Abstract
Objective
Alzheimer’s disease (AD) associated dementia is due to tissue damage caused by amyloid β (Aβ) deposition within the brain and by accompanying neuroinflammation. The NAD glycohydrolase, CD38, which is expressed by neurons, astrocytes and microglial cells, regulates inflammatory and repair processes in the brain and other tissues by degrading NAD and repressing the activity of other NAD-consuming enzymes and by producing NAD-derived metabolites that regulate calcium signaling and migration of inflammatory cells. Given the role of CD38 in neuroinflammation and repair, we examined the effect of CD38 deletion on AD pathology.
Methods
We crossed APPswePS1ΔE9 (APP.PS) mice with Cd38−/− mice to generate AD-prone CD38 deficient animals (APP.PS.Cd38−/−) and examined AD-related phenotypes in both groups.
Results
APP.PS.Cd38−/− mice exhibited significant reductions in Aβ plaque load and soluble Aβ levels compared to APP.PS mice and this correlated with improved spatial learning. Although CD38 deficiency resulted in decreased microglia/macrophage (MM) accumulation, the transcription profile of the Cd38−/− and Cd38+/+ MM was similar, suggesting that the decreased Aβ burden in APP.PS.Cd38−/− mice was not due to alterations in MM activation/function. Instead, APP.PS.Cd38−/− neuronal cultures secreted less Aβ and this reduction was mimicked when APP.PS neuronal cultures were treated with inhibitors that blocked CD38 enzyme activity or the signaling pathways controlled by CD38-derived metabolites. Furthermore, β and γ-secretase activity was decreased in APP.PS.Cd38−/− mice, which correlated with decreased Aβ production.
Interpretation
CD38 regulates AD pathology in the APP.PS model of AD, suggesting that CD38 may be a novel target for AD treatment.
Introduction
Alzheimer’s disease (AD), the most common form of dementia in the elderly, is characterized by amyloid β (Aβ) deposition, synaptic dysfunction, and neuroinflammation.1 The deposition and accumulation of Aβ42, either as plaques or as smaller soluble Aβ oligomers, is a major pathogenic factor in AD. Aβ is produced following cleavage of amyloid precursor protein (APP) by two enzymes, β-secretase [β-site APP-cleaving enzyme 1] and γ-secretase.2 Resident microglia/infiltrating macrophages (MM) become activated and accumulate following Aβ deposition in the brain3 and can play a protective role in AD by facilitating Aβ clearance through phagocytosis of Aβ and the production of Aβ-degrading enzymes.4 However, MM also produce a range of inflammatory mediators, including potentially neurotoxic mediators.5 Thus, MM can play dual but opposing roles in AD pathogenesis.
CD38 is a pro-inflammatory ectoenzyme that catalyzes the formation of the calcium-mobilizing metabolites adenosine diphosphoribose (ADPR), cyclic ADPR (cADPR) and nicotinic acid adenine dinucleotide phosphate (NAADP) from its substrates NAD(P). CD38 is the major constitutively active NAD-degrading enzyme expressed in mammalian cells6, 7 and mice deficient in CD38 exhibit increased NAD levels in many tissues, including the brain.6 CD38-dependent changes in NAD homeostasis can influence cell metabolism8 and the activity of other NAD-dependent enzymes like SIRT1.9 In addition to its ability to modulate NAD homeostasis, CD38, through production of cADPR and ADPR, can also regulate the calcium-dependent activation and migration of myeloid-derived inflammatory cells.10 In the brain, CD38 is expressed by microglia11 neurons12 and astrocytes13 and is known to influence the function of all three cell types.14–16 We11 and others17 previously showed that CD38 regulates microglia activation, controls the migration of monocytes/macrophages and microglia to inflammatory mediators including Aβ peptide18, and affects recovery from head trauma19. Given the important role that CD38 plays in neuroinflammation and brain repair processes, we hypothesized that CD38 might alter the development or progression of AD. To test this hypothesis we examined the effect of loss of CD38 on AD pathology using the APPswePS1ΔE9 (herein APP.PS) mouse model for AD.20 Our results demonstrate that Aβ burden was significantly reduced and learning performance was significantly improved in APP.PS.Cd38−/− mice. These effects were accompanied by a reduction in β- and γ-secretase activity and in MM accumulation in the brain. Furthermore, less Aβ was detected in primary neuronal cultures from APP.PS.Cd38−/− mice compared to APP.PS mice. Likewise, Aβ levels were decreased in APP.PS neuronal cultures treated with a CD38 enzyme inhibitor or an antagonist of one of the CD38-generated Ca2+-mobilizing metabolites, cADPR. Taken together, these results show that loss of CD38 is neuroprotective in the APP.PS AD mouse model.
Materials and Methods
All reagents were purchased from Sigma (St. Louis, MO) unless otherwise stated. Antibodies list (Table S1), primers’ sequences (Table S2) and a table summarizing the number of mice in the different experiments (Table S3) are available in the Supplementary Materials.
Mice
Hemizygous APPswePS1ΔE9 transgenic mice20 (APP.PS) were purchased from Jackson Laboratory; B6.Cg-Tg(APPswePSEN1dE9)85Dbo/Mmjax stock # 005864 and crossed to C57BL/6J mice to generate hemizygous APP.PS mice that were used in experiments. The hemizygous APP.PS transgenic mice were also intercrossed with C57BL/6J.Cd38−/− mice21 to generate Cd38−/− mice that expressed the APP.PS transgene in the hemizygous form (APP.PS.Cd38−/−). The APP.PS.Cd38−/− animals were bred to the parental Cd38−/− strain, and APP.PS.Cd38−/− offspring were identified by genotyping and used in experiments.
C57BL/6J.Cd38−/− (Cd38−/−) and C57BL/6J [wild-type (WT)] (Harlan, catalog number 2BL/610) animals were also used in some experiments. Male mice were used in the experiments. All experiments with mice followed the applicable rules and guidelines of the Animal Care and Use Committee of Tel Aviv University and the University of Rochester. All studies were conducted in accordance with the United States Public Health Service’s Policy on Humane Care and Use of Laboratory Animals.
Brain Processing
At the indicated ages, experimentally-naïve mice were anesthetized with a mixture of ketamine (Ketaset, Fort Dodge, IA) and xylazine and perfused transcardially with saline. For immunoblot and immunohistochemistry experiments, the two cerebral hemispheres were separated, and snap frozen or transferred to 4% paraformaldehyde phosphate buffer saline, respectively.
Morris Water Maze (MWM)
On each day over an 8 day period, mice were placed into a tank (1 meter diameter) filled with opaque water. In each trial, mice were placed at a different starting point and the latency to locate a fixed-location submerged platform was recorded. Each mouse performed four 60-seconds trials per day, with an inter-trial interval of 1 h. At the end of the trial, mice remained on the platform for 30 seconds. Trials in which the mouse floated motionless for more than half the trial were omitted from analysis. A probe trial was performed on day 9, in which the platform was removed and the duration of time the mice spent in the platform quadrant was recorded. To exclude artifacts resulting from differences in swimming abilities, the latency to climb a visible platform was recorded on the 10th day.
Preparation of Brain Sections for Immunohistochemistry
Brain hemispheres were processed, and sections were prepared and immunostained as previously described.22 Sections were photographed and image analysis performed using Image-Pro Plus v.5.1 (Media Cybernetics).
Image Analysis for Immunohistochemistry
For Aβ analysis, images of the entire brain were captured with 2× magnification. The plaque number and size, as well as the relative Aβ stained areas were analyzed using ImagePro. First, a threshold for specific Aβ staining was set using the select ranges tool, which discriminates the signal from the background. Using the Select Measurements tool, an area range was selected to include plaques of separate size categories. For determination of plaque number, the number of plaques in each size category was determined using the Count function. For determination of the area covered by plaques for each size category, the Area (Sum) option in the Data Collector was used, and expressed relatively to the section area.
For analysis of Iba1 staining, hippocampus images (bregma −1.34 to −2.06) were captured frame by frame using a 10× magnification. Two different intensity thresholds were selected to differentiate between cell bodies (high intensity) and extensions (low intensity).
Immunoblot Analysis
For Aβ analysis, frozen hemispheres were homogenized in TBS (1:4 weight:volume) and centrifuged at 186,000 × g for 30 min at 4°C. The supernatant was used as the soluble protein fraction. The pellet was homogenized in 1% Triton X-100 in TBS, sonicated, suspended using needles and centrifuged. The supernatant was used as the detergent-soluble protein fraction, and the pellet was homogenized and sonicated in 5M Guanidine hydrochloride. Then samples were suspended using needles and after overnight incubation and centrifugation the supernatant was used as the insoluble protein fraction.
