Abstract
Background: Long non-coding RNAs (lncRNAs) constitute an important component of the regulatory apparatus that controls stem cell pluripotency. However, the specific mechanisms utilized by these lncRNAs in the control of pluripotency are not fully characterized.
Methods: We utilized a RNA reverse transcription-associated trap sequencing (RAT-seq) approach to profile the mouse genome-wide interaction targets for lncRNAs that are screened by RNA-seq.
Results: We identified Peblr20 (Pou5F1 enhancer binding lncRNA 20) as a novel lncRNA that is associated with stem cell reprogramming. Peblr20 was differentially transcribed in fibroblasts compared to induced pluripotent stem cells (iPSCs). Notably, we found that Peblr20 utilized a trans mechanism to interact with the regulatory elements of multiple stemness genes. Using gain- and loss-of-function experiments, we showed that knockdown of Peblr20 caused iPSCs to exit from pluripotency, while overexpression of Peblr20 activated endogenous Pou5F1 expression. We further showed that Peblr20 promoted pluripotent reprogramming. Mechanistically, we demonstrated that Peblr20 activated endogenous Pou5F1 by binding to the Pou5F1 enhancer in trans, recruiting TET2 demethylase and activating the enhancer-transcribed RNAs.
Conclusions: Our data reveal a novel epigenetic mechanism by which a lncRNA controls the fate of stem cells by trans-regulating the Pou5F1 enhancer RNA pathway. We demonstrate the potential for leveraging lncRNA biology to enhance the generation of stem cells for regenerative medicine.
Keywords: Stem Cell, pluripotency, enhancer RNA, long noncoding RNA, epigenetics, DNA demethylation
INTRODUCTION
Pluripotent stem cells (PSCs) are vital tools for developmental biology and regenerative medicine due to their unlimited self-renewal capacity and pluripotency 1, 2. PSCs can be isolated as embryonic stem cells (ESCs) from the inner cell mass (ICM) of mammalian blastocysta 3, or somatic cells can be reprogrammed as induced pluripotent stem cells (iPSCs) by exposure to a cocktail of transcription factors (Pou5F1, Sox2, Klf-4, c-Myc) 4. The reprogramming process to make iPSCs, however, is extremely inefficient and time-consuming, hindering their potential clinical applications for regenerative medicine. Therefore, it is important to understand the regulatory network that controls their pluripotent state and the reprogramming process.
Long noncoding RNAs (lncRNAs) may play a role in regulating the induction and maintenance of pluripotency 5-8. LncRNAs are a group of >200 ribonucleotide transcripts that do not encode protein products 9. The number of lncRNAs is correlated with the complexity of an organism 10 and lncRNA diversity is restricted to specific cell lineages 11, and it has been postulated that lncRNAs play a role in cell fate determination, including stem cell differentiation and the somatic reprogramming process 7, 8, 12. An understanding of lncRNA physiology could yield novel RNA-based strategies for manipulating ESCs or iPSCs to advance regenerative medicine.
In this communication, we utilized a recently reported RNA reverse transcription-associated capture sequencing (RAT-seq) and RNA-seq strategy to characterize functional lncRNA candidates that are associated with pluripotency 13, 14. By integrating these two approaches, we identified a Pou5F1 enhancer binding lncRNA, Peblr20, that is essential for the maintenance of stem cell pluripotency. We show that Peblr20 harnesses a novel epigenetic mechanism to control pluripotency in trans by recruiting TET2 to Pou5F1 enhancer loci, thereby activating the enhancer.
MATERIAL AND METHODS
Identification of differentially expressed lncRNAs in reprogramming by RNA-seq
As previously reported 13, 14, a combined RNA-seq and RAT-seq strategy was employed to identify pluripotency-associated lncRNAs. The conventional RNA-seq approach was initially used to identify lncRNAs that are differentially expressed during the process of pluripotent reprogramming. Mouse fibroblasts were reprogrammed into iPSCs by using lentiviral Pou5F1- Sox2-Klf4-c-Myc-GFP (OSKMN) 15, 16. The selected iPSC colony cells were characterized by immunostaining for stem cell markers, alkaline phosphatase staining, karyotype analysis, and teratoma formation 15, 16.
For RNA-seq, total RNA was isolated from fibroblasts and iPSCs using TRIzol (Invitrogen, Carlsbad, CA), and indexed libraries were prepared using Illumina's TruSeq RNA Sample Prep Kit v2. Paired-end sequencing was performed by Shanghai Biotechnology (Shanghai, PRC) using a HiSeq4000 (Illumina). After Seqtk filtering, clean reads were mapped to the mouse genome (genome version: mm10, GRCm38.p4 (ftp://ftp.ensembl.org/pub/release-83/fasta/mus_musculus/dna/Mus_musculus.GRCm38.dna.primary_assembly.fa.gz) for mRNAs and lncRNAs using the STAR software 17. Gene counts were normalized to the values of Reads Per Kilobase of transcript per Million mapped reads (RPKM). Cuffdiff was used to calculate RNAs that are differentially expressed in reprogramming, using the fold-change > 2 and p < 0.05 with an unpaired two-sided t-test 13.
Profiling the Peblr20 genome-wide gene targets by RAT-seq
A RAT-seq approach 18, 19 was modified to map the genome wide interacting target genes for lncRNA candidates 13. Briefly, 1.0 × 107 cells were cross-linked with 2% formaldehyde and lysed with cell lysis buffer (10 mM Tris [pH 8.0], 10 mM NaCl, 0.2% NP-40, 1X protease inhibitors). Nuclei were collected, suspended in 1X reverse transcription buffer in the presence of gene-specific primer, biotin-14-dCTP, RNase inhibitor and Maxima Reverse Transcriptase (Thermo Fisher Scientific, CA). After 30 min of reverse transcription of Peblr20 lncRNA labeled by biotin-14-dCTP with Maxima Reverse Transcriptase at 65◦C, the reaction was stopped by adding 4ul 0.5M EDTA. After nuclear lysis, the complex was subjected to sonication for 180 s (10 s on and 10 s off) on ice with a Branson sonicator with a 2-mm microtip at 40% output control and 90% duty cycle settings. The biotin-cDNA/chromatin DNA complex was pulled down with biotin-streptavidin magic beads (Invitrogen, CA). After reversing the cross-links and washing with 10 mg/ml proteinase K at 65°C overnight and treatment with 0.4 μg/ml RNase A for 30 min at 37°C, the genomic DNA that interacts with the lncRNA was extracted and digested by MboI, and ligated with the NEBNext adaptors (NEBNext® ChIP-Seq Library Prep Master Mix Set for Illumina) to construct the library. The library DNAs were subjected to Illumina sequencing (Shanghai Biotechnology, Shanghai) and PCR with primers shown in Table S1. For RAT-seq control, we performed a RAT assay by replacing Peblr20 complementary primers with random primers and constructed a control library for sequencing using the same protocol.
After RAT sequencing, the low quality reads were filtered using Fastx (version: 0.0.13) software (http://hannonlab.cshl.edu/fastx_toolkit/index.htm). Clean reads were mapped to the mouse genome (genome version: mm10) using the Bowtie (version: 0.12.8) software with default parameters 20. Enriched regions of the genome were identified by comparing the RAT-seq peaks to input samples using MACS2 (version: 2.1.1) and q-value of 0.05 was used as the initial cutoff threshold to minimize peak caller bias 21. The upstream 2 k of the transcription start sites and the downstream 5k of the transcription termination region were defined as the gene regions. The significant GO terms of biological processes with a p-value < 0.05 were selected. We also used the MEME suite 22 for the discovery and analysis of the peaks' sequence motifs. The resulting coverage tracks (bedgraph file) were visualized in UCSC genome browser. To reduce the background, the RAT-seq data were further normalized over the peaks of the control RAT-seq data that were generated by using random oligonucleotide primers in the RAT assay. Differential binding analysis was performed with the DiffBind package using parameters of fold change difference ≥2 and p-value < 0.05, with false discovery rate (FDR) <0.1. The adjusted RAT-seq data were used for mapping the lncRNA target gene interaction network 14.
RNA extraction and cDNA synthesis
To examine the role of lncRNAs, we collected cells at different stages of reprogramming, including fibroblasts and iPSCs. For comparison, the fibroblast-like cells that expressed OSKMN but failed to complete reprogramming were also collected as the non-iPSCs and used in parallel with iPSCs in the study 23. Total RNA was extracted by Trizol reagent (Invitrogen) according to the manufacturer's guide. Concentration and quality of all RNA samples were evaluated by Nanodrop 1000 (Thermo Scientific,CA), and the 260/280 and 260/230 values of all samples were more than 1.8 and 1.9, respectively. The extracted RNA samples were stored at -80°C. cDNA was synthesized using M-MLV reverse transcriptase (Invitrogen) after genomic DNA digestion. Briefly, 400-800ng total RNA were added to 12ul liquid wax and genomic DNA contamination was removed by DNase I (Millipore Sigma, MA). The reverse transcription reaction was performed with M-MLV reverse transcriptase at 37°C 1h, followed by 95°C 10min. After 10-fold dilution, cDNA was stored at -20°C and ready for PCR.
Quantitation of gene expression by Q-PCR
Real time PCR was carried out using 3 X Klen-Taq I Mix with a Bio-Rad Thermol Cycler. PCR amplification was performed by PCR of 1 cycle at 95°C for 5 min, 32 cycles at 95°C for 20s, 62°C for15s and 72°C for 15s, and 1 cycle at 72 °C for 10 min. β-Actin was used as PCR input. Quantitative real-time PCR (RT-Q-PCR) was performed using the FastStart Universal SYBR Green Master mix (Millipore Sigma, MA) with a StepOnePlus real-time PCR system (ABI Prism 7900HT; Applied Biosystems, USA). For quantitative real-time PCR, the threshold cycle (Ct) values of target genes were normalized over the Ct of the β-Actin control. Primers used for real-time PCR and Q-PCR are listed in Table S1.
Preparation of cytoplasmic and nuclear fractions
iPSCs were briefly treated with Trypsin-EDTA and gently resuspended in DMEM. Cells were spun down and washed with PBS. After completely aspirating the PBS, 800 μL hypotonic buffer (10 mM Hepes, pH 7.9, 1.5 mM MgCl2, 10 mM KCl) were added and placed in ice for 2 min. 10% Nonidet P-40 was added to a final concentration of 0.4% (35 μL). Samples were inverted a few times and spun at 3,000 rpm for 7 min. Supernatants (cytoplasmic fractions) were collected for processing, and the pellet (nuclear fraction) was gently resuspended in 500 μL hypotonic buffer and spun at 3,000 rpm for 2 min. This washing step was repeated three to four times. Both the cytoplasmic and nuclear fractions were processed for RNA extraction, cDNA synthesis and real-time PCR. To verify that the cytoplasmic and nuclear fractions were completely separated, we used U6 as a nuclear control and β-Actin as cytoplasmic control. The primers for PCR are listed in Table S1.
5'- and 3'-RACE of Peblr20 lncRNA
The full length of Peblr20 lncRNA was characterized by 3'- and 5'-. The 3'-end was raced using the 3'-race system for rapid amplification of cDNA ends kit (Invitrogen) according to instruction manual. 1st PCR and 2nd PCR were performed by 3' gene-specific primer (GSP) and 3' nested GSP, respectively. The 5'-end was raced using RAT library sample which contained Peblr20 cDNA. Universal primer from Library Prep Master Mix Set was used as forward primer and 5' GSP and 5' nest GSP were reverse primers for 1st and 2nd PCR respectively. The 5′ - and 3′ -RACE 2nd PCR products were cloned into pJet vector (Thermo Fisher Scientific, CA) and sequenced. All these primers are shown in Table S1.
