Abstract
Background
Individuals with cocaine addiction are characterized by under-responsiveness to natural reinforcers. As part of the dopaminergic pathways, the hypothalamus supports motivated behaviors. Rodent studies suggested inter-related roles of the hypothalamus in regulating drug and food intake. However, few studies have investigated hypothalamic responses to drugs and food or related cues in humans.
Methods
We examined regional responses in 20 cocaine-dependent and 24 healthy control participants exposed to cocaine/food (cocaine dependent) and food (healthy control) vs neutral cues during functional magnetic resonance imaging. We examined the relationship between imaging findings and clinical variables and performed mediation analyses to examine the inter-relationships between cue-related activations, tonic cocaine craving, and recent cocaine use.
Results
At a corrected threshold, cocaine-dependent participants demonstrated higher activation to cocaine than to food cues in the hypothalamus, inferior parietal cortex, and visual cortex. Cocaine-dependent participants as compared with healthy control participants also demonstrated higher hypothalamic activation to food cues. Further, the extent of these cue-induced hypothalamic activations was correlated with tonic craving, as assessed by the Cocaine Craving Questionnaire, and days of cocaine use in the prior month. In mediation analyses, hypothalamic activation to cocaine and food cues both completely mediated the relationship between the Cocaine Craving Questionnaire score and days of cocaine use in the past month.
Conclusions
The results were consistent with the proposition that the mechanisms of feeding and drug addiction are inter-linked in the hypothalamus and altered in cocaine addiction. The findings provide new evidence in support of hypothalamic dysfunction in cocaine addiction.
Keywords: cocaine, hypothalamus, drug cue, food cue, fMRI
Significance Statement.
The hypothalamus is an evolutionally old brain region that regulates motivated behavior such as eating and mating. Chronic cocaine use is known to lead to hypothalamic dysfunction and diminished response to natural rewards. We employed brain imaging to investigate how the hypothalamus responds to cocaine vs food cues in chronic cocaine users. Cocaine users showed higher hypothalamic activation to cocaine vs food cues, and, in contrast with non-drug users, higher hypothalamic activation to food cues too. The extent of these cue-induced hypothalamic activations was associated with cocaine craving. Further, statistical analyses suggested hypothalamic activation as an important neural mediator linking cocaine craving to cocaine consumption. Together, the results are consistent with the proposition that the mechanisms of feeding and drug addiction are inter-linked in the hypothalamus. The findings provide new evidence in support of hypothalamic dysfunction as a neural marker of altered motivations in cocaine-addicted individuals.
Introduction
Both eating and drug consumption are motivated behaviors that can be reinforced by contextual cues and depend critically on dopaminergic signaling (Volkow et al., 2012, 2017). Many addictive drugs engage the dopaminergic circuits and quantitatively scale the reward responses, which eventually lead to compulsive drug seeking (Kelley and Berridge, 2002). In humans, use of cocaine and other psycho-stimulants is widely associated with suppression of appetite and reduced body weight (Cochrane et al., 1998). It is possible that chronic cocaine exposure results in diminished response of the reward circuits to food and, as a result, less motivation for food intake. On the other hand, an earlier study demonstrated that, despite reduced body weight, cocaine-dependent men consumed more high-fat and -carbohydrate food (Ersche et al., 2013). Further, cocaine-dependent men showed a decrease in plasma leptin, a hormone secreted by adipose tissue to suppress hunger, compared with non-drug–using controls (Ersche et al., 2013). Thus, contrary to the common conception, reduced body weight in cocaine users does not appear to result from a decrease in appetite and food intake, and overeating during early abstinence may simply reflect the use of food as a drug substitute (Cowan and Devine, 2008; Ersche et al., 2013; Billing and Ersche, 2015). These findings raise the important questions of how cocaine-addicted and non-drug–using individuals may differ in the responses of the motivational circuits to food intake or exposure to food cues and how cocaine-addicted individuals differ in the responses to drug vs food intake or exposure to drug vs food cues.
Examining brain activations to food and cocaine cues in 20 individuals with cocaine dependence (CD) with both functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) imaging, a recent work reported that cocaine and food cues elicited similar circuit responses and that striatal dopamine D2/D3 receptor binding potentials modulated these responses (Tomasi et al., 2015). Specifically, the authors observed “deactivations” of the ventral striatum (VS) and hypothalamus to food and cocaine as compared with neutral cues, with less deactivation in the hypothalamus to cocaine than to food cues. The study highlighted hypothalamic responses to cue exposure; on the other hand, as acknowledged by the authors, the findings of lower hypothalamus and VS activations were surprising and in contrast to the literature of enhanced dopaminergic circuit activation to drug cues (Berthoud, 2007; Volkow et al., 2017). The authors suggested that the findings might reflect the use of videos rather than pictures in their cue reactivity paradigms. Using picture cues, we previously examined regional responses in 23 cocaine-dependent individuals exposed to cocaine vs neutral cues during fMRI, and, at a corrected threshold, observed higher hypothalamic activation to cocaine vs neutral cues (Zhang et al., 2018a). Importantly, the extent of hypothalamic but not other regional response was correlated with craving and cocaine addiction severity, assessed by the Cocaine Craving Questionnaire (CCQ) and Cocaine Selective Severity Assessment (CSSA), respectively. The findings suggested the differences in using pictures and videos in cue-induced craving paradigms, and, more importantly, highlighted the importance of hypothalamic dysfunction in cocaine craving and addiction severity.
Studies showed that feeding, drug consumption, and electrical stimulation of the lateral hypothalamus all increased dopamine release in the nucleus accumbens (Hoebel and Teitelbaum, 1962; Hull, 2011). The hypothalamus receives heavy dopaminergic projections from the midbrain and represents a key brain region in regulating arousal, food intake, and sexual drive and in supporting reward and affective responses during a wide range of goal-directed behaviors (DiLeone et al., 2003; Watabe-Uchida et al., 2012; Sternson, 2013; Verschure et al., 2014; Castro et al., 2015; Poeppl et al., 2016; Stuber and Wise, 2016). For instance, the hypothalamus has been observed to respond during exposure to high-calorie vs low-calorie/non-food stimuli (Cornier et al., 2007, 2009; Fletcher et al., 2010; Frank et al., 2010; St-Onge et al., 2012; de Araujo et al., 2013), during exposure to erotic vs neutral visual stimulation (Beauregard et al., 2001; Arnow et al., 2002; Ferretti et al., 2005; Stark et al., 2005; Kim et al., 2006; Ponseti et al., 2006; Brunetti et al., 2008; Sundaram et al., 2010; Wehrum et al., 2013; Kim and Jeong, 2014; Lee et al., 2015), and during gain vs no-gain scenarios in the monetary incentive delay task (Knutson et al., 2008; Luo et al., 2011; Enzi et al., 2012; Helfinstein et al., 2013; Montoya et al., 2014; Pfabigan et al., 2014). Rodent studies have implicated the hypothalamus in the etiological processes of drug addiction (Marchant et al., 2012). Reinstatement of cocaine seeking was associated with increased c-Fos expression in the hypothalamus (Hamlin et al., 2008), and the transition from controlled to compulsive cocaine self-administration was associated with substantial remodeling and altered gene expressions in the hypothalamic circuitry (Ahmed et al., 2005; Zhou et al., 2008; Chen et al., 2013). In humans CD showed altered hypothalamic activation viewing erotic vs neutral pictures, compared with healthy controls (HC) (Asensio et al., 2010). Hypothalamic response to monetary reward vs non-reward was associated with the duration of abstinence in CD (Bustamante et al., 2014). Together, these studies suggest hypothalamic dysfunction and altered goal-directed behavior in cocaine addiction.