For analysis of APP and synaptophysin in the brain cortex and the secretase subunits in cerebral hemispheres, total protein was extracted and 2–100 μg protein were separated by 10%, 12.5% or 15% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS PAGE), electroblotted, blocked and then incubated with the appropriate Abs as previously described.22 For Aβ immunoblot, the blotted membranes were boiled before antibody incubation. Signals were detected using the Supersignal West Pico Chemiluminescent Substrate (Pierce Biotechnology) and normalized to internal protein controls (cytochrome c band or 12 kDa Ponceau-stained band). The normalized signal was quantified using EZQuant-Gel software. Using the Define Background tool, an area in the blot where no bands were visible was selected and utilized by the software to distinguish signal from background. Bands were marked using the Band Select tool and a value representing the band area and signal density was calculated by the software and used for analysis.
Analysis of NAD Levels in Brain Extracts
Brains from 2, 4, and 8 month old APP.PS, APP.PS.Cd38−/−, WT and Cd38−/− mice were harvested and flash frozen. Brains were thawed on ice, weighed, and homogenized in perchloric acid (0.1g tissue/1mL PCA) using glass dounce homogenizers. Samples were centrifuged at 14,000 rpm for 10 min and 500 μL of supernatant was transferred to a tube. Samples were neutralized to pH 6.8 – 6.95 using potassium phosphate buffer and centrifuged to remove precipitated salts. To measure NAD levels, 50 μL of each sample were loaded to a 96 well plate and analyzed using a NAD enzymatic cycling assay.23 Absorbance was read at 540 nm and values were normalized to mg of tissue.
Isolation of MM
CD11b+ MM were isolated from the brains and purified as previously described.22 The purity of the MM preparations was ~90% as assessed by flow cytometry (CD11b+CD45.2+).
RT-qPCR Analysis
Total RNA prepared from the isolated MM or from cerebral hemispheres of 10 week old mice was reverse-transcribed. The cDNA (10 ng) was used as a template for RT-qPCR using an ABI 7300 or 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) and ABsolute™ Blue SYBR Green (ABgene, Epsom, UK) or Fast SYBR Green Master Mix (Applied Biosystems). Relative levels of the different transcripts were quantified using the 2−ΔΔCT method24 and samples were normalized to GAPDH or β-actin.
Determination of α- β- and γ-Secretase Activities
Purified mouse brain membranes prepared25 from 10 week old mice were used for the determination of α- β- and γ-secretase activities as described26. The enzymatic activity of each secretase was plotted over time and the slope of the reaction’s linear range, representing the enzymatic activity rate, was calculated for each mouse.
Preparation of Mouse Primary Neuronal Cultures
Mouse neuronal primary cultures were prepared from WT, Cd38−/−, APP.PS and APP.PS.Cd38−/− mice as previously described27 with minor modifications. Cerebral cortices from 1- 2-day-old neonates were dissected, minced in sterile Hank’s balanced salt solution (HBSS), and digested in 0.025% trypsin. The tissue was then dispersed by repeated pipetting with DMEM supplemented with 0.02% DNase I, washed and triturated by polished Pasteur pipettes. The cells were pelleted and resuspended in plating medium [Neurobasal-A medium (Invitrogen) containing 5% Horse serum, 5% FCS, 1% GlutaMAX-I (Invitrogen), 2% B-27 supplement (Invitrogen), 2 μM HEPES and 0.5% penicillin–streptomycin, (Invitrogen)]. On the following day and twice a week thereafter, half of the medium volume was exchanged with growth medium (plating medium without serum). 106 cells were seeded in the middle of an 18 mm2 glass slide pre-coated with polyethyleneimine. Cultures were kept in an atmosphere of 5% CO2, 95% air at 37°C for 17 days and then medium was collected for Aβ measurements. In the experiments assessing the effect of inhibitors on Aβ, the cells were treated after 17 days in culture with NAD (100 μM), 8Br-cADPR (50 μM), Luteolin (3′-4′-5′-7′-Terahydroxyflavone 97%, Alfa Aesar) (20 μM) or DMSO (0.25%) as vehicle. Four days later, media were collected, cells were scraped on ice-cold PBS, centrifuged, and pellets were snap-frozen in liquid nitrogen. The cell pellets were used to determine protein concentration in each primary culture sample and to determine NAD levels.
NAD Measurements in Primary Neuronal Cultures
Total NAD levels were measured using a bioluminescent-based NAD cycling assay [NAD-NADH Glo assays (Promega)]. Luminescence in the samples was measured using a Spectramax L luminometer (Molecular Devices). Total NAD levels were quantitated based on a NAD standard curve and normalized to protein content which was determined using a Bradford Protein Assay.
Aβ42 Measurements
Aβ42 level in the neuronal culture medium was determined using the Beta-Amyloid X-42 ELISA kit (Covance #SIG-38952). Chemoluminescence was detected using Veritas™ microplate luminometer 1.5 (Turner Biosystems) and the values obtained were normalized to the protein content in the corresponding cell pellets.
Results
CD38 Regulates Aβ Levels in Brains of AD-prone Mice
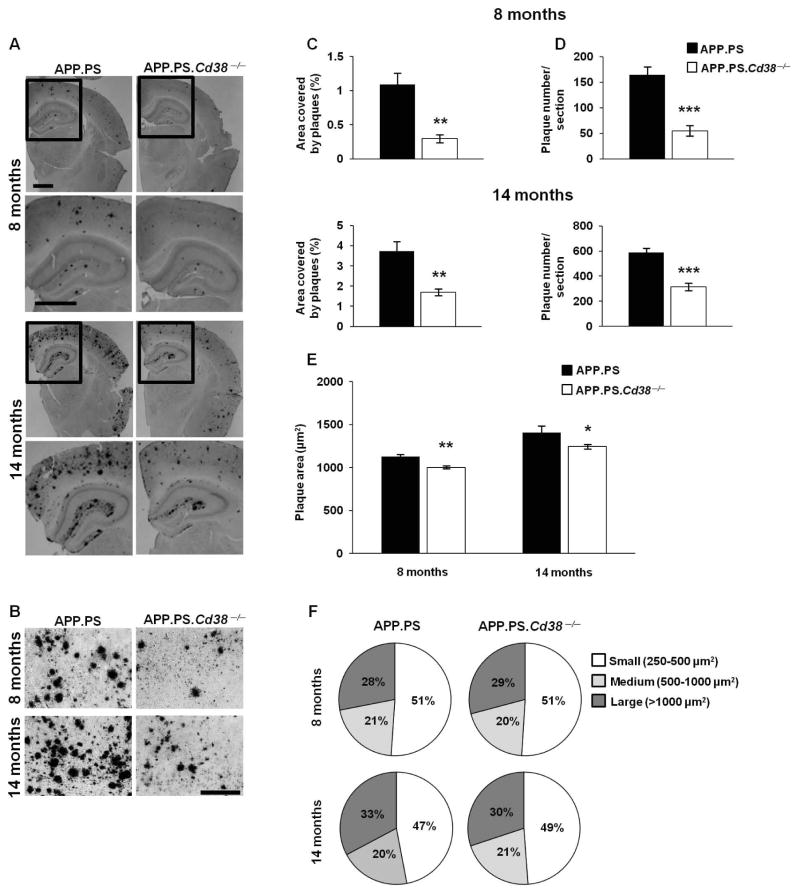
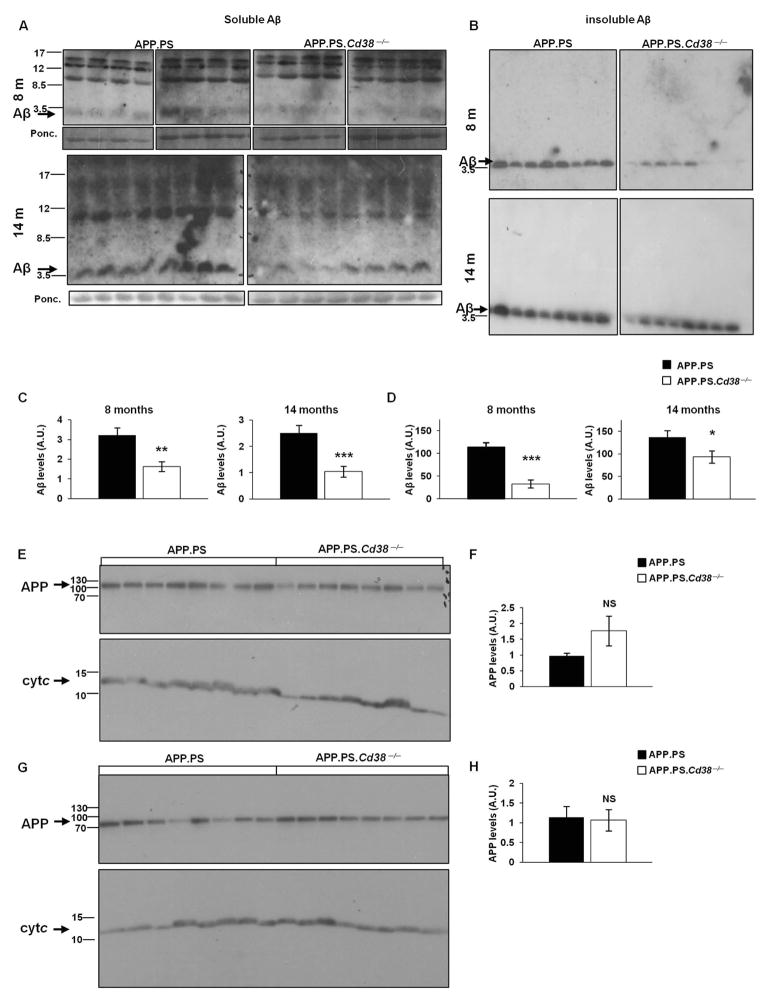
To determine whether CD38 affects the development or progression of AD, we intercrossed C57BL/6J Cd38−/− mice with AD-prone mice expressing the APP.PS transgenes20 to generate APP.PS.Cd38−/− mice and measured the accumulation of Aβ plaques in the brains of these mice. We observed a marked decrease in Aβ plaque staining in 8 and 14 month APP.PS.Cd38−/− mice compared to APP.PS mice (Fig. 1A and B). The total area stained by the Aβ antibody was significantly decreased in the APP.PS.Cd38−/− brains at both time points (Fig. 1C). This reduction was due primarily to a decrease in the number of plaques found in the APP.PS.Cd38−/− brains (Fig. 1D) as the average size of individual plaques was only slightly reduced in these mice (Fig. 1E) and the proportions of the total plaques that were categorized as small, medium or large (Fig. 1F) were almost identical between the two groups. Next, we quantitated the amount of soluble and insoluble Aβ peptides present in the brains of these mice. As shown in Figure 2, significantly lower levels of both the soluble (Fig. 2A and C) and insoluble (Fig. 2B and D) forms of Aβ peptides were detected in 8 and 14 month APP.PS.Cd38−/− brains relative to APP.PS brains. The decrease in Aβ peptide levels did not result from reduced expression of the transgene-encoded Aβ peptide precursor, APP, as no significant differences in total APP levels were observed between the two groups of mice (Fig. 2E–H). Thus, accumulation of Aβ in the brains of APP.PS mice appears to be regulated by CD38.
