Abstract
Background
Preeclampsia is a severe obstetric complication affecting the health of pregnant women. The aim of this study was to determine the effect of LAMA4 gene on extravillous trophoblasts (EVTs) in the pathogenesis of preeclampsia and its possible regulatory mechanism.
Material/Methods
HTR-8/SVneo cells were transfected with small-interfering ribonucleic acid (siRNA) targeting LAMA. The LAMA4 protein level was detected via Western blotting. Moreover, the influences of LAMA4 gene on the proliferation, migration and invasion of HTR-8/SVneo cells were detected via cell counting kit-8 (CCK-8) assay and Transwell assay. We also assessed the influences of LAMA4 gene on vascular endothelial growth factor (VEGF) and soluble fms-like tyrosine kinase-1 (sFlt-1) messenger RNA (mRNA) levels in HTR-8/SVneo cells as measured by reverse transcription-polymerase chain reaction (RT-PCR).
Results
The cell lines with downregulation of LAMA4 gene were successfully established by transfection. Compared with those in the normal group, the proliferation, migration, and invasion of HTR-8/SVneo cells declined, the VEGF mRNA level was reduced, and the sFlt-1 mRNA level was increased in the silencing group.
Conclusions
Downregulation of the LAMA4 gene inhibits the proliferation, migration, and invasion of EVT to suppress the expression of vascular factors, leading to the occurrence or development of preeclampsia. Our data provide new insights into modulation of LAMA4 expression as a potential target for therapy against preeclampsia. Further research is needed on placenta sampling from pre-eclamptic pregnancies to validate the effect of LAMA4 expression compared to control pregnancies.
MeSH Keywords: Cell Migration Assays, Neoplasm Invasiveness, Pre-Eclampsia
Background
Preeclampsia is a placental disease presenting as a severe obstetric complication, not only affecting the health of pregnant women, but also seriously threatening the health of fetuses. It is a major cause of mortality among fetuses and pregnant women [1,2]. The pathogenesis of preeclampsia is complex, and the pathogenic factors mainly include an abnormal systemic inflammatory response, placental ischemia and hypoxia, excessive oxidative stress, and vascular substance imbalance. In-depth studies have demonstrated that preeclampsia is related to disorders of apoptosis and invasion ability of extravillous trophoblasts (EVTs). The abnormal apoptosis of trophoblasts reduces their invasion ability in the uterine spiral artery, thus resulting in the occurrence and development of preeclampsia [3–5].
Human trophoblasts are differentiated from extra-embryonic cells, which are the key cells in the maternal-fetal interface. After implantation, the fertilized ovum first differentiates into cytotrophoblasts at the implantation site and then form syncytiotrophoblasts a few days later. At 2 weeks after conception, syncytiotrophoblasts transform into primary villous cells known as EVTs, except for those at other sites. EVTs are divided into proliferative-type and invasive-type. Previous finding showed that, in the case of signal stimuli against invasive-type EVTs, the deficient invasion ability of EVT led to insufficient spiral artery remodeling and reduced placental perfusion, seriously affecting fetal development [6]. In normal pregnancies, the result of normal spiral artery remodeling is the formation of blood vessels that are dilated and have low resistance. However, pre-eclampsia occurs as a result of a poor trophoblast invasion, which leads to abnormal spiral artery remodeling and formation of high-capacitance blood vessels. Spiral artery remodeling favors the continuous formation of new vessels and reduces the occurrence and development of preeclampsia [7].
Blood vessels are composed of multiple types of cells, in which laminin (LN), one of the components of the cell basement membrane, has drawn the attention of researchers. LN alpha 4 (LNα4) is encoded by the LAMA4 gene and is involved in biological processes such as proliferation, apoptosis, and migration of endothelial cells and tumor cells [8,9]. In this study, therefore, we aimed to determine the regulatory role of the LAMA4 gene on EVTs in the early pathogenesis of preeclampsia.
