left ventricular ejection fraction (LVEF). Following adjustment, Cox proportional regression analysis showed that the serum calprotectin level (HR, 2.11; 95% CI, 1.14–6.65; P<0.01), the GRACE score (HR, 2.38; 95% CI, 1.13–9.65; P=0.01), and the LVEF (HR, 0.82; 95% CI, 0.78–0.98; P=0.02) were significant independent predictive factors for MACE in patients with diabetes who underwent PCI for ACS (Table 2).
Table 2.
Univariate and multivariate analysis of predictors for major adverse cardiovascular events (MACE) in patients with diabetes who underwent percutaneous coronary intervention (PCI) for acute coronary syndrome (ACS).
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Age, years | 1.03 | 0.88–1.32 | 0.25 | |||
| BMI, kg/m2 | 0.98 | 0.87–1.35 | 0.66 | |||
| Hypertension | 1.02 | 0.95–1.42 | 0.91 | |||
| Hyperlipidemia | 0.86 | 0.53–1.21 | 0.42 | |||
| Chronic kidney disease | 1.01 | 0.98–1.09 | 0.56 | |||
| Statin use | 0.91 | 0.62–1.61 | 0.74 | |||
| Insulin | 1.61 | 0.84–4.67 | 0.22 | |||
| ACEIs/ARBs use | 1.03 | 0.82–2.52 | 0.48 | |||
| Number of stents, per case | 1.00 | 0.89–1.12 | 0.96 | |||
| GRACE score | 2.68 | 1.01–4.58 | 0.02 | 2.38 | 1.13–9.65 | 0.01 |
| LVEF,% | 0.78 | 0.71–0.92 | 0.03 | 0.82 | 0.78–0.98 | 0.02 |
| LDL-C, mmol/l | 1.01 | 0.97–1.02 | 0.51 | |||
| Calprotectin level | 1.56 | 1.08–4.62 | 0.01 | 2.11 | 1.14–6.65 | <0.01 |
MACE – major adverse cardiovascular events; PCI – percutaneous coronary intervention; HR – Hazard ratio; 95% CI – 95% confidence interval; BMI – body mass index; ACEIs – angiotensin-converting enzyme inhibitors; ARBs – angiotensin receptor blockers; LVEF – left ventricular ejection fraction; LDL-C – low-density lipoprotein cholesterol; GRACE – Global Registry of Acute Coronary Events.
Discussion
The aim of this retrospective study was to evaluate the prognostic role of serum calprotectin in patients with diabetes who underwent percutaneous coronary intervention (PCI) for acute coronary syndrome (ACS). The findings showed high serum levels of calprotectin were significantly associated with levels of plasma glucose, high-density lipoprotein cholesterol (HDL-C), and the Global Registry of Acute Coronary Events (GRACE) risk score for ACS. In the prognostic analysis, serum calprotectin level was also significantly associated with the clinical outcome following PCI. Patients with a higher serum calprotectin level had a significantly increased risk of developing major adverse cardiovascular events (MACE) at 12-month follow-up when compared with patients with a lower serum calprotectin level. Furthermore, the higher incidence of MACE in the patients with poor glycemic control was associated with a significantly increased rate of target vessel revascularization (TVR), or the need for an unplanned repeat PCI or coronary artery procedure. When confounding factors were excluded, the serum calprotectin level was an independent predictor for the clinical outcome in patients with diabetes who underwent PCI for ACS.
Calprotectin, or S100A8/A9, is a heterocomplex of calcium-binding proteins released from neutrophils and monocytes, which is an indicator of severity for several inflammatory diseases [20]. Coronary artery disease (CAD) is due to the local complications of atherosclerosis, which is a chronic inflammatory condition. Calprotectin has previously been shown to be associated with the development of CAD and has a role as a mediator of atherosclerosis. Viemann et al. incubated human microvascular endothelial cells with calprotectin and showed that calprotectin increased platelet aggregation, inflammation, and endothelial permeability [21]. Ehlermann et al. showed that calprotectin had a dose-dependent effect on the activation of proinflammatory responses in the endothelium in diabetes [22]. Calprotectin was shown to stimulate the activation of nuclear factor-κB (NF-κB) activation by interacting with ligands of the receptor for advanced glycation end products (RAGE), which may furtherly contribute to inflammation and vascular complications in patients with CAD [23]. It has been previously reported that calprotectin levels were significantly increased in patients with ACS [24,25]. Also, increased calprotectin levels have been identified in coronary artery thrombus and coronary atherosclerotic plaques from patients with ACS [26,27]. The findings from the present study showed that the serum calprotectin level was significantly associated with the incidence of MACE following PCI, which was consistent with the findings from previously published studies.