Lentiviral overexpression of Peblr20 lncRNA in fibroblasts
Using the 3' and 5' race results, full-length 746bp Peblr20 lncRNA was amplified with PCR primers containing the EcoRI and EcoRV restriction sites. The PCR products were gel-purified, cut by restriction enzymes and ligated into the pCMV-DsRed/Puro vector constructed in our lab. The Peblr20 lncRNA clone was confirmed by sequencing and then packaged in 293T packing cells 24 using the method described in our lab 25. After transfecting fibroblasts, cells were selected by 1ug/µl puromycin. The DsRed reporter in the vector was used to track the lentivirus transfection efficiency. After 10-day selection, cells were collected for RNA quantitation of Peblr20 lncRNA and targeted genes using RT-Q-PCR and for DNA methylation.
Knockdown of Peblr20 lncRNA in iPSCs
Peblr20 lncRNA was knocked down by shRNA lentiviruses. The shRNA vector was constructed by cloning four shRNAs into pGreenPuro vector (#SI505A-1, SBI, CA). shRNAs were designed online (http://katahdin.cshl.edu/homepage/siRNA/RNAi). For cloning, two pairs of shRNAs (5'- GCCGTTGAGAGTTCAAAGGAAGTTG-3'; 5'-CAACTTCCTTTGAACTCTCAACGGC-3' and 5'-CTGGCTTGCTTTGCTTTGCTAAATA-3'; 5'-TATTTAGCAAAGCAAAGCAAGCCAG-3') combined with loop were linked to the H1 and U6 promoter using PCR and were ligated into the EcoR1/BamH1 site in pGreenPuro vector. The copGFP reporter in the vector was used to track the lentivirus transfection in iPSCs. A random shRNA (GCAGCAACTGGACACGTGATCTTAA) was cloned in the same vector as the assay control (shCT). After lentiviral transfection, iPSCs were selected by puromycin. Nanog was stained to show pluripotent differences between Peblr20 down-regulated iPSCs and normal iPSCs. Single colonies (SCs) were selected and cultured for expansion. Cells were collected for RNA quantitation of Peblr20 lncRNA and targeted genes using RT-Q-PCR.
Immunohistochemical staining of stem cell markers
Immunofluorescent staining was used to examine stem cell markers in iPSC colonies15. Cells were briefly rinsed by freshly prepared PBS, fixed by freshly made 4% paraformaldehyde for 10min at RT, washed three times in PBS for 5 min each, permeabilized with freshly made 0.5% v/v Triton X-100/PBS on ice for 5min, then blocked in 1% w/v BSA for 30min at RT. After incubation with primary antibodies diluted in 1% BSA (2ug/ml 1:500) for 1-3h at RT, the samples were washed three times in PBS for 5 min each, incubated with secondary antibody for 1h at RT. The following antibodies were used in the immunostaining: rabbit anti-NANOG (1:100 dilution, Santa Cruz). The cell samples were subsequently incubated with Cy3 or Alexa Fluor 488 labeled secondary antibodies for 1 hour. After washing three times with PBS, samples were counterstained with Hoechst 33258 (Invitrogen). Alternatively, the pluripotency of stem cells was examined by Fluorescent Mouse ES/iPS Cell Characterization kit (Cat.#SCR077, Millipore, MA) following the protocol provided by the manufacturer. Fluorescence images were acquired with a Zeiss AxioCam Camera.
Embryoid body differentiation
E14 cells were cultured by the hanging drop method in a 10cm culture dish without LIF. After 3 days, embryoid bodies (EBs) were transferred to 10cm ultra-low-adherence plates for suspension culture with slow shaking for up to 8 days. The media were replenished by sedimentation every other day. EBs were collected on D0, D2, D4, D6 and D8 for quantitation of Peblr20 lncRNA and targeted genes using RT-Q-PCR.
The Pou5F1 promoter-luciferase assay
The function of Peblr20 in activating the Pou5F1 enhancer was first examined in 293T cells by using a dual-luciferase reporter assay. A 3.9 kb genomic DNA fragment covering the Pou5F1 enhancer, promoter and part of the exon 1 sequence was amplified by PCR using primers: SJ559 5'-TATCGATAGGTACCGTCTGTGAGGAGGTGGCTGAACT-3' and SJ560 5'-ATCGCAGATCTCGAGCTCCTCGGGAGTTGGTTCCAC-3'. The DNA fragment was cloned into pGL3 vector by Kpn1/Xho1.
For the luciferase assay, cells were seeded at a density of 5 × 104 cells/well in 96-well plates. The lentiviral Peblr20 overexpression vector was co-transfected with a Pou5F1-luciferase plasmid and Renilla luciferase control plasmid (Promega) using Lipofectamine 3000 (Invitrogen, CA). The empty lentiviral vector and random lncRNA vector were used as controls. Forty-eight hours after transfection, firefly and Renilla luciferase activities were measured with the dual- luciferase reporter system (Promega) using a luminometer (Turner Biosytem, CA). The relative activity of the Pou5F1 enhancer was calculated by setting the untreated control cells as 1. All luciferase assays were repeated three times with three culture replicates each.
The DOX-OSKM reprogramming assay
Peblr20 cDNA was cloned into pCMV-DsRed-Puro vector and lentiviruses were packaged in 293 cells. Control lentiviruses carried the pCMV-DsRed-Puro empty vector (Vector). OG2 MEFs were transfected with the Peblr20 and control lentiviruses and were selected by puromycin. MEFs were reprogrammed following the method as described 26. Briefly, 15,000 lentivirus-transfected MEFs were seeded in 12-well plates and were cultured in KSR iPS medium containing 2 μg/ml doxycycline (DOX). The medium was changed every other day. The iPSC colonies were immunostained with rabbit anti-NANOG Antibody (A300-397A, Bethyl, 1:500 dilution). Positive iPSC colonies per field were recorded15.
Status of CpG DNA methylation by sodium bisulfite sequencing
Genomic DNA was extracted by solution D from normal fibroblasts, fibroblasts transfected with vector control and fibroblasts that overexpressed Peblr20. DNA bisulfite (BS) conversion was performed using the EZ DNA Methylation-Gold kit (Zymo Research, Orange, CA) according to manufacturer's protocol. PCR was performed using BS-treated DNA samples as template, and BS-specific primers were shown in Table S1. PCR conditions were 95°C for 10 min followed by 38 cycles of 95°C for 20s, 58°C for 20s of annealing, 72°C for 20s of extension and completing the reaction at 72°C for 5 min. PCR products were run on 2% agarose gels to verify product size, and extracted using QIAquick gel extraction kit (QIAGEN). Purified PCR products were cloned into pJet vector (Thermo Fisher Scientific, CA) and sequenced. PCR products from CpG3 were digested by restriction enzymes HpyCH4IV (NEB, MA) and run on 2% agarose gels. The gray values of gel bands were calculated using ImageJ.
Chromatin immunoprecipitation and ChIP Q-PCR assay
Chromatin immunoprecipitation (ChIP) assays were performed using ChIP Assay Kit (Millipore Sigma, MA) according to the manufacturer's protocol. Briefly, Peblr20 overexpressed fibroblasts and vector control transfected fibroblasts were collected respectively and fixed for 10 min at 37 °C with 1% formaldehyde, followed in sequence with SDS lysis and DNA shearing, protein and DNA immuneprecipitation, cross-linked DNA reversal and DNA purification, and finally the immunoprecipitated DNA fragments were detected by real-time Q-PCR. Anti-TET1 (Invitrogen, USA) and anti-TET2 antibodies (Abcam, MA) were immunoprecipitated with sheared DNA from Peblr20 overexpressed fibroblasts and vector control transfected fibroblasts, respectively. Normal mouse IgG (Millipore Sigma, MA) was used as the negative control. The primers for Pou5F1 enhancer loci are listed in Supplementary Table S1. CHIP-Q-PCR was performed with three replicates.
RNA binding protein immunoprecipitation (RIP) assay
A lncRNA-affinity binding precipitation assay (RIP) 27 was performed to examine the binding of TET2 protein with Peblr20 lncRNA. RIP was performed using the Magna RIPTM RNA-Binding Protein Immunoprecipitation Kit (Millipore, Germany) according to the manufacturer's instructions. Generally, Peblr20 overexpressed fibroblasts were collected and lysed using RIP lysis buffer. Then 100ul cell extract was incubated with RIP buffer containing magnetic beads conjugated with anti-TET2 antibody (Abcam,MA). Mouse IgG was used as the negative control and anti-SNRNP70 was as the positive control. The samples were incubated with proteinase K to digest protein and then the immunoprecipitated RNA was isolated. The purified RNAs were detected by reverse transcription Q-PCR. The primers for Q-PCR are listed in in Supplementary Table S1. The CHIP-Q-PCR was performed with three replicates. The Ct values were normalized over the input and compared with IgG control.
Statistical analysis
All experiments were performed in triplicate. The data were expressed as mean ± standard error of mean (SEM) and were analyzed using SPSS software (version16.0, Inc.,IL). The data were analyzed with Student's t-test or by one-way analysis of variance, and statistically significant differences by Student's t test.
RESULTS
Identification of Peblr20 as a pluripotent lncRNA by combining RAT-seq and RNA-seq
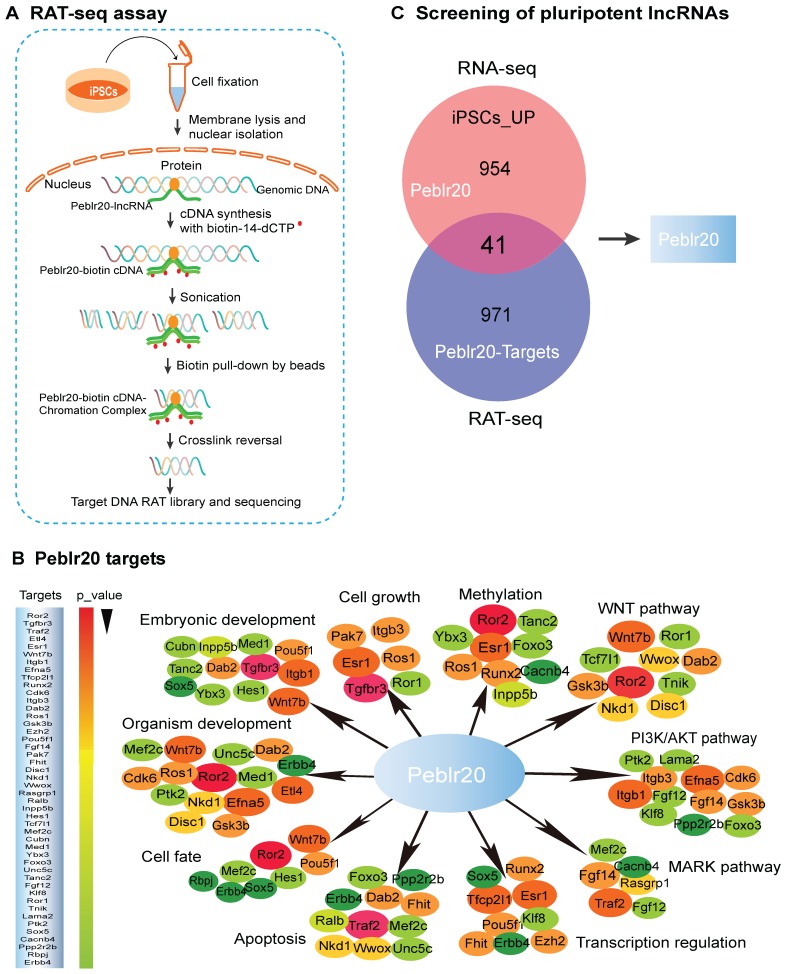
LncRNAs play important roles in the growth and self-renewal of stem cells, but their underlying mechanisms of action remain largely uncharacterized. We propose a novel strategy to identify pluripotency-associated lncRNAs (Fig. S1A). Conventional RNA-seq is used to identify differentially-expressed lncRNAs related to reprogramming. A RAT-seq assay is then employed to map the genome-wide target interactome with which the lncRNAs interact (Fig. 1A-1B). Together, they can identify functional lncRNA candidates that are not only expressed differentially in reprogramming, but are also able to bind to the regulatory elements of multiple core stem cell factor genes (Fig. 1C, Fig. S1A) 13, 14.
