Abstract
T-type channels are low-voltage-activated calcium channels that contribute to a variety of cellular and physiological functions, including neuronal excitability, hormone and neurotransmitter release as well as developmental aspects. Several human conditions including epilepsy, autism spectrum disorders, schizophrenia, motor neuron disorders and aldosteronism have been traced to variations in genes encoding T-type channels. In this short review, we present the genetics of T-type channels with an emphasis on structure-function relationships and associated channelopathies.
Keywords: calcium channels, t-type channels, cav3 channels, mutation, channelopathies, epilepsy, autism spectrum disorders, schizophrenia, amyotrophic lateral sclerosis, aldosteronism
Introduction
Low-voltage-activated Cav3 T-type channels are members of the superfamily of voltage-gated calcium channels.1 T-type channels are widely expressed throughout the nervous, the neuroendocrine and the cardiovascular system2 and are also found in several non-excitable tissues such as osteocytes,3 sperm cells4 and immune cells.5 6 The cellular and physiological processes in which T-type channels are implicated depend primarily on the tissue distribution of the channels. For instance, in the central and peripheral nervous systems, T-type channels play an essential role in shaping neuronal excitability,7–9 whereas they contribute to the release of hormones in the neuroendocrine system.10 11
The essential role of T-type channels in human physiology is emphasised by the existence of channelopathies which are disorders that are caused or enhanced by mutations in genes that encode these channels. Most of T-type channelopathies are transmitted by recessive inheritance or appear sporadic. The clinical manifestations of these disorders depend primarily on dysfunctions of the biophysical characteristics and cell surface trafficking of the channels and can lead to either gain-of-function or loss-of-function.
In this review, we present an overview of human T-type channelopathies and their relationship with the diversity, structure and function of T-type channels. This will be followed by the presentation of the various syndromes for which T-type channels have been linked to, with an emphasis on the structure-function-pathogenicity relationship of mutant Cav3 channels.
Diversity, structure and function of Cav3 channels
Although T-type currents recorded from various native tissues present a common feature illustrated by a low-threshold of activation around −55 mV, they also exhibit several differences in their electrophysiological and pharmacological properties. This heterogeneity is in part explained by the existence of three T-type channel isoforms, Cav3.1,12 Cav3.213 and Cav3.3,14 which in humans are encoded by the genes CACNA1G, CACNA1H and CACNA1I, respectively (figure 1A). This diversity is further enriched by the existence of several channel splice variants. Indeed, alternative splicing of Cav3.1,15–19 Cav3.220–24 and Cav3.325 26 contributes to increase the functional diversity of T-type channels and may also have important pathophysiological implications.
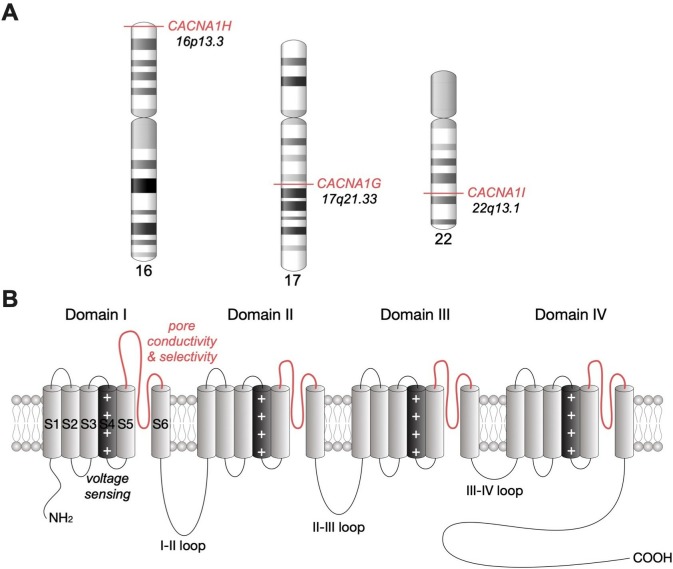
Figure 1.
Chromosomal location of human Cav3 channels and their membrane topology. (A) Chromosomal location of human CACNA1G, CACNA1H and CACNA1I genes encoding Cav3.1, Cav3.2 and Cav3.3 channels, respectively. (B) Secondary membrane topology of Cav3 depicting the main structural channel gating determinants.
T-type channels consist on a single Cav3 pore-forming subunit that contains all the structural determinants of channel gating and ion selectivity and permeability (for review see.27 The Cav3 subunit is a relatively large plasma membrane protein of about 260 kDa organised into four hydrophobic domains (DI to DIV), each of them made of six transmembrane helices (S1 to S6) (figure 1B). The voltage-sensing module of the channel is formed by the positively charged arginine/lysine-rich S4 segments,28 while the ion conductivity and selectivity lie on the re-entrant extracellular linkers connecting S5 and S6 segments of each domain, so-called pore-forming loop (P loop).2 The four transmembrane domains are linked together by several intracellular loops connecting the S6 segment of the upstream domain to the S1 segment of the downstream domain, which in combination with the amino and carboxy termini provide hubs for channel regulation by a variety of signalling molecules and other molecular partners including the G-protein βγ-dimer,29 30 CaMKII,31 32 kelch-like 1,33 calcineurin,34 syntaxin-1A,35 stac1,36 CACHD1,37 38 spectrin α/β and ankyrin B39 as well as several ion channels.40 41 In addition, T-type channels undergo several post-translational modifications such as phosphorylation,42 ubiquitination43 and glycosylation,44–49 which contribute to the expression and activity of the channel.
The contribution of T-type channels in particular cellular processes is partly inherent to their unique electrophysiological properties. Voltage-dependent opening of T-type channels occurs at comparatively negative membrane potentials where calcium influx contributes to the depolarisation of the plasma membrane, therefore increasing the opening probability of voltage-gated sodium channels and the propensity of cells to fire action potentials. This aspect is especially relevant in several central neurons including thalamic and hippocampal cells where T-type channels are particularly abundant in dendrites to enhance subthreshold postsynaptic potentials and facilitate the propagation of the electrical signal to the cell body.50 Another way by which T-type channels contribute to neuronal excitability is by forming functional complexes with several types of voltage-activated and calcium-activated potassium channels that allow these channels to operate at subthreshold membrane potentials.51–55 In addition, their fast recovery from inactivation allows T-type channels to generate calcium spikes on brief periods of hyperpolarisation, which leads to the firing of rebound burst of action potentials that support various forms of neuronal rhythmogenesis.56–59 Although a significant fraction of T-type channels is inactivated at most resting membrane potentials of nerve cells, a small fraction remains open to support the passive influx of calcium (termed window current due to the ‘window’ created by the overlap between the activation and inactivation curves of the channels). In nerve cells, this window current has been implicated in the generation of low frequency oscillations observed during sleep patterns60 and is likely to play additional functions especially in non-excitable cells. T-type channels also contribute to several forms of synaptic plasticity.61 Finally, T-type channels are implicated in the low-threshold release of neurotransmitters and hormones, possibly by virtue of their functional coupling with the vesicular release machinery.10 11 Genetic knockout in mice also provided insightful information on the physiological importance of T-type channels. For instance, knockout of Cacna1g has highlighted the role of Cav3.1 in the generation of sinoatrial node pacemaker activity and atrioventricular conduction62 and also their implication in the development of trigeminal neuropathic pain63 and peripheral pain,64 as well as endothelial dysfunction associated with ageing.65 Mice lacking Cacna1h display abnormal coronary function,66 67 decreased susceptibility to cardiac hypertrophy68 and absence seizure,69 decreased peripheral pain signalling70 as well as several neurological symptoms including elevated anxiety and impaired memory.71 72 Mice lacking Cacna1I have provided important information on the implication of Cav3.3 channels in sleep rhythmogenesis.73 74 Finally, several studies from genetic knock out have uncovered a role for T-type channels in the control of myogenic tone.75
Considering that the cellular and physiological functions in which T-type channels are implicated are directly dependent on their electrophysiological properties, it is anticipated that alteration of channel gating caused by mutations will have deleterious consequences. In the next section, we will cover the current state of knowledge of T-type channelopathies and illustrate the links between the structure-function of mutant channels and the pathophysiological features of the associated human syndromes.
CACNA1H (Cav3.2) channelopathies
Idiopathic generalised epilepsy
It is well established that T-type channels play an essential role in the functioning of the thalamocortical circuitry and underlie spike-and-wave discharges that occur during absence seizures.7 69 76–82 This notion is further supported by the observation that thalamic T-type currents are enhanced in several rodent models of absence epilepsy83–85 and genetic overexpression of Cav3.1 channels produces pure epilepsy in rodents.86 Conversely, pharmacological inhibition of T-type currents using pan T-type channel blockers reduces thalamic burst firing and suppresses seizures.87–89 In addition, several pan T-type channel blockers are effective in the treatment of absence seizures in humans90–92 (for recent review see Ref. 81).
Genetic association studies have identified more than 200 missense variants in the human CACNA1H gene that segregate in patients presenting a range of epilepsy syndromes including childhood absence, juvenile absence, juvenile myoclonic and myoclonic astatic epilepsies as well as febrile seizures and temporal lobe epilepsy that fall under the umbrella term of idiopathic generalised epilepsies (IGE).93–98 It is worth noting that most of these variants have been reported in the Exome Aggregation Consortium (ExAC) suggesting that their contribution to human epilepsies may be rather low or might be dependent on additional genetic and/or environmental factors. Electrophysiological analysis of several of these variants (figure 2) using exogenous expression of mutated Cav3.2 channels in human embryonic kidney cells (HEK293) revealed that these mutations generally produce mild biophysical changes and in some cases do not alter the gating of the channel at all (table 1). This is not completely surprising since the variants examined so far do not concentrate in specific loci that are known to be essential for the gating of the channel, but are rather scattered across the entire channel sequence. However, an interesting study by Zhong and colleagues21 revealed that some of the mutations in Cav3.2 may affect alternative splicing of the channel, which in turn may affect the behaviour of native T-type currents.
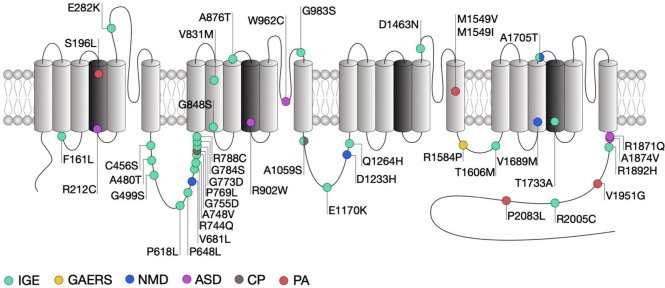
Figure 2.
