Abstract
Background
Highly complex tasks generally benefit from increases in cognitive control, which has been linked to dopamine. Yet, the same amount of control may actually be detrimental in tasks with low complexity so that the task-dependent allocation of cognitive control resources (also known as “metacontrol”) is key to expedient and adaptive behavior in various contexts.
Methods
Given that dopamine D1 and D2 receptors have been suggested to exert opposing effects on cognitive control, we investigated the impact of 2 single nucleotide polymorphisms in the DRD1 (rs4532) and DRD2 (rs6277) genes on metacontrol in 195 healthy young adults. Subjects performed 2 consecutive tasks that differed in their demand for control (starting with the less complex task and then performing a more complex task rule).
Results
We found carriers of the DRD1 rs4532 G allele to outperform noncarriers in case of high control requirements (i.e., reveal a better response accuracy), but not in case of low control requirements. This was confirmed by Bayesian analyses. No effects of DRD2 rs6277 genotype on either task were evident, again confirmed by Bayesian analyses.
Conclusions
Our findings suggest that higher DRD1 receptor efficiency improves performance during high, but not low, control requirements, probably by promoting a “D1 state,” which is characterized by highly stable task set representations. The null findings for DRD2 signaling might be explained by the fact that the “D2 state” is thought to enhance flexible switching between task set representations when our task only featured 1 task set at any given time.
Keywords: cognitive control, metacontrol, dopamine, DRD1, DRD2, dual state theory
Significance Statement.
Efficient goal-directed behavior requires dynamical control allocation and an estimation of how much control is invested in a particular situation. The neurobiological mechanisms that are associated with this dynamical adjustment are widely elusive. To the best of our knowledge, this study is the first to investigate the relevance of the dopaminergic system with a focus on dopamine D1 and D2 receptors. Our findings suggest that only the DRD1 receptor system seems important for dynamical adjustments of how much cognitive control is invested in a particular situation. Theoretical implications of this finding are discussed.
Introduction
The ability to flexibly exert cognitive control in order to adapt to dynamic environments is key to successful goal-directed behavior (Botvinick et al., 2004; Gruber and Goschke, 2004; Bocanegra and Hommel, 2014; Larson et al., 2014). It is not, however, the best strategy to always invest the maximal possible amount of cognitive control irrespective of the situation at hand (Rubinstein et al., 2001; Hommel and Wiers, 2017; Pieczykolan and Huestegge, 2017). Complex tasks require cognitive control, with increases in complexity usually translating to increases in control requirements (Miller, 2000; Miller and Cohen, 2001; Botvinick et al., 2004; Ochsner and Gross, 2005; Botvinick and Braver, 2015). Simple tasks with low complexity, however, often seem to benefit from decreases in cognitive control. In such tasks, automatization is more expedient than cognitive control in that it makes information processing as well as response generation faster and less error-prone (Bocanegra and Hommel, 2014). Upregulating cognitive control can hence have adverse effects on performance in situations with low task complexity or where automatized processes would be optimal (Olivers and Nieuwenhuis, 2005; Taatgen et al., 2009; Bocanegra and Hommel, 2014; Stock et al., 2016; Zink et al., 2018a). Therefore, goal-directed behavior is best driven by a dynamical allocation of control (Bocanegra and Hommel, 2014; Hommel and Wiers, 2017), which matches the complexity or demands of a given situation, rather than always exerting high levels of control (Hommel and Wiers, 2017). Another factor that further adds to this need for “metacontrol” (Goschke and Bolte, 2014; Hommel and Wiers, 2017; Beste et al., 2018) is that cognitive control processes require effort and are capacity-limited (Meyer and Kieras, 1997; Engle and Kane, 2003; Kane and Engle, 2003; Tombu and Jolicoeur, 2003; Lavie et al., 2004; Marois and Ivanoff, 2005; Sigman and Dehaene, 2008; Engle, 2010; Lavie, 2010; Yildiz et al., 2013; Yildiz and Beste, 2015). The central question here is how metacontrol is achieved, that is, how cognitive control is adapted or regulated in situations with different control requirements (Doya, 2008; Goschke and Bolte, 2014).
The neurobiological mechanisms that drive and modulate metacontrol, especially the adaptation of response selection to varying cognitive control demands, have, however, remained largely elusive. Yet, it is already known that the dopaminergic system may play a key role in this process (Goschke and Bolte, 2014). In this context, the dual-state theory (Seamans and Yang, 2004; Durstewitz and Seamans, 2008; Durstewitz et al., 2010) describes the interplay of 2 relevant, functionally opposing “states” that are brought about by antagonistic effects of dopamine D1 and D2 receptors: Dopamine D1 receptor activation fosters a so-called “D1 state” that is characterized by stable mental representations. This rigidity makes it hard to switch between different (neuronal) activations, but at the same time allows to shield task-related activation against distractions and competing input (Seamans and Yang, 2004; Durstewitz and Seamans, 2008). The D1 state should therefore be optimal in situations with high demands on cognitive control that require stable processing. In contrast to this, dopamine D2 receptor activation fosters a so-called “D2 state” that allows for easy and flexible shifting between different neuronal representations. However, it comes at the cost of lower representation stability, which impairs the ability to effectively shield task-related activation against distractions and competing input, thus making it prone to interference. When interpreting this state as a reduction in cognitive control (Kornblum et al., 1990; Hommel, 1993; Botvinick et al., 2001; Ridderinkhof, 2002; Ulrich et al., 2015), it should make the D2 state optimal in situations that do not impose high demands on cognitive control so that information processing can be fairly automatic. To examine how D1 and D2 receptor processes modulate metacontrol, we genotyped 2 single-nucleotide polymorphisms (SNPs) in the DRD1 (rs4532) and DRD2 (rs6277) receptor genes.
