Abstract
Objective
Recently, 3‐Tesla magnetic resonance imaging (MRI) with intravenous gadolinium injection has been used to reveal endolymphatic hydrops (EH). In the present study, we aimed to evaluate EH in patients with Meniere's disease (MD) objectively and quantitatively, and compared the endolymphatic space (ELS) in individuals with MD and healthy controls, to gain understanding of the characteristics of MD.
Methods
Eighty‐two patients with unilateral MD (uMD), 16 patients with bilateral MD (bMD), and 47 healthy volunteers were enrolled. All participants underwent 3‐T MRI at 4 hours after intravenous gadolinium injection. The volumes of the total fluid space (TFS) and ELS were measured semiautomatically using our workstation, and the percentage of ELS to TFS (ELS percentage) was calculated.
Results
The ELS percentage was 13.9 in the ears of controls, 18.2 in the contralateral ear of individuals with uMD, 26.1 in the affected ears of these individuals, and 23.0 in both ears of individuals with bMD. The ELS percentages in the affected ear of uMD and the ears of bMD individuals were significantly higher than that in the ears of control individuals (P < .01, one‐way analysis of variance (ANOVA), Tukey's test).
Conclusion
The ELS is significantly larger in the affected ears of uMD and in both ears of bMD individuals. Accurate diagnosis of MD can be facilitated by using 3‐T MRI 4 hours after intravenous gadolinium injection and performing volumetric measurements of the ELS.
Level of Evidence
2b
Keywords: Endolymphatic hydrops, healthy controls, magnetic resonance imaging, Meniere's disease
INTRODUCTION
Meniere's disease (MD) is a chronic condition involving recurrent vertigo, tinnitus, aural fullness, and fluctuating hearing loss. In 1983, Hallpike and Cairns, in England and Yamakawa, in Japan independently but simultaneously used temporal bone studies to show that endolymphatic hydrops (EH) is primarily implicated in MD pathology.1, 2 Since then, many neurotologists have attempted to reveal the mechanisms underlying the development of EH and to observe EH in living patients. The glycerol test, furosemide test, electrocochleography, and glycerol vestibular‐evoked myogenic potentials have been used for the estimation of EH.3, 4, 5 However, it has long been regarded to be difficult to detect EH by morphological examinations.
In 2007, Nakashima et al reported a new method for visualizing EH using 3‐Tesla magnetic resonance imaging (MRI) with linear gadolinium (Gd) enhancement (inner ear MRI).6 Linear contrast agents accumulate in the brain much more than macrocyclic agents7, 8, 9; Kanda et al reported that hyperintensity in the dentate nucleus was associated with previous administration of linear but not of macrocyclic Gd.10 Thus, we attempted and succeeded in visualizing EH using macrocyclic agents in 2016.11 Since then, we have used only macrocyclic agents to minimize the side effects of Gd enhancement. To observe the inner ear objectively and quantitively, we have previously used 3‐T MRI to measure the volume of the inner ear and succeeded in objective and quantitative observation of the inner ears. We also confirmed that our methods yielded results that were concordant with pathological observations.12, 13 Combining our method of volumetric measurement of inner ears with inner ear MRI, we could evaluate the endolymphatic space (ELS) and EH objectively, quantitively, and precisely.
In the present study, we aimed to characterize EH in individuals with MD, by comparing the ELS in unilateral MD (uMD)/bilateral MD (bMD) with that in healthy volunteers (controls) using 3‐T MRI and volumetric measurement of the inner ears.
MATERIALS AND METHODS
The Medical Ethics Committee of our university hospital approved this study (certificate number: 0889). All subjects provided written informed consent in accordance with the Declaration of Helsinki.
Patients and Controls
Eighty‐two patients who were diagnosed with uMD and 16 patients who were diagnosed with bMD according to the Bárány Society criteria were enrolled in the present study.14 As control subjects, we enrolled 47 healthy volunteers. They had no history of hearing loss, vertigo, middle or inner ear diseases, cranial disease, head trauma, renal disease, or heart disease. They took no daily medications and had no history of allergy to Gd.