Figure 1. Loss of CD38 reduces Aβ plaque load in APP.PS mice.
Brain sections of APP.PS and APP.PS.Cd38−/− mice, 8 and 14 months of age were stained with 4G8 anti-Aβ mAb. (A) Representative images of APP.PS and APP.PS.Cd38−/− mice at 8 and 14 months of age. Lower panels represent magnifications of the selected areas in the upper panels marked by rectangles. Scale bar = 1 mm and 200 μm for higher and lower panels, respectively. (B) Representative high magnification (20×) images of 8 and 14 month old APP.PS and APP.PS.Cd38−/− mice. Scale bar = 200 μm. (C, D) Quantification of the stained area representing Aβ plaques (C) or the number of Aβ plaques (D) (**p < 0.005, ***p < 0.0005, Student’s t test). (E) Analysis of the average plaque area (*p < 0.05, **p < 0.005, Student’s t test). (F) Plaques were categorized according to their size to three groups: small (250–500 μm2), medium (500–1000 μm2) and large (> 1000 μm2) plaques. Similar distribution of the plaques in APP.PS and APP.PS.Cd38−/− mice was observed, both at 8 and 14 months of age. The quantified values shown are presented as mean ± SEM (bars) (n = 8 and 6 for APP.PS and APP.PS.Cd38−/− aged 8 months, respectively and n = 8 for APP.PS and APP.PS.Cd38−/− aged 14 months).
Figure 2. Loss of CD38 reduces soluble and insoluble Aβ peptide but not APP levels in APP.PS mice.
(A,B) Immunoblot analysis of Aβ peptide levels. Soluble (A) and insoluble (B) protein extracts were prepared as described in Materials and Methods. Proteins (175 and 2 μg for soluble and insoluble respectively) were separated by SDS-PAGE and membranes were probed with anti-Aβ Abs. Aβ levels in the soluble fraction were normalized to a ~12 KDa band in the Ponceau staining (lower panels). (C, D) Quantification of immunoblot results of soluble (C) and insoluble (D) Aβ levels (*p < 0.05, **p < 0.005, ***p < 0.0005, Student’s t test). (n = 8). (E, G) Immunoblot analysis of APP levels. Cortical protein extracts were prepared from brains of APP.PS and APP.PS.Cd38−/− mice at 8 (E) and 14 (G) months of age. Proteins (50 μg) were separated on SDS-PAGE and membranes were probed with anti-APP Abs as described in Materials and Methods. The membranes were cut into two parts and each part was stained separately for either APP or for cytochrome c for normalization. APP levels were normalized to cytochrome c (Cyt c). (F, H) Quantification of APP immunoblot results at 8 (F) and 14 (H) months of age. (NS, not significant; Student’s t test) The quantified values shown are presented as the mean ± SEM (bars). (n = 8 for each genotype).
CD38 Regulates the Activity of α-, β- and γ-secretases at the Post-transcriptional Level
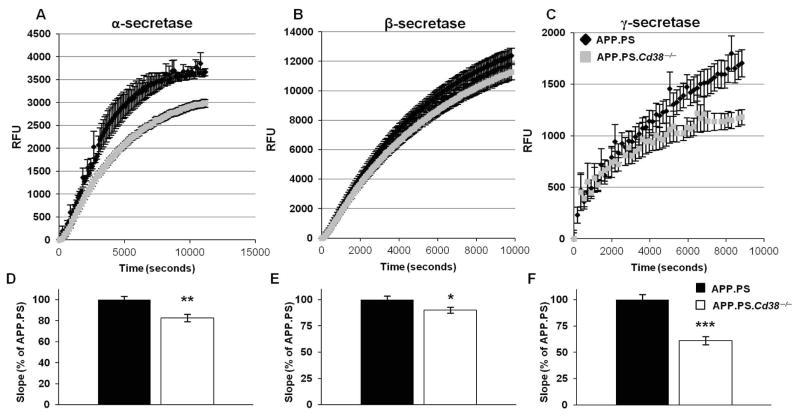
Production of amyloidogenic Aβ peptides can be reduced when the α-secretase, ADAM10, which cleaves APP into non-pathogenic peptides, is activated or when β- and γ-secretases, which are involved in producing the pathogenic Aβ peptides, are inhibited.2 Given the reduced accumulation of plaques and Aβ peptides in APP.PS.Cd38−/− brains, we hypothesized that either the α-secretase activity was increased in the APP.PS.Cd38−/− brains or that the activities of the β - and γ-secretases were decreased in the brains of these mice. To test this hypothesis, we measured α-, β- and γ-secretases activity in brain extracts isolated from 10 week old APP.PS and APP.PS.Cd38−/− mice. In contrast to our prediction, α-secretase activity was lower in APP.PS.Cd38−/− brain extracts compared to APP.PS brain extracts (Fig. 3A and D), indicating that the reduction in production of pathogenic Aβ peptides observed in the APP.PS.Cd38−/− mice was not due to enhanced cleavage of the APP precursor into non-amyloidogenic peptides by ADAM10/α-secretase. However, β-secretase (Fig. 3B and E) and γ-secretase (Fig. 3C and F) activities were also both significantly lower in the APP.PS.Cd38−/− mice, suggesting that less of the pathogenic Aβ peptides were produced in the APP.PS.Cd38−/− brains.
Figure 3. Reduction in α-, β- and γ-secretase activity in APP.PS.Cd38−/− mice.
Brain hemispheres were isolated from 10 week old APP.PS and APP.PS.Cd38−/− mice, membranes where purified and used for secretase activity measurements as described in Materials and Methods. α-secretase (A) β-secretase (B) and γ-secretase (C) activities were measured over time using fluorometric assays and the enzymatic activities were calculated as described in Materials and Methods. (D–F) The slope of the linear range of the reaction representing the α-secretase (D), β-secretase (E) or γ-secretase (F) enzyme activity rates was calculated for each mouse. Displayed are the calculated slopes, normalized to APP.PS mice. (*p < 0.05, **p < 0.005, ***p < 0.0005, student’s t-test). The values are presented as the mean ± SEM (bars). (n = 8).
