Abstract
Background
Transcranial direct current stimulation (tDCS) is a noninvasive brain stimulation (NIBS) technique designed to improve cognitive and physical function of stroke survivors. There are many studies being conducted in the search for an effective intervention of tDCS. This study focused on cognitive motor learning in relation to hand function of stroke survivors.
Material/Methods
We enrolled 30 subjects with cognitive and hand function disorders. The participants in each group were inpatients at a hospital in Korea and had undergone neurorehabilitation training (60 min). Groups 1 and 3 had tDCS applied for 20 min, while group 2 received sham tDCS for the same duration. Afterwards, groups 1 and 2 played Nintendo games for 20 min, but group 3 did not. The total intervention period was 40 min/day, 2 days/week, for 8 weeks. The cognitive and hand function of the subjects were assessed using the Trail Making Test (TMT-A, TMT-B), Grip strength, Box and Block Test (BBT), and the Manual Function Test (MFT) before and after intervention.
Results
The tDCS + Nintendo Switch game group showed significant differences in TMT-A, TMT-B, Grip strength, MFT, and BBT results compared to the other groups between before and after intervention (p<.05).
Conclusions
Our results suggest that inclusion of motor tasks with the application of tDCS may be effective in improving cognitive and hand function of stroke survivors.
MeSH Keywords: Cognition, Hand Strength, Stroke, tDSC
Background
Normal movement is vital to human beings. In order to participate in social interactions, social productivity, and personal hobbies, human movement must incorporate elements of the domains of the individual, task, and environment [1]. However, when normal movement is impaired, the degrees of freedom between nerves, muscles, and joints become restricted [2]. Motor control via environmental and spatial sensory/perceptual systems, along with cognitive processing, is also compromised [1,3]. Therefore, it is important to consider the physical, mental, and social environment and task performance aspects as criteria of the International Classification of Functioning, Disability and Health (ICF) for treating stroke survivors [4].
For effective recovery of these complex rehabilitation processes, clinicians teach normal movement patterns and elicit motor learning through creative motor tasks [5]. However, despite systematic and scientific clinical interventions, stroke can cause permanent damage. The complexity and challenges of the rehabilitation process depend on the grade of injury, cognition, perception, sensation, strength, balance, and age of the person affected by stroke. Effective and intensive treatment, research, and strategies are needed to transform abnormal movement to normalized functional and social movements in these patients.
In addition to rehabilitation of the existing motor control and motor learning abilities, stimulation of the brain region involved in the facilitation of motor control and optimal motor learning has become prevalent [6]. This method is commonly referred to as noninvasive brain stimulation (NIBS), which is effective in acquiring motor skills by activating the plasticity of the neural junctions or reorganizing neuronal connectivity. Generally, NIBS techniques that are clinically applied to persons with stroke include transcranial direct current stimulation (tDCS), paired associative stimulation, and transcranial magnetic stimulation. tDCS is a device that increases by anode or decreases by cathode the sensitivity of the cortex by using a very weak electrical current (0.5–2 mA) through an electrode plate attached directly to the scalp, which can change the cortical cell membrane potential [7]. In previous studies, tDCS was applied to subjects with neurological diseases such as Alzheimer’s disease for cognitive impairment [8], Parkinson’s Disease for dyskinesia [9], and stroke for motor dysfunction [10].
Simple direct current (DC) electrical stimulation positively affects the nervous system defects because it allows for easier nerve transmission and the white matter acts as a type of wire that improves the transmission of signals between the nerve cells. DC electrical stimulation has a positive effect on the anisotropy of the white matter caused by brain injury, creating a physiological phenomenon that activates neuronal interactions [11].