Figure 1.
Identification of pluripotent lncRNAs by RNA-seq and RAT-seq. (A) Schematic diagram of the RNA reverse transcription-associated trap sequencing (RAT-seq) assay. RNA was in situ reverse transcribed using Peblr20 lncRNA-specific antisense oligo primers and biotin-14-dCTP. The biotin- cDNA/chromatin DNA complex was pulled down by biotin-streptavidin magic bead after sonication. The Peblr20-binding chromatin DNAs were isolated for library sequencing. (B) The Peblr20 interaction network targets identified by RAT-seq. Peblr20 binds to many pathway genes that are involved in embryonic development, transcription regulation, cell fate, signaling pathways, etc. Target genes were listed in the order of p-value. (C) Profiling pluripotent lncRNAs by integrating RNA-seq and RAT-seq. The conventional RNA-seq approach identifies thousands of lncRNAs that are differentially expressed during pluripotent reprogramming. A modified RAT-seq approach was performed to map the genome wide interacting target genes for the selected lncRNA. Using this strategy, we identified Peblr20 as a pluripotent lncRNA, because it is activated in iPSCs and binds to multiple stemness gene targets.
Using this strategy, we identified a Pou5F1 enhancer binding lncRNA that matches to 1700097N02Rik. We refer it to as Peblr20 (Pou5F1 enhancer binding lncRNA 20). 5'-RACE and 3'-RACE suggested the presence of two variants. Peblr20 variant 1 shares the same sequences as the four-exon 1700097N02Rik, and variant 2 has two exons, with the 5-end differing from 1700097N02Rik (Fig. S2).
RAT-seq and RNA-seq data showed that Peblr20 is a pluripotency-associated lncRNA. It was silenced in fibroblasts and upregulated in iPSCs in parallel with the stem marker genes, Pou5F1 and Sox2 (Fig. S3). Notably, Peblr20 bound to the regulatory elements of multiple stemness genes (Fig. 1B), including the Pou5F1 enhancer (Fig. S1C), the Sox2 enhancer (Fig. S1D), and Klf4 CpG islands (Fig. S1E). These data suggest that Peblr20 might play important roles in pluripotency initiation and maintenance by regulating these core factor genes in trans.
Peblr20 lncRNA is required for the maintenance of the pluripotent state
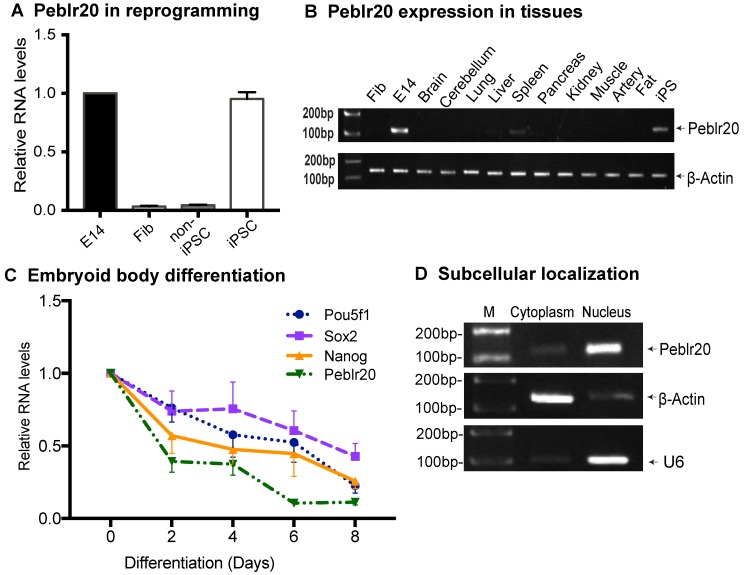
In order to verify the role of Peblr20 in stem cell maintenance, we first examined the dynamic expression of Peblr20 during pluripotent reprogramming. The expression of Peblr20 was closely correlated with the pluripotency status during reprogramming (Fig. 2A). Peblr20 was only minimally transcribed in fibroblasts and in non-iPSCs, which carry the OSKM transgenes but fail to complete reprogramming 23. However, Peblr20 became activated as the cells were fully reprogrammed into iPSCs. Like other pluripotency-associated lncRNAs, Peblr20 was not expressed in terminally differentiated organs and tissues (Fig. 2B).
Figure 2.
Characterization of Peblr20. (A) Differential expression of Peblr20 in reprogramming. Cells were collected at different stages of reprogramming and the dynamic expression of Peblr20 was measured by RT-Q-PCR. Fib: fibroblast; non-iPSC: un-reprogrammed cells that express four OSKM cocktail factors, but failed to complete reprogramming; iPSC: induced pluripotent stem cells; E14: mouse pluripotent stem cell line used as a positive control. M: marker. Gene expression was normalized to β-Actin. For comparison, positive control E14 was set as 1. (B) Peblr20 lncRNA in differentiated tissues. Ten mouse tissues were collected and cDNAs were synthesized and used for RT-PCR. Fib was used as a negative control, E14 and iPSC were positive controls and β-Actin was used as the internal control. Note that Peblr20 was not expressed in terminally differentiated tissues. (C) Embryoid body differentiation. E14s were collected at D0, D2, D4, D6 and D8 of EB formation for Q-PCR. Peblr20 was dynamically associated with core pluripotent factors, including Pou5F1, Sox2 and Nanog. (D) Subcellular localization of Peblr20 lncRNA. Cytoplasmic and nuclear RNAs were isolated and used to quantitate the subcellular distribution of Peblr20. β-Actin was used as the cytoplasmic control and U6 was used as the nuclear control.
We also examined the expression of Peblr20 during the process of embryonic body differentiation, and found that Peblr20 became down-regulated in parallel with the decreased transcription of the core stem cell factors, Pou5F1, Sox2 and Nanog (Fig. 2C). In iPSCs, the cytoplasmic-nuclear fractionation assay revealed that Peblr20 was predominantly located in the cell nucleus (Fig. 2D).
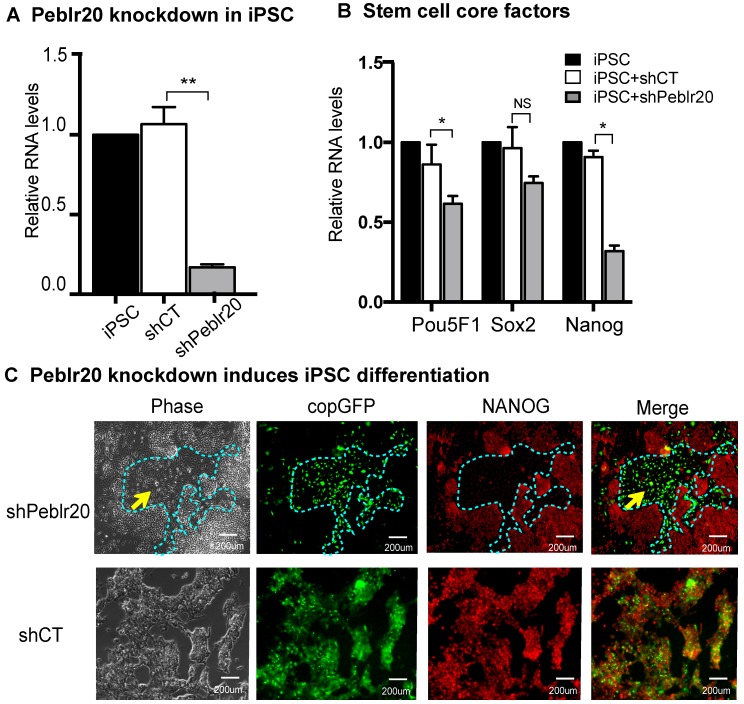
We then performed a loss-of function experiment to study the role of Peblr20 in pluripotent stem cells. Two Peblr20 shRNAs were constructed under the control of the H1 and U6 promoters, respectively, in a lentiviral vector (pCMV-copGFP/Puro) (Fig. S4A). After lentiviral transfection, iPSCs were selected by puromycin. Single colonies emitting the copGFP green signal were selected, expanded and collected for RT- Q-PCR (Fig. S4B). Peblr20 shRNA knockdown reduced Peblr20 expression by 80% (Fig. 3A). After Peblr20 knockdown, the expression of Pou5F1, Sox2, and Nanog, were also down-regulated (Fig. 3B). The shPeblr20-transfected iPSCs exited from pluripotency, as demonstrated by their differentiated morphology and the loss of immunostaining staining for the pluripotent marker protein NANOG (Fig. 3C, top panel, yellow arrow). As the control, the treatment with random shRNA (shCT) did not affect the pluripotency of iPSCs (Fig. 3C, bottom panel). These data suggest that Peblr20 is a crucial molecular factor required for maintenance of pluripotency.
Figure 3.
Peblr20 lncRNA is required for pluripotent state maintenance. (A) Peblr20 knockdown in iPSCs. After lentiviral shRNA transfection and puromycin selection, iPSCs colonies were selected, expanded and collected for Q-PCR. shCT: random shRNA control; shPeblr20: Peblr20 shRNA. For comparison, the abundance of Peblr20 in iPSCs was set as 1. ** p<0.01 as compared with iPSCs and random shCT controls. (B) Peblr20 knockdown downregulates stem cell core factors in iPSCs. * p<0.05 as compared with iPSC and random shCT controls. NS: not significant. (C) Peblr20 knockdown induces iPSC differentiation. The Obelr20 shRNA and random shRNA lentiviral vectors carry the copGFP reporter gene (green). After lentiviral transfection, cells were fixed and immunostained by an antibody against the stem cell pluripotent marker NANOG (red). Compared to the control group, shPeblr20 transfected iPSCs were negative for NANOG immunostaining and were differentiated morphologically (blue dotted line).