Patients with diabetes have specific clinical characteristics that include insulin resistance, obesity, and dyslipidemia [28]. In 2011, Catalan et al. showed a potential role for calprotectin as a chemotactic factor in macrophages recruitment in inflammation and the development of co-morbidities associated with obesity [29]. Ortega et al. recently showed that serum levels of calprotectin were associated with inflammation independent of obesity in patients with diabetes mellitus [30]. In the present study, we also found that patients with diabetes had increased serum levels of calprotectin, which were significantly associated with levels of high-density lipoprotein cholesterol (HDL-C) and fasting plasma glucose (FBG), but not with the body mass index (BMI). Pedersen et al. reported that patients with diabetes mellitus had higher concentrations of plasma calprotectin, which were associated with myocardial ischemia, which was consistent with the findings of the present study [13]. Furthermore, hyperglycemia, hyperinsulinemia, chronic inflammation, abnormal platelet function, and insulin resistance may promote vascular neointimal hyperplasia following stent placement in patients with diabetes, resulting in the development of restenosis following PCI [31]. In this study, we found that a high rate of TVR was a major contributor to the high incidence of MACE following PCI in patients with diabetes, which supports the relationship between the risk of restenosis following PCI and diabetes mellitus.
In the present study, we found that increased serum levels of calprotectin were significantly associated with MACE in patients with diabetes and ACS treated with PCI. This retrospective study did not investigate the underlying mechanisms for the relationship between serum calprotectin levels and clinical outcome following PCI. However, it is possible that in patients with diabetes and ACS, inflammation and atherosclerosis may be promoted by calprotectin. These possible associations require further study. The findings from this study also showed that patients with high serum calprotectin levels had a higher GRACE risk score for ACS, indicating that patients with a higher calprotectin might have had more severe cardiac disease. This hypothesis is supported by the findings from a study by Larsen et al., which showed that calprotectin could increase platelet aggregation, which might result in increased cardiac ischemia from coronary artery occlusion [32].
This study had several limitations. The single-center study design and short duration of follow-up may have introduced bias into the study analysis or interpretation of the results. The cutoff level of serum calprotectin was based on values derived from receiver operating characteristic (ROC) curve analysis of the data used in this study. However, optimal predictive cutoff levels of serum calprotectin for clinical outcome after PCI should be evaluated from meta-analysis data. Finally, this study included a small patient samples size, and the findings should be interpreted cautiously until they can be supported with future large-scale, prospective, multi-center studies.