Rs4532 is located in the 5′ untranslated region of DRD1, and the minor G allele has been associated with higher D1 receptor efficiency (Dolžan et al., 2007; Novak et al., 2010), which has previously been linked to increased cognitive control (Kehagia et al., 2010; Stock et al., 2014; Beste et al., 2016). Therefore, DRD1 G allele carriers should be more inclined to the dopamine D1 state than A allele carriers (Stock et al., 2014).
Rs6277 is a synonymous SNP (Pro319Pro) where homozygous T allele carriers have been shown to display greater striatal dopamine D2 receptor density (Hirvonen et al., 2004; Frank and Hutchison, 2009), which has previously been associated with decreased cognitive control (Kehagia et al., 2010; Stock et al., 2014; Beste et al., 2016). It can be assumed that homozygous T allele carriers are more inclined towards a dopamine D2 state (Durstewitz and Seamans, 2002, 2008) than CT and CC carriers (Stock et al., 2014).
In summary, our study investigated the effects of DRD1 and DRD2 receptor genotypes in 195 healthy individuals, who were assessed with an experiment consisting of 2 complementary tasks (Bocanegra and Hommel, 2014; Zink et al., 2018a, 2018b). Subjects responded to the same set of stimuli in both tasks, but the demand for cognitive control was manipulated by asking them to either follow a less complex rule (easy task/low cognitive control requirement) or a more complex rule (hard task/high cognitive control requirement). We hypothesized that DRD1 (rs4532) G allele carriers should be better able than noncarriers to exert cognitive control in situations with high cognitive control demand (i.e., when task rules are complex). This should be reflected by better behavioral performance (i.e., higher accuracy/faster response times [RTs]) in the task version with a complex rule but not necessarily in case of low control requirements (i.e., the easy task). We further hypothesized that DRD2 (rs6277) T allele carriers should have lower control than noncarriers. This might impair performance in high control situations (i.e., in the high demand task) but could also yield a benefit in the low demand task. However, we were less certain about our hypotheses on DRD2 effects, as we previously observed DRD2 effects on task set switching under high cognitive control demands (Stock et al., 2014). Against this background, it could also be possible that there are no effects of DRD2 receptor genotypes in the easy task, since the task used for this study requires neither to flexibly switch between several task goal representations, nor high levels of cognitive control.
Materials and Methods
Sample
A total of 195 healthy young participants (137 females, 57 males) between 18 and 32 years of age (mean 23.9, SD 3.2) were included in this study. All participants were of Caucasian descent, reported no psychiatric or neurologic diseases, and stated to have normal or corrected-to-normal vision. Depression was ruled out using the Beck Depression Inventory scores (mean = 4.4, SD = 4.2). Each participant received a financial reimbursement of 25 €. All participants gave written informed consent and were treated in accordance with the Declaration of Helsinki. The study was approved by the local ethics committee of the TU Dresden, Germany.
Please note that while our work group has used the same SNPs to investigate other functionally unrelated questions in previous studies (Stock et al., 2014; Beste et al., 2016), none of the individuals included in the current sample took part in these previous studies.
Genotyping
Genomic DNA was extracted from whole blood samples. The DRD1 rs4532 SNP and DRD2 rs6277 SNP were genotyped using polymerase chain reaction-restriction fragment-length polymorphism. SNP rs4532 is located in the 5′ untranslated region of DRD1 and rs6277 is a synonymous SNP (Pro319Pro) within the coding sequence of DRD2. Primers were designed with Primer Express 2.0 software (Applied Biosystems). Details of the methodology and primer sequences are available on request.
Task and Experimental Setup
During the experiment, the participants sat in front of a 17-inch CRT computer monitor at a viewing distance of 57 cm. Participants were asked to respond with 2 keys (left and right Ctrl key) on a regular QWERTZ keyboard with their left and right index fingers, respectively. The software Presentation (version 14.9. of Neurobehavioral Systems, Inc.) was used for stimulus presentation and response recording.
A modified version of an experimental paradigm developed by Bocanegra and Hommel (2014) was used in this study. The paradigm consists of 2 separate tasks, called “automatic” and “control” task. However, since they differ in the complexity of the task rules and thus in the level of cognitive control required, we decided to refer to them as “easy” and “hard” task, respectively.
Following the protocol by Bocanegra and Hommel (2014) and to keep possible order effects constant across genotype groups, all participants first performed the hard and then the easy task version. As mentioned above, we defined metacontrol as the adaptive and demand-specific allocation of cognitive control as a function of task complexity (Goschke and Bolte, 2014; Hommel and Wiers, 2017; Beste et al., 2018). This implies that metacontrol is an adaptive (i.e., flexible) process but also responsive to the demand on cognitive control. Using a fixed consecutive order design, we decided to focus on the demand on cognitive control rather than the adaptability processes to (1) replicate the findings by Bocanegra and Hommel (2014) and (2) avoid confounding metacontrol with task switching processes. Thus, the fixed consecutive task order in our block-wise design measures the ability to up- and downregulate cognitive control depending on the demand on cognitive control capacities rather than the ability to flexibly switch between varying cognitive control demands.