In the current study, we examined cue-elicited brain activations in a new sample of 20 CDs engaged in both cocaine craving and food craving tasks and 24 HC matched in demographics and body mass index (BMI) in the food craving task. First, we aimed to replicate higher hypothalamic activations to cocaine vs neutral cues in CD. Second, we posited that, because cocaine cues are a stronger incentive than food cues, the hypothalamus would show higher cocaine- than food cue-elicited activation in CD. Third, we will examine whether and how CD and HC may differ in food cue-elicited hypothalamic activation. Following whole-brain group analyses, we will identify regions of interest and examine how regional cue-related activations relate to clinical measures of cocaine craving, addiction severity, and recent cocaine use.
Materials and Methods
Participants, Informed Consent, and Assessment
Twenty recently abstinent participants with cocaine dependence (CD, 17 men) and 24 age-, gender-, and BMI-matched healthy controls (HC, 19 men) participated in the study (Table 1). CD met criteria for current cocaine dependence as diagnosed by the Structured Clinical Interview for DSM-IV (First et al., 1995). Recent cocaine use was confirmed by urine toxicology screens. They were drug-free for about 7 to 10 days while staying in the Clinical Neuroscience Research Unit of the Connecticut Mental Health Center, a locked treatment facility, prior to the current fMRI study. All participants were physically healthy with no major medical illnesses or current use of prescription medications. None reported having a history of head injury or neurological illness. Other exclusion criteria included dependence on another psychoactive substance (except nicotine) and current or past history of Axis I disorders. The Human Investigation Committee at Yale University School of Medicine approved the study procedures, and all participants signed an informed consent prior to the study.
Table 1.
Demographics and Clinical Measures of Participants
| Characteristic | CD (n = 20) | HC (n = 24) | P value |
|---|---|---|---|
| Age (y) | 46.8 ± 5.3 | 46.3 ± 5.9 | .72a |
| Gender (M/F) | 17/3 | 19/5 | .91b |
| Race (EA/AA/others) | 5/15/0 | 8/13/3 | .61b |
| BMI | 25.5 ± 4.1 | 26.2 ± 3.0 | .51a |
| Years of drinking | 29.8 ± 7.3 | 27.3 ± 9.7 | .34a |
| Years of smoking | 18.4 ± 13 | 3.8 ± 8.0 | .001a |
| CCQ score | 50.1 ± 13.9 | N/A | N/A |
| CSSA score | 35.7 ± 18.8 | N/A | N/A |
| Monthly cocaine use (g, average, prior year) | 37.3 ± 49.8 | N/A | N/A |
| Cocaine amount per use (g, prior month) | 1.6 ± 1.6 | N/A | N/A |
| Days of cocaine use (prior month) | 19.9 ± 8.4 | N/A | N/A |
| Years of cocaine use | 18.8 ± 10.1 | N/A | N/A |
Abbreviations: AA, African American; BMI, body mass index; CCQ, Cocaine Craving Questionnaire; CSSA, Cocaine Selective Severity Assessment; EA, European American.
Values are mean ± SD.
a 2-tailed 2-sample t test.
b χ 2 test.
CD participants were interviewed with the 18-item CSSA (Kampman et al., 1998) to evaluate cocaine withdrawal signs and symptoms. CSSA scores were highly correlated with recent cocaine use and with severity measures of the Addiction Severity Index, including the interviewer severity rating and composite score in the drug section (Kampman et al., 1998). For cocaine-dependent individuals, initial CSSA scores were higher for those who failed to achieve abstinence or who subsequently dropped out of treatment (Kampman et al., 1998). Cocaine craving was assessed with the Cocaine Craving Questionnaire-Brief version for all CDs every 2 to 3 days (Sussner et al., 2006). The CCQ-Brief version is a 10-item questionnaire abbreviated from the CCQ-Now (Tiffany et al., 1993) and highly congruent with the CCQ-Now and other cocaine craving measures (Sussner et al., 2006). Each item was rated on a scale from 1 to 7, with a higher total score (ranging from 10 to 70) indicating greater craving. CCQ score was averaged across all assessments during the 2- to 3-week inpatient stay to index a tonic level of craving.
Behavioral Tasks
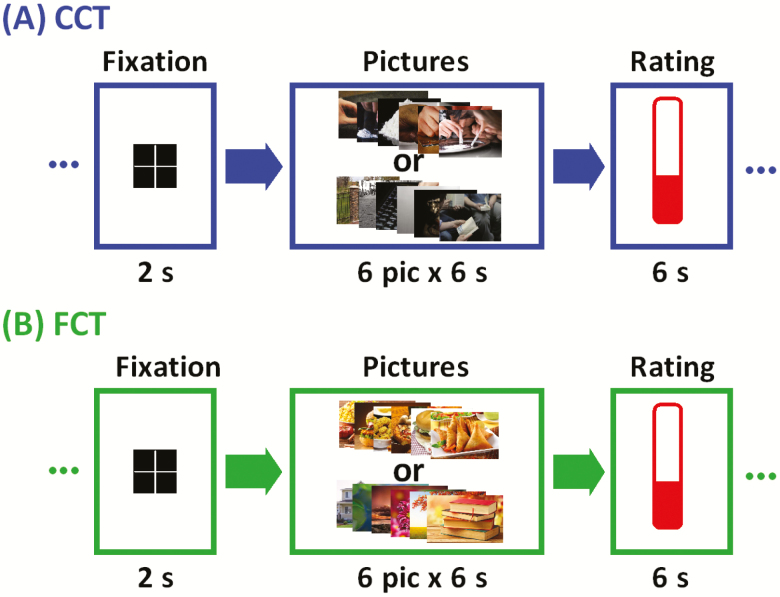
We employed a cue-induced cocaine craving task (CCT) for CD only and a cue-induced food craving task (FCT) for both CD and HC (Figure 1). The order of CCT and FCT was counter-balanced across subjects for CD. Details can be found in the Supplement.
Figure 1.
Block design of (A) cue-induced cocaine craving task (CCT) and (B) cue-induced food craving task (FCT). Briefly, a fixation cross appeared on the screen to engage attention at the beginning of each block. After 2 seconds, 6 pictures displaying cocaine/food-related cues (cocaine/food block) or neutral scenes (neutral block) were shown for 6 seconds each. Participants were asked to look at the pictures and think about how they might relate to the scenes. At the end of each block participants were asked to report how much they craved cocaine/food on a visual analog scale from 0 (no craving) to 10 (highest craving ever experienced) by pressing a button on the hand-held box in the scanner. A total of 12 cocaine/food and 12 neutral picture blocks were interleaved in two 9-minute runs of fMRI.
Imaging Protocol and Data Preprocessing
The imaging protocol is described in detail in the Supplement. Data were analyzed with SPM (Statistical Parametric Mapping) software following established routines (Zhang et al., 2018a; Zhornitsky et al., 2019), as in the Supplement.
Imaging Data Modeling
Data blocks were first distinguished by “cocaine/food picture” and “neutral picture” for CCT/FCT. A statistical analytical block design was constructed for each participant using a general linear model. Because each block was associated with a craving rating, we included a column of block onset parametrically modulated by its corresponding craving score as a regressor in the model. Realignment parameters in all 6 dimensions were also entered in the model. Serial autocorrelation caused by aliased cardiovascular and respiratory effects was corrected by a first-degree autoregressive or AR(1) model. The general linear model estimated the component of variance that could be explained by each of the regressors.
