Abstract
Objective
Reports on sinonasal oncocytic papilloma (SNOP) are scarce. The aim of this retrospective study was to evaluate the clinical features of this rarest form of sinonasal papilloma with special emphasis on the pattern of recurrences and on the potential factors predicting them.
Study Design
Retrospective study.
Methods
Between the years 1994 and 2016, 20 patients (mean age 66 years; range 30–87) were diagnosed with SNOP at the Department of Otorhinolaryngology–Head and Neck Surgery, HUS Helsinki University Hospital (Helsinki, Finland). Hospital charts were reviewed to record various medical and sociodemographic patient characteristics, and the archived histological specimens were re‐evaluated. Postoperative follow‐up time varied between 26 days and 167 months.
Results
Maxillary sinus was the most common (60%) tumor location. None of the tissue samples showed dysplasia. Recurrence rate was 39% and the median time span to the first recurrence was 25 months (range 7–71). Smokers had more often a recurrence than nonsmokers (75% vs. 31%). Patients with perioperative purulent rhinosinusitis during the primary surgery had a higher recurrence rate compared with those without (60% vs. 31%). Tumors located in the sinuses recurred more often than those located in the nasal cavity (45% vs. 29%). However, all these findings remained statistically nonsignificant. None of the cases showed malignant transformation during the follow‐up.
Conclusion
SNOP has a propensity to recur. History of smoking, purulent rhinosinusitis during the primary surgery, and tumor location in the sinuses outside the nasal cavity seem to contribute to an increased trend in the risk of recurrence.
Level of Evidence
4
Keywords: Nasal cavity, nasal neoplasms, papilloma, paranasal sinuses, recurrence
INTRODUCTION
A great variety of both benign and malignant neoplasms may occur in the sinonasal tract.1 Sinonasal papillomas form a clinically important group for differential diagnostics. They arise from the ectodermally derived ciliated columnar, respiratory epithelium (Schneiderian membrane) lining nasal cavity, and paranasal sinuses, and are therefore also called as Schneiderian papillomas.2 Sinonasal papillomas are rare tumors, with an overall annual incidence of 0.74 cases per 100,000 people.3 They are divided into three subtypes according to their morphology. The terminology has previously been variable but the latest version (4th edition) of the WHO Classification of Head and Neck Tumours applies the names sinonasal exophytic papilloma (SNEP), sinonasal inverted papilloma (SNIP), and sinonasal oncocytic papilloma (SNOP).1 The proportions of these three sinonasal papilloma subtypes vary in the existing reports but SNOP remains the most uncommon. An American study by Hyams on 315 cases reported 50% SNEPs (fungiform), 47% SNIPs, and 3% SNOPs.4 In a Canadian series of 72 cases, the percentages were 18%, 76%, and 5.5%, respectively,5 and in a Danish series of 82 patients, 23%, 70%, and 6%, respectively.3
Due to the rarity of SNOP, the literature is also scarce and mostly consists of case reports. Typically these patients are presented as part of a larger series of sinonasal papillomas.2, 3, 6 Only one previous study (n = 33) focuses on SNOPs7 and therefore, there is an obvious need for reports on this rare histological subtype.
The aim of this retrospective study was to evaluate the clinical features of this rarest form of sinonasal papillomas with special emphasis on the pattern of recurrences and on the potential factors predicting them. In addition, rate of dysplasia in the surgical specimens and rate of metachronous malignancies (ie, not occurring at the time of SNOP diagnosis) during the follow‐up were investigated.
MATERIALS AND METHODS
This retrospective study comprised 20 patients treated with a diagnosis of SNOP between 1994 and 2016 at the Department of Otorhinolaryngology–Head and Neck Surgery, Helsinki University Hospital (Helsinki, Finland). The SNOP diagnosis was available from the year 1994 onward in the data register of Department of Pathology. Hospital charts were reviewed to record various patient, disease, diagnostic method, treatment, and postoperative follow‐up parameters. As there is no established official SNOP classification available, all cases were classified according to the Krouse staging system for SNIP (Table 1). Classification was made based on endoscopic, radiological, and perioperative findings. One patient (#4) had no preoperative imaging available and his lesion was classified according to the perioperative findings only. Seven (35%) patients were diagnosed with perioperative purulent rhinosinusitis and microbiological samples were taken. The factors potentially impacting the recurrence rate were analyzed by using SPSS Version 22 (SPSS, Inc., Chicago, IL, http://www-01.ibm.com/software/uk/analytics/spss/, https://www.ibm.com, RRID:SCR_002865). The chi‐square test was used for evaluation of differences between groups. Two‐sided P value was calculated using either Pearson chi‐square test or Fisher's exact test depending on the respective study setting. An institutional study permission was granted for the study.