Magnetic Resonance Imaging
Naganawa et al found that MRI performed 4 hours after intravenous Gd injection is useful for imaging EH.15 Thus, briefly, in the present study, MRI measurements were acquired 4 hours after intravenous administration of a single dose (0.2 mL/kg or 0.1 mM/kg body weight) of Gd‐diethylenetriaminepentaacetic acid dimethylamide (Magnescope; Guerbet, Tokyo, Japan). A 3‐T MRI unit (MAGNETOM Verio; Siemens, Erlangen, Germany) with a 32‐channel array head‐coil was used. The special sequences proposed by Naganawa et al,15 which reveal the endolymphatic and perilymphatic fluids, were adopted.
Heavily T2‐weighted (hT2W) MR cisternography was used to obtain an anatomical total lymph fluid reference. hT2W 3D fluid‐attenuated inversion recovery sequences with an inversion time of 2,250 msec yielded positive perilymph images (PPI) and hT2W 3D inversion recovery with an inversion time of 2,050 msec yielded positive endolymph images (PEI). Then, a hybrid of the reversed image of the positive endolymph signal and the negative image of the positive perilymph signal was obtained by subtracting PEI from PPI, as proposed by Naganawa et al.15
Volumetric Measurement of Total Fluid Space and ELS
In 2016, we reported a method for measuring the volume of total fluid space (TFS) and the ELS.12, 13 Recently, we also described a method for precise evaluation of the ELS or EH in MD.16 Briefly, the area of the ELS was first identified on our workstation (Virtual Place; AZE, Ltd., Tokyo, Japan) (Fig. 1). In this protocol, ELS voxels have negative signal values and the perilymph space voxels have positive signal values. PPI and PEI were transferred, and the PEI were subtracted from the PPI using the fusion program included in the software in our workstation. The borderline between the gray area and the green area of the color bar in our subtracted image was considered as a zero‐value in our program (Fig. 1). After activation of the SPACE sequence image and the “PPI‐PEI” image on our workstation (Virtual Place), components of the inner ear were identified on the SPACE sequence image by the borderlines between the inner ear and the peripheral side of the acoustic nerve, between the end of the cochlea and the vestibule, between the three ampullae of the semicircular canals (SCCs) and the vestibule, and between the distal side of the common crus and the vestibule, using anatomical drawings.12 The volume of the TFS was acquired by automatically counting voxels on our workstation. Then, the volumes of the ELS were also measured by counting the voxels of the minus signals on the “PPI‐PEI” image. Finally, the percentage of the volume of the ELS to that of the TFS was calculated (defined as the ELS percentage). We performed these measurements three times, and the average was utilized in the present study. The measurements were performed in both ears of all individuals, and that the values for both ears of control individuals were compared with those of both ears of individuals with bMD, and with those of the affected and the unaffected ears of individuals with uMD.
Figure 1.
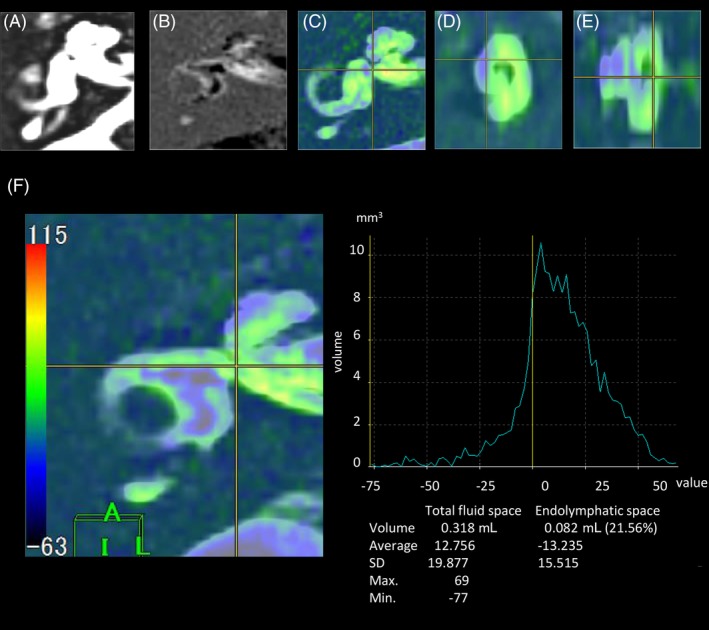
Reconstruction procedure for color rendering and fusion of three‐dimensional images of the inner ear fluid space and of the endolymphatic space (ELS). (A) Image of the total inner ear fluid space; (B) Image of the ELS; (C) Fusion images; axial view of the inner ear; (D) Fusion images; sagittal view of the inner ear; (E) Fusion images; coronal view of the inner ear; (F) A graph used for the measurement of the volume of the total inner ear fluid space and the ELS. The image of the total inner ear fluid space obtained using the T2‐SPACE sequence was fused with the image of the ELS by subtracting the positive endolymph image from the positive perilymph image. The gray area on the color bar (F) indicates a value of 0 on the graph (yellow line); A negative value indicates the volume of the ELS.
