Abstract
Accumulating evidence suggests that natural medicines have notable curative effects on neurological conditions, such as migraine, that are mediated by regulating the gut microbial flora. A natural medicine pair used in traditional Chinese medicine, Gastrodia elata Blume and Uncaria rhynchophylla (Miq.) Miq. ex Havil. (GU), have shown excellent effect in treating migraine, yet the role of gut microbes in the therapeutic effect of GU in chronic migraine (CMG) is unknown. Here, we performed a 16S rRNA gene sequencing and metabolomics study of the effects of GU in a nitroglycerin (NTG)-induced rat model of CMG. Our results showed that the gut microbial community structure changed significantly and was similar to that of control rats after GU administration in CMG rats. Specifically, GU increased the relative abundance of Bacteroides and Coprococcus and reduced the abundance of Prevotella_1 and Escherichia-Shigella in CMG rats. The metabolomics profiles of the plasma and ileum contents of CMG rats obtained with an ultra-performance liquid chromatography-mass spectrometer (UPLC-MS) revealed similar biomarkers in both samples, and GU treatment reduced 3-indoxyl sulfate, glutamic acid, L-tyrosine, and L-arginine levels, and increased 5-HIAA, L-tryptophan, and linoleic acid levels in plasma. Correlation analysis showed that the affected bacteria were closely related to amino acid metabolism. Most importantly, GU treatment hardly affected biomarkers in feces samples after inhibiting the activity of gut microbes. Collectively, these findings indicate that structural changes in gut flora are closely related to host metabolism and that regulating the gut microbial community structure and function may be one of the important mechanisms underlying the therapeutic effects of GU in migraine.
Keywords: migraine, Gastrodia-Uncaria, 16S rRNA-seq, plasma metabolomics, pharmacodynamic
Introduction
Migraine, a severe type of headache, is a common chronic neurovascular disorder with recurrent episodes that seriously affects the lives of patients [Headache Classification Committee of the International Headache Society (IHS), 2013]. Though anti-inflammatory drugs, ergot-type preparations, triptans, and calcitonin gene-related peptide (CGRP) receptor antagonists have shown therapeutic effects on migraine in the clinic (Lipton et al., 2000; Olesen et al., 2004), their clinical use has been limited by their side-effects, such as cardiovascular and gastrointestinal disorders (Katsarava et al., 2018), which calls for a rethink of the therapeutic strategy for migraine. Recently, several studies have shown that disturbances of intestinal flora may be associated with neurological disorders, including migraine headache (Foster et al., 2016; Tang et al., 2019). The dysbiosis of gut flora could affect the brain–gut axis, which would promote neurotransmitter disorders, and this appears to be true for migraine-associated neurotransmitters (Grover and Kashyap, 2014).
Natural products, food supplements, and traditional medicine (TM) have been shown to have therapeutic benefits on migraine (Al-Hashel et al., 2018). Gastrodia elata Blume and Uncaria rhynchophylla (Miq.) Miq. ex Havil. (GE and UR, [GU]) are the main herbs in the GU decoction, which has shown significant efficacy in vascular headache (Jiang, 2007). The prescription preparation, GU granules, in particular, are commonly used for treating neurological conditions such as migraine (Zhang et al., 2017). Extracts of GE and UR have been reported to exert neuroprotective effects (Xian et al., 2016; Liu et al., 2018); however, the oral absorption bioavailability of GE and UR extract is poor (Ge et al., 2014). Many compounds, in particular, natural products taken orally, reach the intestine and are readily metabolized by gut flora. In general, they are decomposed into metabolites with polarity and lower molecular weight, thus having higher bioavailability. Therefore, to study the mechanism of GU in treating migraine, we need to study the function of intestinal flora and metabolism in vivo. In this study, omics synthesis was applied to study the treatment mechanism of GU on migraine (Lionetto et al., 2013; Gasparini et al., 2017).
Recently, several studies have indicated that DNA sequence variants affect the risk of migraine subtypes (Sutherland et al, 2019; van den et al, 2019; Grieco et al, 2018), and 16S rRNA gene sequencing and metabolomics have been used as effective tools for studying disease and drug treatment mechanisms (Estruel-Amades et al., 2019). 16S rRNA gene sequencing studies can accurately identify the structure and metabolic functions of the gut microbe. Metabolomics technology provides a unique and novel technique for disease and pharmacodynamics characterization that facilitates systematic analysis of metabolic pathways and related metabolites (Zhang et al., 2018). In recent years, many diseases and drug interventions have been found to cause changes in the structure of the gut microbial flora. Metabolomics techniques have been extensively used in studies of disease and drug mechanisms. However, the mechanism by which GU affects host plasma metabolism through gut microbes remains to be clarified.
This study aims to investigate the effect of GU administration on intestinal flora and biomarkers in migraine rats and to explore the potential linkage. To this end, we probed the changes in gut microbiota, plasma biomarkers, and intestinal biomarkers after GU administration and investigated the possible links between them. The differences in gut microbial community structure and plasma metabolomics were analyzed by 16S rRNA sequencing technology and LC/MS analysis, respectively. In particular, we conducted validation tests consisting of short-term in vitro incubation of feces with the drug to prove the effect of GU on the metabolism of the flora. These studies provide a more comprehensive and detailed understanding of the mechanism by which GU mediates therapeutic effects in NTG-induced chronic migraine by regulating the host gut microbial flora.
Materials and Methods
Chemicals and Drugs
HPLC grade methanol and acetonitrile were obtained from Fisher Scientific (Fair Lawn, NJ, USA). Pure distilled water was purchased from Wahaha (Hangzhou, China). HPLC grade formic acid was obtained from Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China. 2-Chloro-L-phenylalanine, which was used as the internal standard (IS), was provided by Shanghai Macklin Biochemistry Company, Shanghai, China. Standard 3-indoxyl sulfate was obtained from Beijing LeBo Biotech Co., Ltd., Beijing, China. L-Arginine, glutamic acid, L-valine, linoleic acid, L-phenylalanine, L-tryptophan, and L-tyrosine were purchased from Shanghai Yuanye Biotechnology Co. Ltd., Shanghai, China.
Extraction Methods
The pieces of GE and UR used (purchased from Jiangxi Jiangzhong Chinese Herbal Pieces Co. Ltd.) accorded with the standard in the Chinese Pharmacopoeia. To prepare the GU extract, GU (exact amounts of GE and UR in a weight ratio of 9:12) was extracted by reference to the boiling steps for GU Granules in Volume I of the Pharmacopoeia of the People’s Republic of China (Edition 2015). The extraction process was that herbs were decocted twice with water and filtered, then the decoctions were merged, and the filtrate was concentrated. The detailed method is shown in Figure S1 . The concentrated extract was dried in a water bath at 60°C. The final yield was powdered and then stored in a desiccator at room temperature. Water extracts of GU were analyzed with a Waters ACQUITY UPLC System (Waters Corp. Milford, USA). The extract was dissolved in water and then filtered through a 0.22-µm filter before being separated in an Acquity UPLC HSS T3 column (100 mm × 2.1 mm, 1.8 µm) at 40°C. The mobile phase was composed of 0.1% formic acid water (A) and acetonitrile (B), and the gradient program was as follows: 0–3 min, 2% B; 3–9 min, 2–12% B; 9–24 min, 12–32% B; 24–29 min, 32–75% B; 29–29.1 min, 75–100% B; 29.1–32 min, 100% B. The flow rate was 0.3 mL/min, and the injection volume was 1 µL. To determine the composition of the compounds in the GU extract, the effluent was introduced into an MS for mass spectrometry analysis, using a Waters SYNAPT G2 system with an electrospray ionization (ESI) source operating in both positive and negative ion modes. Briefly, the parameters of the positive ion mode were set as follows: capillary voltage, 4.0 kV; source temperature, 120°C; cone gas rate, 40 L/h; desolvation gas rate and temperature, 800 L/h and 400°C; cone voltage, 19 V; collision energy 25–40 V; scan time and inter-scan delay, 0.15 and 0.02 s. The parameters used in the negative ion mode were the same as those used in the positive ion mode except: capillary voltage, 2.5 kV; cone voltage, 25 V. The data were collected from m/z 50 to 1,200 Da.
Animals and Treatments
Specific pathogen-free (SPF) female Sprague–Dawley rats (weight: 220 ± 20 g, purchased from the Hunan Slack Jingda Laboratory Animal Co., Ltd., Hunan, China) were acclimated for 5 days under standard laboratory conditions at Jiangxi University of Traditional Chinese Medicine, with free access to food and water. Subsequently, 60 rats were randomly divided into Control, Model, Sumatriptan, and GU groups. Rats in the Sumatriptan and GU groups were orally administered Sumatriptan (25 mg/Kg/d) or GU (4 g/Kg/d), respectively, for 10 days. The dose was determined by the pre-experiment results. Rats in the Control and Model groups received distilled water orally. At the same time, rats in the Control group rats received saline (intraperitoneal [i.p.] on days 3, 5, 7, and 9), while the other groups were injected with NTG (10 mg/Kg, i.p.) (Farajdokht et al., 2018).
All experimental procedures used in this study were based on the ethical principles for laboratory animals of the State Key Laboratory (Reference number: BCTG-2016-18).
Sample Collection and Preparation
One hour after the final oral administration, feces samples were collected in sterile conical tubes and stored at -80°C. Subsequently, all rats in each group were anesthetized (i.p.) with 10% chloral hydrate, blood samples were taken from the abdominal aorta and collected in heparin-sodium tubes, and intestinal lavage fluid was collected by instilling the intestines with 30% ethanol. Plasma and intestinal lavage fluid samples were collected by centrifugation (4°C, 5,702 g, 5 min) and stored at -80°C prior to analysis.
16S rRNA Microbial Community Analysis
The differences in the gut microbial communities between the Control and Model groups were determined by collecting fecal samples from 10 randomly selected rats in each group for 16S rRNA gene analysis. Total DNA was extracted using the E.Z.N.A. Stool DNA kit (Omega Bio-Tek, Norcross, GA, USA).
The V3–V4 region of the bacterial 16S rRNA gene was polymerase chain reaction (PCR)-amplified using the following primers: 338F 5’ -ACTCCTACGGGAGGCAGCA-3’ and 806R 5’- GGACTACHVGGGT WTC TAAT-3’. PCR was performed under the following conditions: 95°C for 3 min, followed by 27 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 45 s, with a final step at 72°C for 10 min. Amplification was confirmed by 2% agarose gel electrophoresis. PCR products were purified with the AxyPrep DNA kit (AXYGEN, Tewksbury, MA, USA) and sequenced on the Illumina HiSeq platform. The PE reads obtained by MiSeq sequencing were first spliced according to the overlap relationship, and the quality of the sequences was controlled and filtered simultaneously. After distinguishing the samples, community bar plot analysis was performed at the phylum and genus levels. β-Diversity was estimated by the Bray–Curtis distance algorithm and visualized by principal coordinate analysis (PCoA). The Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) was used to predict function and obtain COG family information corresponding to Operational Taxonomic Units (OUT). The description and function of each COG were searched in the eggNOG database to obtain a functional abundance spectrum. The data were analyzed on the free online Majorbio I-Sanger Cloud Platform (www.i-sanger.com).