Peblr20 enhances iPSC reprogramming efficiency
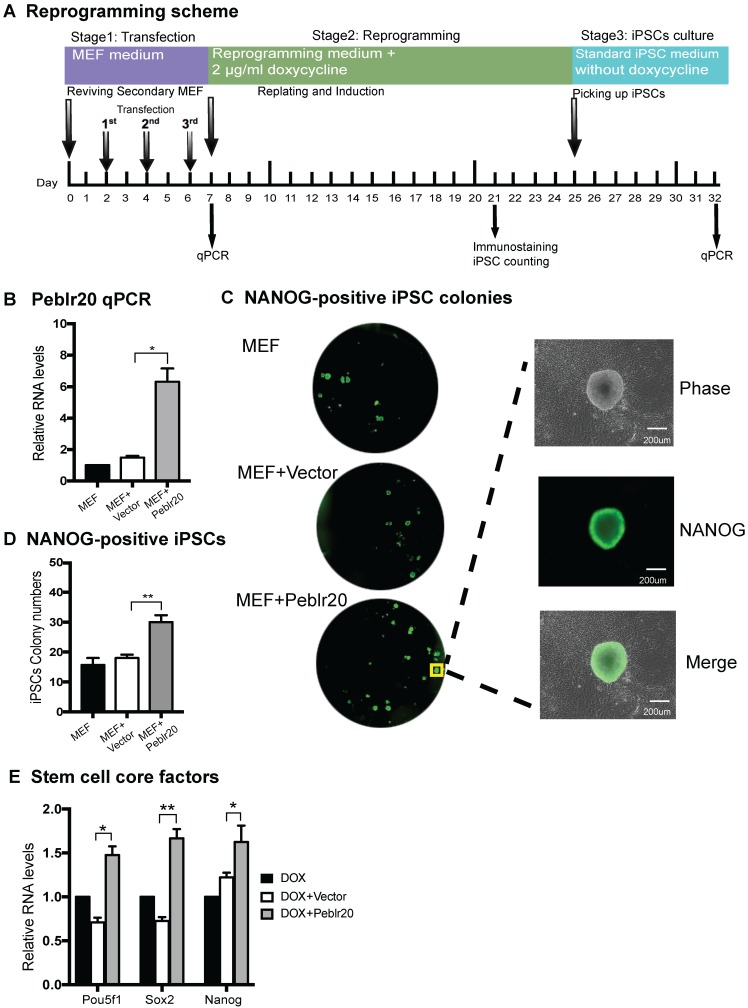
To determine whether high levels of Peblr20 transcripts affect reprogramming, we performed a reprogramming assay using secondary mouse embryonic fibroblasts (MEFs) that carry the DOX-inducible lentiviral OSKM 28. MEFs were administered three doses of lentivirus carrying the pCMV-DsRed/Puro-Peblr20 cassette (Fig. 4A). An empty lentiviral vector was used as the control. After puromycin selection, cells were collected to assess the expression of Peblr20 by RT-Q-PCR (Fig. 4B). After it was determined that the abundance of Peblr20 was increased, we incubated the cells in a medium containing 2µg/ml doxycycline (DOX) as a reprogramming inducer. In MEF cells transfected with the lentivirus containing the Peblr20 cassette, typical iPSC colonies began to appear on day 15 (eight days after DOX addition). On day 21, reprogramming efficiencies were examined by immunostaining for the stem cell marker NANOG (green fluorescence) (Fig. 4C). The number of NANONG-positive colonies was significantly increased in the Peblr20-overexpressing group (Fig. 4D).
Figure 4.
Peblr20 improves reprogramming efficiency. (A) Reprogramming scheme. Cells were collected at three separate stages for Q-PCR. After reprogramming, colonies were stained by anti-NANOG. (B) Overexpression of Peblr20. After transfection, MEFs were collected and used for quantitation of Peblr20 lncRNA. (C) NANOG-positive colonies after immunostaining. Cells from D21 colonies were fixed and stained with NANOG antibody. NANOG-positive colonies/well (left, under 4x objective lens) A single NANOG-positive colony is shown in the right panel (20x objective lens). (D) NANOG-positive colonies per well. (E) Upregulation of core pluripotent factors in Peblr20 transfected secondary MEF cells. * P < 0.05; ** P < 0.01.
At the conclusion of reprogramming, iPSCs colonies were selected and cultured on mitomycin C-inactivated MEF feeder cells using standard iPSC medium without DOX. iPSCs were collected after the third passage on day 32 for RNA quantitation of Pou5F1, Sox2 and Nanog. Peblr20-overexpressing cells contained significantly higher abundance of stemness genes (Fig. 4E). Taken together, these results indicate that Peblr20 lncRNA improves reprogramming efficiency.
Peblr20 activates stem cell core factors in trans
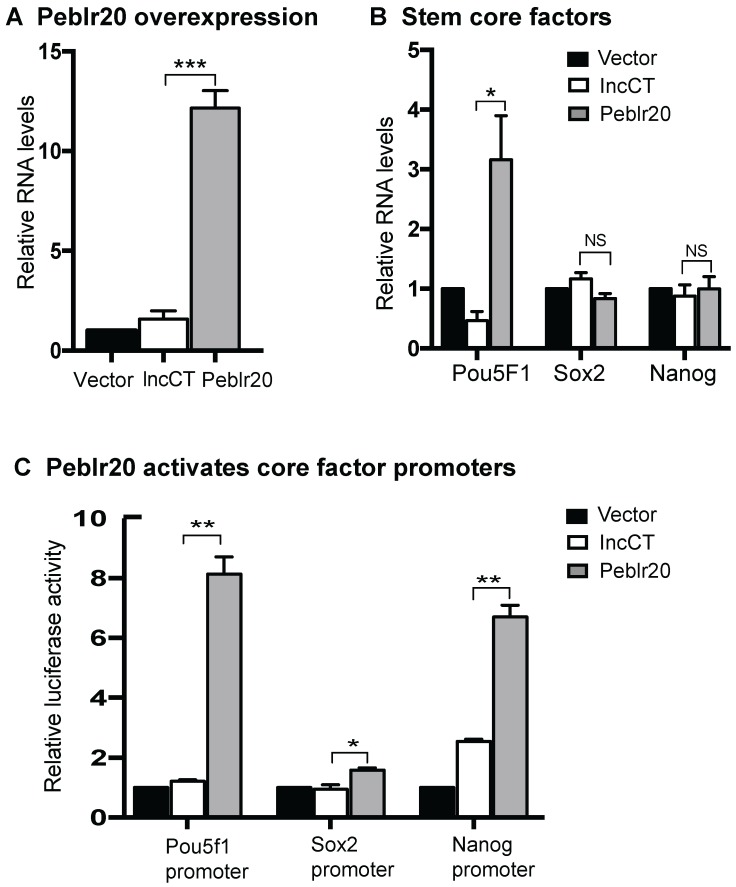
We then initiated a series of studies to delineate the molecular mechanisms by which Peblr20 regulates reprogramming. First, we examined if Peblr20 was able to activate the expression of core stem cell factor genes in fibroblasts. We synthesized a lenti pCMV-DsRed/Puro-Peblr20 plasmid (Fig. S4C) and packaged the lentivirus in 293T cells. Fibroblasts were transfected with Peblr20 lentiviruses. After puromycin selection, ~90% of fibroblasts overexpressed Peblr20 using DsRed as a tracking marker (Fig. S4D). Using RT-Q-PCR, we confirmed a >12 fold overexpression of Peblr20 as compared with the vector control (Fig. 5A). Pou5F1, but not Sox2 or Nanog, was significantly upregulated in cells in which Peblr20 was overexpressed (Fig. 5B).
Figure 5.
Peblr20 activates stem cell core factors. (A) Peblr20 overexpression in fibroblasts. (B) The effect of Peblr20 on stem cell core factor genes. (C) Peblr20 activates promoter activities of core pluripotent factors. 293T cells were co-transfected by reporter plasmids and Peblr20 plasmid. Forty-eight hours after transfection, cells were collected for luciferase activity measurements. Reporter plasmid empty vector and random lncRNA (lncCT) vectors were used as the controls.
Dual-luciferase reporter systems have been widely used to study the activation of specific core regulatory elements in pluripotent stem cells 29-31. We used this system to confirm whether Peblr20 lncRNA could enhance the promoter activity of Pou5F1, Sox2 and Nanog. We constructed promoter reporter plasmids of these three genes and co-transfected them with a Peblr20 lncRNA overexpression plasmid and Renilla luciferase control plasmid into 293T cells. Forty-eight hours after transfection, cells were harvested and the luciferase activity was measured using the Dual-Luciferase reporter assay system. As compared with the vector control (Vector) and the random lncRNA control (lncCT), Peblr20 lncRNA significantly enhanced the activities of the Pou5F1 and Nanog promoters (Fig. 5C).
Peblr20 facilitates Pou5F1 enhancer RNA transcription
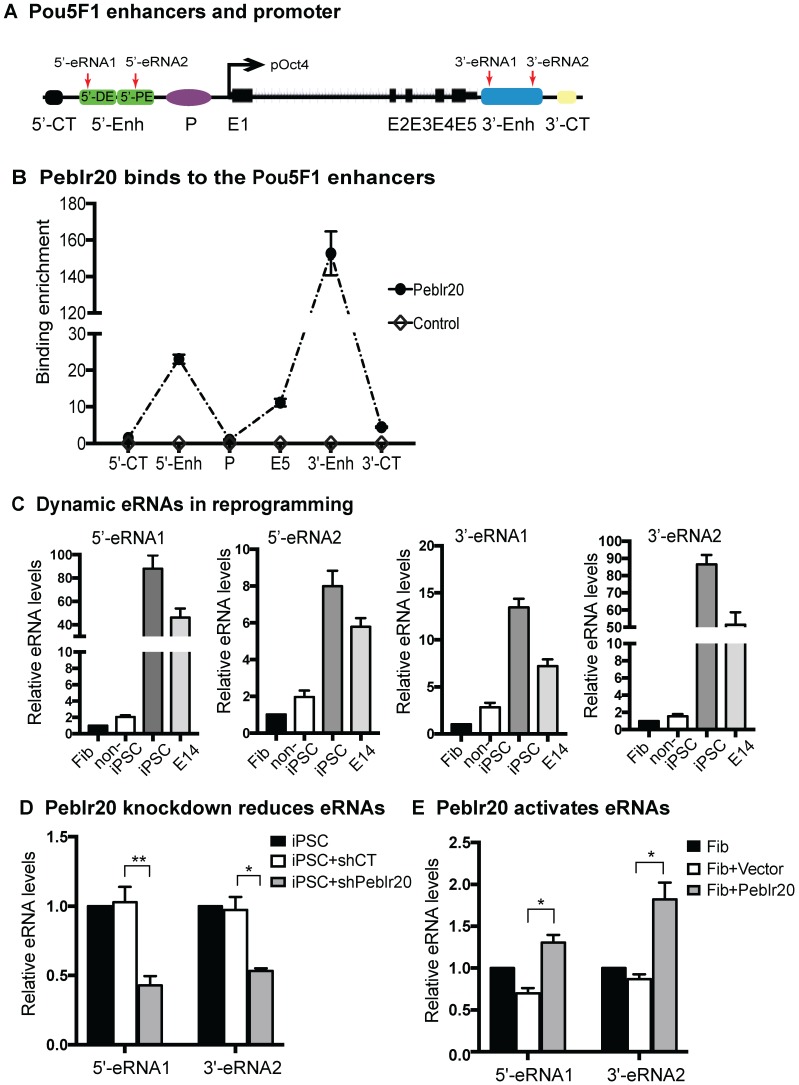
To further delineate the mechanism underlying the activation of Pou5F1, we used quantitative PCR to map Peblr20 binding at the Pou5F1 locus (Fig. 6A). Using RAT-seq, we found that Peblr20 bound to the Pou5F1 5'- and 3'-enhancer elements. No such interaction signals were detected in the RAT random control library products (CTL). As expected, we did not detect the RAT signals at the 5'- and the 3'- control sites (5'-CT, 3'-CT). Interestingly, Peblr20 did not bind to the Pou5F1 promoter (pPou5F1) (Fig. 6B).