Table 1.
Krouse Staging System for Inverted Papilloma.8
| Type 1 | Tumor totally confined to the nasal cavity, without extension into the sinuses. The tumor can be localized to one wall or region of the nasal cavity, or can be bulky and extensive within the nasal cavity, but must not extend into the sinuses or into any extranasal compartment. There must be no concurrent malignancy. |
| Type 2 | Tumor involving the ostiomeatal complex, and ethmoid sinuses, and/or the medial portion of the maxillary sinus, with or without involvement of the nasal cavity. There must be no concurrent malignancy. |
| Type 3 | Tumor involving the lateral, inferior, superior, anterior, or posterior walls of the maxillary sinus, the sphenoid sinus, and/or the frontal sinus, with or without involvement of the medial portion of the maxillary sinus, the ethmoid sinuses, or the nasal cavity. There must be no concurrent malignancy. |
| Type 4 | All tumors with any extranasal/extrasinus extension to involve adjacent, contiguous structures such as the orbit, the intracranial compartment, or the pterygomaxillary space. All tumors associated with malignancy. |
RESULTS
Classification, Diagnosis, and Treatment
There were altogether 20 SNOP patients and the basic characteristics of this study population are presented in Table 2. Nine (45%) of the patients were female and 11 (55%) were male. Four (20%) patients were current smokers. All tumors were unilateral and the side of the disease was nearly equally distributed. Maxillary sinus was the most usual location (60%). Seven (35%) patients had perioperative purulent rhinosinusitis during the primary surgery. All six available bacterial samples were positive. Fungal samples were available for three patients. One patient had a positive fungal culture and another had a positive finding in the fungal swab sample. One of the microbiological reports was not available for review. Preoperative computed tomography was performed in 95% of the cases. Magnetic resonance imaging was used in the diagnostics of one patient. One patient (patient #4) with tumor location only in the nasal septum was not imaged preoperatively. Endoscopic technique was used in 55% of the cases, and in one of them in combination with open surgery (patient #19). This patient had tumor in maxillary sinus and a partly endoscopically assisted Caldwell‐Luc procedure was performed. The nine cases with open surgery consisted of four Caldwell‐Luc approaches, four external medial maxillectomies, one frontal sinus osteoplastic flap approach, and one removal of the septal tumor. First endoscopic surgery was done in 2005 and this technique was then used in most cases (9/12, 75%). Five (25%) patients received further treatment for the tumor site as follows: laser cauterization (N = 1), bone drilling (N = 1), bone resection (N = 1), electrocauterization (N = 1), and combination of bone resection and electrocauterization of the surrounding mucous membrane (N = 1).
Table 2.
Characteristics Related to the Patients and Their Disease and Treatment.