It is more difficult to rehabilitate for improvement of motor control and motor learning in stroke survivors due to their adverse physical conditions. There are 3 advantages of tDCS that can improve these conditions. The first advantage is selective activation of the brain hemispheres. Stroke survivors, unlike healthy individuals, activate both the affected and less affected sides when attempting to move the affected hand [12]. The second advantage to tDCS is the ability to facilitate for optimal motor learning conditions. The application of tDCS causes cortical excitability and long-term potentiation; therefore, tDCS is recommended to be used before and during therapeutic exercises [13]. Lastly, according to a Cochrane Review, tDCS restores cognitive function in individuals with stroke [14].
Cognitive function is crucial in motor control because its process includes a task-oriented purpose. Intact cognition adds efficiency to individual movements in the environment and is required in motor learning to understand a task’s purpose [1]. If tDCS activates the brains of stroke survivors and creates optimal motor learning conditions, it is important to improve the patient’s function by training with appropriate motor tasks.
Recently, research on stroke rehabilitation using Nintendo games have been conducted in Korea and internationally. A positive feature of Nintendo use is that virtual reality games are fun and interesting and can act as a catalyst to active participation in rehabilitation [15]. The recently developed 1-2-Switch game consists of various themes in virtual reality. In this study, based on several previous studies, the themes were classified into activities of daily living, cognitive training, rhythm training, aerobic training.
Our hypothesis was that tDCS would create an optimal environment for the body and for brain plasticity after brain injury via selective activation of the brain hemispheres, optimal motor learning conditions, and recovery of cognitive function. The purpose of this study was to investigate the effects of active participation using the 1–2-Switch Nintendo game on the hand and cognitive function of chronic stroke survivors.
Material and Methods
Subjects
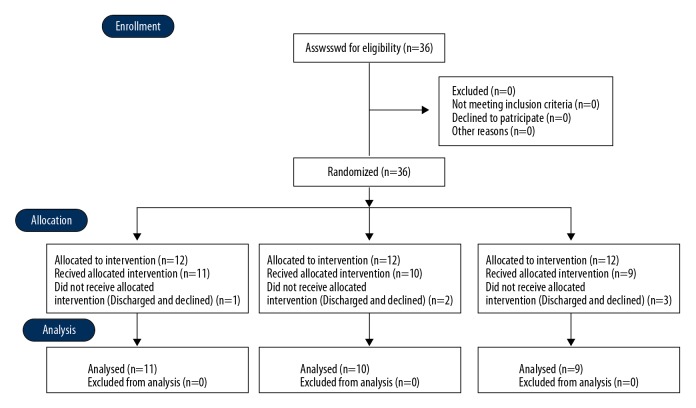
This study was conducted on 36 subjects with chronic stroke who were hospitalized in G hospital, located in Incheon Korea, between July 25 and September 22, 2019. All 36 subjects were randomly and equally divided into either the tDCS + Nintendo Switch group (group 1), the sham tDCS + Nintendo Switch group (group 2), or the tDCS-only group (group 3). The study concluded with 11 participants in group 1, 10 in group 2, and 9 in group 3 due to 6 early discharges. The flow diagram of the experiment is shown in Figure 1. This study was approved by the Institutional Review Board of Sahmyook University (2-7001793-AB-N-012018028HR). Inclusion criteria were: able to understand the experimental method, no orthopedic problems, able to walk 10 meters independently, chronic patients 6 months after onset, and signed the consent form.
Figure 1.
Flow diagram of the experimental procedure.
Procedure
All 3 groups participated in a 40-min intervention twice a week for a total of 8 weeks. Prior to the start of this study, preliminary tests were performed on the dependent variables, which included cognitive and hand functions. The same dependent variables were examined after the intervention.
To ensure accurate testing, a physical therapist with more than 5 years of experience and who understood the evaluation techniques performed the assessment for each dependent variable. The interventions were conducted in a quiet, private room that was secluded from the external environment, and all subjects underwent neurorehabilitation training with mat activities and gait training.
Application of tDCS focused on the activation of the anode on the affected side. The tDCS used in this study could not be applied to the cathode since it was made of equipment that could only be applied to the anode.
