Abstract
Background
The reconstruction of segmental mandibular defects remains a challenge for the reconstructive surgeon, from both a functional and an esthetic point of view.
Methods
This clinical review examines the different techniques currently in use for mandibular reconstruction as related to a range of etiologies, including the different bone donor sites, the alternatives to free flaps (FFs), as well as the contribution of computer‐assisted surgery. Recent progress and the perspectives in bone tissue engineering (BTE) are also discussed.
Results
Osseous FF allows reliable and satisfying outcomes. However, locoregional flap, distraction osteogenesis, or even induced membrane techniques are other potential options in less favorable cases. Obtaining an engineered bone with satisfactory mechanical properties and sufficient vascular supply requires further investigations.
Conclusions
Osseous FF procedure remains the gold standard for segmental mandible reconstruction. BTE strategies offer promising alternatives.
Keywords: bone tissue engineering, CAD/CAM, free flap, mandible reconstruction, microvascular
1. INTRODUCTION
Segmental mandibular substance loss is frequently encountered in maxillofacial surgery. To limit the functional consequences and their sociopsychological impact, the objective of reconstruction is to reestablish mandibular continuity and functionality, with the ultimate goal of returning the patient to a predisease state.1 The surgeon has a wide range of techniques available that has continually grown and improved over the past few years. Among these techniques, tissue‐free transfer has become a reliable gold standard for extensive defects2, 3 with an overall success rate uniformly greater than 95% in high‐volume reconstructive centers.2, 4 The variety of donor sites and technical refinements (eg, computer‐assisted surgery [CAS]) have optimized the functional and esthetic outcomes.5 However, the limited amount of available bone and the morbidity of the donor site remain the major limits of this technique.6, 7
Consequently, the current challenge is to find an alternative procedure that is not subject to the drawbacks of autologous bone harvesting with a similar or even higher success rate. Bone tissue engineering (BTE) strategies have demonstrated promising results for segmental defects over the past few years. The use of calcium phosphate (CaP) biomaterials as scaffolds associated with osteogenic cells and cell modulation factors have been described in several cases in which free flap (FF) use was compromised. However, despite these encouraging results, obtaining vascularized scaffold providing reliable bone formation with mechanical properties adapted to the mandibular functions remains a major challenge.8, 9, 10
The objective of this review is to present the current techniques for segmental mandibular defect reconstruction as well as the current state of the research in BTE. The main processes, their recent progress, and the promising results of preclinical studies are exposed.
2. CLINICAL CONTEXT AND OBJECTIVES OF SEGMENTAL MANDIBULAR DEFECT RECONSTRUCTION
Segmental mandibular loss can be caused by a number of etiologies3, 11, 12, 13, 14, 15, 16 as detailed in Table 1. Among them, tumor ablative surgery is the most common, followed by facial trauma and more rarely inflammatory or infection diseases.17 Even though the objective is to restore mandibular continuity, the time and reconstructive method can vary.
Table 1.
The most frequent etiologies of segmental mandible defects
| Origin |
|---|
| Tumor resection: Malignancies: squamous cell carcinoma (95%), sarcoma, salivary gland carcinoma; benign tumor: ameloblastoma |
| Trauma: Gunshot, road accident |
| Osteonecrosis of jaws: Osteoradionecrosis, medication‐related osteonecrosis |
| Osteomyelitis: Dental infection, autoimmune disease |
The particularity in oncological surgery is that it involves several tissues (bone, mucosa, and muscle) and must be anticipated by the reconstructive surgeon. Mandibular reconstruction is therefore planned according to the surgical margins to optimize the results of the procedure. In addition, success and healing are essential because adjuvant irradiation must be performed within 6 weeks of surgery to improve patient survival.2, 18 Thus, the vascularized tissue transfer is the most widely used technique for primary segmental bone reconstruction.
Substance loss due to injury (eg, traffic accidents, gunshot) can cause tear injuries and uncontrolled loss of soft and hard tissues. Several surgical stages are frequently required, and the functional and esthetic results are often uncertain or even disappointing. Nonetheless, many technical possibilities exist for these cases (vascularized tissue free transfer, bone grafting, bone distraction).12, 19
The osteonecrosis of the jaws can also be responsible for mandible loss requiring segmental reconstruction. The particularity of osteoradionecrosis (ORN) is that it occurs in cases with a poor healing potential due to the bone and the soft tissue irradiation.13 Medication‐related osteonecrosis of the jaw (MRONJ) differs in its clinical presentation. Related to the systemic intake of bone antiresorptives (bisphosphonate, denosumab) or antiangiogenics, damage is often less clearly limited than an ORN but with a more favorable surrounding tissue. However, surgery in cases of MRONJ should take into consideration the prognosis of the patient, who is often suffering from metastatic disease. The healing complications usually encountered in ORN and MRONJ make FF the best therapeutic option if segmental reconstruction is required when the medical treatment no longer controls the underlying disease.13, 14, 20
The main objectives of mandibular reconstruction are first to restore chewing, phonation, and breathing functions. Reconstruction should also preserve dental occlusion and the temporomandibular joint, and it should allow dental rehabilitation.21
Finally, the face is an individual's interface with society and the esthetic result should be the best possible. Deformities of the lower part of the facial contour can have major social and psychological repercussions. The stakes of the reconstruction are therefore considerable to limit psychological distress that can lead to isolation and depression.3
3. CLASSIFICATIONS OF SEGMENTAL MANDIBULAR SUBSTANCE LOSS
The main use of the classifications is to assess the characteristics of the defect to define a therapeutic algorithm for the reconstruction.
In 1989, Boyd described the HCL classification for hemimandibular (H), central (C), and lateral (L) defects. He modified his classification in 1993 to integrate mucous and/or cutaneous damage (“o” no mucosal or skin defect, “m” mucosal defect, “s” skin defect, “sm” skin and mucosal defect).22 However, the length of a defect was not included in this classification. In 1991, Urken described the CRBS classification using the following anatomical terms: condyle, ramus, body, and symphysis.23 It considered oromandibular composite defects (lip, buccal, soft palate, floor of the mouth, the tongue, and the skin) and also the neurological deficit (inferior alveolar, lingual, hypoglossal nerve, and facial nerve). As a result, the nomenclature provides a more detailed description but includes up to 3500 possibilities.