Location of CACNA1H mutations within the secondary structure of Cav3.2 along with their associated syndromes. Only mutations that have been functionally characterised are indicated. Protein reference: UniProt O95180. ASD, autism spectrum disorder; CP, chronic pain; GAERS, genetic absence epilepsy rat from Strasburg; IGE, idiopathic generalised epilepsy; NMD, neuromuscular disorder; PA, primary aldosteronism.
Table 1.
Summary of the gating properties of T-type channel variants
| Gene | Mutation | Disease | Biophysical effect | Settings | Reference |
|
CACNA1G
(Ca v 3.1) |
A570V | IGE | None | HEK293, 2Ca | 144 |
| A961T | CA | Gain-of-function | HEK293T, 2Ca | 142 | |
| A1089S | IGE | None | HEK293, 2Ca | 144 | |
| M1531V | CA | Gain-of-function | HEK293T, 2Ca | 142 | |
| R1715H | CA | Loss-of-function | HEK293T | 138 | |
|
CACNA1H
(Ca v 3.2) |
F161L | IGE | Gain-of-function | tsA-201, 5Ba | 99 |
| S196L | PA | Gain-of-function | tsA-201, 2Ca | 134 | |
| R212C | ASD | Loss-of-function | HEK293T, 2Ca | 110 | |
| E282K | IGE | Gain-of-function | tsA-201, 5Ba | 99 | |
| C456S | IGE | None | tsA-201, 5Ba | 99 | |
| C456S | IGE | Gain-of-function | Hippo, 1.8Ca | 105 | |
| A480T | IGE | None | tsA-201, 5Ba | 100 | |
| G499S | IGE | None | tsA-201, 5Ca | 102 | |
| P618L | IGE | Gain-of-function | tsA-201, 5Ba | 100 | |
| P648L | IGE | None | tsA-201, 5Ca | 102 | |
| V681L | NMD | Loss-of-function | tsA-201, 5Ba | 123 | |
| R744Q | IGE | None | tsA-201, 5Ca | 102 | |
| A748V | IGE | None | tsA-201, 5Ca | 102 | |
| G755D | IGE | Gain-of-function | tsA-201, 5Ba | 100 | |
| P769L | CP | None | tsA-201, 10Ba | 130 | |
| G773D | IGE | None | tsA-201, 5Ca | 102 | |
| G784S | IGE | None | tsA-201, 5Ca | 102 | |
| R788C | IGE | Gain-of-function | HEK293, 5Ca | 101 | |
| V831M | IGE | Gain-of-function | tsA-201, 5Ba | 99 | |
| G848S | IGE | Gain-of-function | tsA-201, 5Ca | 102 | |
| A876T | IGE | Gain-of-function | tsA-201, 2Ca | 95 | |
| R902W | ASD | Loss-of-function | HEK293T, 2Ca | 110 | |
| W962C | ASD | Loss-of-function | HEK293T, 2Ca | 110 | |
| G983S | IGE | Loss-of-function | tsA-201, 2Ca | 95 | |
| A1059S | IGE+CP | Loss-of-function | tsA-201, 2-10Ca | 95 130 | |
| E1170K | IGE | None | tsA-201, 2Ca | 95 | |
| D1233H | NMD | Loss-of-function | tsA-201, 5Ba | 123 | |
| Q1264H | IGE | None | tsA-201, 2Ca | 95 | |
| D1463N | IGE | None | tsA-201, 5Ba | 99 | |
| M1549V | PA | Gain-of-function | HEK293T, 5Ca | 132 | |
| M1549I | PA | Mix effect | tsA-201, 2Ca | 134 | |
| T1606M | IGE | Gain-of-function | tsA-201, 2Ca | 95 | |
| V1689M | NMD | Loss-of-function | tsA-201, 5Ba | 118 | |
| A1705T | IGE+NMD | Gain-of-function | tsA-201, 2Ca | 95 | |
| A1705T | IGE+NMD | Loss-of-function | tsA-201, 5Ba | 118 | |
| T1733A | IGE | Gain-of-function | tsA-201, 2Ca | 95 | |
| R1871Q | ASD | Gain-of-function | HEK293T, 2Ca | 110 | |
| A1874V | ASD | ND | HEK293T, 2Ca | 110 | |
| R1892H | IGE | Gain-of-function | tsA-201, 2Ca | 95 | |
| V1951G | PA | Gain-of-function | tsA-201, 2Ca | 134 | |
| R2005C | IGE | Gain-of-function | tsA-201, 2Ca | 95 | |
| P2083L | PA | Gain-of-function | tsA-201, 2Ca | 134 | |
|
CACNA1I
(Ca v 3.3) |
T797M | SCZ | None | HEK293, 2Ca | 149 |
| R1346H | SCZ | Loss-of-function | HEK293, 2Ca | 149 |
The biophysical effects produced by each mutation is summarised as None (blue), Gain-of-function (green) and Loss-of-function (red). Recording conditions are also indicated (cell type, nature and concentration of the permeating cation).
Green colour: gain-of-function mutation; red colour: loss-of-function mutation; blue colour: no biophysical change.
ASD, autism spectrum disorder; CA, cerebellar ataxia; CP, chronic pain; Hippo, hippocampal neuron; IGE, idiopathic generalised epilepsy; NMD, neuromuscular disorder; PA, primary aldosteronism; SCZ, schizophrenia.
Among the mutations that do affect channel gating, the alterations observed are in general consistent with a gain-of-function of the channel, although in rare exceptions a loss-of-function was observed.99–103 In addition, cell surface expression of the channels may be affected by a subset of mutations within the domain I-II linker region of the channel.104 Intuitively, gain-of-function mutations would increase the propensity of neurons to fire action potentials. This notion is in part supported by computer simulations predicting that several of these mutations would increase neuronal firing and induce neuronal oscillations at similar frequencies as observed during absence seizures.101 In addition, cultured hippocampal neurons expressing the gain-of-function C456S mutation indeed showed increased firing.105 However, studies investigating the influence of Cav3.2 variants in native conditions are too rare to draw a general conclusion on the impact of CACNA1H variants on neuronal excitability.
Despite evident functional effects on Cav3.2 gating which in general are expected to increase neuronal excitability and to potentially drive seizures, only few of the CACNA1H variants identified so far segregate with specific epilepsy phenotypes within families.95 A clear causal mutation linking Cav3.2 to genetic epilepsies was found in the genetic absence epilepsy rat from Strasburg (GAERS) (figure 2).106 This mutation segregates with the occurrence of seizures and causes a gain-of-function of Cav3.2, on the one hand by enhancing the recovery from inaction of the channel23 and on the other hand by enhancing expression of Cav3.2 at the cell surface due to altered association with calnexin.107 Interestingly, biophysical gain of function effects of this mutation selectively manifested themselves in a Cav3.2 splice variant that contained exon 25 which is expressed at high levels in thalamic tissue.23 This may account for the observation that GAERS rats exhibit seizures but do not have other physiological dysfunctions that would be expected from increased T-type channel activity.
Altogether, it remains unclear to which extent CACNA1H variants contribute to human epilepsies. It is likely that these variants only represent a low-risk factor for genetic epilepsies and may only contribute to the disease in combination with other genetic or environmental factors.
Autism spectrum disorder
Autism spectrum disorders (ASD) are neurodevelopmental conditions characterised by impaired social interaction, communication and unusual behaviour. Despite an exceptionally diverse genetic aetiology with hundreds of risk genes identified,108 a subset of high-risk mutations is recurrently found in about 5% of individuals with ASD.109 Several missense mutations in CACNA1H were identified in patients with ASD (figure 2) and were functionally characterised using heterologous expression of mutated Cav3.2 channels.110 Although all these mutations produced several alterations of the channel gating consistent with a loss-of-channel function (table 1), the severity of these alterations appears to be correlated with the location of the mutations in the channel protein. Indeed, and consistent with the observation that the R212C and R920W mutations are located within the voltage sensing region of the channel and neutralise an arginine residue, they produced a depolarising shift of the voltage-dependence of activation of the channel, along with a decreased T-type current. In contrast, the W962C mutation located within the pore-forming loop of the channel did not affect the voltage sensitivity but produced a dramatic decrease of the T-type current, which likely resulted from an alteration of the ionic permeability of the channel. Finally, the R1871Q and A1874V mutations are located in the proximal region of the carboxy terminal region of the channel, a region that is not particularly known to contribute to the gating of the channel and produced only a mild decrease of the T-type current.
As for CACNA1H variants associated with epilepsy syndromes, variants associated with ASD do not segregate with the ASD phenotype, but instead may modify the phenotypic expression. In contrast, several rare de novo gain-of-function mutations with high penetrance were recently identified in CACNA1D and are considered as high-risk factor for ASD and more generally neurodevelopmental conditions.111–114
Neuromuscular disorder
Neuromuscular disorders encompass a wide range of conditions characterised by progressive muscle degeneration and weakness that primarily or secondary impair skeletal muscles and their innervation. For instance, amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig’s disease, is a neurodegenerative disorder characterised by the progressive loss of cortical, brain stem and spinal motor neurons, eventually leading to muscle weakness and paralysis. ALS is regarded as a complex genetic disorder with a Mendelian pattern of inheritance in approximately 5%–10% of familial cases,115 but most patients have no discernable family history of the disease which is then referred to being ‘sporadic’ or ‘isolated’ in nature (sALS). However, several genes and loci in apparent sALS cases have been proposed to be associated with an increased risk of the disease or to modify the onset or progression of the disease, which highlights the importance of genetic risk factors.116 Recently, whole exome sequence analysis of case-unaffected-parent trios identified two compound heterozygous recessive missense mutations in CACNA1H (figure 2).117 Functional analysis revealed that these mutations cause a mild alteration of Cav3.2 activity that is consistent with a loss-of-function of the channel (table 1).118 In addition, computer simulations suggested decreased neuronal activity of nerve cells expressing the channel variants.