| Number of Patients (%) | |
|---|---|
| Sex | |
| Female | 9 (45%) |
| Male | 11 (55%) |
| Smoking | |
| Current | 4 (20%) |
| Former | 3 (15%) |
| Never | 12 (60%) |
| Data missing | 1 (5%) |
| Side of the disease | |
| Right | 11 (55%) |
| Left | 9 (45%) |
| Main location | |
| Nasal septum | 1 (5%) |
| Nasal cavity | 6 (30%) |
| Maxillary sinus | 12 (60%) |
| Frontal sinus | 1 (5%) |
| Krouse stage | |
| Type 1 | 4 (20%) |
| Type 2 | 3 (15%) |
| Type 3 | 13 (65%) |
| Type 4 | 0 (0%) |
| Perioperative purulent rhinosinusitis | |
| Yes | 7 (35%) |
| No | 13 (65%) |
| Preoperative imaging | |
| Computed tomography | 19 (95%) |
| Without contrast | 10 (50%) |
| With contrast | 9 (45%) |
| Magnetic resonance imaging | 1 (5%) |
| Without contrast | 1 (5%) |
| With contrast | 0 |
| Surgical technique | |
| Open surgery | 10 (50%) |
| Endoscopic surgery | 9 (45%) |
| Combined | 1 (5%) |
| Further treatment of the primary site (eg, cauterization, bone drilling/resection) | |
| Yes | 5 (25%) |
| No | 15 (75%) |
Follow‐Up
The sex and age of the patients, and their follow‐up details including data on the recurrences are shown in Table 3. The age of the patients ranged between 30 and 87 years and the mean age was 66 years. Postoperative follow‐up time varied between 26 days and 167 months. Two patients died of other causes within 1 year after the first operation for SNOP. Patient #3 had chronic lymphocytic leukemia and he died in less than 1 month after the operation. Patient #10 had carcinoma in his transverse colon and he died 10 months after the operation. At the time of data analysis, 18 patients had been followed up for more than 24 months and they were all included in the recurrence evaluation. For these 18 patients, the median follow‐up time was 44 months (range 24–167). Seven (39%) patients had at least one recurrence. The median time for the first recurrence was 25 months (range 7–71). Two (29%) out of the seven primary recurrences were found during the first follow‐up year and three (43%) recurrences during the first two follow‐up years. Three (43%) of the seven patients with a recurrence had a second recurrence later during their follow‐up. The interval between the first and the second recurrence varied between 12 and 45 months. None of the patients had more than two recurrences. All recurrences were found in the same location as the primary tumor.
Table 3.
Twenty Patients With Oncocytic Papilloma.
| Patient Number | Age at Diagnosis Day (yr) | Sex | The First Recurrence* | The Second Recurrence* | Interval Between the First and Second Recurrence† | Follow‐Up Time* | Disease‐Free Follow‐Up Time From the Last Surgery† | Death From Other Causes* |
|---|---|---|---|---|---|---|---|---|
| 1 | 63 | F | 7.1 | 51.9 | 44.9 | 117.9 | 66 | |
| 2 | 30 | M | 71.1 | 89.9 | 18.9 | |||
| 3 | 81 | M | 0.9 | 0.9 | 0.9 | |||
| 4 | 40 | M | 167 | 167 | ||||
| 5 | 78 | F | 40.3 | 40.3 | ||||
| 6 | 55 | M | 15.2 | 39.4 | 22.4 | |||
| 7 | 76 | F | 48.3 | 48.3 | ||||
| 8 | 58 | M | 152 | 152 | ||||
| 9 | 75 | M | 25.4 | 37.8 | 12.4 | 79.6 | 41.9 | |
| 10 | 86 | M | 8.6 | 8.6 | 9.6 | |||
| 11 | 51 | F | 39.3 | 63.7 | 24.4 | 87.2 | 23.5 | |
| 12 | 62 | F | 35.9 | 35.9 | ||||
| 13 | 57 | M | 69.3 | 69.3 | ||||
| 14 | 87 | F | 6.9 | 40.7 | 33.7 | |||
| 15 | 66 | F | 50.3 | 50.3 | ||||
| 16 | 85 | F | 33.6‡ | 33.6 | ||||
| 17 | 63 | M | 35.3 | 35.3 | ||||
| 18 | 59 | F | 27.6 | 31.2‡ | 0.3 | |||
| 19 | 76 | M | 31.8‡ | 31.8 | ||||
| 20 | 69 | M | 24.6‡ | 24.6 |
Data on time intervals during follow‐up.
Time in months after the first surgery.
Time in months.
Follow‐up will continue.
Possible Factors Related to the Recurrence Rate
Table 4 shows the patients divided into two subgroups; those with a recurrence and those without. The distribution of the different factors among these groups is presented. Smokers had more recurrences (75%) than nonsmokers (31%) (former smokers and never smokers combined), but the difference did not reach statistical significance (P = .445). Recurrences were most uncommon in cases primarily located in the nasal cavity, but this finding was not statistically significant (P = .603). Patients with purulent rhinosinusitis during the primary surgery showed a higher recurrence rate than patients without, but the difference was not statistically significant (P = .326). Figure 1 shows the contrast enhanced computer tomography image of a 87‐year‐old female patient (patient #14) with SNOP in the right maxillary sinus and nasal cavity. Patient had an acute infection at the time of tumor removal, and she later had a tumor recurrence. Patients with Krouse stage 1 or 3 had recurrences during the follow‐up (50% and 45%, respectively), but patients with Krouse stage 2 had not (P = .421). Surgical technique and further treatment modalities to the tumor site showed no effect on the recurrence rate.