Statistical Analysis
For statistical analysis, we used one‐way analysis of variance (ANOVA) for multiple comparisons followed by pair‐wise comparison with Tukey's post hoc test and the chi‐squared (χ 2) test or paired Student's t tests for two‐group comparisons. P values lower than .05 were considered statistically significant. All data were statistically analyzed in SPSS version 25.0 (IBM Corp., Armonk, NY).
RESULTS
Patients' background data are shown in Table 1. There were no significant differences among three groups (control, uMD, and bMD groups).
Table 1.
Background of Controls and Patients.
| Control | uMD | bMD | ||
|---|---|---|---|---|
| Sex | ||||
| Male | 25 | 31 | 9 | NS |
| Female | 22 | 51 | 7 | χ 2 test |
| Age | ||||
| Years | 22–83 | 22–83 | 21–81 | NS |
| Mean ± SD | 58.4 ± 16.3 | 57.2 ± 14.9 | 61.0 ± 15.3 | One‐way ANOVA |
Controls consisted of 25 males and 22 females, uMDs consisted of 31 males and 51 females, and bMDs consisted of 9 males and 7 females. The age of controls was 58.4 ± 16.3 yr (mean ± SD), that of uMD patients was 57.2 ± 14.9 yr, and that of bMD patients was 61.0 ± 15.3 yr. There were no significant differences among control, uMD, and bMD individuals.
ANOVA = analysis of variance; bMD = bilateral Meniere's disease; uMD = unilateral Meniere's disease; NS = not significant.
ELS Percentage in Control, uMD, and bMD Groups
Figure 2 shows the ELS percentage in the inner ear as a whole (cochlea + vestibule + SCCs), and for the cochlea, vestibule, and SCC separately. In the inner ear, cochlea, vestibule, and SCC, the ELS percentages of the affected ear of individuals with uMD were significantly higher than those of the controls (P < .01, one‐way ANOVA, Tukey's test). The ELS percentages of the inner ear, cochlea, and vestibule of both ears of individuals with bMD were also significantly higher than those of controls (P < .01, one‐way ANOVA, Tukey's test). The ELS percentage of the inner ear, cochlea, and vestibule of the affected ear of individuals with uMD was significantly higher than those of their unaffected, contralateral ear (P < .01, one‐way ANOVA, Tukey's test). For SCCs, the ELS percentage of the affected ears of individuals with uMD was significantly higher than that of the ears of controls and the contralateral ears of individuals with uMD.
Figure 2.
