Abstract
Background
Polyphenols are phytochemicals that have been associated with therapeutic effects in stress-related disorders. Indeed, studies suggest that polyphenols exert significant neuroprotection against multiple neuronal injuries, including oxidative stress and neuroinflammation, but the mechanisms are unclear. Evidence indicates that polyphenol neuroprotection may be mediated by activation of Nrf2, a transcription factor associated with antioxidant and cell survival responses. On the other hand, in stress-linked disorders, Fkbp5 is a novel molecular target for treatment because of its capacity to regulate glucocorticoid receptor sensitivity. However, it is not clear the role Fkbp5 plays in polyphenol-mediated stress modulation. In this study, the neuroprotective effects and mechanisms of the naturally derived polyphenols xanthohumol and quercetin against cytotoxicity induced by corticosterone were investigated in primary cortical cells.
Methods
Primary cortical cells containing both neurons and astrocytes were pre-incubated with different concentrations of quercetin and xanthohumol to examine the neuroprotective effects of polyphenols on cell viability, morphology, and gene expression following corticosterone insult.
Results
Both polyphenols tested prevented the reduction of cell viability and alterations of neuronal/astrocytic numbers due to corticosterone exposure. Basal levels of Bdnf mRNA were also decreased after corticosterone insult; however, this was reversed by both polyphenol treatments. Interestingly, the Nrf2 inhibitor blocked xanthohumol but not quercetin-mediated neuroprotection. In contrast, we found that Fkbp5 expression is exclusively modulated by quercetin.
Conclusions
These results suggest that naturally derived polyphenols protect cortical cells against corticosterone-induced cytotoxicity and enhance cell survival via modulation of the Nrf2 pathway and expression of Fkbp5.
Keywords: cortical neurons, corticosterone, polyphenols, stress
Significance Statement.
Stress-related mental disorders, including depression and anxiety, are currently a major public health concern. Thus, research and drug discovery focused on treatment for mental illnesses linked with stress are of substantial interest. A number of novel natural compounds have been associated with potential therapeutic effect for depression and anxiety. For example, naturally derived polyphenols have demonstrated positive effects against stress-induced mood despair; however, the mechanisms are unclear. On the other hand, cellular models represent a practical approach to assess gene expression and protein trafficking after drug exposure. In this study, we examined the protective capacity of polyphenols against chronic exposure to corticosterone (the main stress hormone) on cortical neurons in vitro. The results of this work provide new evidence about the neuroprotective properties of polyphenols and a novel insight into the potential mechanisms underlying polyphenol-mediated improvement of stress-related disorders.
Introduction
Alterations in hypothalamic-pituitary-adrenal (HPA) axis function have been associated with many neuropsychiatric disorders including depression, anxiety, anorexia nervosa, and posttraumatic stress disorder (de Kloet et al., 2006; Pariante and Lightman, 2008). It is well known that the HPA axis is activated in response to stress, leading to increased concentration of glucocorticoids in the circulation. These steroid hormones are critically involved in the homeostatic regulation of metabolism, development, and immune function (Sapolsky, 2000). Moreover, preclinical studies have shown that chronic exposure to glucocorticoids produces deleterious effects to the structure and functional plasticity of the hippocampus and amygdala of adult rats (Mitra and Sapolsky, 2008; Zhu et al., 2013). In addition, stress-induced increase of glucocorticoids has also been associated with impairment in the structure and function of the prefrontal cortex (PFC), a brain region that has been implicated in executive function (Arnsten, 2009). Indeed, chronic stress exposure induces loss of dendrites and spines in pyramidal cells of the PFC in rodents, which correlates with impaired working memory in the delayed alternation task (Hains et al., 2009; Shansky et al., 2009). In humans, chronic stress has been shown to weaken functional connectivity in the PFC and regulation of the amygdala, which correlates with loss of grey matter (Arnsten, 2015). Moreover, brain-derived neurotrophic factor (BDNF), which maintains neuronal survival and plasticity, is decreased in chronically stressed animals and patients with major depressive disorder and stress-related disorders (Murakami et al., 2005; Brunoni et al., 2008). Based on these observations, a drug that can attenuate the effects of chronic exposure to glucocorticoids on brain function may have therapeutic potential in preventing and treating stress-related disorders.
Polyphenolic compounds are natural phytochemicals found abundantly in plant food sources and characterized by the presence of multiple hydroxyl structural units on aromatic rings (Vauzour, 2012). Several in vivo studies have focused on the potential of dietary polyphenols in protecting cognitive function and reducing risk for developing neurodegenerative and neuropsychiatric disorders, including depression and anxiety (Bhutada et al., 2010; Hurley et al., 2014; Lopresti et al., 2014). Indeed, increasing data support the neuromodulatory potential of polyphenols via their ability to protect vulnerable neurons and improve neuronal function and regeneration (Spencer, 2008). In particular, several in vitro studies have confirmed the neuroprotective capacity of polyphenols mainly against cytotoxicity by both oxidative stress and amyloid peptide (Aβ) neuronal insult (Godoy et al., 2017).
Polyphenols vary in their chemical structure and functional activity (Vauzour et al., 2010). Xanthohumol, for example, is a prenylated chalcone isolated from the female hop plant, Humulus lupulus, and has been shown to possess anticancer, antiinflammatory, and neuroprotective effects in vitro (Liu et al., 2015; Yao et al., 2015). On the other hand, quercetin, a member of the flavonoid family, is one of the most prominent and widely distributed polyphenols in nature and is found in many fruits, vegetables, leaves, and grains (Manach et al., 2004). Quercetin has been associated with countless beneficial health effects such as protection against certain types of cancer and cardiovascular and neurodegenerative disorders (Boots et al., 2008; Godoy et al., 2017).
It is well known that polyphenols can activate the nuclear factor erythroid 2-related factor-2 (Nrf2), a redox-sensitive transcription factor related to cell survival and antioxidant responses (Ungvari et al., 2010; Gopinath and Sudhandiran, 2012). On the other hand, there is evidence showing that polyphenols are able to modulate the glucocorticoid receptor (GR) function. Indeed, the polyphenolic flavonoid icariin is able to alter the expression of the GR and the FK506 binding protein 5 (FKBP5), which promotes GR stability and reduces GR sensitivity to GC in vivo (Wei et al., 2016). Actually, modulation of the GR regulatory system is currently an interesting target for treatment for stress-related disorders; therefore, more research is needed in this area (O’Leary et al., 2013).
The purpose of the present study was to explore through an in vitro approach new pharmacological strategies to treat stress-related disorders. In particular, we examined whether xanthohumol and quercetin, two naturally derived polyphenols, protect against corticosterone-induced toxicity in neurons derived from the brain cortex, a key brain area involved in the pathophysiology of stress-related disorders. In addition, we explored the molecular mechanisms potentially implicated in such an effect. Specifically, we examined the interaction between the Nrf2 pathway and the GR signaling pathway to elucidate the key molecular mechanisms through which polyphenols may protect neuronal integrity and improve stress-related disorders.
METHODS
Chemical and Reagents
Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), poly-L-lysine, corticosterone (CORT), trypsin, L-glutamine, penicillin/streptomycin, D-glucose, 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), dimethyl sulfoxide, Triton X-100, RU486, HEPES, NaCl, trigonelline, and quercetin were purchased from Sigma. B-27 supplement was obtained from Thermo Fisher Scientific. Xanthohumol (purity 65–85%) was provided by Hopsteiner.
Animals
All procedures on live animals were performed under license from the Government of Ireland Department of Health (B100/3774) in accordance with National and European Union directive 2010/63/EU, with prior ethical approval by University College Cork (AEEC #2012/045). Experiments were conducted in accordance with guidelines established by University College Cork’s Animal Welfare Body.
Cell Culture
Primary cortical cell cultures, consisting of both neurons and astrocytes, were prepared as described previously (Pusceddu et al., 2016) with some modifications. Briefly, post-natal day 1 (PND1) Sprague Dawley male rats were euthanized, brains removed, and cerebral cortices dissected. Tissue was enzymatically dissociated with trypsin (0.25 µg/mL) and then triturated using a glass Pasteur pipette in warm DMEM-F12 with 10% FBS and 100 µg/mL DNase. Cell suspension was passed through 40-µm strainer and then centrifuged at 1000 rpm for 10 minutes at room temperature. The pellet was resuspended in warm culture media (DMEM-F12 supplemented with B-27, 1% FBS, 100 U/mL penicillin, 100 µg/mL streptomycin, L-glutamine 2 mM, and D-glucose 55 mM). The suspended cells were cultured at 37°C with 5% CO2.