Although recent studies have reported the expression of T-type channels in motor neurons,119 120 the functional implication of T-type channels in these neurons has yet to be analysed. Increased neuronal excitability has been reported as a hallmark in ALS, where an increase of the sodium conductance and a decrease of axonal potassium currents is observed.121 Considering the role of T-type channels in the control of calcium-activated potassium channels, it is a possibility that decrease of T-type channel activity caused by ALS-associated mutations may contribute to the alteration of potassium currents. In addition, a recent finding demonstrated the role of T-type channels in the maintenance of neuronal progenitor cell viability.122 This aspect will deserve particular attention, especially in the context on neurodegenerative disorders such as ALS. Additionally, a recent study reported the case of a patient with severe infantile onset amyotrophy carrying two inherited heterozygous CACNA1H mutations.123 Functional analysis of Cav3.2 variants were consistent with a loss-of-channel function particularly evidenced by a decreased window current, therefore expending the possible association of CACNA1H with motor neuron diseases.
Chronic pain
It is well established that T-type channels play an essential role in the processing of peripheral nociception and altered expression and altered expression of Cav3.2 has been documented in several chronic pain conditions. For instance, increased activity of Cav3.2 channels in primary afferent fibres is observed in diabetic neuropathy,124 nerve injury,125 irritable bowel syndrome126 and peripheral inflammation43 and is believed to be causally related to the development and maintenance of chronic pain. These gain of function effects in Cav3.2 calcium channels are not linked to mutations in the channel sequence, but instead mediated by altered post-translational modification by deubiqutination43 127–129 and glycosylation processes.45 Recently, two heterozygous missense mutations in the CACNA1H gene were identified in a patient presenting with paediatric chronic pain (figure 2).130 Functional characterisation of these variants using heterologous expression of mutant Cav3.2 channels provided inconclusive results as to the impact of these mutations on the functioning of the channel, mainly due to the observation that the phenotypic manifestations appeared to be dependent on the experimental conditions.
Primary aldosteronism
Primary aldosteronism (PA) is the most common form of secondary hypertension. T-type calcium channels have been implicated in the secretion of aldosterone secretion from the adrenal zona glomerulosa and in situ hybridisation studies combined functional and pharmacological analysis have revealed that Cav3.2 is the main channel isoform generating the T-type current.131 Whole exome sequencing of patients with PA has identified several germline mutations in CACNA1H. Despite an incomplete penetrance, these variants often segregate with the disease132–134 (figure 2). Heterologous expression of the mutated Cav3.2 channels in HEK-293 cells revealed several alterations of the gating of the channel generally consistent with a gain-of-channel function (table 1). In addition, potassium-induced aldosterone secretion is enhanced in several aldosterone-producing adrenocortical cell lines expressing Cav3.2 variants.133 134 This effect may be attributed to a direct potentiation of aldosterone release and/or to an augmented aldosterone production since an increased expression of genes encoding for enzymes involved in the metabolism of aldosterone was also observed in cells expressing Cav3.2 variants. It is important to note that in contrast to CACNA1D mutations that are often associated with severe neurodevelopmental and endocrine disorders,135 136 PA-associated CACNA1H mutations are typically not accompanied with other conditions.
CACNA1G (Cav3.1) channelopathies
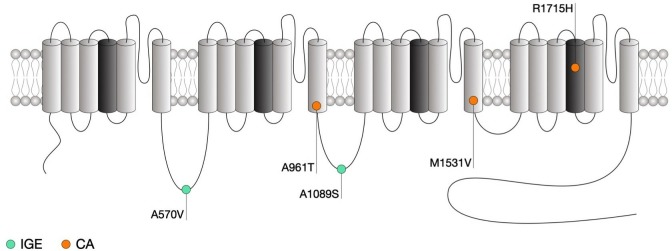
CACNA1G has been associated with both cerebellar ataxia and epilepsy. Cerebellar ataxias are clinically heterogenous disorders affecting the cerebellum and cerebellar pathways resulting in impaired coordination. While non-genetic ataxias are caused by acquired conditions or sporadic neurodegenerative disorders, several genes have been associated with hereditary cerebellar ataxias where ion channels are largely represented.137 Among these genes, CACNA1G encoding for the Cav3.1 T-type channel has emerged as a potential contributor and whole exosome sequencing identified a common R1715H variant that segregates in several families with autosomal dominant cerebellar ataxia.138–141 This mutation is located in the IVS4 voltage sensing region of Cav3.1 (figure 3). Consistent with the notion that positively charged residues within the voltage sensing region of the channel are essential for the gating, electrophysiological analysis of the mutant Cav3.1 channel in HEK-293 cells revealed a variety of alterations consistent with a loss-of-channel function (table 1), which is corroborated by computer simulations in deep cerebellar nuclei neurons that suggest a decreased neuronal excitability. Importantly, altered T-type currents were also confirmed in iPSC-derived Purkinje cells from patient carrying the R1715H variant.139 Gain-of-function mutations in CACNA1G have also been identified in patients with childhood-onset cerebellar atrophy.142 Three subjects showed an A961T variant, and one patient had an M1531V substitution. Both of these mutations induced gains-of-function by impairing the inactivation of the channel. A recent case report has implicated CACNA1G mutations in spinocerebellar ataxia type 42. An M1574L mutation was found in three patients from a Chinese family. In addition to ataxia, the clinical phenotype included cerebellar atrophy and brainstem defects.143
Figure 3.
Location of CACNA1G mutations within the secondary structure of Cav3.1 along with their associated syndromes. Only mutations that have been functionally characterised are indicated. Protein reference: UniProt O43497. CA, cerebellar ataxia; IDCN, deep cerebellar nuclei; GE, idiopathic generalised epilepsy; TRN, thalamic reticular nucleus.
Mutations in Cav3.1 have been associated with the development of epilepsy. In a cohort of 123 patients with IGEs, 13 CACNA1G variants were identified, including five that led to amino acid substitutions.144 In this study, an A570V substitution was found in a sporadic case of juvenile myoclonic epilepsy, as was an A1089S substitution that was detected in three family members. A biophysical analysis of the biophysical consequences of these mutations did not identify statistically significant effects. Interestingly, it has recently been reported that the CACNA1G gene can act as a modifier of Dravet syndrome induced by defects in the sodium channel Nav1.2,145 146 suggesting that previously identified CACNA1G mutations may also interact phenotypically with other genes, rather than being pathogenic per se.
CACNA1I (Cav3.3) channelopathies
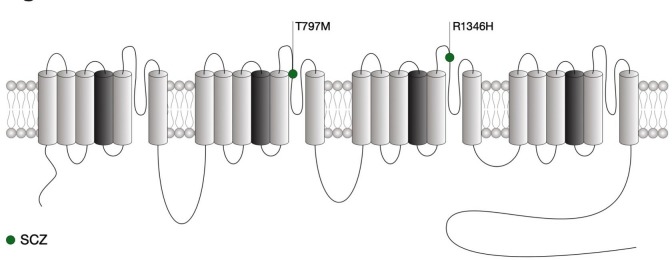
As for CACNA1G, the channelopathies associated with CACNA1I are yet limited and only recently two de novo missense variations were identified in individual with schizophrenia,147 a psychiatric disorder for which the genetic includes a variety of common and rare variants.148 Both mutations are located in the pore-forming region of Cav3.3 (figure 4). While these two variants had no biophysical effect, the A1346H mutation caused a significant decrease of the expression of the channel, possibly by altering glycosylation of Cav3.3.149 In contrast, the T797M variant had no impact on the channel (table 1). Computer simulations in thalamic reticular nucleus neurons also support a decreased neuronal excitability caused by schizophrenia-associated R1346H mutation. In contrast with Cav3.1 and Cav3.2, mutations in Cav3.3 have so far not been associated with epilepsy.150
Figure 4.
Location of CACNA1I mutations within the secondary structure of Cav3.3 along with their associated syndromes. Only mutations that have been functionally characterised are indicated. Protein reference: UniProt Q9P0×4. SCZ, schizophrenia.
Functional prediction of Cav3 mutations
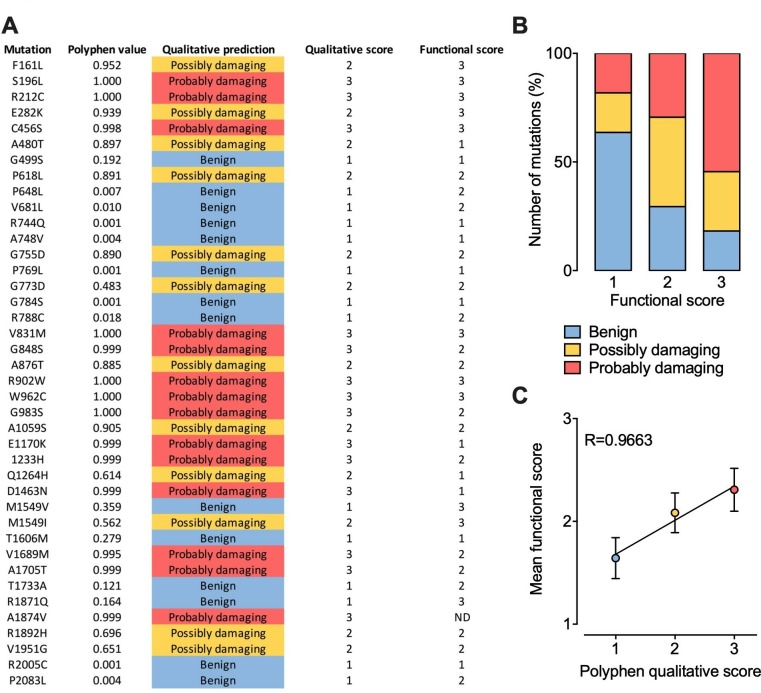
Although the effect of several Cav3 mutations on the functioning of the channel has already been characterised, hundreds of additional variants have yet to be analysed. The question then arises as to whether we could predict their functional impact. Therefore, we collected all functional data currently available for Cav3.2 in order to attribute a functional score for each mutation. The functional score was established as follow: score 1 for mutations producing less than a 2 mV alteration of the voltage-dependence of activation or inactivation or less than a 20% alteration of the kinetics (activation, inaction, recovery from inactivation) or current density compared with the wild type channel; score 2 for mutations producing a between 2 mV and 5 mV alteration of the voltage-dependence or a between 20% and 50% alteration of the kinetics or current density; score 3 for mutations producing more than a 5 mV alteration of the voltage-dependence or more than a 50% alteration of the kinetics or current density. Because functional analyses are often performed using different experimental conditions such as the concentration and nature of the permeating cation which directly affect the gating properties of the channel, the functional score attributed for each mutation is based on the relative biophysical effect of the mutation compared with the gating properties of the wild type channel recorded in the same experimental settings. In parallel, each mutation was probed using PolyPhen-2 algorithm151 that predicts the possible impact of an amino acid substitution on the structure and function of a human protein, providing a qualitative score 1 for benign, 2 for possibly damaging and 3 for probably damaging) (figure 5A). Qualitatively, we observed that higher PolyPhen scores tend to associated with higher functional scores, although a small fraction of mutations predicted to be damaging are not associated with functional alteration and vice versa (figure 5B). When plotting the average functional scores against Polyphen predicted scores, we observed a very strong correlation (R=0.9663), suggesting that in average it may be possible to predict to which extent a mutation might alter the functioning of the channel with a relative degree of certainty (figure 5C).