Material and Methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM)/F12 medium, fetal bovine serum (FBS), double antibody, and trypsin were purchased from Gibco (USA). LAMA4 primary antibody and secondary antibody were bought from CST (USA). The cell counting kit-8 (CCK-8) kit was obtained from Dojindo (Japan). Transwell chambers were bought from Corning (USA). Vascular endothelial growth factor (VEGF) and soluble fms-like tyrosine kinase-1 (sFlt-1) primers were from Invitrogen, USA. Small-interfering RNA (siRNA) targeting LAMA was acquired from Shanghai GenePharma Co. Lipofectamine 2000 transfection reagent was from Thermo (USA). Human EVT HTR-8/SVneo cell lines were purchased from BeNa.
Instruments
We used a multifunctional microplate reader purchased from Gene (USA). The gel electrophoresis apparatus and membrane transfer device were bought from BioRad (USA).
Cell culture and transfection
HTR-8/SVneo cells were cultured in the DMEM/F12 medium containing 15% FBS and 1% double antibody in an incubator with 5% CO2 at 37°C. When the growth density reached 80–90%, the cells were digested with 3 mL pre-heated trypsin for 5 min, and the medium was added to terminate the digestion, followed by centrifugation at 1500 rpm for 5 min. The cells were cultured for subsequent experiments. LAMA4 siRNA and empty vector were transfected into HTR-8/SVneo cells using Lipofectamine 2000 reagent, and the LAMA4 protein level was detected after 72 h.
Detection of LAMA4 protein level via Western blotting
The protein was extracted from HTR-8/SVneo cells transfected with LAMA4 siRNA and empty vector, and the protein concentration was detected using Bradford’s method. The protein was loaded with SDS-PAGE concentration gel at a constant voltage of 80 V, and separation gel at a constant voltage of 100 V. After that, the protein was transferred onto the membrane, blocked for 2 h, and incubated with the LAMA4 primary antibody at 4°C overnight. On the next morning, the protein was incubated with the secondary antibody for 1 h and washed with washing buffer. The color was developed using DAB developing solution, and the optical density value of the band was calculated using Image J software.
Detection of cell proliferation via CCK-8 assay
Cells in logarithmic growth phase were inoculated into a 96-well plate (1×103 cells/well) and cultured in an incubator overnight. On the next day, 10 μL CCK-8 solution was added into the plate, followed by culturing for another 4 h. The absorbance was measured (λ=450 nm) using a multifunctional microplate reader, and 6 repeated wells were set-up in each group.
Detection of cell migration via Transwell assay
Cells in logarithmic growth phase were digested and then resuspended in serum-free medium. We added 200 μL cell suspension into the Transwell chamber, and the cell medium was added into the lower chamber (avoiding bubbles), followed by culturing in an incubator for 24 h. Cells in the upper chamber were washed with PBS, and the cells in the lower chamber were fixed with 4% paraformaldehyde and stained with crystal violet working solution for 10 min. Finally, the number of migrated cells was counter under a microscope.
Detection of cell invasion via Transwell assay
Matrigel was diluted with serum-free medium, mixed evenly, and used to coat the Transwell chamber. We added 40 μL diluent and the cells were then incubated in an incubator. The cells were added into the upper chamber, while the serum-containing medium was added into the lower chamber, followed by culturing for another 48 h. After the medium in the chamber was removed and the culture was terminated, the cells were fixed with 4% paraformaldehyde and stained with crystal violet working solution. Finally, the number of invaded cells was counter under a microscope.