The Nintendo Switch game (1–2-Switch) is a virtual reality game played in conjunction with a television screen using a recognition controller referred to as a joy-con. There were 28 types of games that could be divided into the domains of activities of daily living, cognitive training, rhythm training, and aerobic training according to the characteristics of each game. If the game was too difficult, we proceeded to an easier game with similar themes, and the games required both seated and standing positions, depending on the style of the game. There were 7 cognitive games (Telephone, Ball count, Treasure chest, Safe crack, Sneaky dice, Joy-con rotation, and Fake draw), 12 aerobic exercises (Zen, Quick draw, Signal flags, Soda shake, Table tennis, Baseball, Beach flag, Wizard, Sword fight, Samurai Training, Boxing, and Plate spin), 4 ADL exercises (Milk, Shave, Baby, and Eating contest), and 5 rhythm exercises (Copy dance, Runway, Air guitar, Dance off, and Gorilla).
Outcome measures
Cognitive function
The Trail Making Test (TMT-A and TMT-B) is a symbolic connection test. TMT-A consists of 25 numbered circles and requires the subject to draw a line connecting them in sequential order beginning at 1. TMT-B consists of both numbers (1–13) and letters (A–L), which requires the patient to draw a line connecting them in sequential order again but incorporating both numbers and letters (e.g., 1-A-2-B-3-C…). The Trail Making tests were performed with both parts A and B in this study. These tests require visual exploration, visual perception, motor skills, agility, and complex visual scanning. The Korean version was used, which was shortened to accommodate elderly subjects [16].
Hand function
Box and Block Test
The Box and Block Test (BBT) is a tool that measures hand dexterity where the number of 1-inch blocks moved from one box to another in 10 minutes is assessed [17].
Manual Function Test
The Manual Function Test (MFT) consists of 4 tasks of upper-extremity ability in which 2 items assess grasp and 2 items assess manipulation ability [18].
Grip strength
Grip strength was measured using a Jamar dynamometer. The participant’s posture was assumed in adduction of the shoulder without rotation, 90° of elbow flexion, and neutral positioning of the forearm and wrist [19]. Three measurements were taken to determine the mean value.
Interventions
The subjects received the intervention twice a week for 8 weeks, and the study was conducted according to a predetermined schedule. Subjects in group 1 received 20 min of tDCS for the activation of optimal motor learning conditions and then participated in the Nintendo Switch game for 20 min. Subjects in group 2 sat in chairs and performed the same game after 20 min of sham tDCS. Subjects in group 3 received only tDCS.
The tDCS device used was the Halo Sport developed by Halo Neuroscience, USA, and is a safe, FDA-approved device. It is a headset designed with a maximum current of 2.2 mA, maximum voltage of 36 V, and weighs 340 g. It is an easy-to-use device, which, when placed on the head, is localized to the motor cortex of the brain [20]. Prior to wearing the device, the subjects had to sufficiently wet their heads to ensure accurate electrode conduction. Afterwards, the application program on a cell phone was paired with the Halo, and the ‘Legs, Core, and Arms’ mode was selected for a 20-min set. Electrode positioning is not arbitrarily adjustable and is designed to target the motor cortex in the vertical direction from the ear. According to the study’s purpose, the electrode was placed only on the affected hemisphere. Application of the Halo was in accordance with the official manual.
The Nintendo Switch game was divided into 4 themes that were changed every week. If the subject had difficulty during a game, they proceeded to the easier version of the same theme.
Statistical analysis
The GPower 3.1 software was used to calculate the sample size required so that a reasonable expectation would be likely to detect an expected effect size of 0.3 between the 3 different groups, with an alpha error probability of 0.05 and a power of 0.80. A sample size of 30 participants was required to provide a statistical power of 80%.
To verify the intermediary effect of this study, a normality test was performed on the general characteristics of subjects and the dependent variables. However, because it was not normally distributed, a nonparametric test was used. The Kruskal-Wallis test analyzed the statistical differences among the 3 groups, and the Mann-Whitney test was performed for post hoc analysis. The Wilcoxon test was used to identify the before and after differences within the groups. This study used SPSS version 20.0. Except for the Mann-Whitney test, the statistical significance level was below p<0.05. The Mann-Whitney test used for the post hoc analysis was 5%/3=1.66%, or below p<0.016 using Bonferroni method.