In the first-level analysis, we constructed for each participant statistical contrasts of “cocaine picture” vs “neutral picture” for CCT and “food picture” vs “neutral picture” for FCT. These contrasts allowed us to evaluate brain regions that responded differently to viewing of cocaine and food pictures, compared with viewing of neutral pictures, in CCT and FCT, respectively. The contrast images of the first-level analysis were then used for the second-level group statistics. We conducted paired t test to examine differences between “cocaine picture” vs “neutral picture” and “food picture” vs “neutral picture” in CD and 2-sample t test to examine differences between CD and HC for contrast of “food picture” vs “neutral picture.” In addition to whole-brain analyses, we examined cue-related responses of the hypothalamus with a mask as in earlier studies (Breen et al., 2016). Following current reporting standards (Poldrack et al., 2017), all imaging results were evaluated with voxel P < .001, uncorrected, in combination with cluster P < .05, FWE or family-wise error corrected for the whole brain or small volume correction for a hypothalamus mask obtained from the WFU Pick Atlas (http://fmri.wfubmc.edu/software/pickatlas) (Breen et al., 2016), on the basis of Gaussian random field theory as implemented in SPM. With small volume correction, one applied standard voxel level statistics (e.g., FWE) to voxels within the small region instead of within the whole brain. On the basis of our previous study (Zhang et al., 2018a), we had a very strong hypothesis that the hypothalamus would show differences in cue-related activities in the study. Thus, we tested the hypothesis using small volume correction.
In region of interest (ROI) analysis, we used MarsBaR (http://marsbar.sourceforge.net/) to derive for each participant the activity difference (β contrast) for the ROIs. Functional ROIs were defined based on clusters obtained from whole brain analysis. All voxel activations were presented in Montreal Neurological Institute coordinates.
Mediation Analysis
Owing to space limitations, mediation analyses are presented in the Supplement.
Results
Cue-Induced Craving
CD reported higher cocaine craving during viewing of cocaine pictures (t = 2.09, P = .00006, 2-tailed paired sample t test; cocaine cue: mean ± SD = 3.7 ± 2.0, neutral cue: 1.3 ± 0.8) as well as food craving during viewing of food pictures (t = 2.09, P = .0000002; food cue: 6.4 ± 2.7, neutral cue: 2.4 ± 1.6) compared with viewing of neutral pictures each during the CCT and FCT. CD showed a greater difference in food craving rating during viewing of food vs neutral pictures as compared with cocaine craving rating during viewing of cocaine vs neutral pictures (t = 2.02, P = .01, 2-tailed paired sample t test; 2.4 ± 2.1 for cocaine craving and 4.0 ± 2.2 for food craving).
HC reported higher food craving too during exposure to food vs neutral cues (t = 2.07, P = .0000001, 2-tailed paired sample t test; food cue: mean ± SD = 3.9 ± 2.1, neutral cue: 2.0 ± 1.6). Compared with HC, CD reported a greater difference in food craving rating during viewing of food vs neutral pictures (t = 2.05, P = .001, 2-tailed 2-sample t test; 4.0 ± 2.2 for CD and 2.2 ± 1.5 for HC). CD showed a positive correlation between food craving rating and BMI (r = 0.44, P = .048, Pearson regression). HC showed a trend toward significance in correlation between food craving rating and BMI (r = 0.39, P = .058).
Cue-Induced Brain Activations
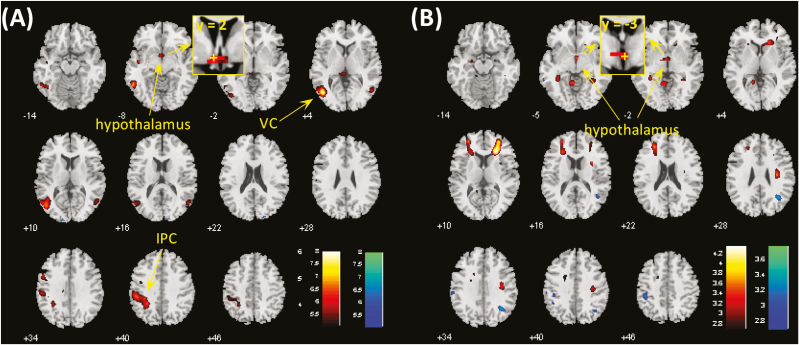
In the CCT, CD showed higher activations to cocaine vs neutral cues in bilateral visual cortex, bilateral inferior parietal gyri, bilateral middle frontal gyri, and hypothalamus at voxel P < .001, uncorrected, in combination with cluster-level P < .05, FWE corrected (Supplementary Figure 1A). No brain regions showed higher activation to neutral vs cocaine pictures. The findings replicated what we observed previously in a different cohort (Zhang et al., 2018a). At the same threshold, no brain regions showed differences in activation to food vs neutral cues in the FCT (Supplementary Figure 1B). We compared CCT and FCT directly in cue-induced activation using paired t test in CD. Compared with food vs neutral, cocaine vs neutral cues involved higher activations in the left visual cortex (VC), left inferior parietal cortex (IPC) and, with small volume correction, the hypothalamus (Figure 2A; Table 2). Conversely, no brain regions showed higher activation during exposure to food vs neutral as compared with cocaine vs neutral cues.
Figure 2.
(A) Brain regions showing differences between cocaine and food cue-induced activation in cocaine-dependent participants (CD) at voxel P < .001. Clusters meeting cluster-level P < .05 whole-brain corrected for familywise error of multiple comparisons or P < .05 familywise error corrected for the hypothalamus mask are summarized in Table 2. Hot color represents clusters showing higher responses to cocaine vs neutral cues as compared with food vs neutral cues, and blue color represents clusters of the opposite contrast. (B) Hypothalamus showed differences in food cue-induced activations between CD and healthy controls (HC) at voxel P < .001 and P < .05 familywise error corrected for the hypothalamus mask. IPC, inferior parietal cortex; VC, visual cortex. The insets showed coronal sections of the brain to highlight the location of the hypothalamic cluster, with “+” indicating the location of voxel peak.
Table 2.
Regions Showing Differences in Cocaine vs Neutral as Compared With Food vs Neutral Cue-induced Activations
| Volume | Peak voxel | MNI coordinates (mm) | Side | Identified brain region | ||
|---|---|---|---|---|---|---|
| (mm3) | (Z) | x | y | z | ||
| CD: Cocaine—Neutral > Food – Neutral | ||||||
| 8559 | 4.75 | -45 | -67 | 7 | L | Visual cortex |
| 7047 | 4.14 | -30 | -49 | 37 | L | Inferior parietal cortex |
| *540 | 3.98 | -3 | 2 | -8 | L/R | Hypothalamus |
| CD: Food - Neutral > Cocaine - Neutral | ||||||
| None | ||||||
| Food—Neutral: CD > HC | ||||||
| *621 | 3.49 | 3 | -3 | -5 | L/R | Hypothalamus |
| Food—Neutral: HC > CD | ||||||
| None | ||||||
Abbreviations: CD, cocaine-dependent participants; HC, healthy control participants; L, left; R, right.
Voxel P < .001 uncorrected and cluster-level P < .05 FWE for the whole brain (or *small volume correction for the hypothalamus mask).
HC showed higher activations to viewing of food pictures compared with neutral pictures in bilateral visual cortex as well as lower activations in bilateral parahippocampal gyrus and, with small volume correction, the hypothalamus, at voxel P < .001, uncorrected, in combination with cluster-level P < .05 (FWE corrected) (Supplementary Figure 1C). We then compared CD and HC directly in food cue-induced activation using 2-sample t test with age, gender, BMI, years of drinking, and years of smoking as covariates. Compared with HC, CD showed higher activations in hypothalamus at voxel P < .001, uncorrected, in combination with cluster-level P < .05 corrected for the hypothalamus mask (Figure 2B; Table 2). Conversely, no brain regions showed higher activation in HC compared with CD. Further, we reran the analysis without the rating as parametric modulator in first-level model, and the results were nearly identical (Supplementary Figure 2).