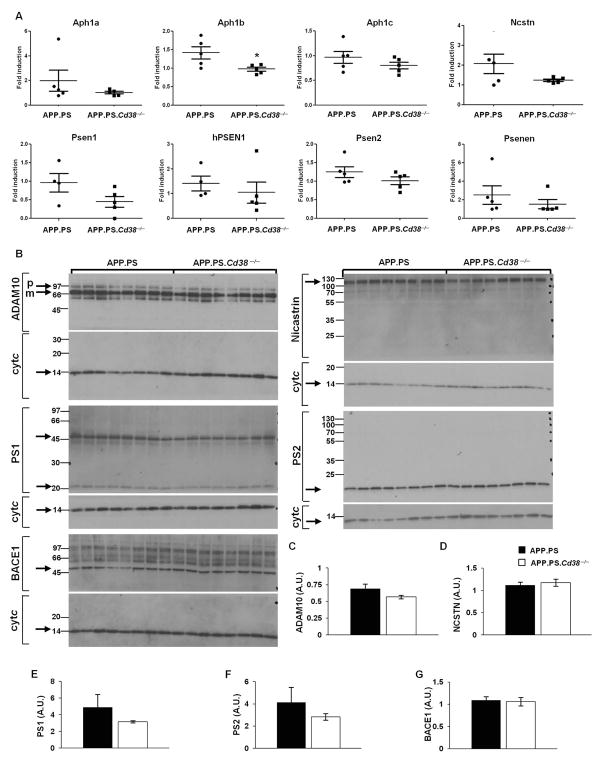
To determine whether the decreased activity of the α-, β- and γ-secretases observed in the APP.PS.Cd38−/− brains was due to changes in expression of these enzymes, we measured mRNA and protein levels of the different secretases. No significant differences were observed in the mRNA levels of genes encoding the proteins in the γ-secretase complex in brains of APP.PS and APP.PS.Cd38−/− mice, with the exception of Aph1b, which was expressed at lower levels in the APP.PS.Cd38−/− brains relative to control APP.PS brains (Fig. 4A). No significant differences were observed in protein levels of ADAM10, nicastrin, PS1, PS2 and β-secretase enzyme (BACE1) in brains of 10 week old APP.PS and APP.PS.Cd38−/− mice (Fig. 4B–G). Taken together, the data suggest that the loss of CD38 attenuated production of Aβ peptides by repressing the activity, rather than the expression, of the Aβ generating β- and γ-secretases.
Figure 4. mRNA and protein levels’ measurements of the different secretases.
(A) Total RNA samples, prepared from brain hemispheres, were analyzed by RT-qPCR as described in Materials and Methods to determine relative mRNAs levels of the γ-secretase components; human PS1 transgene (PSEN1), mouse PS1 (Psen1), Psen2, Ncstn, Psenen, Aph1a, Aph1b and Aph1c. The results are expressed as fold induction for each animal in both groups relative to one APP.PS mouse. The data are presented as individual value plots displaying the relative expression level of the indicated gene for each mouse. (n = 4–5). (*p < 0.05, Student’s t test). (B–G) Protein levels of ADAM10, β and γ-secretase components. Brain hemispheres from 10 week old APP.PS and APP.PS.Cd38−/− mice were dissected, and protein extracts were prepared as described in Materials and Methods. (B) Proteins (50 μg) were separated on SDS-PAGE and probed with antibodies against the indicated proteins. Cytochrome c (cyt c) was used as loading control. The membranes were cut into two parts and each part was stained separately for either the corresponding protein or for cytochrome c for normalization. (C–G) Densitometry was used to normalize protein levels in the samples to cytochrome c. Quantification of the normalized results did not reveal a difference in ADAM10 (C), nicastrin (D), PS1 (E), PS2 (F) and BACE1 (G) protein levels between the groups. The values are presented as the mean ± SEM (bars). (n = 8).
Aβ Accumulation in Neurons is Regulated by the CD38 Substrate NAD and the CD38-derived Metabolite, cADPR
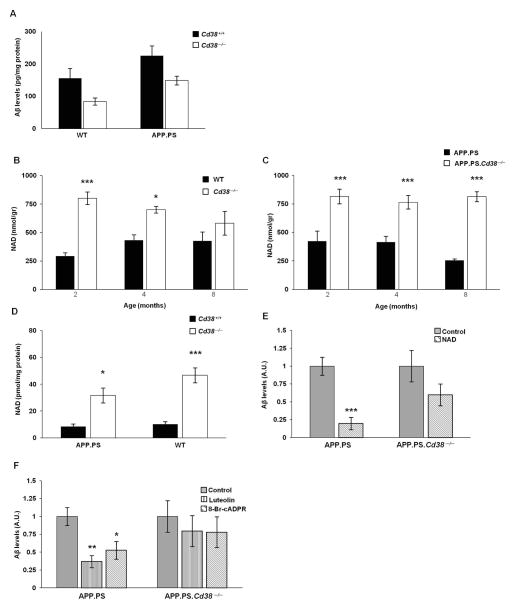
To further study the mechanism underlying the reduced Aβ levels in APP.PS.Cd38−/− mice and to assess whether this effect was dependent on the APP.PS transgene, we measured Aβ42 levels in primary neuronal cultures established from the brains of newborn APP.PS, APP.PS.Cd38−/−, WT and Cd38−/− mice. As expected, Aβ42 levels were higher in APP.PS neuronal cultures compared with WT neuronal cultures (Fig. 5A). Moreover, the amount of Aβ42 present in the APP.PS.Cd38−/− and Cd38−/− neuronal cultures was decreased relative to the APP.PS and WT controls, suggesting that loss of CD38 in neurons is sufficient to repress Aβ accumulation and that the reduction in Aβ accumulation in the APP.PS.Cd38−/− mice may be due, at least in part, to the loss of neuronal-derived CD38.
Figure 5. Regulation of neuronal Aβ production by CD38, NAD and the CD38 generated metabolite, cADPR.
(A) Aβ levels in primary neuronal cultures. Primary neuronal cultures were prepared from brains isolated from APP.PS, APP.PS.Cd38−/−, WT and Cd38−/− neonatal mice and the amount of secreted Aβ present in the cultures was measured as described in Materials and Methods. (p = 0.01 for transgene and p = 0.005 for CD38, two-way ANOVA).(n = 16, 15, 22 and 17 cultures for WT, Cd38−/−, APP.PS and APP.PS.Cd38−/−, respectively). (B, C) Determination of NAD levels. Brains from 2, 4, and 8 month old APP.PS, APP.PS.Cd38−/−, WT and Cd38−/− mice were homogenized and intracellular NAD levels were measured as described in Materials and Methods. (B) NAD levels in WT and Cd38−/− mice (p = 3×10−6 for CD38, Two-way ANOVA, *p = 0.006 and ***p = 8×10−6, Fisher’s LSD post hoc test; n = 5). (C) NAD levels in APP.PS and APP.PS.Cd38−/− mice (p = 4×10−8 for CD38, Two-way ANOVA, ***p < 5×10−4, Fisher’s LSD post hoc test; n = 4). (D) Determination of NAD levels in primary neuronal cultures. Intracellular NAD levels were measured, as described in Materials and Methods, in cells isolated from the primary neuronal cultures. (p = 5×10−5 for CD38, Two-way ANOVA, *p < 0.05 and ***p < 0.0005, Fisher’s LSD post hoc test) (n = 15 for APP.PS and APP.PS.Cd38−/− mice; n = 16 for WT and Cd38−/− mice. 1–2 samples from each mouse). (E) The effect of NAD on Aβ levels. Primary neuronal cultures were treated with 100 μM NAD and Aβ levels were measured as described in Materials and Methods. (***p = 0.0001; p = 0.07 in WT cultures, Student’s t test). (n = 8 and 7 for APP.PS control and NAD, respectively, n = 8 for WT cultures). Results are expressed relative to the control (untreated) of the relevant genotype. (F) Effect of CD38 enzymatic inhibitors and cADPR antagonists on Aβ levels. Primary neuronal cultures were treated with the cADPR antagonist, 8Br-cADPR (50 μM), or the CD38 inhibitor, luteolin (20 μM), for 96 hours and Aβ levels were measured as described in Materials and Methods. (in APP.PS cultures, p = 0.002, one-way ANOVA, *p = 0.007 and **p = 0.0007 for 8Br-cADPR and luteolin, respectively, Fisher’s LSD post hoc test). In APP.PS.Cd38−/− cultures, no significant effect was observed for any of the treatments (n = 8). Results are shown relative to the control (untreated) of the relevant genotype.