Schultz et al and Brown et al have recently proposed simplified classifications that do not include soft tissue loss, which are intended to be more “functional” so that an algorithm for reconstruction can be proposed.5, 24 Schultz et al defined four types of defects (type 1 for unilateral dentoalveolus; type 2 for unilateral dentoalveolus + ramus; type 3 for bilateral dentoalveolus; type 4 for bilateral dentoalveolus + ramus) and included the laterality of the donor vessels (viable ipsilateral vasculature, A; nonviable ipsilateral vasculature, B) and the involvement of the condyle to define the best‐adapted bone FF (fibula or iliac crest). Brown et al defined four classes of segmental mandibulectomy (class I as lateral, class II as hemimandibulectomy, class III as anterior, class IV as extensive, c when condylectomy is required). This classification is intended to be simpler and more detailed (size and location of the defect, functional morbidity) to define the most adapted FF type.
Finally, lateral and anterior defects are the two main types of these classifications playing a major role for the functional outcomes: lateral resections are better tolerated than resections involving the anterior symphyseal region which present greater functional (eg, swallowing, mastication, phonation, and breathing) and esthetic morbidity.2, 25
However, there is no ideal system, especially given that these classifications, sometimes complicated, can be difficult to apply in routine practice. Actually, no consensus exists, and the choice of reconstruction mainly depends on the experience and habits of the surgical team and the associated soft tissues to reconstruct.
4. CURRENT SURGICAL PROCEDURES: INDICATIONS AND LIMITATIONS
4.1. Osseous FFs: the gold standard
Osseous FF is currently the choice treatment for segmental mandibular defect reconstruction due to its low failure rate (<5%) and the possibility to restore complex multitissue loss of substance with mechanical properties adapted to mandibular functions. Technical refinements such as chimeric FF or CAS have also optimized the esthetic and functional outcomes over the past few years.26, 27
The available osseous donor sites are the fibula, the iliac crest, the scapula, and the radius as detailed in Table 2.28 The choice depends on the length and the shape of the bone defect, the pedicle length, the type of soft tissue to repair, and the team's experience.
Table 2.
Free flap characteristics for segmental mandible reconstruction
| Fibula | Iliac crest | Scapula | Radius | ||
|---|---|---|---|---|---|
| Lateral border | Tip | ||||
| Bone | |||||
| Length | 20‐25 cm | 10‐15 cm | 10–12 cm | 6–8 cm | 10–12 cm |
| Shaping | Simple | Moderate | Simple | Not adapted | Simple |
| Reconstruction site | All parts | Angle and body | All parts | Symphyse | All parts |
| Dental implant | Yes | Yes | Yes, ± bone graft | Not adapted, + bone graft | Yes, ± bone graft |
| Pedicle length | Long | Short | Moderate | Long | |
| Skin paddle | |||||
| Thinness | Moderate | Moderate | Thick | Thin | |
| Number | Various | Unique | Various | Various | |
| Bone fixation | Fixed | Fixed | Free | Free from bone, fixed to pedicle | |
| Available | Soleus muscle | Internal oblique | Latissimus dorsi, serratia | No | |
| Muscle | Fixed to bone | Fixed to bone | Free from bone | ||
| Donor site morbidity and disadvantages |
|
|
|
|
|
Abbreviation: CI, contraindicated.
The fibula is the donor site that presents the greatest number of favorable criteria: its reliability and easy harvesting, the available bone and its easy shaping, bone allowing dental implant placement, and a low donor site morbidity.6, 7, 23, 29 However, alternative FF is needed in case of obstructive arterial disease of the lower limbs or when the paddles fixity or its limited volume are not adapted.
Thus, the location and length of the segment defect, associated soft tissue loss, and the type of dental rehabilitation planned (implant, prosthesis) are the main criteria guiding the flap choice.
4.2. Alternatives to osseous FF
4.2.1. Absence of osseous reconstruction
Sometimes, reconstruction options can be limited for multioperated and/or irradiated patients presenting a vessel‐depleted neck, for those with comorbidities contraindicating heavy surgery, or in case of repeated failures of mandibular reconstruction.30, 31
Thus, the functional result of the posterolateral defects only reconstructed by soft tissue (FF or regional musculocutaneous flap) is described as acceptable by several authors despite mandibular deviation and potential social repercussions.32, 33
4.2.2. Rigid fixation plates
Rigid fixation plates are an alternative for cases poorly suited to FF reconstruction. A musculocutaneous or fasciocutaneous flap cover (free or locoregional) must be associated to limit the risk of exposure. Reconstruction plates give acceptable functional results in lateral segmental defects and prevent mandibular lateral deviation. However, the risk of fracture, secondary loosening, or exposure remains significant (50%‐80%) and limits this reconstruction to fragile and/or toothless patients with limited chewing strength, for whom there is no dental rehabilitation project (implant or prosthesis).34, 35, 36
4.2.3. Autologous bone grafting
Nonvascularized autologous bone grafting has shown its efficacy in limited bone defects with preservation of the periosteum (<5 cm) and without history of radiotherapy.37 Cortical and trabecular bone harvesting from the iliac crest secured with a fixation plate system is a therapeutic possibility for lateral or anterior segmental reconstructions. For ramus reconstructions including the condyle, chondrocostal bone graft has also been described, notably in children given its growth potential.38, 39
4.2.4. Induced membrane technique
The induced membrane technique (IMT) is a variant of autologous bone grafting and its aim is to improve graft vascularization via a two‐phase procedure. The osteoinductive potential promotes the bone regeneration and provides a simple and short approach that can be adapted to fragile patients or less favorable cases (infected or irradiated bone, ORN).
At the first surgical time, the extremities of the bone defect are freshened and the substance loss is filled with a spacer (eg, polymethyl methacrylate, cement) inducing an inflammatory reaction and the formation of the “induced membrane” with high cellularity and angiogenic capacity. A second surgery is then performed 2 months later: the membrane is incised, the spacer is removed, the defect is filled with cancellous bone graft, and membrane is sutured to contain the graft and encourage its vascularization.