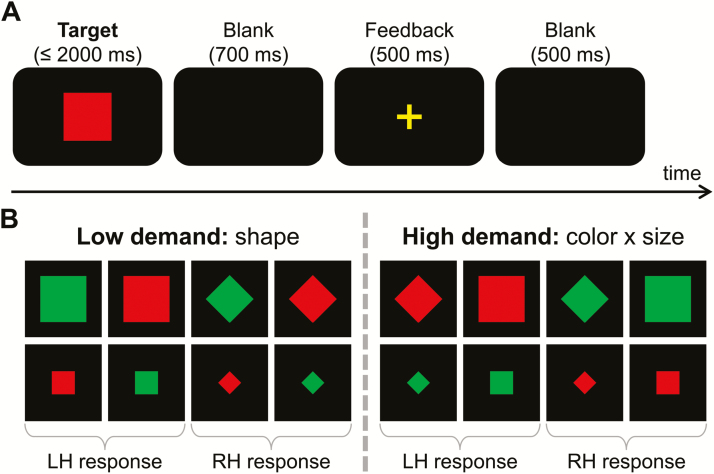
Each trial began with the presentation of a single visual stimulus in the middle of a black screen for 2000 ms or until a response was given (see Figure 1A). The stimuli varied in shape (square or diamond), color (green or red), and size (small: about 2.5 cm in diameter or large: about 5 cm in diameter). In both tasks, all 8 possible combinations of stimulus characteristics were presented equally often (see Figure 1B). In the easy task, participants were instructed to react only to the shape of the stimuli. Whenever the target stimulus was a diamond, the left Ctrl key had to be pressed with the left index finger. Whenever the target stimulus was a square, the right Ctrl key had to be pressed with the right index finger. In the hard task, participants were asked to respond to a combination of target size and color (see Figure 1B). The left Ctrl key had to be pressed when the target stimulus was large and red or when it was small and green. The right Ctrl key had to be pressed when the target stimulus was large and green or when it was small and red. If no response was given within 2000 ms, stimulus presentation was stopped and the trial was coded as “miss.” A 500-millisecond feedback was given 700 milliseconds after the target stimulus offset to tell participants whether their response was correct (“+”) or incorrect (“−”). After the feedback, there was another fixation cross for 500 milliseconds before the next trial was presented. Each task was divided into 5 equal blocks 96 trials each. Hence, each task (easy and hard) comprised a total of 480 trials. Behavioral measures (accuracy and mean response times of correct answers) were collected separately for the easy and hard task version.
Figure 1.
(A) Illustration of the task. Each trial started with a target presentation, which was either terminated by the first response or after 2000 milliseconds had elapsed (in this case, the trial was coded as a “miss”). The target was followed by a 700-millisecond blank screen, a 500-millisecond feedback (“+” for correct and “−” for incorrect or missed responses), and a second 500-millisecond blank screen. (B) Illustration of employed target stimuli. Targets could vary in shape (square vs diamond), size (small vs large), and color (green vs red). The easy low demand task required left hand (“LH”) button presses for squares and right hand (“RH”) button presses for diamonds (see left bottom graph). The harder high demand task required left hand button presses for targets that were either large and red OR small and green while right hand button presses were required for targets that were either large and green OR small and red (see right bottom graph). Hence, the 4 stimuli on the left of each graph required a left hand response while the 4 stimuli on the right of each graph required a right hand response.
Statistical Analyses
The behavioral data (RTs and accuracy) were analyzed using SPSS Statistics 24. We ran separate mixed-effects ANOVAs with the between-subject factor “genotype” (AA vs AG vs GG for DRD1 rs4532; CC vs CT vs TT for DRD2 rs6277) and the within-subject factor “task” (easy task vs hard task). Greenhouse-Geisser corrections were applied whenever necessary. In the results section, the reported mean values are followed by the SEM as a measure of variance.
Results
Genotype Groups
Of the n = 195 participants, 86 were homozygous for the DRD1 rs4532 A allele, 86 were A/G carriers, and 23 were homozygous for the G allele. Due to the rather low frequency of GG genotype carriers, the AG and GG genotypes were combined into 1 group for further analyses. The observed frequencies for A and G allele were 66.2%. and 33.8%, respectively. Six subjects could not be genotyped with respect to the DRD2 rs6277 SNP. Of the remaining 189 participants, 49 were homozygous for the C allele, 98 were C/T carriers, and 42 were homozygous for the T allele. The observed frequencies for C and T allele were 51.9% and 48.1%, respectively. The distribution of genotypes did not significantly differ from the Hardy–Weinberg equilibrium (P = .596).
Task Performance Data
Behavioral performance is illustrated in Figure 2.