Figure 6.
Peblr20 activates the Pou5F1 enhancer RNA pathway. (A) Location of Pou5F1 enhancer RNAs. 5'-eRNA1: the 5-distal enhancer (5'-DE); 5'-eRNA2: the 5'-proximal enhancer (5'-PE); 3'-eRNA1 and 2: the 3'-enhancer (3'-Enh); P: the Pou5F1 promoter; 5'-CT, 3'-CT: the RAT 5'- and 3'- control sites; E1-E5: Pou5F1 exons 1-5. (B) Peblr20 lncRNA-Pou5F1 DNA interactions by Q-PCR. RAT library samples were used to perform Q-PCR to quantitate binding intensity. The results were normalized to the value of the streptavidin bead pulldown control. Control: the RAT-seq library was constructed using random oligo primers; Obelr20: the RAT-seq library was constructed using Peblr20-speccific primers. (C) Dynamic expression of Pou5F1 eRNAs in reprogramming. Cells were collected at various stages of reprogramming and the expression of eRNAs was measured by RT-Q-PCR. Fib: fibroblast; URC: non-iPSC: fibroblasts that carried the lentiviral OSKM but failed to complete reprogramming; iPSC: induced pluripotent stem cells from secondary MEFs; E14: mouse pluripotent stem cell line as a positive control. Gene expression was normalized to β-Actin internal control. (D) Peblr20 knockdown inhibits Pou5F1 eRNA. iPS+shCT: iPSCs transfected with the random shRNA vector control; iPS +shPeblr20: iPSCs transfected with shPeblr20. Gene expression was normalized to β-Actin control. (E) Peblr20 overexpression activates Pou5F1 enhancer RNAs in fibroblasts. Cells were collected from gain-of-function experiments and eRNAs expressions were measured by RT-Q-PCR. Fib+Vector: fibroblasts transfected with vector control; Fib+Peblr20: fibroblasts overexpressed Peblr20. All experiments were performed in triplicate and statistically significant differences by Student's t test. * P < 0.05; ** < 0.01.
Enhancers are defined as DNA elements that act over a distance to positively regulate expression of target genes in a spatial and temporal fashion 32. Recent studies have shown that active enhancer loci can recruit RNA polymerase II (RNA Pol II) and express enhancer RNAs (eRNAs) and these eRNAs correlate with the enhancer activity. The eRNA-transcribed enhancers are more active than the non-transcribed enhancers in activating their target genes.
To determine if Peblr20 lncRNA might activate the Pou5F1 through eRNA transcription, we collected cells during pluripotent reprogramming and used Q-PCR to map the eRNA transcripts at the Pou5F1 locus, including the 5'- distal enhancer, 5'- proximal enhancer, and 3'- enhancer (Fig. 6A). We found that the abundance of eRNA transcripts was closely associated with pluripotency (Fig. 6C). The 5'- and 3'- eRNAs, particularly 5'-eRNA1 and 3'-eRNA2, were actively transcribed in iPSCs and embryonic stem cell E14 line, but were expressed at very low levels in fibroblasts and non-iPSCs that expressed the lentiviral OSKM but failed to complete reprogramming 23.
We then knocked down Peblr20 in iPSCs and performed Q-PCR to measure the Pou5F1 eRNAs. After Peblr20 knockdown, we found that the eRNAs at both the Pou5F1 5'- and 3'- enhancer sites were significantly downregulated (Fig. 6D). The shRNA control (gCT) did not interfere with the eRNA transcription. We also examined whether ectopic expression of Peblr20 would affect the eRNAs in fibroblasts. As seen in Figure 6E, overexpression of Peblr20 significantly increased the expression of eRNAs at the Pou5F1 enhancer loci. Taken together, these data suggest that Peblr20 may regulate the Pou5F1 gene activity by promoting the eRNA synthesis.
Peblr20 activates Pou5F1 enhancer RNA expression by inducing DNA demethylation
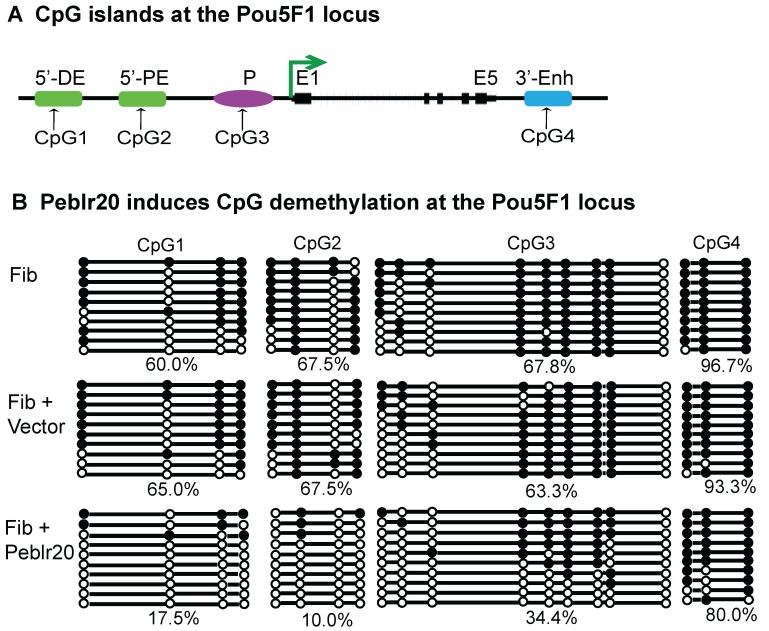
To determine if Peblr20 induced Pou5F1 eRNAs by altering epigenetic modifications at the Pou5F1 enhancer and promoter loci (Fig. 7A), we isolated genomic DNAs from fibroblasts that overexpressed Peblr20 and used sodium bisulfite sequencing to examine the status of DNA methylation. The lentiviral vector control did not affect DNA methylation in these loci, however, after ectopic expression of Peblr20, we observed an extensive decrease in DNA methylation (Fig. 7B, panel 3). Using a restriction enzyme digestion assay, we also confirmed a 40% reduction of DNA methylation in the Pou5F1 promoter CpG island in the Peblr20-overexpressing fibroblasts (Fig. S5). These data suggest that the overexpression of Peblr20 may trigger the activation of the Pou5F1 and its enhancer RNAs through DNA demethylation.
Figure 7.
Peblr20 induces DNA demethylation at the Pou5F1 enhancer loci. (A) CpG islands at the Pou5F1 locus. CpG1: 5'-distal enhancer (5'-DE); CpG2:5' proximal enhancer (5'-PE); CpG3: promoter region (P); CpG4: 3'-enhancer (3'-Enh). (B) Peblr20 induces CpG demethylation at the Pou5F1 locus. DNAs were extracted from fibroblasts from gain-of-function experiments, treated by sodium bisulfite, amplified with the Pou5F1-specific primers, cloned into the pJET vector, and sequenced. Solid circle: methylated CpG; open circle: un-methylated CpG. Each line represents sequencing from one clone. Number under the last line represents the methylation level of each CpG island.
Peblr20 recruits TET2 to the Pou5F1 enhancer locus
The Ten eleven translocation (TET) family of enzymes (TET1/2/3) are critical dioxygenases in DNA demethylation, and they have emerged as potential drivers of epigenetic reprogramming 33, 34. We thus asked if TET family members were involved in DNA demethylation in Peblr20-overexpressing fibroblasts. We focused on TET1/TET2, which are correlated with the status of pluripotency in mouse ESCs 35, 36.
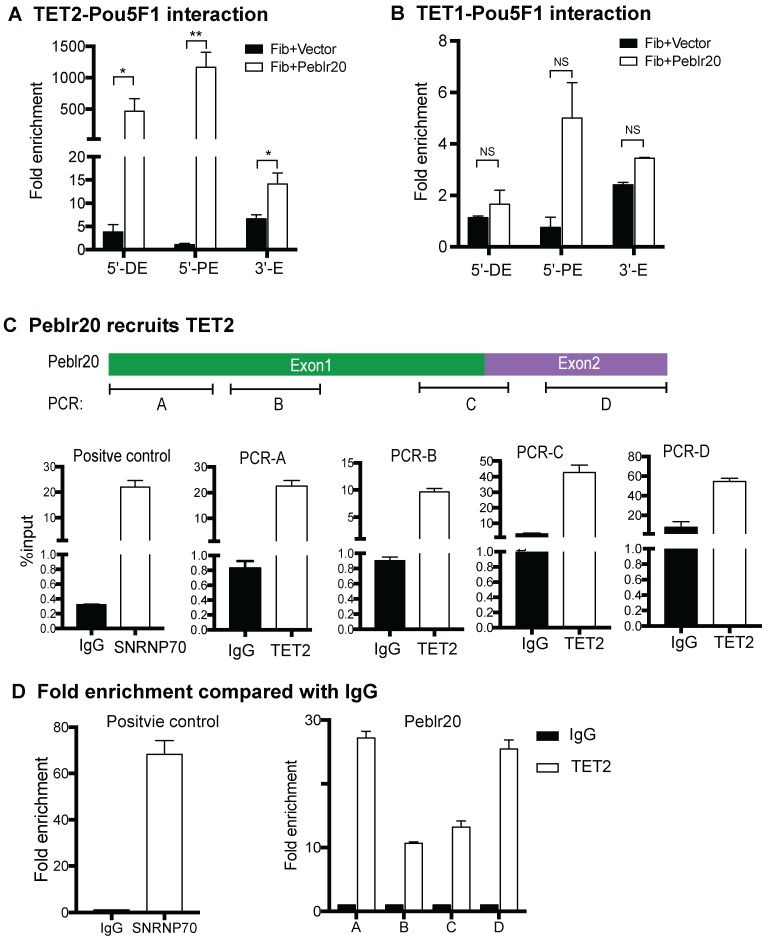
First, we examined if TET1/2 interacted with the regulatory elements of the Pou5F1 gene. Fibroblasts were transfected with lentiviruses carrying Peblr20 or empty vector control. After puromycin selection, cells were collected and chromatin immunoprecipitation (ChIP) was performed with anti-TET1, anti-TET2 and mouse IgG control antibodies. Real-time PCR was used to measure immunoprecipitation enrichment at the 5'-distal enhancer, 5'-proximal enhancer, and 3'-enhancer of Pou5F1 after adjusting over the IgG control. TET2, but not TET1, bound to the Pou5F1 enhancer loci in Peblr20-overexpressing fibroblasts (Fig. 8A-8B).
Figure 8.
Peblr20 recruits TET2 to the Pou5F1 enhancer loci. (A) Interaction of TET2 at the Pou5F1 enhancer loci in the Peblr20 overexpressing fibroblasts. Enrichments of TET2 to the Pou5F1 enhancers were measured from TET2 ChIP samples and normalized to the IgG control samples. Compared to vector control group, there were remarkable enrichments of Pou5F1 enhancer loci with TET2 in Peblr20 overexpressed fibroblasts. 5'-DE: 5'-distal enhancer; 5'-PE: 5'-proximal enhancer; 3'E: 3'-enhancer. (B) No binding of TET1 with Pou5F1 enhancer loci. Fold enrichments of Pou5F1 enhancer loci were measured using TET1 ChIP samples relative to IgG samples. (C) Peblr20 recruits TET2. Fibroblasts transfected with Peblr20 were collected and immunoprecipitated with anti-TET2 antibodies, with mouse IgG as negative control and with anti-SNRNP70 as positive control. Purified binding RNAs were reverse transcribed and used as Q-PCR templates. RIP data are represented by fold enrichments relative to the input. (D) RIP data represented by fold enrichments relative to IgG showed that TET2 interacted primarily with the 3'- and 5'-fragment of Peblr20 lncRNA.