CD38, a constitutively active NAD glycohydrolase, regulates NAD homeostasis in multiple tissues including the brain6. Since NAD has been suggested to repress Aβ levels in AD-prone mice28, we hypothesized that CD38 may control Aβ accumulation by modulating NAD availability in the brain. To test this, we measured NAD levels in brain extracts of APP.PS and APP.PS.Cd38−/− mice at 2, 4, and 8 months of age. For controls, we also examined NAD levels in WT and Cd38−/− brains. As expected from prior studies6, NAD levels were significantly higher in the brains of CD38 deficient mice compared to WT controls at almost all timepoints (Fig. 5B). Likewise, NAD levels were elevated in the brains of APP.PS.Cd38−/− mice compared to APP.PS mice (Fig. 5C). Since we showed that neuronal Aβ levels were regulated by neuronal CD38, we next asked whether CD38 regulated NAD levels in primary neuronal cultures. We found that the loss of CD38 resulted in increased NAD levels in neuronal cultures derived from APP.PS.Cd38−/− and Cd38−/− mice compared to control APP.PS and WT mice (Fig. 5D). To determine whether NAD inhibited Aβ accumulation in the cultures, we treated APP.PS and APP.PS.Cd38−/− neuronal cultures with NAD and measured Aβ levels. NAD exposure significantly decreased Aβ levels in the neuronal cultures derived from APP.PS mice (Fig. 5E). Addition of exogenous NAD to the APP.PS.Cd38−/− cultures, which already exhibited increased NAD levels, resulted in a non-significant further decline in the amount of Aβ present in the cultures.
To examine whether blocking CD38 enzyme activity or the activity of cADPR, the Ca2+-mobilizing metabolite generated by CD38, affected Aβ accumulation in neurons, we treated the APP.PS and APP.PS.Cd38−/− neuronal cultures with either luteolin, a known CD38 inhibitor29 or with the potent cADPR antagonist, 8Br-cADPR.10 Both luteolin and 8Br-cADPR decreased Aβ levels in APP.PS neuronal cultures, but not in APP.PS.Cd38−/− neuronal cultures (Fig. 5F), suggesting that these compounds blocked neuronal Aβ accumulation in a CD38-dependent manner. Collectively, these results suggested that Aβ accumulation in neurons was regulated by the CD38 substrate, NAD, and the CD38 product, cADPR, and that CD38 regulated Aβ accumulation in neurons in an enzyme dependent manner.
Microglia/macrophage Accumulation is Attenuated in the Brains of APP.PS.Cd38−/− Mice
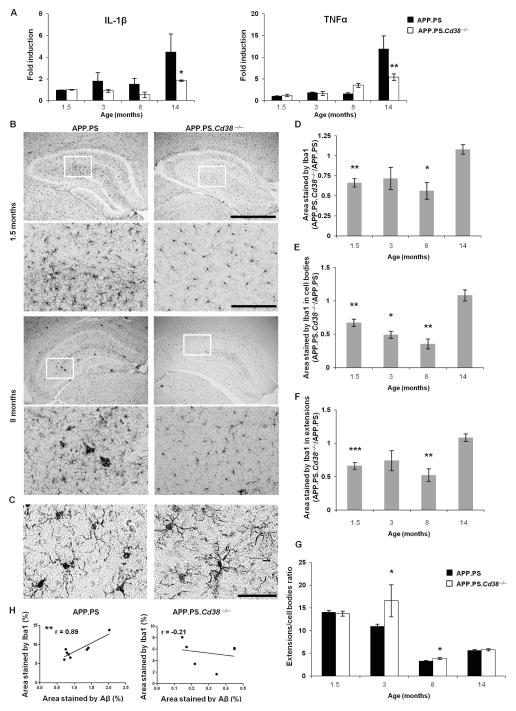
As MM can play significant roles in AD and prior data from our groups showed that CD38 regulates microglia activation11 and controls the chemotaxis of primary myeloid-derived cells and N9 microglial cells to Aβ peptide18, we hypothesized that loss of CD38 either enhanced expression of Aβ-degrading enzymes by the MM or impaired their activation and/or migration in the brain. To test this hypothesis, we isolated MM from brains of 1.5, 3, 8 and 14 month old APP.PS and APP.PS.Cd38−/− mice and examined mRNA expression levels of genes (Table S2) involved in MM activation, Aβ degradation, Aβ binding/phagocytosis, neuroprotection and immunosupression. Surprisingly, only three out of the 15 tested genes were differentially expressed between MM isolated from the two different groups of mice. Importantly, the loss of CD38 in MM did not affect transcript levels of the genes involved in Aβ phagocytosis or degradation, with the exception of the Aβ-degrading enzyme IDE, which was decreased rather than increased in the CD38 deficient MM (data not shown). IL-1β and TNFα transcript levels were also significantly lower in MM from APP.PS.Cd38−/− mice, but only at the 14 month time point (Fig. 6A). Thus, it seemed unlikely that the reduced Aβ burden in APP.PS.Cd38−/− mice was due to either enhanced Aβ degradation/phagocytosis or to decreased expression of neurotoxic cytokines by the MM.
Figure 6. CD38 regulates MM accumulation in the brains of APP.PS AD-prone mice.
(A) Analysis of IL-1β and TNFα mRNA levels in MM isolated from APP.PS and APP.PS.Cd38−/−. MM were isolated from the brains of the two groups at 1.5, 3, 8 and 14 months of age, RNA was extracted and gene expression was examined by qRT-PCR as described in Materials and Methods. (For IL-1β, p = 0.04 for genotype and p = 0.03 for age, two-way ANOVA, *p < 0.05, Fisher’s LSD post-hoc test. For TNFα, p = 0.0005 for age and p = 0.02 for the age × genotype interaction, two-way ANOVA, **p < 0.005, Fisher’s LSD post-hoc test). The values are presented as the mean ± SEM (bars) (n = 2 pools of 2–3 mice for each group). (B) Representative images of Iba1 staining in hippocampi of APP.PS and APP.PS.Cd38−/− mice at 1.5 and 8 months of age. Images were captured at a 4× (upper panel) and 20× magnification (lower panel). Rectangles indicate areas taken for 20× magnification. Scale bar = 1000 μm or 200 μm for upper and lower panels, respectively. Higher resolution representative (60×) images in the in hippocampi of 8 month old APP.PS and APP.PS.Cd38−/− mice are shown in (C). Scale bar = 50 μm. (D) Quantification of the total area stained by Iba1. The results are expressed as the ratio between APP.PS.Cd38−/− and APP.PS. (*p < 0.05 and **p < 0.005, student’s t-test). (E) Quantification of the area occupied by the MM cell bodies in the hippocampus. Differentiation between cell bodies and extension was based on higher Iba1 staining intensity in cell bodies than in extensions. Thus, implementing differential range of intensity of the Iba1 staining, cell bodies were identified as high intensity staining areas and extensions were identified as low intensity staining areas. The results are expressed as the ratio between APP.PS.Cd38−/− and APP.PS. (*p < 0.05 and **p < 0.005, student’s t-test), (F) Quantification of the area stained by the MM extensions in the hippocampus. The results are expressed as the ratio between APP.PS.Cd38−/− and APP.PS. (**p < 0.005 and ***p < 0.0005, student’s t-test). (G) Quantification of the ratio between the area of the MM extensions and the MM cell bodies. The results are expressed as the ratio between APP.PS.Cd38−/− and APP.PS. (*p < 0.05, student’s t-test).The values are presented as the mean ± SEM (bars). (n = 8 for all groups except for APP.PS.Cd38−/− 3 months of age, n = 6). (H) Correlation between the extent of Aβ and Iba-1 staining at 8 months of age. For each mouse, the average total area stained by Aβ was plotted against the average total area stained by Iba1 in the hippocampus. A significant correlation was found only in APP.PS mice (pearson’s r = 0.89, **p < 0.005). (n = 8 for APP.PS and n = 6 for APP.PS.Cd38−/− mice).
Aβ can induce MM activation and accumulation30 and Aβ burden was decreased in the brains of the APP.PS.Cd38−/− mice. Therefore, we postulated that the accumulation of MM in the brain would be decreased in the APP.PS.Cd38−/− mice because of the reduced Aβ burden and/or because CD38 controls chemotaxis of myeloid lineage cells to Aβ.18 To test this hypothesis, we stained hippocampus brain sections with anti-Iba1 antibodies to identify MM (Fig. 6B and C). The area of the hippocampus stained by Iba1 antibody was significantly lower in APP.PS.Cd38−/− mice at 1.5 and 8 months (Fig. 6D). Separate analysis of the Iba1 staining in MM cell bodies (Fig. 6E) and extensions (Fig. 6F) revealed a significant decrease in Iba1 staining in the APP.PS.Cd38−/− mice at 1.5, 3 and 8 months and at 1.5 and 8 months, respectively. The morphology of the MM, as reflected by the ratio between the area of MM extensions and cell bodies (Fig. 6G) was significantly different between the two groups at 3 and 8 months. Collectively, these results suggest that loss of CD38 altered the number and morphology of MM, even before Aβ plaques could be detected.
