Abstract
Background and Aims
Faecal diversion is associated with improvements in Crohn’s disease but not ulcerative colitis, indicating that differing mechanisms mediate the diseases. This study aimed to investigate levels of systemic mediators of inflammation, including fibrocytes and cytokines, [1] in patients with Crohn’s disease and ulcerative colitis preoperatively compared with healthy controls and [2] in patients with Crohn’s disease and ulcerative colitis prior to and following faecal diversion.
Methods
Blood samples were obtained from healthy individuals and patients with Crohn’s disease or ulcerative colitis. Levels of circulating fibrocytes were quantified using flow cytometric analysis and their potential relationship to risk factors of inflammatory bowel disease were determined. Levels of circulating cytokines involved in inflammation and fibrocyte recruitment and differentiation were investigated.
Results
Circulating fibrocytes were elevated in Crohn’s disease and ulcerative colitis patients when compared with healthy controls. Smoking, or a history of smoking, was associated with increases in circulating fibrocytes in Crohn’s disease, but not ulcerative colitis. Cytokines involved in fibrocyte recruitment were increased in Crohn’s disease patients, whereas patients with ulcerative colitis displayed increased levels of pro-inflammatory cytokines. Faecal diversion in Crohn’s disease patients resulted in decreased circulating fibrocytes, pro-inflammatory cytokines, and TGF-β1, and increased IL-10, whereas the inverse was observed in ulcerative colitis patients.
Conclusions
The clinical effect of faecal diversion in Crohn’s disease and ulcerative colitis may be explained by differing circulating fibrocyte and cytokine responses. Such differences aid in understanding the disease mechanisms and suggest a new therapeutic strategy for inflammatory bowel disease.
Keywords: Inflammatory bowel disease, faecal diversion, circulating fibrocytes, cytokines
1. Introduction
Inflammatory bowel diseases [IBD] are chronic, relapsing, inflammatory conditions mediated by concerted immunological, environmental, and genetic processes.1,2 They include both Crohn’s disease [CD] and ulcerative colitis [UC], and are believed to result from an overly aggressive immune response in individuals genetically susceptible to an environmental factor, such as gut commensals.1,2
Faecal diversion is an approach to management of IBD patients whereby the stream of luminal contents is reduced or eradicated through the formation of an ostomy. Faecal diversion has not shown efficacy in the management of UC, but it has been used to great effect in CD.3–11 In that setting, faecal diversion is associated with induction of clinical remission, mucosal healing, and maintenance of mucosal architecture.4,7,12 The differential effect of faecal diversion in CD and UC implies that patients’ microbiomes, alone or in combination with other faeces-borne factors, may be influential. We have demonstrated previously that patients with CD have a distinctly different mesenteric lymph node [MLN] microbiome compared with that observed in patients with UC.13 Similarly, differences between the CD and UC gut microbiome have been reported.14,15 In summary, the MLN microbiota of CD patients is more dysregulated than that of UC patients when compared with the reported healthy gut microbiome, and reflects increases in Proteobacteria. In contrast, the microbiome of MLNs from UC resections exhibits similarity to the reported normal gut microbiome, albeit comprising elevated Firmicutes.13,16
MLNs are involved in the initiation and progression of immunological processes, which occur in response to bacterial translocation.17–20 The differences observed in MLN microbiomes of patients with CD or UC could aid in understanding the mechanisms mediating each disease. Specifically, Proteobacteria, encompassing numerous pathogenic bacteria, may trigger more aggressive immune responses than bacteria present in the MLNs of UC patients. In contrast, MLNs from UC patients display an abundance of Faecalibacterium.13Faecalibacterium is associated with anti-inflammatory effects and can induce IL-10 production by dendritic cells.21–24 An influential anti-inflammatory cytokine, IL-10, can reduce production of pro-inflammatory cytokines while increasing levels of anti-inflammatory cytokines.25–27
Circulating fibrocytes have also been implicated in the pathogenesis of IBD [in addition to inflammatory and fibrotic diseases], through their differentiation to fibroblasts, myofibroblasts, and adipocytes at sites of inflammation.28–40 These cells mediate mucosal and mesenteric fibrosis and inflammation in IBD, through increased proliferation and cytokine and extracellular matrix production.28,41–46 They are the cellular basis of many manifestations of IBD, particularly CD, including stricturing, fat wrapping, and mesenteric thickening.43,44,47 To our knowledge, only two studies have investigated circulating fibrocytes in CD, with no published literature relevant to the UC setting. In CD, levels of circulating fibrocytes are increased when compared with healthy controls.46,48 Recruited to sites of mucosal inflammation early in the inflammatory phase of CD,46 circulating fibrocytes are present in diseased, but not normal mesentery. Levels of circulating fibrocytes increase as disease severity (mucosal, mesenteric, and the Crohn’s Disease Activity Index [CDAI]) increases,48 therefore suggesting a pathobiological role for fibrocytes in IBD.
Given this potential role in disease pathogenesis, we wished to investigate levels of circulating fibrocytes in patients with CD and, for the first time, UC. This study further attempted to explore the relationship between circulating fibrocytes and known risk factors of IBD. The majority of studies investigating faecal diversion in IBD have focused on clinical parameters and potential mucosal healing. However, here, faecal diversion facilitated an appraisal of systemic disease mechanisms and manifestations of IBD following removal [or considerable reduction] of the microbe-rich faecal stream, with emphasis on circulating fibrocytes and plasma-borne cytokines.
2. Methods
2.1. Ethical approval
Ethical approval was obtained from the Research Ethics Committee of University Hospital Limerick [UHL].
2.2. Inclusion criteria
All patients admitted to UHL for an operation for CD [n = 35] or UC [n = 15] from March 2013 to January 2018 were included. Pathological controls, including patients with colorectal cancer [n = 49] and patients with diverticular disease [n = 6], and self-declared healthy volunteers [n = 20], were recruited from UHL and the University of Limerick, respectively. A sub-cohort of the recruited patients with CD were admitted to hospital for an emergency resection to manage a disease-related surgical indication, such as an obstruction, abscess, or perforation. These patients were not suitable for a resection due to the extent of their disease observed at the time of their exploratory laparotomy, and were defunctioned with the creation of an ostomy to divert the faecal stream away from the remaining intestine, and were also included.
2.3. Faecal diversion in Crohn’s disease and ulcerative colitis
2.3.1. Crohn’s disease
Following admittance to hospital for an emergency resection, an exploratory laparotomy was conducted to assess the extent of disease in a sub-cohort of recruited patients with CD. It was determined that a resection which includes the mesentery,48 or a classic conservative resection, could not be safely or successfully completed. Therefore, these patients were defunctioned through the creation of a loop ileostomy to divert the faecal stream away from the remaining intestine. An average of 7.2 months later, a second exploratory laparotomy was performed to once again evaluate the extent of disease. Here, it was decided whether to resect the diseased intestine and mesentery or to leave the defunctioning loop ileostomy in situ to allow further healing. Previous work by our group has demonstrated the efficacy of including the mesentery during resections for CD in reducing rates of surgical recurrence.48 Blood samples were taken from patients with CD before their initial exploration laparatomy with loop ileostomy creation, and again before their second exploratory laparotomy with or without resection [7.2 months later].
2.3.2. Ulcerative colitis
Patients with UC underwent a total colectomy followed by a completion proctectomy. An end ileostomy was created to divert the faecal stream away from the remaining rectum following the patient’s total colectomy, as per the standard of care at the Department of Surgery, UHL. Following an average of 13.7 months where the faecal stream was diverted, the end ileostomy was reversed and a completion proctectomy was performed with the formation of an ileal pouch-anal anastomosis. In this study, the term faecal diversion in patients with UC refers to the time period with the end ileostomy in situ [where the faecal stream was diverted away from the remaining rectum]. Blood samples were taken from patients with UC before their total colectomy with end ileostomy creation, and again before their completion proctectomy [13.7 months later].
2.4. Demographics and information collected
Retrieved data included patient’s medical therapy, cigarette smoking status, age, and disease location and behaviour [Montreal classification system] at the time of their operation[s], in addition to their age at the time of diagnosis and a family history of IBD [if any].49 Preoperative white blood cell, lymphocyte, monocyte, eosinophil, basophil, neutrophil, and platelet counts were obtained for patients in addition to their preoperative C-reactive protein [CRP] levels. Patient data were generated by a combination of direct contact, chart reviews, operation and endoscopy notes, and pathology reports.