Cell Treatment
Quercetin and xanthohumol were selected after we detected their positive effects in a preliminary in vitro screen testing the potential of several polyphenolic compounds, including resveratrol, naringin, and astaxanthin, against CORT cytotoxicity in cortical cells (data not shown). Quercetin (0.03, 0.3, and 3 µM) and xanthohumol (0.2, 1, and 5 µM) were added to primary cortical cell cultures at day in vitro (DIV) 5 and maintained for 24 hours. The media was replaced with fresh media containing 200 µM CORT at DIV6 and kept for 96 hours (Figure 2A). The doses of polyphenols were chosen based on previous publications, which showed neuroprotective effects in vitro (Yao et al., 2015; Godoy et al., 2017). To investigate the involvement of Nrf2 signaling in quercetin and xanthohumol-mediated neuroprotective effects, cells were co-treated with a specific Nrf2 inhibitor (5 µM trigonelline) along with quercetin or xanthohumol prior to CORT treatment. The dose of trigonelline was based on previous studies of Nrf2 inhibition in cellular models (Arlt et al., 2013; Rizza et al., 2015).
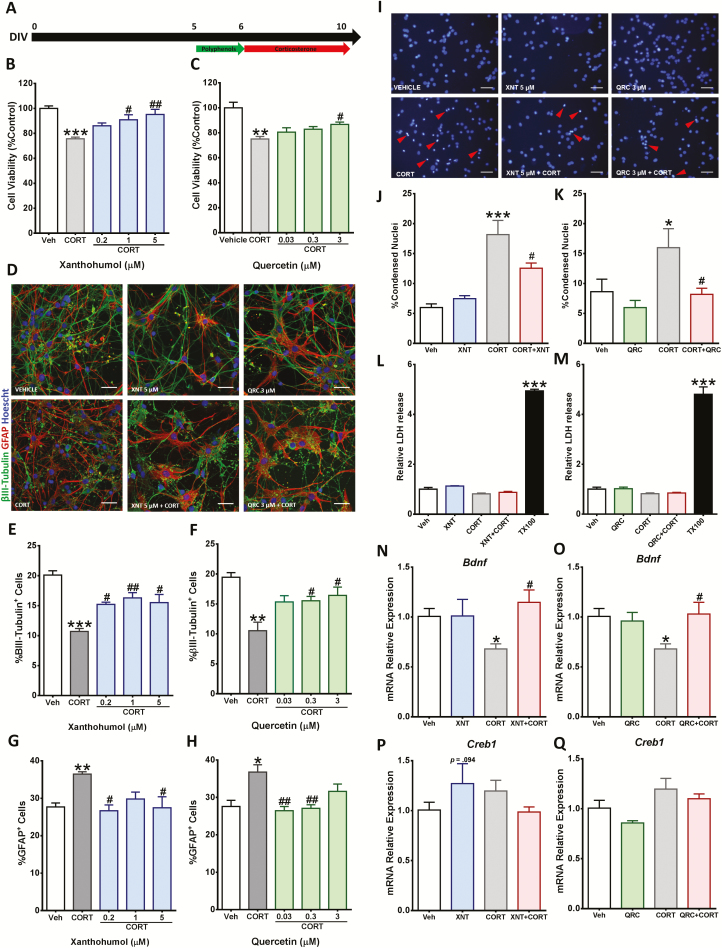
Figure 2.
Xanthohumol and quercetin prevented corticosterone (CORT)-induced changes in cortical cells. (A) Schematic representing the experiment timeline. (B–C) Cortical cells were pretreated with the indicated concentrations of xanthohumol and quercetin for 24 hours and then treated with 200 µM CORT for 96 hours. Cell viability was measured by 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay. (D–H) Quantitative analysis of neurons and astrocytes was performed by immunostaining of βIII-tubulin+ and GFAP+ cells. Scale bar = 50 µm. (I–K) Cortical cells were pretreated with 5 µM xanthohumol or 3 µM quercetin for 24 hours and then exposed for 96 hours to 200 µM CORT. Morphological changes of the nuclei were determined by Hoechst 33258 staining. Scale bar = 50 µm. Red arrows indicate representative condensed nuclei. (L–M) Lactate dehydrogenase (LDH) activity was measured in cell culture media after polyphenol and CORT treatment. (N–Q) The gene expression was quantitatively measured using real time reverse transcription polymerase chain reaction (RT-PCR) for Bdnf and Creb1 mRNA relative expression. Results are expressed as the mean ± SEM of 3 independent experiments performed in triplicate (*P < .05; **P < .01; ***P < .001 vs vehicle groups; #P < .05; ##P < .01 vs CORT groups).
Cell Viability Measurement
Cell viability was determined by an MTT assay based on mitochondrial dehydrogenase activity of viable cells as previously described (Mosmann, 1983). Briefly, cortical cells were cultured in a 24-well plate at a density of 4 × 104 cells per well. After treatment, medium was removed and replaced with fresh culture medium containing MTT (500 µg/mL) and then incubated at 37°C for 3 hours. Afterwards, 100 µL of dimethyl sulfoxide was added to each well to dissolve the resulting formazan. The absorbance values were measured by spectrophotometry at 570 nm with a microplate reader (BioTek Synergy HT). The results were expressed as a percentage of vehicle control group.
LDH Release Determination
Lactate dehydrogenase (LDH) release is usually quantified as a marker for cell lysis and necrosis (Brito et al., 2006). LDH release was measured using the Cytotoxicity Detection Kit LDH (Roche) according to the manufacturer’s instructions. Briefly, cortical cells were cultured in a 24-well plate at a density of 4 × 104 cells per well. After treatment, medium was transferred to a 96-well plate. Mix solution from the kit containing both catalyst and dye solution was added to the wells and incubated for 30 minutes at room temperature protected from light. The absorbance values were measured by spectrophotometry at 490 nm with a microplate reader (BioTek Synergy HT).
Immunocytochemistry
Cellular staining for βIII-tubulin and Glial Fibrillary Acidic Protein (GFAP) proteins, molecular markers for neurons and astrocyte, respectively, was assessed through immunofluorescence detection as previously described (Pusceddu et al., 2016). Briefly, cortical cells were fixed with ice-cold methanol for 10 minutes and then blocked overnight in 5% horse serum at 4°C. Subsequently, the cells were incubated in primary antibody solution (mouse anti-βIII-tubulin 1:300, Promega; rabbit anti-GFAP 1:300, Dako) overnight at 4°C. The following day, the cells were incubated with the appropriate secondary antibody (Alexa Fluor 594 donkey anti-mouse 1:2000, Thermo Fisher; Alexa Fluor 488 donkey anti-rabbit 1:2000, Thermo Fisher) for 1 hour at room temperature. Finally, cellular counterstaining was assessed with Hoechst 33258 (Sigma) for 5 minutes. The cells were viewed using an Olympus BX53 upright fluorescence microscope.
Quantitative RT-PCR
Total RNA was isolated from primary cortical cells using the High Pure RNA isolation kit (Roche). Briefly, cells were seeded at a density of 1.5 × 106 cells per well in 6-well plates followed by pretreatments with quercetin and xanthohumol before CORT insult. Cells were harvested and the lysate transferred into a filter tubes. RNA concentration was assessed using the ND-1000 spectrophotometer. Subsequently, isolated RNA was reverse transcribed into cDNA using the ExiLERATE LNA qPCR, cDNA synthesis kit (Exiqon). Following reverse transcription, mRNA expression levels of respective genes (Table 1) were measured using a lightcycler 480 II (Roche). Amplification was performed using ExiLERATE LNA qPCR, SYBR Green master mix kit (Exiqon). Each sample was analyzed in triplicate for both target gene and reference gene (β-actin), and the relative mRNA expressions were calculated using the 2-ΔΔCt method (Livak and Schmittgen, 2001).
Table 1.