Results
The general characteristics of the study subjects are shown in Table 1. There were 17 men (56.6%) and 13 women (43.3%). The diagnoses included 12 (40%) cerebral hemorrhages and 18 (60%) cerebral infarctions. The mean age of the subjects was 63.5±7.45 years, the mean height was 161.93±7.21 cm, the mean weight was 61.4±12.20 kg, and the mean stroke onset was 24.53±11.56 months.
Table 1.
General characteristics.
| General characteristics | Subjects(n) | Percent (%) | |
|---|---|---|---|
| Sex | Male | 17 | 56.6% |
| Female | 13 | 43.3% | |
| Diagnosis | Hemorrahge | 12 | 40.0% |
| Infarction | 18 | 60.0% | |
| Affected Side | Rt | 19 | 63.3% |
| Lt | 11 | 36.6% | |
| Age (year) | 63.5±7.45 | ||
| Height (cm) | 161.93±7.21 | ||
| Weight (Kg) | 61.4±12.20 | ||
| Duration (month) | 24.53±11.56 | ||
Comparison of the differences between the 3 groups
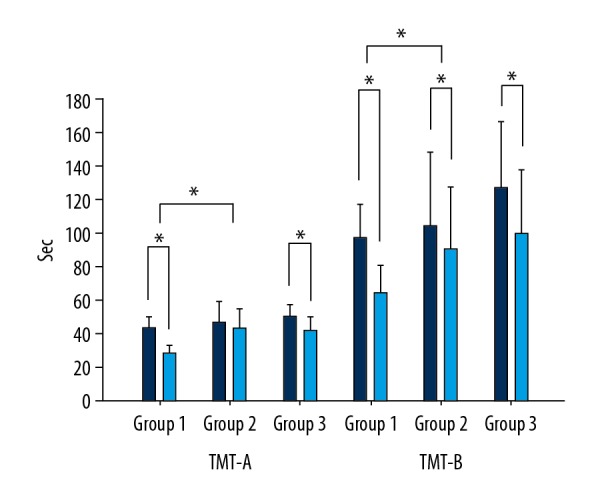
Comparison of the dependent variables is shown in Table 2. TMT-A was the lowest in group 1, with a mean of −14.32 s (10.45), followed by −7.68 s (16.22) in group 3 and −3.36 s (20.40) in group 2. The statistical difference between the 3 groups was 0.03 (p<0.05), and the post hoc analysis showed that group 1 was significantly lower than in group 2 by 0.013 (p<0.016). TMT-B was the lowest in group 1, with a mean of −30.54 s (11.82), followed by −27.33 s (13.89) in group 3 and −13.39 s (21.00) in group 2. The statistical difference between the 3 groups was 0.04 s (p<0.05), and the post hoc analysis showed that group 1 was significantly lower than group 3 by 0.016 s (p<0.016) (Figure 2).
Table 2.
Compare the differences between the three groups.