Plasma and Intestinal Lavage Fluid Metabolomics
Sample Preparation
A working internal standard (IS) solution of 2-chloro-l-phenylalanine (10 µg/mL) was prepared in methanol. Plasma samples (50 µL) were added into 200 µL working IS solution. The mixture was vortexed for 3 min and centrifuged (17,108 g, 15 min, 4°C). After centrifugation, the supernatant was collected and stored at -20°C. Intestinal lavage fluid samples (5 mL) were condensed to half the original volume by nitrogen flow. Concentrated samples (200 µL) were almost completely dried under nitrogen flow, then re-dissolved in IS solution (200 µL), vortexed and centrifuged (17,108 g, 15 min, 4°C). The collected supernatant was analyzed by UPLC-Q-TOF/MS. Furthermore, significant differences between groups were analyzed based on community abundance data.
UPLC-Q-TOF/MS Analysis
Chromatographic analysis was performed on the Waters ACQUITY UPLC System (Waters Corp. Milford, USA). The samples were separated in an Acquity UPLC HSS T3 column (100 mm × 2.1 mm, 1.8 µm) at 40°C and a flow rate of 0.35 mL/min with a mobile phase composed of 0.1% formic acid water (A) and acetonitrile (B). The gradient program for plasm samples was optimized as follows: 0–0.2 min, 5% B; 0.2–3 min, 5% B to 20% B; 3–5 min, 20% B to 45% B; 5–7 min, 45% B to 55% B; 7–13 min, 55% B to 65% B; 13–16min, 55% B to 65% B; 16–21min, 65% B to 80% B; 21–23min, 80% B to 95% B; and 23–26 min, equilibration with 5% B. The gradient program for intestinal lavage fluid samples was optimized as follows: 0–0.5 min, 1% B; 0.5–3 min, 1% B to 30% B; 3–8 min, 30% B to 40% B; 8–20 min, 40% B to 60% B; 20–22 min, 60% B to 99%, 22–23 min, washing with 99% B, and 23–26 min, equilibration with 1% B. Mass spectrometry analysis was performed using the Waters SYNAPT G2 system with an ESI source operating in both positive and negative ion modes. Briefly, the parameters of the positive ion mode were set as follows: capillary voltage, 4.0 kV; source temperature, 120°C; cone gas rate, 40 L/h; desolvation gas rate and temperature, 800 L/h and 400°C; cone voltage, 19 V; collision energy 20–40 V; scan time and inter-scan delay, 0.15 and 0.02 s. The parameters used in the negative ion mode were the same as those used in the positive ion mode except: capillary voltage, 2.5 kV; cone voltage, 25 V. For quality calibration, leucine-enkephalin (0.5 µg/mL) was used as the lockmass in all analyses ([M+H]+ = 556.2771, [M-H]- = 554.2615) with a flow rate of 5 µL/min. Profile data were collected in MSE mode from m/z 50 to 1,250 Da.
Method Validation
To ensure the stability and repeatability of our sequence analysis, equal volumes (10 µL) of each plasma sample and intestinal lavage fluid sample were pooled as quality control (QC) samples and then prepared in the same way as the test samples. The QC sample was injected every 10 samples during the batch analysis. Subsequently, 10 typical peaks (including the IS peak) were extracted for method validation. The relative standard deviation (R.S.D.%) values of the intensity are shown in Tables S1 , S2 , and the base peak intensity (BPI) chromatograms of the QC samples showed good concordance ( Figures S2 and S3 ).
Data Processing and Multivariate Analysis
All of the raw LC-MS files of the Control and Model groups were imported to Progenesis QI V 2.0 software for data processing. The converted files were subjected to alignment, peak-picking, and deconvolution. The filtered data were exported to EZinfo 3.0 for principal component analysis (PCA) and orthogonal partial least square discriminate analysis (OPLS-DA). Combined VIP-plots (VIP >1) were generated from the OPLS analysis to select distinct variables as potential markers. Tags (VIP >1 and P < 0.05) were established to screen and identify differentially expressed compounds. ChemSpider (http://www.chemspider.com/), the Human Metabolome Database (HMDB, http://www.hmdb.ca/), and the Kyoto Encyclopedia of Genes and Genomes (KEGG, http://www.kegg.jp/) database were selected for metabolite identification based on a combination of database queries using the exact mass measurements (mass error <5 ppm) and MS/MS patterns measured by the Q-TOF platform. Other parameter settings were designated as default for automatic data processing.
Thermal Sensitivity Test
Thermal sensitivity tests were performed at 1, 3, 5, 7, and 9 days between 10:00 a.m. and 12:00 p.m. in a quiet room using the ZH-200 thermal stabbing pain instrument (Anhui Zhenghua Instrument Equipment Co., Ltd.). All animals were acclimatized to the test room. After the rats stop moving, the right hind paw of rats was stimulated by infrared radiation stimulated with a cut-off time for 15 s to prevent burns. When the rats lifted or licked the hind foot, the light was switched off manually, and the duration of the stimulus was recorded. This procedure was repeated three times (at 5-min intervals) for each rat, and the average value was calculated as the photorespiration threshold time.
Determination of Plasma Biochemical Indicators
The plasma concentrations of calcitonin gene-related peptide (CGRP), endothelin 1 (ET 1), serotonin (5-HT), and nitric oxide (NO) were determined using a rat-specific enzyme-linked immunosorbent assay (ELISA) kit and NO assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China) according to the manufacturer’s instructions. All samples were tested in duplicate.
Co-Incubation of Feces and GUW In Vitro
To verify that the in vivo effects of GUW on metabolism are mediated via intestinal bacteria, we collected feces from rats in the Control and Model groups. Samples were divided into four groups (control group (CG), model group (MG), model+GUW group (MGG), and model +GUW+ antibiotic group (MGA); n = 4 samples per group). Stool samples were weighed and added to Eppendorf (EP) tubes. Three volumes of water were added to the feces samples of CG and MG. GUW (2 mg/mL) was added to the MGG samples. GUW (2 mg/mL) and antibiotics (Metronidazole, 2 mg/L, Gentamicin Sulfate, 4 mg/L, and Ampicillin, 1 mg/L) were added to fecal samples of MGA. The samples were placed in a strong plastic anaerobic box, and an anaerobic gas bag was placed in the box to simulate an anaerobic environment. The box was then placed in a Constant Temperature Foster Box (Sanyo Electric Co., Ltd., Japan). The samples were incubated in the anaerobic box at 37°C for 24 h. They were then centrifuged (7,128 g, 5 min, 4°C), and the supernatants were collected and stored at -20°C.
Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics 21.0 (Chicago, USA). Differences between groups were evaluated by one-way analysis of variance (ANOVA). Multi-omics data were analyzed by Pearson’s correlation analysis. The significance threshold was set at P < 0.05 for all tests.
Results
Identification of Chemical Constituents in Extracts of Gastrodia-Uncaria
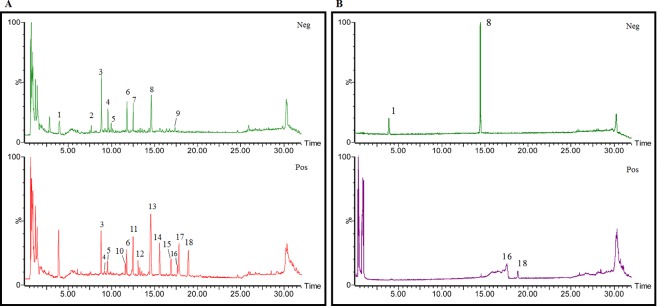
The total ion chromatogram (TIC) profiles of the compounds are shown in Figure 1 , and detailed information on these chemical compounds is given in Table 1 . Eighteen compounds were identified in the GU extract, of which four major active ingredients, gastrodin, parishin A, rhynchophylline, and isorhynchophylline, were identified using standards (Herbpurify Co., Ltd, Chengdu, China) and others were identified by examining their characteristic product ions. Peak 8 was identified as Parishin A by comparison with the standard and observing its fragment ions at m/z727.2248, 441.1115, and 423.0990. Similar to Peak 8, Peaks 3, 6, and 7 were identified as Parishin E, Parishin B, and Parishin C, respectively (Li et al., 2015). Peak 14 presented a characteristic product ion [M+H]+ at m/z 385.2134. It was identified as rhynchophylline by using standards, and the main fragment ions included m/z353.1861, 267.1454, 241.1368, 187.0890, and 160.0741. The characteristic ions of Peaks 12, 14, 15, 17, and 18 were similar to Peak 14, and these peaks were identified as demethyl rhynchophylline, demethyl isorhynchophylline, dehydrogen rhynchophylline, dehydrogen isorhynchophylline, and isorhynchophylline (Pan et al., 2017).
Figure 1.
UPLC-Q-TOF chromatograms of GU extraction and Standers (A); Standers (B).
Table 1.
Identification of the main components in GU extract based on UPLC-Q-TOF-MS.