Real Time Polymerase Chain Reaction (PCR) Primers
| Target mRNA | Forward primer 5’-3’ | Reverse primer 5’-3’ |
|---|---|---|
| β-actin | TGTCACCAACTGGGACGATA | GGGGTGTTGAAGGTCTCAAA |
| Bdnf | GGACATATCCATGACCAGAAAGAAA | GCAACAAACCACAACATTATCGAG |
| Creb1 | CGTCATCTGCTCCCACTGTA | CCTTCGTTTTTGGGAATCAG |
| Nqo-1 | TCACCACTCTACTTTGCTCCAA | TTTTCTGCTCCTCTTGAACCTC |
| Ho-1 | GCCCTGGAAGAGGAGATAGAG | TAGTGCTGTGTGGCTGGTGT |
| Gclc | CAAGGACAAGAACACACCATCT | CAGCACTCAAAGCCATAACAAT |
| Nr3c1 | GAAAAGCCATCGTCAAAAGGG | TGGAAGCAGTAGGTAAGGAGA |
| Gilz | CAGGCCATGGATCTAGTGAA | AGCGTCTTCAGGAGGGTATT |
| Fkbp5 | ACATGCAGGCCGTGATTCAGTA | TTGTCACAGCACTCGACAGCTT |
| Sgk1 | TGAAATAGCCAGTGCCTTGGGT | AAGGTGGACGTTGTCCCATTGT |
| Foxo3 | TGTCAGCAACATGGGCTTGAGT | GGGAAGGTTTGCACTGGCTGAATA |
| Btg1 | TTCAGGCTTCTCCCAAGTGAACTC | CCATTTGCACGTTGGTGCTGTT |
Reverse-Phase Protein Array (RPPA)
High-throughput RPPA for 249 proteins (antibody list provided in Supplementary Information) was performed following the protocol and directions of MD Anderson Center (Houston, TX), where RPPA was performed. Briefly, cells were seeded at a density of 1.5 × 106 cells per well in 6-well plates followed by pretreatment with quercetin and xanthohumol before CORT insult. Cells were harvested and then lysed using the lysis buffer suggested by MD Anderson (1% Triton X-100, 50 mM HEPES, pH 7.4, 150 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 100 mM NaF, 10 mM Na pyrophosphate, 1 mM Na3VO4, and 10% glycerol). Total protein concentration in lysates was determined by performing a BCA assay (Thermo Scientific), and samples were adjusted to a concentration of 1–1.5 μg/μL. Samples were then denatured using the sodium dodecyl sulfate (SDS) sample buffer recommended by the core facility, boiled for 5 minutes, and then stored at −80°C before being shipped to MD Anderson Center on dry ice. Samples were then serially diluted and arrayed onto nitrocellulose-coated slides. Slides were then probed with the core facility’s collection of antibodies, and signal was generated using a 3, 3′-diaminobenzidine colorimetric reaction-based system. Results provided by MD Anderson Center were then analyzed using the functional annotation tool DAVID 6.8 to detect up- or downregulation of signaling pathways within the KEGG database.
Statistical Analysis
Statistical analysis was performed using the software SPSS 24.0, and the results were presented as mean ± SEM. Data were analyzed using 1- or 2-way ANOVA as appropriate. A P value of .05 was considered statistically significant.
RESULTS
CORT-Induced Changes in Cortical Cells were Mediated by the GR
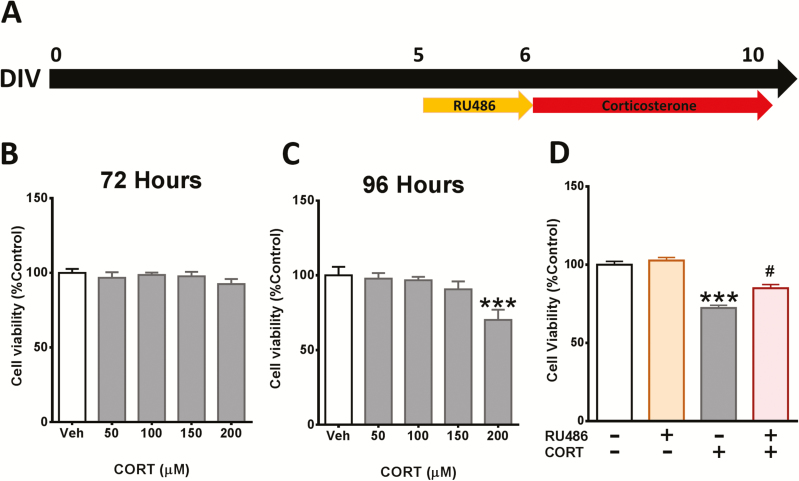
To investigate the role of the GR in CORT-elicited cytotoxicity in cortical cells, dose and time curve responses of CORT were determined by MTT assay. At DIV5 the cells were treated with CORT for 72 and 96 hours (Figure 1A). As expected, stimulation with CORT caused a significant reduction in cell viability at 96 hours (Figure 1B–C). Pre-incubation with the GR antagonist RU486 ameliorated the reduction of cell viability caused by CORT (Figure 1D).
Figure 1.
Corticosterone (CORT)-induced cytotoxicity in cortical cells is mediated by the glucocorticoid receptor (GR). (A) Schematic representing the experiment timeline. (B–C) Cortical cells were treated with various concentrations of CORT for 72 and 96 hours. (D) Cortical cells were pretreated with 50 nM of RU486 for 24 hours and then treated with 200 µM CORT for 96 hours. Cell viability was measured by 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay. Results are expressed as the mean ± SEM of 3 independent experiments performed in triplicate (***P < .001 vs vehicle groups; #P < .05 vs CORT groups).
Xanthohumol and Quercetin Prevented CORT-Induced Cytotoxicity in Cortical Cells
To determine the protective effects of xanthohumol and quercetin from CORT-induced cytotoxicity, cortical cells were pre-incubated with different concentrations of xanthohumol and quercetin (Figure 2A). The reduction of cell viability in cortical cells induced by 96 hours of incubation with 200 µM CORT was significantly prevented by 24-hour pretreatment with both xanthohumol (1 or 5 µM) and quercetin (3 µM) (Figure 2B–C).
Xanthohumol and Quercetin Prevented CORT-Induced Alterations in Neuron and Astrocyte Proportion
To further determine the potential protective effects of xanthohumol and quercetin against CORT-induced cytotoxicity, we explored whether these polyphenols prevented the alteration in neuron and astrocyte composition caused by CORT in cortical cells. As illustrated in Figure 2D–H, pretreatment with both xanthohumol and quercetin prevented the decrease in the number of neurons and the increase in astrocytes induced by treatment with 200 µM CORT.
Xanthohumol and Quercetin Prevented CORT-Induced Apoptotic Morphological Changes in Cortical Cells
To define the type of cell death triggered by CORT, apoptotic and necrotic features were measured in cortical cells after CORT exposure. Apoptosis is characterized by morphological changes including chromatin condensation and reduction of cell membrane permeability. On the other hand, membrane integrity is completely lost during necrosis (Brito et al., 2006). A 24-hour pre-incubation with 5 µM xanthohumol and 3 µM quercetin reduced the number of condensed nuclei in cortical cells after 200 µM CORT treatment for 96 hours (Figure 2I–K). Extracellular LDH activity was not increased after CORT treatment, suggesting that plasmatic membrane did not lose integrity, similarly to apoptosis. In contrast, the necrotic/cell lysis inductor Triton X-100 produced a significant increase of extracellular LDH activity. (Figure 2L–M)
Xanthohumol and Quercetin Prevented CORT-Induced Downregulation of Bdnf Gene Expression in Cortical Cells
Modulation of BDNF expression is one of the mechanisms through which neuroprotective agents exert their effects (Zhao et al., 2017). Thus, the effect of xanthohumol and quercetin on CORT-induced reduction in mRNA Bdnf levels was investigated in cortical cells. After 96 hours of treatment, 200 µM CORT triggered a considerable reduction of Bdnf gene expression in cortical cells as previously reported (Pusceddu et al., 2016). However, this Bdnf reduction was prevented by a pre-incubation with 5 µM xanthohumol and 3 µM quercetin for 24 hours (Figure 2N–O). To investigate whether CORT-induced reduction of Bdnf expression is mediated by downregulation of Creb1, we first explored whether CORT regulates the Creb1 mRNA expression in cortical cells (Figure 2P–Q). Treatment with 200 µM CORT for 96 hours did not induce changes in Creb1 expression in cortical cells. Moreover, neither 5 µM xanthohumol nor 3 µM quercetin was able to upregulate Creb1 gene expression.