| Group 1 (n=11) | Group 2 (n=10) | Group 3 (n=9) | P (<0.05)* | Post hoc | ||
|---|---|---|---|---|---|---|
| Mean value | Mean value | Mean value | ||||
| TMT-A (sec) | Pre | 42.86 | 46.63 | 49.64 | 0.03* | |
| Post | 28.54 | 43.27 | 41.95 | |||
| Difference | −14.32 | −3.36 | −7.68 | |||
| p (<0.05)* | 0.00* | 0.13 | 0.00* | 1>2 | ||
| Average rank | 10.45 | 20.40 | 16.22 | |||
| TMT-B (sec) | Pre | 96.84 | 103.39 | 126.80 | 0.04* | |
| Post | 64.30 | 90.00 | 99.47 | |||
| Difference | −30.54 | −13.39 | −27.33 | |||
| p (<0.05)* | 0.00* | 0.03* | 0.00* | 1>2 | ||
| Average rank | 11.82 | 21.00 | 13.89 | |||
| Grip A/S (Kg) | Pre | 10.22 | 10.90 | 14.11 | 0.00* | |
| Post | 13.36 | 10.15 | 15.00 | |||
| Difference | 3.15 | −0.75 | 0.89 | |||
| p (<0.05) | 0.00* | 0.49 | 0.16 | 1>2, 3 | ||
| Average rank | 22.14 | 9.65 | 13.89 | |||
| MFT A/S (score) | Pre | 17.27 | 17.40 | 22.77 | 0.04* | |
| Post | 20.00 | 18.50 | 23.77 | |||
| Difference | 2.27 | 1.10 | 1.00 | |||
| p (<0.05) | 0.00* | 0.10 | 0.02* | 1>3 | ||
| Average rank | 20.86 | 12.75 | 12.00 | |||
| BBT A/S (score) | Pre | 21.00 | 23.20 | 28.66 | 0.03* | |
| Post | 27.54 | 24.70 | 30.00 | |||
| Difference | 6.54 | 1.50 | 1.33 | |||
| p (<0.05) | 0.00* | 0.05* | 0.15 | |||
| Average rank | 20.50 | 13.60 | 11.50 |
Kruskal-Wallis test, Wilcoxon test, Mann-Whitney test,
TMT – Trial Making Test, A/S – Affected Side, MFT – Manual Function Test, BBT – Box and Block Test.
Figure 2.
Comparison of TMT-A and -B between groups.
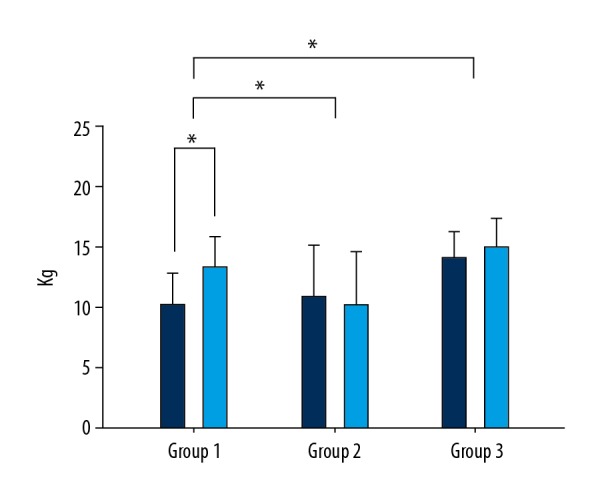
Grip strength (affected side) was the highest in group 1, with a mean of 3.15 kg (22.14), followed by 0.89 kg (13.89) in group 3 and −0.75 kg (9.65) in group 2. The statistical difference between the 3 groups was 0.00 kg (p<0.05), and the post hoc analysis showed that group 1 was significantly higher than groups 2 and 3 by 0.002 kg (p<0.016) and 0.016 kg (p<0.016), respectively (Figure 3).
Figure 3.
Comparison of Grip Strength between groups.
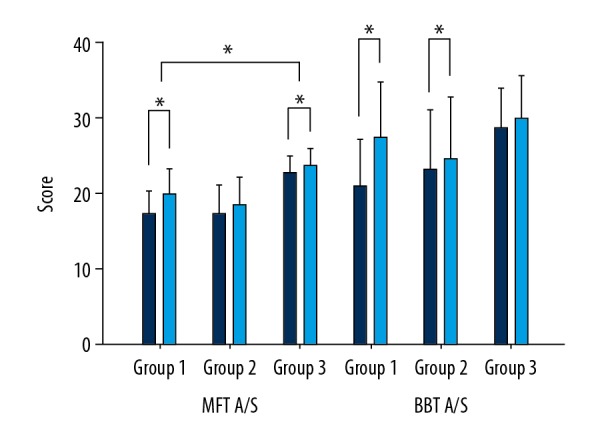
The MFT (affected side) scores were the highest in group 1, with a mean of 2.27 (20.86), followed by group 2 with 1.10 (12.75) and group 3 with 1.00 (12.00). The statistical difference between the 3 groups was 0.04 (p<0.05), and the post hoc analysis showed that group 1 scores were higher than group 3 by 0.004 (p <0.016) (Figure 4).