In CD hypothalamic responses to cocaine vs neutral cues and to food vs neutral cues were highly correlated (r = 0.75, P = .0001). The VC and IPC, which also showed higher activation to cocaine (vs neutral) cues vs food (vs neutral) cues did not show significant correlation in activations to cocaine and to food cues (VC: r = 0.37, P = .10; IPC: r = 0.28, P = .23).
Relationship of Cue-Induced Craving With Cocaine Use Characteristics
We examined whether “acute,” cue-induced craving was related to clinical characteristics with a linear regression of craving rating (cocaine vs neutral in the CCT and food vs neutral in the FCT) against years of cocaine use, days of cocaine use in the past month, amount per cocaine use in grams, amount of average monthly cocaine use (g) in the prior year, tonic craving (CCQ score), and addiction severity (CSSA score). We examined the results with a corrected P value of .05/(2 × 6) = .0042.
The results showed that no correlations were significant (Supplementary Table 1). Overall, acute, cue-induced craving during the behavioral tasks did not appear to reflect characteristics of cocaine use behavior.
Relationship of Cue-Induced Brain Activations With Cocaine Use Characteristics
We examined whether the cue-related regional responses were correlated with clinical characteristics with a linear regression of the beta contrast of each ROI against years of cocaine use, days of cocaine use in the past month, amount per cocaine use in grams, amount of average monthly cocaine use (g) in the prior year, tonic craving (CCQ score), and addiction severity (CSSA score). Three clusters—hypothalamus, visual cortex (VC), and inferior parietal cortex (IPC)—were identified from the contrast [(cocaine – neutral) – (food – neutral)] in CD. Thus, with the 3 clusters and 6 clinical measures, we evaluated the results at a corrected P = .05/(3 × 6) = .0028.
For all 3 ROIs, differences in activation between the CCT and FCT [i.e., (cocaine – neutral) – (food – neutral)] were not correlated with any of these clinical measures (all P > 0.27).
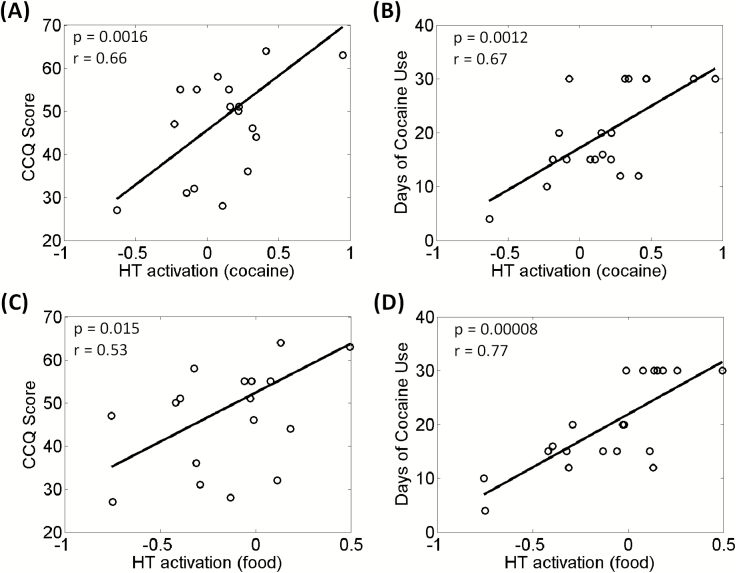
We next examined whether the cue-related activities of the 3 ROIs each during the CCT and FCT were correlated with clinical variables. In the CCT, hypothalamic activation to cocaine vs neutral cues showed a positive correlation with CCQ score (r = 0.66, P = .0016) and days of cocaine use in the past month (r = 0.67, P = .0011) (Figure 3A–B). None of the other correlations were significant (supplementary Table 2).
Figure 3.
Cocaine/food cue-elicited hypothalamic activation was positively correlated with (A, C) Cocaine Craving Questionnaire (CCQ) score and with (B, D) days of cocaine use in the past month across cocaine-dependent (CD) participants. Each data point represents one participant.
In the FCT, hypothalamic activation to food vs neutral cues showed a positive correlation with days of cocaine use in the past month (r = 0.77, P = .00008) (Figure 3D). None of the other correlations were significant (supplementary Table 2). However, we show the results of regression with the CCQ score (r = 0.53, P = .015) in Figure 3C to contrast with the correlation between cocaine cue-evoked response and CCQ score.
Neither cocaine nor food cue-elicited hypothalamic activation was correlated with age (P = .72 for CCT and P = .36 for FCT), sex (P = .99 for CCT and P = .98 for FCT), BMI (P = .11 for CCT and P = .16 for FCT), years of drinking (r = −0.04, P = .85 and r = −0.08, P = .74), or years of smoking (r = −0.35, P = .12 and r = −0.31, P = .19). Nonetheless, we conducted additional analyses to account for age, sex, BMI, years of drinking, and years of smoking. With these variables as covariates, cocaine cue-elicited hypothalamic activation remained positively correlated with CCQ score (r = 0.66, P = .0028) and with days of cocaine use in the past month (r = 0.57, P = .013). The food cue-elicited hypothalamic activations remained positively correlated with days of cocaine use in the past month (r = 0.73, P = .0022).
Thus, in CD, hypothalamic responses to cocaine (vs neutral) and to food (vs neutral) both showed a significant or a trend toward significant correlation with the CCQ score and days of cocaine use in the prior month. The inter-relationships between these neural and clinical measures were examined with mediation analyses next.
Mediation Analysis
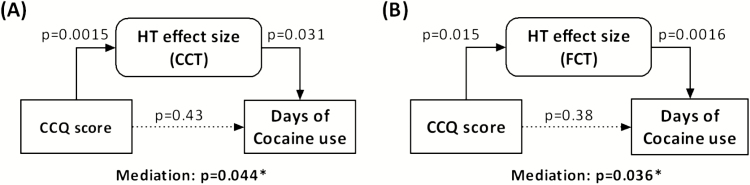
With mediation analysis we further examined the inter-relationships between hypothalamic activation to cocaine/food (vs neutral) cues, days of cocaine use in the past month, and CCQ score. The CCQ score, which was averaged from multiple days of assessment as an index of tonic cocaine craving, was correlated with days of cocaine use in the past month (r = 0.55, P = .01). The hypothalamic activation was positively correlated with both CCQ score and days of cocaine use in the past month, as shown in the above. Thus, we performed mediation analyses to test whether hypothalamic cue activities mediated the relationship between cocaine craving and days of cocaine use in the past month. The results showed that hypothalamic activation to both cocaine and food (vs neutral) cue exposure significantly mediated the correlation between the CCQ score and days of cocaine use in the past month (Figure 4). Without the mediation of hypothalamic activation, CCQ score was not correlated with days of cocaine use in the past month (P = .43 for CCT and P = .38 for FCT). None of the other 5 models showed significant mediation (supplementary Table 3).
Figure 4.
Mediation analysis. (A) Cocaine cue-elicited hypothalamic (HT) activation completely mediated the correlation between tonic cocaine craving (Cocaine Craving Questionnaire [CCQ] score) and days of cocaine use in the past month. (B) Food cue-elicited hypothalamic (HT) activation completely mediated the correlation between tonic cocaine craving (CCQ score) and days of cocaine use in the past month. The P values associated with mediation are for the path “a × b” (see Methods).
