Abstract
Objective
To report current knowledge on the topic of intracochlear fibrosis and the foreign body response following cochlear implantation (CI).
Methods
A literature search was performed in PubMed to identify peer‐reviewed articles. Search components included “cochlear implant,” “Foreign body response (FBR),” and “fibrosis.” Original studies and review articles relevant to the topic were included.
Results
Ninety peer‐reviewed articles describing the foreign body response or intracochlear fibrosis following CI were included.
Conclusions
Intracochlear fibrosis following CI represents a significant limiting factor for the success of CI users. Several strategies have been employed to mitigate the foreign body response within the cochlea including drug delivery systems and modifications in surgical technique and electrode design. A better understanding of the FBR has the potential to improve CI outcomes and the next generation of cochlear prostheses.
Keywords: biomaterials, cochlear implant, fibrosis, foreign body response, impedance
Intracochlear fibrosis following CI represents a significant limiting factor for the success of CI users. Several strategies have been employed to mitigate the foreign body response within the cochlea including drug delivery systems and modifications in surgical technique and electrode design. A better understanding of the FBR has the potential to improve CI outcomes and the next generation of cochlear prostheses.
1. INTRACOCHLEAR TISSUE RESPONSES AFTER COCHLEAR IMPLANTATION
Cochlear implants (CIs) provide successful auditory rehabilitation to patients with severe to profound sensorineural hearing loss. Conventional CIs are indicated in patients with moderate‐to‐severe to severe‐to‐profound hearing loss. With the shift in focus to preservation of native acoustic hearing and the advent of the hybrid CI, the population of patient candidates for CI has expanded to include those with mid‐to‐high frequency severe‐to‐profound hearing loss, yet normal‐to‐moderate hearing loss in the low‐frequencies. CI recipients with preserved hearing are thus able to combine low‐frequency acoustic hearing with high‐frequency electrical stimulation, resulting in significant enhancements in performance such as speech understanding in noise, music appreciation, and sound localization.1, 2, 3, 4 The candidacy for both conventional and hearing preservation CIs is projected to continue to increase over the next 40 years with the aging population and continued advancements in electrode design and optimization of surgical techniques.5
While CIs are generally considered biocompatible with low complication rates, an inflammatory/fibrotic response occurs after implantation of an electrode array into the cochlea.6, 7 This inflammatory response involves formation of a densely organized fibrous sheath surrounding the electrode track that can expand to include loose areolar fibrotic tissue, granulomas, or new bone formation (neo‐ossification). Several histopathologic temporal bone studies from CI recipients confirm this inflammatory response.8, 9, 10, 11, 12, 13, 14, 15 For example, Seyyedi and Nadol described a chronic inflammatory/fibrotic reaction involving inflammatory cells, fibrosis, and neo‐ossification in each temporal bone examined (n = 28, 100%) from patients with CIs during life.12 Similarly, Benatti and Castiglione examined the severity and location of the inflammatory response within the cochlea in 28 temporal bones. All electrodes in the study were surrounded by a fibrous sheath.11 Several studies confirm that the fibrotic response is most pronounced in the basal turn of the cochlea, near the site of electrode insertion, and decreases in severity with increasing distance from the cochleostomy site. In some cases, fibrosis and neo‐ossification extend apically beyond the distal end of the electrode.6, 9, 11, 16, 17 Several animal studies across multiple species have likewise demonstrated a similar reaction involving intracochlear fibrosis following CI.18, 19, 20, 21 The severity of the inflammatory response to CI varies amongst patients, from mild fibrosis and neo‐ossification to severe granulomatous processes.22 Several studies have demonstrated that the fibrosis and new bone formation does not correlate with the duration of implantation.6, 16, 17, 23 However, electrical stimulation may play a role in modulating the foreign body response, as demonstrated by Shepherd et al.24, 25
The tissue response to CI has both an immediate and a delayed component. The acute response is attributed to insertion trauma, which violates the normal cochlear anatomy. Insertion trauma may include damage to the lateral wall with disruption of the intracochlear endosteum, fracture of the osseous spiral lamina, displacement of the basilar membrane, damage to the stria vascularis, or disruption of cochlear fluids.16, 17, 26 Studies have shown that damage to the lateral wall of the cochlea correlates with augmented fibrosis and neo‐ossification, implicating lateral wall damage as an initiator of the inflammatory response.6, 16 Additionally, some studies have shown an association between fracture of the osseous spiral lamina or displacement of the basilar membrane and increased fibrosis, while others do not support this correlation.6, 23
The delayed component of the inflammatory response is attributed to the host‐mediated foreign body response (FBR) within the cochlea (Figure 1). The FBR occurs in response to nearly all biomaterials. It begins with immediate plasma protein (eg, albumin, fibrinogen) adsorption onto the biomaterial surface and formation of a provisional matrix.27, 28 An infiltration of neutrophils characterizes the acute phase of the response. This develops into a chronic inflammatory reaction, as monocytes/macrophages and lymphocytes migrate to the site of tissue injury in response to chemokines and chemoattractants. Following adhesion to the biomaterial surface, macrophages fuse to form foreign body giant cells (FBGCs). Macrophages and FBGCs release mediators of degradation including reactive oxygen species, enzymes, and acids. In response to macrophage activation, fibroblasts migrate to the site of the implant, proliferate, and lay down extracellular matrix (ECM) proteins, including collagen. Macrophages and fibroblasts contribute to the formation of granulation tissue. In the presence of a foreign body, fibroblast proliferation and ECM deposition becomes dysregulated, resulting in an irreversible fibrotic response, which progresses to formation of a fibrous capsule surrounding the implant.27, 28, 29
Figure 1.