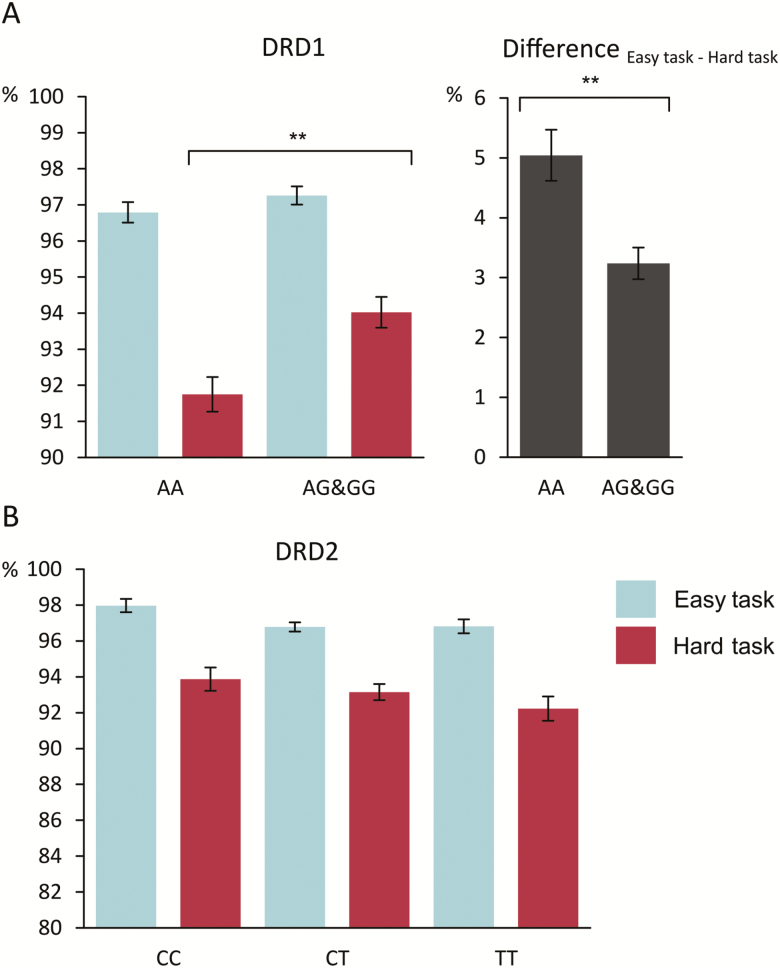
Figure 2.
(A) Illustration of response accuracy for the different genotype groups of the DRD1 (rs4532) single nucleotide polymorphism (SNP). G allele carriers (AG&GG) showed a smaller performance difference between the easy and hard task than homozygous A allele carriers (AA) (right graph). This performance difference was mainly driven by higher accuracy in the hard task for the AG&GG group compared to the AA group. (B) Illustration of response accuracy for the different genotype groups of the DRD2 (rs6277) SNP. There were no accuracy differences between the 3 genotype groups (CC, CT, and TT) in the easy task or the hard task. Error bars indicate SEMs, and significant group differences are denoted with asterisks (**P < .001).
DRD1
The analysis of the hit RTs showed a main effect of task (F(1,193) = 1647.96; P < .001; ηp2 = 0.895), with longer RTs in the hard task (678 ms ± 8) compared with the easy task (463 ms ± 5). No other main or interaction effects were significant for hit RTs (all F < 0.498, P > .481).
The accuracy analysis revealed a main effect of task (F(1,193) = 295.49; P < .001; ηp2 = 0.605), with higher accuracy in the easy task (97.0% ± 0.2) compared with the hard task (92.9% ± 0.3). There was also a main effect of genotype (F(1,193) = 8.54; P = .004; ηp2 = 0.042), with higher accuracy in the AG&GG group (95.6% ± 0.3) compared with the AA group (94.3% ± 0.4). Importantly, there was an interaction of task × genotype (F(1,193) = 14.05; P < .001; ηp2 = 0.068). Post-hoc t tests showed that both genotype groups had a significant task effect (i.e., significantly higher accuracy in the easy task than in the hard task; all t < 11.83, P < .001). However, the task difference (easy minus hard) was significantly larger for the AA group (5.0% ± 0.4), than for the AG&GG group (3.2% ± 0.3) (t(145.96) = 3.60, P < .001). Importantly, this effect was driven by a significant genotype difference that could be found in the hard task (t(193) = −3.54, P = .001; AA group: 91.7% ± 0.6 vs AG&GG group: 94.0% ± 0.4) but not in the easy task (t(193) = −1.23, P = .219; AA group: 96.8% ± 0.3 vs AG&GG group: 97.3% ± 0.3). To confirm that there was indeed no main effect of genotype group in the easy task, we conducted additional Bayesian analyses as suggested by Wagenmakers (2007) using the template by Masson (2011). With this analysis, the probability of the null hypothesis being true, given the observed data, p(H0|D), can be determined. Values <0.5 indicate that the alternative hypothesis is more likely to be true than the null hypothesis. Values between 0.5 and 0.75 provide weak evidence, values between 0.75 and 0.95 give positive evidence, and values between 0.95 and 0.99 give strong evidence for the null hypothesis being true (Raftery, 1995). Regarding the main effect of genotype group in the easy task, a value of p(H0|D) = 0.951 was obtained for the accuracy data. This provides strong evidence that the DRD1 genotype did not modulate performance in the easy task.