Figure 4.
Comparison of Grip MFT and BBT between groups.
The Box and Block Test (affected side) scores were the highest in group 1, with a mean of 6.54 (20.54), followed by group 2 with 1.50 (13.60) and group 3 with 1.33 (11.50). The statistical difference between the 3 groups was 0.03 (p <0.05), and there was no significant difference in the post hoc analysis (Figure 4).
Discussion
This study aimed to verify the effectiveness of tDCS on cognitive and hand function of chronic stroke survivors. While existing studies have independently applied each of the Nintendo games with tDCS, our study creatively investigated the selective activation of brain hemispheres, optimal motor learning conditions, and cognitive recovery of participants. We investigated the effectiveness of the game using a Nintendo Switch game, which can be entertaining for the participants.
When comparing the before and after results, the differences between cognition and hand function was obvious in the tDCS+Nintendo Switch group, whereas in the sham tDCS+Nintendo Switch group, there was only a difference in TMT-B and BBT. In the tDCS-only group, there was a difference between cognition and MFT before and after the study.
Comparing the tDCS+Nintendo Switch group and the sham tDCS+Nintendo Switch group, there were differences in the TMT-A, TMT-B, and grip strength; in the tDCS+Nintendo Switch group and the tDCS-only group, there were differences in grip strength and MFT. Finally, there was no significant difference between the sham tDCS+Nintendo Switch group and the tDCS-only group.
The effect of tDCS was seen most dramatically in the comparison between the tDCS+Nintendo Switch group and the sham tDCS+Nintendo Switch group. Based on improved cognitive function, tDCS had a positive effect for achieving optimal motor learning conditions, resulting in positive outcomes on grip strength. The tDCS+Nintendo Switch group also demonstrated improvements in both cognitive and hand functions, as well as grip strength. In contrast, the sham tDCS+Nintendo Switch group revealed only changes in TMT-B and BBT. However, there was no significant difference in cognitive function when comparing the tDCS+Nintendo Switch group and the tDCS-only group, indicating that tDCS had a clear effect on cognitive function. No significant change was noted in the sham tDCS+Nintendo Switch group regarding before and after results. A greater effect was seen when tDCS was used. For example, both the tDCS+Nintendo Switch group and the tDCS-only group showed changes in grip strength and MFT; therefore, tDCS may have produced cognitive improvement and optimal motor learning conditions.
Our findings show tDCS had a clear influence on cognitive function of chronic stroke survivors and can create an ideal environment in the body for optimal motor learning. In addition, if a task could be performed, tDCS could be an effective intervention for treating deficits in cognition and hand functions in chronic stroke survivors.
In previous studies, tDCS produced optimal motor learning conditions for participants with chronic stroke and demonstrated effects on motor performance and cognitive function. Chronic stroke participants were subjected to cathode tDCS for 20 min on the less affected side and were then evaluated at 90 min and 24 h after application. In the experimental group, motor performance improved and optimal motor learning conditions were found [21]. Another study applied dual-tDCS to subjects with chronic stroke, and a circuit game involving complex visual perception techniques was performed during tDCS, 30 min after tDCS, 60 min after, and 1 week after application. As a result, tDCS improved conditions for optimal motor learning in the stroke survivors, including significant improvements in qualitative and quantitative factors for all temporal conditions and motor skills [22].