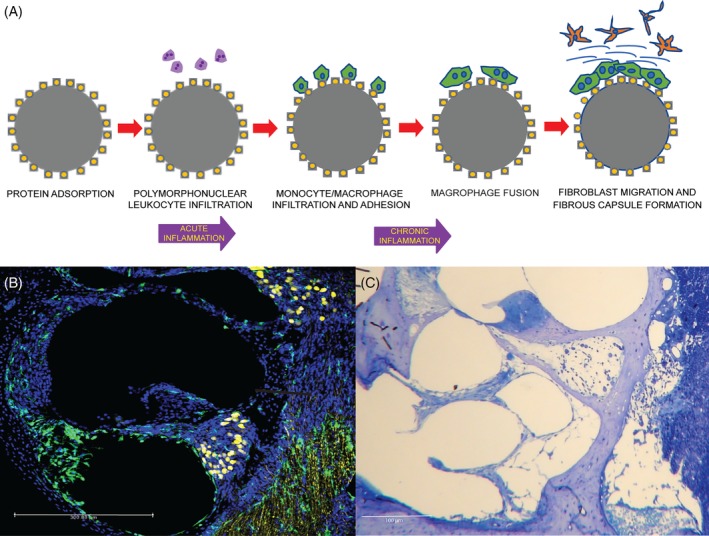
A, Schematic of the foreign body response to cochlear implantation. Cochlear section from a CX3CR1/Thy1‐YFP C57BL6 mouse euthanized at day 8 post cochlear implantation, showing increased total cell and macrophage counts in the scala tympani. B, Cell nuclei are labeled bleu (Hoechst stain), macrophages green (CX3CR1‐GFP), and neurons yellow (Thy1‐YFP). C, Richardson's stain demonstrating the FBR after CI in a mouse cochlear section. Inflammatory cells and fibrosis can be seen along the electrode tract in the scala tympani. CI, cochlear implantation; FBR, foreign body response
The FBR can lead to degradation of CI biomaterials. CIs are comprised of platinum (Pt)‐iridium (90/10) electrode arrays embedded in a polydimethylsiloxane (PDMS, silicone) carrier. Both platinum and silicone particles have been demonstrated both extracellularly and intracellularly within macrophages from temporal bone sections of CI recipients.7, 30 It is presumed that these biomaterials are subject to mediators of degradation released by macrophages and FBGCs and undergo phagocytosis in an attempt to clear the debris. FBGCs are typically seen at the interface between the electrode and the fibrous capsule.9, 31 In the cochlea, an exaggerated FBR often progresses to involve neo‐ossification.
2. IMPACT OF INTRACOCHLEAR FIBROSIS/NEO‐OSSIFICATION ON CLINICAL OUTCOMES IN CI PATIENTS
Although CIs have been markedly successful in restoring hearing to patients with profound hearing loss, the FBR and intracochlear fibrosis could be a significant limiting factor to the success and outcomes of CI users. It is hypothesized that this fibro‐osseous reaction could be a contributing factor to delayed loss of residual acoustic hearing after hearing preservation CI. Although the goal is to preserve low frequency hearing in hybrid CI recipients, there is a subpopulation of these patients in which initially preserved low frequency hearing is lost 3 months to 3 years after successful implant surgery.32, 33, 34 While many hypotheses aim to explain this delayed loss of residual hearing, the cause remains largely unknown. Some possible explanations include inflammation,35 oxidative stress and apoptosis within hair cells,36, 37 excitotoxicity due to acoustic and electrical stimulation,38 alterations in cochlear fluid homeostasis,32 vascular injury,39 or the foreign body response. The FBR as a cause of delayed hearing loss is implicated in a recent histopathologic report of a temporal bone from a hybrid CI recipient who experienced delayed loss of functional residual hearing 4 to 18 weeks after CI.13 The basal turn of the implanted cochlea demonstrated fibrous tissue and neo‐ossification of the scala tympani and scala vestibuli, speculated to cause an “inner ear conductive” hearing loss.13, 40 Importantly, hair cell and spiral ganglion neuron counts were comparable between the patient's implanted and unimplanted ears. These observations imply that progressive fibrotic tissue growth contributes to hearing loss following initial hearing preservation CI.