Next, to determine whether the number of Aβ plaques correlated with the abundance of MM in the hippocampus, we plotted the area occupied by the Iba1-expressing MM against the area occupied by the Aβ plaques in each 8 month old animal. A significant correlation was found between the two parameters in APP.PS mice (pearson’s r = 0.89, p = 0.003), whereas no correlation was observed in the APP.PS.Cd38−/− mice (Fig. 6H), suggesting that the decrease in the number of MM present in the brains of the APP.PS.Cd38−/− mice did not result from the reduction in Aβ load. Instead, the data suggest that the decreased accumulation of MM in the brains of the APP.PS.Cd38−/− mice was likely due to the inability of CD38 deficient MM to migrate/accumulate in response to Aβ. Regardless of the mechanism, the data clearly showed that loss of CD38 controls the accumulation of MM in the brains of AD-prone mice and in this way may also attenuate MM-mediated pathology.
Improved Spatial Learning and Elevated Synpatophysin Levels in CD38 Deficient AD-prone Mice
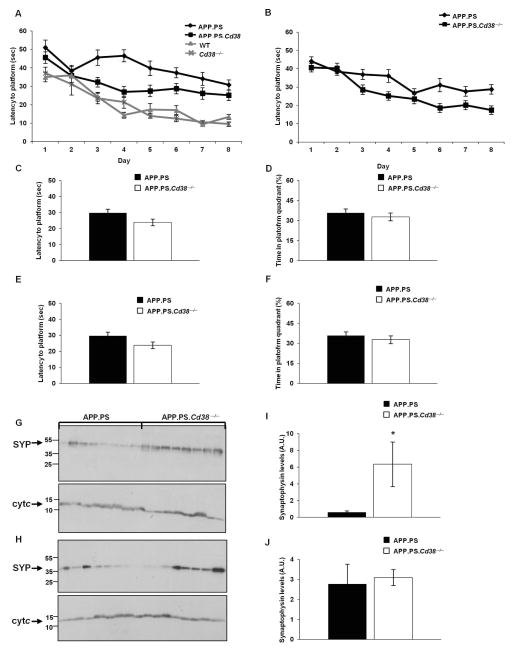
Given the link between Aβ burden and poor cognitive performance31 and the clear reduction in Aβ peptides and plaques in APP.PS.Cd38−/− brains, we hypothesized that CD38 deficient AD-prone mice might be spared from developing some of the cognitive deficits observed in AD animals and humans. To test this hypothesis, we assessed spatial learning using the Morris Water Maze (MWM) test, as this test is known to reveal learning defects in the APP.PS mice32. As shown in Figure 7, the performance of the 10 (Fig. 7A) and 14 (Fig. 7B) month old APP.PS.Cd38−/− mice was significantly better than that of the APP.PS mice, suggesting that the loss of CD38 protected cognitive ability and spatial learning in the AD-prone animals. Both groups of mice performed similarly in the visible platform test (Fig. 7C and E) and the rotarod and open field tests (not shown), indicating that the differences in learning between the two groups were not due to differences in swimming/motor skills or anxiety levels. The regulation by CD38 was limited to the learning phase of the task, as both groups performed similarly in a probe trial, assessing memory consolidation (Fig. 7D and F). The learning-specific effect was dependent on the presence of the APP.PS transgenes, as no significant difference was observed in the performance of WT and Cd38−/− mice at 10 months of age in the learning phase (Fig. 7A). Notably, while the APP.PS.Cd38−/− mice performed significantly better than the APP.PS mice in the learning tests, the APP.PS.Cd38−/− mice still exhibited mild cognitive defects relative to the non-AD-prone control WT or Cd38−/− mice (Fig. 7A). Thus, deleting CD38 was not sufficient, in and of itself, to fully prevent the deleterious effects of APP.PS transgenes on learning.
Figure 7. Loss of CD38 improves spatial learning in APP.PS mice.
(A) Morris Water Maze Test. 10 month old APP.PS, APP.PS.Cd38−/−, WT and Cd38−/− mice were tested. The results presented are the average latency of 4 daily trials to reach the hidden platform for each of the 8 days of the learning phase of the task. (p < 10−17 for day and p = 2×10−12 for transgene, repeated measures ANOVA, p = 5×10−5 between APP.PS and APP.PS.Cd38−/− mice, p = 0.66 between WT and Cd38−/− mice, p = 10−5 between APP.PS.Cd38−/− and WT mice, p = 2×10−6 between APP.PS.Cd38−/− and Cd38−/− mice, Fisher’s LSD post hoc test). (n = 8, 11, 9, 10 for APP.PS, APP.PS.Cd38−/−, WT and Cd38−/− mice respectively). (B) APP.PS and APP.PS.Cd38−/− were tested at 14 months of age. The results presented are the average latency of 4 daily trials to reach the hidden platform for each of the 8 days of the learning phase of the task. (p < 10−17 for day and p = 0.0017 for CD38, repeated measures ANOVA). (n = 20). (C, E) Visible platform test. The results shown are latencies to climb a visible platform. No significant difference (student’s t-test) was observed between the latencies of APP.PS mice and APP.PS.Cd38−/− mice at 10 (C) or at 14 months of age (E). (D, F) Single probe trial test. The test was conducted 24 h after the last learning trial. The results shown are the percentage of the time that the mice spent in the quadrant in which the platform was previously located. No significant difference (student’s t-test) was observed between APP.PS and APP.PS.Cd38−/− mice at 10 (D) or 14 months of age (F). The values are presented as the mean ± SEM (bars). (G–J) Expression of synaptophysin. The cortex of APP.PS and APP.PS.Cd38−/− mice at 8 (G) and 14 (H) months of age was dissected, and protein extracts prepared as described in Materials and Methods. Proteins (50 μg) were separated on SDS-PAGE and probed with anti-synaptophysin (SYP) or with anti-cytochrome c (Cyt c) Abs as loading control. (I, J) Quantification of the normalized results at 8 (I) and 14 (J) months of age. (*p = 0.02, Student’s t test). The values are presented as the mean ± SEM (bars). (n = 8).
Since one of the hallmarks of AD in humans and mice is synaptic deficits33 and the expression level of the synaptic marker synaptophysin is reduced in APP.PS mice34, we examined the effect of loss of CD38 on synaptophysin levels. The results showed (Fig. 7G–J) that synaptophysin levels in cortical samples were substantially and significantly higher in the APP.PSCd38−/− mice at 8 months of age, but not at 14 months of age. These results suggested that loss of CD38 can affect also synaptic properties, with potential implications for brain function.
Discussion
Our data show that loss of CD38 attenuates AD pathology by markedly reducing Aβ load in the brain, which correlates with increased synaptophysin levels and improved spatial learning. Furthermore, reduced Aβ42 levels were also observed in primary neuronal cultures from CD38 deficient mice and in APP.PS neuronal cultures that were treated with a CD38 enzyme inhibitor or an antagonist of cADPR-dependent Ca2+ signaling.
Loss of CD38 decreases Aβ load most likely by inhibiting Aβ production since: i) Loss of CD38 led to a robust reduction in the number but not the size of plaques; ii) Loss of CD38 reduced both soluble and insoluble Aβ levels; iii) Loss of CD38 had no effect on the expression of genes involved in MM-mediated Aβ phagocytosis or degradation and iv) Loss of CD38 significantly lowered the activity of the Aβ generating β- and γ-secretases.
The data showing that CD38 deficiency results in decreased Aβ levels in primary neuronal cultures suggest that, at least in vitro, this reduction is mediated by neurons without the aid of additional cells (e.g., microglia or astrocytes) or systems (e.g., clearance via interstitial fluid). However, since residual astrocytes and microglia are present in the neuronal cultures, we cannot exclude the possibility that these other cell types may also contribute to the reduction in Aβ levels observed in the primary neuronal cultures as well as the brains of the aged APP.PS.Cd38−/− mice. Similarly, while we believe that the cognitive improvements observed in the aged APP.PS.Cd38−/− mice are due, in large part, to the reduction in Aβ levels, it is certainly possible that the reduced accumulation or inflammatory activity of MM in the brains of APP.PS.Cd38−/− mice also contribute to the improved cognitive function observed in these mice. In support of this possibility, MM from APP.PS.Cd38−/− mice did not accumulate to the same extent in the hippocampus (before obvious changes in Aβ deposition or cognitive function), exhibited altered morphology and expressed fewer IL-1β and TNFα transcripts.