Detection of VEGF and sFlt-1 mRNA levels via RT-PCR
The cells in both groups were lysed with pre-cooled TRIzol reagent, the total RNA was extracted from cells, and the RNA concentration was measured. The RNA was qualified if its concentration was 1.8–2.0. Then, RNA was reverse-transcribed into cDNA according to the instructions of the reverse transcription kit, followed by PCR amplification based on the amplification system (a total of 35 cycles). In brief, after determining the RNA concentration, in vitro reverse transcription was performed in a volume of 20 μL with a system including 4 μl 25 mM MgCl2, 2 μL reverse transcription 10×buffer, 2 μL 104 mM dNTP mixture, 0.5 μL ribonuclease inhibitor, 15U AMV reverse transcriptase, 0.5 μg random primers, 1μ total RNA and nuclease-free water to a final volume of 20 μL at 42°C for 15 min and 85°C denaturing (Promega, USA). PCR was then performed by using the Ex Taq kit (Takara, Japan) (7.5 μL 2×premix, 10 mM forward and reverse primers, dH2O to a final volume of 15 μL) in the following conditions: 94°C denature for 3 min, followed by 35 cycles each with 94°C denaturing for 30 s, 58°C annealing for 30 s, and extension for 30 s with PCR Cycler T100 (BioRad, USA). Primer sequences were:
VEGF-F, 5′-TGCAGATTATGCGGATCAAACC-3′.
VEGF-R, 5′-TGCATTCACATTTGTTGTGCTGTAG-3′.
sFLT-1-F, 5′-ACAATCAGAGGTGAGCACTGCAA-3′.
sFLT-1-R, 5′-TCCGAGCCTGAAAGTTAGCAA-3.
β-actin-F, 5′-CGTACCACTGGCATCGTGAT-3′.
β-actin-R 5′-GTGTTGGCGTACAGGTCTTTG-3′.
Finally, gel electrophoresis was performed, and the optical density value of bands was analyzed using Image J software, with β-actin as an internal reference.
Statistical processing
GraphPad 5.0 software was used for statistical analysis and processing of all data. The t test was used to assess differences in data between 2 groups, and one-way analysis of variance was used among multiple groups. All data are expressed as mean±standard deviation, and p<0.05 suggests that the difference was statistically significant.
Results
Transfection of LAMA4 siRNA into cells
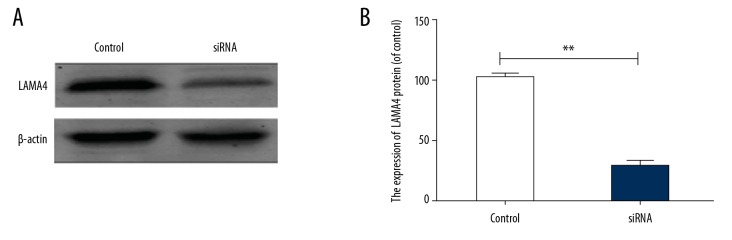
To detect whether LAMA4 siRNA was successfully transfected, the LAMA4 protein level was detected via Western blotting (Figure 1A). The results revealed that the expression of LAMA4 in the silencing group was significantly lower than in the empty vector group (p<0.01) (Figure 1B).
Figure 1.
LAMA4 protein level. (A) Western blotting band, (B) LAMA4 protein level. ** p<0.01, compared to control group.
Influence of changes in LAMA4 gene expression on proliferation of HTR-8/SVneo cells
Cell proliferation was detected using the CCK-8 kit, and the absorbance value was converted into the proliferation rate. The results showed that the absorbance value of HTR-8/SVneo cells was lower and the proliferation rate was significantly reduced in the silencing group compared with the empty vector group (p<0.05) (Figure 2), indicating that downregulation of LAMA4 expression inhibited the proliferation of HTR-8/SVneo cells.
Figure 2.
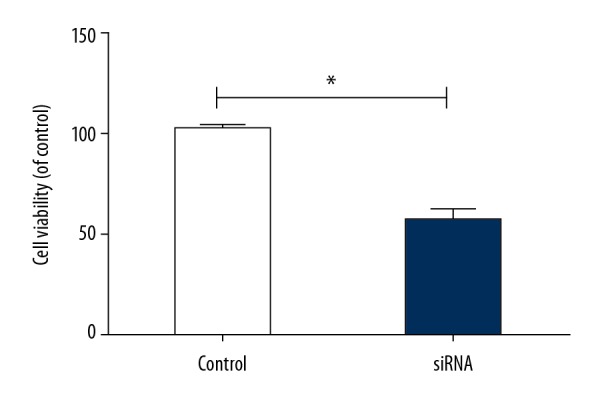
Survival rate of HTR-8/SVneo cells. * p<0.05, compared to control group.