We then performed a RNA-binding protein immunoprecipitation (RIP) assay to examine if Peblr20 interacts with TET2. The TET2-RNA chromatin complex was immunoprecipitated with anti-TET2 antibody. An anti-SNRNP70 antibody was used as the positive control and a mouse IgG was used as the negative control. Immunoprecipitated RNA fragments were reverse transcribed and detected by real-time Q-PCR using primers from Peblr20 (Fig. 8 C). As compared with the IgG control, it seemed that TET2 interacted primarily with the 3'- and 5'-fragment of Peblr20 lncRNA (Fig. 8D). These data suggest that Peblr20 may help recruit TET2 to the Pou5F1 locus, where it induces DNA demethylation and promotes eRNA expression.
DISCUSSION
The specific role of lncRNAs in cell fate determination, particularly the stem cell reprogramming process, remains unclear. Using combined RNA-seq and RAT-seq assays, we have identified Peblr20 as a critical lncRNA component that helps maintain the pluripotent status of iPSCs. Peblr20 is differentially transcribed in pluripotent reprograming, being transcriptionally silenced in terminally differentiated fibroblasts but activated in iPSCs. Knockdown of Peblr20 induces exit from pluripotency in iPSCs. In contrast, overexpression of Peblr20 activates stemness genes in fibroblasts and enhances pluripotent reprogramming. Most importantly, we demonstrate that Peblr20 is the first lncRNA that controls the pluripotency of stem cells by epigenetically activating the enhancer RNA pathway.
It is generally believed that lncRNAs may act as decoys, guides and scaffolds to suppress or enhance the function of other regulators, including DNA, RNA and proteins 37, depending on their intracellular localization 38, 39. Nuclear lncRNAs can guide chromatin modification complexes to specific genomic loci and/or serve as molecular scaffolds that tether together distinct functionally related complexes 40. Due to their intrinsic ability to base-pair with other nucleic acids, lncRNAs can exert repressive or promoting activities on target genes by cis-acting on neighboring genes or trans-acting on genes at distant loci 41. In contrast, some lncRNAs are localized in the cytoplasm, where they can regulate phenotypes through base-pairing complementary regions on RNA targets 42. Depending on their target partners, lncRNAs can regulate chromatin modifications 43, RNA binding proteins 44, miRNA activity 45 and signaling pathways 46. LncRNA binding to target DNA can initiate the formation of heterochromatin by recruitment of DNA or histone methyltransferases, resulting in repression of gene expression 47. Conversely, transcriptional activation can be induced by recruitment of different chromatin modifiers, such as histone H3 lysine 4 (H3K4) and methyltransferase MLL1, or by changing the 3D chromatin conformation 40, 48, 49. Most importantly, we demonstrate in this study that Peblr20 functions through a unique enhancer RNA regulatory pathway. Peblr20 specifically interacts with the Pou5F1 enhancers and facilitates Pou5F1 eRNA expression. By activating this complicated eRNA regulatory network, Peblr20 controls the pluripotent state and iPSC reprogramming process.
DNA methylation status at regulatory elements of pluripotency regulator genes, such as Pou5F1, is correlated with stable silencing of these genes during development. Overcoming this barrier is believed to be a key step to initiate cellular reprogramming 50-53. Recently, hypomethylation has also been found at active enhancers 54, 55. Using whole-genome bisulfite sequencing (BisSeq), Stadler 55 generated methylomes in mouse ESCs and in neuronal progenitors (NP). Using an analytical approach that quantifies DNA methylation locally, they identified a novel epigenomic feature defined by localized reduced levels of methylation. These low-methylated regions (LMRs) show an average methylation of 30% and occur distal to promoters with little overlap with CpG islands. Contrasting LMRs with maps of histone modifications, DNase I hypersensitivity and several DNA-binding factors revealed that LMRs represent distal regulatory regions, such as enhancers. This is in agreement with several single-gene studies of methylation dynamics at localized distal regulatory regions 56, 57. Most recently, Hon et al 58 identified 302,864 tissue-specific differentially methylated regions (tsDMRs) by profiling the methylomes of a diverse panel of 17 normal adult mouse tissues. The identified tsDMRs are generally hypomethylated in a tissue-specific manner. Most notably, these sequences predominantly correspond with distal regulatory elements in the genome. Moreover, some tsDMRs mark enhancers that were dormant in adult tissues but active in embryonic development. In our study, Peblr20 promoted DNA demethylation at the Pou5F1 locus, especially the 5'-enhancer region. After Peblr20 overexpression, the originally silent Pou5F1 enhancers became highly demethylated in fibroblasts. Consequently, the Pou5F1 eRNAs are transcribed, and they activate the Pou5F1 promoter to promote pluripotent reprogramming.
DNA and chromatin-modifying enzymes establish an ESC/iPSC-specific epigenomic landscape that is linked to a network of pluripotency genes that influences differentiation potential. These enzymes contain an active C-terminal catalytic domain that converts 5-methylcytosine (5mC) sequentially to 5-hydroxymethylcytosine (5hmC), 5-formylcytosine (5fC), and 5-carboxylcytosine (5caC) 59. TET-driven 5mC oxidation provides a direct mechanistic route for both passive and active DNA demethylation. Both Tet1 and Tet2 are expressed in mouse ESCs, while Tet3 is not expressed in ESCs and is only induced upon differentiation, consistent with its presence in various differentiated cell types 35, 36. Tet1 and Tet2 are key players in this network of coordinated genetic and epigenetic control. The generation of Tet1, Tet2, and Tet3 single mutant 60, 61 and Tet1/2 double-knockout (DKO) 35 and Tet1/2/3 triple-knockout (TKO)62 mESCs has shed light on the roles of these proteins in pluripotency as well as embryonic and adult development. Depletion of Tet1 or Tet2 significantly reduces iPSC reprogramming efficiency in conventional reprogramming medium 63-65, and triple knockout Tet1/2/3 (Tet-TKO) mouse embryonic fibroblasts completely fail to generate iPSC colonies following OKSM overexpression 65. Whether Tet1 is implicated in promoter and enhancer demethylation and gene activation during iPSC reprogramming has been partially addressed in a study reporting that Tet1 is necessary for the demethylation of the Pou5F1 enhancer and promoter regions followed by the transcriptional reactivation of this gene 66. In our study, using both ChIP-PCR and RIP-PCR assays, we confirmed that Peblr20 lncRNA recruits TET2 to the Pou5F1 enhancers, where DNA demethylation promotes eRNA expression.
It should be noted that not all enhancers are able to transcribe eRNAs. Kim et al 67 estimated that only about half of the intergenic enhancers can transcribe eRNAs. Enhancer transcription is a widespread phenomenon observed across multiple cell types in different species 68-71. Using an integrated epigenomic screening, Ounzain and colleagues 72 recently established a catalogue of enhancer RNAs that are dynamically expressed in ESCs during cardiac differentiation. The expression of these transcripts correlated with the expression of target genes in their genomic proximity. The expression of the eRNAs was inhibited when the target mRNA abundance reached maximal levels. Using genome-wide analysis of eRNAs in gene regulation across 12 mouse tissues, Cheng 73 demonstrated that the state of enhancer activity is tissue-specific, as the same enhancer can differentially transcribe eRNAs across tissues. Overall, these data are an important contribution to the functional impact of eRNAs on pluripotency and differentiation. In our study, the transcription of Pou5F1 eRNAs correlated with the pluripotent state, and transcription changed in parallel with Peblr20 lncRNA levels.
Recent studies suggest that eRNAs appear to function through three distinct mechanisms. First, eRNAs may facilitate chromosomal looping formation between enhancers and promoters. Chromatin interaction studies demonstrated that enhancers engaged in looping with promoters of protein-coding genes possess higher expression of eRNAs 74. Numerous studies 71, 75, 76 have suggested a potential role of eRNAs in the process of proper formation of chromosomal looping between enhancers and promoters. The potential role of eRNA in the modulation of chromosomal looping was also suggested by the observation that eRNAs could interact with the SMC3 (structural maintenance of chromosomes 3) and RAD21 (double-strand-break repair protein rad21 homolog) subunits of the cohesin complex, which has been shown to control enhancer-promoter looping in stem cells 76. Second, eRNAs play a role in the eviction of transcriptional repressors. A study 77 showed that eRNAs facilitated the transition of paused RNA polymerase II (RNAPII) into active elongation by acting as decoys for the negative elongation factor (NELF) complex upon induction of immediate early genes (IEGs) in neurons. Third, eRNAs recruit transcriptional activators. Mousavi et al 78 found that knockdown of eRNA from core enhancer of MyoD locus decreased RNA PolII recruitment at the promoter and gene body of MyoD, but not at the core enhancer itself, suggesting that eRNA transcripts facilitate RNA PolII recruitment to the promoter of the target gene. In this study, we show that Peblr20 recruits TET2 and facilitates eRNA transcription by inducing Pou5F1 enhancer demethylation.
Transcriptional-regulatory circuitries may exhibit notable differences between human and mouse pluripotent stem cells. For example, human endogenous retrovirus subfamily H (HERVH) can produce lncRNAs that are highly expressed in human pluripotent cells. HERVH lncRNAs interact with Pou5F1 and coactivators in the regulatory network of pluripotency 5, 6. By genome browser alignment, it appears that Peblr20 is conserved between mouse and rat, but not in human. Thus, Peblr20 may function as a rodent-specific chromatin lncRNA to maintain the pluripotency identity. Whether there are pluripotency-associated lncRNAs that function using a similar mechanism specifically in human would need to be answered in the future.
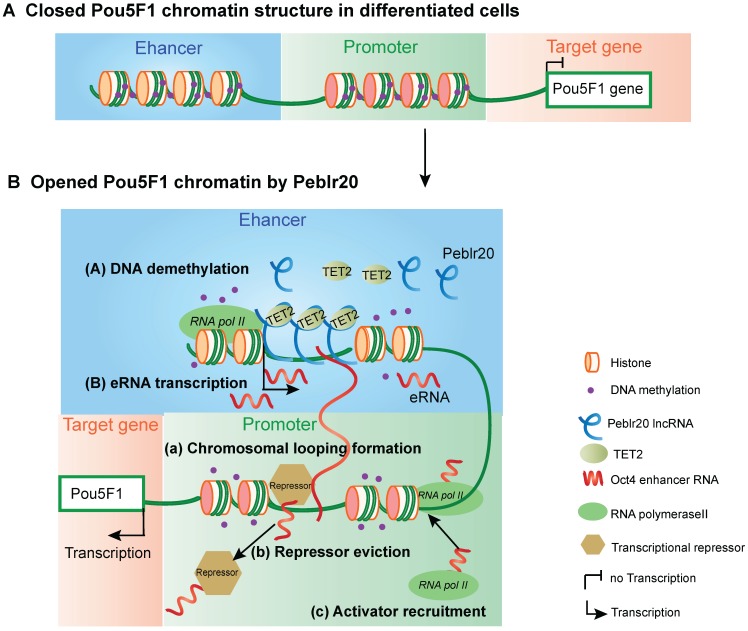
In conclusion, this study describes Peblr20 as a critical pluripotent lncRNA that functions by epigenetically activating the enhancer RNA pathway (Fig. 9). Peblr20 is specifically activated during the process of reprogramming. Once transcribed, Peblr20 interacts in trans with regulatory elements of multiple stemness genes. Peblr20 binds to the Pou5F1 enhancers and recruits TET2 demethylase, leading to the activation of the enhancer RNA (eRNA) pathway. Thus, Peblr20 utilizes a novel trans epigenetic eRNA mechanism to control the fate of stem cells.