Figure 5.
Prediction of CACNA1H variants on Cav3.2 channel gating. (A) Summary of PolyPhen-2 qualitative prediction and functional experimental score for each Cav3.2 mutations characterised experimentally. (B) Chart representing the proportion of Cav3.2 variants according to Polyphen-2 prediction as a function of their functional score. (C) Plot of the mean functional score as a function of Polyphen-2 prediction.
Conclusion and perspectives
A number of genetic association studies have identified variations in the genes encoding different T-type channels and associated with several neuronal, neuroendocrine and psychiatric syndromes. However, because of the absence of traditional segregation patterns in families in part due to reduced penetrance in adults with the absence of large multiplex families, de novo mutations and/or variable expressivity, many Mendelian traits are likely overlooked. In addition, functional analysis of Cav3 mutants indicate that these mutations generally produce mild alterations of the channel activity, which may be interpreted as a weak evidence of the implication of the channel in the disease. However, several considerations need to be made. First, functional studies are largely predominated by the use of heterologous expression systems that may not entirely reflect the extent to which the mutations affect the functioning of the channel. For instance, although the cellular and physiological aspects controlled by T-type channels are largely dependent on their intrinsic gating properties, recent studies have shown that T-type channels are far more complex than anticipated in terms of their regulation and association with other signalling molecules. The impact of the mutations on these aspects is likely to be overlooked in heterologous expression systems. Second, several Cav3 splice variants have been documented. Therefore, it is worth considering the possibility that the functional expression of the mutations may vary depending on the channel splice variant in which it is reintroduced. For instance, as noted earlier, this notion is supported by the observation that the biophysical expression of the GAERS mutation depends on the Cav3.2 splice variant used.23 Third, it is currently complicated to fully apprehend the long-term impact of small alterations of the channel gating on neurodevelopmental aspects which may have an important impact on the pathogenesis of the disease. Indeed, in addition to their role in neuronal excitability, T-type channels also contribute to several developmental aspects. Finally, the observation that T-type channel mutations are not confined to a particular structural determinant but are rather scattered across the entire protein highlight the need for additional structure-function relationship studies. It is also important to note that mutations in a given gene and producing virtually identical biophysical alterations can lead to different disorders typically without overlap between the diseases. This is, for instance, the case for CACNA1H mutations where a gain-of-function phonotype leads to PA without conferring other disease risk, while similar gain-of-function mutations are associated with increased risk for IGE without concomitant endocrine disorders. This phenotypic heterogeneity suggests that several additional factors such as modifier genes, environmental aspects, allelic variations or complex genetic and environmental interactions are likely to contribute to penetrance and expressivity of these mutations.152
In addition to disorders for which T-type channels have already been implicated, it is likely that other disorders could be caused or influenced by mutations or polymorphisms in T-type channel genes. Indeed, besides being expressed in electrically excitable tissues, T-type channels are also present in several non-excitable cells. For instance, Cav3.1 channels are functionally expressed in immune T cells where they shape the immune response.5 6 T-type channels are also expressed in sperm cells where they participate to the fertilisation of the egg.4 Similarly, T-type channels contribute to calcium signalling in osteocytes.3 This suggests that additional human T-type channelopathies might exist. An important step forward in our understanding of T-type channelopathies will be the identification of modifier genes that are likely to play an important role in the phenotypic expressivity of T-type channel variants.
Footnotes
Contributors: NW and GWZ analysed the literature and wrote the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Ertel EA, Campbell KP, Harpold MM, Hofmann F, Mori Y, Perez-Reyes E, Schwartz A, Snutch TP, Tanabe T, Birnbaumer L, Tsien RW, Catterall WA. Nomenclature of voltage-gated calcium channels. Neuron 2000;25:533–5. 10.1016/S0896-6273(00)81057-0 [DOI] [PubMed] [Google Scholar]
- 2. Perez-Reyes E. Molecular physiology of low-voltage-activated T-type calcium channels. Physiol Rev 2003;83:117–61. 10.1152/physrev.00018.2002 [DOI] [PubMed] [Google Scholar]
- 3. Brown GN, Leong PL, Guo XE. T-type voltage-sensitive calcium channels mediate mechanically-induced intracellular calcium oscillations in osteocytes by regulating endoplasmic reticulum calcium dynamics. Bone 2016;88:56–63. 10.1016/j.bone.2016.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arnoult C, Cardullo RA, Lemos JR, Florman HM. Activation of mouse sperm T-type Ca2+ channels by adhesion to the egg zona pellucida. Proc Natl Acad Sci U S A 1996;93:13004–9. 10.1073/pnas.93.23.13004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang H, Zhang X, Xue L, Xing J, Jouvin M-H, Putney JW, Anderson MP, Trebak M, Kinet J-P. Low-voltage-activated CaV3.1 calcium channels shape T helper cell cytokine profiles. Immunity 2016;44:782–94. 10.1016/j.immuni.2016.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lacinova L, Weiss N. It takes two T to shape immunity: emerging role for T-type calcium channels in immune cells. Gen Physiol Biophys 2016;35:393–6. 10.4149/gpb_2016034 [DOI] [PubMed] [Google Scholar]
- 7. Huguenard JR, Prince DA. A novel T-type current underlies prolonged Ca(2+)-dependent burst firing in GABAergic neurons of rat thalamic reticular nucleus. J Neurosci 1992;12:3804–17. 10.1523/JNEUROSCI.12-10-03804.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Molineux ML, McRory JE, McKay BE, Hamid J, Mehaffey WH, Rehak R, Snutch TP, Zamponi GW, Turner RW. Specific T-type calcium channel isoforms are associated with distinct burst phenotypes in deep cerebellar nuclear neurons. Proc Natl Acad Sci U S A 2006;103:5555–60. 10.1073/pnas.0601261103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cain SM, Snutch TP. Contributions of T-type calcium channel isoforms to neuronal firing. Channels 2010;4:475–82. 10.4161/chan.4.6.14106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weiss N, Zamponi GW. Control of low-threshold exocytosis by T-type calcium channels. Biochim Biophys Acta 1828;2013:1579–86. [DOI] [PubMed] [Google Scholar]
- 11. Carbone E, Calorio C, Vandael DHF. T-type channel-mediated neurotransmitter release. Pflugers Arch - Eur J Physiol 2014;466:677–87. 10.1007/s00424-014-1489-z [DOI] [PubMed] [Google Scholar]
- 12. Perez-Reyes E, Cribbs LL, Daud A, Lacerda AE, Barclay J, Williamson MP, Fox M, Rees M, Lee J-H. Molecular characterization of a neuronal low-voltage-activated T-type calcium channel. Nature 1998;391:896–900. 10.1038/36110 [DOI] [PubMed] [Google Scholar]
- 13. Cribbs LL, Lee JH, Yang J, Satin J, Zhang Y, Daud A, Barclay J, Williamson MP, Fox M, Rees M, Perez-Reyes E. Cloning and characterization of alpha1H from human heart, a member of the T-type Ca2+ channel gene family. Circ Res 1998;83:103–9. 10.1161/01.RES.83.1.103 [DOI] [PubMed] [Google Scholar]
- 14. Lee JH, Daud AN, Cribbs LL, Lacerda AE, Pereverzev A, Klöckner U, Schneider T, Perez-Reyes E. Cloning and expression of a novel member of the low voltage-activated T-type calcium channel family. J Neurosci 1999;19:1912–21. 10.1523/JNEUROSCI.19-06-01912.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shcheglovitov A, Vitko I, Bidaud I, Baumgart JP, Navarro-Gonzalez MF, Grayson TH, Lory P, Hill CE, Perez-Reyes E. Alternative splicing within the I-II loop controls surface expression of T-type Ca(v)3.1 calcium channels. FEBS Lett 2008;582:3765–70. 10.1016/j.febslet.2008.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nie L, Zhu J, Gratton MA, Liao A, Mu KJ, Nonner W, Richardson GP, Yamoah EN. Molecular identity and functional properties of a novel T-type Ca2+ channel cloned from the sensory epithelia of the mouse inner ear. J Neurophysiol 2008;100:2287–99. 10.1152/jn.90707.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chemin J, Monteil A, Bourinet E, Nargeot J, Lory P, alpha Aspliced. Alternatively spliced alpha(1G) (Ca(V)3.1) intracellular loops promote specific T-type Ca(2+) channel gating properties. Biophys J 2001;80:1238–50. 10.1016/S0006-3495(01)76100-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Latour I, Louw DF, Beedle AM, Hamid J, Sutherland GR, Zamponi GW. Expression of T-type calcium channel splice variants in human glioma. Glia 2004;48:112–9. 10.1002/glia.20063 [DOI] [PubMed] [Google Scholar]