Intracochlear fibrosis, particularly of the basal turn of the cochlea, is theorized to modify the vibrations of the basilar membrane apically, which may impact native low‐frequency hearing.40 Others theorize that insertion of an electrode through a cochleostomy may lead to endosteal damage to the scala vestibuli and resultant intracochlear fibrosis. The trauma to the endosteum causes proliferation of spiral ligament cells and impaired function of the ductus reuniens, leading to endolymphatic hydrops as a cause of delayed loss of residual low‐frequency hearing.41, 42 Ishiyama et al demonstrated fibrosis of all three scala and resultant endolymphatic hydrops in 17 of 29 temporal bones from patients who underwent CI using a cochleostomy approach.41
Additionally, fibrosis within the cochlea is associated with elevated electrical impedances.43, 44 Electrode impedance reflects the resistance between the stimulating electrode and the return electrode. The presence of protein and cellular adhesion, fibrous tissue, or new bone formation can be expected to increase electrode impedance.23, 45, 46, 47 Further, recent work suggests that fibrosis results in a reduction and changes in composition of perilymph or extracellular fluid adjacent to the electrodes leading to elevated impedance.48 As a result of the increased impedance at the electrode‐tissue interface, higher voltages are required leading to decreased dynamic range of stimulation and decreased CI battery life.9, 43, 49 Further, hearing loss after CI has been associated with increased impedances, presumably reflecting increased fibrosis.18, 45, 50
It has been suggested that neo‐ossification as a result of the FBR following CI may also be correlated with poorer word recognition scores post‐operatively. Takefumi et al demonstrated a negative correlation between Consonant‐Vowel Nucleus‐Consonant Word Test (CNC) word scores and the volume of new bone in the scala media/vestibule in a study of 17 temporal bones.6 However, this and other studies16, 23 have shown no correlation between word recognition score and the volume of fibrous tissue in the cochlea.
Formation of granulomas and other granulomatous reactions have also been reported following CI.11, 51, 52 One histopathologic study of a revision CI following “soft failure” of the device demonstrated a necrotizing granulomatous process of the entire cochlea bilaterally, consisting of FBGCs and lymphocytes.22, 53 The reaction was attributed to a delayed hypersensitivity reaction.22 Another case report described endosteal erosion of the upper basal turn of the cochlea with an area of focal osteomyelitis.54 In more extreme cases (approximately 1%), device extrusion has been reported and is thought to be associated with a severe soft tissue reaction. Device failure occurs rarely, accounting for 1.53% of cases.11
Along with the perceived unfavorable outcomes for current CI users, it is theorized that neo‐ossification could also decrease the efficacy of future surgeries (eg, explantation and reimplantation) and may limit future therapies aimed at hair cell or neuron regeneration.26, 55 Intracochlear tissue growth including fibrosis and neo‐ossification could dramatically hinder efforts to develop next generation cochlear prostheses (eg, thin film arrays), optical “trodes,” and cell based therapies that require bioscaffolding.