DRD2
The analysis of the hit RTs showed a main effect of task (F(1,190) = 1442.91; P < .001; ηp2 = 0.884), with longer RTs in the hard task (676 ms ± 8) compared with the easy task (462 ms ± 5). No other main or interaction effects were significant for hit RTs (all F < 0.960, P > .385). The accuracy analysis revealed a main effect of task (F(1,190) = 247.14; P < .001; ηp2 = 0.565), with higher accuracy in the easy task (97.2% ± 0.2) compared with the hard task (93.1% ± 0.4). No other main or interaction effects were significant for accuracy (all F < 2.25, P > .108). To confirm that there was indeed no main or interaction effect of genotype, we conducted additional Bayesian analysis. For the main effect of genotype, we obtained a value of p(H0|D) = 0.987 for hit RTs and p(H0|D) = 0.954 for accuracy. For the interaction of task × genotype, we obtained a value of p(H0|D) = 0.993 for hit RTs and p(H0|D) = 0.983 for accuracy. This constitutes strong evidence for the null hypothesis.
Discussion
In the current study, we examined the relevance of the dopamine D1 and D2 receptor system for cognitive control processes using a molecular genetic approach. More specifically, we examined whether D1 and D2 receptor-related processes play different or opposing roles for metacontrol, which is defined as the adaptive and demand-specific allocation of cognitive control as a function of task complexity (Goschke and Bolte, 2014; Hommel and Wiers, 2017; Beste et al., 2018). This kind of metacontrol is an important competence, as a cognitive control system that always exerts the same level of cognitive control (i.e., irrespective of how much control is optimal or needed) is highly dysfunctional and has been proven detrimental to performance in situations with low control requirements (Hommel and Wiers, 2017; Stock et al., 2019). In short, the obtained results show that DRD1 genotypes modulated performance in the harder version of the applied task, which requires a higher level of cognitive control. DRD1 genotypes did not, however, modulate performance in the easier version of the applied task, which requires a lower level of cognitive control. In contrast to this, DRD2 genotypes modulated performance in neither the easy task nor the harder task. This lack of effects was supported by Bayesian analyses. This pattern of results has important implications for our understanding of the effect of dopamine receptor functions on cognitive control.
The DRD1 rs4532 G allele has been associated with higher D1 receptor efficiency (Dolžan et al., 2007; Novak et al., 2010), which has previously been linked to improved cognitive control (Kehagia et al., 2010; Stock et al., 2014; Trampush et al., 2014; Beste et al., 2016). Based on this, we had hypothesized that DRD1 G allele carriers should be more inclined to the dopamine D1 state than A allele carriers and, as a consequence, perform better in the high demand task (but not necessarily in the low demand task). Our results of improved performance in the high demand task only are hence well in line with previous reports on the control-promoting effects of the G allele as well as our hypotheses. In the hard task version, participants were asked to respond to a combination of 2 independent stimulus features. This imposes rather high demands on working memory processes, as several aspects need to be considered for successful response selection. Matching this, the maintenance of stable representations in neural networks has been attributed to be a major aspect of the D1 state (Durstewitz and Seamans, 2008). The results on the DRD1 genotype effects are thus well in line with the predictions of the dual state theory. This finding allows to draw the conclusion that improved DRD1 signaling is associated with improved cognitive control in cases of high control demands. Inferences about meta-control should not, however, be based on this finding alone, as the lack of DRD1 effects in the low demand task means that we did not demonstrate any (detrimental) effects of DRD1 on the disengagement of control, which has previously been argued to be beneficial in tasks with low control demands (Bocanegra and Hommel, 2014; Stock et al., 2016).
The DRD2 rs6277 T allele has been associated with greater striatal dopamine D2 receptor density (Hirvonen et al., 2004; Frank and Hutchison, 2009), which has previously been linked to decreased cognitive control (Kehagia et al., 2010; Stock et al., 2014; Beste et al., 2016). We therefore hypothesized that T allele carriers should be more inclined towards a dopamine D2 state (Durstewitz and Seamans, 2002, 2008; Stock et al., 2014). We further reasoned that they should have lower levels of control and therefore show worse performance in the high demand task, but potentially beneficial effects in the low demand task. However, we did not observe either of these 2 effects, and Bayes statistics provided strong evidence for the null hypothesis in both cases.
When trying to understand the lack of DRD effects on the hard task, it could be helpful to look at how dopamine is thought to promote cognitive control. Evidence suggests that D1 receptor-mediated effects in the prefrontal cortex and the striatum are promoted by medium DA concentrations (West and Grace, 2002; Lavin et al., 2005; Durstewitz and Seamans, 2008), while very low (and also very high) DA concentrations foster a D2 state (Durstewitz and Seamans, 2008). According to the inverted U-shape function between dopamine concentrations and working memory performance (Goldman-Rakic, 1995; Cools and D’Esposito, 2011), increases in working memory or cognitive control demands necessitate higher DA levels for optimal performance. It is thus likely that optimal performance in the hard task requires higher DA concentrations than optimal performance in the easier task, because the latter has comparatively lower control or working memory demands. Within a medium range, higher DA concentrations likely lead to a stronger modulation of D1 compared with D2 receptors. This suggests that genetic variations in DRD1, rather than DRD2, seem to modulate performance in the hard task with high control requirements.