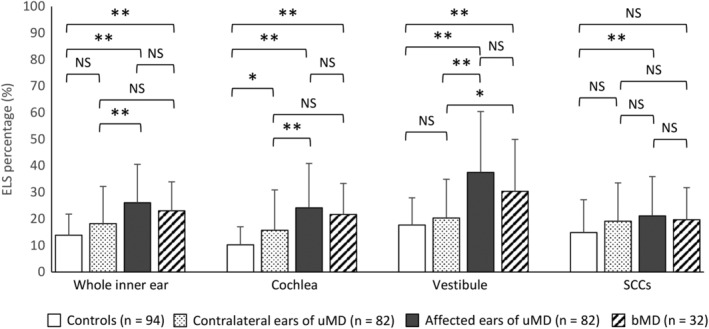
The ELS percentage of controls, the contralateral ears and the affected ears of patients with uMD, and both ears of patients with bMD. In whole inner ears, the ELS percentage of controls was 13.9 ± 7.9% (mean ± SD), that of the contralateral ears in patients with uMD was 18.2 ± 14.0%, that of the affected ears of patients with uMD was 26.1 ± 14.5%, and that of patients with bMD was 23.0 ± 10.9%. The ELS percentages of the affected ears of patients with uMD and of both ears of patients with bMD were significantly higher than that of controls. The ELS percentage of the affected ears of patients with uMD was significantly higher than that of their contralateral ears. In the cochlea, the ELS percentage of controls was 10.2 ± 6.8%, that in the contralateral ears of patients with uMD was 15.7 ± 15.2%, that in the affected ears of patients with uMD was 24.1 ± 1 6.7%, and that in bMDs was 21.7 ± 11.7%. The ELS percentage of the affected ears of patients with uMD and the ears of patients with bMD was significantly higher than that in controls. The ELS percentage of the affected ears of patients with uMD was significantly higher than that in the contralateral ears of patients with uMD. In the vestibule, the ELS percentage of controls was 17.7 ± 10.2%, that in the contralateral ears of patients with uMD was 20.3 ± 14.6%, that in the affected ears of patients with uMD was 37.5 ± 23.0%, and that in patients with bMD was 30.4 ± 23.0%. The ELS percentages of the affected ears of patients with uMD and bMD were significantly higher than that in controls. The ELS percentage of the affected ears of patients with uMD was significantly higher than that in the contralateral ears of patients with uMD. In SCCs, the ELS percentage of controls was 14.8 ± 12.4%, that of the contralateral ears of patients with uMD was 19.1 ± 14.4%, that of the affected ears of patients with uMD was 21.1 ± 14.8%, and that in patients with bMDs was 19.6 ± 12.1%. The percentage in the affected ears of patients with uMD was significantly higher than that in controls. **P < .01, one‐way ANOVA, Tukey's test. *P < .05, one‐way ANOVA = analysis of variance, Tukey's test. bMD = bilateral Meniere's disease; ELS = endolymphatic space; NS = not significant; SCC = semicircular canal; uMD = unilateral Meniere's disease.
ELS Percentages of the Active and Stable Ears of Individuals With bMD
Figure 3 shows the ELS percentages of the active ears (the side that presented cochlear symptoms when MRI was performed) and the stable ears (the contralateral ear) among individuals with bMD. In the inner ears and cochleae, the ELS percentages of the active ears tended to be higher than those of the stable ears. In the vestibule, the ELS percentage of the active ears was significantly higher than that of the stable ears (P < .05, paired t test).
Figure 3.
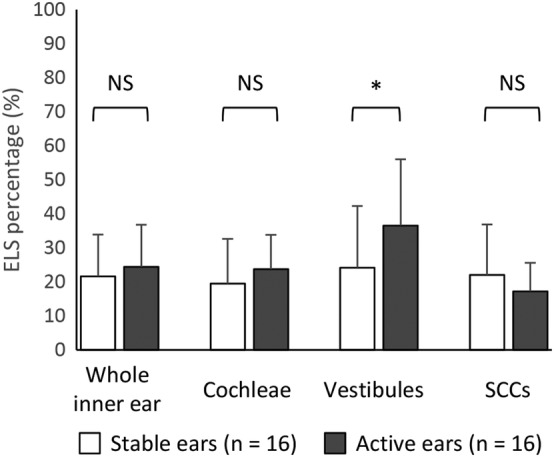
The ELS percentage of stable ears and active ears of patients with bilateral Meniere's disease (bMD). In the inner ears, the ELS percentage of the stable ears of patients with bMD was 21.6 ± 12.4% and that of their active ears was 24.4 ± 13.4%. In the cochlea, the ELS percentage of the stable ears was 19.5 ± 13.1% and that of the active ears was 23.8 ± 10.0%. In the vestibule, the ELS percentage of the stable ears of patients with bMD was 24.2 ± 18.1% and that of the active ears was 36.5 ± 19.5. In the SCCs, the ELS percentage of the stable ears of patients with bMD was 22.0 ± 14.9% and that of their active ears was 17.3 ± 8.4%. In the vestibules, the ELS percentage of active ears was significantly higher than that of the stable ears of patients with bMD. The active ear refers to the side that had cochlear symptoms and/or hearing loss at the time that magnetic resonance imaging was performed. The stable ear refers to the contralateral ear. ELS = endolymphatic space; NS = not significant; SCC = semicircular canal.