3. STRATEGIES TO MITIGATE THE TISSUE RESPONSE IN THE COCHLEA FOLLOWING CI
Several strategies to mitigate the inflammatory/fibrotic response following CI have been proposed and represent an area of ongoing research. Pharmacological approaches include local or systemic glucocorticoids,56, 57 other immune/inflammatory modulating drugs,58, 59, 60 anti‐apoptotic compounds,61, 62, 63, 64 and anti‐oxidants.65 Use of glucocorticoids systemically, locally (intratympanic, intracochlear), as well as glucocorticoid‐emitting or glucocorticoid‐coated electrodes have been shown to decrease the inflammatory reaction and provide some degree of otoprotection following CI.56, 57, 66, 67, 68, 69, 70 For example, in a guinea pig model, a 7‐day infusion of dexamethasone sodium phosphate through the round window after CI resulted in a significant decrease in the total area of the tissue response and fibrosis when compared to the control group.45 Dexamethasone (DEX) eluding CIs have also been shown to reduce fibrosis surrounding the electrode array and decrease electrode impedance levels in a guinea pig model.49, 71 Additionally, DEX eluding CIs have been shown to decrease the volume of fibrosis surrounding electrode arrays and show promise in hearing preservation in humans.49, 57, 72
Surgical modifications, or “soft” electrode insertion techniques, can also help minimize intraoperative trauma to the cochlea and mitigate the inflammatory reaction to CI and subsequent fibrosis within the cochlea.73, 74 Use of slow insertion of the electrode array, angled superior‐to‐inferior, with minimal pressure applied can reduce surgical trauma.74, 75 Additionally, choice of surgical entry into the cochlea may affect the degree of intracochlear fibrosis. CI electrode arrays can be inserted through the round window membrane or through a bony cochleostomy anterior and inferior in the basal turn of the scala tympani. The round window approach is thought to minimize damage to cochlear structures thus decreasing secondary intracochlear fibrosis and neo‐ossification.41, 44, 76, 77, 78, 79 Additionally, studies have shown that insertion using the round window approach may be associated with a higher likelihood of successful placement of the electrode into the scala tympani and higher hearing preservation rates.80, 81, 82 Surgical entry through a more traditional cochleostomy may prevent damage to the osseous spiral lamina.16, 76, 83 However, adverse outcomes associated with cochleostomy may include perilymph loss, acoustic trauma from drilling, and the introduction of bone dust into the perilymphatic space, which may promote neo‐ossification and further amplify the host response to the foreign material.16, 77 Maintaining a clean surgical field with irrigation of bone dust and pate is another way to minimize entry of foreign debris into the scala tympani. Bone dust may promote new bone formation in the cochlea, as demonstrated in a study by McElveen et al.84 However, there is limited direct evidence in support of this conclusion. Additionally, intracochlear bleeding intraoperatively may increase fibrosis and ossification.85, 86
Another strategy involves development of new electrode arrays that are short, thin, flexible, and/or directed to specific regions of the scala tympani (lateral wall, mid‐scala, perimodiolar) in an effort to limit insertional trauma and consequent inflammation. While shorter electrodes may minimize trauma to the cochlea, this must be balanced with how effectively they can stimulate neurons more apically in the cochlea. Of note, Ishai et al demonstrated that different electrode designs can result in different patterns of fibrosis.23 Finally, modifications to CI biomaterials themselves, such as surface thin‐film hydrogel coatings that reduce biofouling and/or promote water retention around the electrode array, have been suggested as methods to reduce the fibrosis and increase in impedances that occur following CI.48, 87
4. CONCLUSIONS
The inflammatory FBR following CI represents a significant limiting factor for the success of CI users. Several strategies have been employed in an attempt to mitigate the FBR within the cochlea including modifications in surgical technique and electrode design and pharmacotherapy. A more detailed understanding of the FBR, including the effects of electrical stimulation and the response to specific components of the CI (platinum and PDMS), has the potential to improve CI outcomes and impact the design of the next generation of cochlear prostheses.
CONFLICT OF INTEREST
Dr Hansen is a co‐founder and Chief Medical Officer of Iotamotion, Inc. All other authors of this work declare no conflict of interest.