With respect to the lack of DRD2 effects on the easy task, it could be helpful to look at the dual-state state theory. The D2 state confers the advantage of easier access of information to neural networks, which should improve flexibility in terms of switching between different task set representations (Durstewitz and Seamans, 2008), but is also a relevant prerequisite to become processed quite automatically or with low cognitive control (Kornblum et al., 1990; Hommel, 1993; Botvinick et al., 2001; Ridderinkhof, 2002; Ulrich et al., 2015). Against this background, we had expected DRD2 T allele carriers to thrive in the easy task due to having higher striatal dopamine D2 receptor density (Hirvonen et al., 2004; Frank and Hutchison, 2009), which has previously been suggested to decrease cognitive control (Kehagia et al., 2010; Stock et al., 2014; Beste et al., 2016). Yet, enhanced access to task rule representations in the D2 state might be especially advantageous in situations that require to flexibly switch between several (task rule) representations (Durstewitz and Seamans, 2008). While the stimuli used in this study did have task-irrelevant features, the task itself did not require the participants to actively switch back and forth between different task set representations. Instead, the task adapted from Bocanegra and Hommel (2014) focused more on the general difference between high and low control requirements in a block-wise design. Thus, the paradigm only assesses the general ability to up- or downregulate cognitive control capacities depending on the demand on cognitive control load. It is hence possible that the factor that determines the functional relevance of DRD2 signaling is the question of whether flexibility is necessary, rather than whether the updating threshold is lowered. This is also supported by findings showing that DRD2 genotype effects are evident in situations with high control demands that also require cognitive flexibility (Stock et al., 2014). It could therefore be possible that DRD2 effects would only emerge if a “mixed” block was introduced to the paradigm, which cues the participants to flexibly switch between the low and high demand task. As we did not anticipate this and therefore included no such experimental block, this will however remain speculative until tested in future studies.
Nevertheless, the current findings have important implications for the ongoing debate on how dynamical adjustments of cognitive control emerge in the process of metacontrol (Bocanegra and Hommel, 2014; Goschke and Bolte, 2014; Hommel and Wiers, 2017). It has been suggested that the updating threshold represents a metacontrol parameter, that is, a factor that facilitates dynamical adjustments of cognitive control (Goschke and Bolte, 2014). Modulations of the updating threshold, which determine how easily information gets access to response selection processes, have been suggested to play a central role (Goschke and Bolte, 2014). While the updating threshold clearly differs between dopamine D1 and D2 states (Durstewitz and Seamans, 2008), the current data show that this factor does not seem to modulate performance in situations with low control demands, which should benefit from a low updating threshold. It therefore seems that a simple updating threshold does not provide a comprehensive explanation of how dynamical adjustments in cognitive control emerge in situations with little demand for switching between multiple representations. In other words, modulations of the updating threshold alone seem insufficient to enable dynamics akin to metacontrol. It has however been suggested that the dopamine-dependent calculation of costs and benefits plays an important role in deciding how to select a response and how much control needs to be invested (Cools, 2016; Shenhav et al., 2016; Hommel and Wiers, 2017).
It should furthermore be noted that even though we found solid evidence for both the presence of DRD1 effects and the absence of DRD2 effects on the assessed behavior, the assessed data do not allow to draw any conclusion about the interaction of these 2 dopaminergic transmitter systems. The main reason for this limitation is that our sample lacks the power (i.e., is not sufficiently large) to properly investigate potential epistasis effects of the interaction of the 2 SNPs. Therefore, we cannot provide information on how the alleles of the DRD1 rs4532 and DRD2 rs6277 SNPs interact in the assessed meta-control measures.
In summary, we investigated the relevance of dopamine D1 and D2 receptor signaling for cognitive control allocation or metacontrol by assessing performance in 2 experimental blocks, which differed in the degree of required cognitive control. Given that dopamine D1 and D2 receptors have been suggested to exert opposing effects on cognitive control, we investigated the impact of 2 SNPs in the DRD1 (rs4532) and DRD2 (rs6277) genes on metacontrol in 195 healthy young adults. Matching previous reports of higher receptor efficiency, we found carriers of the DRD1 G allele to outperform noncarriers in cases of high control requirements. Yet, we found no effects of DRD2 genotypes on high control requirements and no effects of either SNP on low control requirements (as confirmed by add-on Bayesian analyses). Our findings suggest that higher DRD1 receptor efficiency may improve performance when a high degree of cognitive control is required, probably by promoting a “D1 state,” which is characterized by highly stable task set representations. The lack of effects for DRD2 signaling might be explained by the fact that the “D2 state” is thought to enhance flexible switching between task set representations, when our task only featured 1 task set at any given time. Given the overall lack of effects on the easy task, the obtained data do not, however, allow for strong conclusions on complementary DRD1 and DRD2 effects on metacontrol, that is, control allocation in situations with different control requirements. Hence, future studies should explore more elaborate or alternative explanations by putting them to the test.
Acknowledgments
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (DFG) SFB 940 project B8.
Statement of Interest
None.