| NO | Name | Formula | tR | Mass Observed | Mass Errol (ppm) | Fragment ions | Structure |
|---|---|---|---|---|---|---|---|
| 1 | Gastrodin | C13H18O7 | 3.87 | 331.1020 [M+COOH]- | -0.3 | 123.0440 | 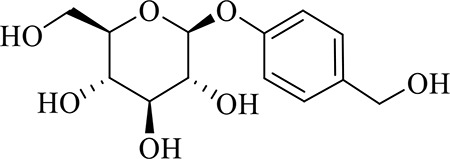 |
| 2 | 5-caffeoylquinic acid | C16H18O9 | 7.63 | 353.0844 [M-H]- | -4.5 | 191.0593, 179.0350, 173.0487, 135.0433 | 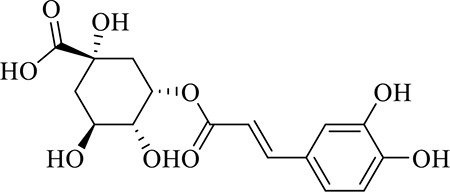 |
| 3 | Parishin E | C19H24O13 | 8.84 | 459.1150 [M-H]- | 3.7 | 173.0085, 190.9929, 123.0440 | 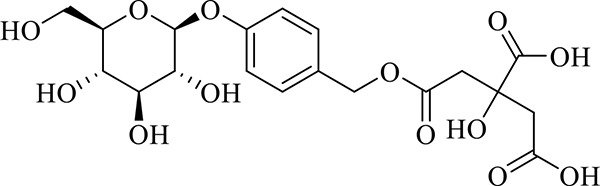 |
| 4 | 3-caffeoylquinic acid | C16H18O9 | 9.58 | 353.0844 [M-H]- | -4.5 | 191.0593, 179.0350, 173.0487, 161.0266 | 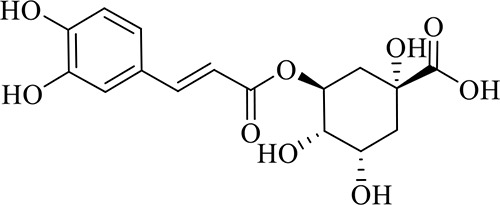 |
| 5 | 4-caffeoylquinic acid | C16H18O9 | 9.95 | 353.0844 [M-H]- | -4.5 | 191.0593, 179.0350, 173.0487, 161.0266, 135.0484 | 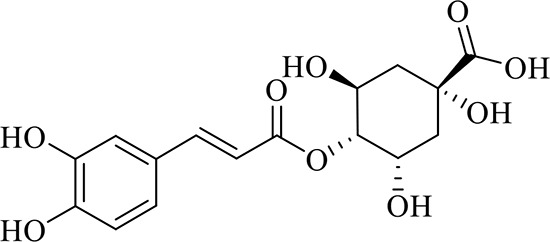 |
| 6 | Parishin B | C32H40O19 | 11.80 | 727.2087 [M-H]- | 0.6 | 441.1115, 423.0990, 161.0487 | 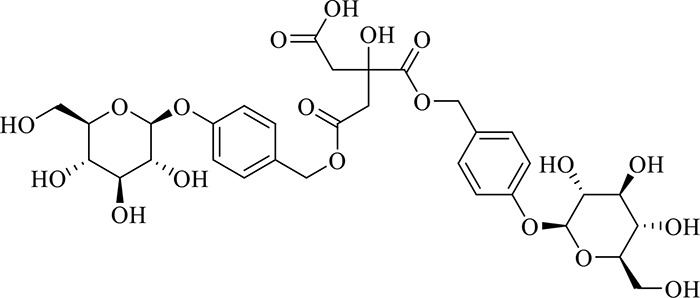 |
| 7 | Parishin C | C32H40O19 | 12.48 | 727.2087 [M-H]- | 0.6 | 441.1115, 423.0990, 161.0487 | 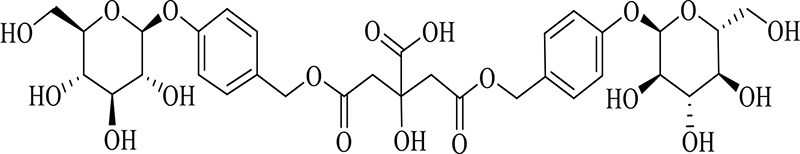 |
| 8 | Parishin A | C25H56O25 | 14.58 | 995.3038 [M-H]- | 0.7 | 727.2248, 441.1115, 423.0990 | 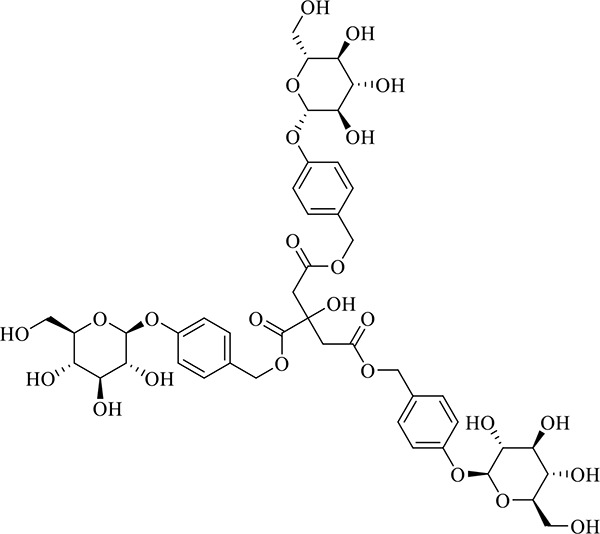 |
| 9 | 4,5-dicaffeoylquinic acid | C25H24O12 | 17.34 | 515.1204 [M-H]- | 1.3 | 353.0944, 335.0769, 191.0593, 179.0350, 173.0487, 135.0484 | 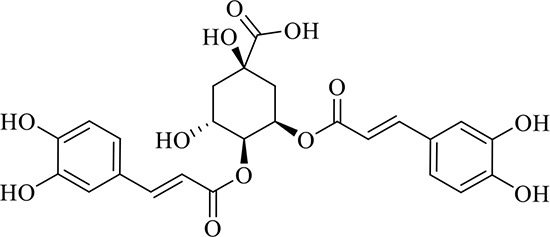 |
| 10 | Strictosidine | C27H34N2O9 | 11.58 | 531.2352 [M+H]+ | 1.9 | 369.1826, 301.1386, 267.1454, 193.0497, 160.0741 | 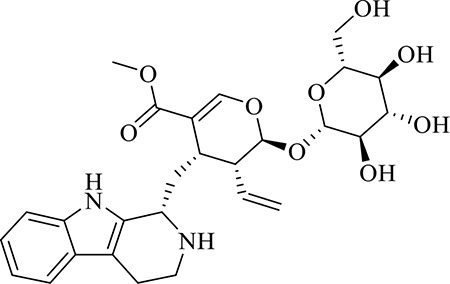 |
| 11 | uncarine A | C21H24N2O4 | 12.45 | 369.1826 [M+H]+ | 3.6 | 351.1706, 267.1525, 160.0741 | 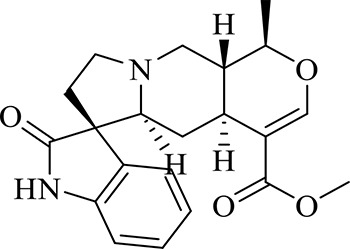 |
| 12 | Demethyl Rhynchophylline | C21H26N2O4 | 13.07 | 371.1978 [M+H]+ | 2.3 | 267.1454, 241.1368, 187.0890, 160.0741 | 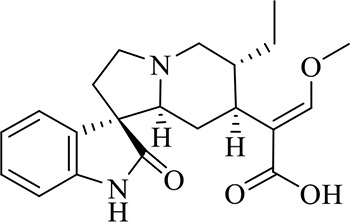 |
| 13 | uncarine C | C21H24N2O4 | 14.55 | 369.1826 [M+H]+ | 3.6 | 367.1525, 192.1027, 160.0741 | 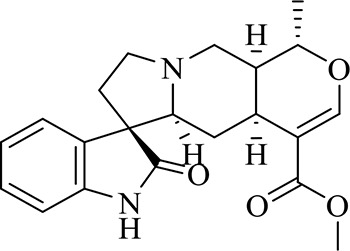 |
| 14 | Demethyl Isorhynchophylline | C21H26N2O4 | 15.57 | 371.1978 [M+H]+ | 2.3 | 269.1616, 353.1861, 241.1368, 160.0741 | 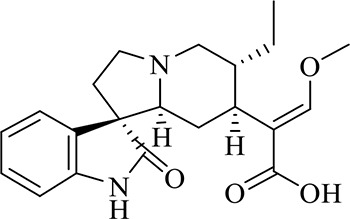 |
| 15 | Dehydrogen Rhynchophylline | C22H26N2O4 | 16.87 | 383.1967 [M+H]+ | -1.1 | 351.1706, 267.1454, 187.0890, 160.0741 | 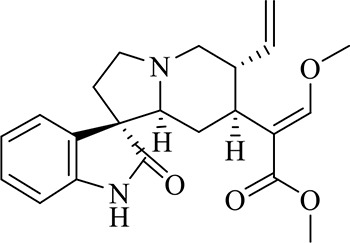 |
| 16 | Rhynchophylline | C22H28N2O4 | 17.70 | 385.2134 [M+H]+ | 2.5 | 353.1861, 267.1454, 241.1368, 187.0890, 160.0741 | 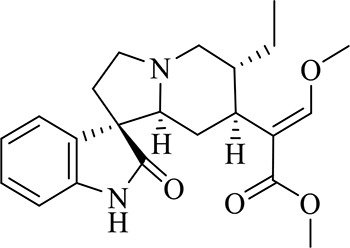 |
| 17 | Dehydrogen Isorhynchophylline | C22H26N2O4 | 17.79 | 383.2053 [M+H]+ | 2.1 | 351.1706, 267.1525, 160.0741 | 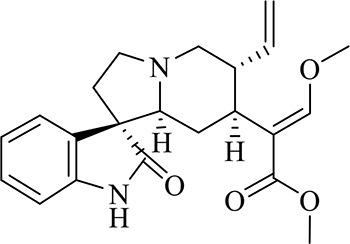 |
| 18 | Isorhynchophylline | C22H28N2O4 | 18.87 | 385.2134 [M+H]+ | 2.5 | 353.1861, 269.1616, 241.1300, 160.0741 | 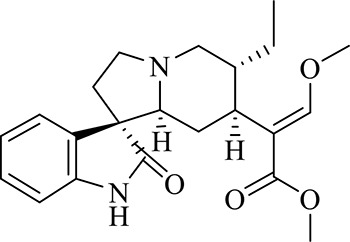 |
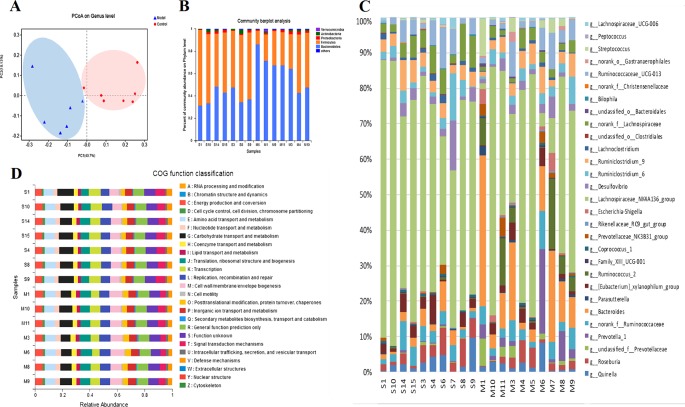
Gut Microbiota Disorder in NTG-Induced CMG
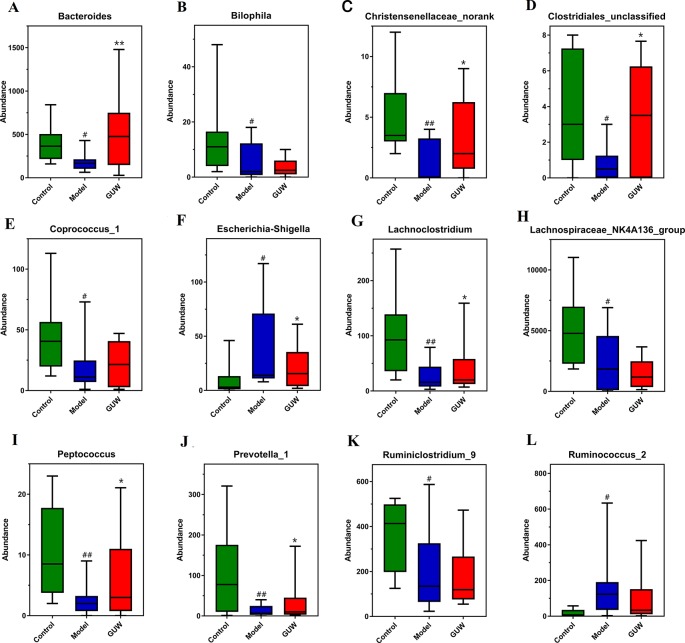
The principal co-ordinate analysis (PCoA) of the Bray–Curtis genus horizontal distance showed significant separation between the Control and Model groups ( Figure 2A ); the first principal coordinate explained 40.7% of the variation, and the second principal coordinate explained 18.13% of the variation. Identification at the phylum level showed that the proportion of Bacteroidetes and Firmicutes exceeded 90%. Compared to the Control group, the abundance of Firmicutes in the Model group decreased significantly (P < 0.05), whereas the abundance of Bacteroides increased ( Figure 2B ). In addition, comparisons of the Model and Control groups revealed statistically significant differences among the 30 genus-level bacteria (P < 0.05, Mann-Whitney U test) ( Figure 2C ). In migraine model rats, metagenomic data indicated increases in the relative abundance of Prevotella_1, Prevotellaceae_unclassified, Ruminococcaceae_norank, Bacteroides, Parasutterella, Bacteroidales_unclassified, Family_XIII_UCG-001, Gastranaerophilales_norank, Lachnospiraceae_UCG-006, Ruminococcus_2, Prevotellaceae_NK3B31_group, Rikenellaceae_RC9_gut_group, Streptococcus, Escherichia-Shigella, and decreases in the relative abundance of other differential intestinal bacteria, such as Desulfovibrio, Ruminococcaceae_UCG-013, Coprococcus_1, Lachnospiraceae_NK4A136_group, Christensenellaceae_norank, Ruminiclostridium_6, Ruminiclostridium_9, Bilophila, Lachnospiraceae_norank, Lachnoclostridium, Clostridiales_unclassified, and Peptococcus.