Foggia MJ, Quevedo RV, Hansen MR. Intracochlear fibrosis and the foreign body response to cochlear implant biomaterials. Laryngoscope Investigative Otolaryngology. 2019;4:678–683. 10.1002/lio2.329
Funding information National Institute on Deafness and Other Communication Disorders, Grant/Award Numbers: R01 DC012578, 5T32DC000040, P50 DC00242
REFERENCES
- 1. Gantz BJ, Turner C, Gfeller KE, Lowder MW. Preservation of hearing in cochlear implant surgery: advantages of combined electrical and acoustical speech processing. Laryngoscope. 2005;115:796‐802. [DOI] [PubMed] [Google Scholar]
- 2. Gfeller KE, Olszewski C, Turner C, Gantz B, Oleson J. Music perception with cochlear implants and residual hearing. Audiol Neurootol. 2006;11(Suppl 1):12‐15. [DOI] [PubMed] [Google Scholar]
- 3. Turner CW, Reiss LA, Gantz BJ. Combined acoustic and electric hearing: preserving residual acoustic hearing. Hear Res. 2008;242:164‐171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Woodson EA, Reiss LA, Turner CW, Gfeller K, Gantz BJ. The hybrid cochlear implant: a review. Adv Otorhinolaryngol. 2010;67:125‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goman AM, Dunn CC, Gantz BJ, Lin FR. Prevalence of potential hybrid and conventional cochlear implant candidates based on audiometric profile. Otol Neurotol. 2018;39:515‐517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kamakura T, Nadol JB Jr. Correlation between word recognition score and intracochlear new bone and fibrous tissue after cochlear implantation in the human. Hear Res. 2016;339:132‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clark GM, Clark J, Cardamone T, et al. Biomedical studies on temporal bones of the first multi‐channel cochlear implant patient at the University of Melbourne. Cochlear Implants Int. 2014;15(Suppl 2):S1‐S15. [DOI] [PubMed] [Google Scholar]
- 8. Marsh MA, Jenkins HA, Coker NJ. Histopathology of the temporal bone following multichannel cochlear implantation. Arch Otolaryngol Head Neck Surg. 1992;118:1257‐1265. [DOI] [PubMed] [Google Scholar]
- 9. Nadol JB Jr, Shiao JY, Burgess BJ, et al. Histopathology of cochlear implants in humans. Ann Otol Rhinol Laryngol. 2001;110:883‐891. [DOI] [PubMed] [Google Scholar]
- 10. Schindler RA, Bjorkroth B. Traumatic intracochlear electrode implantation. Laryngoscope. 1979;89:752‐758. [DOI] [PubMed] [Google Scholar]
- 11. Benatti A, Castiglione A, Trevisi P, et al. Endocochlear inflammation in cochlear implant users: case report and literature review. Int J Pediatr Otorhinolaryngol. 2013;77:885‐893. [DOI] [PubMed] [Google Scholar]
- 12. Seyyedi M, Nadol JB Jr. Intracochlear inflammatory response to cochlear implant electrodes in humans. Otol Neurotol. 2014;35:1545‐1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Quesnel AM, Nakajima HH, Rosowski JJ, Hansen MR, Gantz BJ, Nadol JB Jr. Delayed loss of hearing after hearing preservation cochlear implantation: human temporal bone pathology and implications for etiology. Hear Res. 2016;333:225‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. deTorres A, Olszewski RT, Lopez IA, Ishiyama A, Linthicum FH Jr, Hoa M. Supporting cell survival after cochlear implant surgery. Laryngoscope. 2019;129:E36‐E40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Linthicum FH Jr, Fayad J, Otto SR, Galey FR, House WF. Cochlear implant histopathology. Am J Otol. 1991;12:245‐311. [PubMed] [Google Scholar]
- 16. Li PM, Somdas MA, Eddington DK, Nadol JB Jr. Analysis of intracochlear new bone and fibrous tissue formation in human subjects with cochlear implants. Ann Otol Rhinol Laryngol. 2007;116:731‐738. [DOI] [PubMed] [Google Scholar]
- 17. Fayad JN, Makarem AO, Linthicum FH Jr. Histopathologic assessment of fibrosis and new bone formation in implanted human temporal bones using 3D reconstruction. Otolaryngol Head Neck Surg. 2009;141:247‐252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. O'Leary SJ, Monksfield P, Kel G, et al. Relations between cochlear histopathology and hearing loss in experimental cochlear implantation. Hear Res. 2013;298:27‐35. [DOI] [PubMed] [Google Scholar]
- 19. Huang CQ, Tykocinski M, Stathopoulos D, Cowan R. Effects of steroids and lubricants on electrical impedance and tissue response following cochlear implantation. Cochlear Implants Int. 2007;8:123‐147. [DOI] [PubMed] [Google Scholar]
- 20. Araki S, Kawano A, Seldon HL, Shepherd RK, Funasaka S, Clark GM. Effects of intracochlear factors on spiral ganglion cells and auditory brain stem response after long‐term electrical stimulation in deafened kittens. Otolaryngol Head Neck Surg. 2000;122:425‐433. [DOI] [PubMed] [Google Scholar]