CD38 appears to regulate Aβ levels in neurons, at least in vitro, in an enzyme-dependent mechanism, as Aβ levels in primary WT neurons were decreased when the neurons were treated with a CD38 enzyme inhibitor. One of the consequences of blocking CD38 enzyme activity (or deleting CD38) is prevention of cADPR generation. Importantly, 8Br-cADPR, a cADPR antagonist also significantly decreased Aβ levels in neuronal cultures, suggesting that cADPR-dependent Ca2+ signaling likely influences Aβ production by neurons. Ca2+ signaling is reported to play a role in AD pathology35 and previous studies showed that CD38-generated Ca2+ mobilizing metabolites also regulate synaptic properties of neurons.36
Another consequence of blocking CD38 enzyme activity (or deleting CD38) is that the CD38 substrate, NAD, can accumulate to higher levels in the brain. In fact, similar to prior studies using WT and Cd38−/− mice6, 7, the loss of CD38 resulted in increased NAD levels in the brains of APP.PS.Cd38−/− mice and in primary neuronal cultures derived from APP.PS.Cd38−/− brains. Moreover, addition of NAD to APP.PS primary neuronal cultures reduced Aβ levels, suggesting that NAD can regulate Aβ generation in neurons. These data are in line with a previous report showing that exogenous NAD treatment decreases Aβ levels in primary neuronal cultures derived from Tg2576 AD model mice.28 Furthermore, treatment of Tg2576 mice with an NAD biosynthetic precursor attenuated cognitive deficits37, supporting the notion that increased NAD levels contribute to protection in AD and that deleting CD38 may preserve a pool of neuroprotective NAD.
It is known that the activity of the NAD-dependent deacetylase SIRT1 is increased in CD38 deficient tissues38 and in the brains of APP.PS.Cd38−/− mice (unpublished observations). Interestingly, SIRT1 regulates the expression and activity of the α-secretase, ADAM1039 and is responsible for increased α-secretase activity and decreased Aβ levels in caloric-restricted Tg2576 mice.28 Alternatively, the SIRT1/PGC-1α pathway is reported to reduce β-secretase activity by promoting BACE1 degradation.37 However, as we did not observe higher α-secretase activity or higher ADAM10 level nor reduced BACE1 levels in APP.PS.Cd38−/− mice, the data suggest that the protective effects of CD38 loss/inhibition and NAD in the AD model are not simply due to SIRT1-dependent changes in α- and/or β-secretase expression and activity. Interestingly, published data show that NAD analogs can directly suppress γ-secretase activity.40 These data suggest that the γ-secretase complex contains a (di)nucleotide binding site40 that, when bound, inhibits the protease activity of the complex. It is therefore tempting to speculate that NAD, which is found in larger quantities in the brain extracts of APP.PS.Cd38−/− mice may repress γ-secretase activity by binding to and inhibiting the activity of γ-secretase.
Notably, deletion of CD38 negatively affected the activity of all three APP secretases. This may be indicative of an increased need of the CD38 deficient cells to preserve full length APP, at least within some sub-cellular locations, due to a global reduction in APP expression or increased degradation of the full length APP through non α-, β-, or γ-secretase mediated cleavage. Alternatively, reduced levels of APP ligands or altered trafficking of APP within the Cd38−/− cells may have resulted in global downregulation of secretases’ activity. It is also possible that the changes in the activities of the secretases observed in the APP.PS.Cd38−/− mice are a secondary consequence of the attenuated disease course in these mice. Indeed, previous studies showed that β-secretase activity increases with age and the onset of AD.41 Although we cannot exclude this possibility, we think it unlikely as the activity of all the secretases, including β-secretase, was decreased in the APP.PS.Cd38−/− mice as early as 1.5 months of age.
Collectively, our results suggest that targeting CD38 may provide a novel therapeutic approach for AD and that targeting CD38 may allow for therapeutic modulation of both neuroinflammation and Aβ production. Loss of CD38 also decreased Aβ levels in non-transgenic neuronal cultures, suggesting that this approach may not be limited to familial mutations driven AD. Notably, γ-secretase inhibitors have been shown to attenuate Aβ levels in mice and humans42–45, however side effects have led to the halt of clinical trials testing such compounds46. Further studies are required to establish whether inhibition of CD38 activity can overcome these limitations and provide an effective and safe approach for modulation of Aβ levels.
Supplementary Material
Acknowledgments
We thank Nurit Geva, Noam Welder and Bar Ben Baruch for technical assistance. This work is supported in part by The U.S.-Israel Binational Science Foundation (2007061 and 2011054 to R.S. and F.L.), Adams Super-Center for Brain Research at Tel-Aviv University and the National Network of Excellence in Neuroscience (NNE) (to R.S.) and National Institutes of Health (R01 AI063399 to F.L.), the EU FP7 project LipiDiDiet, Grant Agreement No. 211696 (TH), the DFG (TH), and the HOMFORexzellent 2011 (MG) (Saarland University research grants).
Footnotes
Authorship
R.S., A. L., and F.L. conceived the study, designed experiments, and wrote the article. E.B., A.L., and T.D. performed the experiments. V.J.H., M.O.W.G., and T.H preformed the secretases experiments. A.B., performed the primary neuronal culture experiments. All authors were participants in the discussion of results, determination of conclusions, and review of the manuscript.
Potential Conflicts of Interest
R.S., A.L., E.B., and A.B.,: grant, Teva
F.L., None declared
T.D., None declared
T.H., None declared
M.G., None declared
V.H., None declared
References
- 1.Serrano-Pozo A, Frosch MP, Masliah E, et al. Neuropathological alterations in Alzheimer disease. Cold Spring Harbor perspectives in medicine. 2011;1:a006189. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Brien RJ, Wong PC. Amyloid precursor protein processing and Alzheimer’s disease. Annual review of neuroscience. 2011;34:185–204. doi: 10.1146/annurev-neuro-061010-113613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Itagaki S, McGeer PL, Akiyama H, et al. Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. Journal of neuroimmunology. 1989;24:173–182. doi: 10.1016/0165-5728(89)90115-x. [DOI] [PubMed] [Google Scholar]
- 4.El Khoury J, Toft M, Hickman SE, et al. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nature medicine. 2007;13:432–438. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- 5.Kettenmann H, Hanisch UK, Noda M, et al. Physiology of microglia. Physiological reviews. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 6.Aksoy P, White TA, Thompson M, et al. Regulation of intracellular levels of NAD: a novel role for CD38. Biochemical and biophysical research communications. 2006;345:1386–1392. doi: 10.1016/j.bbrc.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 7.Young GS, Choleris E, Lund FE, et al. Decreased cADPR and increased NAD+ in the Cd38−/− mouse. Biochemical and biophysical research communications. 2006;346:188–192. doi: 10.1016/j.bbrc.2006.05.100. [DOI] [PubMed] [Google Scholar]
- 8.Chini EN. CD38 as a regulator of cellular NAD: a novel potential pharmacological target for metabolic conditions. Curr Pharm Des. 2009;15:57–63. doi: 10.2174/138161209787185788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aksoy P, Escande C, White TA, et al. Regulation of SIRT 1 mediated NAD dependent deacetylation: a novel role for the multifunctional enzyme CD38. Biochemical and biophysical research communications. 2006;349:353–359. doi: 10.1016/j.bbrc.2006.08.066. [DOI] [PubMed] [Google Scholar]
- 10.Partida-Sanchez S, Goodrich S, Kusser K, et al. Regulation of dendritic cell trafficking by the ADP-ribosyl cyclase CD38: impact on the development of humoral immunity. Immunity. 2004;20:279–291. doi: 10.1016/s1074-7613(04)00048-2. [DOI] [PubMed] [Google Scholar]
- 11.Mayo L, Jacob-Hirsch J, Amariglio N, et al. Dual role of CD38 in microglial activation and activation-induced cell death. J Immunol. 2008;181:92–103. doi: 10.4049/jimmunol.181.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mizuguchi M, Otsuka N, Sato M, et al. Neuronal localization of CD38 antigen in the human brain. Brain Res. 1995;697:235–240. doi: 10.1016/0006-8993(95)00885-t. [DOI] [PubMed] [Google Scholar]