However, the use of this approach requires two surgical times and presents a high risk of infection or exposure of the nonresorbable biomaterial that may require premature removal and thus failure of the procedure.40, 41
4.2.5. Distraction osteogenesis
Distraction osteogenesis (DO) technique potentially allows the reconstruction of extensive segmental defects.42, 43
The procedure begins with osteotomy at the mandibular stump followed by daily distraction of the mandibular fragment (0.5‐1 mm). The distraction device is removed at the end of bone ingrowth, and the bone reconstruction is maintained by a fixation plate. DO is a valuable option for segmental reconstruction avoiding autologous bone harvest and allowing the soft tissues to adapt on the newly formed bone. In addition, several cases of dental implant rehabilitation have been described after DO.43
However, DO can be a prolonged procedure in case of extensive defect. In addition, DO cannot be applied when periosteum is removed or in case of irradiation. Finally, an external distractor is generally preferred to internal device for largest defects and results in facial scarring and burdensome procedure often limiting this technique in patients after gunshot injuries.42, 44, 45
4.2.6. Input of CAS surgery for segmental mandibular reconstruction
CAS is a tool and its objective is to simplify the intraoperative surgical stages and to optimize the functional and esthetic results.4, 46 Using the computer‐assisted design/computer‐assisted manufacturing (CAD/CAM) process and three‐dimensional (3D) printing, CAS makes individualized mandibular reconstruction possible. It implies prior collaboration between the surgeon and an engineer to plan the different reconstruction stages using software designed specifically for the mirroring technique.
The main contribution of CAS is the fabrication of cutting and bone drilling guides as well as customized plates to optimize the reconstruction and shorten surgical time47 (Figure 1). This allows better shaping of the mandibular contour and better positioning of the condyle. CAS thus facilitates bone conformations and complex flap placement and can even be used to plan dental implant placement in the same surgical time through guided surgery.
Figure 1.
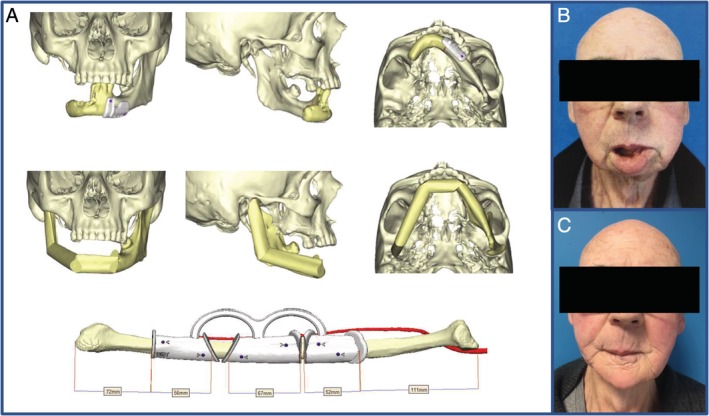
Input of computer‐assisted surgery for the shaping of osseous free flap. A, Images from a surgical planning showing the cutting guide for the shaping of the fibula (Materialise, Louvain, and Belgium). B, The preoperative results. C, The postoperative results
Source: Giannoudis et al.48
CAS is also useful for alternative reconstruction techniques to FFs such as customized distractor fabrication,44 positioning of chondrocostal grafts, and fabrication of porous titanium reconstruction plates49 (Figure 2A‐C).
Figure 2.
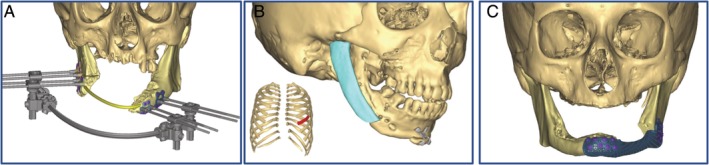
Virtual surgical planning for alternative techniques to osseous free flap. Surgical planning for, A, a distraction osteogenesis, B, a chondrocostal graft and, C, a custom made rigid fixation plate (Materialise, Louvain, and Belgium)
CAS has a wide and exponential scope of application that continues to be developed in mandibular reconstruction.
5. BTE: CURRENT AND FUTURE TECHNIQUES
For many years regenerative medicine teams have attempted to set up alternative bone reconstruction procedures based on tissue engineering. The advantage of BTE is defining a reproducible procedure that is technically simple, adaptable to bone defects and each type of patient (comorbidity, infection, and postoperative radiotherapy) using biomaterials to prevent the morbidity associated with autologous bone harvesting. In extensive bone defects, Giannoudis et al48, 50 diagrammatically described the well‐known “diamond concept” for BTE that is based on five entities including an osteoconductive support or “scaffold” playing the role of extracellular matrix, this matrix's mechanical stability, osteogenic cells that are capable of differentiation, osteoinductive regulation factors, and vascular supply providing nutrition and oxygen input (Figure 3).
Figure 3.
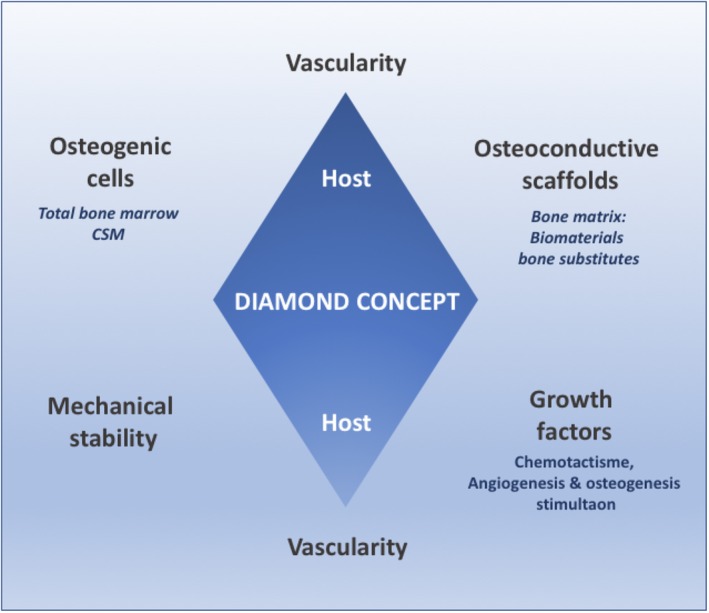
Diamond concept describing the required characteristics for bone tissue engineering. Desirables features of a bone construct including the bone matrix (scaffold) able to guide the bone ingrowth, osteogenic cells, cell modulators as well as the oxygen and nutrient supply
5.1. The choice of bone matrices or scaffolds
The scaffold is a porous 3D support that plays the role of an extracellular matrix that should serve as a guide to bone growth stemming from the host tissue. It should be suitable for the local mechanical properties, be the vector for osteogenic cells, and allow cellular exchanges and vascular colonization.51, 52 As detailed in Table 3, the desirable properties of the “perfect” scaffold must include no immune response, consolidation with the host tissue and new bone formation. In addition, biodegradation and the newly formed bone should also occur concurrently at a matching rate.51, 53, 54, 55, 56, 57
Table 3.