Discussion
The current findings showed higher hypothalamic activation in recently abstinent CDs viewing cocaine vs neutral picture cues as contrasted to viewing food vs neutral cues. The extent of hypothalamic activation to viewing of both cocaine vs neutral and food vs neutral cues was significantly correlated with the CCQ score and days of cocaine use in the past month, with greater hypothalamic activation reflecting stronger daily cocaine craving and more frequent, recent cocaine use. Hypothalamic response to cocaine/food (vs neutral) cue exposure completely mediated the correlation between the CCQ score and days of cocaine use in the past month. The latter finding suggested hypothalamic activation as an important neural mediator linking cocaine craving to cocaine consumption. Further, compared with HC, CD showed higher hypothalamic activation during viewing food vs neutral pictures. Together, these findings provided new evidence in support of hypothalamic dysfunction as a neural marker of cocaine addiction.
Food Cue Activations in CD vs HC
Consistent with an earlier report of consumption of higher levels of dietary fat and carbohydrates, despite a reduced fat mass, in CD as compared with their non-drug using peers (Ersche et al., 2013), we observed higher food craving rating as well as food cue-induced activation in the hypothalamus in CD compared with age, sex, and BMI- matched HC. These findings along with those reported by Ersche and colleagues suggest that cocaine misuse is associated with diminished response to natural reinforcers perhaps only relatively to response to cocaine. Higher hypothalamic activation to food cues may represent plasticity of the dopaminergic circuits in response to chronic exposure to cocaine. Importantly, as with cocaine cue responses, food cue responses of the hypothalamus were correlated with tonic cocaine craving and recent cocaine use, suggesting that altered hypothalamic activations to natural reinforcers may reflect critical psychological and clinical dimensions of cocaine misuse.
It is worth noting that we observed decreases in hypothalamic activation in HC during viewing food vs neutral pictures, seemingly at odds with previous reports of healthy people showing higher hypothalamic activity during exposure to food vs nonfood cues (St-Onge et al., 2012; Wang et al., 2014), high vs low calorie food images (Cornier et al., 2007; Ulrich et al., 2016), and food vs non-food odors (Eiler et al., 2015). Other studies reported higher hypothalamic activations in obese but not in normal-weight participants during exposure to food vs nonfood cues (Page et al., 2011; Jastreboff et al., 2013). A recent study examined the neutral correlates of basic tastes in healthy adults and observed higher hypothalamic activation in young adults (18–30 years of age) but lower hypothalamic activation in older adults (60–72 years) to sweet, salty, and bitter stimuli vs visual fixation on the screen (Hoogeveen et al., 2015). Another fMRI study of 36 children (7–10 years) reported lower activation for high- vs low-calorie food images in the left hypothalamus (English et al., 2016). Thus, one may consider the age of the participants to account for these discrepancies in findings. Most of the aforementioned studies that reported higher hypothalamic activation involved participants 19 to 40 years old, whereas the participants of the current study ranged from 39 to 54 years in age. More research is needed to determine how age may engage regional brain activations differently during food cue exposures.
Cocaine vs Food Cue Activations in CD
We did not observe food cue-elicited brain activations, despite higher craving rating for food vs neutral images, in CD. Although reporting higher food craving in the FCT than cocaine craving in the CCT, CD showed higher cocaine-elicited compared with food cue-elicited activation in the hypothalamus, inferior parietal cortex, and visual cortex. Subjective reports of craving are known to be heavily influenced by accessibility of cocaine (Sayette et al., 2000; Wertz and Sayette, 2001; Parvaz et al., 2016). As the participants were treatment-seeking inpatients residing in a locked unit, inaccessibility of cocaine may have accounted for the discrepancy between craving ratings and imaging findings. In support of the imaging findings, animal studies suggested that preferences for cocaine over palatable food reward identified individuals who are relatively vulnerable to addiction (Ahmed, 2010). Cocaine-preferring rats showed increased motivation for cocaine, but attenuated motivation for food, as well as greater cocaine and cue-induced reinstatement of drug seeking (Perry et al., 2013).
Both cocaine and food cue-elicited hypothalamic activation were significantly correlated with the CCQ score and days of cocaine use in the past month, consistent with the proposition that the cellular and molecular mechanisms of feeding and drug addiction are inter-linked in the hypothalamus (DiLeone et al., 2003). In rodents, hypothalamic hypocretin-1/orexin-A activity facilitated cocaine reinstatement (Kallupi et al., 2010) and approach behavior for food (Cason et al., 2010), suggesting that motivational drive as entrained by the hypothalamus goes in the same direction for both drug of abuse and natural incentives.
We observed that hypothalamic activation elicited by both cocaine and food cue was an important mediator linking cocaine craving to recent cocaine use. The findings highlighted hypothalamic responses to incentive stimuli as a critical marker of cocaine addiction. Longitudinal studies are warranted to examine whether hypothalamic cue responses may predict the severity of drug use or relapse to drug use in treatment-seeking individuals.
Increases in activations were also observed in the VC and IPC during viewing of cocaine (vs neutral) compared with viewing of food (vs neutral) cues. The finding was broadly consistent with attentional bases toward drug cues and parietal dysfunction in chronic cocaine users (Garavan et al., 2000; Hester and Garavan, 2004; Kubler et al., 2005; Tomasi et al., 2007; Bustamante et al., 2011). Interestingly, in obese individuals, hypothalamus IPC connectivity increased during exposure to food vs non-food stimuli, and both food craving and hypothalamus IPC connectivity decreased after leptin administration, in support of a role of these 2 brain regions in food “wanting” (Hinkle et al., 2013). Using resting state fMRI, we previously showed higher hypothalamic connectivity with the IPC in CD compared with HC, with HC but not CD showing significant negative connectivity (Zhang et al., 2018b). The VC was often reported to be activated during exposure to drug cues (Chase et al., 2011; Hanlon et al., 2014; Tomasi et al., 2015). A meta-analysis showed that about 86% of published imaging studies reported significant drug cue-induced activity in the VC (Hanlon et al., 2014). Notably, treatment-seeking cocaine-using participants as well as participants with strong motivation to quit demonstrated diminished cue-induced VC activation compared with non-treatment–seeking and less motivated participants (Prisciandaro et al., 2014). Together, higher IPC and VC activations suggested that cocaine cues were more salient than food cues for individuals engaged in cocaine misuse.
Limitations of the Study and Conclusions
A number of limitations should be considered. First, the study comprised a small sample size. However, we wish to emphasize that both the imaging findings and the correlations between regional activations and clinical variables were significant with full consideration of multiple comparisons. Second, CD and HC differed in years of cigarette smoking. Although we included years of smoking as a covariate when comparing CD and HC, we could not entirely rule out the effects of cigarette smoking on the current findings. Third, participants were instructed to fast after midnight on the day of MR scan, and all reported not eating or drinking anything but water before scans. However, we did not have an objective measure to confirm their reports. Fourth, the whole-brain analyses did not reveal striatal responses to drug or food cues. The striatum has been implicated in cue-induced craving (Chase et al., 2011; Kühn and Gallinat, 2011) and reward-related processing (Filbey et al., 2016; Luijten et al., 2017; Zimmermann et al., 2019), whereas some studies of cue-induced craving did not demonstrate striatal activation (Garavan et al., 2000; Wexler et al., 2001; Duncan et al., 2007; Lee et al., 2013). Although not a primary aim of the current study, we conducted a ROI analysis focusing on the VS and dorsal striatum. The VS showed significant increases in activation for “cocaine vs neutral” compared with “food vs neutral” in CD (P = .01) but not between CD and HC for “food vs neutral (P = .89).” These findings are consistent with an earlier meta-analysis suggesting that cue-elicited VS activation is largely significant only in ROI analysis (Schacht et al., 2013). The dorsal striatum did not show significant differences for either contrast (P = .32 and P = .24, respectively). Thus, the role of striatal activity and striatal hypothalamic interaction in supporting cue-related responses remains to be clarified. A related consideration is that in block design of fMRI studies, investigators have used varying block duration, depending on the type of stimulus. The great majority of studies employed 10–60 seconds (Kao et al., 2014), although some researchers suggest an optimal duration of 15 seconds (Maus and van Breukelen, 2013). In the current study, we followed our previous studies of cue-elicited craving (Wang et al., 2019; Zhornitsky et al., 2019) and employed 36-second blocks. It is possible that the striatal cue responses may be more transient and readily visible in experiments with shorter block duration. Fifth, CCQ scores were obtained every 2 to 3 days during the 2- to 3-week inpatient stay with the intention to understand how cocaine craving may evolve during early abstinence. However, we did not notice any consistent patterns of change (perhaps due to the small sample size and short duration of assessment), and across participants the day-to-day variation was quite limited. We averaged the CCQ scores across assessments to reflect tonic craving (Zhang et al., 2018a). Nonetheless, the hypothalamus activation was also significantly correlated with the CCQ score obtained on the day closest to MRI (P = .0013, r = 0.67). Finally, the study was cross-sectional, and it remains to be seen whether the hypothalamic cue response may predict relapse to drug use in a longitudinal setting.