Inhibiting the Nrf2 Pathway Attenuated Only the Protective Effect of Xanthohumol Against Cort-Induced Cytotoxicity in cortical cells
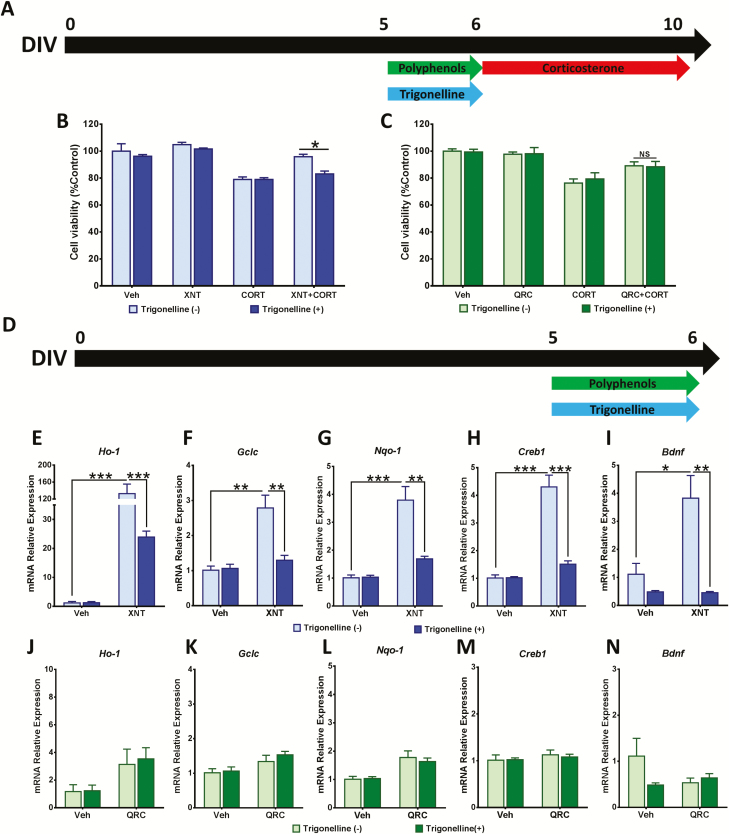
The activation of Nrf2 pathway has been shown to be involved in the protective mechanisms of polyphenols against CORT in neuronal models (Freitas et al., 2015; Sun et al., 2018). Thus we used a pharmacological approach to determine the mediatory role of the Nrf2 pathway in the protective effects of xanthohumol and quercetin against CORT-elicited cytotoxicity. We investigated whether trigonelline, an inhibitor of Nrf2 nuclear import (Arlt et al., 2013), could abolish the cytoprotective effect of these polyphenols against CORT-dependent reduction in cell viability in cortical cells. At DIV5 the cells received a treatment with both polyphenols and 5 µM trigonelline for 24 hours and subsequently CORT stimulus for 96 hours (Figure 3A). Trigonelline treatment ameliorated the increase in cell viability induced by treatment with xanthohumol, which suggested that inhibition of the Nrf2 pathway blocked the protective effect of xanthohumol against CORT-induced cytotoxicity in cortical cells. In contrast, trigonelline did not affect the protective effects of quercetin against CORT-induced cytotoxicity (Figure 3B–C).
Figure 3.
Xanthohumol neuroprotection is mediated by the Nrf2 pathway. (A–C) Cortical cells were co-treated with 5 µM trigonelline and polyphenols (5 µM xanthohumol or 3 µM quercetin) for 24 hours before 96-hour exposure to 200 µM corticosterone (CORT). Cell viability was detected by 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay. (D) Cortical cells were co-treated with 5 µM trigonelline and 5 µM xanthohumol or 3 µM quercetin for 24 hours. Relative expression of Nrf2-activated genes (Ho-1, Nqo-1, and Gclc), Creb1, and Bdnf induced by (E–I) xanthohumol and (J–N) quercetin was determined through real time reverse transcription polymerase chain reaction (RT-PCR). Data were expressed as the mean ± SEM of 3 independent experiments performed in triplicate (***P < .001; **P < .01; ***P < .001). Gclc glutamate—cysteine ligase catalytic subunit; Ho-1, heme oxygenase 1; Nqo-1, NAD(P)H dehydrogenase (quinone 1).
Xanthohumol Induced the Expression of Nrf2 Target Genes in Cortical Cells
We further investigated whether xanthohumol or quercetin induced the expression of Nrf2-driven cytoprotective/antioxidant genes in cortical cells. At DIV5 the cells received a treatment with either xanthohumol or quercetin along with 5 µM trigonelline for 24 hours (Figure 3D). After 24-hour treatment with 5 µM xanthohumol, the mRNA expression of Nrf2-activated genes (Ho-1, Nqo-1, and Gclc) was significantly increased. Moreover, 5 µM trigonelline co-treatment blocked xanthohumol-induced upregulation of Nrf2-activated genes, which confirmed the activation of Nrf2-pathway by xanthohumol (Figure 3E–G). On the other hand, 3 µM quercetin treatment did not induce expression of Nrf2-driven genes in cortical cells (Figure 3J–L).
Xanthohumol-Induced Bdnf Gene Expression Was Dependent of Nrf2 Activation
The effect of xanthohumol and quercetin on gene expression of Bdnf before CORT exposure was studied in cortical cells. Treatment with 5 µM xanthohumol for 24 hours remarkably induced the gene expression of Bdnf and Creb1. Interestingly, 5 µM trigonelline prevented the increase in Bdnf and Creb1 gene expression induced by treatment with xanthohumol (Figure 3H–I). In contrast, 3 µM quercetin treatment for 24 hours did not induce gene expression of Bdnf and Creb1 (Figure 3M–N). These data indicate that xanthohumol-induced Creb1 and Bdnf gene expression may be mediated by the Nrf2 pathway.
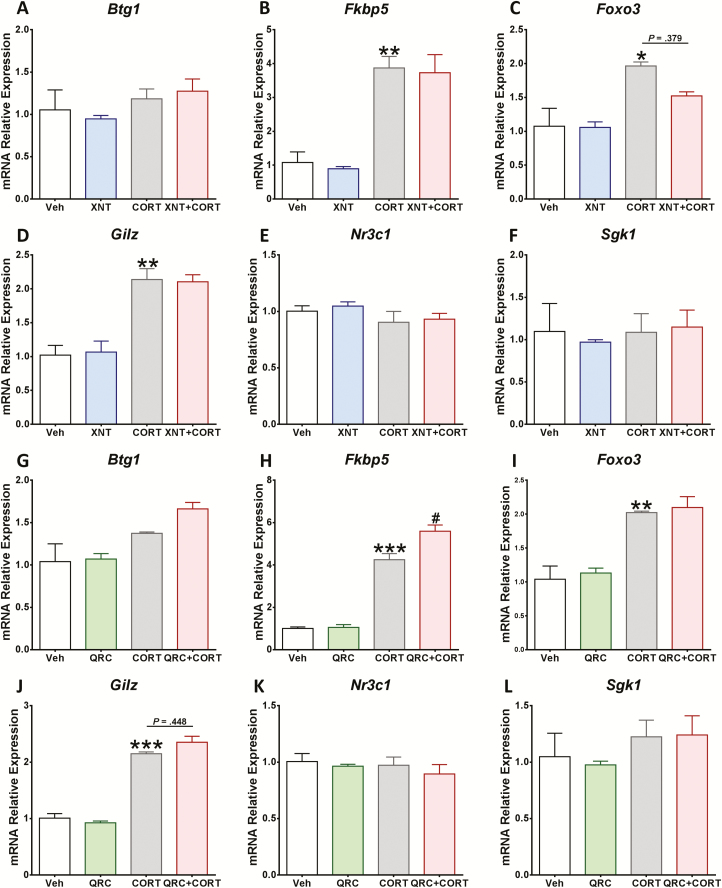
Quercetin Enhanced FKBP5 Gene Expression After CORT Stimulus in Cortical Cells
Given that the Nrf2 pathway might not be involved in quercetin neuroprotection, we examined the GR signaling. The effect of xanthohumol and quercetin on GR-regulated gene expression after CORT treatment was studied in cortical cells. Although we did not find changes in Btg1, N3c1, and Sgk1 mRNA levels after CORT exposure, the gene expression of Fkbp5, Foxo3, and Gilz was significantly increased (Figure 4). Moreover, Fkbp5 expression was further increased by pre-incubation with 3 µM quercetin followed by CORT treatment for 96 hours compared with CORT group alone (Figure 4H). Pre-treatment with 5 µM xanthohumol did not affect the expression of GR-regulated genes (Figure 4A–F).