Conclusions
This retrospective study was conducted to evaluate the prognostic role of serum calprotectin in patients with diabetes who underwent percutaneous coronary intervention (PCI) for acute coronary syndrome (ACS). The findings showed that patients with diabetes who had lower serum levels of calprotectin were at lower risk of restenosis and showed improved clinical outcome following PCI during the 12-month follow-up.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Dauerman HL. Five years of coronary artery disease. Coron Artery Dis. 2018;29(5):366–67. doi: 10.1097/MCA.0000000000000634. [DOI] [PubMed] [Google Scholar]
- 2.Waldeyer C, Brunner FJ, Braetz J, et al. Adherence to mediterranean diet, high-sensitive C-reactive protein, and severity of coronary artery disease: Contemporary data from the INTERCATH cohort. Atherosclerosis. 2018;275:256–61. doi: 10.1016/j.atherosclerosis.2018.06.877. [DOI] [PubMed] [Google Scholar]
- 3.Mahmud E, Ben-Yehuda O. Percutaneous coronary intervention in acute coronary syndrome: Completing the job saves lives. J Am Coll Cardiol. 2018;72(17):2000–2. doi: 10.1016/j.jacc.2018.08.2129. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Ping Yen Yan B, et al. Clinical and economic analysis of lipid goal attainments in Chinese patients with acute coronary syndrome who received post-percutaneous coronary intervention. J Atheroscler Thromb. 2018;25(12):1255–73. doi: 10.5551/jat.44818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tscharre M, Herman R, Rohla M, et al. Uric acid is associated with long-term adverse cardiovascular outcomes in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Atherosclerosis. 2018;270:173–79. doi: 10.1016/j.atherosclerosis.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Katsiki N, Mikhailidis DP. Management of patients with type 2 diabetes mellitus and acute coronary syndrome: Better be safe than sorry! J Diabetes Complications. 2019;33(7):465–67. doi: 10.1016/j.jdiacomp.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Selvin E, Coresh J, Golden SH, et al. Glycemic control and coronary heart disease risk in persons with and without diabetes: The atherosclerosis risk in communities study. Arch Intern Med. 2005;165(16):1910–16. doi: 10.1001/archinte.165.16.1910. [DOI] [PubMed] [Google Scholar]
- 8.Mellen PB, Cefalu WT, Herrington DM. Diabetes, the metabolic syndrome, and angiographic progression of coronary arterial disease in postmenopausal women. Arterioscler Thromb Vasc Biol. 2006;26(1):189–93. doi: 10.1161/01.ATV.0000191656.71812.7c. [DOI] [PubMed] [Google Scholar]
- 9.John JM, Bhatt DL. Management of acute coronary syndrome in diabetes mellitus. Herz. 2004;29(5):532–41. doi: 10.1007/s00059-004-2614-0. [DOI] [PubMed] [Google Scholar]
- 10.Katsiki N, Papanas N. Diabetes mellitus and acute coronary syndrome: A lethal combination requiring better therapeutic strategies. Curr Vasc Pharmacol. :2019. doi: 10.2174/1570161117666190328095249. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Ma CY, Xu ZY, Wang SP, et al. Change of inflammatory factors in patients with acute coronary syndrome. Chin Med J (Engl) 2018;131(12):1444–49. doi: 10.4103/0366-6999.233953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hessian PA, Edgeworth J, Hogg N. MRP-8 and MRP-14, two abundant Ca(2+)-binding proteins of neutrophils and monocytes. J Leukoc Biol. 1993;53(2):197–204. [PubMed] [Google Scholar]
- 13.Pedersen L, Nybo M, Poulsen MK, et al. Plasma calprotectin and its association with cardiovascular disease manifestations, obesity and the metabolic syndrome in type 2 diabetes mellitus patients. BMC Cardiovasc Disord. 2014;14:196. doi: 10.1186/1471-2261-14-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morrow DA, Wang Y, Croce K, et al. Myeloid-related protein 8/14 and the risk of cardiovascular death or myocardial infarction after an acute coronary syndrome in the Pravastatin or Atorvastatin Evaluation and Infection Therapy: Thrombolysis in Myocardial Infarction (PROVE IT-TIMI 22) trial. Am Heart J. 2008;155(1):49–55. doi: 10.1016/j.ahj.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dominguez-Rodriguez A, Abreu-Gonzalez P. [Inflammatory biomarkers in ischemic acute coronary syndrome]. Emergencias. 2015;27(5):319–24. [in Spanish] [PubMed] [Google Scholar]