Relationship of the ELS Percentage to the Interval Between Onset of MD and MRI
Figure 4 shows the relationship between the ELS percentage and the interval from the onset of uMD to MRI. There was no correlation between this interval and the ELS percentage.
Figure 4.
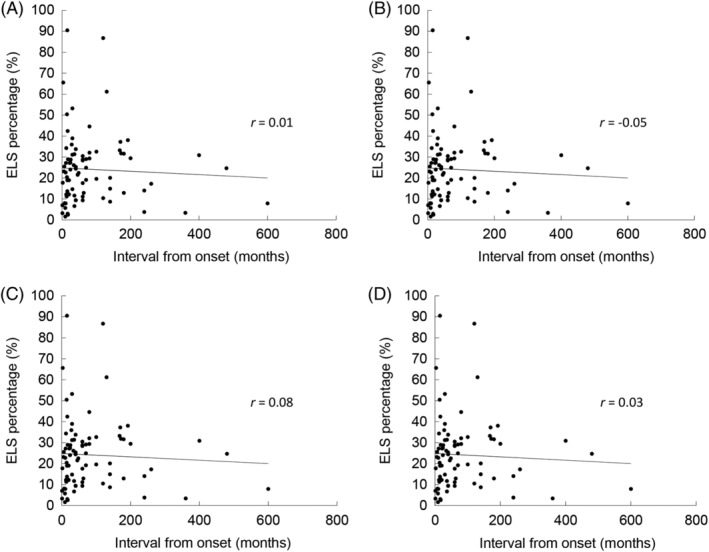
The relationship between the ELS percentage of unilateral Meniere's disease (uMD) and the interval from onset to magnetic resonance imaging (MRI). (A) Whole inner ear, (B) cochlea, (C) vestibule, (D) semicircular canals (SCCs). There was no correlation between the interval from the onset of uMD to MRI and the ELS percentages of the whole inner ear (Pearson's correlation coefficient, r = 0.01, P = .93), cochlea (r = −0.05, P = .64), vestibule (r = 0.08, P = .50), and SCCs (r = 0.03, P = .77). ELS = endolymphatic space.
DISCUSSION
In the present study, we characterized EH in patients with MD by volumetric measurement of the ELS using 3‐T MRI, in comparison with that in healthy controls. The volumes of ELS in patients with MD had not been compared with that in healthy controls to date. The ELS percentage of the affected ears of individuals with uMD was significantly higher than that in the ears of controls and in the contralateral ears of individuals with uMD. The ELS percentage of individuals with bMD was also higher than that in controls.
In 2016, we first reported the comparison of ELS in patients with MD (uMD and bMD) with that in healthy controls using 3‐T MRI at 4 hours after intravenous administration of Gd.11 In the previous study, we adopted the criteria for the evaluation of EH proposed by Nakashima et al.17 Using these criteria, it is possible to diagnose EH rapidly, although qualitatively. For precise evaluation or analysis of EH, simple and quantitative methods are required. In addition, Nakashima's criteria are based on only one MRI slice, and the plane for image analysis can differ depending on the head position and anatomical differences in the skulls of patients. Our methods of volumetric measurement after three‐dimensional reconstruction of MRI can address these issues, because the total inner ear volume is measured.
In 2015, Gürkov et al reported that the ELS percentage could be reliably measured in patients with MD, and reported a mean value of 15% for the cochlea and 28% for the vestibule.18 In the present study, the ELS percentage was 24.1% for the cochlea and 37.5% for the vestibule of the affected ear of patients with uMD, consistent with the results of Gürkov et al. In addition, our findings for the inner ear were consistent with the volumes of the inner ear of cadavers.12 Thus, our approach can be used to evaluate EH quantitatively and precisely.