Figure 2.
(A) The gut microbiota patterns of Control group and Model group differentiated by PCoA. The gut microbiota composition profiles at the phylum (B) and genus (C) levels in the Control and Model group as revealed by 16S rRNA gene sequencing; each color represents one bacterial phylum or genus. The x-axis represents different samples. The y-axis represents the percentage abundance of bacteria in each sample. (D) Abundances of PICRUSt-inferred function in the Control and Model samples.
Prediction of function using the Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) indicated that the main changes occurred in the amino metabolism, energy metabolism, and lipid metabolism pathways. The clusters of orthologous groups (COG) function classification is shown in Figure 2D . Compared with the Control group, the abundance of defense mechanisms, energy production and conversion, and amino acid transport and metabolism were decreased in the CMG group.
Plasma Metabolic Profile of the Rat CMG Model and Identification of Potential Metabolite Biomarkers
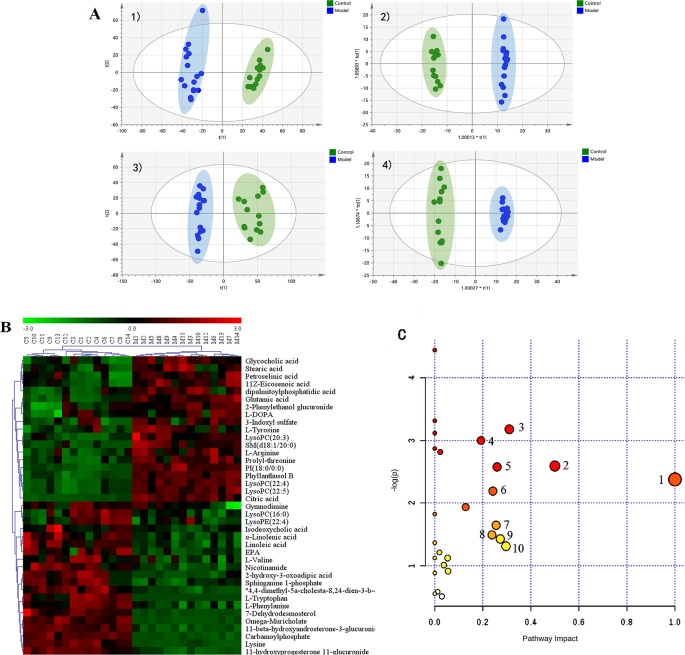
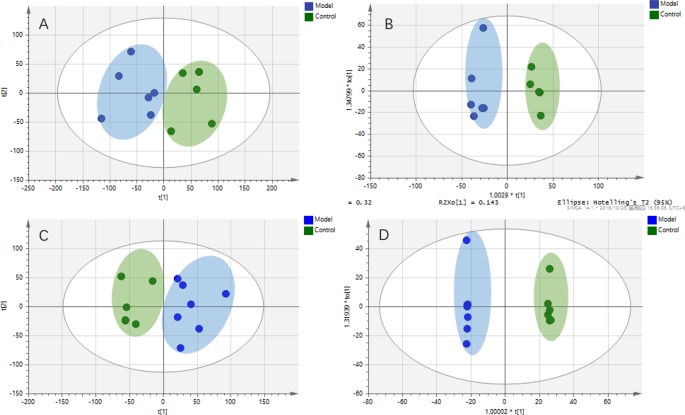
The metabolic profiles of plasma samples in each group were analyzed by UPLC-Q-TOF-MS/MS in both the positive and negative ion modes. All the groups tested were discriminated in the principal component analysis (PCA) model and orthogonal partial least square discriminate analysis (OPLS-DA), and a separation between the CMG and Control groups was clearly shown both in the PCA score plot and the OPLS-DA analysis ( Figure 3A ). Furthermore, the parameters obtained in the permutation of 200 tests indicated that the OPLS-DA models were reliable, with good predictability ( Figure S4 ).
Figure 3.
Metabolic profiles of plasma samples in rats with chronic migraine induced by nitroglycerin (A) 1) PCA score plot in negative mode; 2) OPLS-DA score plot in negative mode (R2Y = 0.997, Q2Y = 0.98); 3) PCA score plot in positive mode; 4) OPLS-DA score plot in positive mode (R2Y = 0.995, Q2Y = 0.973). (B) Significant changes in plasma metabolites are expressed as a heatmap showing metabolite changes in the Control (C) and Model (M) treatment groups, detected by UPLC-Q-TOF/MS. (C) Overview of metabolic pathway analysis: 1. Linoleic acid metabolism 2. Phenylalanine, tyrosine, and tryptophan biosynthesis 3. Tryptophan metabolism 4. Arginine and proline metabolism 5. Alanine, aspartate, and glutamate metabolism 6. Glycerophospholipid metabolism 7. Tyrosine metabolism 8. Nicotinate and nicotinamide metabolism 9. Pentose and glucuronate interconversions 10. Glyoxylate and dicarboxylate metabolism.
The OPLS-DA method was used to refine the separation between the CMG and Control groups already established via PCA. Dissimilar metabolites (VIP >1) were then imported into the QI program and tagged as contributive variables. Those filtered metabolites were selected to create a new quick tag (ANOVA P < 0.05). Metabolites that satisfied the parameters of VIP >1 and P < 0.05 were identified using the online databases (e.g., HMDB, ChemSpider, KEGG) with Progenesis QI, and the identification results were then exported. The accurate mass and MSE spectral measurements of the identification results, collected by UHPLC-Q-TOF/MS, were then matched with standards ( Figure S5 ), and the identification was confirmed using the UNIFI Scientific Information System. In comparisons of the CMG Model and Control groups, 14 metabolites showed significantly different levels in the positive ion mode and 26 in the negative ion mode ( Table 2 ). Differentially expressed biomarkers mainly consisted of indoles, carbohydrates, amines, organic acids, amino acids, neurotransmitters, bile acids, free fatty acids, and lipids. Variation in the identified plasma biomarkers related to NTG-induced CMG is depicted in the heatmap for each treatment group ( Figure 3B ). All 40 potential biomarkers were subjected to metabolic pathway analysis (MetPA) using the KEGG online database and MetaboAnalyst 3.0 (Xia and Wishart, 2010). An overview of the pathway analysis shown in Figure 3C reflects the metabolic network associated with the CMG model. For the rat plasma samples, the metabolic pathways were identified as linoleic acid metabolism, phenylalanine, tyrosine and tryptophan biosynthesis, tryptophan metabolism, arginine and proline metabolism, alanine, aspartate and glutamate metabolism, glycerophospholipid metabolism, tyrosine metabolism, nicotinate and nicotinamide metabolism, pentose and glucuronate interconversions, and glyoxylate and dicarboxylate metabolism.
Table 2.
Identification of potential biomarkers of rat plasma samples between control group and chronic migraine module group.
| NO | Retention Time | Name | Formula | Experimental Mass | Ion Mode | Mass Error | MS/MS |
|---|---|---|---|---|---|---|---|
| P1 | 7.12 | omega-Muricholate | C24H40O5 | 407.2803 | [M-H]- | 1.7 | 389.2695, 346.2837, 289.2176, 209.1544, 195.1758 |
| P2 | 11.44 | Gymnodimine | C32H45NO4 | 506.3276 | [M-H]- | -4.3 | 488.3161 |
| P3 | 3.81 | 2-hydroxy-3-oxoadipic acid | C6H8O6 | 175.0248 | [M-H]- | -1.1 | 157.0135, 129.0194,113.0245, 85.0297, 59.0142 |
| P4 | 9.38 | Glycocholic acid | C26H43NO6 | 500.2784 | [M+Cl]- | -0.6 | 464.3020, 402.3008 |
| P5 | 19.47 | Petroselinic acid | C18H34O2 | 281.2486 | [M-H]- | -0.7 | 281.2486, 263.2380 |
| P6 | 21.27 | Stearic acid | C18H36O2 | 283.2637 | [M-H]- | -2.0 | 269.2487, 255.2329, 241.2180, 227.2017 |
| P7 | 21.51 | 11Z-Eicosenoic acid | C20H38O2 | 309.2799 | [M-H]- | -0.6 | 265.2895 |
| P8 | 9.34 | Isodeoxycholic acid | C24H40O4 | 391.2854 | [M-H]- | -0.5 | 373.2738, 329.2844 |
| P9 | 8.58 | sphinganine 1-phosphate | C18H40NO5P | 380.2571 | [M-H]- | -1.1 | 253.2170, 224.0692, 163.1127 |
| P10 | 2.65 | L-Tryptophan | C11H12N2O2 | 203.0826 | [M-H]- | -1.5 | 186.0568, 130.0666, 116.0510, 74.0254 |
| P11 | 0.58 | Lysine | C6H14N2O2 | 145.0982 | [M-H]- | -0.7 | 131.0826, 113.0732 |
| P12 | 0.73 | glutamic acid | C5H9NO4 | 146.0465 | [M-H]- | -1.6 | 128.0355, 102.0546 |
| P13 | 0.62 | L-Arginine | C6H14N4O2 | 173.1044 | [M-H]- | -0.1 | 131.0826, 156.0732, 68.9956 |
| P14 | 8.34 | LysoPC(14:0) | C22H46NO7P | 512.2994 | [M+HCOO]- | -0.8 | 452.2784, 227.2017, 168.0430, 78.9592 |
| P15 | 12.12 | LysoPC(16:0) | C24H50NO7P | 494.3252 | [M-H]- | -0.8 | 462.2978, 421.2724, 269.2484, 224.0689, 78.9593 |
| P16 | 9.81 | LysoPE(22:4) | C27H48NO7P | 528.309 6 | [M-H]- | -0.8 | 483.2499, 457.2362, 303.2331, 259.2429, |
| P17 | 3.35 | 3-Indoxyl sulfate | C8H7NO4S | 212.0023 | [M-H]- | -1.9 | 132.0454, 80.9655 |
| P18 | 4.83 | 2-Phenylethanol glucuronide | C14H18O7 | 297.098 | [M-H]- | -0.7 | 204.0664, 175.0243, 121.0661 |
| P19 | 9.49 | Phyllanthusol B | C35H49NO18 | 816.2932 | [M+HCOO]- | -1.5 | 628.2337, 507.2115, 346.1273, 168.0429 |
| P20 | 10.33 | LysoPC(20:3) | C28H52NO7P | 590.3463 | [M+HCOO]- | -1.4 | 530.3249, 460.2833, 305.2486 |
| P21 | 11.99 | LysoPC(22:4) | C30H54NO7P | 616.362 | [M+HCOO]- | 0.2 | 556.3398, 331.2634 |