Desirable scaffolds characteristics for bone tissue engineering
| Biocompatibility | Bioactivity | Osteoinduction | Osteoconduction | Bioresorption | Mechanical resistance | Porosity |
|---|---|---|---|---|---|---|
| No immune rejection; no releasing of toxic components | Binding and consolidation to host bone | Pluripotent cells stimulation and osteogenesis | Passive conduction of bone growth | Scaffold degradation replaced by bone formation | Similar elastic and compressive strength to host bone | Architecture allowing vascular invasion and cell‐scaffold interaction |
The main bone substitutes that have been described in preclinical animal model or in human for craniofacial repair are CaP ceramics, polymers, and allogenic bone substitutes51, 55:
CaP ceramics can be made of hydroxyapatite (HA), beta‐tricalcium phosphate (βTCP), or both (biphasic calcium phosphate, BCP). Close to the inorganic fraction of bone, they are particularly interesting for their biocompatibility, osteoconduction, and bioactivity. They also have the advantage of good mechanical resistance. HA has a long bioresorption time but provides mechanical resistance to the scaffold. βTCP degrades more rapidly and improves bioresorption and biocompatibility but at the expense of mechanical properties. As a result, combining the two (BCP) makes it possible to accumulate the qualities of each of the biomaterials and obtain a balance between mechanical properties and their bioresorption qualities.
Polymer bone substitutes can be either natural or synthetic. Natural polymers such as collagen have excellent osteoconduction but low biocompatibility and mechanical properties. Consequently, synthetic polymers are more widely used. Polylactic acid (PLA), polyglycolic acid (PGA), poly(lactic‐co‐glycolic acid) (PLGA), which associates PGA and PLA, and polycaprolactones present excellent biocompatibility at a low price. However, the risk of an inflammatory reaction related to long bioresorption, limited bioactivity, and low mechanical resistance are the main weak points of these scaffolds, severely limiting their compatibility for mandibular reconstruction and their use for clinical settings.51
Allogenic bone substitutes are natural deproteinized and decellularized human bone from bone banks. Allografts present good osteoconduction properties, but the results obtained in cases where large mandibular defects need filling are disappointing because of their low bioactivity as well as slow revascularization and new bone formation. Moreover, the potential risk of transmissible disease or an immune response remains a significant limitation in the use of this type of biomaterial in human.55
Finally, use of CaP bioceramic seems to be the best compromise and the current trend for the segmental mandibular reconstruction in preclinical studies or in some experimental cases in human.58, 59 From a surgical point of view, the scaffold should be able to adapt perfectly to the mandibular defect. Construction of a scaffold that is anatomically close to the mandible seems possible using CAD/CAM processes and additive methods (eg, 3D printing). Nevertheless, in practice, scaffold construction remains a technical challenge, notably in terms of controlling the macroarchitecture and microarchitecture, making colonization by osteogenic and endothelial cells unreliable.60 In addition, adding osteogenic cell elements, growth factors (GFs), vascular supply, and the control of materials degradation are indispensable for bone regeneration in a bone construct.52
5.2. Cell modulator and vascularization of mandibular scaffolds
GFs play a key role in osteoinduction, proliferation, differentiation of osteoprogenitor cells as well as in angiogenesis. Bone morphogenetic proteins (BMPs) showed the most promising results for segmental engineered mandible.61, 62, 63 However, BMPs use in human expose to risk of wound complication such as breakdown, infection, or important local swelling with potential acute respiratory failure when it is used too close to airways.64, 65, 66 In addition, BMPs can also be responsible for ectopic bone formation in the surrounding soft tissues as described in numerous preclinical studies55, 61, 67, 68 limiting its extrapolation for clinical practice.
Osteogenic cell impregnation of the scaffold is the cornerstone to ensure bone formation and its renewal. The main types of cells that can be used to impregnate mandible scaffolds and provide an osteogenic capacity are mesenchymal stem cells (MSCs) or total bone marrow (TBM). The advantage of TBM is the presence of MSCs and diverse GFs as well as the possibility of extemporaneous harvesting during a surgical procedure. The use of MSCs requires an in vitro stage beforehand but makes it possible to control the cell rate better during scaffold impregnation.61, 67, 69, 70, 71, 72
The final indispensable element for segmental mandibular reconstruction with BTE is to ensure adapted nutrition and oxygen supply.51, 52, 73Within the scaffold, although the cells present on the edges of the biomaterial can easily receive oxygen and nutrients from the surrounding tissues through simple diffusion, the cells seeded at the center do not receive this supply, which is limited to a few micrometers deep. Use of bioreactors is therefore a procedure aiming to impregnate adjuvants and ensure the nutrient supply. The scaffold is either cultured in vitro before implantation or it is nurtured in vivo before being explanted and then reimplanted at the mandibular defect. Even though in vitro bioreactors with continuous flow of perfusate mimic vascularization and initially allow sufficient perfusion to the bone matrix, today they cannot ensure long‐term, sufficient vascular supply to cells once they have been implanted in segmental defects.58, 59
In vivo bioreactors have shown, however, more promising results in animal experiments and in cases of segmental mandibular defect reconstruction in patients who could not undergo classic bone FF reconstruction. To mimic the tissue's physiological blood supply, the strategy is to use the patient as a bioreactor. In several cases, this method has made it possible to establish neovascularization that has persisted beyond implantation.
The scaffolds first described in animal model and then in rare human cases were CaP substitutes impregnated with cells (TBM) with or without GF (BMPs), stabilized by osteosynthesis screws or preformed titanium cages. The scaffolds were placed in a heterotopic situation in a muscle to ensure it's prevascularization. The latissimus dorsi muscle remains the most frequently described implantation site for implantations lasting 2‐6 months. Once they have been vascularized, scaffolds are implanted as a FF or pedicled flap toward the mandibular reconstruction site.8, 9, 10 However, the absence of mechanical properties adapted to mandibular functions resulted in a middle‐term failure in most cases (eg, fracture, osteointegration defect, infection). The mechanical resistance and immediate stability of the reconstructions are therefore another crucial point in the success of this type of procedure.