- 21. Claussen AD, Vielman Quevedo R, Mostaert B, Kirk JR, Dueck WF, Hansen MR. A mouse model of cochlear implantation with chronic electric stimulation. PLoS One. 2019;14:e0215407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nadol JB Jr, Eddington DK, Burgess BJ. Foreign body or hypersensitivity granuloma of the inner ear after cochlear implantation: one possible cause of a soft failure? Otol Neurotol. 2008;29:1076‐1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ishai R, Herrmann BS, Nadol JB Jr, Quesnel AM. The pattern and degree of capsular fibrous sheaths surrounding cochlear electrode arrays. Hear Res. 2017;348:44‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shepherd RK, Matsushima J, Martin RL, Clark GM. Cochlear pathology following chronic electrical stimulation of the auditory nerve: II. Deafened kittens. Hear Res. 1994;81:150‐166. [DOI] [PubMed] [Google Scholar]
- 25. Shepherd RK, Wise AK, Enke YL, Carter PM, Fallon JB. Evaluation of focused multipolar stimulation for cochlear implants: a preclinical safety study. J Neural Eng. 2017;14:046020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Somdas MA, Li PM, Whiten DM, Eddington DK, Nadol JB Jr. Quantitative evaluation of new bone and fibrous tissue in the cochlea following cochlear implantation in the human. Audiol Neurootol. 2007;12:277‐284. [DOI] [PubMed] [Google Scholar]
- 27. Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sheikh Z, Brooks PJ, Barzilay O, Fine N, Glogauer M. Macrophages, foreign body giant cells and their response to implantable biomaterials. Materials. 2015;8:5671‐5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44:450‐462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. O'Malley JT, Burgess BJ, Galler D, Nadol JB Jr. Foreign body response to silicone in cochlear implant electrodes in the human. Otol Neurotol. 2017;38:970‐977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nadol JB Jr, O'Malley JT, Burgess BJ, Galler D. Cellular immunologic responses to cochlear implantation in the human. Hear Res. 2014;318:11‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kopelovich JC, Reiss LA, Oleson JJ, Lundt ES, Gantz BJ, Hansen MR. Risk factors for loss of ipsilateral residual hearing after hybrid cochlear implantation. Otol Neurotol. 2014;35:1403‐1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jurawitz MC, Buchner A, Harpel T, et al. Hearing preservation outcomes with different cochlear implant electrodes: nucleus(R) hybrid‐L24 and nucleus freedom CI422. Audiol Neurootol. 2014;19:293‐309. [DOI] [PubMed] [Google Scholar]
- 34. Van Abel KM, Dunn CC, Sladen DP, et al. Hearing preservation among patients undergoing cochlear implantation. Otol Neurotol. 2015;36:416‐421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rizer FM, Arkis PN, Lippy WH, Schuring AG. A postoperative audiometric evaluation of cochlear implant patients. Otolaryngol Head Neck Surg. 1988;98:203‐206. [DOI] [PubMed] [Google Scholar]
- 36. Eshraghi AA, Polak M, He J, Telischi FF, Balkany TJ, Van De Water TR. Pattern of hearing loss in a rat model of cochlear implantation trauma. Otol Neurotol. 2005;26:442‐447. discussion 447. [DOI] [PubMed] [Google Scholar]
- 37. Eshraghi AA, He J, Mou CH, et al. D‐JNKI‐1 treatment prevents the progression of hearing loss in a model of cochlear implantation trauma. Otol Neurotol. 2006;27:504‐511. [DOI] [PubMed] [Google Scholar]
- 38. Tanaka C, Nguyen‐Huynh A, Loera K, Stark G, Reiss L. Factors associated with hearing loss in a normal‐hearing guinea pig model of hybrid cochlear implants. Hear Res. 2014;316:82‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wright CG, Roland PS. Vascular trauma during cochlear implantation: a contributor to residual hearing loss? Otol Neurotol. 2013;34:402‐407. [DOI] [PubMed] [Google Scholar]
- 40. Choi CH, Oghalai JS. Predicting the effect of post‐implant cochlear fibrosis on residual hearing. Hear Res. 2005;205:193‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ishiyama A, Doherty J, Ishiyama G, Quesnel AM, Lopez I, Linthicum FH. Post hybrid Cochlear implant hearing loss and Endolymphatic Hydrops. Otol Neurotol. 2016;37:1516‐1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Linthicum FH Jr, Doherty JK, Lopez IA, Ishiyama A. Cochlear implant histopathology. World J Otorhinolaryngol Head Neck Surg. 2017;3:211‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Clark GM, Shute SA, Shepherd RK, Carter TD. Cochlear implantation: osteoneogenesis, electrode‐tissue impedance, and residual hearing. Ann Otol Rhinol Laryngol Suppl. 1995;166:40‐42. [PubMed] [Google Scholar]
- 44. Shepherd RK, Clark GM, Xu SA, Pyman BC. Cochlear pathology following reimplantation of a multichannel scala tympani electrode array in the macaque. Am J Otol. 1995;16:186‐199. [PubMed] [Google Scholar]