| P22 | 14.11 | PI(18:0/0:0) | C27H53O12P | 599.3202 | [M-H]- | 0.3 | 419.2563, 315.0487, 241.0117, 152.8859, 78.9592 |
| P23 | 17.62 | dipalmitoylphosphatidic acid | C35H69O8P | 629.4552 | [M-H2O-H]- | -0.2 | 629.4542 |
| P24 | 11.19 | LysoPC(22:5) | C30H52NO7P | 614.3463 | [M+HCOO]- | -2.8 | 554.3245, 497.2881, 329.2483, 285.2598 |
| P25 | 7.12 | SM(d18:1/20:0) | C43H87N2O6P | 837.5491 | [M+Br]- | 1.0 | 429.2628, 407.2809, 251.2020, 195.1758 |
| P26 | 3.48 | L-DOPA | C9H11NO4 | 178.051 | [M-H2O-H]- | -1.1 | 178.0507, 134.0613 |
| P27 | 2.65 | L-Tryptophan | C11H12N2O2 | 205.0972 | [M+H]+ | -3.5 | 159.0952, 144.0831, 118.0683, 90.0482, 75.0249 |
| P28 | 16.63 | Linolenic acid | C18H30O2 | 279.2397 | [M+H]+ | -5.3 | 261.2286 |
| P29 | 18.04 | Linoleic acid | C18H32O2 | 263.2443 | [M-H20+H]+ | -0.6 | 263.2463, 121.1044 |
| P30 | 1.94 | L-Phenylalanine | C9H11NO2 | 166.0909 | [M+H]+ | -0.7 | 119.9480, 103.0005 |
| P31 | 1.14 | L-Tyrosine | C9H11NO3 | 182.0859 | [M+H]+ | -0.6 | 135.8930, 95.0005 |
| P32 | 0.8 | L-Valine | C5H11NO2 | 118.0862 | [M+H]+ | -1.1 | 118.0862 |
| P33 | 1.07 | Nicotinamide | C6H6N2O | 123.0549 | [M+H]+ | -1.5 | 108.0439, 80.0490 |
| P34 | 5.43 | prolyl-threonine | C9H16N2O4 | 217.1261 | [M+H]+ | -0.7 | 102.0550 |
| P35 | 20.17 | 11-beta-hydroxyandro-sterone-3-glucuronide | C25H38O9 | 483.2654 | [M+H]+ | -1.6 | 289.2164, 177.0396 |
| P36 | 8.53 | 4,4-dimethyl-5a-cholesta-8,24-dien-3-b-ol | C29H50O | 437.3815 | [M+Na]+ | 0.0 | 463.2347, 203.1849, 133.1047, 105.0726 |
| P37 | 1.05 | Carbamoylphosphate | CH4NO5P | 141.9642 | [M+H]+ | -0.8 | 98.9847 |
| P38 | 1.96 | Citric acid | C6H8O7 | 210.0590 | [M+NH4]+ | -0.8 | 193.0348, 133.0133 |
| P39 | 11.43 | 7-Dehydrodesmosterol | C27H42O | 400.3555 | [M+NH4]+ | -0.8 | 365.3203 |
| P40 | 9.98 | 11-hydroxyprogesterone 3-glucuronide | C27H38O9 | 507.2661 | [M+H]+ | -1.9 | 331.2273, 177.0395 |
Relevance Analysis Between Plasma Biomarkers and Gut Microbiota
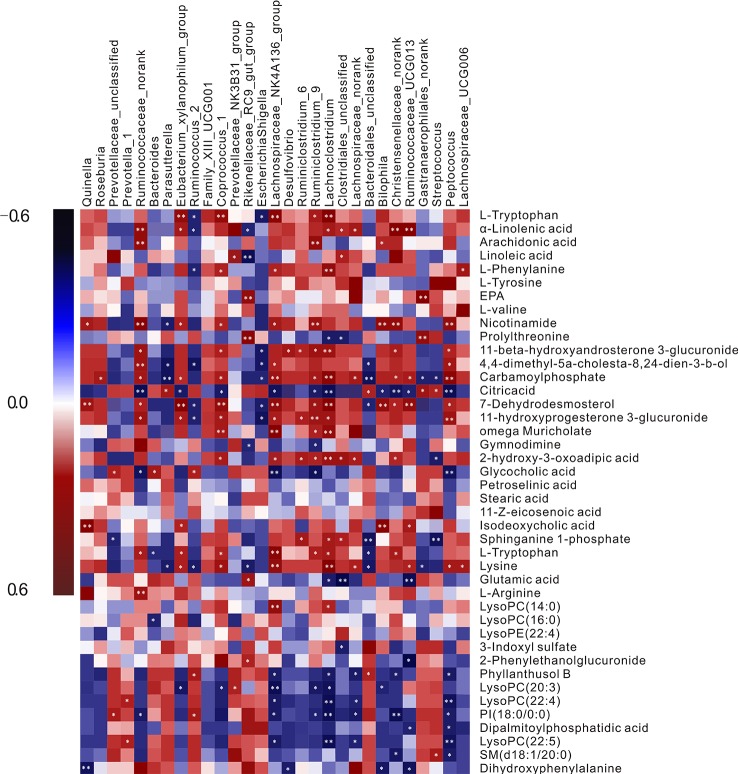
Pearson’s correlation analysis was performed to evaluate potential links between gut microbiota genera and plasma biomarkers (range for correlation, r >0.4 or r < −0.4; P < 0.05). In the correlation analysis, associations between the perturbed gut microbiota and altered plasma metabolites were identified. As was shown in Figure 4 L-tryptophan (P10) was positively related to Ruminiclostridium_9, Coprococcus_1, Lachnospiraceae_NK4A136_group, and Lachnoclostridium but negatively related to Bacteroides, Ruminococcus, and Escherichia-Shigella. L-Arginine (P13) showed highly positive correlations with Lachnoclostridium and Clostridiales_unclassified, whereas glutamic acid (P12) had the opposite correlation with the two bacteria. L-Tyrosine (P31) correlated positively with Prevotella_1 and negatively with Christensenellaceae_norank. Significant negative correlations were discerned between L-DOPA (P26) and Bilophila. The intensity of 3-indoxyl sulfate (P17) was negatively correlated with the abundance of Clostridiales_unclassified. Nicotinamide (P33) was correlated positively with Coprococcus_1, Bilophila, Ruminiclostridium_9, and Peptococcus. These metabolites were involved in four key metabolic pathways, namely tryptophan metabolism (P10 and P17), arginine and proline metabolism (P12 and P13), nicotinate and nicotinamide metabolism (P33), and tyrosine metabolism (P31 and P26), and also represented a complete metabolome contributing to the formation of gut microbiota symbiosis.
Figure 4.
A correlation heatmap is used to represent significant statistical correlation values (r) between perturbed gut microbiota genera and altered plasma metabolites in the Model group and Control group. Blue squares indicate negative correlations, and red squares indicate positive correlations; the significance of correlation is closely related to the depth of color.
Intestinal Lavage Fluid Metabolic Profile of the Rat CMG Model and Identification of Potential Metabolite Biomarkers
Intestinal metabolites are directly influenced by gut microbial composition and affect plasma metabolomics by absorption into the blood (Windmueller and Spaeth, 1976); therefore, the correlation between intestinal flora and plasma metabolism may be caused by altered intestinal metabolism. Metabolic profiles of intestinal lavage fluid samples in the Control and Model groups were analyzed by UPLC-Q-TOF-MS/MS in both the positive and negative ion modes. The PCA and OPLS-DA ( Figure 5 ) model indicated a complete separation between the metabolic profiles of rats in the two groups. The differential components of the intestinal lavage fluid were identified using the same methods used to identify the plasma biomarkers. In total, 12 metabolites were identified in intestinal lavage fluid, which is similar to the components of plasma metabolites identified ( Table 3 ).
Figure 5.
Metabolic profiles of intestinal lavage fluid samples in rats with chronic migraine induced by nitroglycerin. (A) PCA score plot in negative mode; (B) OPLS-DA score plot in negative mode (R2Y = 0.945, Q2Y = 0.748); (C) PCA score plot in positive mode; (D) OPLS-DA score plot in positive mode (R2Y = 0.999, Q2Y = 0.840).
Table 3.
Potential intestinal lavage fluid biomarkers in the rat model of chronic migraine induced by nitroglycerin.
| NO | Retention Time | Name | Formula | Ion Mode | Error (ppm) | MS/MS |
|---|---|---|---|---|---|---|
| I1 | 11.03 | omega-Muricholate | C24H40O5 | [M-H]- | -0.12 | 389.2695, 289.2176, 209.1544, 195.1758 |
| I2 | 0.88 | Lysine | C6H14N2O2 | [M-H]- | 0.14 | 131.0826, 113.0732 |
| I3 | 0.81 | glutamic acid | C5H9NO4 | [M-H]- | -0.07 | 128.0355, 102.0546 |
| I4 | 0.74 | L-Arginine | C6H14N4O2 | [M-H]- | -0.12 | 131.0826, 156.0732, 68.9956 |
| I5 | 3.54 | 3-Indoxyl sulfate | C8H7NO4S | [M-H]- | -0.14 | 132.0454, 80.9655 |
| I6 | 11.03 | SM(d18:1/20:0) | C43H87N2O6P | [M+Br]- | -0.20 | 429.2628, 407.2809, 251.2020, 195.1758 |
| I7 | 3.59 | dihydroxyphenylalanine | C9H11NO4 | [M-H2O-H]- | 0.11 | 178.0507, 134.0613 |
| I8 | 3.04 | L-Tryptophan | C11H12N2O2 | [M+H]+ | -1.12 | 159.0952, 144.0831, 118.0683, 90.0482, 75.0249 |
| I9 | 2.65 | L-Phenylalanine | C9H11NO2 | [M+H]+ | 1.81 | 119.9480, 103.0005 |
| I10 | 2.22 | L-Tyrosine | C9H11NO3 | [M+H]+ | -0.77 | 135.8930, 95.0005 |
| I11 | 0.83 | L-Valine | C5H11NO2 | [M+H]+ | -0.85 | 118.0862, 78.0805 |
| I12 | 15.69 | 11-beta-hydroxyan- drosterone-3-glucuronide | C25H38O9 | [M+Na]+ | 2.47 | 289.2164, 177.0396 |
Effect of GUW Treatment on Gut Microbiota Dysbiosis
After GUW treatment, chronic NTG-induced dysregulation of bacterial abundance was improved at the genus level. Changes in the abundance of bacteria are shown in Figure 6 . Compared with the Model group, the abundances of Ruminococcus_2 and Escherichia-Shigella were reduced in the GUW group. The abundances of Bacteroides, Lachnoclostridium, Clostridiales_unclassified, Bilophila, Peptococcus, and Christensenellaceae_norank were similar to those in the Control group. There were no obvious changes in the abundances of Prevotella_1, Coprococcus_1, Ruminiclostridium_9, and Lachnospiraceae_NK4A136_group.