Endolymphatic sac drainage is regarded as an optional treatment for intractable MD when conservative medical treatments fail to control the symptoms.19, 20 In cases of bMD, the surgical side is important, but it is sometimes difficult to select the side because the selection depends on the patients' complaints of cochlear symptoms. Morimoto et al reported that EH was observed in both the active ears and stable ears among patients with bMDs.21 In the present study, the ELS percentage of the cochlea in the active ears of such patients tended to be higher than that in the stable ear, and the ELS percentage of the vestibule in the active ear was significantly higher than that in the stable ear. The volumetric measurement of EH may thus help us to decide the side for surgical endolymphatic sac drainage.
Wu et al reported the incidence of EH in the cochleae and vestibules of 54 patients with uMD, and showed correlations with the interval from onset to MRI, using MRI with intratympanic Gd administration for enhancement.22 However, the ELS percentage of 82 patients with uMD and the interval from onset to MRI showed no correlation in the present study. The difference in study findings may be due to the methods used for Gd administration and evaluation of EH. To verify the existence of a correlation, it is necessary to study more patients using different intervals. If there is no correlation, 3‐T MRI will be useful for the diagnosis of MD because the ELS percentage of patients with MD is significantly higher than that in controls.
At present, 3‐T MRI is the useful morphological examination that can reveal EH. It is a minimally invasive examination, which could be performed repeatedly. Regular 3‐T MRI during treatment of MD can be used to evaluate the effect of treatments and can shed light on the features of MD.
CONCLUSION
The ELS percentages of the affected ears of patients with uMD and both ears of patients with bMD were significantly higher than those of controls. The ELS percentage of the affected ears of patients with uMD was also significantly higher than that of their unaffected, contralateral ear. 3‐T MRI and volumetric measurement may contribute to the accurate diagnosis of MD.
AUTHOR CONTRIBUTIONS
T. I., H. I., T. M., T. S., and A. H. performed the research. T. I. analyzed the data and wrote the paper.
ACKNOWLEDGMENTS
We would like to thank Professors Shinji Naganawa and Toshiaki Taoka (Department of Radiology, Nagoya University Graduate School of Medicine, Nagoya, Aichi, Japan) and Mr. Tsuyoshi Sakamoto (PixSpace Ltd., Kokura, Fukuoka, Japan) for technical support. We also thank Editage (http://www.editage.jp) for English language editing.
Editor's Note: This Manuscript was accepted for publication on September 13, 2019.
Conflict of Interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding: This work was supported by JSPS KAKENHI Grant Number 18K09354 Grant‐in‐Aid for Scientific Research (C), AMED under Grant Number 18dk0310092h000, and Health and Labor Sciences Research Grant for Research on Rare and Intractable Diseases (H29‐Nanchito (Nan)‐Ippan‐031) from the Ministry of Health, Labor, and Welfare of Japan.
BIBLIOGRAPHY
- 1. Hallpike CS, Cairns H. Observations on the pathology of Meniere's syndrome: section of otology. Proc R Soc Med 1938;31(11):1317–1336. [PMC free article] [PubMed] [Google Scholar]
- 2. Yamakawa K. Hearing organ of a patient who showed Meniere's symptoms. J Otolaryngol Soc Jpn 1938;44:2310–2312. [Google Scholar]
- 3. Futaki T, Kitahara M, Morimoto M. The furosemid test for Meniere's disease. Acta Otolaryngol 1975;79(5–6):419–424. [DOI] [PubMed] [Google Scholar]
- 4. Ferraro JA, Durrant JD. Electrocochleography in the evaluation of patients with Meniere's disease/endolymphatic hydrops. J Am Acad Audiol 2006;17(1):45–68. [DOI] [PubMed] [Google Scholar]