- 16.Roffi M, Patrono C, Collet JP, et al. [2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC)]. G Ital Cardiol (Rome) 2016;17(10):831–72. doi: 10.1714/2464.25804. [DOI] [PubMed] [Google Scholar]
- 17.American Diabetes Association. Standards of Medical Care in Diabetes-2016 Abridged for Primary Care Providers. Clin Diabetes. 2016;34(1):3–21. doi: 10.2337/diaclin.34.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu D, Huang Z, Chan TM, et al. Acute coronary syndrome risk prediction based on GRACE risk score. Stud Health Technol Inform. 2017;245:398–402. [PubMed] [Google Scholar]
- 19.Gong IY, Goodman SG, Brieger D, et al. GRACE risk score: Sex-based validity of in-hospital mortality prediction in Canadian patients with acute coronary syndrome. Int J Cardiol. 2017;244:24–29. doi: 10.1016/j.ijcard.2017.06.055. [DOI] [PubMed] [Google Scholar]
- 20.Ricciuto A, Griffiths AM. Clinical value of fecal calprotectin. Crit Rev Clin Lab Sci. 2019;56(5):307–20. doi: 10.1080/10408363.2019.1619159. [DOI] [PubMed] [Google Scholar]
- 21.Viemann D, Strey A, Janning A, et al. Myeloid-related proteins 8 and 14 induce a specific inflammatory response in human microvascular endothelial cells. Blood. 2005;105(7):2955–62. doi: 10.1182/blood-2004-07-2520. [DOI] [PubMed] [Google Scholar]
- 22.Ehlermann P, Eggers K, Bierhaus A, et al. Increased proinflammatory endothelial response to S100A8/A9 after preactivation through advanced glycation end products. Cardiovasc Diabetol. 2006;5:6. doi: 10.1186/1475-2840-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghavami S, Rashedi I, Dattilo BM, et al. S100A8/A9 at low concentration promotes tumor cell growth via RAGE ligation and MAP kinase-dependent pathway. J Leukoc Biol. 2008;83(6):1484–92. doi: 10.1189/jlb.0607397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montagnana M, Danese E, Lippi G. Calprotectin and cardiovascular events. A narrative review. Clin Biochem. 2014;47(12):996–1001. doi: 10.1016/j.clinbiochem.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 25.Kruzliak P, Novak J, Novak M, Fodor GJ. Role of calprotectin in cardiometabolic diseases. Cytokine Growth Factor Rev. 2014;25(1):67–75. doi: 10.1016/j.cytogfr.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 26.Altwegg LA, Neidhart M, Hersberger M, et al. Myeloid-related protein 8/14 complex is released by monocytes and granulocytes at the site of coronary occlusion: A novel, early, and sensitive marker of acute coronary syndromes. Eur Heart J. 2007;28(8):941–48. doi: 10.1093/eurheartj/ehm078. [DOI] [PubMed] [Google Scholar]
- 27.Miyamoto S, Ueda M, Ikemoto M, et al. Increased serum levels and expression of S100A8/A9 complex in infiltrated neutrophils in atherosclerotic plaque of unstable angina. Heart. 2008;94(8):1002–7. doi: 10.1136/hrt.2007.121640. [DOI] [PubMed] [Google Scholar]
- 28.Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83(2):461s–65s. doi: 10.1093/ajcn/83.2.461S. [DOI] [PubMed] [Google Scholar]
- 29.Catalan V, Gomez-Ambrosi J, Rodriguez A, et al. Increased levels of calprotectin in obesity are related to macrophage content: Impact on inflammation and effect of weight loss. Mol Med. 2011;17(11–12):1157–67. doi: 10.2119/molmed.2011.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ortega FJ, Sabater M, Moreno-Navarrete JM, et al. Serum and urinary concentrations of calprotectin as markers of insulin resistance and type 2 diabetes. Eur J Endocrinol. 2012;167(4):569–78. doi: 10.1530/EJE-12-0374. [DOI] [PubMed] [Google Scholar]
- 31.Indolfi C, Torella D, Cavuto L, et al. Effects of balloon injury on neointimal hyperplasia in streptozotocin-induced diabetes and in hyperinsulinemic nondiabetic pancreatic islet-transplanted rats. Circulation. 2001;103(24):2980–86. doi: 10.1161/01.cir.103.24.2980. [DOI] [PubMed] [Google Scholar]
- 32.Larsen SB, Grove EL, Pareek M, et al. Calprotectin and platelet aggregation in patients with stable coronary artery disease. PLoS One. 2015;10(5):e0125992. doi: 10.1371/journal.pone.0125992. [DOI] [PMC free article] [PubMed] [Google Scholar]