Figure 4.
Quercetin enhanced the expression of FK506 binding protein 5 (Fkbp5) after corticosterone (CORT) treatment in cortical cells. After 24-hour exposure to (A–F) 5 µM xanthohumol or (G–L) 3 µM quercetin, cortical cells were treated for 96 hours with 200 µM CORT. The gene expression of Btg1, Fkbp5, Foxo3, Gilz, Nr3c1, and Sgk1 was quantitatively measured using real time reverse transcription polymerase chain reaction (RT-PCR). Results are expressed as the mean ± SEM of 3 independent experiments performed in triplicate (***P < .001; **P < .01; *P < .05 vs vehicle groups; #P < .05 vs CORT groups). Btg1, B-cell translocation gene 1; Foxo3, forkhead box O3; Gilz, glucocorticoid-induced leucine zipper; Nr3c1, nuclear receptor subfamily 3 group C member 1; Sgk1, serum and glucocorticoid-regulated kinase 1.
CORT-Induced Changes in Akt/mTOR/AMPK Signaling Pathways
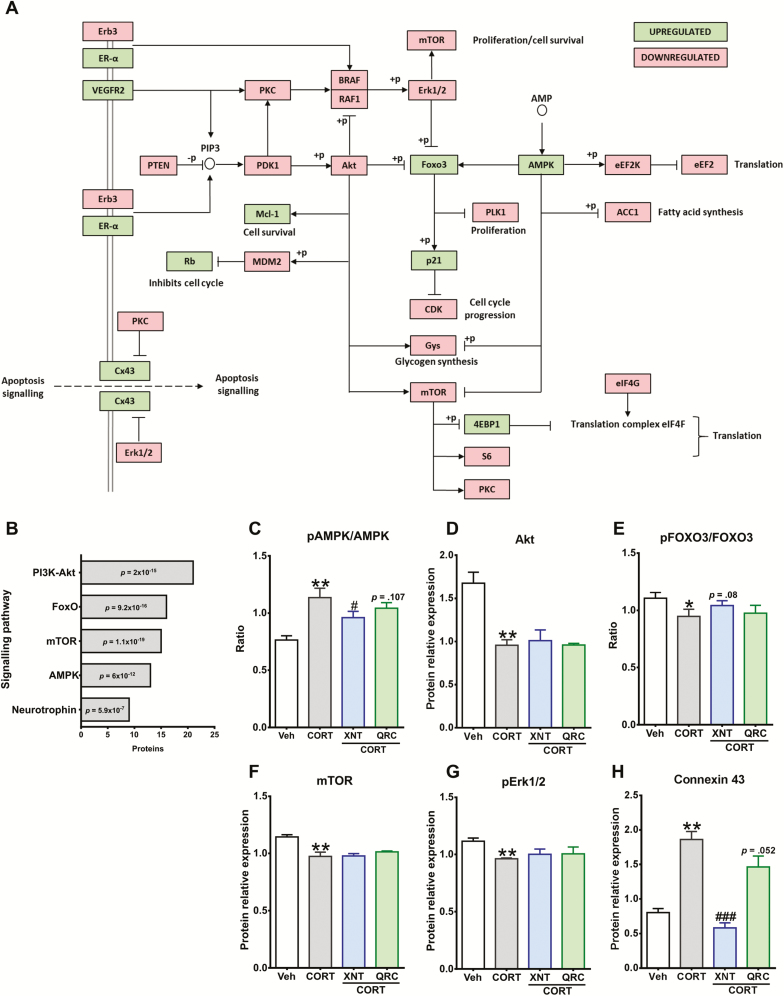
To investigate the impact of CORT treatment in signaling pathways associated with proliferation, cell growth, and apoptosis in cortical cells, we screened the activation or downregulation of proteins involved in these physiological processes using the RPPA approach. After treatment with 200 µM CORT for 96 hours, we found 46 proteins with altered expression (Figure 5A), and further analysis revealed that Akt, FOXO, mTOR, AMPK, and Neurotrophin signaling pathways were significantly affected (Figure 5B; KEGG codes: Akt map04151, FoxO map04068, mTOR map04150, AMPK map04152, and Neurotrophin map04722).
Figure 5.
Impact of xanthohumol and quercetin on corticosterone (CORT)-induced changes in Akt/mTOR, AMPK, and Erk1/2 signaling pathways. (A) Schematic interaction map of proteins altered by CORT; upregulated expression of proteins is shown in green and downregulated proteins are highlighted in red. Cortical cells were treated with 200 µM CORT for 96 hours. The relative expression of proteins involved in proliferation, cell growth and apoptosis was determined using the reverse-phase protein array (RPPA) approach. (B) Kyoto encyclopedia of genes and genomes (KEGG) signaling pathways affected by CORT were detected using the functional annotation tool DAVID 6.8. (C–H) After 24-hour exposure to 5 µM xanthohumol or 3 µM quercetin, cortical cells were treated for 96 hours with 200 µM CORT. The levels of protein expression of AMP-activated protein kinase (AMPK), protein kinase B (Akt), forkhead box O3 (FOXO3), mammalian target of rapamycin (mTOR), extracellular signal regulated kinase 1/2 (Erk1/2), and connexin 43 were quantitatively measured using RPPA and provided by MD Anderson. Results are expressed as the mean ± SEM of 3 independent experiments (***P < .001; **P < .01; *P < .05 vs vehicle groups; ###P < .001; #P < .05 vs CORT groups).
Impact of Xanthohumol and Quercetin on CORT-Induced Changes in Akt/mTOR, AMPK, and Erk1/2 Signaling Pathways
The effect of xanthohumol and quercetin on CORT-induced alteration in signaling pathways associated with proliferation and apoptosis was next investigated in cortical cells. Pre-incubation with xanthohumol or quercetin did not interfere significantly against CORT-induced changes in Akt, mTOR, and Erk1/2 signaling pathways (Figure 5D, F, G). Nevertheless, xanthohumol treatment prevented the increase of the pAMPK/AMPK ratio caused after CORT insult (Figure 5C). Surprisingly, xanthohumol abolished CORT-induced protein overexpression of connexin 43 (Cx43), which is not involved directly with these signaling pathways (Figure 5H).
Discussion
In the present study, we demonstrated that administration of naturally derived polyphenols xanthohumol and quercetin significantly protects primary cortical cells against CORT-elicited cytotoxicity, altered neuronal/astrocytic ratio, and reduced Bdnf expression. In addition, our results establish that trigonelline, an inhibitor of Nrf2, abolishes the neuroprotective effects of xanthohumol but not quercetin against CORT-induced cell death. Taken together, our findings reveal that although both xanthohumol and quercetin are neuroprotective agents, they recruit different cellular signaling pathways to induce such effects.
Several reports have demonstrated that the glucocorticoid hormone corticosterone exerts a toxic effect on neurons (Gao et al., 2015; Zhao et al., 2018). Additionally, increasing levels of CORT are associated with depressive and anxious-like behaviors in rodent models (Rosa et al., 2014; Mendez-David et al., 2017). Therefore, preventing the neurotoxic effects of glucocorticoids could represent a novel therapeutic approach for treatment of stress-related disorders. On the other hand, polyphenols have attracted considerable interest due to their multiple health benefits including antioxidant, anticancer, and neuroprotective effects (Vauzour et al., 2010). However, the molecular mechanisms underlying polyphenol-mediated neuroprotection in neurons are not fully understood. Thus, the aim of the present study was to investigate whether the naturally derived polyphenolic compounds xanthohumol and quercetin prevent CORT-induced neurotoxicity in cortical cells.