Influence of changes in LAMA4 gene expression on migration of HTR-8/SVneo cells
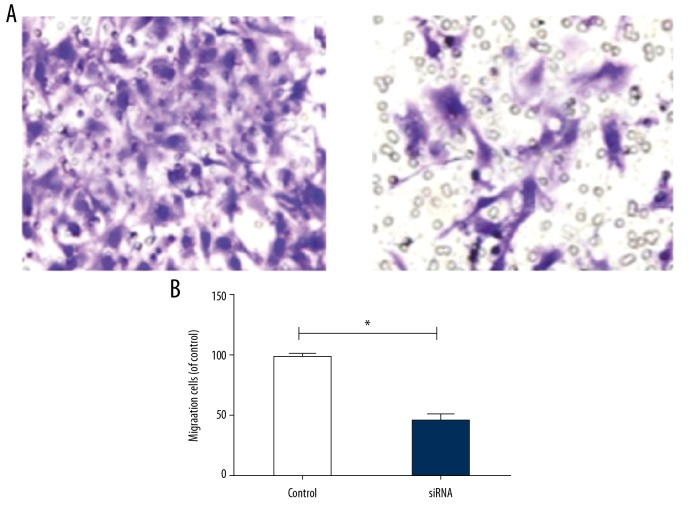
The results of Transwell migration assay showed that the number of migrating HTR-8/SVneo cells in the silencing group was significantly lower than in the empty vector group (p<0.05) (Figure 3A, 3B), suggesting that the reduction of LAMA4 level reduced the migration rate of HTR-8/SVneo cells.
Figure 3.
Migration rate of HTR-8/SVneo cells. (A) Number of migrating HTR-8/SVneo cells, (B) Migration rate of HTR-8/SVneo cells. * p<0.05, compared to control group.
Influence of changes in LAMA4 gene expression on invasion of HTR-8/SVneo cells
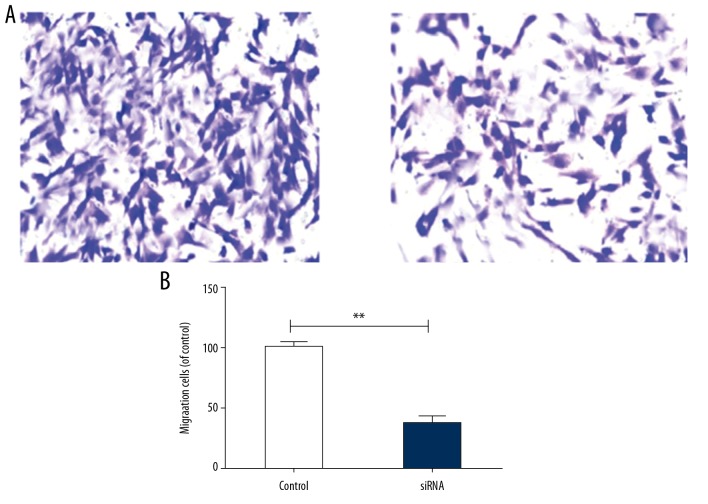
According to the results of the Transwell invasion assay, the number of invading HTR-8/SVneo cells in the silencing group was significantly lower than in the empty vector group (** p<0.01) (Figure 4A, 4B), indicating that the decrease in LAMA4 downregulated the invasion rate of HTR-8/SVneo cells.
Figure 4.
Invasion rate of HTR-8/SVneo cells. (A) Number of invading HTR-8/SVneo cells, (B) Invasion rate of HTR-8/SVneo cells. ** p<0.01, compared to control group.