Figure 6.
Regulation of GU on intestinal bacteria in the Model group, *P < 0.05, **P < 0.01 compared with the Model group; # P < 0.05, ## P < 0.01 compared with the Control group. (Control, Control group; Model, Model group; GUW, GU administration of rats in the Model group.) (A) Bacteroides; (B) Bilophila; (C) Christensenellaceae_norank; (D) Clostridiales_unclassified; (E) Coprococcus_1; (F) Escherichia-Shigella; (G) Lachnoclostridium; (H) Lachnospiraceae_NK4A136_group; (I) Peptococcus; (J) Prevotella_1; (K) Ruminiclostridium_9; (L) Ruminococcus_2.
Effect of GUW Treatment on Plasma Metabolomics
To show the effect of GUW on the plasma metabolomics of the rat CMG model, the data for all groups (Control, Model, and CMG groups) were imported into SIMCA-P 14.1 to obtain a comprehensive metabolic profile. PCA and OPLS-DA analysis were performed to show the effect of GUW administration ( Figure S6 ). The profiles of the three groups were obviously distinct, and the Model group was far from the Control group. The GUW group was between the Model group and the Control group in the x-axis direction, indicating differences between the groups. To avoid overfitting and random effects, a permutation of 200 tests was also performed to confirm that the OPLS-DA models were reliable with good predictability ( Figure S6 ).
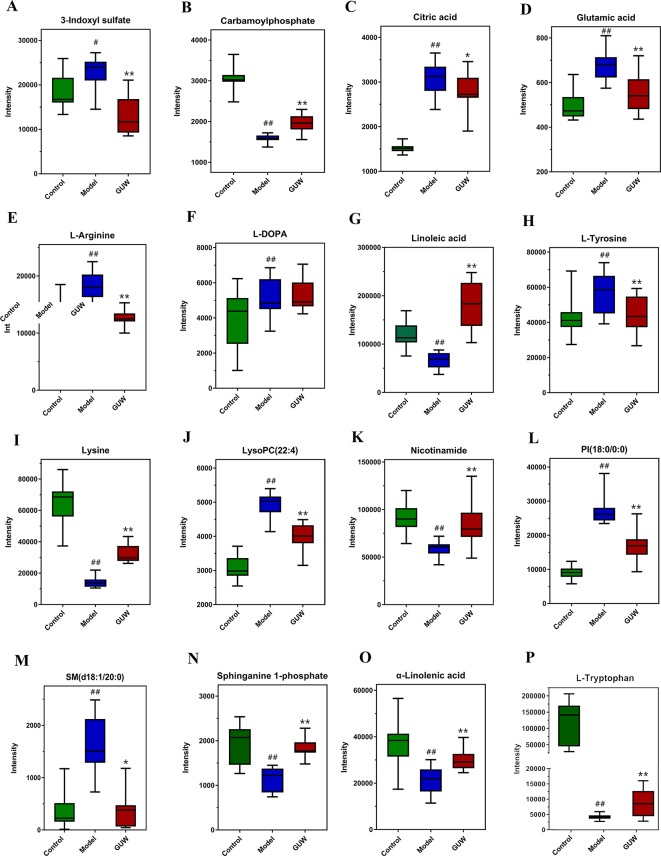
After GUW treatment, 28 endogenous metabolites that were disordered in CMG rats recovered to almost normal levels. The changes in 16 metabolites are listed in Figure 7 , including L-tryptophan, L-arginine, glutamic acid, L-tyrosine, L-DOPA, 3-indoxyl sulfate, linoleic acid, and nicotinamide. These potential biomarkers are related to tryptophan metabolism, arginine and proline metabolism, nicotinate and nicotinamide metabolism, tyrosine metabolism, and linoleic acid metabolism. The other biomarkers are listed in Figure S7 .
Figure 7.
Expression of plasma biomarkers in all groups, *P < 0.05, **P < 0.01 compared with the Model group; # P < 0.05, ## P < 0.01 compared with the Control group. (Control, Control group; Model, Model group; GUW, GU administration of rats in model group.) (A) 3-Indoxyl sulfate; (B) Carbamoylphosphate; (C) Citric acid; (D) Glutamic acid; (E) L-Arginine; (F) L-DOPA; (G) Linolenic acid; (H) L-Tyrosine; (I) Lysine; (J) LysoPC(22:4); (K) Nicotinamide; (L) PI(18:0/0:0); (M) SM(d18:1/20:0); (N) Sphinganine 1-phosphate; (O) α-Linolenic acid; (P) L-Tryptophan.
Validation Study
The Effect of GUW Treatment Thermal Hyperalgesia Induced by Chronic NTG
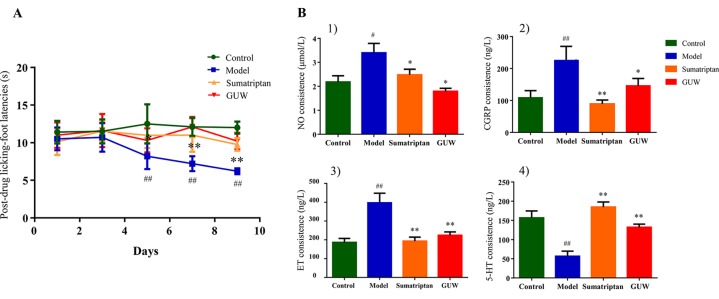
Some studies have shown that CMG model rats exhibited thermal allodynia (Bates et al., 2010). Thermal sensitivity tests showed that NTG induced significant thermal hyperalgesia after the third day of treatment (on days 5, 7, and 9). Treatment with Sumatriptan and GUW for 10 days significantly increased thermal thresholds, and no significant differences were observed compared with the Control group ( Figure 8A ).
Figure 8.
Chronic NTG-evoked hyperalgesia, which was significantly attenuated by GUW treatment. Data are expressed as mean ± SD (n = 10), *P < 0.05, **P < 0.01 compared with the Model group; # P < 0.05, ## P < 0.01 compared with the Control group (A). Regulation of plasma biochemical indicators levels induced by NTG administration and its reduction by GUW treatment. 1) NO, 2) CGRP, 3) ET, 4) 5-HT. Data are expressed as mean ± SD (n = 10), *P < 0.05, **P < 0.01 compared with the Model group; # P < 0.05, ## P < 0 .01 compared with the Control group (B).
Effect of GUW Treatment on Plasma Concentration of 5-HT, CGRP, ET, and NO
Plasma biochemical indicators (5-HT, CGRP, ET, and NO) are closely related to chronic migraine models and are commonly used to indicate disease progression (Cui et al., 2017). Our study demonstrated that, compared with the Control group, chronic intermittent NTG administration increased plasma levels of NO (P < 0.05), CGRP (P < 0.01), and ET (P < 0.01) and reduced 5-HT levels (P < 0.01). After Sumatriptan and GUW treatment, there were no significant differences in plasma levels of NO, CGRP, ET, and 5-HT compared with those in the Control group ( Figure 8B ).
Co-Incubation of Feces and GUW In Vitro
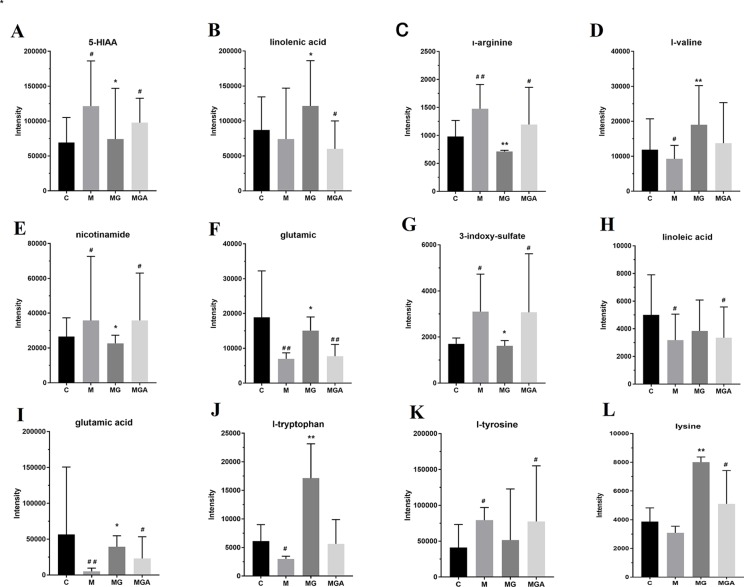
To verify the in vivo effects of GUW on metabolism via intestinal bacteria, samples were divided into three groups (model group (MG), model+GUW group (MGG), and model +GUW+ antibiotic group (MGA); n = 4 samples per group) for short-term in vitro incubation. The samples were analyzed using UPLC-Q-TOF MS, and 19 components were identified in the feces samples that were associated with biomarkers in plasma. Since the metabolism of bacteria in the feces was inhibited after the addition of antibiotics, there were no significant differences in the metabolism of fecal bacteria in the MGA (P > 0.05) compared with MG ( Figure 9 ). Levels of 3-indoxyl sulfate, glutamic acid, L-tyrosine, and L-arginine were reduced in MGG compared with in MG, and there were no significant differences between MG and MGA. Compared with MG, the levels of 5-HIAA, L-tryptophan, linoleic acid, and L-phenylalanine were increased after GUW treatment, and there were minimal changes in MGA.
Figure 9.
Effect of in vitro co-incubation of fecal samples on fecal bacterial metabolite levels, *P < 0.05, **P < 0.01 compared with the Model group; # P < 0.05, ## P < 0.01 compared with the Control group. (C, feces of Control group; M, feces of Model group; MG, water extraction of GU added into feces of Model group; MGA, water extraction of GU and antibiotic mixed solution added into feces of Model group.) (A) 5-HIAA; (B) linolenic acid; (C) l-arginine; (D) l-valine; (E) nicotinamide; (F) glutamic; (G) 3-Indoxyl sulfate; (H) linoleic acid; (I) glutamic acid; (J) l-tryptophan; (K) l-tyrosine; (L) lysine.
Discussion
Studying the response of microbial communities in the face of interference is critical to health (Costello et al., 2012), not only because the gut microbiota may change under disease conditions but also because the microbiota is affected by drugs administered to maintain host health (Zhang et al., 2019). It is increasingly recognized that the therapeutic effects of natural products on disease may be achieved through drug-microbial interactions, indicating the importance of research into the effects of drugs on microbial metabolism (Genda et al., 2018). In this study, we first determined the changes in the structure and function of the gut microbiota in a rat model of CMG. We found differences in the structure and function of the microbiota between the Model and Control groups. After GUW administration, dysregulation of the structure and function of the microbiota in the CMG rats was improved. We also observed that the significant changes in plasma metabolites in the Model group and drug-administered group varied with the changes in the intestinal microbiota.