2.5. Blood sample collection, preparation, and processing
Blood [7.5 mL] was collected from patients and healthy volunteers via peripheral, upper extremity venepuncture, in K3 EDTA S-Monovette® [Sarstedt, Nümbrecht, Germany]. The various components of blood, i.e., red blood cells, white blood cells, and plasma, were separated by density gradient centrifugation, using Histopaque®-1077 [Sigma-Aldrich, Wicklow, Ireland], at 1500 rpm for 30 min. Following separation, plasma was removed and transferred to 1.5-mL tubes for storage in 500-µL aliquots. White blood cells were subsequently washed in phosphate-buffered saline (PBS; pH 7.4 [137 mM NaCl; 2.68 mM KCl; 9.94 mM Na2HPO4; 1.76 mM KH2PO4]) and re-suspended in freezing medium [50% foetal bovine serum, 40% RPMI medium, and 10% dimethyl sulphoxide] prior to transfer to cryogenic vials in 1-mL aliquots. Samples were cooled in a cryogenic temperature control rate container to -80 °C until processing for flow cytometry.
2.6. Flow cytometric quantification of circulating fibrocytes
Circulating fibrocytes were quantified as described previously.48 Cells [1 × 106] were re-suspended in flow cytometry buffer [RPMI-1640 medium supplemented with 10% horse serum, 0.1% sodium azide, and 25 mM HEPES] following thawing. Cells were fixed and permeabilised using BD Cytofix/Cytoperm™ solution [BD Biosciences, Oxford, UK] and blocked using flow cytometry buffer prior to intracellular staining of collagen I with mouse anti-human Collagen-I antibody [Millipore, Cork, Ireland]. Collagen I was subsequently tagged with Alexa-Fluor 488 goat anti-mouse secondary antibody [Jackson ImmunoResearch Europe, Suffolk, UK]. Cells were then stained for cell surface antigen CD45 using PerCP anti-human CD45 [Biolegend, London, UK]. Finally, cells were re-suspended in micron-filtered PBS for subsequent analysis on a BD FACSVerse™ [BD Biosciences]. Data were analysed using BD FACSuite v1.0.5 [BD Biosciences] and results were displayed as a percentage of the total white blood cell population. A suitable protocol was established to detect fibrocytes by running relevant controls, including fluorescence minus one controls. The 488 blue laser was used to excite both stains, with the green detector reading Alexa-Fluor 488 [Ex/Em 493/519 nm] and the red detector reading PerCP [Ex/Em 482/667 nm]. A total of 10 000 monocyte cellular events were recorded with a flow rate of approximately 0.24 µL per second. Cells were gated and those positive for CD45 and Collagen I were regarded as fibrocytes. These markers have been used by several groups in identifying and quantitating fibrocytes.36,46,50,51
2.7. Cytokine array technology to assess fold change in systemic inflammatory and fibrotic cytokines
Levels of 23 cytokines involved in inflammation, fibrocyte recruitment, and fibrocyte differentiation were determined using RayBio® Human Custom Antibody Array G Series [RayBiotech, Georgia, USA] [Supplementary Table 1, available as Supplementary data at ECCO-JCC online] and compared as per manufacturer’s instructions. In brief, G Series glass slides were incubated with whole plasma overnight at 4°C following blocking for 30 min with RayBio® blocking buffer. G Series slides were then washed and incubated with a custom biotinylated antibody cocktail overnight at 4 °C. Subsequently, G Series slides were washed once again before incubation with IRDye 800CW Streptavidin [LI-COR Biosciences, UK] and visualisation on an Odyssey® SA [LI-COR]. Background signal was subtracted from values and data were normalised to the average of the positive controls within the replicate. Data were analysed as the fold change of the cytokine levels in preoperative CD or UC plasma when compared with the levels in healthy controls, or the fold change of cytokine levels in CD or UC patients following faecal diversion compared with the levels before faecal diversion. Levels of preoperative cytokines were assessed in a representative number of patients from our CD and UC cohorts and healthy controls. Sample selection was completed based on fibrocyte levels, ensuring that plasma from individuals with a range of fibrocyte levels was used.
2.8. Statistical analysis
Data are presented as mean ± standard error of the mean [SE] unless otherwise stated. All statistical analyses were completed using SPSSv24 [SPSS Inc., Chicago, USA]. A one-way analysis of variance [ANOVA] with Bonferroni post-hoc tests were used to compare the levels of circulating fibrocytes in patients with colorectal diseases and healthy controls. Two-tailed independent samples t tests were used to compare non-related parametric variables, and a Mann-Whitney U test was used to compare non-related non-parametric variables. A two-tailed paired t test was used to compare related parametric variables, and a Wilcoxon test was used to compare related non-parametric variables. Chi square tests and Z test for proportions were used to compare nominal data. A 5% level of significance was used for all statistical tests.
3. Results
3.1. Patient and operation information
A full description of total patient demographics and operation information is available in Table 1. Seven of the recruited patients with CD underwent faecal diversion. Following faecal diversion for an average of 7.2 months, five patients were suitable for a resection that included the mesentery and the formation of an anastomosis, and two patients had diseased intestine and mesentery which remained unsuitable for a resection and the defunctioning loop ileostomy was left in situ. Demographics of all patients who underwent faecal diversion are provided in Supplementary Table 2, available as Supplementary data at ECCO-JCC online.
Table 1.
Demographics of recruited patients with Crohn’s disease and ulcerative colitis.
| Variable | CD [n = 35] | UC [n = 15] | p-value |
|---|---|---|---|
| Gender | 0.849 [overall chi 2 ] | ||
| Male | 13 [37%] | 6 [40%] | 0.849 [Z test] |
| Female | 22 [63%] | 9 [60%] | 0.849 [Z test] |
| Age at operation [years] | 36.9 ± 2.27 | 37.8 ± 3.10 | 0.814 [t test] |
| Age at diagnosis [years] | 29.7 ± 2.05 | 35.3 ± 3.99 | 0.228 [t test] |
| Disease duration [months] | 91.1 ± 16.11 | 63.6 ± 22.19 | 0.779 [t test] |
| Family history | 0.198 [overall chi 2 ] | ||
| Yes | 11 [31%] | 3 [20%] | 0.412 [Z test] |
| No | 17 [49%] | 12 [80%] | 0.039 [Z test] |
| Data not available | 7 [20%] | 0 [0%] | 0.061 [Z test] |
| Smoking status | 0.779 [overall chi 2 ] | ||
| Active/history | 22 [63%] | 10 [59%] | 0.281 [Z test] |
| Non-smoker | 13 [37%] | 7 [41%] | 0.281 [Z test] |
| Operation type | |||
| Ileocolic resection | 20 [57%] | N/A | N/A |
| Ileocolic resection + other | 1 [3%] | N/A | N/A |
| Ileocolic resection + defunction | 1 [3%] | N/A | N/A |
| Small bowel resection | 2 [6%] | N/A | N/A |
| Sigmoid colectomy | 1 [3%] | N/A | N/A |
| Panproctocolectomy | 2 [6%] | N/A | N/A |
| Washout | 1 [3%] | N/A | N/A |
| Adhesiolysis | 1 [3%] | N/A | N/A |
| Defunction | 6 [16%] | N/A | N/A |
| Total colectomy | N/A | 11 [69%] | N/A |
| Completion proctecomy | N/A | 5 [31%] | N/A |
| Medications at time of surgery | 28 [80%] | 13 [77%] | 0.925 [overall chi 2 ] |
| Aminosalicylates | 9 [26%] | 7 [41%] | 0.222 [chi2 test] |
| Steroid | 14 [40%] | 6 [35%] | 0.804 [chi2 test] |
| Antibiotics | 3 [9%] | 4 [24%] | 0.124 [chi2 test] |
| Immunosuppressants | 8 [29%] | 3 [18%] | 0.704 [chi2 test] |
| Thiopurines | 7 [20%] | 3 [18%] | 0.842 [z test] |
| Methotrexate | 1 [3%] | 0 [0%] | 0.484 [Z test] |
| Biologics | 16 [46%] | 10 [59%] | 0.308 [chi2 test] |
| anti-TNF | 15 [43%] | 9 [53%] | 0.497 [Z test] |
| anti-α 4β 7 | 1 [3%] | 1 [6%] | 0.596 [Z test] |
| None | 6 [17%] | 3 [18%] | 0.412 [chi2 test] |
| Data not available | 1 [3%] | 1 [6%] | 0.412 [chi2 test] |
| Montreal Classification of disease | |||
| Age at diagnosis | 0.591 [overall chi 2 ] | ||
| A1 ≤16 years old | 2 [6%] | 0 [0%] | 0.347 [Z test] |
| A2 = 17–40 years old | 24 [69%] | 8 [53%] | 0.303 [Z test] |
| A3 >40 years old | 8 [29%] | 4 [27%] | 0.772 [Z test] |
| Data not available | 1 [3%] | 1 [7%] | 0.529 [Z test] |
| Location | |||
| L1 Terminal ileum | 19 [54%] | N/A | N/A |
| L2 Colonic | 3 [9%] | N/A | N/A |
| L3 Ileocolic | 7 [20%] | N/A | N/A |
| L4 Upper GI | 4 [11%] | N/A | N/A |
| L1 + L4 | 1 [3%] | N/A | N/A |
| E1 proctitis | N/A | 1 [7%] | N/A |
| E2 left-sided colitis | N/A | 6 [40%] | N/A |
| E3 pan- | N/A | 8 [53%] | N/A |
| Data not available | 1 [3%] | N/A | N/A |
| Disease behaviour | |||
| B1 non-stricturing, non-penetrating | 5 [14%] | N/A | N/A |
| B2 stricturing | 14 [40%] | N/A | N/A |
| B3 penetrating | 15 [43%] | N/A | N/A |
| P perianal involvement | 3 [9%] | N/A | N/A |
| Data not available | 1 [3%] | N/A | N/A |
Data are presented as mean ± SE. Bold text indicates statistically significant results. Italicised and underlined text indicates results for overall statistical tests. Biologic medication consists of anti-tumour necrosis factor [TNF] [adalimumab, infliximab, certolizumab, or golimumab] or anti-α 4β 7 integrin [vedolimumab] agents.