References
- Beste C, Moll CKE, Pötter-Nerger M, Münchau A (2018) Striatal microstructure and its relevance for cognitive control. Trends Cogn Sci 22:747–751. [DOI] [PubMed] [Google Scholar]
- Beste C, Stock AK, Epplen JT, Arning L (2016) Dissociable electrophysiological subprocesses during response inhibition are differentially modulated by dopamine D1 and D2 receptors. Eur Neuropsychopharmacol 26:1029–1036. [DOI] [PubMed] [Google Scholar]
- Bocanegra BR, Hommel B (2014) When cognitive control is not adaptive. Psychol Sci 25:1249–1255. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Braver T (2015) Motivation and cognitive control: from behavior to neural mechanism. Annu Rev Psychol 66:83–113. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD (2001) Conflict monitoring and cognitive control. Psychol Rev 108:624–652. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS (2004) Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci 8:539–546. [DOI] [PubMed] [Google Scholar]
- Cools R. (2016) The costs and benefits of brain dopamine for cognitive control. Wiley Interdiscip Rev Cogn Sci 7:317–329. [DOI] [PubMed] [Google Scholar]
- Cools R, D’Esposito M (2011) Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry 69:e113–e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolžan V, Plesničar BK, Serretti A, Mandelli L, Zalar B, Koprivšek J, Breskvar K (2007) Polymorphisms in dopamine receptor DRD1 and DRD2 genes and psychopathological and extrapyramidal symptoms in patients on long-term antipsychotic treatment. Am J Med Genet B Neuropsychiatr Genet 144B:809–815. [DOI] [PubMed] [Google Scholar]
- Doya K. (2008) Modulators of decision making. Nat Neurosci 11:410–416. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK (2002) 2002 Special issue. The computational role of dopamine D1 receptors in working memory. Neural Netw 15:561–572. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK (2008) The dual-state theory of prefrontal cortex dopamine function with relevance to catechol-o-methyltransferase genotypes and schizophrenia. Biol Psychiatry 64:739–749. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Vittoz NM, Floresco SB, Seamans JK (2010) Abrupt transitions between prefrontal neural ensemble states accompany behavioral transitions during rule learning. Neuron 66:438–448. [DOI] [PubMed] [Google Scholar]
- Engle RW. (2010) Role of working-memory capacity in cognitive control. Curr Anthropol 51:S17–S26. [Google Scholar]
- Engle RW, Kane MJ (2003) Executive attention, working memory capacity, and a two-factor theory of cognitive control. Psychol Learn Motiv 44:145–199. [Google Scholar]
- Frank MJ, Hutchison K (2009) Genetic contributions to avoidance-based decisions: striatal D2 receptor polymorphisms. Neuroscience 164:131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. (1995) Cellular basis of working memory. Neuron 14:477–485. [DOI] [PubMed] [Google Scholar]
- Goschke T, Bolte A (2014) Emotional modulation of control dilemmas: the role of positive affect, reward, and dopamine in cognitive stability and flexibility. Neuropsychologia 62:403–423. [DOI] [PubMed] [Google Scholar]
- Gruber O, Goschke T (2004) Executive control emerging from dynamic interactions between brain systems mediating language, working memory and attentional processes. Acta Psychol (Amst) 115:105–121. [DOI] [PubMed] [Google Scholar]
- Hirvonen M, Laakso A, Någren K, Rinne JO, Pohjalainen T, Hietala J (2004) C957T polymorphism of the dopamine D2 receptor (DRD2) gene affects striatal DRD2 availability in vivo. Mol Psychiatry 9:1060–1061. [DOI] [PubMed] [Google Scholar]
- Hommel B. (1993) The relationship between stimulus processing and response selection in the Simon task: evidence for a temporal overlap. Psychol Res 55:280–290. [Google Scholar]
- Hommel B, Wiers RW (2017) Towards a unitary approach to human action control. Trends Cogn Sci 21:940–949. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Engle RW (2003) Working-memory capacity and the control of attention: the contributions of goal neglect, response competition, and task set to Stroop interference. J Exp Psychol Gen 132:47–70. [DOI] [PubMed] [Google Scholar]
- Kehagia AA, Murray GK, Robbins TW (2010) Learning and cognitive flexibility: frontostriatal function and monoaminergic modulation. Curr Opin Neurobiol 20:199–204. [DOI] [PubMed] [Google Scholar]
- Kornblum S, Hasbroucq T, Osman A (1990) Dimensional overlap: cognitive basis for stimulus-response compatibility–a model and taxonomy. Psychol Rev 97:253–270. [DOI] [PubMed] [Google Scholar]
- Larson MJ, Clayson PE, Clawson A (2014) Making sense of all the conflict: a theoretical review and critique of conflict-related ERPs. Int J Psychophysiol 93:283–297. [DOI] [PubMed] [Google Scholar]
- Lavie N. (2010) Attention, distraction, and cognitive control under load. Curr Dir Psychol Sci 19:143–148. [Google Scholar]
- Lavie N, Hirst A, de Fockert JW, Viding E (2004) Load theory of selective attention and cognitive control. J Exp Psychol Gen 133:339–354. [DOI] [PubMed] [Google Scholar]
- Lavin A, Nogueira L, Lapish CC, Wightman RM, Phillips PE, Seamans JK (2005) Mesocortical dopamine neurons operate in distinct temporal domains using multimodal signaling. J Neurosci 25:5013–5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marois R, Ivanoff J (2005) Capacity limits of information processing in the brain. Trends Cogn Sci 9:296–305. [DOI] [PubMed] [Google Scholar]