- 13.Banerjee S, Walseth TF, Borgmann K, et al. CD38/cyclic ADP-ribose regulates astrocyte calcium signaling: implications for neuroinflammation and HIV-1-associated dementia. J Neuroimmune Pharmacol. 2008;3:154–164. doi: 10.1007/s11481-008-9105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higashida H, Bowden SE, Yokoyama S, et al. Overexpression of human CD38/ADP-ribosyl cyclase enhances acetylcholine-induced Ca2+ signalling in rodent NG108-15 neuroblastoma cells. Neurosci Res. 2007;57:339–346. doi: 10.1016/j.neures.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Bruzzone S, Verderio C, Schenk U, et al. Glutamate-mediated overexpression of CD38 in astrocytes cultured with neurones. Journal of neurochemistry. 2004;89:264–272. doi: 10.1111/j.1471-4159.2003.02326.x. [DOI] [PubMed] [Google Scholar]
- 16.Verderio C, Bruzzone S, Zocchi E, et al. Evidence of a role for cyclic ADP-ribose in calcium signalling and neurotransmitter release in cultured astrocytes. Journal of neurochemistry. 2001;78:646–657. doi: 10.1046/j.1471-4159.2001.00455.x. [DOI] [PubMed] [Google Scholar]
- 17.Franco L, Bodrato N, Moreschi I, et al. Cyclic ADP-ribose is a second messenger in the lipopolysaccharide-stimulated activation of murine N9 microglial cell line. Journal of neurochemistry. 2006;99:165–176. doi: 10.1111/j.1471-4159.2006.04031.x. [DOI] [PubMed] [Google Scholar]
- 18.Partida-Sanchez S, Iribarren P, Moreno-Garcia ME, et al. Chemotaxis and calcium responses of phagocytes to formyl peptide receptor ligands is differentially regulated by cyclic ADP ribose. J Immunol. 2004;172:1896–1906. doi: 10.4049/jimmunol.172.3.1896. [DOI] [PubMed] [Google Scholar]
- 19.Levy A, Bercovich-Kinori A, Alexandrovich AG, et al. CD38 facilitates recovery from traumatic brain injury. Journal of neurotrauma. 2009;26:1521–1533. doi: 10.1089/neu.2008.0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jankowsky JL, Fadale DJ, Anderson J, et al. Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase. Hum Mol Genet. 2004;13:159–170. doi: 10.1093/hmg/ddh019. [DOI] [PubMed] [Google Scholar]
- 21.Cockayne DA, Muchamuel T, Grimaldi JC, et al. Mice deficient for the ecto-nicotinamide adenine dinucleotide glycohydrolase CD38 exhibit altered humoral immune responses. Blood. 1998;92:1324–1333. [PubMed] [Google Scholar]
- 22.Levy A, Blacher E, Vaknine H, et al. CD38 deficiency in the tumor microenvironment attenuates glioma progression and modulates features of tumor-associated microglia/macrophages. Neurooncology. 2012;14:1037–1049. doi: 10.1093/neuonc/nos121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobson EL, Lange RA, Jacobson MK. Pyridine nucleotide synthesis in 3T3 cells. Journal of cellular physiology. 1979;99:417–425. doi: 10.1002/jcp.1040990316. [DOI] [PubMed] [Google Scholar]
- 24.Lukasiak S, Breuhahn K, Schiller C, et al. Quantitative analysis of gene expression relative to 18S rRNA in carcinoma samples using the LightCycler instrument and a SYBR GreenI-based assay: determining FAT10 mRNA levels in hepatocellular carcinoma. Methods Mol Biol. 2008;429:59–72. doi: 10.1007/978-1-60327-040-3_5. [DOI] [PubMed] [Google Scholar]
- 25.Grimm MO, Haupenthal VJ, Rothhaar TL, et al. Effect of Different Phospholipids on alpha-Secretase Activity in the Non-Amyloidogenic Pathway of Alzheimer’s Disease. International journal of molecular sciences. 2013;14:5879–5898. doi: 10.3390/ijms14035879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothhaar TL, Grosgen S, Haupenthal VJ, et al. Plasmalogens inhibit APP processing by directly affecting gamma-secretase activity in Alzheimer’s disease. The Scientific World Journal. 2012;2012:141240. doi: 10.1100/2012/141240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seibenhener ML, Wooten MW. Isolation and culture of hippocampal neurons from prenatal mice. Journal of visualized experiments : JoVE. 2012 doi: 10.3791/3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin WP, Yang TL, Ho L, et al. Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J Biol Chem. 2006;281:21745–21754. doi: 10.1074/jbc.M602909200. [DOI] [PubMed] [Google Scholar]
- 29.Kellenberger E, Kuhn I, Schuber F, et al. Flavonoids as inhibitors of human CD38. Bioorg Med Chem Lett. 2011;21:3939–3942. doi: 10.1016/j.bmcl.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 30.Rogers J, Strohmeyer R, Kovelowski CJ, et al. Microglia and inflammatory mechanisms in the clearance of amyloid beta peptide. Glia. 2002;40:260–269. doi: 10.1002/glia.10153. [DOI] [PubMed] [Google Scholar]
- 31.Savonenko A, Xu GM, Melnikova T, et al. Episodic-like memory deficits in the APPswe/PS1dE9 mouse model of Alzheimer’s disease: relationships to beta-amyloid deposition and neurotransmitter abnormalities. Neurobiology of disease. 2005;18:602–617. doi: 10.1016/j.nbd.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 32.Jankowsky JL, Melnikova T, Fadale DJ, et al. Environmental enrichment mitigates cognitive deficits in a mouse model of Alzheimer’s disease. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:5217–5224. doi: 10.1523/JNEUROSCI.5080-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 34.Perez-Gonzalez R, Alvira-Botero MX, Robayo O, et al. Leptin gene therapy attenuates neuronal damages evoked by amyloid-beta and rescues memory deficits in APP/PS1 mice. Gene Ther. 2014;21:298–308. doi: 10.1038/gt.2013.85. [DOI] [PubMed] [Google Scholar]
- 35.Berridge MJ. Calcium hypothesis of Alzheimer’s disease. Pflugers Archiv : European journal of physiology. 2010;459:441–449. doi: 10.1007/s00424-009-0736-1. [DOI] [PubMed] [Google Scholar]
- 36.Reyes-Harde M, Potter BV, Galione A, et al. Induction of hippocampal LTD requires nitric-oxide-stimulated PKG activity and Ca2+ release from cyclic ADP-ribose-sensitive stores. J Neurophysiol. 1999;82:1569–1576. doi: 10.1152/jn.1999.82.3.1569. [DOI] [PubMed] [Google Scholar]
- 37.Gong B, Pan Y, Vempati P, et al. Nicotinamide riboside restores cognition through an upregulation of proliferator-activated receptor-gamma coactivator 1alpha regulated beta-secretase 1 degradation and mitochondrial gene expression in Alzheimer’s mouse models. Neurobiology of aging. 2013;34:1581–1588. doi: 10.1016/j.neurobiolaging.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barbosa MT, Soares SM, Novak CM, et al. The enzyme CD38 (a NAD glycohydrolase, EC 3.2.2.5) is necessary for the development of diet-induced obesity. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2007;21:3629–3639. doi: 10.1096/fj.07-8290com. [DOI] [PubMed] [Google Scholar]
- 39.Wang J, Fivecoat H, Ho L, et al. The role of Sirt1: at the crossroad between promotion of longevity and protection against Alzheimer’s disease neuropathology. Biochimica et biophysica acta. 2010;1804:1690–1694. doi: 10.1016/j.bbapap.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 40.Wu F, Schweizer C, Rudinskiy N, et al. Novel gamma-secretase inhibitors uncover a common nucleotide-binding site in JAK3, SIRT2, and PS1. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2010;24:2464–2474. doi: 10.1096/fj.09-148031. [DOI] [PubMed] [Google Scholar]
- 41.Fukumoto H, Rosene DL, Moss MB, et al. Beta-secretase activity increases with aging in human, monkey, and mouse brain. The American journal of pathology. 2004;164:719–725. doi: 10.1016/s0002-9440(10)63159-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bateman RJ, Siemers ER, Mawuenyega KG, et al. A gamma-Secretase Inhibitor Decreases Amyloid-beta Production in the Central Nervous System. Ann Neurol. 2009;66:48–54. doi: 10.1002/ana.21623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siemers ER, Dean RA, Friedrich S, et al. Safety, tolerability, and effects on plasma and cerebrospinal fluid amyloid-beta after inhibition of gamma-secretase. Clin Neuropharmacol. 2007;30:317–325. doi: 10.1097/WNF.0b013e31805b7660. [DOI] [PubMed] [Google Scholar]
- 44.Dovey HF, John V, Anderson JP, et al. Functional gamma-secretase inhibitors reduce beta-amyloid peptide levels in brain. Journal of neurochemistry. 2001;76:173–181. doi: 10.1046/j.1471-4159.2001.00012.x. [DOI] [PubMed] [Google Scholar]
- 45.Abramowski D, Wiederhold KH, Furrer U, et al. Dynamics of A beta Turnover and Deposition in Different beta-Amyloid Precursor Protein Transgenic Mouse Models Following gamma-Secretase Inhibition. J Pharmacol Exp Ther. 2008;327:411–424. doi: 10.1124/jpet.108.140327. [DOI] [PubMed] [Google Scholar]
- 46.De Strooper B, Chavez Gutierrez L. Learning by Failing: Ideas and Concepts to Tackle gamma-Secretases in Alzheimer Disease and Beyond. Annual review of pharmacology and toxicology. 2014 doi: 10.1146/annurev-pharmtox-010814-124309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.