Influence of changes in LAMA4 gene expression on VEGF and sFlt-1 mRNA levels
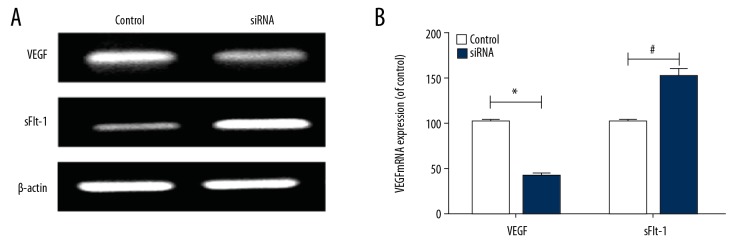
To further study the influences of LAMA4 gene expression on the proliferation, migration, and invasion abilities of HTR-8/SVneo cells, the VEGF and sFlt-1 mRNA levels in HTR-8/SVneo cells were detected via RT-PCR (Figure 5A). It was found that compared with those in the empty vector group, the VEGF mRNA level in HTR-8/SVneo cells was remarkably decreased (* p<0.05) and the sFlt-1 mRNA level was markedly increased in the silencing group (# p<0.05) (Figure 5B), suggesting that decreasing the level of LAMA4 inhibited angiogenesis and induced the occurrence and development of preeclampsia.
Figure 5.
VEGF and sFlt-1 mRNA levels. (A) RT-PCR band, (B) VEGF and sFlt-1 mRNA levels. * p<0.05, # p<0.05, compared to control group.
Discussion
Pregnancy is a complex process, and preeclampsia is a unique disease due to its complex and unclear pathogenesis in pregnancy. Recent research suggests that preeclampsia is caused by a variety of vascular diseases, in which pathological changes in EVTs lead to mother–infant placental vascular dysfunction [10–12]. During normal pregnancy, EVTs invade the uterus and gradually replace smooth muscle cells and arterial endothelial cells in remodeling of the uterine spiral artery, thus increasing the placental blood volume and ensuring fetal development. However, it is noteworthy that EVTs have the characteristics of proliferation, migration and invasion, but their migration and invasion abilities are reduced under stress, leading to insufficient remodeling of the uterine spiral artery and inducing abortion and preeclampsia [13,14]. Therefore, the proliferation, migration, and invasion of EVTs affect the pregnancy outcome, and an in-depth understanding of the biological functions of EVTs could provide a solid foundation for the prevention and treatment of preeclampsia.
The important role of physiological function of EVTs in the pathogenesis of preeclampsia has received research attention. JARID2 protein has significant influences on the survival and invasion ability of EVTs. After EVTs are transfected with JARID2, their survival, migration, and invasion abilities were significantly impaired in the case of JARID2 gene silencing, the mechanism of which may be related to inhibition of the PI3K/Akt signaling pathway [15]. Notably, by testing the serum of pregnant women, the abnormal expression of miR-141 was found, and in-depth research showed that after transfection with miR-141-mimic, trophoblastic cells secreted EVs with increased miR-141 content. These vesicles did not exert effects on trophoblastic cell invasion, but reduced Jurkat T cell proliferation. The elevated levels of miR-141 can be transferred from trophoblasts to immune cells by release and internalization of EVs, suggesting their role in the immune regulation of normal and pathologic pregnancies [16]. In addition, it has been reported that the apoA-1 protein inhibits TNF-α in the invasion of EVTs, and the apoptosis of HTR-8/SVneo cells caused by TNF-α can be inhibited after treatment with apoA-1 protein [17]. The above research results indicate that EVTs play an important role in the occurrence and development of preeclampsia.
It is reported that the LAMA4 gene plays a key role in proliferation, migration, and invasion of tumor cells. A study enrolling colon cancer patients demonstrated that the LAMA4 protein expression was significantly increased in tumor patients [18]. In a study on 48 patients with liver cancer, it was found that the expression level of LAMA4 in liver cancer tissues was significantly higher than that in tumor-free tissues, suggesting that the tumor infiltration was closely related to upregulation of LAMA4 gene expression. In the analysis of tumor vascular basement membrane signaling, it was found that LAMA4 gene expression can affect angiogenesis. Therefore, it is speculated that LAMA4 may be a new marker for the auxiliary diagnosis of liver cancer [19]. The importance of the LAMA4 gene has attracted extensive attention, but there are few reports on the role of LAMA4 in the pathogenesis of preeclampsia. A study on the regulatory effect of the LAMA4 gene on EVTs in placental development showed that the growth of EVTs can be obviously inhibited, and the levels of MMP2 and MMP9 can also be lowered by the inhibition of LAMA4 in negative mediation of the p38 signaling pathway [20], indicating that the LAMA4 gene is involved in regulating the occurrence and development of preeclampsia.