CD, Crohn’s disease; UC,ulcerative colitis; GI, gastrointestinal; N/A, not available.
3.2. Circulating fibrocytes are elevated in inflammatory bowel diseases
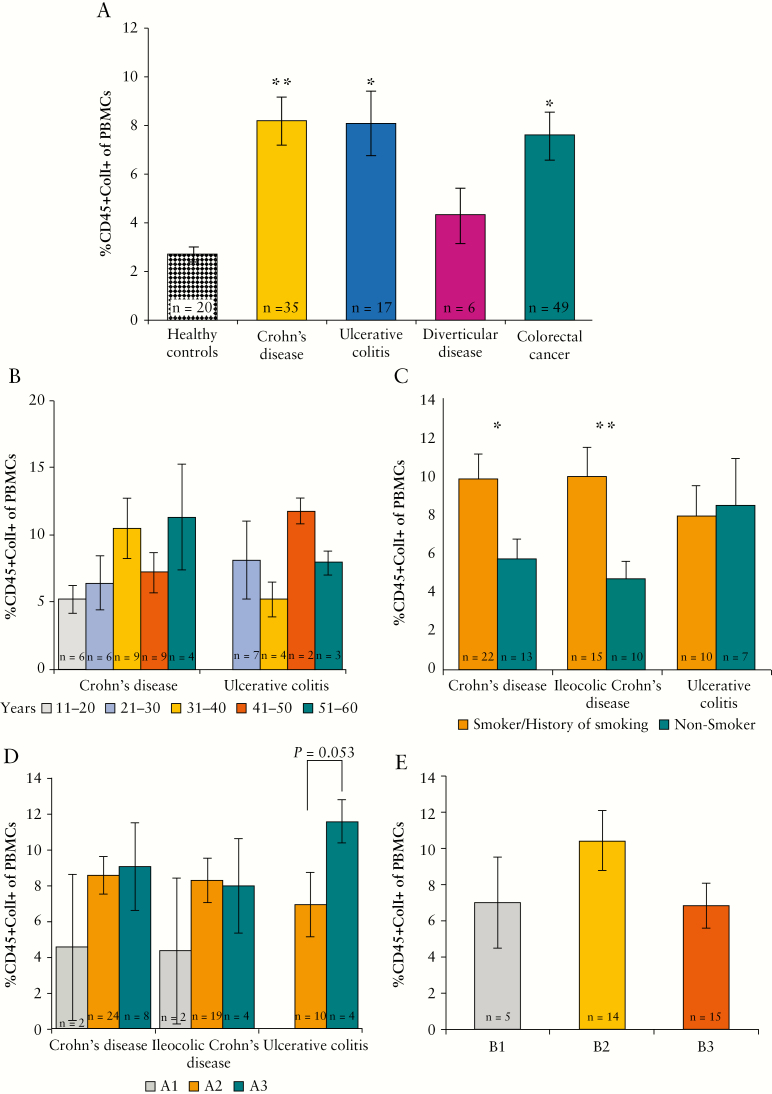
Circulating fibrocytes were significantly elevated in patients with CD [n = 35] and UC [n = 17] when compared with levels in healthy controls [n = 20] [CD: 8.2 ± 0.95% vs. 2.7 ± 0.34, p = 0.005, Bonferroni test; UC: 8.1 ± 1.32% vs. 2.7 ± 0.34, p = 0.037, Bonferroni test] [Figure 1A]. Levels of circulating fibrocytes were also elevated significantly in patients with colorectal cancer [n = 49] when compared with healthy controls [n = 20] [7.6 ± 0.96% vs. 2.7 ± 0.34%, p = 0.013, Bonferroni test]. Although circulating fibrocytes were elevated in patients with diverticular disease [n = 6] when compared with healthy controls [n = 20], this was not significant [4.3 ± 1.13% vs. 2.7 ± 0.34%, p = 1.000, Bonferroni test]. However, the circulating fibrocyte level for patients with diverticular disease was not reliable, due to the low number of patients recruited. Levels of circulating fibrocytes were similar in CD, UC, and colorectal cancer [Figure 1A].
Figure 1.
Quantification and analysis of circulating fibrocytes in inflammatory bowel disease [IBD]. [A] Circulating fibrocytes were increased in various colorectal diseases when compared with healthy controls, including Crohn’s disease [CD], ulcerative colitis [UC], and colorectal cancer. Levels of circulating fibrocytes were elevated in patients with diverticular disease but this was not significant. [B] Circulating fibrocytes trend towards increasing in patients with CD as their age at the time of surgery increases. [C] Circulating fibrocytes are increased in patients with CD who smoke or with a history of smoking when compared with those who never smoked. Smoking has no effect on levels of circulating fibrocytes in UC patients. [D] There was a trend of increasing circulating fibrocytes as the age of diagnosis increased in patients with UC. [E] Levels of circulating fibrocytes are highest in patients with CD with a stricturing [B2] behaviour type. Text at base of bars indicates number of samples analysed [n].
The level of circulating fibrocytes in CD patients increased as their age at the time of surgery increased [11–20: n = 6; 21–30: n = 6; 31–40: n = 9; 41–50: n = 9; 51–60: n = 4]. The same trend was not observed within the UC cohort [11–20: n = 0; 21–30: n = 7; 31–40: n = 4; 41–50: n = 2; 51–60: n = 3] [Figure 1B]. Patients with CD who smoked and those with a history of smoking [n = 22] had higher levels of circulating fibrocytes compared with those who had never smoked [n = 13] [smoker/history of smoking vs. non-smoker: 9.8 ± 1.28% vs. 5.7 ± 1.03%, p = 0.017, independent samples t test]. Smoking had no effect on levels of circulating fibrocytes in patients with UC [smoker/history of smoking: n = 10; non-smoker: n = 7] [Figure 1C].