- 19. Bertolesi GE, Walia Da Silva R, Jollimore CAB, Shi C, Barnes S, Kelly MEM, Ca KME. CaV3.1 splice variant expression during neuronal differentiation of Y-79 retinoblastoma cells. Neuroscience 2006;141:259–68. 10.1016/j.neuroscience.2006.03.067 [DOI] [PubMed] [Google Scholar]
- 20. Ohkubo T, Inoue Y, Kawarabayashi T, Kitamura K. Identification and Electrophysiological Characteristics of Isoforms of T-type Calcium Channel Ca<sub>v</sub>3.2 Expressed in Pregnant Human Uterus. Cell Physiol Biochem 2005;16:245–54. 10.1159/000089850 [DOI] [PubMed] [Google Scholar]
- 21. Zhong X, Liu JR, Kyle JW, Hanck DA, Agnew WS. A profile of alternative RNA splicing and transcript variation of CACNA1H, a human T-channel gene candidate for idiopathic generalized epilepsies. Hum Mol Genet 2006;15:1497–512. 10.1093/hmg/ddl068 [DOI] [PubMed] [Google Scholar]
- 22. Swayne LA, Bourinet E. Voltage-gated calcium channels in chronic pain: emerging role of alternative splicing. Pflugers Arch - Eur J Physiol 2008;456:459–66. 10.1007/s00424-007-0390-4 [DOI] [PubMed] [Google Scholar]
- 23. Powell KL, Cain SM, Ng C, Sirdesai S, David LS, Kyi M, Garcia E, Tyson JR, Reid CA, Bahlo M, Foote SJ, Snutch TP, O'Brien TJ. A Cav3.2 T-type calcium channel point mutation has splice-variant-specific effects on function and segregates with seizure expression in a polygenic rat model of absence epilepsy. J Neurosci 2009;29:371–80. 10.1523/JNEUROSCI.5295-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. David LS, Garcia E, Cain SM, Thau E, Tyson JR, Snutch TP. Splice-variant changes of the Ca(V)3.2 T-type calcium channel mediate voltage-dependent facilitation and associate with cardiac hypertrophy and development. Channels 2010;4:375–89. 10.4161/chan.4.5.12874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Murbartián J, Arias JM, Lee J-H, Gomora JC, Perez-Reyes E. Alternative splicing of the rat Ca(v)3.3 T-type calcium channel gene produces variants with distinct functional properties(1). FEBS Lett 2002;528:272–8. 10.1016/S0014-5793(02)03341-0 [DOI] [PubMed] [Google Scholar]
- 26. Murbartián J, Arias JM, Perez-Reyes E. Functional impact of alternative splicing of human T-type Cav3.3 calcium channels. J Neurophysiol 2004;92:3399–407. 10.1152/jn.00498.2004 [DOI] [PubMed] [Google Scholar]
- 27. Zamponi GW, Striessnig J, Koschak A, Dolphin AC. The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol Rev 2015;67:821–70. 10.1124/pr.114.009654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jurkovicova-Tarabova B, Mackova K, Moravcikova L, Karmazinova M, Lacinova L. Role of individual S4 segments in gating of Cav3.1 T-type calcium channel by voltage. Channels 2018;12:378–87. 10.1080/19336950.2018.1543520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wolfe JT, Wang H, Howard J, Garrison JC, Barrett PQ. T-type calcium channel regulation by specific G-protein βγ subunits. Nature 2003;424:209–13. 10.1038/nature01772 [DOI] [PubMed] [Google Scholar]
- 30. DePuy SD, Yao J, Hu C, McIntire W, Bidaud I, Lory P, Rastinejad F, Gonzalez C, Garrison JC, Barrett PQ. The molecular basis for T-type Ca2+ channel inhibition by G protein beta2gamma2 subunits. Proc Natl Acad Sci U S A 2006;103:14590–5. 10.1073/pnas.0603945103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Welsby PJ, Wang H, Wolfe JT, Colbran RJ, Johnson ML, Barrett PQ. A Mechanism for the Direct Regulation of T-Type Calcium Channels by Ca 2+ /Calmodulin-Dependent Kinase II. J. Neurosci. 2003;23:10116–21. 10.1523/JNEUROSCI.23-31-10116.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Asmara H, Micu I, Rizwan AP, Sahu G, Simms BA, Zhang F-X, Engbers JDT, Stys PK, Zamponi GW, Turner RW. A T-type channel-calmodulin complex triggers αCaMKII activation. Mol Brain 2017;10 10.1186/s13041-017-0317-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aromolaran KA, Benzow KA, Cribbs LL, Koob MD, Piedras-Rentería ES. T-type current modulation by the actin-binding protein Kelch-like 1. Am J Physiol Cell Physiol 2010;298:C1353–C1362. 10.1152/ajpcell.00235.2009 [DOI] [PubMed] [Google Scholar]
- 34. Huang C-H, Chen Y-C, Chen C-C. Physical interaction between calcineurin and Cav3.2 T-type Ca2+ channel modulates their functions. FEBS Lett 2013;587:1723–30. 10.1016/j.febslet.2013.04.040 [DOI] [PubMed] [Google Scholar]
- 35. Weiss N, Hameed S, Fernández-Fernández JM, Fablet K, Karmazinova M, Poillot C, Proft J, Chen L, Bidaud I, Monteil A, Huc-Brandt S, Lacinova L, Lory P, Zamponi GW, De Waard M, A Ca DWM. A Ca(v)3.2/syntaxin-1A signaling complex controls T-type channel activity and low-threshold exocytosis. J Biol Chem 2012;287:2810–8. 10.1074/jbc.M111.290882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rzhepetskyy Y, Lazniewska J, Proft J, Campiglio M, Flucher BE, Weiss N. A Ca v 3.2/Stac1 molecular complex controls T-type channel expression at the plasma membrane. Channels 2016;10:346–54. 10.1080/19336950.2016.1186318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cottrell GS, Soubrane CH, Hounshell JA, Lin H, Owenson V, Rigby M, Cox PJ, Barker BS, Ottolini M, Ince S, Bauer CC, Perez-Reyes E, Patel MK, Stevens EB, Stephens GJ. CACHD1 is an α2δ-Like Protein That Modulates CaV3 Voltage-Gated Calcium Channel Activity. J Neurosci 2018;38:9186–201. 10.1523/JNEUROSCI.3572-15.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stephens GJ, Cottrell GS. CACHD1: a new activity-modifying protein for voltage-gated calcium channels. Channels 2019;13:120–3. 10.1080/19336950.2019.1600968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Garcia-Caballero A, Zhang F-X, Hodgkinson V, Huang J, Chen L, Souza IA, Cain S, Kass J, Alles S, Snutch TP, Zamponi GW. T-type calcium channels functionally interact with spectrin (α/β) and ankyrin B. Mol Brain 2018;11 10.1186/s13041-018-0368-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Turner RW, Zamponi GW. T-type channels buddy up. Pflugers Arch - Eur J Physiol 2014;466:661–75. 10.1007/s00424-013-1434-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Garcia-Caballero A, Gandini MA, Huang S, Chen L, Souza IA, Dang YL, Stutts MJ, Zamponi GW. Cav3.2 calcium channel interactions with the epithelial sodium channel ENaC. Mol Brain 2019;12 10.1186/s13041-019-0433-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Blesneac I, Chemin J, Bidaud I, Huc-Brandt S, Vandermoere F, Lory P. Phosphorylation of the Cav3.2 T-type calcium channel directly regulates its gating properties. Proc Natl Acad Sci USA 2015;112:13705–10. 10.1073/pnas.1511740112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. García-Caballero A, Gadotti VM, Stemkowski P, Weiss N, Souza IA, Hodgkinson V, Bladen C, Chen L, Hamid J, Pizzoccaro A, Deage M, François A, Bourinet E, Zamponi GW. The deubiquitinating enzyme USP5 modulates neuropathic and inflammatory pain by enhancing Cav3.2 channel activity. Neuron 2014;83:1144–58. 10.1016/j.neuron.2014.07.036 [DOI] [PubMed] [Google Scholar]
- 44. Weiss N, Black SAG, Bladen C, Chen L, Zamponi GW. Surface expression and function of Cav3.2 T-type calcium channels are controlled by asparagine-linked glycosylation. Pflugers Arch - Eur J Physiol 2013;465:1159–70. 10.1007/s00424-013-1259-3 [DOI] [PubMed] [Google Scholar]
- 45. Orestes P, Osuru HP, McIntire WE, Jacus MO, Salajegheh R, Jagodic MM, Choe W, Lee J, Lee S-S, Rose KE, Poiro N, DiGruccio MR, Krishnan K, Covey DF, Lee J-H, Barrett PQ, Jevtovic-Todorovic V, Todorovic SM. Reversal of neuropathic pain in diabetes by targeting glycosylation of Cav3.2 T-type calcium channels. Diabetes 2013;62:3828–38. 10.2337/db13-0813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lazniewska J, Weiss N. The "sweet" side of ion channels. Rev Physiol Biochem Pharmacol 2014;167:67–114. 10.1007/112_2014_20 [DOI] [PubMed] [Google Scholar]
- 47. Lazniewska J, Rzhepetskyy Y, Zhang F-X, Zamponi GW, Weiss N. Cooperative roles of glucose and asparagine-linked glycosylation in T-type calcium channel expression. Pflugers Arch - Eur J Physiol 2016;468:1837–51. 10.1007/s00424-016-1881-y [DOI] [PubMed] [Google Scholar]
- 48. Ondacova K, Karmazinova M, Lazniewska J, Weiss N, Lacinova L. Modulation of Ca v 3.2 T-type calcium channel permeability by asparagine-linked glycosylation. Channels 2016;10:175–84. 10.1080/19336950.2016.1138189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lazniewska J, Weiss N. Glycosylation of voltage-gated calcium channels in health and disease. Biochim Biophys Acta Biomembr 2017;1859:662–8. 10.1016/j.bbamem.2017.01.018 [DOI] [PubMed] [Google Scholar]
- 50. Crandall SR, Govindaiah G, Cox CL. Low-threshold Ca2+ current amplifies distal dendritic signaling in thalamic reticular neurons. Journal of Neuroscience 2010;30:15419–29. 10.1523/JNEUROSCI.3636-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Anderson D, Mehaffey WH, Iftinca M, Rehak R, Engbers JDT, Hameed S, Zamponi GW, Turner RW. Regulation of neuronal activity by Cav3-Kv4 channel signaling complexes. Nat Neurosci 2010;13:333–7. 10.1038/nn.2493 [DOI] [PubMed] [Google Scholar]
- 52. Anderson D, Rehak R, Hameed S, Mehaffey WH, Zamponi GW, Turner RW. Regulation of the Kv4.2 complex by CaV3.1 calcium channels. Channels 2010;4:163–7. 10.4161/chan.4.3.11955 [DOI] [PubMed] [Google Scholar]