Mixed cortical cell cultures containing both neurons and astrocytes have been extensively used as a model to study the neurotoxic effects of numerous stimulants in vitro (Wie et al., 1997; Trackey et al., 2001; Huang et al., 2009). As expected, our data show that CORT treatment decreased the cell viability of cortical cells. In addition, further analysis indicated that CORT-exerted cell death is mediated through activation of the GR, resulting in the induction of apoptosis. In accordance with our findings, it has been shown before that CORT induces apoptosis via activation of the GR cascade in different neuronal models such as hippocampal neurons (Latt et al., 2018), PC12 cells (Li et al., 2014), hypothalamic neurons (Zhang et al., 2012), and cortical cells (Pusceddu et al., 2016).
We next demonstrated that treatment with xanthohumol and quercetin significantly reduced CORT-induced cytotoxicity. In addition, these compounds reduced the alteration of cellular composition in cortical cells produced by CORT. Indeed, our study shows that the reduction in neuronal percentage and increased astrocytic concentration caused by CORT treatment are prevented by these polyphenols. In neural tissue, astrocytes play an important role in the regulation of synapse, energy, and neurotransmitter balance (Sofroniew and Vinters, 2010). Accordingly, astrocytic overgrowth or astrogliosis has been described as a physiological process critical for neural protection and repair (Zhang et al., 2007). This is in line with previous studies showing similar effects on astrocytes after CORT insult (Bridges et al., 2008; Pusceddu et al., 2016).
To further investigate the possible mechanisms underlying the protective effect of xanthohumol and quercetin against CORT-induced cytotoxicity, we examined the BDNF gene expression. BDNF is a neurotrophin involved in neuroplasticity and neuronal survival, which has been associated with neuroprotective effects in vitro and in vivo (Almeida et al., 2005; Motaghinejad et al., 2017). Here we demonstrated that both polyphenols prevent CORT-induced reduction of Bdnf mRNA levels. Previous studies have demonstrated that several polyphenols, including xanthohumol and quercetin, induce the activation of Nrf2, which contributes significantly to cytoprotective and antioxidant responses in different cellular models (Tanigawa et al., 2007; Ungvari et al., 2010; Gopinath and Sudhandiran, 2012; Yao et al., 2015). We found that trigonelline, an inhibitor of Nrf2, significantly reversed the protective effect of xanthohumol but not quercetin. Moreover, blocking Nrf2 activation abolished the xanthohumol-mediated increase of Bdnf mRNA levels, confirming previous studies that also highlight the crosstalk between BDNF and Nrf2 signaling (Mendez-David et al., 2015; Bouvier et al., 2017; Ishii and Mann, 2018).
Surprisingly, quercetin treatment did not induce Bdnf gene expression, suggesting that quercetin prevented CORT-induced depletion of BDNF in cortical cells through an alternative mechanism. Interestingly, there is some evidence indicating that quercetin is able to activate Nrf2 signaling in other in vitro studies (Tanigawa et al., 2007; Granado-Serrano et al., 2012). However, the reason as to why quercetin fails to activate Nrf2 in cortical cells remains unknown.
To elucidate the mechanisms underlying the neuroprotection of quercetin, we examined whether quercetin attenuates activation of the GR cascade following CORT stimulation. We hypothesized that downregulation of the GR signaling pathway could decrease CORT-induced cytotoxicity and eventually reverse BDNF depletion in cortical cells. GR blockade strategies have been proposed to be a novel therapeutic approach to prevent the negative effects of chronic stress (Oomen et al., 2007; Revsin et al., 2009). In this regard, analysis of FKBP5, a protein that regulates GR sensitivity, is considered an interesting target for the prevention and treatment of stress-related psychiatric disorders (Guidotti et al., 2013). When FKBP5 is bound to the receptor complex, glucocorticoids bind with lower affinity and the nuclear translocation of the receptor is less efficient (Binder, 2009). Our data demonstrate that quercetin treatment significantly increased expression of Fkbp5 after CORT stimuli in cortical cells, suggesting that the GR negative feedback system could be activated, thus preventing BDNF depletion.
BDNF-mediated neuroprotective mechanisms are associated with the activation of different molecular pathways related to proliferation and cell growth processes such as Akt, mTOR, and Erk1/2 (Han and Holtzman, 2000; Chen et al., 2013). Therefore, we analyzed components of these signaling pathways in cortical cells upon CORT stimulation. In this study, we established that CORT treatment significantly influences the proliferative pathways Akt and mTOR as well as metabolic signaling pathways that possibly trigger apoptosis, such as AMPK and FOXO3. Indeed, downregulation of Akt and mTOR is associated with decreased proliferation in neuronal models (Peltier et al., 2007; Bhaskar et al., 2009), while activation of AMPK/FOXO3 pathways indirectly lead to apoptotic signaling (Davila et al., 2012). Our results demonstrate that xanthohumol treatment reduced AMPK phosphorylation after CORT stimulus, indicating downregulation of this pathway. However, treatment with either xanthohumol or quercetin did not prevent CORT-induced changes at protein levels of Akt, mTOR, and Erk1/2. These data suggest that activation of these proliferative pathways are not critical for BDNF-mediated neuroprotection in cortical cells.
Additionally, we have demonstrated that xanthohumol significantly blocked CORT-induced overexpression of Cx43 in cortical cells. The evidence suggests that Cx43 plays a proapoptotic role in different cytotoxic stimuli, which has been suggested before (Huang et al., 2001; Dong et al., 2015; Yahiro et al., 2015), although the mechanisms responsible for Cx43-mediated apoptosis are unknown. Because Cx43 is the structural component of gap junctions and it is responsible for the transfer of water‐soluble molecules directly from one cell to another without passing through the membrane, the enhancement of cytotoxic effects on Cx43‐transfected cells may be due to increased transfer of drugs or proapoptotic molecules from one cell to another (Kameritsch et al., 2013).
In conclusion, our present work confirmed that naturally derived polyphenols xanthohumol and quercetin attenuate CORT-exerted neurotoxicity in primary cortical cultures. We further found that blocking the Nrf2 pathway could abolish the protective effect of xanthohumol, while quercetin neuroprotection may be mediated by FKBP5 modulation. Our data reveal that xanthohumol and quercetin mediate BDNF restoration upon CORT treatment in cortical cells, indicating the possible role of this neurotrophin in the neuroprotective mechanisms of these polyphenols. In addition, our findings provide a novel insight into the mechanisms underlying polyphenol-mediated neuroprotective effects in vitro and potential health benefits for stress-related disorders.
Supplementary Material
Acknowledgments
We thank Dr Matteo Pusceddu and Ms Loreto Olavarría for their assistance in performing the cell culture.
The APC Microbiome Ireland has conducted research funded by many pharmaceutical and food companies. T.G.D. and J.F.C. have received research funding from Mead Johnson, Cremo, Suntory Wellness, Nutricia, 4D Pharma, and DuPont. This work was supported by Science Foundation Ireland (grant no. SFI/12/RC/2273) and the Department of Agriculture, Food and the Marine, Irish government (grant no. 13F 411).
Interest Statement
We declare no competing interests. However, in the interest of full disclosure, J.F.C. and T.G.D. have received research funding from Dupont Nutrition Biosciences APS, Cremo SA, Alkermes Inc., 4D Pharma PLC, Mead Johnson Nutrition, Nutricia Danone, and Suntory Wellness and have spoken at meetings sponsored by food and pharmaceutical companies.