Circulating fibrocytes increased in patients with UC, but not CD, as their age at the time of diagnosis increased [UC: A2 [n = 10] vs. A3 [n = 4]: 6.9 ± 1.78% vs. 11.5 ± 1.18%, p = 0.053, independent samples t test] [Figure 1D, n located on base of bars]. There was no relationship between disease location at the time of operation and the level of circulating fibrocytes in CD [L1: n = 19; L2: n = 3; L3: n = 7; L4: n = 4; L1+L4: n = 1] or UC [E1: n = 1; E2: n = 8; E3: n = 8] [data not presented]. Patients with stricturing behavioural type [B2] CD [n = 14] were found to have higher levels of circulating fibrocytes than other CD behavioural types (i.e., inflammatory [B1] [n = 5] and penetrating [B3] [n = 15]) [B1: 7.0 ± 2.51%; B2: 10.4 ± 1.66%; B3: 6.8 ± 1.24%] [Figure 1E]. Gender, family history of IBD, duration of disease, and perianal involvement had no effect on circulating fibrocyte levels in CD or UC [data not presented].
3.3. Common classes of medications used to manage inflammatory bowel disease have no effect on circulating fibrocytes in IBD
The majority of patients with CD and UC included in the study were undergoing medical therapy to manage disease at the time of surgery [Table 1]. Information on the types of medications used and prescribed dosage ranges is provided in Supplementary Table 3, available as Supplementary data at ECCO-JCC online. Biologic agents provided to our cohort of patients comprised anti-tumour necrosis factor [TNF] [adalimumab, infliximab, certolizumab, and golimumab] and anti-integrin α 4β 7 [vedolimumab] [Table 1; Supplementary Table 3]. Although these are anti-inflammatory, for the purposes of this study we have reported their effects separately from non-biologic anti-inflammatory medications [e.g., mesalazine and sulphasalazine] [Table 1].
Medications commonly used to treat IBD had no effect on levels of circulating fibrocytes in CD patients [aminosalicylate: n = 9; steroid: n = 14; antibiotic: n = 4; immunosuppressive: n = 8; biologic: n = 16] [Supplementary Figure 1A, 1B, n located on base of bars, available as Supplementary data at ECCO-JCC online]. Similarly, antibiotics [n = 4], aminosalicylate [n = 7], steroid [n = 6], and biologic [n = 10] agents did not alter levels of circulating fibrocytes in UC [Supplementary Figure 1C]. The use of immunosuppressive medications [n = 3] decreased levels of circulating fibrocytes in UC [on immunosuppressive medications vs. not on immunosuppressive medications: 4.1 ± 1.77% vs. 9.6 ± 1.47%, p = 0.059, independent samples t test] [Supplementary Figure 1C, n located on base of bars, and Supplementary Table 3]. However, patients with UC undergoing immunosuppressive therapy were also administered biologic agents in combination therapeutic strategies [two patients on thiopurines and anti-TNF agents, one patient on thiopurine and an anti-α 4β 7 agent].
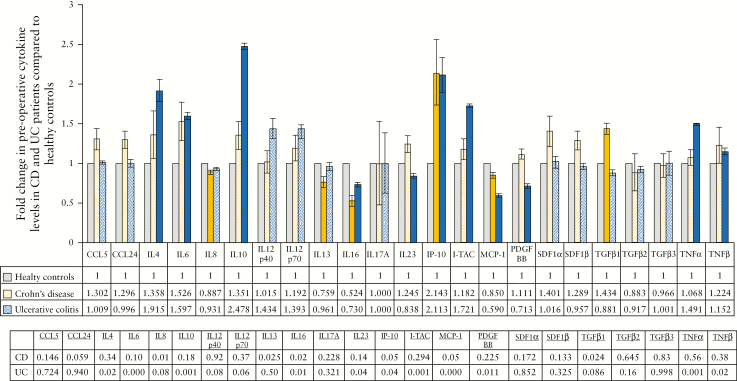
3.4. Cytokines associated with fibrocyte recruitment and differentiation are increased in Crohn’s disease but not ulcerative colitis
Preoperative levels of 23 plasma-borne cytokines in CD [n = 9] and UC [n = 7] patients were compared with their respective levels in healthy controls [n = 4]. Demographics of patients whose plasma was used for this study are provided in Supplementary Table 4, available as Supplementary data at ECCO-JCC online.
Fibrocyte recruitment is mediated by stromal derived factor 1 [SDF-1] (also known as C-X-C chemokine ligand 12 [CXCL12]), C-C ligand 5 [CCL5] (also known as regulated on activation, normal T cell expressed and secreted [RANTES]) and CCL24 [also known as Eotaxin-2].30,36,46,52,53 Levels of these cytokines were increased in CD, but not UC, when compared with healthy controls [Figure 2, associated p-values on table]. TGF-β1 was also increased in patients with CD [p = 0.024] but not UC. TGF-β1 mediates differentiation of fibrocytes to fibroblasts and myofibroblasts.29,40,54
Figure 2.
Levels of circulating cytokines involved in inflammation and fibrosis in patients with inflammatory bowel disease [IBD]. The average fold change in cytokines in patients with Crohn’s disease [CD] and ulcerative colitis [UC] before their operation compared with levels in healthy controls. Data are presented as fold changes when compared with the healthy controls [data table on graph]. Solid colour bars indicate cytokines significantly different in CD or UC compared with healthy controls. Associated p-values are presented in the bottom table. All experiments were completed in triplicate.
Within various behavioural sub-types of CD (inflammatory [B1]: n = 1; stricturing [B2]: n = 5; penetrating [B3]: n = 3), only CCL5 was increased in all behavioural subtypes [Supplementary Figure 2, associated p-values in table, available as Supplementary data at ECCO-JCC online]. CCL24 was increased in patients with inflammatory and stricturing CD only [B1: p = 0.076, B2: p = 0.027]. Increases in SDF-1 and TGF-β1 were associated with patients with more complicated behavioural types of CD (SDF-1α [B2: p = 0.143, B3: p = 0.257]; SDF-1β [B2: p = 0.004, B3: p = 0.257]; TGF-β1 [B2: p = 0.043, B3: p = 0.017] [Supplementary Figure 2].
3.5. Inflammation-associated cytokines are increased in ulcerative colitis
Preoperative levels of cytokines associated with inflammation were assessed in the same cohort of CD [n = 9] and UC [n = 7] patients [Supplementary Table 4, available as Supplementary data at ECCO-JCC online] and compared with their respective levels in healthy controls [n = 4].
UC was associated with increases in levels of pro-inflammatory cytokines when compared with healthy controls, including interleukin [IL]-6 [p = 0.001], IL-12p40 [p = 0.078], IL-12p70 [p = 0.055], interferon gamma-induced protein [IP-10] [also known as CXCL10; p = 0.037], interferon inducible T-cell alpha chemoattractant [I-TAC] [also known as CXCL11; p = 0.001], and tumour necrosis factor alpha [TNFα] [p = 0.001]. However, of these cytokines, only IL-6 [p = 0.096] and IP-10 [p = 0.05] were increased in patients with CD and, of those, patients with a stricturing [IL-6: p = 0.188, IP-10: p = 0.047] or penetrating [IL-6: p = 0.021, IP-10: p = 0.089] behavioural type only [B2: n = 5; B3: n = 3] [Figure 2; Supplementary Figure 2].
Decreases in pro-inflammatory IL-16 and monocyte chemoattractant protein 1 [MCP-1] [also known as CCL2] were common to both CD and UC (CD [IL-16: p = 0.020, MCP-1: p = 0.05], UC [IL-16: p = 0.014; MCP-1: p < 0.001]. Interestingly, anti-inflammatory IL-10 was significantly increased in patients with UC [p = 0.001] but not CD [Figure 2].