5.3. Perspectives in tissue engineering
Even though the segmental mandibular defect reconstructions described using BTE in humans are a technical feat, this type of reconstruction does not respond to the depleted‐vessel neck problem and the procedure remains complex: it requires cell and molecular impregnation of the scaffold followed by a long prevascularization and maturation period followed by a second surgical intervention always causing donor site morbidity. In response to the vascular issue, intrinsic vascularization procedure is BTE strategies generating axially vascularized scaffold by directly placing an arteriovenous loop within the mandible scaffold. It allows spontaneous vessels sprouting from the loop and subsequent revascularization of the bone construct.67, 73, 74, 75, 76, 77 Consequently, this method could decrease the donor site morbidity by simple transfer of the tissue construct with its pedicle or even completely avoid the donor site morbidity if the mandible defect is used as a primary recipient site for the CaP scaffold.70, 77, 78, 79, 80 Constructing a vascular loop may also simplify the problem of a lack of neck vessels stemming from the use of a venous graft.76, 81
6. CONCLUSIONS
A large number of innovative techniques in mandibular reconstruction have emerged over the past few years. Refinement of surgical techniques such as CAS have greatly improved the functional and esthetic results stemming from osseous FF, which today remain the gold standard. However, BTE methods are continually developing new combinations of biomaterials and adjuvants, aiming to form an implant that is perfectly adapted to the defect, which would stimulate new bone formation without causing sequelae and morbidity secondary to autologous bone harvesting. Nonetheless, constructing a scaffold with satisfactory mechanical properties as well as the necessary oxygen and nutrients to the survival of osteogenic cells once implanted remains the main challenge in mandibular reconstructive surgery.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
ACKNOWLEDGMENT
This study was supported by the Fondation des Gueules Cassées (No. 66‐2017).
Paré A, Bossard A, Laure B, Weiss P, Gauthier O, Corre P. Reconstruction of segmental mandibular defects: Current procedures and perspectives. Laryngoscope Investigative Otolaryngology. 2019;4:587–596. 10.1002/lio2.325
Funding information Fondation des Gueules Cassées, Grant/Award Number: 66‐2017
REFERENCES
- 1. Namaki S, Matsumoto M, Ohba H, Tanaka H, Koshikawa N, Shinohara M. Masticatory efficiency before and after surgery in oral cancer patients: comparative study of glossectomy, marginal mandibulectomy and segmental mandibulectomy. J Oral Sci. 2004;46(2):113‐117. [DOI] [PubMed] [Google Scholar]
- 2. Kakarala K, Shnayder Y, Tsue TT, Girod DA. Mandibular reconstruction. Oral Oncol. 2018;77:111‐117. 10.1016/j.oraloncology.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 3. Batstone MD. Reconstruction of major defects of the jaws. Aust Dent J. 2018;63(Suppl 1):S108‐S113. 10.1111/adj.12596. [DOI] [PubMed] [Google Scholar]
- 4. Toto JM, Chang EI, Agag R, Devarajan K, Patel SA, Topham NS. Improved operative efficiency of free fibula flap mandible reconstruction with patient‐specific, computer‐guided preoperative planning: preoperative modeling for free fibula flaps. Head Neck. 2015;37(11):1660‐1664. 10.1002/hed.23815. [DOI] [PubMed] [Google Scholar]
- 5. Schultz BD, Sosin M, Nam A, et al. Classification of mandible defects and algorithm for microvascular reconstruction. Plast Reconstr Surg. 2015;135(4):743e‐754e. 10.1097/PRS.0000000000001106. [DOI] [PubMed] [Google Scholar]
- 6. Ling XF, Peng X. What is the Price to pay for a free fibula flap? A systematic review of donor‐site morbidity following free fibula flap surgery. Plast Reconstr Surg. 2012;129(3):657‐674. 10.1097/PRS.0b013e3182402d9a. [DOI] [PubMed] [Google Scholar]
- 7. Momoh AO, Yu P, Skoracki RJ, Liu S, Feng L, Hanasono MMA. Prospective cohort study of fibula free flap donor‐site morbidity in 157 consecutive patients. Plast Reconstr Surg. 2011;128(3):714‐720. 10.1097/PRS.0b013e318221dc2a. [DOI] [PubMed] [Google Scholar]
- 8. Warnke PH, Wiltfang J, Springer I, et al. Man as living bioreactor: fate of an exogenously prepared customized tissue‐engineered mandible. Biomaterials. 2006;27(17):3163‐3167. 10.1016/j.biomaterials.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 9. Warnke PH, Springer ING, Wiltfang J, et al. Growth and transplantation of a custom vascularised bone graft in a man. Lancet Lond Engl. 2004;364(9436):766‐770. 10.1016/S0140-6736(04)16935-3. [DOI] [PubMed] [Google Scholar]
- 10. Heliotis M, Lavery KM, Ripamonti U, Tsiridis E, di Silvio L. Transformation of a prefabricated hydroxyapatite/osteogenic protein‐1 implant into a vascularised pedicled bone flap in the human chest. Int J Oral Maxillofac Surg. 2006;35(3):265‐269. 10.1016/j.ijom.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 11. Paré A, Joly A. Oral cancer: risk factors and management. Presse Medicale Paris Fr. 2017;46:320‐330. 10.1016/j.lpm.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 12. Khatib B, Gelesko S, Amundson M, et al. Updates in management of craniomaxillofacial gunshot wounds and reconstruction of the mandible. Facial Plast Surg Clin North Am. 2017;25(4):563‐576. 10.1016/j.fsc.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 13. Rommel N, Kesting MR, Rohleder NH, Wolff K‐D, Weitz J. Surgical management of severe osteoradionecrosis of the mandibular bone by using double free flap reconstruction. J Craniomaxillofac Surg. 2018;46(1):148‐154. 10.1016/j.jcms.2017.09.025. [DOI] [PubMed] [Google Scholar]