Motor learning can be defined as a sustainable change in repetitive training based on a specific goal and is a steady state facilitated by a conscious-to-unconscious feedback process [23]. Motor learning via effective intervention programs may be necessary for individuals with stroke who lack motor control and for whom functional recovery is essential. In this study, the Nintendo- and tDCS-combined groups showed significantly better results compared to the independent tDCS group, indicating that tDCS effectively restored cognitive function and maximized the optimal learning condition.
After learning a new skill, it can be retained by explicit memory, which is a conscious-memory response of the sensory association area, medial temporal lobe, and hippocampus. Repeated learning leads to unconscious and reflexive habitual learning, which is governed by the amygdala, cerebellum, basal ganglia, and the supplementary motor area. In many studies of explicit and implicit memory, tDCS activated primary explicit memory [24]. Therefore, it can be concluded that by playing the Nintendo game immediately after tDCS, participants demonstrated effective and conscious learning. Stimulation with tDCS produces an increase in calcium transport in the astrocytes, which can lead to acceleration of brain plasticity [25]. A series of procedures in this study may have helped repair the neuronal damaged by activation of astrocytes and may have assisted in the production of new neurons.
Finally, effective motor learning can be explained by using the Hebbian Learning Rule. Transcranial direct current stimulation produces a response similar to the long-term potentiation and long-term depression of the Hebbian Learning Rule through the regulation of N-methyl-D-aspartic acid receptors. Subsequently, amplification of the mRNA expression associated with the dependent synaptic plasticity of the brain-derived neurotrophic factor could be used to create optimal motor learning conditions [26].
Conclusions
Although therapeutic exercise is important for individuals with stroke, it is imperative to promote effective motor learning with tDCS via motor tasks, as evidenced by the intervention program in this study. Stroke can cause permanent brain damage, have detrimental effects on socialization, and increase healthcare costs. Development of various creative and efficient training tasks is essential in clinical fields.
Therefore, this study was conducted and revealed the necessity of optimal motor learning conditions with the use of tDCS for effective treatment of cognitive and physical functioning in chronic stroke survivors. Due to the small sample size, this study could not include parametric tests. A large sample size and assessment of variables related to quality of life and daily living should be considered for future studies.
Footnotes
Source of support: This paper was supported by the Fund of the Sahmyook University in 2019
Conflicts of interest
None.
References
- 1.Shumway-Cook A, Woollacott MH. Motor control: translating research into clinical practice. Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 2.Bernstein N. The co-ordination and regulation of movements. The co-ordination and regulation of movements. 1966 [Google Scholar]
- 3.Rosenbaum DA. Human motor control. Academic press; 2009. [Google Scholar]
- 4.World Health Organization. International classification of functioning, disability and health: ICF. Geneva: World Health Organization; 2001. [Google Scholar]
- 5.Zimerman M, Hummel FC. Non-invasive brain stimulation: enhancing motor and cognitive functions in healthy old subjects. Front Aging Neurosci. 2010;2:149. doi: 10.3389/fnagi.2010.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang N, Summers JJ, Cauraugh JH. Transcranial direct current stimulation facilitates motor learning post-stroke: A systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2016;87(4):345–55. doi: 10.1136/jnnp-2015-311242. [DOI] [PubMed] [Google Scholar]
- 7.Nitsche MA, Cohen LG, Wassermann EM, et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008;1(3):206–23. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Flöel A, Suttorp W, Kohl O, et al. Non-invasive brain stimulation improves object-location learning in the elderly. Neurobiol Aging. 2012;33(8):1682–89. doi: 10.1016/j.neurobiolaging.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Pascual-Leone A, Valls-Sole J, Brasil-Neto J, et al. Akinesia in Parkinson’s disease. II. Effects of subthreshold repetitive transcranial motor cortex stimulation. Neurology. 1994;44(5):892–98. doi: 10.1212/wnl.44.5.892. [DOI] [PubMed] [Google Scholar]