Figure 9.
The putative model of Peblr20 in regulating pluripotency. (A) Dormant Pou5F1 gene locus in differentiated cells. The CpG islands of Pou5F1 enhancer and promoter region are methylated leading to closed chromatin structure. (B) Active Pou5F1 gene locus after Peblr20 overexpression. Overexpressed Peblr20 lncRNA recruits TET to Pou5F1 enhancer loci, causes enhancer demethylation, leads to active enhancers which can transcribe functional enhancer RNAs. Subsequently, eRNAs activate the promoter by facilitating loop formation of enhancer and promoter, eviction of transcriptional repressors and recruitment of transcriptional activators. eRNA: enhancer RNA, RNA pol II: RNA polymerase II.
Data availability
The RNA-seq data generated in this study have been deposited in NIH GEO databases with accession number GSE116605, including 1). PSC RNA-seq.fq.gz (GSM3243668, iPSC RNA sequencing fastq data); 2). FIB RNA-seq.fq.gz (GSM3243669, Fibroblast RNA sequencing fastq data). The RAT-seq data generated in this study have been deposited in NIH GEO databases with accession number GSE101765, including 1). Peblr20 RAT.fq.gz (Peblr20-specific primer RAT-seq library fastq data in iPSC); 2). PSC RAT Ct34.fq (GSM3244899, random control RAT-seq library fastq data in iPSC); 3). Palr35 RAT-seq.fq (GSM3244900, control lncRNA Palr35 RAT-seq library fastq data in iPSC). Palr35 was used as a control lncRNA as it is differentially expressed in reprogramming, but shows no interaction with core stem cell factor genes.
Acknowledgments
This work was supported by the National Key R&D Program of China (2018YFA0106902), National Natural Science Foundation of China (31430021, 81874052, 81672275, 31871297, 81670143, 81900701, 81900327), the Key Project of Chinese Ministry of Education grant (311015), the National Basic Research Program of China (973 Program)(2015CB943303), Nation Key Research and Development Program of China grant (2016YFC13038000), Research on Chronic Noncommunicable Diseases Prevention and Control of National Ministry of Science and Technology (2016YFC1303804), National Health Development Planning Commission Major Disease Prevention and Control of Science and Technology Plan of Action, Cancer Prevention and Control (ZX-07-C2016004), Natural Science Foundation of Jilin Province (20150101176JC, 20180101117JC, 20130413010GH), Provincial Science Fund of Jilin Province Development and Reform Commission (2014N147 and 2017C022), and California Institute of Regenerative Medicine (CIRM) grant (RT2-01942); and the Biomedical Research Service of the U.S. Department of Veterans Affairs (BX002905).
Supplementary Material
Supplementary figures and tables.
References
- 1.Neyrinck K, Breuls N, Holvoet B, Oosterlinck W, Wolfs E, Vanbilloen H. et al. The human somatostatin receptor type 2 as an imaging and suicide reporter gene for pluripotent stem cell-derived therapy of myocardial infarction. Theranostics. 2018;8:2799–813. doi: 10.7150/thno.22980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xiang M, Lu M, Quan J, Xu M, Meng D, Cui A. et al. Direct in vivo application of induced pluripotent stem cells is feasible and can be safe. Theranostics. 2019;9:290–310. doi: 10.7150/thno.28671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nichols J, Smith A. Pluripotency in the embryo and in culture. Cold Spring Harb Perspect Biol. 2012;4:a008128. doi: 10.1101/cshperspect.a008128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 5.Lu X, Sachs F, Ramsay L, Jacques PE, Goke J, Bourque G. et al. The retrovirus HERVH is a long noncoding RNA required for human embryonic stem cell identity. Nat Struct Mol Biol. 2014;21:423–5. doi: 10.1038/nsmb.2799. [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Xie G, Singh M, Ghanbarian AT, Rasko T, Szvetnik A. et al. Primate-specific endogenous retrovirus-driven transcription defines naive-like stem cells. Nature. 2014;516:405–9. doi: 10.1038/nature13804. [DOI] [PubMed] [Google Scholar]
- 7.Sherstyuk VV, Medvedev SP, Zakian SM. Noncoding RNAs in the Regulation of Pluripotency and Reprogramming. Stem Cell Rev. 2018;14:58–70. doi: 10.1007/s12015-017-9782-9. [DOI] [PubMed] [Google Scholar]
- 8.Chen L, Zhang S. Long noncoding RNAs in cell differentiation and pluripotency. Cell Tissue Res. 2016;366:509–21. doi: 10.1007/s00441-016-2451-5. [DOI] [PubMed] [Google Scholar]
- 9.Okazaki Y, Furuno M, Kasukawa T, Adachi J, Bono H, Kondo S. et al. Analysis of the mouse transcriptome based on functional annotation of 60,770 full-length cDNAs. Nature. 2002;420:563–73. doi: 10.1038/nature01266. [DOI] [PubMed] [Google Scholar]
- 10.Taft RJ, Pheasant M, Mattick JS. The relationship between non-protein-coding DNA and eukaryotic complexity. Bioessays. 2007;29:288–99. doi: 10.1002/bies.20544. [DOI] [PubMed] [Google Scholar]
- 11.Derrien T, Johnson R, Bussotti G, Tanzer A, Djebali S, Tilgner H. et al. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res. 2012;22:1775–89. doi: 10.1101/gr.132159.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flynn RA, Chang HY. Long noncoding RNAs in cell-fate programming and reprogramming. Cell Stem Cell. 2014;14:752–61. doi: 10.1016/j.stem.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du Z, Jia L, Wang Y, Wang C, Wen X, Chen J. et al. Combined RNA-seq and RAT-seq mapping of long noncoding RNAs in pluripotent reprogramming. Sci Data. 2018;5:180255. doi: 10.1038/sdata.2018.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang S, Wang Y, Jia L, Du Z, Wen X, Wang C. et al. Profiling the long noncoding RNA interaction network in the regulatory elements of target genes by chromatin in situ reverse transcription sequencing. Genome Res. 2019;29:1521–1532. doi: 10.1101/gr.244996.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen M, Zhang H, Wu J, Xu L, Xu D, Sun J. et al. Promotion of the induction of cell pluripotency through metabolic remodeling by thyroid hormone triiodothyronine-activated PI3K/AKT signal pathway. Biomaterials. 2012;33:5514–23. doi: 10.1016/j.biomaterials.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang A, Zhou N, Huang J, Liu Q, Fukuda K, Ma D. et al. The human long non-coding RNA-RoR is a p53 repressor in response to DNA damage. Cell Res. 2013;23:340–50. doi: 10.1038/cr.2012.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun J, Li W, Sun Y, Yu D, Wen X, Wang H. et al. A novel antisense long noncoding RNA within the IGF1R gene locus is imprinted in hematopoietic malignancies. Nucleic Acids Res. 2014;42:9588–601. doi: 10.1093/nar/gku549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H, Li W, Guo R, Sun J, Cui J, Wang G. et al. An intragenic long noncoding RNA interacts epigenetically with the RUNX1 promoter and enhancer chromatin DNA in hematopoietic malignancies. Int J Cancer. 2014;135:2783–94. doi: 10.1002/ijc.28922. [DOI] [PubMed] [Google Scholar]
- 20.Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biology; 2009. p. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Liu T, Meyer C, Eeckhoute J, Johnson D, Bernstein B. et al. Model-based analysis of ChIP-Seq (MACS) Genome Biol. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Machanick P, Bailey T. MEME-ChIP: motif analysis of large DNA datasets. Bioinformatics. 2011;27:1696–7. doi: 10.1093/bioinformatics/btr189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang H, Jiao W, Sun L, Fan J, Chen M, Wang H. et al. Intrachromosomal looping is required for activation of endogenous pluripotency genes during reprogramming. Cell Stem Cell. 2013;13:30–5. doi: 10.1016/j.stem.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 24.Broussau S, Jabbour N, Lachapelle G, Durocher Y, Tom R, Transfiguracion J. et al. Inducible packaging cells for large-scale production of lentiviral vectors in serum-free suspension culture. Mol Ther. 2008;16:500–7. doi: 10.1038/sj.mt.6300383. [DOI] [PubMed] [Google Scholar]
- 25.Zhang H, Zeitz MJ, Wang H, Niu B, Ge S, Li W. et al. Long noncoding RNA-mediated intrachromosomal interactions promote imprinting at the Kcnq1 locus. J Cell Biol. 2014;204:61–75. doi: 10.1083/jcb.201304152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhuang Q, Li W, Benda C, Huang Z, Ahmed T, Liu P. et al. NCoR/SMRT co-repressors cooperate with c-MYC to create an epigenetic barrier to somatic cell reprogramming. Nat Cell Biol. 2018;20:400–12. doi: 10.1038/s41556-018-0047-x. [DOI] [PubMed] [Google Scholar]
- 27.Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA. et al. Functional Demarcation of Active and Silent Chromatin Domains in Human HOX Loci by Noncoding RNAs. Cell. 2007;129:1311–23. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wernig M, Lengner CJ, Hanna J, Lodato MA, Steine E, Foreman R. et al. A drug-inducible transgenic system for direct reprogramming of multiple somatic cell types. Nat Biotechnol. 2008;26:916–24. doi: 10.1038/nbt1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun Q, Zhang Y, Yang G, Chen X, Zhang Y, Cao G. et al. Transforming growth factor-beta-regulated miR-24 promotes skeletal muscle differentiation. Nucleic Acids Res. 2008;36:2690–9. doi: 10.1093/nar/gkn032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiong K, Zhou Y, Blichfeld KA, Hyttel P, Bolund L, Freude KK. et al. RNA-Guided Activation of Pluripotency Genes in Human Fibroblasts. Cell Reprogram. 2017;19:189–98. doi: 10.1089/cell.2017.0006. [DOI] [PubMed] [Google Scholar]
- 31.Bae KB, Yu DH, Lee KY, Yao K, Ryu J, Lim DY. et al. Serine 347 Phosphorylation by JNKs Negatively Regulates OCT4 Protein Stability in Mouse Embryonic Stem Cells. Stem Cell Reports. 2017;9:2050–64. doi: 10.1016/j.stemcr.2017.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Banerji J, Rusconi S, Schaffner W. Expression of a beta-globin gene is enhanced by remote SV40 DNA sequences. Cell. 1981;27:299–308. doi: 10.1016/0092-8674(81)90413-x. [DOI] [PubMed] [Google Scholar]
- 33.Ito S, D'Alessio AC, Taranova OV, Hong K, Sowers LC, Zhang Y. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature. 2010;466:1129–33. doi: 10.1038/nature09303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hill PW, Amouroux R, Hajkova P. DNA demethylation, Tet proteins and 5-hydroxymethylcytosine in epigenetic reprogramming: an emerging complex story. Genomics. 2014;104:324–33. doi: 10.1016/j.ygeno.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 35.Dawlaty MM, Breiling A, Le T, Raddatz G, Barrasa MI, Cheng AW. et al. Combined deficiency of Tet1 and Tet2 causes epigenetic abnormalities but is compatible with postnatal development. Dev Cell. 2013;24:310–23. doi: 10.1016/j.devcel.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koh KP, Yabuuchi A, Rao S, Huang Y, Cunniff K, Nardone J. et al. Tet1 and Tet2 regulate 5-hydroxymethylcytosine production and cell lineage specification in mouse embryonic stem cells. Cell Stem Cell. 2011;8:200–13. doi: 10.1016/j.stem.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43:904–14. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fatica A, Bozzoni I. Long non-coding RNAs: new players in cell differentiation and development. Nat Rev Genet. 2014;15:7–21. doi: 10.1038/nrg3606. [DOI] [PubMed] [Google Scholar]