Table 4.
Potential Factors Related to Recurrence Rate.
| Patients With a Recurrence | Patients With No Recurrence | P Value | |
|---|---|---|---|
| Smoking | |||
| Current | 3 (75%) | 1 (25%) | |
| Former | 1 (33%) | 2 (67%) | |
| Never | 3 (30%) | 7 (70%) | |
| Data missing | 0 (0%) | 1 (100%) | .445 |
| Surgical technique | |||
| Open surgery | 3 (43%) | 4 (57%) | |
| Endoscopic surgery | 4 (40%) | 6 (60%) | |
| Combined | 0 (0%) | 1 (100%) | 1.0 |
| Main location | |||
| Nasal cavity | 2 (29%) | 5 (71%) | |
| Maxillary sinus | 4 (40%) | 6 (60%) | |
| Frontal sinus | 1 (100%) | 0 (0%) | .603 |
| Krouse stage | |||
| Type 1 | 2 (50%) | 2 (50%) | |
| Type 2 | 0 (0%) | 3 (100%) | |
| Type 3 | 5 (45%) | 6 (55%) | .421 |
| Perioperative purulent rhinosinusitis | |||
| Yes | 3 (60%) | 2 (40%) | .326 |
| No | 4 (31%) | 9 (69%) | |
| Further treatment of the primary site | |||
| Yes | 2 (40%) | 3 (60%) | |
| No | 5 (38%) | 8 (62%) | 1.0 |
Patients with at least 2 yr of follow‐up were included (N = 18).
Figure 1.
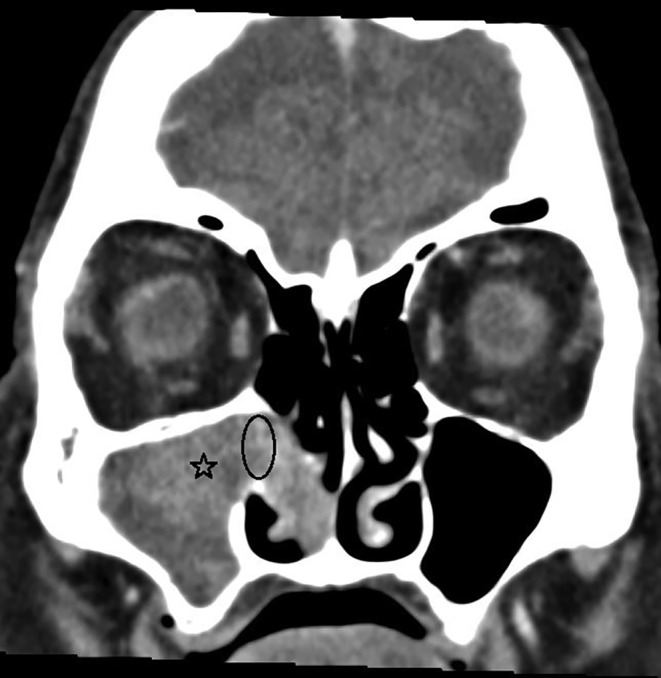
The contrast enhanced computer tomography picture of the 87‐year‐old female patient (#14) with sinonasal oncocytic papilloma (asterisk) in the right maxillary sinus and nasal cavity. The middle part of the mass showed contrast enhancement and also lamellar structures, which raised the suspicion of sinonasal papilloma. The tumor had caused bone remodellation at the region of ostiomeatal complex (ellipse). Perioperatively several pus accumulations were found among tumor mass.
Dysplasia and Malignancy
Altogether, there were 44 tissue samples available for these 20 patients for histological analysis and none of them showed dysplasia. None of the patients developed a malignant tumor in the nasal cavity or paranasal sinuses during the follow‐up.
DISCUSSION
We report on a comprehensive series of 20 consecutive SNOP cases treated at a single university hospital during 22 years. The mean age (66 years) of our patients corresponds to that in previous reports.2, 7, 9 Only two patients were younger than 50 years of age. Nine out of the 20 patients were female, which is in accordance with previous literature showing that SNOP is equally distributed between the sexes.7, 9
Recurrences
The recurrence rate in the present series was 39%. This finding is well in line with previous reports showing recurrence rates between 17% and 40%.2, 4, 6 Karligkiotis et al observed a much lower recurrence rate of 6% in a series of 33 SNOP patients.7 Due to the high frequency of recurrences, careful follow‐up is generally recommended.6 Some of our patients experienced late recurrences and therefore, we currently advise them to contact our hospital if any sinonasal symptoms recur after the last endoscopic follow‐up examination.