Chronic NTG administration disturbed the microbial population, resulting in a significant decrease in the abundance of thick-walled bacteria and an increase in the relative abundance of Bacteroides. This result is consistent with the changes in the intestinal flora of autistic children (Finegold et al., 2010). In CMG model rats, metagenomic data indicated an increased relative abundance of Prevotellaceae_unclassified, Prevotella_1, Ruminococcaceae_norank, Bacteroides, Parasutterella, Bacteroidales_unclassified, Family_XIII_UCG-001, Prevotellaceae_NK3B31_group, Ruminococcus_2, Gastranaerophilales_norank, Rikenellaceae_RC9_gut_group, Escherichia-Shigella, and Streptococcus, while decreases were observed in the relative abundance of other differential intestinal bacteria, such as Coprococcus_1, Lachnospiraceae_NK4A136_group, Ruminococcaceae_UCG-013, Desulfovibrio, Ruminiclostridium_6, Ruminiclostridium_9, Lachnoclostridium, Clostridiales_unclassified, Lachnospiraceae_norank, Lachnospiraceae_UCG-006, Bilophila, Christensenellaceae_norank, and Peptococcus. It has been reported that women with high intestinal levels of Prevotella_1 show more negative emotions, such as anxiety and pain (Tillisch et al., 2017), which is consistent with our results. Lack of Coprococcus is found in most patients with depression, reflecting a link between Coprococcus and brain disease (Valles-Colomer et al., 2019). Desulfovibrio is associated with amino acid breakdown and ammonia production (Eschenlauer et al., 2002). The results of the present study indicate that the function of some bacterial strains may be related to chronic migraine; however, the function of most strains needs further verification.
Compared with the Control group, our metabolomics analysis showed differences in the levels of metabolites in the plasma and ileum of rats in the Model group. We found metabolites of interest in the Model group (P < 0.05, VIP >1 compared to the Control group). Previous metabolomics studies in this field have focused on transgenic migraine models and clinical patients (Shyti et al., 2015) and have shown disruption of biological metabolic pathways and changes in common metabolites, including free fatty acids and amino acids (Ren et al., 2018). Our study differs from previous studies in that we used a rat model of CMG induced by NTG for plasma metabolomics and intestinal lavage fluid analyses. Although model rats or clinical metabolomics are particularly relevant for the analysis of migraine biomarkers, exploring the potential correlation between plasma metabolites and endogenous markers in the ileum using this rat model of CMG is more indicative of the changes in the intestinal tract and the effect of changes in the flora on the overall metabolic level of the host. In addition, some intestinal strains have previously been shown to affect the progression of migraine disorders (Gonzalez et al., 2016). Analysis of differential markers of gut content allows us to focus on the differences in the compounds produced by changes in gut microbiota in the Model group and analyze their effects on host metabolism. Clostridiales_unclassified affects glutamate metabolism (Buckel, 2001), Pseudomonas affects plasma levels of nitrate (Gonzalez et al., 2016), Coprococcus seems to have a pathway associated with dopamine metabolism, and Ruminococcus metabolizes tryptophan to tryptamine (Williams et al., 2014). Although we have speculated that some strains are associated with plasma markers based on our data and earlier studies, whether these compounds can be regulated by the gut flora needs further verification.
GU is recognized as a natural medicine pair for the treatment of migraine. Currently, GU Granules (GUG) are marketed for the treatment of migraine. Studies have shown that few constituents of GUG migrate to the blood (Zhang et al., 2019), and some of the active ingredients, such as gastrodin, have a short half-life (Wu et al., 2017). Therefore, we consider that, in addition to the active ingredients into the blood, GU acts indirectly via another pathway. Intestinal flora analysis, a new method for studying the mechanism of action of natural medicines, was employed in our study. Using this approach, we validated the effect of GU in the rat model of CMG and determined the changes in the dysregulated flora and biomarkers in response to GU administration.
In vitro incubation of feces with antibiotics is the key to verifying whether the effects of GU are mediated via the intestinal flora. Intestinal bacteria are mostly anaerobic; therefore, we used an anaerobic box to simulate the internal environment. We did not perform these studies with a commonly used culture medium because these contain amino acids and other components that would interfere with the subsequent determination of endogenous components. Inhibition of the metabolism of intestinal bacteria by antibiotics showed that GU did not regulate the levels of components in the feces of the CMG model rats. In contrast, in the samples without antibiotics, the contents of some biomarkers were similar to those in the Control group after in vitro culture with GU, indicating that GU regulates the endogenous components in the intestine via the intestinal flora.
Conclusion
The main contribution of this study is that clarification of the pathogenesis of migraine and the therapeutic effect of GUW is provided by the combination of 16S rRNA sequencing with metabolomics based on UPLC-MS. Our study shows that the therapeutic effect of GUW treatment on NTG-induced CMG rats is driven by the intestinal flora, which in turn regulates intestinal metabolism and the absorption of intestinal metabolites into the blood. After GUW administration, the abundance of disordered intestinal bacteria, including Ruminococcus_2, Escherichia-Shigella, Bacteroides, Lachnoclostridium, Bilophila, and Peptococcus, was adjusted, thereby affecting the metabolism of tryptophan, tyrosine, arginine, and niacin and nicotinamide to achieve therapeutic effects in NTG-induced CMG. Our findings provide new evidence of the role of the brain–gut axis in the mechanism by which food and natural medicines can be used to modulate the intestinal flora and achieve therapeutic effects in migraine. In future studies, we will conduct a flora transplantation experiment to confirm the role of characteristic intestinal flora in the treatment of migraine.
Data Availability Statement
The datasets generated for this study can be found in the Sequence Read Archive, https://www.ncbi.nlm.nih.gov/sra/?term= SRP214751.
Ethics Statement
The animal study was reviewed and approved by the Experimental Animal Ethics Committee of the State Key Laboratory (Reference number: BCTG-2016-18).
Author Contributions
ZW wrote this main manuscript text and performed the data analysis. The animal experiment was conducted by ZW and CP together. In addition, MH, YR, and JL gave the contribution to plasma sample detection. As for data process, it is conducted by MH, ZL, and MZ. LD and YL contributed to manuscript preparation. HO designed the work that led to the submission, acquired data, and played an important role in interpreting the results. YF revised the manuscript. And SY contributed to the conception of the study.
Funding
The study was funded by a grant (No. 81660651, 81560638) from National Natural Science Foundation of China, China Postdoctoral Science Foundation (No. 2017M612159), Postdoctoral Science Foundation of Jiangxi Province (No. 2017KY07), Natural Science Foundation of Jiangxi Province (No. 20171ACB21029, 20165BCB19009), Health and Family Planning Commission of Jiangxi Province (No. 2016A009), Science and Technology Project of Jiangxi Province (No. 20161BBH80002), Nanchang Innovative Talent Team (No. [2016]173), Education Department of Jiangxi Province (No.GJJ180669).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2019.01425/full#supplementary-material
Extraction protocols of GU water extract. (GE = Gastrodia, UR = Uncaria, h = hour, min = minute).
Base peak intensity (BPI) chromatograms of ten plasma QC sample injections: (A) for negative ion mode; (B) for positive ion mode.
Base peak intensity (BPI) chromatograms of ten intestinal lavage fluid QC sample injections: (A) for negative ion mode; (B) for positive ion mode.
Overview of permutations test plot for OPLS-DA (200 tests) for rat plasma samples. (A) Permutations test of OPLS-DA in negative ion mode for Control and Model groups; (B) Permutations test of OPLS-DA in positive ion mode for Control and Model groups.
Base peak intensity (BPI) chromatograms of seven standards: (A) for negative ion mode; (B) for positive ion mode. (P10) L-tryptophan; (P12) glutamic acid; (P13) L-arginine; (P17) 3-indoxyl sulfate; (P27) L-tryptophan; (P30) L-phenylalanine; (P31) L-tyrosine; (P32) L-valine.
Overview of metabolic profile after GU treatment: (A) ESI+, PCA; (B) ESI+, OPLS-DA; (C) ESI-, PCA; (D) ESI-, OPLS-DA. (E) Permutations test plot for OPLS-DA (200 tests) in positive ion mode for rat plasma samples; (F) Permutations test plot for OPLS-DA (200 tests) in negative ion mode for rat plasma samples.
Expression of 24 plasma biomarkers in all groups, *P < 0.05, **P < 0.01 compared with the model group; #P < 0.05, ##P < 0.01 compared with the control group (Control = Control group; Model = Model group; GUW = GU administration of rats in model group).
RSD of typical peaks of plasma QC sample (ESI-.n=10; ESI+,n=10).
RSD of typical peaks of intestinal lavage fluid QC sample (ESI-.n=10; ESI+,n=10).