- 14. Neto T, Horta R, Balhau R, et al. Resection and microvascular reconstruction of bisphosphonate‐related osteonecrosis of the jaw: the role of microvascular reconstruction. Head Neck. 2016;38(8):1278‐1285. 10.1002/hed.24395. [DOI] [PubMed] [Google Scholar]
- 15. Paré A, Joly A, Laure B, Bonin Goga B, Kün‐Darbois J‐D, Goga D. A chronic swelling of the mandible in a child. J Stomatol Oral Maxillofac Surg. 2018;119(1):81‐82. 10.1016/j.jormas.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 16. Baur DA, Altay MA, Flores‐Hidalgo A, Ort Y, Quereshy FA. Chronic osteomyelitis of the mandible: diagnosis and management—an institution's experience over 7 years. J Oral Maxillofac Surg. 2015;73(4):655‐665. 10.1016/j.joms.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 17. Kumar BP, Venkatesh V, Kumar KAJ, Yadav BY, Mohan SR. Mandibular reconstruction: overview. J Maxillofac Oral Surg. 2016;15(4):425‐441. 10.1007/s12663-015-0766-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koyfman SA, Ismaila N, Crook D, et al. Management of the neck in squamous cell carcinoma of the oral cavity and oropharynx: ASCO clinical practice guideline. J Oncol Pract. 2019;15(5):273‐278. 10.1200/JCO.18.01921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gurunluoglu R, Gatherwright J. Microsurgical reconstruction of complex maxillofacial gunshot wounds: outcomes analysis and algorithm. Microsurgery. 2019;39:384‐394. 10.1002/micr.30418. [DOI] [PubMed] [Google Scholar]
- 20. Caldroney S, Ghazali N, Dyalram D, Lubek JE. Surgical resection and vascularized bone reconstruction in advanced stage medication‐related osteonecrosis of the jaw. Int J Oral Maxillofac Surg. 2017;46(7):871‐876. 10.1016/j.ijom.2017.01.023. [DOI] [PubMed] [Google Scholar]
- 21. Kumar VV, Jacob PC, Ebenezer S, et al. Implant supported dental rehabilitation following segmental mandibular reconstruction‐quality of life outcomes of a prospective randomized trial. J Craniomaxillofac Surg. 2016;44(7):800‐810. 10.1016/j.jcms.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 22. Boyd JB, Gullane PJ, Rotstein LE, Brown DH, Irish JC. Classification of mandibular defects. Plast Reconstr Surg. 1993;92(7):1266‐1275. [PubMed] [Google Scholar]
- 23. Urken ML, Weinberg H, Vickery C, Buchbinder D, Lawson W, Biller HF. Oromandibular Reconstruction Using Microvascular Composite Free Flaps: Report of 71 Cases and a New Classification Scheme for Bony, Soft‐Tissue, and Neurologic Defects. Arch Otolaryngol Head Neck Surg. 1991;117(7):733‐744. 10.1001/archotol.1991.01870190045010. [DOI] [PubMed] [Google Scholar]
- 24. Brown JS, Barry C, Ho M, Shaw R. A new classification for mandibular defects after oncological resection. Lancet Oncol. 2016;17(1):e23‐e30. 10.1016/S1470-2045(15)00310-1. [DOI] [PubMed] [Google Scholar]
- 25. Cordeiro PG, Henderson PW, Matros E. A 20‐year experience with 202 segmental mandibulectomy defects: a defect classification system, algorithm for flap selection, and surgical outcomes. Plast Reconstr Surg. 2018;141(4):571e‐581e. 10.1097/PRS.0000000000004239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Silva AK, Humphries LS, Maldonado AA, Gottlieb LJ. Chimeric vs composite flaps for mandible reconstruction. Head Neck. 2019;41:1597‐1604. 10.1002/hed.25606. [DOI] [PubMed] [Google Scholar]
- 27. De Maesschalck T, Courvoisier DS, Scolozzi P. Computer‐assisted versus traditional freehand technique in fibular free flap mandibular reconstruction: a morphological comparative study. Eur Arch Otorhinolaryngol. 2017;274(1):517‐526. 10.1007/s00405-016-4246-4. [DOI] [PubMed] [Google Scholar]
- 28. Kim R, Sokoya M, Ducic Y, Williams F. Free‐flap reconstruction of the mandible. Semin Plast Surg. 2019;33(01):046‐053. 10.1055/s-0039-1677791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huang S‐T, Liu W‐C, Chen L‐W, Yang K‐C. Oromandibular reconstruction with chimeric double‐skin paddle flap based on peroneal vessel axis for synchronous opposite double oral cancer. Ann Plast Surg. 2015;74(Suppl 2):S132‐S138. 10.1097/SAP.0000000000000454. [DOI] [PubMed] [Google Scholar]
- 30. Frohwitter G, Rau A, Kesting MR, Fichter A. Microvascular reconstruction in the vessel depleted neck ‐ a systematic review. J Craniomaxillofac Surg. 2018;46(9):1652‐1658. 10.1016/j.jcms.2018.05.051. [DOI] [PubMed] [Google Scholar]
- 31. Tsao C‐K, Loh CYY, Barrera JM. Free tissue transfer as a vascular source for the vessel‐depleted neck. Head Neck. 2016;38(11):E2515‐E2518. 10.1002/hed.24539. [DOI] [PubMed] [Google Scholar]
- 32. Mosahebi A, Chaudhry A, McCarthy CM, et al. Reconstruction of extensive composite posterolateral mandibular defects using nonosseous free tissue transfer. Plast Reconstr Surg. 2009;124(5):1571‐1577. 10.1097/PRS.0b013e3181b98b78. [DOI] [PubMed] [Google Scholar]
- 33. Hanasono MM, Zevallos JP, Skoracki RJ, Yu P. A prospective analysis of bony versus soft‐tissue reconstruction for posterior mandibular defects. Plast Reconstr Surg. 2010;125(5):1413‐1421. 10.1097/PRS.0b013e3181d62aef. [DOI] [PubMed] [Google Scholar]
- 34. Blackwell KE, Buchbinder D, Urken ML. Lateral mandibular reconstruction using soft‐tissue free flaps and plates. Arch Otolaryngol Head Neck Surg. 1996;122(6):672‐678. 10.1001/archotol.1996.01890180078018. [DOI] [PubMed] [Google Scholar]
- 35. Chepeha DB, Teknos TN, Fung K, et al. Lateral oromandibular defect: when is it appropriate to use a bridging reconstruction plate combined with a soft tissue revascularized flap? Head Neck. 2008;30(6):709‐717. 10.1002/hed.20776. [DOI] [PubMed] [Google Scholar]