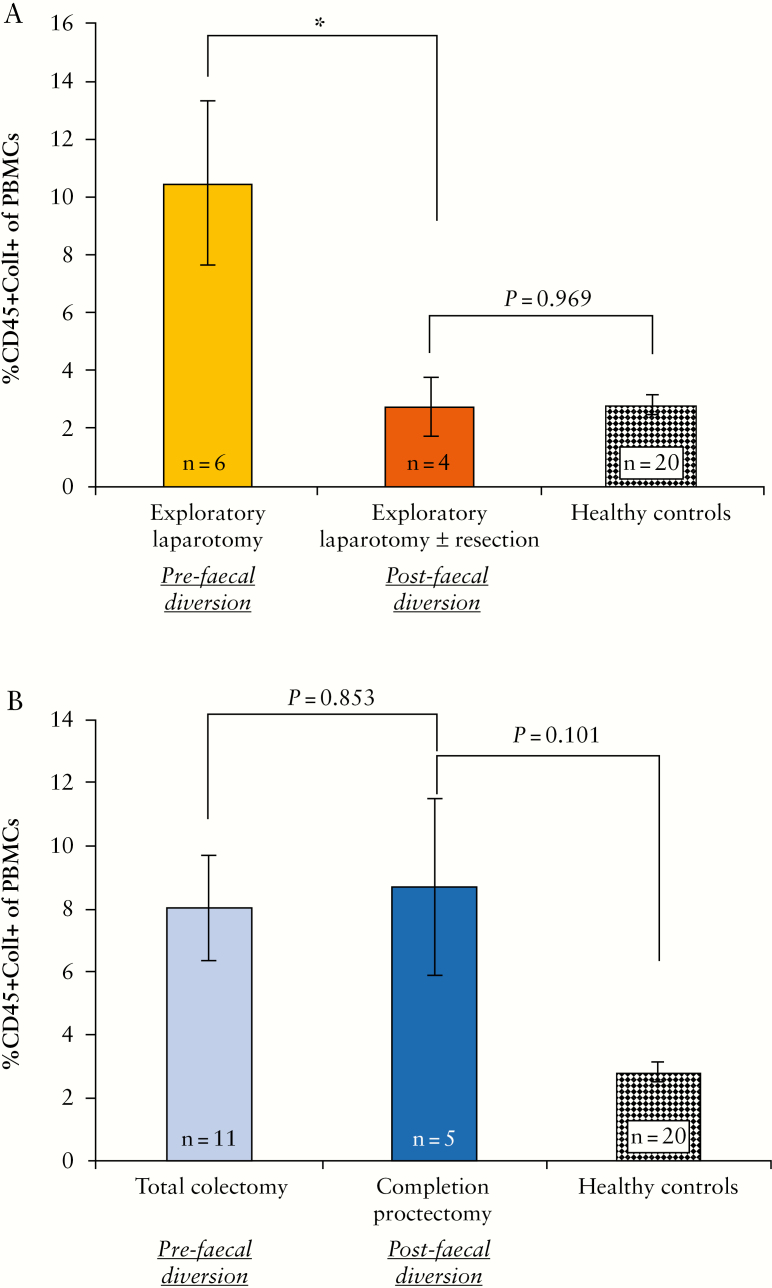
3.6. Faecal diversion is associated with a decrease in fibrocytes in patients with Crohn’s disease but not in ulcerative colitis
Preoperative levels of circulating fibrocytes were determined in CD and UC patients before [CD: n = 6; UC: n = 11] and after [CD: n = 4; UC: n = 5] faecal diversion. Circulating fibrocytes in patients with CD decreased following faecal diversion [10.5 ± 2.89% vs. 2.8 ± 0.98%, p = 0.044, independent samples t test] [Figure 3A]. Levels of circulating fibrocytes in CD patients following faecal diversion were similar to the levels observed in healthy controls [n = 20] [2.8 ± 0.98% vs. 2.8 ± 0.34%, p = 0.969, independent samples t test] [Figure 3A].
Figure 3.
The effect of faecal diversion on levels of circulating fibrocytes in patients with inflammatory bowel disease [IBD]. [A] Circulating fibrocytes decreased in patients with Crohn’s disease [CD] following faecal diversion. Levels of circulating fibrocytes were measured before the patient’s initial exploratory laparotomy [n = 6] and again before their second exploratory laparotomy ± resection. [B] There was no change in levels of circulating fibrocytes in patients with UC following faecal diversion. Circulating fibrocytes were quantified before their total colectomy, and again, before their completion proctectomy [n = 5]. Text at base of bars indicates number of samples analysed [n].
In contrast, there was no difference in the percentage of circulating fibrocytes in patients with UC following faecal diversion [8.1 ± 1.67% vs. 8.7 ± 2.83%, p = 0.853, independent samples t test] [Figure 3B]. Following faecal diversion, levels of circulating fibrocytes in UC patients remained higher than those in healthy controls [n = 20] [8.7 ± 2.83% vs. 2.7 ± 0.34%, p = 0.101, independent samples t test].
3.7. Faecal diversion is associated with changes in levels of haematopoietic cells and C-reactive protein in patients with inflammatory bowel disease
Preoperative haemotopoietic cell counts [including total white blood cells, lymphocytes, monocytes, eosinophils, basophils, neutrophils, and platelets] were available for all patients who underwent faecal diversion [CD: n = 7; UC: n = 11]. Faecal diversion was associated with decreases in monocytes, neutrophils, platelets, and total white blood cell count, but increases in eosinophils, in both CD and UC [Supplementary Figure 3, available as Supplementary data at ECCO-JCC online]. Notably, although both basophils and lymphocytes increased in patients with CD following faecal diversion, in patients with UC, basophils decreased and levels of lymphocytes did not change following faecal diversion [Supplementary Figure 3].
CRP levels were measured in patients with CD [n = 5] prior to both exploratory laparotomies. Levels of CRP significantly decreased in patients with CD following faecal diversion [231.6 ± 45.31 mg/mL vs. 7.8 ± 2.87 mg/mL, p = 0.008, paired samples t test]. Similar CRP results were not available for patients with UC who underwent faecal diversion.
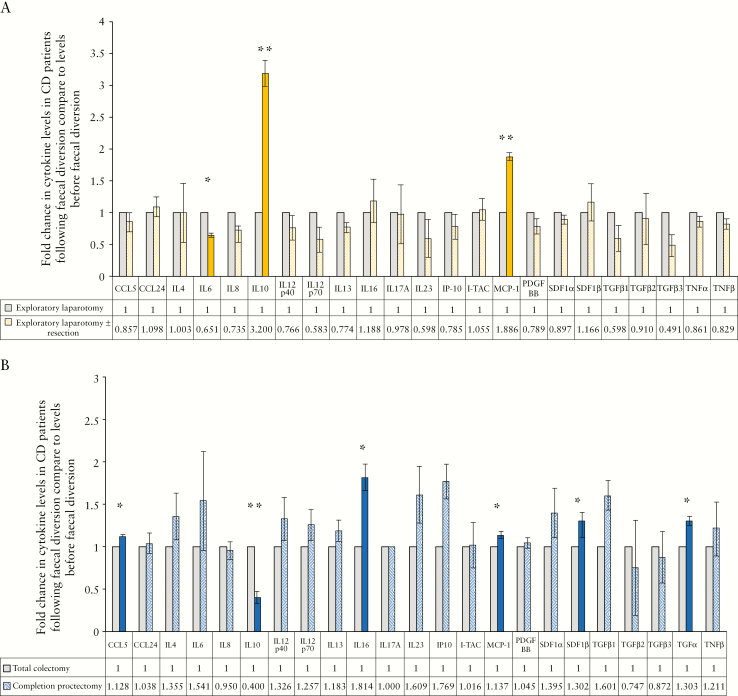
3.8. Faecal diversion results in a reduction of systemic inflammatory and fibrotic processes in Crohn’s disease but not in ulcerative colitis
Levels of post-faecal diversion plasma-borne cytokines in patients with CD [n = 4] and UC [n = 2] were compared with their respective levels in the same patients prior to faecal diversion and to levels in healthy controls [n = 4]. Demographics of patients who underwent faecal diversion, whose plasma was used for this study, are provided in Supplementary Table 5, available as Supplementary data at ECCO-JCC online.
Pro-inflammatory cytokines, including IL-6 [p = 0.012], IL-8 [p = 0.064], IL-12 [p40: p = 0.287; p70: p = 0.162], IL-23 [p = 0.247], and IP-10 [p = 0.331] decreased following faecal diversion in patients with CD [Figure 4A]. The same cytokines increased following faecal diversion in patients with UC [IL-6: p = 0.452; IL-8: p = 0.683; IL-12, p40: p = 0.329, p70: p = 0.296; IL-23: p = 0.210; IP-10: p = 0.065] [Figure 4B]. MCP-1 increased in patients with CD and, to a lesser extent, in UC, following faecal diversion [CD: p <0.001, UC: p = 0.024].