In summary, the current findings replicated the observations in a different sample of CDs of higher hypothalamic activations to cocaine versus neutral cues (Zhang et al., 2018a). We demonstrated higher hypothalamic activation during exposure to cocaine vs food cues in CDs and during exposure to food cues in CD compared with HC. Hypothalamic response to both cocaine and food cues was correlated with daily cocaine craving and days of cocaine use in the past month. The findings suggested the importance in characterizing hypothalamic dysfunction as a neural marker of cocaine addiction.
Supplementary Material
Acknowledgments
We thank the medical and nursing staff at the Clinical Neuroscience Research Unit, Connecticut Mental Health Center for medical care of the participants. We declare no financial interests in the current work.
Supported by National Institute on Drug Abuse at the National Institutes of Health (grant nos.: DA040032, DA045743, DA044749, and DA023248) as well as the Department of Mental Health and Addiction Services (DMHAS) of the State of Connecticut. The funding agencies otherwise have no roles in the conceptualization of the study, data collection and analysis, or the decision to publish these results.
References
- Ahmed SH. (2010) Validation crisis in animal models of drug addiction: beyond non-disordered drug use toward drug addiction. Neurosci Biobehav Rev 35:172–184. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Lutjens R, van der Stap LD, Lekic D, Romano-Spica V, Morales M, Koob GF, Repunte-Canonigo V, Sanna PP (2005) Gene expression evidence for remodeling of lateral hypothalamic circuitry in cocaine addiction. Proc Natl Acad Sci U S A 102:11533–11538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnow BA, Desmond JE, Banner LL, Glover GH, Solomon A, Polan ML, Lue TF, Atlas SW (2002) Brain activation and sexual arousal in healthy, heterosexual males. Brain 125:1014–1023. [DOI] [PubMed] [Google Scholar]
- Asensio S, Romero MJ, Palau C, Sanchez A, Senabre I, Morales JL, Carcelen R, Romero FJ (2010) Altered neural response of the appetitive emotional system in cocaine addiction: an fMRI Study. Addict Biol 15:504–516. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Lévesque J, Bourgouin P (2001) Neural correlates of conscious self-regulation of emotion. J Neurosci 21:RC165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR. (2007) Interactions between the “cognitive” and “metabolic” brain in the control of food intake. Physiol Behav 91:486–498. [DOI] [PubMed] [Google Scholar]
- Billing L, Ersche KD (2015) Cocaine’s appetite for fat and the consequences on body weight. Am J Drug Alcohol Abuse 41:115–118. [DOI] [PubMed] [Google Scholar]
- Breen DP, Nombela C, Vuono R, Jones PS, Fisher K, Burn DJ, Brooks DJ, Reddy AB, Rowe JB, Barker RA (2016) Hypothalamic volume loss is associated with reduced melatonin output in Parkinson’s disease. Mov Disord 31:1062–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunetti M, Babiloni C, Ferretti A, Del Gratta C, Merla A, Olivetti Belardinelli M, Romani GL (2008) Hypothalamus, sexual arousal and psychosexual identity in human males: a functional magnetic resonance imaging study. Eur J Neurosci 27:2922–2927. [DOI] [PubMed] [Google Scholar]
- Bustamante JC, Barrós-Loscertales A, Ventura-Campos N, Sanjuán A, Llopis JJ, Parcet MA, Avila C (2011) Right parietal hypoactivation in a cocaine-dependent group during a verbal working memory task. Brain Res 1375:111–119. [DOI] [PubMed] [Google Scholar]
- Bustamante JC, Barrós-Loscertales A, Costumero V, Fuentes-Claramonte P, Rosell-Negre P, Ventura-Campos N, Llopis JJ, Ávila C (2014) Abstinence duration modulates striatal functioning during monetary reward processing in cocaine patients. Addict Biol 19:885–894. [DOI] [PubMed] [Google Scholar]
- Cason AM, Smith RJ, Tahsili-Fahadan P, Moorman DE, Sartor GC, Aston-Jones G (2010) Role of orexin/hypocretin in reward-seeking and addiction: implications for obesity. Physiol Behav 100:419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro DC, Cole SL, Berridge KC (2015) Lateral hypothalamus, nucleus accumbens, and ventral pallidum roles in eating and hunger: interactions between homeostatic and reward circuitry. Front Syst Neurosci 9:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase HW, Eickhoff SB, Laird AR, Hogarth L (2011) The neural basis of drug stimulus processing and craving: an activation likelihood estimation meta-analysis. Biol Psychiatry 70:785–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Repunte-Canonigo V, Kawamura T, Lefebvre C, Shin W, Howell LL, Hemby SE, Harvey BK, Califano A, Morales M, Koob GF, Sanna PP (2013) Hypothalamic proteoglycan syndecan-3 is a novel cocaine addiction resilience factor. Nat Commun 4:1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane C, Malcolm R, Brewerton T (1998) The role of weight control as a motivation for cocaine abuse. Addict Behav 23:201–207. [DOI] [PubMed] [Google Scholar]
- Cornier MA. (2009) The effects of overfeeding and propensity to weight gain on the neuronal responses to visual food cues. Physiol Behav 97:525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornier MA, Von Kaenel SS, Bessesen DH, Tregellas JR (2007) Effects of overfeeding on the neuronal response to visual food cues. Am J Clin Nutr 86:965–971. [DOI] [PubMed] [Google Scholar]
- Cowan J, Devine C (2008) Food, eating, and weight concerns of men in recovery from substance addiction. Appetite 50:33–42. [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Lin T, Veldhuizen MG, Small DM (2013) Metabolic regulation of brain response to food cues. Curr Biol 23:878–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLeone RJ, Georgescu D, Nestler EJ (2003) Lateral hypothalamic neuropeptides in reward and drug addiction. Life Sci 73:759–768. [DOI] [PubMed] [Google Scholar]
- Duncan E, Boshoven W, Harenski K, Fiallos A, Tracy H, Jovanovic T, Hu X, Drexler K, Kilts C (2007) An fMRI study of the interaction of stress and cocaine cues on cocaine craving in cocaine-dependent men. Am J Addict 16:174–182. [DOI] [PubMed] [Google Scholar]
- Eiler WJ 2nd, Džemidžić M, Case KR, Soeurt CM, Armstrong CL, Mattes RD, O’Connor SJ, Harezlak J, Acton AJ, Considine RV, Kareken DA (2015) The apéritif effect: alcohol’s effects on the brain’s response to food aromas in women. Obesity (Silver Spring) 23:1386–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English LK, Fearnbach SN, Lasschuijt M, Schlegel A, Anderson K, Harris S, Wilson SJ, Fisher JO, Savage JS, Rolls BJ, Keller KL (2016) Brain regions implicated in inhibitory control and appetite regulation are activated in response to food portion size and energy density in children. Int J Obes (Lond) 40:1515–1522. [DOI] [PubMed] [Google Scholar]
- Enzi B, Edel MA, Lissek S, Peters S, Hoffmann R, Nicolas V, Tegenthoff M, Juckel G, Saft C (2012) Altered ventral striatal activation during reward and punishment processing in premanifest Huntington’s disease: a functional magnetic resonance study. Exp Neurol 235:256–264. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Stochl J, Woodward JM, Fletcher PC (2013) The skinny on cocaine: insights into eating behavior and body weight in cocaine-dependent men. Appetite 71:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti A, Caulo M, Del Gratta C, Di Matteo R, Merla A, Montorsi F, Pizzella V, Pompa P, Rigatti P, Rossini PM, Salonia A, Tartaro A, Romani GL (2005) Dynamics of male sexual arousal: distinct components of brain activation revealed by fMRI. Neuroimage 26:1086–1096. [DOI] [PubMed] [Google Scholar]