- 45. Chang MY, Rah YC, Choi JJ, et al. The effect of systemic steroid on hearing preservation after Cochlear implantation via round window approach: a Guinea pig model. Otol Neurotol. 2017;38:962‐969. [DOI] [PubMed] [Google Scholar]
- 46. Tykocinski M, Cohen LT, Cowan RS. Measurement and analysis of access resistance and polarization impedance in cochlear implant recipients. Otol Neurotol. 2005;26:948‐956. [DOI] [PubMed] [Google Scholar]
- 47. Shaul C, Bester CW, Weder S, et al. Electrical impedance as a biomarker for inner ear pathology following Lateral Wall and Peri‐modiolar Cochlear implantation. Otol Neurotol. 2019;40:E518‐E526. [DOI] [PubMed] [Google Scholar]
- 48. Duan YY, Clark GM, Cowan RS. A study of intra‐cochlear electrodes and tissue interface by electrochemical impedance methods in vivo. Biomaterials. 2004;25:3813‐3828. [DOI] [PubMed] [Google Scholar]
- 49. Wilk M, Hessler R, Mugridge K, et al. Impedance changes and fibrous tissue growth after cochlear implantation are correlated and can be reduced using a dexamethasone eluting electrode. PLoS One. 2016;11:e0147552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Scheperle RA, Tejani VD, Omtvedt JK, et al. Delayed changes in auditory status in cochlear implant users with preserved acoustic hearing. Hear Res. 2017;350:45‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ho EC, Dunn C, Proops D, Warfield A. Case report: explantation of a cochlear implant secondary to chronic granulating labyrinthitis. Cochlear Implants Int. 2003;4:191‐195. [DOI] [PubMed] [Google Scholar]
- 52. Bertuleit H, Groden C, Schafer HJ, Leuwer R. Removal of a cochlea implant with chronic granulation labyrinthitis and foreign body reaction. Laryngorhinootologie. 1999;78:304‐306. [DOI] [PubMed] [Google Scholar]
- 53. Balkany TJ, Hodges AV, Buchman CA, et al. Cochlear implant soft failures consensus development conference statement. Cochlear Implants Int. 2005;6:105‐122. [DOI] [PubMed] [Google Scholar]
- 54. Doherty JK, Linthicum TH. Temporal bone histopathology case of the month cochlear endosteal erosion with focal osteomyelitis induced by cochlear implantation. Otol Neurotol. 2004;25:1029‐1030. [DOI] [PubMed] [Google Scholar]
- 55. Alexiades G, Roland JT Jr, Fishman AJ, Shapiro W, Waltzman SB, Cohen NL. Cochlear reimplantation: surgical techniques and functional results. Laryngoscope. 2001;111:1608‐1613. [DOI] [PubMed] [Google Scholar]
- 56. Liu Y, Jolly C, Braun S, et al. Effects of a dexamethasone‐releasing implant on cochleae: a functional, morphological and pharmacokinetic study. Hear Res. 2015;327:89‐101. [DOI] [PubMed] [Google Scholar]
- 57. Bas E, Bohorquez J, Goncalves S, et al. Electrode array‐eluted dexamethasone protects against electrode insertion trauma induced hearing and hair cell losses, damage to neural elements, increases in impedance and fibrosis: a dose response study. Hear Res. 2016;337:12‐24. [DOI] [PubMed] [Google Scholar]
- 58. Kikkawa YS, Nakagawa T, Ying L, et al. Growth factor‐eluting cochlear implant electrode: impact on residual auditory function, insertional trauma, and fibrosis. J Transl Med. 2014;12:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yamahara K, Nishimura K, Ogita H, et al. Hearing preservation at low frequencies by insulin‐like growth factor 1 in a guinea pig model of cochlear implantation. Hear Res. 2018;368:92‐108. [DOI] [PubMed] [Google Scholar]
- 60. Choong JK, Lo J, Chambers SA, Hampson AJ, Eastwood HT, O'Leary SJ. Intracochlear tPA infusion may reduce fibrosis caused by cochlear implantation surgery. Acta Otolaryngol. 2019;139:396‐402. [DOI] [PubMed] [Google Scholar]
- 61. Rejali D, Lee VA, Abrashkin KA, Humayun N, Swiderski DL, Raphael Y. Cochlear implants and ex vivo BDNF gene therapy protect spiral ganglion neurons. Hear Res. 2007;228:180‐187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Warnecke A, Sasse S, Wenzel GI, et al. Stable release of BDNF from the fibroblast cell line NIH3T3 grown on silicone elastomers enhances survival of spiral ganglion cells in vitro and in vivo. Hear Res. 2012;289:86‐97. [DOI] [PubMed] [Google Scholar]
- 63. Eshraghi AA, Lang DM, Roell J, et al. Mechanisms of programmed cell death signaling in hair cells and support cells post‐electrode insertion trauma. Acta Otolaryngol. 2015;135:328‐334. [DOI] [PubMed] [Google Scholar]
- 64. Pirvola U, Xing‐Qun L, Virkkala J, et al. Rescue of hearing, auditory hair cells, and neurons by CEP‐1347/KT7515, an inhibitor of c‐Jun N‐terminal kinase activation. J Neurosci. 2000;20:43‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Eastwood H, Pinder D, James D, et al. Permanent and transient effects of locally delivered n‐acetyl cysteine in a Guinea pig model of cochlear implantation. Hear Res. 2010;259:24‐30. [DOI] [PubMed] [Google Scholar]