References
- Almeida RD, Manadas BJ, Melo CV, Gomes JR, Mendes CS, Grãos MM, Carvalho RF, Carvalho AP, Duarte CB (2005) Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ 12:1329–1343. [DOI] [PubMed] [Google Scholar]
- Arlt A, Sebens S, Krebs S, Geismann C, Grossmann M, Kruse ML, Schreiber S, Schäfer H (2013) Inhibition of the Nrf2 transcription factor by the alkaloid trigonelline renders pancreatic cancer cells more susceptible to apoptosis through decreased proteasomal gene expression and proteasome activity. Oncogene 32:4825–4835. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. (2009) Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci 10:410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF. (2015) Stress weakens prefrontal networks: molecular insults to higher cognition. Nat Neurosci 18:1376–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskar K, Miller M, Chludzinski A, Herrup K, Zagorski M, Lamb BT (2009) The PI3K-Akt-mTOR pathway regulates Abeta oligomer induced neuronal cell cycle events. Mol Neurodegener 4:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutada P, Mundhada Y, Bansod K, Ubgade A, Quazi M, Umathe S, Mundhada D (2010) Reversal by quercetin of corticotrophin releasing factor induced anxiety- and depression-like effect in mice. Prog Neuropsychopharmacol Biol Psychiatry 34:955–960. [DOI] [PubMed] [Google Scholar]
- Binder EB. (2009) The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology 34 (Suppl 1):S186–S195. [DOI] [PubMed] [Google Scholar]
- Boots AW, Haenen GR, Bast A (2008) Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol 585:325–337. [DOI] [PubMed] [Google Scholar]
- Bouvier E, Brouillard F, Molet J, Claverie D, Cabungcal JH, Cresto N, Doligez N, Rivat C, Do KQ, Bernard C, Benoliel JJ, Becker C (2017) Nrf2-dependent persistent oxidative stress results in stress-induced vulnerability to depression. Mol Psychiatry 22:1701–1713. [DOI] [PubMed] [Google Scholar]
- Bridges N, Slais K, Syková E (2008) The effects of chronic corticosterone on hippocampal astrocyte numbers: a comparison of male and female Wistar rats. Acta Neurobiol Exp (Wars) 68:131–138. [DOI] [PubMed] [Google Scholar]
- Brito PM, Mariano A, Almeida LM, Dinis TC (2006) Resveratrol affords protection against peroxynitrite-mediated endothelial cell death: a role for intracellular glutathione. Chem Biol Interact 164:157–166. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Lopes M, Fregni F (2008) A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: implications for the role of neuroplasticity in depression. Int J Neuropsychopharmacol 11:1169–1180. [DOI] [PubMed] [Google Scholar]
- Chen A, Xiong LJ, Tong Y, Mao M (2013) Neuroprotective effect of brain-derived neurotrophic factor mediated by autophagy through the PI3K/Akt/mTOR pathway. Mol Med Rep 8:1011–1016. [DOI] [PubMed] [Google Scholar]
- Davila D, Connolly NM, Bonner H, Weisová P, Dussmann H, Concannon CG, Huber HJ, Prehn JH (2012) Two-step activation of FOXO3 by AMPK generates a coherent feed-forward loop determining excitotoxic cell fate. Cell Death Differ 19:1677–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet CS, Vermetten E, Geuze E, Kavelaars A, Heijnen CJ, Westenberg HG (2006) Assessment of HPA-axis function in posttraumatic stress disorder: pharmacological and non-pharmacological challenge tests, a review. J Psychiatr Res 40:550–567. [DOI] [PubMed] [Google Scholar]
- Dong L, Yang X, Gu W, Zhao K, Ge H, Zhou J, Bai X (2015) Connexin 43 mediates PFOS-induced apoptosis in astrocytes. Chemosphere 132:8–16. [DOI] [PubMed] [Google Scholar]
- Freitas AE, Egea J, Buendía I, Navarro E, Rada P, Cuadrado A, Rodrigues AL, López MG (2015) Agmatine induces Nrf2 and protects against corticosterone effects in hippocampal neuronal cell line. Mol Neurobiol 51:1504–1519. [DOI] [PubMed] [Google Scholar]
- Gao S, Li W, Zou W, Zhang P, Tian Y, Xiao F, Gu H, Tang X (2015) H2S protects PC12 cells against toxicity of corticosterone by modulation of BDNF-TrkB pathway. Acta Biochim Biophys Sin (Shanghai) 47:915–924. [DOI] [PubMed] [Google Scholar]
- Godoy JA, Lindsay CB, Quintanilla RA, Carvajal FJ, Cerpa W, Inestrosa NC (2017) Quercetin exerts differential neuroprotective effects against H2O2 and Aβ aggregates in hippocampal neurons: the role of mitochondria. Mol Neurobiol 54:7116–7128. [DOI] [PubMed] [Google Scholar]
- Gopinath K, Sudhandiran G (2012) Naringin modulates oxidative stress and inflammation in 3-nitropropionic acid-induced neurodegeneration through the activation of nuclear factor-erythroid 2-related factor-2 signalling pathway. Neuroscience 227:134–143. [DOI] [PubMed] [Google Scholar]
- Granado-Serrano AB, Martín MA, Bravo L, Goya L, Ramos S (2012) Quercetin modulates Nrf2 and glutathione-related defenses in HepG2 cells: involvement of p38. Chem Biol Interact 195:154–164. [DOI] [PubMed] [Google Scholar]
- Guidotti G, Calabrese F, Anacker C, Racagni G, Pariante CM, Riva MA (2013) Glucocorticoid receptor and FKBP5 expression is altered following exposure to chronic stress: modulation by antidepressant treatment. Neuropsychopharmacology 38:616–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains AB, Vu MA, Maciejewski PK, van Dyck CH, Gottron M, Arnsten AF (2009) Inhibition of protein kinase C signaling protects prefrontal cortex dendritic spines and cognition from the effects of chronic stress. Proc Natl Acad Sci U S A 106:17957–17962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han BH, Holtzman DM (2000) BDNF protects the neonatal brain from hypoxic-ischemic injury in vivo via the ERK pathway. J Neurosci 20:5775–5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang RP, Hossain MZ, Huang R, Gano J, Fan Y, Boynton AL (2001) Connexin 43 (cx43) enhances chemotherapy-induced apoptosis in human glioblastoma cells. Int J Cancer 92:130–138. [PubMed] [Google Scholar]
- Huang YN, Wu CH, Lin TC, Wang JY (2009) Methamphetamine induces heme oxygenase-1 expression in cortical neurons and glia to prevent its toxicity. Toxicol Appl Pharmacol 240:315–326. [DOI] [PubMed] [Google Scholar]
- Hurley LL, Akinfiresoye L, Kalejaiye O, Tizabi Y (2014) Antidepressant effects of resveratrol in an animal model of depression. Behav Brain Res 268:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T, Mann GE (2018) When and how does brain-derived neurotrophic factor activate Nrf2 in astrocytes and neurons? Neural Regen Res 13:803–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameritsch P, Khandoga N, Pohl U, Pogoda K (2013) Gap junctional communication promotes apoptosis in a connexin-type-dependent manner. Cell Death Dis 4:e584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latt HM, Matsushita H, Morino M, Koga Y, Michiue H, Nishiki T, Tomizawa K, Matsui H (2018) Oxytocin inhibits corticosterone-induced apoptosis in primary hippocampal neurons. Neuroscience 379:383–389. [DOI] [PubMed] [Google Scholar]
- Li ZY, Jiang YM, Liu YM, Guo Z, Shen SN, Liu XM, Pan RL (2014) Saikosaponin D acts against corticosterone-induced apoptosis via regulation of mitochondrial GR translocation and a GR-dependent pathway. Prog Neuropsychopharmacol Biol Psychiatry 53:80–89. [DOI] [PubMed] [Google Scholar]
- Liu M, Hansen PE, Wang G, Qiu L, Dong J, Yin H, Qian Z, Yang M, Miao J (2015) Pharmacological profile of xanthohumol, a prenylated flavonoid from hops (Humulus lupulus). Molecules 20:754–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta C(T)) method. Methods 25:402–408. [DOI] [PubMed] [Google Scholar]
- Lopresti AL, Maes M, Maker GL, Hood SD, Drummond PD (2014) Curcumin for the treatment of major depression: a randomised, double-blind, placebo controlled study. J Affect Disord 167:368–375. [DOI] [PubMed] [Google Scholar]
- Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L (2004) Polyphenols: food sources and bioavailability. Am J Clin Nutr 79:727–747. [DOI] [PubMed] [Google Scholar]