Figure 4.
The effect of faecal diversion on plasma-borne cytokine levels in patients with Crohn’s disease [CD] and patients with ulcerative colitis [UC]. [A] The average fold change in cytokines in patients with CD following faecal diversion when compared with their levels before faecal diversion. [B] The average fold change in cytokines in patients with UC following faecal diversion when compared with their levels before faecal diversion. All data are presented as fold changes when levels following faecal diversion were compared with levels before faecal diversion [data table on graph]. Solid colour bars and asterisks indicate cytokines significantly different in patients with CD or patients with UC following faecal diversion when compared with before faecal diversion. All experiments were completed in triplicate.
Of note, IL-10 significantly increased in patients with CD following faecal diversion [p = 0.008]. In contrast, IL-10 decreased in patients with UC in the same setting [p = 0.015] [Figure 4A, B]. Patients with CD displayed significantly increased [4-fold] levels of IL-10 following faecal diversion when compared with levels in healthy individuals [Supplementary Figure 4, available as Supplementary data at ECCO-JCC online]
Faecal diversion had no effect on levels of cytokines involved in fibrocyte recruitment in CD patients [Figure 4A], whereas it allowed increases in CCL5 [p = 0.012], SDF-1α [p = 0.306], and SDF-1β [p = 0.034] in patients with UC [Figure 4B]. TGF-β1 similarly increased following faecal diversion in patients with UC [p = 0.075] but decreased in patients with CD [p = 0.185] [Figure 4A, B].
4. Discussion
This is the first study to investigate and quantify levels of circulating fibrocytes in patients with UC [and is one of few studies to quantify their levels in CD] while, uniquely, determining the relationship between levels of circulating fibrocytes and known risk factors of IBD. Elevated circulating fibrocytes and distinct circulating cytokine profiles were associated with CD and UC when compared with healthy control samples. Patients with CD had increased levels in cytokines involved in fibrocyte recruitment and differentiation, whereas patients with UC displayed increased levels of pro-inflammatory cytokines.
It has been reported previously that circulating fibrocytes are elevated in patients with CD, and may have a role in its pathogenesis.28,46,48 We found that circulating fibrocytes are also increased in UC patients, suggesting a potential pathobiological role in that setting also. Fibrocytes, through differentiation to fibroblasts, myofibroblasts, and adipocytes, are capable of contributing to inflammation and fibrosis.28,41–46 However, although CD patients exhibited increases in plasma-borne cytokines associated with fibrocyte recruitment to sites of inflammation [e.g., CCL5, CCL24, and CXCL12] when compared with healthy controls, this was not replicated in UC patients. Therefore, although circulating fibrocytes are elevated in both CD and UC, it is reasonable to suggest that their recruitment to sites of inflammation in UC, such as the intestine and mesentery, may be compromised. Our data show that levels of circulating fibrocytes are highest in patients with stricturing CD, in agreement with previous reports of fibrocyte recruitment to sites of mucosal inflammation early in the inflammatory phase of CD, where they differentiate to fibroblasts and myofibroblasts and mediate stricturing.43,46
In our patient cohort, smoking or a history of smoking was associated with increased circulating fibrocytes in CD, but not UC, when compared with those who had never smoked. The differential effect of smoking in CD and UC remains to be fully elucidated. Smoking has a protective effect for patients with UC, while detrimental for patients with CD.55,56 Our data indicate that increased circulating fibrocytes may be implicated.
This study also represents the first time that changes in systemic disease mechanisms have been investigated in patients with CD or UC who underwent faecal diversion. Faecal diversion is associated with clinical remission and mucosal healing in CD, but not UC,4–7,12 although a mechanistic basis for this remains to be determined. Systemic responses following diversion of the faecal stream may provide an explanation for its clinical differential. Despite relatively low numbers of patients, we observed distinctly different systemic responses in patients with CD when compared with those with UC, upon removal [or reduction] of the stream of luminal contents. In CD patients, faecal diversion resulted in a decrease of circulating fibrocytes, a reduction of pro-inflammatory cytokines and TGF-β1, and an increase in anti-inflammatory IL-10. Conversely, levels of circulating fibrocytes did not change in UC patients following faecal diversion. Notably, increases were observed in pro-inflammatory cytokines, cytokines associated with fibrocyte recruitment and TGF-β1, in addition to a decrease in IL-10 in patients with UC.
In health, bacteria are maintained at low levels in the MLNs by the host immune system,57 whereas the gut microbiota is implicated in restriction of translocation of pathogenic bacteria to the MLNs.58 These defences may be compromised in IBD, particularly CD.13 The CD MLN microbiota reflects increases in Proteobacteria, whereas MLNs from UC patients have a microbiota similar to the healthy gut microbiome, albeit with increased Firmicutes.13,16,59,60 We have demonstrated previously that the microbial profile of MLNs taken from the same patient was similar, irrespective of sampling location,13 indicating that the MLN microbiome is influenced by disease rather than bacteria residing in the corresponding intestinal location. This suggests that MLN immune responses are disease-specific.
It is reasonable to suggest that the bacterial profile of MLNs from patients with CD or UC may influence their systemic responses. For instance, MLN production of TNFα in response to bacterial translocation has been demonstrated in a number of disease models.61–63 IBD has been associated with increased levels of TNFα,64 where it increases intestinal permeability65,66 and, in doing so, allows increased bacterial translocation. However, diversion of the faecal stream may allow clearance of bacterial DNA from the MLNs.13 Mechanistically, it may be that eradication of living cells or bacterial DNA from MLNs facilitates altered immune responses. If so, it can be postulated that removal of these factors from CD MLNs results in beneficial systemic responses for patients, with the inverse effect observed in UC. In UC MLNs, Firmicutes dominate, a phylum that contains numerous bacteria found in the healthy gut.13Faecalibacterium are abundant in MLNs from UC patients.13 These bacteria exert anti-inflammatory effects and are capable of inducing dendritic cell production of IL-10.23,24 It is possible that the removal of viable Faecalibcaterium and other healthy gut bacteria, and the associated eradication of bacterial DNA, from the intestine and MLNs could reduce levels of IL-10 and prove instrumental in eliciting a pro-inflammatory response in UC.
Furthermore, MCP-1 increased following faecal diversion in patients with CD and, to a lesser extent, UC. In addition to its role as a chemoattractant for macrophages to sites of inflammation, MCP-1 promotes an M2 macrophage phenotype. M2 macrophages are involved in tissue repair, and MCP-1-derived M2 macrophages produce increased levels of IL-10.67 The increase of MCP-1 facilitated by faecal diversion may allow this shift to the M2 macrophage phenotype, leading to tissue repair and a reduction in pro-inflammatory processes. Similarly butyrate, a short-chain fatty acid [SCFA], exerts anti-inflammatory effects in the intestine and is produced directly by members of Clostridia and F. prausnitzii [both Firmicutes] or can be converted from other SCFAs by bifidobacteria. These bacteria, through their role in butyrate production, and supplementation of butyrate itself, have been suggested as therapeutic options for IBD.68–72 Although desirable, it was not possible to assess breaches in intestinal integrity by immunohistochemistry or fluorescent cell staining.
IL-10 reduces production of pro-inflammatory cytokines, while increasing levels of anti-inflammatory cytokines,25–27 mediating its potential in medical therapy for IBD. However, previous clinical trials assessing the effect of IL-10 supplementation in IBD have yielded disappointing results,73,74 and it has been postulated that administered doses of IL-10 were too low to elicit a response.75 Our results suggest that IBD patients may benefit from a threshold level of four times the level of IL-10 present in healthy controls to induce therapeutic effects. The increase of IL-10 observed in patients with CD correlated with a decrease in pro-inflammatory cytokines. Arguably, future studies investigating IL-10 therapy for IBD could usefully consider these threshold levels, and the balance of pro-inflammatory cytokines relative to levels of IL-10.