- 53. Engbers JDT, Anderson D, Asmara H, Rehak R, Mehaffey WH, Hameed S, McKay BE, Kruskic M, Zamponi GW, Turner RW. Intermediate conductance calcium-activated potassium channels modulate summation of parallel fiber input in cerebellar Purkinje cells. Proceedings of the National Academy of Sciences 2012;109:2601–6. 10.1073/pnas.1115024109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Anderson D, Engbers JDT, Heath NC, Bartoletti TM, Mehaffey WH, Zamponi GW, Turner RW. The Cav3-Kv4 complex acts as a calcium sensor to maintain inhibitory charge transfer during extracellular calcium fluctuations. Journal of Neuroscience 2013;33:7811–24. 10.1523/JNEUROSCI.5384-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rehak R, Bartoletti TM, Engbers JDT, Berecki G, Turner RW, Zamponi GW. Low voltage activation of KCa1.1 current by Cav3-KCa1.1 complexes. PLoS One 2013;8:e61844 10.1371/journal.pone.0061844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Crunelli V, Cope D, Hughes S. Thalamic T-type Ca2+ channels and NREM sleep. Cell Calcium 2006;40:175–90. 10.1016/j.ceca.2006.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bal T, McCormick DA. Synchronized Oscillations in the Inferior Olive Are Controlled by the Hyperpolarization-Activated Cation Current i>Ii> h . J Neurophysiol 1997;77:3145–56. 10.1152/jn.1997.77.6.3145 [DOI] [PubMed] [Google Scholar]
- 58. Beurrier C, Congar P, Bioulac B, Hammond C. Subthalamic nucleus neurons switch from single-spike activity to burst-firing mode. J. Neurosci. 1999;19:599–609. 10.1523/JNEUROSCI.19-02-00599.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cain SM, Snutch TP. T-type calcium channels in burst-firing, network synchrony, and epilepsy. Biochim Biophys Acta 2013;1828:1572–8. 10.1016/j.bbamem.2012.07.028 [DOI] [PubMed] [Google Scholar]
- 60. Crunelli V, Tóth TI, Cope DW, Blethyn K, Hughes SW. The ‘window’ T-type calcium current in brain dynamics of different behavioural states. J Physiol 2005;562:121–9. 10.1113/jphysiol.2004.076273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Leresche N, Lambert RC. T-type calcium channels in synaptic plasticity. Channels 2017;11:121–39. 10.1080/19336950.2016.1238992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mangoni ME, Traboulsie A, Leoni A-L, Couette B, Marger L, Le Quang K, Kupfer E, Cohen-Solal A, Vilar J, Shin H-S, Escande D, Charpentier F, Nargeot J, Lory P, Vilar J, Nargeot J. Bradycardia and slowing of the atrioventricular conduction in mice lacking CaV3.1/alpha1G T-type calcium channels. Circ Res 2006;98:1422–30. 10.1161/01.RES.0000225862.14314.49 [DOI] [PubMed] [Google Scholar]
- 63. Choi S, Yu E, Hwang E, Llinás RR. Pathophysiological implication of Ca V 3.1 T-type Ca 2+ channels in trigeminal neuropathic pain. Proc Natl Acad Sci USA 2016;113:2270–5. 10.1073/pnas.1600418113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Na HS, Choi S, Kim J, Park J, Shin H-S. Attenuated neuropathic pain in CaV3.1 null mice. Mol Cells 2008;25:242–6. [PubMed] [Google Scholar]
- 65. Thuesen AD, Andersen K, Lyngsø KS, Burton M, Brasch-Andersen C, Vanhoutte PM, Hansen PBL. Deletion of T-type calcium channels CaV3.1 or Pflugers Arch. Eur J Physiol 2018;470:355–65. 10.1007/s00424-017-2068-x [DOI] [PubMed] [Google Scholar]
- 66. Chen C-C, Lamping KG, Nuno DW, Barresi R, Prouty SJ, Lavoie JL, Cribbs LL, England SK, Sigmund CD, Weiss RM, Williamson RA, Hill JA, Campbell KP. Abnormal coronary function in mice deficient in alpha1H T-type Ca2+ channels. Science 2003;302:1416–8. 10.1126/science.1089268 [DOI] [PubMed] [Google Scholar]
- 67. Hansen PBL. Functional importance of T-type voltage-gated calcium channels in the cardiovascular and renal system: news from the world of knockout mice. Am J Physiol Regul Integr Comp Physiol 2015;308:R227–R237. 10.1152/ajpregu.00276.2014 [DOI] [PubMed] [Google Scholar]
- 68. Chiang C-S, Huang C-H, Chieng H, Chang Y-T, Chang D, Chen J-J, Chen Y-C, Chen Y-H, Shin H-S, Campbell KP, Chen C-C. The Ca(v)3.2 T-type Ca(2+) channel is required for pressure overload-induced cardiac hypertrophy in mice. Circ Res 2009;104:522–30. 10.1161/CIRCRESAHA.108.184051 [DOI] [PubMed] [Google Scholar]
- 69. Kim D, Song I, Keum S, Lee T, Jeong M-J, Kim S-S, McEnery MW, Shin H-S. Lack of the burst firing of thalamocortical relay neurons and resistance to absence seizures in mice lacking α1G T-type Ca2+ channels. Neuron 2001;31:35–45. 10.1016/S0896-6273(01)00343-9 [DOI] [PubMed] [Google Scholar]
- 70. Choi S, Na HS, Kim J, Lee J, Lee S, Kim D, Park J, Chen C-C, Campbell KP, Shin H-S. Attenuated pain responses in mice lacking Ca(V)3.2 T-type channels. Genes Brain Behav 2007;6:425–31. 10.1111/j.1601-183X.2006.00268.x [DOI] [PubMed] [Google Scholar]
- 71. Chen C-C, Shen J-W, Chung N-C, Min M-Y, Cheng S-J, Liu IY. Retrieval of Context-Associated memory is dependent on the Cav3.2 T-type calcium channel. PLoS ONE 2012;7:e29384 10.1371/journal.pone.0029384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gangarossa G, Laffray S, Bourinet E, Valjent E. T-type calcium channel Cav3.2 deficient mice show elevated anxiety, impaired memory and reduced sensitivity to psychostimulants. Front Behav Neurosci 2014;8 10.3389/fnbeh.2014.00092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Astori S, Wimmer RD, Prosser HM, Corti C, Corsi M, Liaudet N, Volterra A, Franken P, Adelman JP, Lüthi A. The Ca(V)3.3 calcium channel is the major sleep spindle pacemaker in thalamus. Proc Natl Acad Sci U S A 2011;108:13823–8. 10.1073/pnas.1105115108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Pellegrini C, Lecci S, Lüthi A, Astori S. Suppression of sleep spindle rhythmogenesis in mice with deletion of Cav3.2 and Cav3.3 T-type Ca2+ channels. Sleep 2016;39:875–85. 10.5665/sleep.5646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jensen LJ, Nielsen MS, Salomonsson M, Sørensen CM. T-type Ca 2+ channels and autoregulation of local blood flow. Channels 2017;11:183–95. 10.1080/19336950.2016.1273997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Destexhe A, Neubig M, Ulrich D, Huguenard J. Dendritic low-threshold calcium currents in thalamic relay cells. J Neurosci 1998;18:3574–88. 10.1523/JNEUROSCI.18-10-03574.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Huguenard JR. Low-threshold calcium currents in central nervous system neurons. Annu Rev Physiol 1996;58:329–48. 10.1146/annurev.ph.58.030196.001553 [DOI] [PubMed] [Google Scholar]
- 78. Huguenard JR, McCormick DA. Simulation of the currents involved in rhythmic oscillations in thalamic relay neurons. J Neurophysiol 1992;68:1373–83. 10.1152/jn.1992.68.4.1373 [DOI] [PubMed] [Google Scholar]
- 79. Huguenard JR. Block of T -Type Ca(2+) Channels Is an Important Action of Succinimide Antiabsence Drugs. Epilepsy Curr 2002;2:49–52. 10.1046/j.1535-7597.2002.00019.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Khosravani H, Zamponi GW. Voltage-gated calcium channels and idiopathic generalized epilepsies. Physiol Rev 2006;86:941–66. 10.1152/physrev.00002.2006 [DOI] [PubMed] [Google Scholar]
- 81. Zamponi GW. Targeting voltage-gated calcium channels in neurological and psychiatric diseases. Nat Rev Drug Discov 2016;15:19–34. 10.1038/nrd.2015.5 [DOI] [PubMed] [Google Scholar]
- 82. Cain SM, Tyson JR, Choi H-B, Ko R, Lin PJC, LeDue JM, Powell KL, Bernier L-P, Rungta RL, Yang Y, Cullis PR, O'Brien TJ, MacVicar BA, Snutch TP. Ca V 3.2 drives sustained burst-firing, which is critical for absence seizure propagation in reticular thalamic neurons. Epilepsia 2018;59:778–91. 10.1111/epi.14018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tsakiridou E, Bertollini L, de Curtis M, Avanzini G, Pape HC. Selective increase in T-type calcium conductance of reticular thalamic neurons in a rat model of absence epilepsy. J Neurosci 1995;15:3110–7. 10.1523/JNEUROSCI.15-04-03110.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zhang Y, Mori M, Burgess DL, Noebels JL. Mutations in high-voltage-activated calcium channel genes stimulate low-voltage-activated currents in mouse thalamic relay neurons. J Neurosci 2002;22:6362–71. 10.1523/JNEUROSCI.22-15-06362.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zhang Y, Vilaythong AP, Yoshor D, Noebels JL. Elevated thalamic low-voltage-activated currents precede the onset of absence epilepsy in the SNAP25-deficient mouse mutant coloboma. J Neurosci 2004;24:5239–48. 10.1523/JNEUROSCI.0992-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ernst WL, Zhang Y, Yoo JW, Ernst SJ, Noebels JL. Genetic enhancement of thalamocortical network activity by elevating alpha 1g-mediated low-voltage-activated calcium current induces pure absence epilepsy. J Neurosci 2009;29:1615–25. 10.1523/JNEUROSCI.2081-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Tringham E, Powell KL, Cain SM, Kuplast K, Mezeyova J, Weerapura M, Eduljee C, Jiang X, Smith P, Morrison J-L, Jones NC, Braine E, Rind G, Fee-Maki M, Parker D, Pajouhesh H, Parmar M, O'Brien TJ, Snutch TP. T-type calcium channel blockers that attenuate thalamic burst firing and suppress absence seizures. Science Translational Medicine 2012;4 10.1126/scitranslmed.3003120 [DOI] [PubMed] [Google Scholar]