- Mendez-David I, Tritschler L, Ali ZE, Damiens MH, Pallardy M, David DJ, Kerdine-Römer S, Gardier AM (2015) Nrf2-signaling and BDNF: a new target for the antidepressant-like activity of chronic fluoxetine treatment in a mouse model of anxiety/depression. Neurosci Lett 597:121–126. [DOI] [PubMed] [Google Scholar]
- Mendez-David I, Boursier C, Domergue V, Colle R, Falissard B, Corruble E, Gardier AM, Guilloux JP, David DJ (2017) Differential peripheral proteomic biosignature of fluoxetine response in a mouse model of anxiety/depression. Front Cell Neurosci 11:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R, Sapolsky RM (2008) Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. Proc Natl Acad Sci U S A 105:5573–5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63. [DOI] [PubMed] [Google Scholar]
- Motaghinejad M, Motevalian M, Fatima S, Hashemi H, Gholami M (2017) Curcumin confers neuroprotection against alcohol-induced hippocampal neurodegeneration via CREB-BDNF pathway in rats. Biomed Pharmacother 87:721–740. [DOI] [PubMed] [Google Scholar]
- Murakami S, Imbe H, Morikawa Y, Kubo C, Senba E (2005) Chronic stress, as well as acute stress, reduces BDNF mRNA expression in the rat hippocampus but less robustly. Neurosci Res 53:129–139. [DOI] [PubMed] [Google Scholar]
- O’Leary JC 3, Zhang B, Koren J 3, Blair L, Dickey CA (2013) The role of FKBP5 in mood disorders: action of FKBP5 on steroid hormone receptors leads to questions about its evolutionary importance. CNS Neurol Disord Drug Targets 12:1157–1162. [PMC free article] [PubMed] [Google Scholar]
- Oomen CA, Mayer JL, de Kloet ER, Joëls M, Lucassen PJ (2007) Brief treatment with the glucocorticoid receptor antagonist mifepristone normalizes the reduction in neurogenesis after chronic stress. Eur J Neurosci 26:3395–3401. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Lightman SL (2008) The HPA axis in major depression: classical theories and new developments. Trends Neurosci 31:464–468. [DOI] [PubMed] [Google Scholar]
- Peltier J, O’Neill A, Schaffer DV (2007) PI3K/Akt and CREB regulate adult neural hippocampal progenitor proliferation and differentiation. Dev Neurobiol 67:1348–1361. [DOI] [PubMed] [Google Scholar]
- Pusceddu MM, Nolan YM, Green HF, Robertson RC, Stanton C, Kelly P, Cryan JF, Dinan TG (2016) The Omega-3 polyunsaturated fatty acid docosahexaenoic acid (DHA) reverses corticosterone-induced changes in cortical neurons. Int J Neuropsychopharmacol 19:pyv130. doi:10.1093/ijnp/pyv130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revsin Y, Rekers NV, Louwe MC, Saravia FE, De Nicola AF, de Kloet ER, Oitzl MS (2009) Glucocorticoid receptor blockade normalizes hippocampal alterations and cognitive impairment in streptozotocin-induced type 1 diabetes mice. Neuropsychopharmacology 34:747–758. [DOI] [PubMed] [Google Scholar]
- Rizza S, Cirotti C, Montagna C, Cardaci S, Consales C, Cozzolino M, Carrì MT, Cecconi F, Filomeni G (2015) S-Nitrosoglutathione reductase plays opposite roles in SH-SY5Y models of Parkinson’s disease and amyotrophic lateral sclerosis. Mediators Inflamm 2015:536238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa PB, Ribeiro CM, Bettio LE, Colla A, Lieberknecht V, Moretti M, Rodrigues AL (2014) Folic acid prevents depressive-like behavior induced by chronic corticosterone treatment in mice. Pharmacol Biochem Behav 127:1–6. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. (2000) Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry 57:925–935. [DOI] [PubMed] [Google Scholar]
- Shansky RM, Hamo C, Hof PR, McEwen BS, Morrison JH (2009) Stress-induced dendritic remodeling in the prefrontal cortex is circuit specific. Cereb Cortex 19:2479–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV (2010) Astrocytes: biology and pathology. Acta Neuropathol 119:7–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JP. (2008) Food for thought: the role of dietary flavonoids in enhancing human memory, learning and neuro-cognitive performance. Proc Nutr Soc 67:238–252. [DOI] [PubMed] [Google Scholar]
- Sun Q, Jia N, Yang J, Chen G (2018) Nrf2 signaling pathway mediates the antioxidative effects of taurine against corticosterone-induced cell death in HUMAN SK-N-SH cells. Neurochem Res 43:276–286. [DOI] [PubMed] [Google Scholar]
- Tanigawa S, Fujii M, Hou DX (2007) Action of Nrf2 and Keap1 in ARE-mediated NQO1 expression by quercetin. Free Radic Biol Med 42:1690–1703. [DOI] [PubMed] [Google Scholar]
- Trackey JL, Uliasz TF, Hewett SJ (2001) SIN-1-induced cytotoxicity in mixed cortical cell culture: peroxynitrite-dependent and -independent induction of excitotoxic cell death. J Neurochem 79:445–455. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Bagi Z, Feher A, Recchia FA, Sonntag WE, Pearson K, de Cabo R, Csiszar A (2010) Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol 299:H18–H24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vauzour D. (2012) Dietary polyphenols as modulators of brain functions: biological actions and molecular mechanisms underpinning their beneficial effects. Oxid Med Cell Longev 2012:914273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vauzour D, Rodriguez-Mateos A, Corona G, Oruna-Concha MJ, Spencer JP (2010) Polyphenols and human health: prevention of disease and mechanisms of action. Nutrients 2:1106–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei K, Xu Y, Zhao Z, Wu X, Du Y, Sun J, Yi T, Dong J, Liu B (2016) Icariin alters the expression of glucocorticoid receptor, FKBP5 and SGK1 in rat brains following exposure to chronic mild stress. Int J Mol Med 38:337–344. [DOI] [PubMed] [Google Scholar]
- Wie MB, Won MH, Lee KH, Shin JH, Lee JC, Suh HW, Song DK, Kim YH (1997) Eugenol protects neuronal cells from excitotoxic and oxidative injury in primary cortical cultures. Neurosci Lett 225:93–96. [DOI] [PubMed] [Google Scholar]
- Yahiro K, Akazawa Y, Nakano M, Suzuki H, Hisatune J, Isomoto H, Sap J, Noda M, Moss J, Hirayama T (2015) Helicobacter pylori VacA induces apoptosis by accumulation of connexin 43 in autophagic vesicles via a Rac1/ERK-dependent pathway. Cell Death Discov 1:15035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Zhang B, Ge C, Peng S, Fang J (2015) Xanthohumol, a polyphenol chalcone present in hops, activating Nrf2 enzymes to confer protection against oxidative damage in PC12 cells. J Agric Food Chem 63:1521–1531. [DOI] [PubMed] [Google Scholar]
- Zhang H, Liu B, Wu J, Xu C, Tao J, Duan X, Cao Y, Dong J (2012) Icariin inhibits corticosterone-induced apoptosis in hypothalamic neurons via the PI3-K/Akt signaling pathway. Mol Med Rep 6:967–972. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang WP, Chen KD, Qian XD, Fang SH, Wei EQ (2007) Caffeic acid attenuates neuronal damage, astrogliosis and glial scar formation in mouse brain with cryoinjury. Life Sci 80:530–537. [DOI] [PubMed] [Google Scholar]
- Zhao H, Alam A, San CY, Eguchi S, Chen Q, Lian Q, Ma D (2017) Molecular mechanisms of brain-derived neurotrophic factor in neuro-protection: recent developments. Brain Res 1665:1–21. [DOI] [PubMed] [Google Scholar]
- Zhao X, Li R, Jin H, Jin H, Wang Y, Zhang W, Wang H, Chen W (2018) Epigallocatechin-3-gallate confers protection against corticosterone-induced neuron injuries via restoring extracellular signal-regulated kinase ½ and phosphatidylinositol-3 kinase/protein kinase B signaling pathways. Plos One 13:e0192083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Zhao Z, Zhou Y, Chen X, Li Y, Liu X, Lu H, Zhang Y, Zhang J (2013) High-dose glucocorticoid aggravates TBI-associated corticosteroid insufficiency by inducing hypothalamic neuronal apoptosis. Brain Res 1541:69–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.