- 5. Ban JH, Lee JK, Jin SM, Lee KC. Glycerol pure tone audiometry and glycerol vestibular evoked myogenic potential: representing specific status of endolymphatic hydrops in the inner ear. Eur Arch Otorhinolaryngol 2007;264(11):1275–1281. [DOI] [PubMed] [Google Scholar]
- 6. Nakashima T, Naganawa S, Sugiura M, et al. Visualization of endolymphatic hydrops in patients with Meniere's disease. Laryngoscope 2007;117(3):415–420. [DOI] [PubMed] [Google Scholar]
- 7. Radbruch A, Weberling LD, Kieslich PJ, et al. Gadolinium retention in the dentate nucleus and globus pallidus is dependent on the class of contrast agent. Radiology 2015;275(3):783–791. [DOI] [PubMed] [Google Scholar]
- 8. Rogosnitzky M, Branch S. Gadolinium‐based contrast agent toxicity: a review of known and proposed mechanisms. Biometals 2016;29(3):365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moser FG, Watterson CT, Weiss S, et al. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1‐weighted MR images: comparison between gadobutrol and linear gadolinium‐based contrast agents. AJNR Am J Neuroradiol 2018;39(3):421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kanda T, Osawa M, Oba H, et al. High signal intensity in dentate nucleus on unenhanced T1‐weighted MR images: association with linear versus macrocyclic gadolinium chelate administration. Radiology 2015;275(3):803–809. [DOI] [PubMed] [Google Scholar]
- 11. Ito T, Kitahara T, Inui H, et al. Endolymphatic space size in patients with Meniere's disease and healthy controls. Acta Otolaryngol 2016;136(9):879–882. [DOI] [PubMed] [Google Scholar]
- 12. Inui H, Sakamoto T, Ito T, Kitahara T. Magnetic resonance volumetric measurement of endolymphatic space in patients without vertiginous or cochlear symptoms. Acta Otolaryngol 2016;136(12):1206–1212. [DOI] [PubMed] [Google Scholar]
- 13. Inui H, Sakamoto T, Ito T, Kitahara T. Volumetric measurements of the inner ear in patients with Meniere's disease using three‐dimensional magnetic resonance imaging. Acta Otolaryngol 2016;136(9):888–893. [DOI] [PubMed] [Google Scholar]
- 14. Lopez‐Escamez JA, Carey J, Chung WH, et al. Diagnostic criteria for Meniere's disease. J Vestib Res 2015;25(1):1–7. [DOI] [PubMed] [Google Scholar]
- 15. Naganawa S, Yamazaki M, Kawai H, Bokura K, Sone M, Nakashima T. Imaging of Meniere's disease after intravenous administration of single‐dose gadodiamide: utility of subtraction images with different inversion time. Magn Reson Med Sci 2012;11(3):213–219. [DOI] [PubMed] [Google Scholar]
- 16. Ito T, Inui H, Miyasaka T, et al. Three‐dimensional magnetic resonance imaging reveals the relationship between the control of vertigo and decreases in endolymphatic hydrops after endolymphatic sac drainage with steroids for Meniere's disease. Front Neurol 2019;10:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nakashima T, Naganawa S, Pyykko I, et al. Grading of endolymphatic hydrops using magnetic resonance imaging. Acta Otolaryngol Suppl 2009;560:5–8. [DOI] [PubMed] [Google Scholar]
- 18. Gürkov R, Berman A, Dietrich O, et al. MR volumetric assessment of endolymphatic hydrops. Eur Radiol 2015;25(2):585–595. [DOI] [PubMed] [Google Scholar]
- 19. Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Meniere's disease. American Academy of Otolaryngology‐Head and Neck Foundation, Inc. Otolaryngol Head Neck Surg 1995;113(3):181–185. [DOI] [PubMed] [Google Scholar]
- 20. Kitahara T, Kubo T, Okumura S, Kitahara M. Effects of endolymphatic sac drainage with steroids for intractable Meniere's disease: a long‐term follow‐up and randomized controlled study. Laryngoscope 2008;118(5):854–861. [DOI] [PubMed] [Google Scholar]
- 21. Morimoto K, Yoshida T, Sugiura S, et al. Endolymphatic hydrops in patients with unilateral and bilateral Meniere's disease. Acta Otolaryngol 2017;137(1):23–28. [DOI] [PubMed] [Google Scholar]
- 22. Wu Q, Dai C, Zhao M, Sha Y. The correlation between symptoms of definite Meniere's disease and endolymphatic hydrops visualized by magnetic resonance imaging. Laryngoscope 2016;126(4):974–979. [DOI] [PubMed] [Google Scholar]