In the present study, we aimed at evaluating the potential factors affecting the recurrence rate, that is, smoking, surgical technique, tumor location, Krouse stage, purulent rhinosinusitis during the primary surgery, and further treatment for the primary site. Unfortunately, the number of patients was too low to show any statistically significant differences.
Smoking
Three out of the four current smokers had a recurrence. The recurrence rate was much lower (31%) among never and former smokers. On the other hand, only one of the three patients with a second recurrence was a smoker. Both previous and current smoking have been reported to be a risk factor for recurrences in SNIP patients and consequent increased mucosal inflammation has been the suggested possible explanation.10, 11 Similar reports regarding SNOP do not exist and we only found a trend without statistical significance in our series.
Surgical Technique
The present study did not demonstrate a statistically significant difference in the recurrence rate between the nonendoscopic surgery group and the endoscopic surgery group. With modern video‐assisted endoscopic approach, it is possible to obtain better visibility and therefore, more accurate removal of the tumor than with open surgery. Majority of SN surgery is currently performed endoscopically and there is increasing evidence for endoscopic removal of SNIP being associated with at least comparable results to external approaches and with less morbidity.12, 13, 14 Many authors now state that endoscopic approach may be considered the preferred technique in managing most inverted papillomas while only a minority of the tumors warrant combined or external approach. In the study of Karligkiotis et al, 33 SNOP patients were successfully managed with endoscopic surgery.7 Only in one case with a frontal sinus lesion, osteoplastic flap technique was combined with endoscopic technique. As mentioned earlier, the recurrence rate was 6% and there were no intraoperative complications, and long‐term complications were found in only three patients.
Tumor Location
In our series, tumor location was most often the maxillary sinus (60%), followed by the nasal cavity, including the ethmoidal area (35%), and the frontal sinus (5%). None of the tumors was located in the sphenoid sinus. This is in accordance with other studies reporting maxillary sinus as the most usual site for these tumors.2, 6, 7 Tumors located in the sinuses recurred more often than those located in the nasal cavity, but the difference was not statistically significant. Complete removal of the tumors originating from maxillary, sphenoid, or frontal sinuses is typically more challenging compared with those in the nasal cavity or ethmoidal sinuses. On the other hand, there are other challenges in the resection of tumors in the ethmoidal area, that is, those originating from lamina papyracea or skull base.
Krouse Stage
Krouse staging was primarily developed for SNIP.8 It may also be useful for SNOP due to its similar clinical behavior and it has been applied in one previous study concerning this entity.7 In our material, the most usual Krouse stage was type 3. There were no type 4 cases with tumor extension to extrasinonasal spaces or association with malignancy. We only found two reported cases in the literature with a benign SNOP extending through normal anatomical borders of the nasal and paranasal cavities.15, 16 The present study showed no statistically significant differences in the recurrence rate between Krouse types. The recent meta‐analysis regarding SNIP patients reported higher likelihood of recurrence in Krouse type 3.17
Perioperative Purulent Rhinosinusitis
The recurrence rate seemed to be higher among the patients with perioperative purulent rhinosinusitis (60%) compared with the patients without (31%). Interestingly, the three shortest intervals between the primary surgery and the first recurrence were in the patient group with purulent rhinosinusitis. Their recurrences appeared after 7–15 months postoperatively. It is possible that infection of the tissues has made surgical radicality more challenging to be assessed perioperatively and that some of these patients rather had residual disease than recurrences. This finding has clinical impact and will guide the future surgical management of patients with perioperative purulent rhinosinusitis at our center. We are not aware of other reports focusing on the coexisting infection at the time of primary SNOP surgery.
Further Treatment for the Primary Site
Some reports highlight the importance of additional treatment for the tumor resection site, that is, removing totally or partly (drilling) the underlying bone.18, 19 Although currently the removing or drilling of the underlying bone of the tumor is recommended as an essential part of adequate primary surgery, in our retrospective material it had been performed only in 3 out of 18 patients. In this study, we used the term further/additional surgery as an umbrella term covering both the removing or drilling of the underlying bone of the tumor and the cauterization (electro/laser) of tumor attachment site. Furthermore, the recurrence rate was even higher among these patients with additional surgery, but the difference was not statistically significant. However, we recognize inadequate primary surgery as one potential explanation for the high recurrence rate in our study.