- 66. Ye Q, Tillein J, Hartmann R, Gstoettner W, Kiefer J. Application of a corticosteroid (Triamcinolon) protects inner ear function after surgical intervention. Ear Hear. 2007;28:361‐369. [DOI] [PubMed] [Google Scholar]
- 67. Eshraghi AA, Adil E, He J, Graves R, Balkany TJ, Van De Water TR. Local dexamethasone therapy conserves hearing in an animal model of electrode insertion trauma‐induced hearing loss. Otol Neurotol. 2007;28:842‐849. [DOI] [PubMed] [Google Scholar]
- 68. Vivero RJ, Joseph DE, Angeli S, et al. Dexamethasone base conserves hearing from electrode trauma‐induced hearing loss. Laryngoscope. 2008;118:2028‐2035. [DOI] [PubMed] [Google Scholar]
- 69. Honeder C, Zhu C, Schopper H, et al. Effects of sustained release dexamethasone hydrogels in hearing preservation cochlear implantation. Hear Res. 2016;341:43‐49. [DOI] [PubMed] [Google Scholar]
- 70. King EB, Hartsock JJ, O'Leary SJ, Salt AN. Influence of cochleostomy and cochlear implant insertion on drug gradients following intratympanic application in Guinea pigs. Audiol Neurootol. 2013;18:307‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Farhadi M, Jalessi M, Salehian P, et al. Dexamethasone eluting cochlear implant: histological study in animal model. Cochlear Implants Int. 2013;14:45‐50. [DOI] [PubMed] [Google Scholar]
- 72. Paasche G, Bockel F, Tasche C, Lesinski‐Schiedat A, Lenarz T. Changes of postoperative impedances in cochlear implant patients: the short‐term effects of modified electrode surfaces and intracochlear corticosteroids. Otol Neurotol. 2006;27:639‐647. [DOI] [PubMed] [Google Scholar]
- 73. Lehnhardt E. Intracochlear placement of cochlear implant electrodes in soft surgery technique. HNO. 1993;41:356‐359. [PubMed] [Google Scholar]
- 74. Banakis Hartl RM, Kaufmann C, Hansen MR, Tollin DJ. Intracochlear pressure transients during cochlear implant electrode insertion: effect of micro‐mechanical control on limiting pressure trauma. Otol Neurotol. 2019;40:736‐744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Friedland DR, Runge‐Samuelson C. Soft cochlear implantation: rationale for the surgical approach. Trends Amplif. 2009;13:124‐138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Adunka OF, Buchman CA. Scala tympani cochleostomy I: results of a survey. Laryngoscope. 2007;117:2187‐2194. [DOI] [PubMed] [Google Scholar]
- 77. Roland PS, Wright CG. Surgical aspects of cochlear implantation: mechanisms of insertional trauma. Adv Otorhinolaryngol. 2006;64:11‐30. [DOI] [PubMed] [Google Scholar]
- 78. Skarzynski H, Lorens A, Piotrowska A, Podskarbi‐Fayette R. Results of partial deafness cochlear implantation using various electrode designs. Audiol Neurootol. 2009;14:39‐45. [DOI] [PubMed] [Google Scholar]
- 79. Briggs RJ, Tykocinski M, Stidham K, Roberson JB. Cochleostomy site: implications for electrode placement and hearing preservation. Acta Otolaryngol. 2005;125:870‐876. [DOI] [PubMed] [Google Scholar]
- 80. Wanna GB, O'Connell BP, Francis DO, et al. Predictive factors for short‐ and long‐term hearing preservation in cochlear implantation with conventional‐length electrodes. Laryngoscope. 2018;128:482‐489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wanna GB, Noble JH, Carlson ML, et al. Impact of electrode design and surgical approach on scalar location and cochlear implant outcomes. The Laryngoscope. 2014;124(Suppl 6):S1‐S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. O'Connell BP, Cakir A, Hunter JB, et al. Electrode location and angular insertion depth are predictors of audiologic outcomes in cochlear implantation. Otol Neurotol. 2016;37:1016‐1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Briggs RJ, Tykocinski M, Saunders E, et al. Surgical implications of perimodiolar cochlear implant electrode design: avoiding intracochlear damage and scala vestibuli insertion. Cochlear Implants Int. 2001;2:135‐149. [DOI] [PubMed] [Google Scholar]
- 84. McElveen JT Jr, Wolford RD Jr, Miyamoto RT. Implications of bone pate in cochlear implant surgery. Otolaryngol Head Neck Surg. 1995;112:457‐460. [DOI] [PubMed] [Google Scholar]
- 85. Ryu KA, Lyu AR, Park H, Choi JW, Hur GM, Park YH. Intracochlear bleeding enhances cochlear fibrosis and ossification: an animal study. PloS One. 2015;10:e0136617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Radeloff A, Unkelbach MH, Tillein J, et al. Impact of intrascalar blood on hearing. Laryngoscope. 2007;117:58‐62. [DOI] [PubMed] [Google Scholar]
- 87. Leigh BL, Cheng E, Xu L, Derk A, Hansen MR, Guymon CA. Antifouling photograftable zwitterionic coatings on PDMS substrates. Langmuir. 2019;35:1100‐1110. [DOI] [PMC free article] [PubMed] [Google Scholar]