- 10.Hesse S, Werner C, Schonhardt E, et al. Combined transcranial direct current stimulation and robot-assisted arm training in subacute stroke patients: A pilot study. Restor Neurol Neurosci. 2007;25(1):9–15. [PubMed] [Google Scholar]
- 11.Shahid S, Wen P, Ahfock T. Numerical investigation of white matter anisotropic conductivity in defining current distribution under tDCS. Comput Methods Programs Biomed. 2013;109(1):48–64. doi: 10.1016/j.cmpb.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Kandel ER, Schwartz JH, Jessell TM, et al. Principles of neural science. Vol. 4. McGraw-hill; New York: 2000. [Google Scholar]
- 13.Liebetanz D, Nitsche MA, Tergau F, et al. Pharmacological approach to the mechanisms of transcranial DC stimulation induced after effects of human motor cortex excitability. Brain. 2002;125(10):2238–47. doi: 10.1093/brain/awf238. [DOI] [PubMed] [Google Scholar]
- 14.Elsner B, Kugler J, Pohl M, et al. Transcranial direct current stimulation (tDCS) for improving activities of daily living, and physical and cognitive functioning, in people after stroke. Cochrane Database Syst Rev. 2016;3:CD009645. doi: 10.1002/14651858.CD009645.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flynn S, Palma P, Bender A. Feasibility of using the Sony PlayStation 2 gaming platform for an individual poststroke: A case report. J Neurol Phys Ther. 2007;31(4):180–89. doi: 10.1097/NPT.0b013e31815d00d5. [DOI] [PubMed] [Google Scholar]
- 16.Park J. Usefulness of the Korean Trail Making Test for the Elderly (K-TMT-e) in detecting the frontal lobe dysfunction. Dement Neurocogn Disord. 2007;6(1):12–17. [Google Scholar]
- 17.Mathiowetz V, Volland G, Kashman N, et al. Adult norms for the Box and Block Test of manual dexterity. Am J Occup Ther. 1985;39(6):386–91. doi: 10.5014/ajot.39.6.386. [DOI] [PubMed] [Google Scholar]
- 18.Miyamoto S, Kondo T, Suzukamo Y, et al. Reliability and validity of the Manual Function Test in patients with stroke. Am J Phys Med. 2009;88(3):247–55. doi: 10.1097/PHM.0b013e3181951133. [DOI] [PubMed] [Google Scholar]
- 19.Fess E. Clinical assessment recommendations. American Society of Hand Therapists. 1981:6–8. [Google Scholar]
- 20.Huang L, Deng Y, Zheng X, et al. Transcranial direct current stimulation with Halo Sport enhances repeated sprint cycling and cognitive performance. Front Physiol. 2019;10:118. doi: 10.3389/fphys.2019.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zimerman M, Heise KF, Hoppe J, et al. Modulation of training by single-session transcranial direct current stimulation to the intact motor cortex enhances motor skill acquisition of the paretic hand. Stroke. 2012;43(8):2185–91. doi: 10.1161/STROKEAHA.111.645382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lefebvre S, Laloux P, Peeters A, et al. Dual-tDCS enhances online motor skill learning and long-term retention in chronic stroke patients. Front Hum Neurosci. 2013;6:343. doi: 10.3389/fnhum.2012.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winters BD, Saksida LM, Bussey TJ. Object recognition memory: Neurobiological mechanisms of encoding, consolidation and retrieval. Neurosci Biobehav Rev. 2008;32(5):1055–70. doi: 10.1016/j.neubiorev.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Sriraman A, Oishi T, Madhavan S. Timing-dependent priming effects of tDCS on ankle motor skill learning. Brain Res. 2014;1581:23–29. doi: 10.1016/j.brainres.2014.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monai H, Ohkura M, Tanaka M, et al. Calcium imaging reveals glial involvement in transcranial direct current stimulation-induced plasticity in mouse brain. Nat Commun. 2016;7:11100. doi: 10.1038/ncomms11100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fritsch B, Reis J, Martinowich K, et al. Direct current stimulation promotes BDNF-dependent synaptic plasticity: Potential implications for motor learning. Neuron. 2010;66(2):198–204. doi: 10.1016/j.neuron.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]