In conclusion, the distinct cytokine profiles associated with CD and UC indicate differing mechanisms for the diseases. The negative effect of smoking observed in CD, but not UC, may be partly explained by increased circulating fibrocytes. Systemic responses to faecal diversion also differ in CD and UC. This may provide us with an opportunity to understand the mechanisms mediating the disease, including the role of the MLN microbiota. Notably, we have identified an association between clinical improvement and increased IL-10, further supporting the potential of IL-10 in therapy for IBD. As per our results, a threshold level of four times the amount of IL-10 present in healthy individuals may be necessary to elicit a beneficial effect in IBD. This putative therapeutic strategy could be employed pragmatically by measuring levels of circulating IL-10 befor and at regular intervals during supplementation, in addition to monitoring the ratio of IL-10 to levels of pro-inflammatory cytokines.
Supplementary Material
Acknowledgments
We thank Dr Patrick Kiely and his laboratory personnel [Graduate Entry Medical School, University of Limerick] for help in use of the Odyssey Sa.
Funding
This work was supported by the Graduate Entry Medical School [University of Limerick] Strategic Research Fund.
Conflict of Interest
The authors declare no competing personal or financial interests and have nothing to disclose.
Author Contributions
MGK collected and processed blood samples, performed experiments, and was involved in study design, clinical data collection, data analysis, and drafting of manuscript. JCC was involved in study design, sample collection, and drafting of manuscript. SMS aided in blood sample collection and processing and was involved in fibrocyte analysis and clinical data collection. PT aided in blood sample collection and processing, and was involved in clinical data collection. EML aided in clinical data collection. EOL aided in blood sample collection and was involved in clinical data collection. FO aided in blood sample collection and processing and fibrocyte analysis, and was involved in clinical data collection. CPD was involved in study design, data analysis, and drafting of manuscript.
References
- 1. Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet 2012;380:1590–605. [DOI] [PubMed] [Google Scholar]
- 2. Sartor RB. Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol 2006;3:390–407. [DOI] [PubMed] [Google Scholar]
- 3. Burman JH, Thompson H, Cooke WT, Williams JA. The effects of diversion of intestinal contents on the progress of Crohn’s disease of the large bowel. Gut 1971;12:11–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Harper PH, Truelove SC, Lee EC, Kettlewell MG, Jewell DP. Split ileostomy and ileocolostomy for Crohn’s disease of the colon and ulcerative colitis: a 20 year survey. Gut 1983;24:106–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Winslet MC, Allan A, Poxon V, Youngs D, Keighley MR. Faecal diversion for Crohn’s colitis: a model to study the role of the faecal stream in the inflammatory process. Gut 1994;35:236–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Edwards CM, George BD, Jewell DP, Warren BF, Mortensen NJ, Kettlewell MG. Role of a defunctioning stoma in the management of large bowel Crohn’s disease. Br J Surg 2000;87:1063–6. [DOI] [PubMed] [Google Scholar]
- 7. Mennigen R, Heptner B, Senninger N, Rijcken E. Temporary fecal diversion in the management of colorectal and perianal Crohn’s disease. Gastroenterol Res Pract 2015;2015:286315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Singh S, Ding NS, Mathis KL, et al. Systematic review with meta-analysis: faecal diversion for management of perianal Crohn’s disease. Aliment Pharmacol Ther 2015;42:783–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McIlrath DC. Diverting ileostomy or colostomy in the management of Crohn’s disease of the colon. Arch Surg 1971;103:308–10. [DOI] [PubMed] [Google Scholar]
- 10. Truelove SC, Ellis H, Webster CU. Place of a double-barrelled ileostomy in ulcerative colitis and Crohn’s disease of the colon: a preliminary report. Br Med J 1965;1:150–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mueller MH, Geis M, Glatzle J, et al. Risk of fecal diversion in complicated perianal Crohn’s disease. J Gastrointest Surg 2007;11:529–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rutgeerts P, Goboes K, Peeters M, et al. Effect of faecal stream diversion on recurrence of Crohn’s disease in the neoterminal ileum. Lancet 1991;338:771–4. [DOI] [PubMed] [Google Scholar]
- 13. Kiernan MG, Coffey JC, McDermott K, et al. The human mesenteric lymph node microbiome differentiates between Crohn’s disease and ulcerative colitis. J Crohns Colitis 2019;13:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pascal V, Pozuelo M, Borruel N, et al. A microbial signature for Crohn’s disease. Gut 2017;66:813–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Forbes JD, Van Domselaar G, Bernstein CN. Microbiome survey of the inflamed and noninflamed gut at different compartments within the gastrointestinal tract of inflammatory bowel disease patients. Inflamm Bowel Dis 2016;22:817–25. [DOI] [PubMed] [Google Scholar]
- 16. Halfvarson J, Brislawn CJ, Lamendella R, et al. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat Microbiol 2017;2:17004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol 2003;3:331–41. [DOI] [PubMed] [Google Scholar]
- 18. Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol 2004;4:478–85. [DOI] [PubMed] [Google Scholar]
- 19. Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science 2004;303:1662–5. [DOI] [PubMed] [Google Scholar]
- 20. Wei B, Velazquez P, Turovskaya O, et al. Mesenteric B cells centrally inhibit CD4+ T cell colitis through interaction with regulatory T cell subsets. Proc Natl Acad Sci U S A 2005;102:2010–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sokol H, Pigneur B, Watterlot L, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A 2008;105:16731–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sokol H, Seksik P, Furet JP, et al. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm Bowel Dis 2009;15:1183–9. [DOI] [PubMed] [Google Scholar]
- 23. Quévrain E, Maubert MA, Michon C, et al. Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn’s disease. Gut 2016;65:415–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rossi O, van Berkel LA, Chain F, et al. Faecalibacterium prausnitzii A2-165 has a high capacity to induce IL-10 in human and murine dendritic cells and modulates T cell responses. Sci Rep 2016;6:18507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Asadullah K, Sterry W, Volk HD. Interleukin-10 therapy – review of a new approach. Pharmacol Rev 2003;55:241–69. [DOI] [PubMed] [Google Scholar]
- 26. de Moreno de Leblanc A, Del Carmen S, Zurita-Turk M, et al. Importance of IL-10 modulation by probiotic microorganisms in gastrointestinal inflammatory diseases. ISRN Gastroenterol 2011;2011:892971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Iyer SS, Cheng G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit Rev Immunol 2012;32:23–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sahebally SM, Burke JP, Chang KH, Kiernan MG, O’Connell PR, Coffey JC. Circulating fibrocytes and Crohn’s disease. Br J Surg 2013;100:1549–56. [DOI] [PubMed] [Google Scholar]
- 29. Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol 2003;171:380–9. [DOI] [PubMed] [Google Scholar]
- 30. Phillips RJ, Burdick MD, Hong K, et al. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest 2004;114:438–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Haudek SB, Xia Y, Huebener P, et al. Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proc Natl Acad Sci U S A 2006;103:18284–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kisseleva T, Uchinami H, Feirt N, et al. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J Hepatol 2006;45:429–38. [DOI] [PubMed] [Google Scholar]
- 33. Maloo M, Abt P, Kashyap R, et al. Nephrogenic systemic fibrosis among liver transplant recipients: a single institution experience and topic update. Am J Transplant 2006;6:2212–7. [DOI] [PubMed] [Google Scholar]
- 34. Quan TE, Cowper SE, Bucala R. The role of circulating fibrocytes in fibrosis. Curr Rheumatol Rep 2006;8:145–50. [DOI] [PubMed] [Google Scholar]