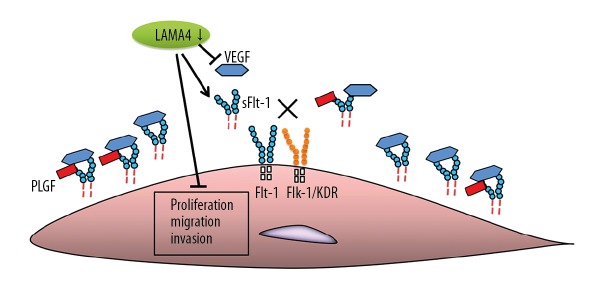
Therefore, in our study, LAMA4 siRNA was transfected into HTR-8/SVneo cells. We found that the proliferation, migration, and invasion of HTR-8/SVneo cells were significantly decreased by LAMA4 gene silencing. To further explore the regulatory mechanism of the LAMA4 gene in preeclampsia, the VEGF and sFlt-1 mRNA levels in HTR-8/SVneo cells were detected, showing that after the LAMA4 level was suppressed, the VEGF mRNA expression level was remarkably decreased, while the sFlt-1 mRNA expression level was clearly increased, indicating that the reduction of LAMA4 affects angiogenesis and inhibits proliferation, migration, and invasion of HTR-8/SVneo cells, thereby inducing and aggravating the development and progression of preeclampsia (Figure 6). A study of the regulatory effects and mechanism of the G protein gamma 7 (GNG7) placental cytotrophoblasts in a rat PE model showed that silencing GNG7 reduced the levels of sFlt1 and sEng, activated the mTOR signaling pathway, and enhanced cell proliferation and differentiation, but inhibited the apoptosis of placental cytotrophoblasts in PE rats [21], suggesting the efficacy of combined use of GNG7 inhibitor and LAMA4 enhancer for treatment of preeclampsia. Further research is needed on placentas from pre-eclamptic pregnancies compared to control pregnancies to validate the effect of LAMA4 expression.
Figure 6.
The interference caused by LAMA4 silencing.
Conclusions
Our preliminary data indicate that the inhibition of LAMA4 expression attenuated the proliferation, migration, and invasion of HTR-8/SVneo cells, suppressed VEGF, and elevated sFlt-1 levels, which opens new avenues for future treatment of preeclampsia.
Footnotes
Source of support: Departmental sources
References
- 1.Phipps E, Prasanna D, Brima W, et al. Preeclampsia: Updates in pathogenesis, definitions, and guidelines. Clin J Am Soc Nephrol. 2016;11(6):1102–13. doi: 10.2215/CJN.12081115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jim B, Karumanchi SA. Preeclampsia: Pathogenesis, prevention, and long-term complications. Semin Nephrol. 2017;37(4):386–97. doi: 10.1016/j.semnephrol.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 3.El-Sayed AAF. Preeclampsia: A review of the pathogenesis and possible management strategies based on its pathophysiological derangements. Taiwan J Obstet Gynecol. 2017;56(5):593–98. doi: 10.1016/j.tjog.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Jido TA, Yakasai IA. Preeclampsia: A review of the evidence. Ann Afr Med. 2013;12(2):75–85. doi: 10.4103/1596-3519.112395. [DOI] [PubMed] [Google Scholar]
- 5.Koual M, Abbou H, Carbonnel M, et al. Short-term outcome of patients with preeclampsia. Vasc Health Risk Manag. 2013;9:143–48. doi: 10.2147/VHRM.S38970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bailey LJ, Alahari S, Tagliaferro A, et al. Augmented trophoblast cell death in preeclampsia can proceed via ceramide-mediated necroptosis. Cell Death Dis. 2017;8(2):e2590. doi: 10.1038/cddis.2016.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lala PK, Nandi P. Mechanisms of trophoblast migration, endometrial angiogenesis in preeclampsia: The role of decorin. Cell Adh Migr. 2016;10(1–2):111–25. doi: 10.1080/19336918.2015.1106669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shan N, Zhang X, Xiao X, et al. The role of laminin α4 in human umbilical vein endothelial cells and pathological mechanism of preeclampsia. Reprod Sci. 2015;22(8):969–79. doi: 10.1177/1933719115570913. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Hou Q, Zhou X. LAMA4 expression is activated by zinc finger E-box-binding homeobox 1 and independently predicts poor overall survival in gastric cancer. Oncol Rep. 2018;40(3):1725–33. doi: 10.3892/or.2018.6564. [DOI] [PubMed] [Google Scholar]