- Filbey FM, Dunlop J, Ketcherside A, Baine J, Rhinehardt T, Kuhn B, DeWitt S, Alvi T (2016) fMRI study of neural sensitization to hedonic stimuli in long-term, daily cannabis users. Hum Brain Mapp 37:3431–3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Williams J, Gibbon M (1995) Structured Clinical Interview for DSM-IV (SCID). Washington, DC: American Psychiatric Association. [Google Scholar]
- Fletcher PC, Napolitano A, Skeggs A, Miller SR, Delafont B, Cambridge VC, de Wit S, Nathan PJ, Brooke A, O’Rahilly S, Farooqi IS, Bullmore ET (2010) Distinct modulatory effects of satiety and sibutramine on brain responses to food images in humans: a double dissociation across hypothalamus, amygdala, and ventral striatum. J Neurosci 30:14346–14355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank TC, Kim GL, Krzemien A, Van Vugt DA (2010) Effect of menstrual cycle phase on corticolimbic brain activation by visual food cues. Brain Res 1363:81–92. [DOI] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA (2000) Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry 157:1789–1798. [DOI] [PubMed] [Google Scholar]
- Hamlin AS, Clemens KJ, McNally GP (2008) Renewal of extinguished cocaine-seeking. Neuroscience 151:659–670. [DOI] [PubMed] [Google Scholar]
- Hanlon CA, Dowdle LT, Naselaris T, Canterberry M, Cortese BM (2014) Visual cortex activation to drug cues: a meta-analysis of functional neuroimaging papers in addiction and substance abuse literature. Drug Alcohol Depend 143:206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfinstein SM, Kirwan ML, Benson BE, Hardin MG, Pine DS, Ernst M, Fox NA (2013) Validation of a child-friendly version of the monetary incentive delay task. Soc Cogn Affect Neurosci 8:720–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Garavan H (2004) Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci 24:11017–11022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkle W, Cordell M, Leibel R, Rosenbaum M, Hirsch J (2013) Effects of reduced weight maintenance and leptin repletion on functional connectivity of the hypothalamus in obese humans. PLoS One 8:e59114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoebel BG, Teitelbaum P (1962) Hypothalamic control of feeding and self-stimulation. Science 135:375–377. [DOI] [PubMed] [Google Scholar]
- Hoogeveen HR, Dalenberg JR, Renken RJ, ter Horst GJ, Lorist MM (2015) Neural processing of basic tastes in healthy young and older adults - an fMRI study. Neuroimage 119:1–12. [DOI] [PubMed] [Google Scholar]
- Hull EM. (2011) Sex, drugs and gluttony: how the brain controls motivated behaviors. Physiol Behav 104:173–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastreboff AM, Sinha R, Lacadie C, Small DM, Sherwin RS, Potenza MN (2013) Neural correlates of stress- and food cue-induced food craving in obesity: association with insulin levels. Diabetes Care 36:394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallupi M, Cannella N, Economidou D, Ubaldi M, Ruggeri B, Weiss F, Massi M, Marugan J, Heilig M, Bonnavion P, de Lecea L, Ciccocioppo R (2010) Neuropeptide S facilitates cue-induced relapse to cocaine seeking through activation of the hypothalamic hypocretin system. Proc Natl Acad Sci U S A 107:19567–19572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampman KM, Volpicelli JR, McGinnis DE, Alterman AI, Weinrieb RM, D’Angelo L, Epperson LE (1998) Reliability and validity of the cocaine selective severity assessment. Addict Behav 23:449–461. [DOI] [PubMed] [Google Scholar]
- Kao MH, Temkit M, Wong WK (2014) Recent developments in optimal experimental designs for functional magnetic resonance imaging. World J Radiol 6:437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC (2002) The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci 22:3306–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GW, Jeong GW (2014) A comparative study of brain activation patterns associated with sexual arousal between males and females using 3.0-T functional magnetic resonance imaging. Sex Health 11:11–16. [DOI] [PubMed] [Google Scholar]
- Kim SW, Sohn DW, Cho YH, Yang WS, Lee KU, Juh R, Ahn KJ, Chung YA, Han SI, Lee KH, Lee CU, Chae JH (2006) Brain activation by visual erotic stimuli in healthy middle aged males. Int J Impot Res 18:452–457. [DOI] [PubMed] [Google Scholar]
- Knutson B, Bhanji JP, Cooney RE, Atlas LY, Gotlib IH (2008) Neural responses to monetary incentives in major depression. Biol Psychiatry 63:686–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kübler A, Murphy K, Garavan H (2005) Cocaine dependence and attention switching within and between verbal and visuospatial working memory. Eur J Neurosci 21:1984–1992. [DOI] [PubMed] [Google Scholar]
- Kühn S, Gallinat J (2011) Common biology of craving across legal and illegal drugs - a quantitative meta-analysis of cue-reactivity brain response. Eur J Neurosci 33:1318–1326. [DOI] [PubMed] [Google Scholar]
- Lee E, Ku J, Jung YC, Lee H, An SK, Kim KR, Yoon KJ, Namkoong K (2013) Neural evidence for emotional involvement in pathological alcohol craving. Alcohol 48:288–294. [DOI] [PubMed] [Google Scholar]
- Lee SW, Jeong BS, Choi J, Kim JW (2015) Sex differences in interactions between nucleus accumbens and visual cortex by explicit visual erotic stimuli: an fMRI study. Int J Impot Res 27:161–166. [DOI] [PubMed] [Google Scholar]
- Luijten M, Schellekens AF, Kühn S, Machielse MW, Sescousse G (2017) Disruption of reward processing in addiction: an image-based meta-analysis of functional magnetic resonance imaging studies. JAMA Psychiatry 74:387–398. [DOI] [PubMed] [Google Scholar]
- Luo S, Ainslie G, Giragosian L, Monterosso JR (2011) Striatal hyposensitivity to delayed rewards among cigarette smokers. Drug Alcohol Depend 116:18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Millan EZ, McNally GP (2012) The hypothalamus and the neurobiology of drug seeking. Cell Mol Life Sci 69:581–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maus B, van Breukelen GJP (2013) Optimal design for functional magnetic resonance imaging experiments methodology, challenges, and future perspectives. Zeitschrift Fur Psychologie-Journal of Psychology 221:174–189. [Google Scholar]
- Montoya ER, Bos PA, Terburg D, Rosenberger LA, van Honk J (2014) Cortisol administration induces global down-regulation of the brain’s reward circuitry. Psychoneuroendocrinology 47:31–42. [DOI] [PubMed] [Google Scholar]