- 35. Sakai N, Wada T, Yokoyama H, et al. Secondary lymphoid tissue chemokine [SLC/CCL21]/CCR7 signaling regulates fibrocytes in renal fibrosis. Proc Natl Acad Sci U S A 2006;103:14098–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mehrad B, Burdick MD, Zisman DA, Keane MP, Belperio JA, Strieter RM. Circulating peripheral blood fibrocytes in human fibrotic interstitial lung disease. Biochem Biophys Res Commun 2007;353:104–8. [DOI] [PubMed] [Google Scholar]
- 37. Keeley EC, Mehrad B, Strieter RM. The role of fibrocytes in fibrotic diseases of the lungs and heart. Fibrogenesis Tissue Repair 2011;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bellini A, Mattoli S. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Invest 2007;87:858–70. [DOI] [PubMed] [Google Scholar]
- 39. Galligan CL, Fish EN. Circulating fibrocytes contribute to the pathogenesis of collagen antibody-induced arthritis. Arthritis Rheum 2012;64:3583–93. [DOI] [PubMed] [Google Scholar]
- 40. Hong KM, Belperio JA, Keane MP, Burdick MD, Strieter RM. Differentiation of human circulating fibrocytes as mediated by transforming growth factor-beta and peroxisome proliferator-activated receptor gamma. J Biol Chem 2007;282:22910–20. [DOI] [PubMed] [Google Scholar]
- 41. Peyrin-Biroulet L, Chamaillard M, Gonzalez F, et al. Mesenteric fat in Crohn’s disease: a pathogenetic hallmark or an innocent bystander? Gut 2007;56:577–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zulian A, Cancello R, Micheletto G, et al. Visceral adipocytes: old actors in obesity and new protagonists in Crohn’s disease? Gut 2012;61:86–94. [DOI] [PubMed] [Google Scholar]
- 43. Burke JP, Mulsow JJ, O’Keane C, Docherty NG, Watson RW, O’Connell PR. Fibrogenesis in Crohn’s disease. Am J Gastroenterol 2007;102:439–48. [DOI] [PubMed] [Google Scholar]
- 44. Coffey JC, O’Leary DP, Kiernan MG, Faul P. The mesentery in Crohn’s disease: friend or foe? Curr Opin Gastroenterol 2016;32:267–73. [DOI] [PubMed] [Google Scholar]
- 45. Takahashi Y, Sato S, Kurashima Y, et al. Reciprocal inflammatory signaling between intestinal epithelial cells and adipocytes in the absence of immune cells. EBioMedicine 2017;23:34–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sazuka S, Katsuno T, Nakagawa T, et al. Fibrocytes are involved in inflammation as well as fibrosis in the pathogenesis of Crohn’s disease. Dig Dis Sci 2014;59:760–8. [DOI] [PubMed] [Google Scholar]
- 47. Borley NR, Mortensen NJ, Jewell DP, Warren BF. The relationship between inflammatory and serosal connective tissue changes in ileal Crohn’s disease: evidence for a possible causative link. J Pathol 2000;190:196–202. [DOI] [PubMed] [Google Scholar]
- 48. Coffey CJ, Kiernan MG, Sahebally SM, et al. Inclusion of the mesentery in ileocolic resection for Crohn’s disease is associated with reduced surgical recurrence. J Crohns Colitis 2018;12:1139–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005;19[Suppl A]:5A–36A. [DOI] [PubMed] [Google Scholar]
- 50. Wang CH, Huang CD, Lin HC, et al. Increased circulating fibrocytes in asthma with chronic airflow obstruction. Am J Respir Crit Care Med 2008;178:583–91. [DOI] [PubMed] [Google Scholar]
- 51. Moeller A, Gilpin SE, Ask K, et al. Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2009;179:588–94. [DOI] [PubMed] [Google Scholar]
- 52. Isgrò M, Bianchetti L, Marini MA, Bellini A, Schmidt M, Mattoli S. The C-C motif chemokine ligands CCL5, CCL11, and CCL24 induce the migration of circulating fibrocytes from patients with severe asthma. Mucosal Immunol 2013;6:718–27. [DOI] [PubMed] [Google Scholar]
- 53. Ishida Y, Kimura A, Kondo T, et al. Essential roles of the CC chemokine ligand 3-CC chemokine receptor 5 axis in bleomycin-induced pulmonary fibrosis through regulation of macrophage and fibrocyte infiltration. Am J Pathol 2007;170:843–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol 2001;166:7556–62. [DOI] [PubMed] [Google Scholar]
- 55. Mahid SS, Minor KS, Soto RE, Hornung CA, Galandiuk S. Smoking and inflammatory bowel disease: a meta-analysis. Mayo Clin Proc 2006;81:1462–71. [DOI] [PubMed] [Google Scholar]
- 56. Tuvlin JA, Raza SS, Bracamonte S, et al. Smoking and inflammatory bowel disease: trends in familial and sporadic cohorts. Inflamm Bowel Dis 2007;13:573–9. [DOI] [PubMed] [Google Scholar]
- 57. Macpherson AJ, Smith K. Mesenteric lymph nodes at the center of immune anatomy. J Exp Med 2006;203:497–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Diehl GE, Longman RS, Zhang JX, et al. Microbiota restricts trafficking of bacteria to mesenteric lymph nodes by CX3CR1hi cells. Nature 2013;494:116–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hong PY, Croix JA, Greenberg E, Gaskins HR, Mackie RI. Pyrosequencing-based analysis of the mucosal microbiota in healthy individuals reveals ubiquitous bacterial groups and micro-heterogeneity. PLoS One 2011;6:e25042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012;486:207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mainous MR, Ertel W, Chaudry IH, Deitch EA. The gut: a cytokine-generating organ in systemic inflammation? Shock 1995;4:193–9. [PubMed] [Google Scholar]
- 62. Genescà J, Martí R, Rojo F, et al. Increased tumour necrosis factor alpha production in mesenteric lymph nodes of cirrhotic patients with ascites. Gut 2003;52:1054–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Deitch EA. Role of the gut lymphatic system in multiple organ failure. Curr Opin Crit Care 2001;7:92–8. [DOI] [PubMed] [Google Scholar]
- 64. Ślebioda TJ, Kmieć Z. Tumour necrosis factor superfamily members in the pathogenesis of inflammatory bowel disease. Mediators Inflamm 2014;2014:325129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Marini M, Bamias G, Rivera-Nieves J, et al. TNF-alpha neutralization ameliorates the severity of murine Crohn’s-like ileitis by abrogation of intestinal epithelial cell apoptosis. Proc Natl Acad Sci U S A 2003;100:8366–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Su L, Nalle SC, Shen L, et al. TNFR2 activates MLCK-dependent tight junction dysregulation to cause apoptosis-mediated barrier loss and experimental colitis. Gastroenterology 2013;145:407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Takada Y, Hisamatsu T, Kamada N, et al. Monocyte chemoattractant protein-1 contributes to gut homeostasis and intestinal inflammation by composition of IL-10-producing regulatory macrophage subset. J Immunol 2010;184:2671–6. [DOI] [PubMed] [Google Scholar]
- 68. von Wright A, Vilpponen-Salmela T, Llopis MP, et al. The survival and colonic adhesion of Bifidobacterium infantis in patients with ulcerative colitis. Int Dairy J 2002;12:197–200. [Google Scholar]
- 69. Canani RB, Costanzo MD, Leone L, Pedata M, Meli R, Calignano A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J Gastroenterol 2011;17:1519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kanai T, Mikami Y, Hayashi A. A breakthrough in probiotics: Clostridium butyricum regulates gut homeostasis and anti-inflammatory response in inflammatory bowel disease. J Gastroenterol 2015;50:928–39. [DOI] [PubMed] [Google Scholar]
- 71. Martín R, Miquel S, Benevides L, et al. Functional characterization of novel Faecalibacterium prausnitzii strains isolated from healthy volunteers: a step forward in the use of F. prausnitzii as a next-generation probiotic. Front Microbiol 2017;8:1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rivière A, Selak M, Lantin D, Leroy F, De Vuyst L. Bifidobacteria and butyrate-producing colon bacteria: importance and strategies for their stimulation in the human gut. Front Microbiol 2016;7:979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Colombel JF, Rutgeerts P, Malchow H, et al. Interleukin 10 [Tenovil] in the prevention of postoperative recurrence of Crohn’s disease. Gut 2001;49:42–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Schreiber S, Fedorak RN, Nielsen OH, et al. Safety and efficacy of recombinant human interleukin 10 in chronic active Crohn’s disease. Crohn’s Disease IL-10 Cooperative Study Group. Gastroenterology 2000;119:1461–72. [DOI] [PubMed] [Google Scholar]
- 75. Marlow GJ, van Gent D, Ferguson LR. Why interleukin-10 supplementation does not work in Crohn’s disease patients. World J Gastroenterol 2013;19:3931–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.