- 10.Palei AC, Granger JP, Tanus-Santos JE. Matrix metalloproteinases as drug targets in preeclampsia. Curr Drug Targets. 2013;14(3):325–34. doi: 10.2174/1389450111314030004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vitoratos N, Hassiakos D, Iavazzo C. Molecular mechanisms of preeclampsia. J Pregnancy. 2012;2012 doi: 10.1155/2012/298343. 298343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zadora J, Singh M, Herse F, et al. Disturbed placental imprinting in preeclampsia leads to altered expression of DLX5, a human-specific early trophoblast marker. Circulation. 2017;136(19):1824–39. doi: 10.1161/CIRCULATIONAHA.117.028110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Craici IM, Wagner SJ, Weissgerber TL, et al. Advances in the pathophysiology of pre-eclampsia and related podocyte injury. Kidney Int. 2014;86(2):275–85. doi: 10.1038/ki.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robillard PY, Dekker G, Chaouat G, et al. Epidemiological studies on primipaternity and immunology in preeclampsia – a statement after twelve years of workshops. J Reprod Immunol. 2011;89(2):104–17. doi: 10.1016/j.jri.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Jiang Y, Chen Y, Chen Y. Knockdown of JARID2 inhibits the viability and migration of placenta trophoblast cells in preeclampsia. Mol Med Rep. 2017;16(3):3594–99. doi: 10.3892/mmr.2017.7011. [DOI] [PubMed] [Google Scholar]
- 16.Ospina-Prieto S, Chaiwangyen W, Herrmann J, et al. MicroRNA-141 is upregulated in preeclamptic placentae and regulates trophoblast invasion and intercellular communication. Transl Res. 2016;172:61–72. doi: 10.1016/j.trsl.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Charlton F, Bobek G, Stait-Gardner T, et al. The protective effect of apolipoprotein in models of trophoblast invasion and preeclampsia. Am J Physiol Regul Integr Comp Physiol. 2017;312(1):R40–48. doi: 10.1152/ajpregu.00331.2016. [DOI] [PubMed] [Google Scholar]
- 18.Wragg JW, Finnity JP, Anderson JA, et al. MCAM and LAMA4 are highly enriched in tumor blood vessels of renal cell carcinoma and predict patient outcome. Cancer Res. 2016;76(8):2314–26. doi: 10.1158/0008-5472.CAN-15-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang X, Ji G, Wu Y, et al. LAMA4, highly expressed in human hepatocellular carcinoma from Chinese patients, is a novel marker of tumor invasion and metastasis. J Cancer Res Clin Oncol. 2008;34(6):705–14. doi: 10.1007/s00432-007-0342-6. [DOI] [PubMed] [Google Scholar]
- 20.Shan N, Zhang X, Xiao X, et al. Laminin α4 (LAMA4) expression promotes trophoblast cell invasion, migration, and angiogenesis and is lowered in pre-eclamptic placentas. Placenta. 2015;36(8):809–20. doi: 10.1016/j.placenta.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Lai W, Ding Y. GNG7 silencing promotes the proliferation and differentiation of placental cytotrophoblasts in preeclampsia rats through activation of the mTOR signaling pathway. Int J Mol Med. 2019;43(5):1939–50. doi: 10.3892/ijmm.2019.4129. [DOI] [PMC free article] [PubMed] [Google Scholar]