- 88. Powell KL, Cain SM, Snutch TP, O'Brien TJ. Low threshold T-type calcium channels as targets for novel epilepsy treatments. Br J Clin Pharmacol 2014;77:729–39. 10.1111/bcp.12205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Casillas-Espinosa PM, Hicks A, Jeffreys A, Snutch TP, O’Brien TJ, Powell KL. Z944, a novel selective T-type calcium channel antagonist delays the progression of seizures in the amygdala kindling model. Plos One 2015;10:e0130012 10.1371/journal.pone.0130012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Capovilla G, Beccaria F, Veggiotti P, Rubboli G, Meletti S, Tassinari CA. Ethosuximide is effective in the treatment of epileptic negative myoclonus in childhood partial epilepsy. J Child Neurol 1999;14:395–400. 10.1177/088307389901400609 [DOI] [PubMed] [Google Scholar]
- 91. Mattson RH, Cramer JA, Williamson PD, Novelly RA. Valproic acid in epilepsy: clinical and pharmacological effects. Ann Neurol. 1978;3:20–5. 10.1002/ana.410030105 [DOI] [PubMed] [Google Scholar]
- 92. Kwan S-Y, Chuang Y-C, Huang C-W, Chen T-C, Jou S-B, Dash A. Zonisamide: review of recent clinical evidence for treatment of epilepsy. CNS Neurosci Ther 2015;21:683–91. 10.1111/cns.12418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Chen Y, Lu J, Pan H, Zhang Y, Wu H, Xu K, Liu X, Jiang Y, Bao X, Yao Z, Ding K, Lo WHY, Qiang B, Chan P, Shen Y, Wu X. Association between genetic variation ofCACNA1H and childhood absence epilepsy. Ann Neurol. 2003;54:239–43. 10.1002/ana.10607 [DOI] [PubMed] [Google Scholar]
- 94. Heron SE, Phillips HA, Mulley JC, Mazarib A, Neufeld MY, Berkovic SF, Scheffer IE. Genetic variation ofCACNA1H in idiopathic generalized epilepsy. Ann Neurol. 2004;55:595–6. 10.1002/ana.20028 [DOI] [PubMed] [Google Scholar]
- 95. Heron SE, Khosravani H, Varela D, Bladen C, Williams TC, Newman MR, Scheffer IE, Berkovic SF, Mulley JC, Zamponi GW. Extended spectrum of idiopathic generalized epilepsies associated with CACNA1H functional variants. Ann Neurol. 2007;62:560–8. 10.1002/ana.21169 [DOI] [PubMed] [Google Scholar]
- 96. Liang J, Zhang Y, Wang J, Pan H, Wu H, Xu K, Liu X, Jiang Y, Shen Y, Wu X. New variants in the CACNA1H gene identified in childhood absence epilepsy. Neuroscience Letters 2006;406:27–32. 10.1016/j.neulet.2006.06.073 [DOI] [PubMed] [Google Scholar]
- 97. Liang J, Zhang Y, Chen Y, Wang J, Pan H, Wu H, Xu K, Liu X, Jiang Y, Shen Y, Wu X. Common Polymorphisms in the CACNA1H Gene Associated with Childhood Absence Epilepsy in Chinese Han Population. Ann Hum Genet 2007;71:325–35. 10.1111/j.1469-1809.2006.00332.x [DOI] [PubMed] [Google Scholar]
- 98. Chourasia N, Ossó-Rivera H, Ghosh A, Von Allmen G, Koenig MK. Expanding the phenotypic spectrum of CACNA1H mutations. Pediatric Neurology 2019;93:50–5. 10.1016/j.pediatrneurol.2018.11.017 [DOI] [PubMed] [Google Scholar]
- 99. Khosravani H, Altier C, Simms B, Hamming KS, Snutch TP, Mezeyova J, McRory JE, Zamponi GW. Gating Effects of Mutations in the Ca v 3.2 T-type Calcium Channel Associated with Childhood Absence Epilepsy. J Biol Chem 2004;279:9681–4. 10.1074/jbc.C400006200 [DOI] [PubMed] [Google Scholar]
- 100. Khosravani H, Bladen C, Parker DB, Snutch TP, McRory JE, Zamponi GW. Effects of Cav3.2 channel mutations linked to idiopathic generalized epilepsy. Ann Neurol 2005;57:745–9. 10.1002/ana.20458 [DOI] [PubMed] [Google Scholar]
- 101. Vitko I, Chen Y, Arias JM, Shen Y, Wu X-R, Perez-Reyes E. Functional characterization and neuronal modeling of the effects of childhood absence epilepsy variants of CACNA1H, a T-Type calcium channel. J Neurosci 2005;25:4844–55. 10.1523/JNEUROSCI.0847-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Peloquin JB, Khosravani H, Barr W, Bladen C, Evans R, Mezeyova J, Parker D, Snutch TP, McRory JE, Zamponi GW. Functional analysis of Ca3.2 T-type calcium channel mutations linked to childhood absence epilepsy. Epilepsia 2006;47:655–8. 10.1111/j.1528-1167.2006.00482.x [DOI] [PubMed] [Google Scholar]
- 103. Arias-Olguín II, Vitko I, Fortuna M, Baumgart JP, Sokolova S, Shumilin IA, Van Deusen A, Soriano-García M, Gomora JC, Perez-Reyes E. Characterization of the gating brake in the I-II loop of Ca(v)3.2 T-type Ca(2+) channels. J Biol Chem 2008;283:8136–44. 10.1074/jbc.M708761200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Baumgart JP, Vitko I, Bidaud I, Kondratskyi A, Lory P, Perez-Reyes E. I-II loop structural determinants in the gating and surface expression of low voltage-activated calcium channels. PLoS One 2008;3:e2976 10.1371/journal.pone.0002976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Eckle V-S, Shcheglovitov A, Vitko I, Dey D, Yap CC, Winckler B, Perez-Reyes E. Mechanisms by which a CACNA1H mutation in epilepsy patients increases seizure susceptibility. J Physiol 2014;592:795–809. 10.1113/jphysiol.2013.264176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Marescaux C, Micheletti G, Vergnes M, Depaulis A, Rumbach L, Warter JM. A model of chronic spontaneous petit mal-like seizures in the rat: comparison with pentylenetetrazol-induced seizures. Epilepsia 1984;25:326–31. 10.1111/j.1528-1157.1984.tb04196.x [DOI] [PubMed] [Google Scholar]
- 107. Proft J, Rzhepetskyy Y, Lazniewska J, Zhang F-X, Cain SM, Snutch TP, Zamponi GW, Weiss N. The Cacna1h mutation in the GAERS model of absence epilepsy enhances T-type Ca2+ currents by altering calnexin-dependent trafficking of Cav3.2 channels. Sci Rep 2017;7 10.1038/s41598-017-11591-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ramaswami G, Geschwind DH. Genetics of autism spectrum disorder. Handb Clin Neurol 2018;147:321–9. 10.1016/B978-0-444-63233-3.00021-X [DOI] [PubMed] [Google Scholar]
- 109. de la Torre-Ubieta L, Won H, Stein JL, Geschwind DH. Advancing the understanding of autism disease mechanisms through genetics. Nat Med 2016;22:345–61. 10.1038/nm.4071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Splawski I, Yoo DS, Stotz SC, Cherry A, Clapham DE, Keating MT. CACNA1H mutations in autism spectrum disorders. J Biol Chem 2006;281:22085–91. 10.1074/jbc.M603316200 [DOI] [PubMed] [Google Scholar]
- 111. Pinggera A, Lieb A, Benedetti B, Lampert M, Monteleone S, Liedl KR, Tuluc P, Striessnig J. Cacna1d de novo mutations in autism spectrum disorders activate Cav1.3 L-type calcium channels. Biol Psychiatry 2015;77:816–22. 10.1016/j.biopsych.2014.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Pinggera A, Mackenroth L, Rump A, Schallner J, Beleggia F, Wollnik B, Striessnig J. New gain-of-function mutation shows Cacna1d as recurrently mutated gene in autism spectrum disorders and epilepsy. Hum Mol Genet 2017;26:2923–32. 10.1093/hmg/ddx175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Pinggera A, Striessnig J. Cav 1.3 (CACNA1D) L-type Ca2+ channel dysfunction in CNS disorders. J Physiol 2016;594:5839–49. 10.1113/JP270672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Pinggera A, Negro G, Tuluc P, Brown MJ, Lieb A, Striessnig J. Gating defects of disease-causing de novo mutations in Cav1.3 Ca2+ channels. Channels 2018;12:388–402. 10.1080/19336950.2018.1546518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Hardiman O, Al-Chalabi A, Chio A, Corr EM, Logroscino G, Robberecht W, Shaw PJ, Simmons Z, van den Berg LH. Amyotrophic lateral sclerosis. Nat Rev Dis Primers 2017;3 10.1038/nrdp.2017.71 [DOI] [PubMed] [Google Scholar]
- 116. Gibson SB, Downie JM, Tsetsou S, Feusier JE, Figueroa KP, Bromberg MB, Jorde LB, Pulst SM. The evolving genetic risk for sporadic ALS. Neurology 2017;89:226–33. 10.1212/WNL.0000000000004109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Steinberg KM, Yu B, Koboldt DC, Mardis ER, Pamphlett R. Exome sequencing of case-unaffected-parents trios reveals recessive and de novo genetic variants in sporadic ALS. Sci Rep 2015;5 10.1038/srep09124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Rzhepetskyy Y, Lazniewska J, Blesneac I, Pamphlett R, Weiss N. CACNA1H missense mutations associated with amyotrophic lateral sclerosis alter Cav3.2 T-type calcium channel activity and reticular thalamic neuron firing. Channels 2016;10:466–77. 10.1080/19336950.2016.1204497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Canto-Bustos M, Loeza-Alcocer E, González-Ramírez R, Gandini MA, Delgado-Lezama R, Felix R. Functional expression of T-type Ca2+ channels in spinal motoneurons of the adult turtle. PLoS One 2014;9:e108187 10.1371/journal.pone.0108187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Zhang Z, David G. Stimulation-induced Ca(2+) influx at nodes of Ranvier in mouse peripheral motor axons. J Physiol 2016;594:39–57. 10.1113/JP271207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Park SB, Kiernan MC, Vucic S. Axonal Excitability in Amyotrophic Lateral Sclerosis : Axonal Excitability in ALS. Neurotherapeutics 2017;14:78–90. 10.1007/s13311-016-0492-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Kim J-W, Oh HA, Lee SH, Kim KC, Eun PH, Ko MJ, Gonzales ELT, Seung H, Kim S, Bahn GH, Shin CY. T-type calcium channels are required to maintain viability of neural progenitor cells. Biomol Ther 2018;26:439–45. 10.4062/biomolther.2017.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Carter MT, McMillan HJ, Tomin A, Weiss N. Compound heterozygous CACNA1H mutations associated with severe congenital amyotrophy. Channels 2019;13:153–61. 