- 36. Wei F‐C, Celik N, Yang W‐G, Chen I‐H, Chang Y‐M, Chen H‐C. Complications after reconstruction by plate and soft‐tissue free flap in composite mandibular defects and secondary salvage reconstruction with osteocutaneous flap. Plast Reconstr Surg. 2003;112(1):37‐42. 10.1097/01.PRS.0000065911.00623.BD. [DOI] [PubMed] [Google Scholar]
- 37. Pogrel MA, Podlesh S, Anthony JP, Alexander J. A comparison of vascularized and nonvascularized bone grafts for reconstruction of mandibular continuity defects. J Oral Maxillofac Surg off J Am Assoc Oral Maxillofac Surg. 1997;55(11):1200‐1206. [DOI] [PubMed] [Google Scholar]
- 38. Imola MJ, Liddell A. Temporomandibular joint reconstruction. Curr Opin Otolaryngol Head Neck Surg. 2016;24(4):336‐342. 10.1097/MOO.0000000000000282. [DOI] [PubMed] [Google Scholar]
- 39. Fauvel F, Pace R, Grimaud F, Marion F, Corre P, Piot B. Costal graft as a support for bone regeneration after mandibular juvenile ossifying fibroma resection: an unusual case report. J Stomatol Oral Maxillofac Surg. 2017;118(5):320‐325. 10.1016/j.jormas.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 40. Masquelet AC, Fitoussi F, Begue T, Muller GP. Reconstruction of the long bones by the induced membrane and spongy autograft. Ann Chir Plast Esthet. 2000;45(3):346‐353. [PubMed] [Google Scholar]
- 41. Zwetyenga N, Fricain J‐C, De Mones E, Gindraux F. Induced membrane technique in oral and maxillofacial reconstruction. Rev Stomatol Chir Maxillofac. 2012;113(4):231‐238. 10.1016/j.stomax.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 42. Labbé D, Nicolas J, Kaluzinski E, et al. Gunshot wounds: reconstruction of the lower face by osteogenic distraction. Plast Reconstr Surg. 2005;116(6):1596‐1603. [DOI] [PubMed] [Google Scholar]
- 43. Zwetyenga N, Siberchicot F, Emparanza A. Reconstruction of large mandibular and surrounding soft‐tissue defects using distraction with bone transport. Int J Oral Maxillofac Surg. 2012;41(10):1215‐1222. 10.1016/j.ijom.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 44. Benateau H, Chatellier A, Caillot A, Labbe D, Veyssiere A. Computer‐assisted planning of distraction osteogenesis for lower face reconstruction in gunshot traumas. J Craniomaxillofac Surg. 2016;44(10):1583‐1591. 10.1016/j.jcms.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 45. Labbé D, Nicolas J, Kaluzinski E, et al. Gunshot wounds: two cases of midface reconstruction by osteogenic distraction. J Plast Reconstr Aesthetic Surg. 2009;62(9):1174‐1180. 10.1016/j.bjps.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 46. Wang YY, Zhang HQ, Fan S, et al. Mandibular reconstruction with the vascularized fibula flap: comparison of virtual planning surgery and conventional surgery. Int J Oral Maxillofac Surg. 2016;45(11):1400‐1405. 10.1016/j.ijom.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 47. Chang EI, Jenkins MP, Patel SA, Topham NS. Long‐term operative outcomes of preoperative computed tomography–guided virtual surgical planning for osteocutaneous free flap mandible reconstruction. Plast Reconstr Surg. 2016;137(2):619‐623. 10.1097/01.prs.0000475796.61855.a7. [DOI] [PubMed] [Google Scholar]
- 48. Giannoudis PV, Einhorn TA, Marsh D. Fracture healing: the diamond concept. Injury. 2007;38(Suppl 4):S3‐S6. [DOI] [PubMed] [Google Scholar]
- 49. Jo Y‐Y, Kim S‐G, Kim M‐K, Shin S‐H, Ahn J, Seok H. Mandibular reconstruction using a customized three‐dimensional titanium implant applied on the lingual surface of the mandible. J Craniofac Surg. 2018;29(2):415‐419. 10.1097/SCS.0000000000004119. [DOI] [PubMed] [Google Scholar]
- 50. Giannoudis PV, Gudipati S, Harwood P, Kanakaris NK. Long bone non‐unions treated with the diamond concept: a case series of 64 patients. Injury. 2015;46(Suppl 8):S48‐S54. 10.1016/S0020-1383(15)30055-3. [DOI] [PubMed] [Google Scholar]
- 51. Konopnicki S, Troulis MJ. Mandibular tissue engineering: past, present, future. J Oral Maxillofac Surg. 2015;73(12):S136‐S146. 10.1016/j.joms.2015.05.037. [DOI] [PubMed] [Google Scholar]
- 52. Tian T, Zhang T, Lin Y, Cai X. Vascularization in craniofacial bone tissue engineering. J Dent Res. 2018;97(9):969‐976. 10.1177/0022034518767120. [DOI] [PubMed] [Google Scholar]
- 53. Bose S, Roy M, Bandyopadhyay A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012;30(10):546‐554. 10.1016/j.tibtech.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Howk D, T‐MG C. Design variables for mechanical properties of bone tissue scaffolds. Biomed Sci Instrum. 2006;42:278‐283. [PubMed] [Google Scholar]
- 55. Turnbull G, Clarke J, Picard F, et al. 3D bioactive composite scaffolds for bone tissue engineering. Bioact Mater. 2018;3(3):278‐314. 10.1016/j.bioactmat.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bignon A, Chouteau J, Chevalier J, et al. Effect of micro‐ and macroporosity of bone substitutes on their mechanical properties and cellular response. J Mater Sci Mater Med. 2003;14(12):1089‐1097. [DOI] [PubMed] [Google Scholar]
- 57. Ishaug SL, Crane GM, Miller MJ, Yasko AW, Yaszemski MJ, Mikos AG. Bone formation by three‐dimensional stromal osteoblast culture in biodegradable polymer scaffolds. J Biomed Mater Res. 1997;36(1):17‐28. [DOI] [PubMed] [Google Scholar]
- 58. Tatara AM, Wong ME, Mikos AG. In vivo bioreactors for mandibular reconstruction. J Dent Res. 2014;93(12):1196‐1202. 10.1177/0022034514547763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tatara AM, Shah SR, Demian N, et al. Reconstruction of large mandibular defects using autologous tissues generated from in vivo bioreactors. Acta Biomater. 2016;45:72‐84. 10.1016/j.actbio.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 60. Murphy WL, Dennis RG, Kileny JL, Mooney DJ. Salt fusion: an approach to improve pore interconnectivity within tissue engineering scaffolds. Tissue Eng. 2002;8(1):43‐52. 10.1089/107632702753503045. [DOI] [PubMed] [Google Scholar]