Dysplasia and Malignancies
SNOP and SNIP may be associated with malignancies, that can be synchronous (ie, occurring at the time of papilloma diagnosis) or metachronous (ie, not occurring at the time of papilloma diagnosis, but found later during the follow‐up).1 None of our cases had malignant transformation during the follow‐up. A total of 43 histological samples were available for re‐evaluation in this series and none of them showed dysplasia. Thus, we could not find any metachronous malignancies in the current series. The fact that we were not able to report synchronous malignancies diagnosed during the same time period is a limitation of our study. These are registered according to a malignant diagnosis and therefore, all sinonasal malignancies during the same time period should be re‐evaluated histologically to identify synchronous cases.
According to the WHO Classification of Head and Neck Tumours around 4%–17% of all SNOPs harbor a carcinoma.1 However, re‐evaluation of the three references to this claim showed that this issue warrants further consideration. The first article by Kaufman et al reports 40 patients with SNIP and SNOP.6 Six out of the 40 patients had SNOP and one of them had a synchronous malignancy, which was not more specifically defined. The second reference was a case report with the SNOP patient having a large lamina papyracea defect without any associated malignancy.15 The third article compared three types of sinonasal papillomas including nine SNOP cases.9 None of them had any association with malignancy or dysplasia. In addition to the previous three articles, there are more reports in the literature concerning the association between SNOP and malignancy. Hyams reported one synchronous squamous cell carcinoma (10%) in a series of 10 SNOP cases.4 Barnes and Bedetti reported one synchronous mucoepidemoid carcinoma among six SNOP cases.2 Karligkiotis et al found one synchronous squamous cell carcinoma among 33 SNOP cases.7 Kapadia et al reported nine cases of carcinoma ex SNOP from two major institutions.20 In eight cases, the malignancy was synchronous and metachronous in one. There were six squamous cell carcinomas, two mucoepidermoid carcinomas, and one undifferentiated carcinoma. Based on the literature findings mentioned above, it seems that nearly all malignancies associated with SNOP are synchronous in nature and metachronous malignancies are rare. This conclusion might be an important addition to patient counseling.
Limitations of the Study
The present study has some limitations. Due to the rarity of oncocytic papilloma, the series had to be collected retrospectively and the size of the material still remained small. The retrospective nature of the study had a negative impact on the quantity and quality of the information and this should be taken into account when interpreting the results. Prospective study setting with systematic data collection would naturally enable more reliable information. Although some clear differences were found, the small sample size influenced the statistical significance of the present results. Furthermore, the null hypothesis of no difference between the groups cannot be excluded. Therefore, the results should be confirmed with a larger sample size in a multicenter setting.
CONCLUSION
We conclude that recurrences are common after primary surgery for SNOP. History of smoking, purulent rhinosinusitis during the primary surgery, and tumor site outside the nasal cavity, that is, in the paranasal sinuses were parameters contributing to an increased tendency for risk of recurrences, but however, these findings did not reach statistical significance. According to the present report and to earlier literature, metachronous malignancies are rare in the patient population with SNOP.
Editor's Note: This Manuscript was accepted for publication on August 22, 2019.
Conflict of interest: No potential conflict of interest was reported by the authors.
Funding: The study was supported by State funding for university‐level health research (TYH2015204).