10.1080/19336950.2019.1614415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Duzhyy DE, Viatchenko-Karpinski VY, Khomula EV, Voitenko NV, Belan PV. Upregulation of T-type Ca2+ channels in long-term diabetes determines increased excitability of a specific type of capsaicin-insensitive DRG neurons. Mol Pain 2015;11 10.1186/s12990-015-0028-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Jagodic MM, Pathirathna S, Joksovic PM, Lee W, Nelson MT, Naik AK, Su P, Jevtovic-Todorovic V, Todorovic SM. Upregulation of the T-type calcium current in small rat sensory neurons after chronic constrictive injury of the sciatic nerve. J Neurophysiol 2008;99:3151–6. 10.1152/jn.01031.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Marger F, Gelot A, Alloui A, Matricon J, Ferrer JFS, Barrère C, Pizzoccaro A, Muller E, Nargeot J, Snutch TP, Eschalier A, Bourinet E, Ardid D. T-type calcium channels contribute to colonic hypersensitivity in a rat model of irritable bowel syndrome. Proc Natl Acad Sci U S A 2011;108:11268–73. 10.1073/pnas.1100869108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Gadotti VM, Caballero AG, Berger ND, Gladding CM, Chen L, Pfeifer TA, Zamponi GW. Small organic molecule disruptors of Cav3.2 - USP5 interactions reverse inflammatory and neuropathic pain. Mol Pain 2015;11 10.1186/s12990-015-0011-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Garcia-Caballero A, Gadotti VM, Chen L, Zamponi GW. A cell-permeant peptide corresponding to the cUBP domain of USP5 reverses inflammatory and neuropathic pain. Mol Pain 2016;12. doi: 10.1177/1744806916642444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Stemkowski P, García-Caballero A, Gadotti VDM, M'Dahoma S, Huang S, Black SAG, Chen L, Souza IA, Zhang Z, Zamponi GW. TRPV1 nociceptor activity initiates USP5/T-type channel-mediated plasticity. Cell Rep 2016;17:2901–12. 10.1016/j.celrep.2016.11.047 [DOI] [PubMed] [Google Scholar]
- 130. Souza IA, Gandini MA, Wan MM, Zamponi GW. Two heterozygous Cav3.2 channel mutations in a pediatric chronic pain patient: recording condition-dependent biophysical effects. Pflugers Arch 2016;468:635–42. 10.1007/s00424-015-1776-3 [DOI] [PubMed] [Google Scholar]
- 131. Schrier AD, Wang H, Talley EM, Perez-Reyes E, Barrett PQ. Alpha1H T-type Ca2+ channel is the predominant subtype expressed in bovine and rat zona glomerulosa. Am J Physiol Cell Physiol 2001;280:C265–C272. 10.1152/ajpcell.2001.280.2.C265 [DOI] [PubMed] [Google Scholar]
- 132. Scholl UI, Stölting G, Nelson-Williams C, Vichot AA, Choi M, Loring E, Prasad ML, Goh G, Carling T, Juhlin CC, Quack I, Rump LC, Thiel A, Lande M, Frazier BG, Rasoulpour M, Bowlin DL, Sethna CB, Trachtman H, Fahlke C, Lifton RP. Recurrent gain of function mutation in calcium channel CACNA1H causes early-onset hypertension with primary aldosteronism. Elife 2015;4:e06315 10.7554/eLife.06315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Reimer EN, Walenda G, Seidel E, Scholl UI. CACNA1H(M1549V) Mutant Calcium Channel Causes Autonomous Aldosterone Production in HAC15 Cells and Is Inhibited by Mibefradil. Endocrinology 2016;157:3016–22. 10.1210/en.2016-1170 [DOI] [PubMed] [Google Scholar]
- 134. Daniil G, Fernandes-Rosa FL, Chemin J, Blesneac I, Beltrand J, Polak M, Jeunemaitre X, Boulkroun S, Amar L, Strom TM, Lory P, Zennaro M-C. CACNA1H mutations are associated with different forms of primary aldosteronism. EBioMedicine 2016;13:225–36. 10.1016/j.ebiom.2016.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Scholl UI, Goh G, Stölting G, de Oliveira RC, Choi M, Overton JD, Fonseca AL, Korah R, Starker LF, Kunstman JW, Prasad ML, Hartung EA, Mauras N, Benson MR, Brady T, Shapiro JR, Loring E, Nelson-Williams C, Libutti SK, Mane S, Hellman P, Westin G, Åkerström G, Björklund P, Carling T, Fahlke C, Hidalgo P, Lifton RP. Somatic and germline Cacna1d calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat Genet 2013;45:1050–4. 10.1038/ng.2695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Flanagan SE, Vairo F, Johnson MB, Caswell R, Laver TW, Lango Allen H, Hussain K, Ellard S. A Cacna1d mutation in a patient with persistent hyperinsulinaemic hypoglycaemia, heart defects, and severe hypotonia. Pediatr Diabetes 2017;18:320–3. 10.1111/pedi.12512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Mancuso M, Orsucci D, Siciliano G, Bonuccelli U. The genetics of ataxia: through the labyrinth of the Minotaur, looking for Ariadne's thread. J Neurol 2014;261 Suppl 2(Suppl 2):528–41. 10.1007/s00415-014-7387-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Coutelier M, Blesneac I, Monteil A, Monin M-L, Ando K, Mundwiller E, Brusco A, Le Ber I, Anheim M, Castrioto A, Duyckaerts C, Brice A, Durr A, Lory P, Stevanin G. A recurrent mutation in CACNA1G alters Cav3.1 T-type calcium-channel conduction and causes autosomal-dominant cerebellar ataxia. Am J Hum Genet 2015;97:726–37. 10.1016/j.ajhg.2015.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Morino H, Matsuda Y, Muguruma K, Miyamoto R, Ohsawa R, Ohtake T, Otobe R, Watanabe M, Maruyama H, Hashimoto K, Kawakami H. A mutation in the low voltage-gated calcium channel CACNA1G alters the physiological properties of the channel, causing spinocerebellar ataxia. Mol Brain 2015;8 10.1186/s13041-015-0180-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Kimura M, Yabe I, Hama Y, Eguchi K, Ura S, Tsuzaka K, Tsuji S, Sasaki H. SCA42 mutation analysis in a case series of Japanese patients with spinocerebellar ataxia. J Hum Genet 2017;62:857–9. 10.1038/jhg.2017.51 [DOI] [PubMed] [Google Scholar]
- 141. Ngo K, Aker M, Petty LE, Chen J, Cavalcanti F, Nelson AB, Hassin-Baer S, Geschwind MD, Perlman S, Italiano D, Laganà A, Cavallaro S, Coppola G, Below JE, Fogel BL. Expanding the global prevalence of spinocerebellar ataxia type 42. Neurol Genet 2018;4:e232 10.1212/NXG.0000000000000232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Chemin J, Siquier-Pernet K, Nicouleau M, Barcia G, Ahmad A, Medina-Cano D, Hanein S, Altin N, Hubert L, Bole-Feysot C, Fourage C, Nitschké P, Thevenon J, Rio M, Blanc P, Vidal C, Bahi-Buisson N, Desguerre I, Munnich A, Lyonnet S, Boddaert N, Fassi E, Shinawi M, Zimmerman H, Amiel J, Faivre L, Colleaux L, Lory P, Cantagrel V. De novo mutation screening in childhood-onset cerebellar atrophy identifies gain-of-function mutations in the CACNA1G calcium channel gene. Brain 2018;141:1998–2013. 10.1093/brain/awy145 [DOI] [PubMed] [Google Scholar]
- 143. Li X, Zhou C, Cui L, Zhu L, Du H, Liu J, Wang C, Fang S. A case of a novel CACNA1G mutation from a Chinese family with SCA42: a case report and literature review. Medicine 2018;97:e12148 10.1097/MD.0000000000012148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Singh B, Monteil A, Bidaud I, Sugimoto Y, Suzuki T, Hamano S-ichiro, Oguni H, Osawa M, Alonso ME, Delgado-Escueta AV, Inoue Y, Yasui-Furukori N, Kaneko S, Lory P, Yamakawa K. Mutational analysis of CACNA1G in idiopathic generalized epilepsy. Mutation in brief #962. Online. Hum Mutat 2007;28:524–5. 10.1002/humu.9491 [DOI] [PubMed] [Google Scholar]
- 145. Calhoun JD, Hawkins NA, Zachwieja NJ, Kearney JA. Cacna1g is a genetic modifier of epilepsy caused by mutation of voltage-gated sodium channel SCN2A. Epilepsia 2016;57:e103–7. 10.1111/epi.13390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Calhoun JD, Hawkins NA, Zachwieja NJ, Kearney JA. Cacna1g is a genetic modifier of epilepsy in a mouse model of Dravet syndrome. Epilepsia 2017;58:e111–5. 10.1111/epi.13811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Gulsuner S, Walsh T, Watts AC, Lee MK, Thornton AM, Casadei S, Rippey C, Shahin H, Nimgaonkar VL, Go RCP, Savage RM, Swerdlow NR, Gur RE, Braff DL, King M-C, McClellan JM, Consortium on the Genetics of Schizophrenia (COGS), PAARTNERS Study Group . Spatial and temporal mapping of de novo mutations in schizophrenia to a fetal prefrontal cortical network. Cell 2013;154:518–29. 10.1016/j.cell.2013.06.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Henriksen MG, Nordgaard J, Jansson LB. Genetics of schizophrenia: overview of methods, findings and limitations. Front Hum Neurosci 2017;11 10.3389/fnhum.2017.00322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Andrade A, Hope J, Allen A, Yorgan V, Lipscombe D, Pan JQ. A rare schizophrenia risk variant of CACNA1I disrupts CaV3.3 channel activity. Sci Rep 2016;6 10.1038/srep34233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Wang J, Zhang Y, Liang J, Pan H, Wu H, Xu K, Liu X, Jiang Y, Shen Y, Wu X. CACNA1I is not associated with childhood absence epilepsy in the Chinese Han population. Pediatr Neurol 2006;35:187–90. 10.1016/j.pediatrneurol.2006.03.006 [DOI] [PubMed] [Google Scholar]
- 151. Adzhubei I, Jordan DM, Sunyaev SR. Predicting functional effect of human missense mutations using PolyPhen-2. Curr Protoc Hum Genet 2013;Chapter 7:7.20.1–7.20.41. 10.1002/0471142905.hg0720s76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Kammenga JE. The background puzzle: how identical mutations in the same gene lead to different disease symptoms. Febs J 2017;284:3362–73. 10.1111/febs.14080 [DOI] [PubMed] [Google Scholar]