- Page KA, Seo D, Belfort-DeAguiar R, Lacadie C, Dzuira J, Naik S, Amarnath S, Constable RT, Sherwin RS, Sinha R (2011) Circulating glucose levels modulate neural control of desire for high-calorie foods in humans. J Clin Invest 121:4161–4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvaz MA, Moeller SJ, Goldstein RZ (2016) Incubation of cue-induced craving in adults addicted to cocaine measured by electroencephalography. JAMA Psychiatry 73:1127–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry AN, Westenbroek C, Becker JB (2013) The development of a preference for cocaine over food identifies individual rats with addiction-like behaviors. PLoS One 8:e79465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfabigan DM, Seidel EM, Sladky R, Hahn A, Paul K, Grahl A, Küblböck M, Kraus C, Hummer A, Kranz GS, Windischberger C, Lanzenberger R, Lamm C (2014) P300 amplitude variation is related to ventral striatum BOLD response during gain and loss anticipation: an EEG and fMRI experiment. Neuroimage 96:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeppl TB, Langguth B, Rupprecht R, Safron A, Bzdok D, Laird AR, Eickhoff SB (2016) The neural basis of sex differences in sexual behavior: a quantitative meta-analysis. Front Neuroendocrinol 43:28–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Baker CI, Durnez J, Gorgolewski KJ, Matthews PM, Munafò MR, Nichols TE, Poline JB, Vul E, Yarkoni T (2017) Scanning the horizon: towards transparent and reproducible neuroimaging research. Nat Rev Neurosci 18:115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponseti J, Bosinski HA, Wolff S, Peller M, Jansen O, Mehdorn HM, Büchel C, Siebner HR (2006) A functional endophenotype for sexual orientation in humans. Neuroimage 33:825–833. [DOI] [PubMed] [Google Scholar]
- Prisciandaro JJ, McRae-Clark AL, Myrick H, Henderson S, Brady KT (2014) Brain activation to cocaine cues and motivation/treatment status. Addict Biol 19:240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, Shiffman S, Tiffany ST, Niaura RS, Martin CS, Shadel WG (2000) The measurement of drug craving. Addiction 95(Suppl 2):S189–S210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Myrick H (2013) Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict Biol 18:121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Onge MP, McReynolds A, Trivedi ZB, Roberts AL, Sy M, Hirsch J (2012) Sleep restriction leads to increased activation of brain regions sensitive to food stimuli. Am J Clin Nutr 95:818–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark R, Schienle A, Girod C, Walter B, Kirsch P, Blecker C, Ott U, Schäfer A, Sammer G, Zimmermann M, Vaitl D (2005) Erotic and disgust-inducing pictures–differences in the hemodynamic responses of the brain. Biol Psychol 70:19–29. [DOI] [PubMed] [Google Scholar]
- Sternson SM. (2013) Hypothalamic survival circuits: blueprints for purposive behaviors. Neuron 77:810–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Wise RA (2016) Lateral hypothalamic circuits for feeding and reward. Nat Neurosci 19:198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram T, Jeong GW, Kim TH, Kim GW, Baek HS, Kang HK (2010) Time-course analysis of the neuroanatomical correlates of sexual arousal evoked by erotic video stimuli in healthy males. Korean J Radiol 11:278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussner BD, Smelson DA, Rodrigues S, Kline A, Losonczy M, Ziedonis D (2006) The validity and reliability of a brief measure of cocaine craving. Drug Alcohol Depend 83:233–237. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Singleton E, Haertzen CA, Henningfield JE (1993) The development of a cocaine craving questionnaire. Drug Alcohol Depend 34:19–28. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Goldstein RZ, Telang F, Maloney T, Alia-Klein N, Caparelli EC, Volkow ND (2007) Widespread disruption in brain activation patterns to a working memory task during cocaine abstinence. Brain Res 1171:83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Wang GJ, Wang R, Caparelli EC, Logan J, Volkow ND (2015) Overlapping patterns of brain activation to food and cocaine cues in cocaine abusers: association to striatal D2/D3 receptors. Hum Brain Mapp 36:120–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich M, Endres F, Kölle M, Adolph O, Widenhorn-Müller K, Grön G (2016) Glucose modulates food-related salience coding of midbrain neurons in humans. Hum Brain Mapp 37:4376–4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verschure PF, Pennartz CM, Pezzulo G (2014) The why, what, where, when and how of goal-directed choice: neuronal and computational principles. Philos Trans R Soc Lond B Biol Sci 369, p. 20130483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Baler R (2012) Food and drug reward: overlapping circuits in human obesity and addiction. Curr Top Behav Neurosci 11:1–24. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wise RA, Baler R (2017) The dopamine motive system: implications for drug and food addiction. Nat Rev Neurosci 18:741–752. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Tomasi D, Volkow ND, Wang R, Telang F, Caparelli EC, Dunayevich E (2014) Effect of combined naltrexone and bupropion therapy on the brain’s reactivity to food cues. Int J Obes (Lond) 38:682–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Zhornitsky S, Le TM, Dhingra I, Zhang S, Krystal JH, Li CR (2019) Cue-elicited craving, thalamic activity, and physiological arousal in adult non-dependent drinkers. J Psychiatr Res 116:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N (2012) Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron 74:858–873. [DOI] [PubMed] [Google Scholar]
- Wehrum S, Klucken T, Kagerer S, Walter B, Hermann A, Vaitl D, Stark R (2013) Gender commonalities and differences in the neural processing of visual sexual stimuli. J Sex Med 10:1328–1342. [DOI] [PubMed] [Google Scholar]
- Wertz JM, Sayette MA (2001) A review of the effects of perceived drug use opportunity of self-reported urge. Exp Clin Psychopharmacol 9:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler BE, Gottschalk CH, Fulbright RK, Prohovnik I, Lacadie CM, Rounsaville BJ, Gore JC (2001) Functional magnetic resonance imaging of cocaine craving. Am J Psychiatry 158:86–95. [DOI] [PubMed] [Google Scholar]
- Zhang S, Wang W, Zhornitsky S, Li CR (2018b) Resting state functional connectivity of the lateral and medial hypothalamus in cocaine dependence: an exploratory study. Front Psychiatry 9:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Zhornitsky S, Angarita GA, Li CR (2018a) Hypothalamic response to cocaine cues and cocaine addiction severity. Addict Biol. doi: 10.1111/adb.12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhornitsky S, Zhang S, Ide JS, Chao HH, Wang W, Le TM, Leeman RF, Bi J, Krystal JH, Li CR (2019) Alcohol expectancy and cerebral responses to cue-elicited craving in adult nondependent drinkers. Biol Psychiatry Cogn Neurosci Neuroimaging 4:493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Cui CL, Schlussman SD, Choi JC, Ho A, Han JS, Kreek MJ (2008) Effects of cocaine place conditioning, chronic escalating-dose “binge” pattern cocaine administration and acute withdrawal on orexin/hypocretin and preprodynorphin gene expressions in lateral hypothalamus of Fischer and Sprague-Dawley rats. Neuroscience 153:1225–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann K, Kendrick KM, Scheele D, Dau W, Banger M, Maier W, Weber B, Ma Y, Hurlemann R, Becker B (2019) Altered striatal reward processing in abstinent dependent cannabis users: social context matters. Eur Neuropsychopharmacol 29:356–364. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.