- 61. Arzi B, Verstraete FJM, Huey DJ, Cissell DD, Athanasiou KA. Regenerating mandibular bone using rhBMP‐2: part 1‐immediate reconstruction of segmental mandibulectomies. Vet Surg. 2015;44(4):403‐409. 10.1111/j.1532-950X.2014.12123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Seto I, Marukawa E, Asahina I. Mandibular reconstruction using a combination graft of rhBMP‐2 with bone marrow cells expanded in vitro. Plast Reconstr Surg. 2006;117(3):902‐908. 10.1097/01.prs.0000200069.81973.49. [DOI] [PubMed] [Google Scholar]
- 63. Ayoub A, Challa SRR, Abu‐Serriah M, et al. Use of a composite pedicled muscle flap and rhBMP‐7 for mandibular reconstruction. Int J Oral Maxillofac Surg. 2007;36(12):1183‐1192. 10.1016/j.ijom.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 64. Neovius E, Lemberger M, Docherty Skogh A, Hilborn J, Engstrand T. Alveolar bone healing accompanied by severe swelling in cleft children treated with bone morphogenetic protein‐2 delivered by hydrogel. J Plast Reconstr Aesthet Surg. 2013;66(1):37‐42. 10.1016/j.bjps.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 65. Woo EJ. Adverse events reported after the use of recombinant human bone morphogenetic protein 2. J Oral Maxillofac Surg. 2012;70(4):765‐767. 10.1016/j.joms.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 66. Shields LBE, Raque GH, Glassman SD, et al. Adverse effects associated with high‐dose recombinant human bone morphogenetic Protein‐2 use in anterior cervical spine fusion. Spine. 2006;31(5):542‐547. 10.1097/01.brs.0000201424.27509.72. [DOI] [PubMed] [Google Scholar]
- 67. Buehrer G, Balzer A, Arnold I, et al. Combination of BMP2 and MSCs significantly increases bone formation in the rat Arterio‐venous loop model. Tissue Eng Part A. 2015;21(1–2):96‐105. 10.1089/ten.tea.2014.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Brooker JE, Camison LB, Bykowski MR, et al. Reconstruction of a calvarial wound complicated by infection: comparing the effects of biopatterned bone morphogenetic protein 2 and vascular endothelial growth factor. J Craniofac Surg. 2019;30(1):260‐264. 10.1097/SCS.0000000000004779. [DOI] [PubMed] [Google Scholar]
- 69. Lopez CD, Diaz‐Siso JR, Witek L, et al. Three dimensionally printed bioactive ceramic scaffold osseoconduction across critical‐sized mandibular defects. J Surg Res. 2018;223:115‐122. 10.1016/j.jss.2017.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kokemueller H, Spalthoff S, Nolff M, et al. Prefabrication of vascularized bioartificial bone grafts in vivo for segmental mandibular reconstruction: experimental pilot study in sheep and first clinical application. Int J Oral Maxillofac Surg. 2010;39(4):379‐387. 10.1016/j.ijom.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 71. Kokemüller H, Jehn P, Spalthoff S, et al. En bloc prefabrication of vascularized bioartificial bone grafts in sheep and complete workflow for custom‐made transplants. Int J Oral Maxillofac Surg. 2014;43(2):163‐172. 10.1016/j.ijom.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 72. Jégoux F, Goyenvalle E, Cognet R, et al. Mandibular segmental defect regenerated with macroporous biphasic calcium phosphate, collagen membrane, and bone marrow graft in dogs. Arch Otolaryngol Head Neck Surg. 2010;136(10):971‐978. 10.1001/archoto.2010.173. [DOI] [PubMed] [Google Scholar]
- 73. Beier JP, Hess A, Loew J, et al. De novo generation of an axially vascularized processed bovine cancellous‐bone substitute in the sheep arteriovenous‐loop model. Eur Surg Res. 2011;46(3):148‐155. 10.1159/000324408. [DOI] [PubMed] [Google Scholar]
- 74. Weigand A, Horch RE, Boos AM, Beier JP, Arkudas A. The arteriovenous loop: engineering of axially vascularized tissue. Eur Surg Res. 2018;59(3–4):286‐299. 10.1159/000492417. [DOI] [PubMed] [Google Scholar]
- 75. Rath SN, Arkudas A, Lam CX, et al. Development of a pre‐vascularized 3D scaffold‐hydrogel composite graft using an arterio‐venous loop for tissue engineering applications. J Biomater Appl. 2012;27(3):277‐289. 10.1177/0885328211402243. [DOI] [PubMed] [Google Scholar]
- 76. Meyer A, Horch RE, Schoengart E, et al. Results of combined vascular reconstruction by means of AV loops and free flap transfer in patients with soft tissue defects. J Plast Reconstr Aesthet Surg. 2016;69(4):545‐553. 10.1016/j.bjps.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 77. Eweida AM, Nabawi AS, Marei MK, Khalil MR, Elhammady HA. Mandibular reconstruction using an axially vascularized tissue‐engineered construct. Ann Surg Innov Res. 2011;5(1):2. 10.1186/1750-1164-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Eweida AM, Horch RE, Marei MK, et al. Axially vascularised mandibular constructs: is it time for a clinical trial? J Craniomaxillofac Surg. 2015;43(7):1028‐1032. 10.1016/j.jcms.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 79. Eweida A, Frisch O, Giordano FA, et al. Axially vascularized tissue‐engineered bone constructs retain their in vivo angiogenic and osteogenic capacity after high‐dose irradiation. J Tissue Eng Regen Med. 2018;12(2):e657‐e668. 10.1002/term.2336. [DOI] [PubMed] [Google Scholar]
- 80. Eweida AM, Nabawi AS, Elhammady HA, et al. Axially vascularized bone substitutes: a systematic review of literature and presentation of a novel model. Arch Orthop Trauma Surg. 2012;132(9):1353‐1362. 10.1007/s00402-012-1550-3. [DOI] [PubMed] [Google Scholar]
- 81. Arkudas A, Horch RE, Regus S, et al. Retrospective cohort study of combined approach for trunk reconstruction using arteriovenous loops and free flaps. J Plast Reconstr Aesthet Surg. 2018;71(3):394‐401. 10.1016/j.bjps.2017.08.025. [DOI] [PubMed] [Google Scholar]