References
- Al-Hashel J. Y., Ahmed S. F., Alshawaf F. J., Alroughani R. (2018). Use of traditional medicine for primary headache disorders in Kuwait. J. Headache Pain. 19, 118. 10.1186/s10194-018-0950-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates E. A., Nikai T., Brennan K. C., Fu Y. H., Charles A. C., Basbaum A. I., et al. (2010). Sumatriptan alleviates nitroglycerin-induced mechanical and thermal allodynia in mice. Cephalalgia 30 (2), 170–178. 10.1111/j.1468-2982.2009.01864.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckel W. (2001). Unusual enzymes involved in five pathways of glutamate fermentation. Appl. Microbiol. Biotechnol. 57, 263–273. 10.1007/s002530100773 [DOI] [PubMed] [Google Scholar]
- Costello E. K., Stagaman K., Dethlefsen L., Bohannan B. J. M., Relman D. A. (2012). The application of ecological theory toward an understanding of the human microbiome. Science 336, 1255–1262. 10.1126/science.1224203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui C., He X., Dong C., Song Z., Ji J., Wan X., et al. (2017). The enhancement mechanism of wine-processed Radix Scutellaria on NTG-induced migraine rats. Biomed. Pharmacother. 91, 138–146. 10.1016/j.biopha.2017.04.067 [DOI] [PubMed] [Google Scholar]
- Eschenlauer S. C. P., McKain N., Walker N. D., McEwan N. R., Newbold C. J., Wallace R. J. (2002). Ammonia production by ruminal microorganisms and enumeration, isolation, and characterization of bacteria capable of growth on peptides and amino acids from the sheep rumen. Appl. Environ. Microbiol. 68, 4925–4931. 10.1128/aem.68.10.4925-4931.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estruel-Amades S., Massot-Cladera M., Pérez-Cano J. F., Franch À., Castell M. (2019). Camps-Bossacoma mariona, hesperidin effects on gut microbiota and gut-associated lymphoid tissue in healthy rats. Nutrients 11, 324–340. 10.3390/nu11020324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farajdokht F., Mohaddes G., Shanehbandi D., Karimi P., Babri S. (2018). Ghrelin attenuated hyperalgesia induced by chronic nitroglycerin: CGRP and TRPV1 as targets for migraine management. Cephalalgia 38, 1716–1730. 10.1177/0333102417748563 [DOI] [PubMed] [Google Scholar]
- Finegold S. M., Dowd S. E., Gontcharova V., Liu C., Henley K. E., Wolcott R. D., et al. (2010). Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe 16, 444–453. 10.1016/j.anaerobe.2010.06.008 [DOI] [PubMed] [Google Scholar]
- Foster J. A., Lyte M., Meyer E., Cryan J. F. (2016). Gut microbiota and brain function: an evolving field in neuroscience. Int. J. Neuropsychopharmacol. 19, 1–7. 10.1093/ijnp/pyv114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini C. F., Smith R. A., Griffiths L. R. (2017). Genetic and biochemical changes of the serotonergic system in migraine pathobiology. J. Headache Pain 18, 20. 10.1186/s10194-016-0711-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Z., Xie Y., Liang Q., Wang Y., Luo G. (2014). Pharmacokinetic comparative study of gastrodin and rhynchophylline after oral administration of different prescriptions of Yizhi Tablets in rats by an HPLC-ESI/MS method. Evid. Based Complement Alternat. Med. 2014, 1–10. 10.1155/2014/167253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genda T., Kondo T., Hino S., Sugiura S., Nishimura N., Morita T. (2018). The Impact of fructo-oligosaccharides on gut permeability and inflammatory responses in the cecal mucosa quite differs between rats fed semi-purified and non-purified diets. J. Nutr. Sci. Vitaminol. 64, 357–366. 10.3177/jnsv.64.357 [DOI] [PubMed] [Google Scholar]
- Gonzalez A., Hyde E., Sangwan N., Gilbert J. A., Viirre E., Knight R., et al. (2016). Migraines are correlated with higher levels of nitrate-, nitrite-, and nitric oxide-reducing oral microbes in the american gut project cohort. mSystems 1, e00105–e00116. 10.1128/mSystems.00105-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieco G. S., Gagliardi S., Ricca I., Pansarasa O., Neri M., Gualandi F. (2018). New CACNA1A deletions are associated to migraine phenotypes. J. Headache Pain 19, 75. 10.1186/s10194-018-0891-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover M., Kashyap P. C. (2014). Germ-free mice as a model to study effect of gut microbiota on host physiology. Neurogastroenterol. Motil. 26, 745–748. 10.1111/nmo.12366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Headache Classification Committee of the International Headache Society (IHS) (2013). The international classification of headache disorders, 3rd edition (beta version). Cephalalgia 33, 629–808. 10.1177/0333102415587704 [DOI] [PubMed] [Google Scholar]
- Jiang B. B. (2007). The new uses of Tianma Gouteng drink. Guangming. J. Chin. Med. 22, 32. 10.3969/j.issn.1003-8914.2007.04.023 [DOI] [Google Scholar]
- Katsarava Z., Mania M., Lampl C., Herberhold J., Steiner T. J. (2018). Poor medical care for people with migraine in europe – evidence from the eurolight study. J. Headache Pain 19, 10. 10.1186/s10194-018-0839-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Wang Y., Ouyang H., Lu Y., Qiu Y., Feng Y., et al. (2015). A novel dereplication strategy for the identification of two new trace compounds in the extract of Gastrodia elata using UHPLC/Q-TOF-MS/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 988, 45–52. 10.1016/j.jchromb.2015.02.020 [DOI] [PubMed] [Google Scholar]
- Lionetto L., Gentile G., Bellei E., Capi M., Sabato D., Marsibilio F. (2013). The omics in migraine. J. Headache Pain 14, 55. 10.1186/1129-2377-14-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton R. B., Stewart W. F., Stone A. M., Láinez M. J., Sawyer J. P. (2000). Disability in strategies of care study group, stratified care vs step care strategies for migraine: the disability in strategies of care (disc) study: a randomized trial. JAMA 284, 2599–2605. 10.1001/jama.284.20.2599 [DOI] [PubMed] [Google Scholar]
- Liu L., Song J., Lu J., Huang Y., Zeng Y., Chen L., et al. (2015). Tianma Gouteng Yin, a traditional chinese medicine decoction, exerts neuroprotective effects in animal and cellular models of Parkinson’s disease. Sci. Rep. 5, 16862–16873. 10.1038/srep16862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Gao J. L., Peng M., Meng H., Ma H., Cai P., et al. (2018). A review on central nervous system effects of gastrodin. Front. Pharmacol. 9, 24. 10.3389/fphar.2018.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen J., Diener H. C., Husstedt I. W., Goadsby P. J., Lesko L. M. (2004). Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N. Engl. J. Med. 350, 1104–1110. 10.1056/NEJMoa030505 [DOI] [PubMed] [Google Scholar]
- Pan H., Yang W., Yao C., Shen Y., Zhang Y., Shi X., et al. (2017). Mass defect filtering-oriented classification and precursor ions list-triggered high-resolution mass spectrometry analysis for the discovery of indole alkaloids from Uncaria sinensis. J. Chromatogr A 1516, 102–113. 10.1016/j.chroma.2017.08.035 [DOI] [PubMed] [Google Scholar]
- Ren C., Liu J., Zhou J., Liang H., Wang Y., Sun Y., et al. (2018). Low levels of serum serotonin and amino acids identified in migraine patients. Biochem. Biophys. Res. Commun. 496, 267–273. 10.1016/j.bbrc.2017.11.203 [DOI] [PubMed] [Google Scholar]
- Shyti R., Kohler I., Schoenmaker B., Derks R. J. E., Ferrari M. D., Tolner E. A., et al. (2015). Plasma metabolic profiling after cortical spreading depression in a transgenic mouse model of hemiplegic migraine by capillary electrophoresis – mass spectrometry. Mol. Biosyst. 11, 1462–1471. 10.1039/c5mb00049a [DOI] [PubMed] [Google Scholar]
- Sutherland H. G., Albury C. L., Griffiths L. R. (2019). Advances in genetics of migraine. J. Headache Pain 20, 72. 10.1186/s10194-019-1017-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., Liu S., Shu H., Yanagisawa L., Tao F. (2019). Gut microbiota dysbiosis enhances migraine-like pain via TNFα U-pregulation. Mol. Neurobiol. 10.1007/s12035-019-01721-7 [DOI] [PMC free article] [PubMed]
- Tillisch K., Mayer E., Gupta A., Gill Z., Brazeilles R., Nevé B. L., et al. (2017). Brain structure and response to emotional stimuli as related to gut microbial profiles in healthy women. Psychosom. Med. 79, 905–913. 10.1097/PSY.0000000000000493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valles-Colomer M., Falony G., Darzi Y., Tigchelaar E. F., Wang J., Tito R. Y., et al. (2019). The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 4, 623–632. 10.1038/s41564-018-0337-x [DOI] [PubMed] [Google Scholar]
- van den Maagdenberg Arn M. J. M., Nyholt Dale R., Anttila V. (2019). Novel hypotheses emerging from GWAS in migraine? J. Headache Pain 20, 5. 10.1186/s10194-018-0956-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B. B., Benschoten A. H. V., Cimermancic P., Donia M. S., Zimmermann M., Taketani M., et al. (2014). Discovery and characterization of gut microbiota decarboxylases that can produce the neurotransmitter tryptamine. Cell Host Microbe 16, 495–503. 10.1016/j.chom.2014.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windmueller H. G., Spaeth A. E. (1976). Metabolism of absorbed aspartate, asparagine, and arginine by rat small intestine in vivo. Arch. Biochem. Biophys. 175 (2), 670–676. 10.1016/0003-9861(76)90558-0 [DOI] [PubMed] [Google Scholar]
- Wu L., Hou J., Wang J., Luo M., Wang L., Wang J., et al. (2017). Effect of combination of Gastrodia and Uncaria on pharmacokinetics of gastrodin and rhynchophylline. J. Tradi. Chin. Med. 42, 2577–2582. 10.19540/j.cnki.cjcmm.20170523.011 [DOI] [PubMed] [Google Scholar]
- Xia J., Wishart D. S. (2011). MetPA: a web-based metabolomics tool for pathway analysis and visualization. Bioinformatics 26, 2342–2344. 10.1093/bioinformatics/btq418 [DOI] [PubMed] [Google Scholar]
- Xian J. W., Choi Y. T., Lau B. S., Leung W. N., Ng C. F., Chan C. W. (2016). Gastrodia and uncaria, (tianma gouteng) water extract exerts antioxidative and antiapoptotic effects against cerebral ischemia in vitro and in vivo. Chin. Med. 11, 27. 10.1186/s13020-016-0097-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. J., Sun T. C., Liu Z. W., Wang F. J., Wang Y. D., Liu J. (2017). Effects of tianmagouteng particles on brain cognitive function in spontaneously hypertensive rats with hyperactivity of liver-yang: a [F-18] FDG micro-pet imaging study. Biomed. Pharmacother. 95, 1838–1843. 10.1016/j.biopha.2017.08.100 [DOI] [PubMed] [Google Scholar]
- Zhang X., Li J., Xie B., Wu B., Lei S., Yao Y., et al. (2018). Comparative metabolomics analysis of cervicitis in human patients and a phenol mucilage-induced rat model using liquid chromatography tandem mass spectrometry. Front. In Pharmacol. 9, 282. 10.3389/fphar.2018.00282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Duan S., Wang L., Liu J., Qi W., Yuan D. (2019). Identification of the absorbed components and their metabolites of Tianma-Gouteng granule in rat plasma and bile using ultra-high-performance liquid chromatography combined with quadrupole time-of-flight mass spectrometry. Biomed. Chromatogr. 33, e4480. 10.1002/bmc.4480 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Zhao J., Tian C., Chen X., Li H., Wei X., et al. (2019). Targeting the gut microbiota to investigate the mechanism of lactulose in negating the effects of a high-salt diet on hypertension. Mol. Nutr. Food Res. 63 (11), e1800941. 10.1002/mnfr.201800941 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Extraction protocols of GU water extract. (GE = Gastrodia, UR = Uncaria, h = hour, min = minute).
Base peak intensity (BPI) chromatograms of ten plasma QC sample injections: (A) for negative ion mode; (B) for positive ion mode.
Base peak intensity (BPI) chromatograms of ten intestinal lavage fluid QC sample injections: (A) for negative ion mode; (B) for positive ion mode.
Overview of permutations test plot for OPLS-DA (200 tests) for rat plasma samples. (A) Permutations test of OPLS-DA in negative ion mode for Control and Model groups; (B) Permutations test of OPLS-DA in positive ion mode for Control and Model groups.
Base peak intensity (BPI) chromatograms of seven standards: (A) for negative ion mode; (B) for positive ion mode. (P10) L-tryptophan; (P12) glutamic acid; (P13) L-arginine; (P17) 3-indoxyl sulfate; (P27) L-tryptophan; (P30) L-phenylalanine; (P31) L-tyrosine; (P32) L-valine.
Overview of metabolic profile after GU treatment: (A) ESI+, PCA; (B) ESI+, OPLS-DA; (C) ESI-, PCA; (D) ESI-, OPLS-DA. (E) Permutations test plot for OPLS-DA (200 tests) in positive ion mode for rat plasma samples; (F) Permutations test plot for OPLS-DA (200 tests) in negative ion mode for rat plasma samples.
Expression of 24 plasma biomarkers in all groups, *P < 0.05, **P < 0.01 compared with the model group; #P < 0.05, ##P < 0.01 compared with the control group (Control = Control group; Model = Model group; GUW = GU administration of rats in model group).
RSD of typical peaks of plasma QC sample (ESI-.n=10; ESI+,n=10).
RSD of typical peaks of intestinal lavage fluid QC sample (ESI-.n=10; ESI+,n=10).
Data Availability Statement
The datasets generated for this study can be found in the Sequence Read Archive, https://www.ncbi.nlm.nih.gov/sra/?term= SRP214751.