- 39.Batista PJ, Chang HY. Long noncoding RNAs: cellular address codes in development and disease. Cell. 2013;152:1298–307. doi: 10.1016/j.cell.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morlando M, Ballarino M, Fatica A, Bozzoni I. The role of long noncoding RNAs in the epigenetic control of gene expression. ChemMedChem. 2014;9:505–10. doi: 10.1002/cmdc.201300569. [DOI] [PubMed] [Google Scholar]
- 41.Wang KC, Yang YW, Liu B, Sanyal A, Corces-Zimmerman R, Chen Y. et al. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature. 2011;472:120–4. doi: 10.1038/nature09819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gong C, Maquat LE. lncRNAs transactivate STAU1-mediated mRNA decay by duplexing with 3' UTRs via Alu elements. Nature. 2011;470:284–8. doi: 10.1038/nature09701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guttman M, Donaghey J, Carey BW, Garber M, Grenier JK, Munson G. et al. lincRNAs act in the circuitry controlling pluripotency and differentiation. Nature. 2011;477:295–300. doi: 10.1038/nature10398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin N, Chang KY, Li Z, Gates K, Rana ZA, Dang J. et al. An evolutionarily conserved long noncoding RNA TUNA controls pluripotency and neural lineage commitment. Mol Cell. 2014;53:1005–19. doi: 10.1016/j.molcel.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Denzler R, Agarwal V, Stefano J, Bartel DP, Stoffel M. Assessing the ceRNA hypothesis with quantitative measurements of miRNA and target abundance. Mol Cell. 2014;54:766–76. doi: 10.1016/j.molcel.2014.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang P, Xue Y, Han Y, Lin L, Wu C, Xu S. et al. The STAT3-binding long noncoding RNA lnc-DC controls human dendritic cell differentiation. Science. 2014;344:310–3. doi: 10.1126/science.1251456. [DOI] [PubMed] [Google Scholar]
- 47.Yu B, Wang S. Angio-LncRs: LncRNAs that regulate angiogenesis and vascular disease. Theranostics. 2018;8:3654–75. doi: 10.7150/thno.26024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA. et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129:1311–123. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li YP, Wang Y. Large noncoding RNAs are promising regulators in embryonic stem cells. J Genet Genomics. 2015;42:99–105. doi: 10.1016/j.jgg.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 50.Feng S, Jacobsen SE, Reik W. Epigenetic reprogramming in plant and animal development. Science. 2010;330:622–7. doi: 10.1126/science.1190614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet. 2012;13:484–92. doi: 10.1038/nrg3230. [DOI] [PubMed] [Google Scholar]
- 52.Meissner A. Epigenetic modifications in pluripotent and differentiated cells. Nat Biotechnol. 2010;28:1079–88. doi: 10.1038/nbt.1684. [DOI] [PubMed] [Google Scholar]
- 53.Polo JM, Anderssen E, Walsh RM, Schwarz BA, Nefzger CM, Lim SM. et al. A molecular roadmap of reprogramming somatic cells into iPS cells. Cell. 2012;151:1617–32. doi: 10.1016/j.cell.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lister R, Pelizzola M, Dowen RH, Hawkins RD, Hon G, Tonti-Filippini J. et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature. 2009;462:315–22. doi: 10.1038/nature08514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stadler MB, Murr R, Burger L, Ivanek R, Lienert F, Scholer A. et al. DNA-binding factors shape the mouse methylome at distal regulatory regions. Nature. 2011;480:490–5. doi: 10.1038/nature10716. [DOI] [PubMed] [Google Scholar]
- 56.Cohen NM, Dighe V, Landan G, Reynisdottir S, Palsson A, Mitalipov S. et al. DNA methylation programming and reprogramming in primate embryonic stem cells. Genome Res. 2009;19:2193–201. doi: 10.1101/gr.096685.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tagoh H, Melnik S, Lefevre P, Chong S, Riggs AD, Bonifer C. Dynamic reorganization of chromatin structure and selective DNA demethylation prior to stable enhancer complex formation during differentiation of primary hematopoietic cells in vitro. Blood. 2004;103:2950–5. doi: 10.1182/blood-2003-09-3323. [DOI] [PubMed] [Google Scholar]
- 58.Hon GC, Rajagopal N, Shen Y, McCleary DF, Yue F, Dang MD. et al. Epigenetic memory at embryonic enhancers identified in DNA methylation maps from adult mouse tissues. Nat Genet. 2013;45:1198–206. doi: 10.1038/ng.2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.He YF, Li BZ, Li Z, Liu P, Wang Y, Tang Q. et al. Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA. Science. 2011;333:1303–7. doi: 10.1126/science.1210944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gu TP, Guo F, Yang H, Wu HP, Xu GF, Liu W. et al. The role of Tet3 DNA dioxygenase in epigenetic reprogramming by oocytes. Nature. 2011;477:606–10. doi: 10.1038/nature10443. [DOI] [PubMed] [Google Scholar]
- 61.Moran-Crusio K, Reavie L, Shih A, Abdel-Wahab O, Ndiaye-Lobry D, Lobry C. et al. Tet2 loss leads to increased hematopoietic stem cell self-renewal and myeloid transformation. Cancer Cell. 2011;20:11–24. doi: 10.1016/j.ccr.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dawlaty MM, Breiling A, Le T, Barrasa MI, Raddatz G, Gao Q. et al. Loss of Tet enzymes compromises proper differentiation of embryonic stem cells. Dev Cell. 2014;29:102–11. doi: 10.1016/j.devcel.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Doege CA, Inoue K, Yamashita T, Rhee DB, Travis S, Fujita R. et al. Early-stage epigenetic modification during somatic cell reprogramming by Parp1 and Tet2. Nature. 2012;488:652–5. doi: 10.1038/nature11333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Costa Y, Ding J, Theunissen TW, Faiola F, Hore TA, Shliaha PV. et al. NANOG-dependent function of TET1 and TET2 in establishment of pluripotency. Nature. 2013;495:370–4. doi: 10.1038/nature11925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hu X, Zhang L, Mao SQ, Li Z, Chen J, Zhang RR. et al. Tet and TDG mediate DNA demethylation essential for mesenchymal-to-epithelial transition in somatic cell reprogramming. Cell Stem Cell. 2014;14:512–22. doi: 10.1016/j.stem.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 66.Gao Y, Chen J, Li K, Wu T, Huang B, Liu W. et al. Replacement of Oct4 by Tet1 during iPSC induction reveals an important role of DNA methylation and hydroxymethylation in reprogramming. Cell Stem Cell. 2013;12:453–69. doi: 10.1016/j.stem.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 67.Kim TK, Hemberg M, Gray JM, Costa AM, Bear DM, Wu J. et al. Widespread transcription at neuronal activity-regulated enhancers. Nature. 2010;465:182–7. doi: 10.1038/nature09033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hah N, Danko CG, Core L, Waterfall JJ, Siepel A, Lis JT. et al. A rapid, extensive, and transient transcriptional response to estrogen signaling in breast cancer cells. Cell. 2011;145:622–34. doi: 10.1016/j.cell.2011.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang D, Garcia-Bassets I, Benner C, Li W, Su X, Zhou Y. et al. Reprogramming transcription by distinct classes of enhancers functionally defined by eRNA. Nature. 2011;474:390–4. doi: 10.1038/nature10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kaikkonen MU, Spann NJ, Heinz S, Romanoski CE, Allison KA, Stender JD. et al. Remodeling of the enhancer landscape during macrophage activation is coupled to enhancer transcription. Mol Cell. 2013;51:310–25. doi: 10.1016/j.molcel.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li W, Notani D, Ma Q, Tanasa B, Nunez E, Chen AY. et al. Functional roles of enhancer RNAs for oestrogen-dependent transcriptional activation. Nature. 2013;498:516–20. doi: 10.1038/nature12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ounzain S, Pezzuto I, Micheletti R, Burdet F, Sheta R, Nemir M. et al. Functional importance of cardiac enhancer-associated noncoding RNAs in heart development and disease. J Mol Cell Cardiol. 2014;76:55–70. doi: 10.1016/j.yjmcc.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cheng JH, Pan DZ, Tsai ZT, Tsai HK. Genome-wide analysis of enhancer RNA in gene regulation across 12 mouse tissues. Sci Rep. 2015;5:12648. doi: 10.1038/srep12648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sanyal A, Lajoie BR, Jain G, Dekker J. The long-range interaction landscape of gene promoters. Nature. 2012;489:109–13. doi: 10.1038/nature11279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lin YC, Benner C, Mansson R, Heinz S, Miyazaki K, Miyazaki M. et al. Global changes in the nuclear positioning of genes and intra- and interdomain genomic interactions that orchestrate B cell fate. Nat Immunol. 2012;13:1196–204. doi: 10.1038/ni.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kagey MH, Newman JJ, Bilodeau S, Zhan Y, Orlando DA, van Berkum NL. et al. Mediator and cohesin connect gene expression and chromatin architecture. Nature. 2010;467:430–5. doi: 10.1038/nature09380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schaukowitch K, Joo JY, Liu X, Watts JK, Martinez C, Kim TK. Enhancer RNA facilitates NELF release from immediate early genes. Mol Cell. 2014;56:29–42. doi: 10.1016/j.molcel.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mousavi K, Zare H, Dell'orso S, Grontved L, Gutierrez-Cruz G, Derfoul A. et al. eRNAs promote transcription by establishing chromatin accessibility at defined genomic loci. Mol Cell. 2013;51:606–17. doi: 10.1016/j.molcel.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures and tables.
Data Availability Statement
The RNA-seq data generated in this study have been deposited in NIH GEO databases with accession number GSE116605, including 1). PSC RNA-seq.fq.gz (GSM3243668, iPSC RNA sequencing fastq data); 2). FIB RNA-seq.fq.gz (GSM3243669, Fibroblast RNA sequencing fastq data). The RAT-seq data generated in this study have been deposited in NIH GEO databases with accession number GSE101765, including 1). Peblr20 RAT.fq.gz (Peblr20-specific primer RAT-seq library fastq data in iPSC); 2). PSC RAT Ct34.fq (GSM3244899, random control RAT-seq library fastq data in iPSC); 3). Palr35 RAT-seq.fq (GSM3244900, control lncRNA Palr35 RAT-seq library fastq data in iPSC). Palr35 was used as a control lncRNA as it is differentially expressed in reprogramming, but shows no interaction with core stem cell factor genes.