- Masson ME. (2011) A tutorial on a practical Bayesian alternative to null-hypothesis significance testing. Behav Res Methods 43:679–690. [DOI] [PubMed] [Google Scholar]
- Meyer DE, Kieras DE (1997) A computational theory of executive cognitive processes and multiple-task performance: part 1. Basic mechanisms. Psychol Rev 104:3–65. [DOI] [PubMed] [Google Scholar]
- Miller EK. (2000) The prefrontal cortex and cognitive control. Nat Rev Neurosci 1:59–65. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD (2001) An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24:167–202. [DOI] [PubMed] [Google Scholar]
- Novak G, LeBlanc M, Zai C, Shaikh S, Renou J, DeLuca V, Bulgin N, Kennedy JL, Le Foll B (2010) Association of polymorphisms in the BDNF, DRD1 and DRD3 genes with tobacco smoking in schizophrenia. Ann Hum Genet 74:291–298. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ (2005) The cognitive control of emotion. Trends Cogn Sci 9:242–249. [DOI] [PubMed] [Google Scholar]
- Olivers CN, Nieuwenhuis S (2005) The beneficial effect of concurrent task-irrelevant mental activity on temporal attention. Psychol Sci 16:265–269. [DOI] [PubMed] [Google Scholar]
- Pieczykolan A, Huestegge L (2017) Cross-modal action complexity: action- and rule-related memory retrieval in dual-response control. Front Psychol 8:529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raftery AE. (1995) Bayesian model selection in social research. Sociol Methodol 25:111. [Google Scholar]
- Ridderinkhof KR. (2002) Micro- and macro-adjustments of task set: activation and suppression in conflict tasks. Psychol Res 66:312–323. [DOI] [PubMed] [Google Scholar]
- Rubinstein JS, Meyer DE, Evans JE (2001) Executive control of cognitive processes in task switching. J Exp Psychol Hum Percept Perform 27:763–797. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Yang CR (2004) The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol 74:1–58. [DOI] [PubMed] [Google Scholar]
- Shenhav A, Cohen JD, Botvinick MM (2016) Dorsal anterior cingulate cortex and the value of control. Nat Neurosci 19:1286–1291. [DOI] [PubMed] [Google Scholar]
- Sigman M, Dehaene S (2008) Brain mechanisms of serial and parallel processing during dual-task performance. J Neurosci 28:7585–7598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock AK, Arning L, Epplen JT, Beste C (2014) DRD1 and DRD2 genotypes modulate processing modes of goal activation processes during action cascading. J Neurosci 34:5335–5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock AK, Steenbergen L, Colzato L, Beste C (2016) The system neurophysiological basis of non-adaptive cognitive control: inhibition of implicit learning mediated by right prefrontal regions. Hum Brain Mapp 37:4511–4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock AK, Rädle M, Beste C (2019) Methamphetamine-associated difficulties in cognitive control allocation may normalize after prolonged abstinence. Prog Neuropsychopharmacol Biol Psychiatry 88:41–52. [DOI] [PubMed] [Google Scholar]
- Taatgen NA, Juvina I, Schipper M, Borst JP, Martens S (2009) Too much control can hurt: a threaded cognition model of the attentional blink. Cogn Psychol 59:1–29. [DOI] [PubMed] [Google Scholar]
- Tombu M, Jolicoeur P (2003) A central capacity sharing model of dual-task performance. J Exp Psychol Hum Percept Perform 29:3–18. [DOI] [PubMed] [Google Scholar]
- Trampush JW, Jacobs MM, Hurd YL, Newcorn JH, Halperin JM (2014) Moderator effects of working memory on the stability of ADHD symptoms by dopamine receptor gene polymorphisms during development. Dev Sci 17:584–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich R, Schröter H, Leuthold H, Birngruber T (2015) Automatic and controlled stimulus processing in conflict tasks: superimposed diffusion processes and delta functions. Cogn Psychol 78:148–174. [DOI] [PubMed] [Google Scholar]
- Wagenmakers EJ. (2007) A practical solution to the pervasive problems of p values. Psychon Bull Rev 14:779–804. [DOI] [PubMed] [Google Scholar]
- West AR, Grace AA (2002) Opposite influences of endogenous dopamine D1 and D2 receptor activation on activity states and electrophysiological properties of striatal neurons: studies combining in vivo intracellular recordings and reverse microdialysis. J Neurosci 22:294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz A, Beste C (2015) Parallel and serial processing in dual-tasking differentially involves mechanisms in the striatum and the lateral prefrontal cortex. Brain Struct Funct 220:3131–3142. [DOI] [PubMed] [Google Scholar]
- Yildiz A, Chmielewski W, Beste C (2013) Dual-task performance is differentially modulated by rewards and punishments. Behav Brain Res 250:304–307. [DOI] [PubMed] [Google Scholar]
- Zink N, Stock AK, Colzato L, Beste C (2018a) Evidence for a neural dual-process account for adverse effects of cognitive control. Brain Struct Funct 223:3347–3363. [DOI] [PubMed] [Google Scholar]
- Zink N, Stock A-K, Vahid A, Beste C (2018b) On the neurophysiological mechanisms underlying the adaptability to varying cognitive control demands. Front Hum Neurosci 12:411. [DOI] [PMC free article] [PubMed] [Google Scholar]