BIBLIOGRAPHY
- 1. El‐Naggar A, Chan J, Grandis J, Takata T, Slootweg P, eds. WHO Classification of Head and Neck Tumours. 4th ed. Lyon, France: IARC; 2017. [Google Scholar]
- 2. Barnes L, Bedetti C. Oncocytic schneiderian papilloma: a reappraisal of cylindrical cell papilloma of the sinonasal tract. Hum Pathol 1984;15(4):344–351. [DOI] [PubMed] [Google Scholar]
- 3. Buchwald C, Franzmann MB, Tos M. Sinonasal papillomas: a report of 82 cases in copenhagen county, including a longitudinal epidemiological and clinical study. Laryngoscope 1995;105(1):72–79. [DOI] [PubMed] [Google Scholar]
- 4. Hyams VJ. Papillomas of the nasal cavity and paranasal sinuses. A clinicopathological study of 315 cases. Ann Otol Rhinol Laryngol 1971;80(2):192–206. [DOI] [PubMed] [Google Scholar]
- 5. Yoskovitch A, Braverman I, Nachtigal D, Frenkiel S, Rochon L, Black MJ. Sinonasal schneiderian papilloma. J Otolaryngol 1998;27(3):122–126. [PubMed] [Google Scholar]
- 6. Kaufman MR, Brandwein MS, Lawson W. Sinonasal papillomas: clinicopathologic review of 40 patients with inverted and oncocytic schneiderian papillomas. Laryngoscope 2002;112(8 pt 1):1372–1377. [DOI] [PubMed] [Google Scholar]
- 7. Karligkiotis A, Bignami M, Terranova P, et al. Oncocytic schneiderian papillomas: clinical behavior and outcomes of the endoscopic endonasal approach in 33 cases. Head Neck 2014;36(5):624–630. [DOI] [PubMed] [Google Scholar]
- 8. Krouse JH. Development of a staging system for inverted papilloma. Laryngoscope 2000;110(6):965–968. [DOI] [PubMed] [Google Scholar]
- 9. Vorasubin N, Vira D, Suh JD, Bhuta S, Wang MB. Schneiderian papillomas: comparative review of exophytic, oncocytic, and inverted types. Am J Rhinol Allergy 2013;27(4):287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moon IJ, Lee DY, Suh MW, et al. Cigarette smoking increases risk of recurrence for sinonasal inverted papilloma. Am J Rhinol Allergy 2010;24(5):325–329. [DOI] [PubMed] [Google Scholar]
- 11. Roh HJ, Mun SJ, Cho KS, Hong SL. Smoking, not human papilloma virus infection, is a risk factor for recurrence of sinonasal inverted papilloma. Am J Rhinol Allergy 2016;30(2):79–82. [DOI] [PubMed] [Google Scholar]
- 12. Lund VJ, Stammberger H, Nicolai P, et al. European position paper on endoscopic management of tumours of the nose, paranasal sinuses and skull base. Rhinol Suppl 2010;22:1–143. [PubMed] [Google Scholar]
- 13. Lombardi D, Tomenzoli D, Butta L, et al. Limitations and complications of endoscopic surgery for treatment for sinonasal inverted papilloma: a reassessment after 212 cases. Head Neck 2011;33(8):1154–1161. [DOI] [PubMed] [Google Scholar]
- 14. Dragonetti A, Gera R, Sciuto A, et al. Sinonasal inverted papilloma: 84 patients treated by endoscopy and proposal for a new classification. Rhinology 2011;49(2):207–213. [DOI] [PubMed] [Google Scholar]
- 15. Liu CY, Tsai TL, Hsu CY, Lin CZ. Oncocytic schneiderian papilloma. J Chin Med Assoc 2004;67(5):255–257. [PubMed] [Google Scholar]
- 16. Bignami M, Pistochini A, Meloni F, Delehaye E, Castelnuovo P. A rare case of oncocytic schneiderian papilloma with intradural and intraorbital extension with notes of operative techniques. Rhinology 2009;47(3):316–319. [DOI] [PubMed] [Google Scholar]
- 17. Lisan Q, Moya‐Plana A, Bonfils P. Association of Krouse classification for sinonasal inverted papilloma with recurrence: a systematic review and meta‐analysis. JAMA Otolaryngol Head Neck Surg 2017;143(11):1104–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Landsberg R, Cavel O, Segev Y, Khafif A, Fliss DM. Attachment‐oriented endoscopic surgical strategy for sinonasal inverted papilloma. Am J Rhinol 2008;22(6):629–634. [DOI] [PubMed] [Google Scholar]
- 19. Healy DY Jr, Chhabra N, Metson R, Holbrook EH, Gray ST. Surgical risk factors for recurrence of inverted papilloma. Laryngoscope 2016;126(4):796–801. [DOI] [PubMed] [Google Scholar]
- 20. Kapadia SB, Barnes L, Pelzman K, Mirani N, Heffner DK, Bedetti C. Carcinoma ex oncocytic schneiderian (cylindrical cell) papilloma. Am J Otolaryngol 1993;14(5):332–338. [DOI] [PubMed] [Google Scholar]


