Abstract
Background and Aims
CD14+ mononuclear phagocytes [MNPs] and T cells infiltrate colon in ulcerative colitis [UC]. Here we investigated how CD14+ MNPs and the cytokines they produce shape the colonic effector T cell profile.
Methods
Colonic or mesenteric lymph node [mLNs] CD4+ T cells isolated from UC or Crohn’s disease [CD] patients were stimulated with cytokines or autologous CD14+ MNPs. Cytokine expression was assessed by intracytoplasmic staining and multiplex ELISA. Unsupervised phenotypic multicolour analysis of colonic CD14+ MNPs was performed using the FlowSOM algorithm.
Results
Among CD14+CD64+HLA-DR+SIRPα + MNPs, only the pro-inflammatory cytokine-producing CD163− subpopulation accumulated in inflamed UC colon and promoted mucosal IL-1β-dependent Th17, Th17/Th1, Th17/Th22 but not Th1 responses. Unsupervised phenotypic analysis of CD14+CD64+ MNPs segregated CD163− monocyte-like cells and CD163+ macrophages. Unexpectedly, IL-12, IL-1β and CD163−, but not CD163+, cells induced IL-8 expression in colonic CD4+ T cells, which co-expressed IFN-γ and/or IL-17 in UC and not CD. The CD163− monocyte-like cells increased the frequency of IL-8+IL-17+/−IFN-γ +/− T cells through IL-1β and IL-12. Finally, colonic IL-8+ T cells co-expressing GM-CSF, TNF-α and IL-6 were detected ex vivo and, promoted by IL-12 in the mucosa and mLNs in UC only.
Conclusions
Our findings established a link between monocyte-like CD163− MNPs, IL-12, IL-1β and the detection of colonic memory IL-8-producing CD4+ T cells, which might all contribute to the pathogenesis of UC.
Keywords: IL8, colonic T cells, Ulcerative colitis, Crohn’s disease, colonic mononuclear phagocytes
1. Introduction
Ulcerative colitis [UC] is a chronic inflammatory disease of the colon. Epithelial cells that produce mucus, the microbiota as well as innate and adaptive immune cells all contribute to the pathophysiology of the disease.1 UC was first considered a Th2 disease because elevated levels of interleukin-5 [IL-5] and IL-13 were detected in the colonic mucosa of patients.2,3 Nonetheless, the view that UC is a type 2 disease distinct from Th1-associated Crohn’s disease [CD], the other common form of inflammatory bowel disease [IBD], has been challenged in the last few years.4,5 First, some studies did not note the increased IL-5 and IL-13 expression in the mucosa of UC patients,6 while others proposed a protective role for IL-13 in paediatric patients with UC.7 Also, two anti-IL-13 monoclonal antibodies failed to improve outcome in UC patients.8,9 Second, the discovery of IL-17-secreting CD4+ T cells [Th17] brought new insights into the pathophysiology of UC. IL-17 and interferon-γ [IFN-γ] mRNA were elevated in biopsies from UC mucosa when compared to normal controls.10–12 Furthermore, Th17 and IL-17+IFN-γ +CD4+ T cells [Th17/Th1] were observed in inflamed mucosa of patients with UC.13–16 However, Th17 cell fate towards a Th17/Th1 profile has been much less well studied in UC than in CD. In inflamed CD mucosa, IL-17+IFN-γ + T cells accumulate in lesional sites and participate in the mucosal inflammatory process.15,17,18 The cytokines that drive the shift from Th17 towards Th17/Th1 cells have been described in CD. CD161+CD4+ T cells isolated from patients with CD express IL-23R, and produce IL-17 and IFN-γ under IL-23 stimulation.19 Moreover, IL-23 was shown to increase the percentage of IFN-γ +IL-17+CD4+ T cells, particularly in an MDR1+IL-23R+ Th17/Th1 population isolated from the blood of healthy donors.17 Recombinant IL-12, which shares a common IL-12p40 chain with IL-23, increases IFN-γ expression while decreasing IL-17 in Th17 clones isolated from the mucosa of patients with CD.18 Although IL-12 augments IFN-γ secretion in the culture supernatant [CSN] of CD3 and CD28 stimulated lamina propria mononuclear cells [LPMCs] from CD and UC patients,10 the regulation of mucosal Th17, Th17/Th1 and Th1 responses remain to be fully investigated in UC.
IL-23 and IL-12 are pro-inflammatory cytokines, which are produced by mononuclear phagocytes [MNPs]. Currently, MNPs are classified as dendritic cells [DCs], macrophages [Mɸ], inflammatory monocytes and/or monocyte-derived cells.20 In the mucosa of patients with UC, a population expressing the monocyte marker CD14 has been reported21; they are considered as Mɸ or monocyte-derived cells.22,23 Also, Magnusson et al. described the accumulation of an HLA-DRdimCD64+ subset in the inflamed mucosa of UC patients compared to normal controls.24 These mucosal MNPs secrete pro-inflammatory cytokines including TNF-α, IL-23, IL-1β and IL-6.21 However, the impact of mucosal CD14+ MNPs on effector memory CD4+ T cell responses remains to be investigated in UC.
In the present study, we examined whether and how CD14+ MNPs that infiltrate the mucosa of patients with UC control the autologous memory colonic Th response. We showed that the pro-inflammatory CD14+ monocyte-like subpopulation, which did not express CD163, accumulated in inflamed colonic UC mucosa and favoured Th17 and Th17/Th1 but not Th1 responses. A few reports have shown that circulating T cells isolated from healthy adults and cord blood secrete IL-8.25–27 This chemokine plays a key role in the of pathogenesis UC.28–30 Unexpectedly, our data further revealed that CD163− but not CD163+ MNPs augmented IL-8 expression in colonic memory CD4+ T cells in UC but not CD.
2. Materials and Methods
2.1. Human clinical samples
All participants signed an informed consent form that has been approved by the Institutional Ethics Research Committee of the Centre Hospitalier de l’Université de Montréal [CHUM]. This study includes 83 patients with UC [median age 42 years], 19 patients with CD [median age 37 years] and six patients undergoing a screening colonoscopy [non-IBD] [median age 60 years] [Table 1]. IBD patient recruitment was based on clinical, endoscopic activity and histological criteria. UC patients presented with bloody stools, diarrhoea and abdominal pain. Endoscopically, they showed continuous inflammation, extending from the rectum to the colon. CD patients presented with diarrhoea, weight loss or abdominal pain. Endoscopically, the mucosa was eroded and exhibited patchy inflammation, deep ulcers and/or strictures. Histologically, the architecture of the crypts was disturbed; the mucosa was infiltrated by mono- or polynuclear cells, with or without pathognomonic granuloma in the case of CD patients. No histological data or bacteriological infections suggested a differential diagnosis. An endoscopic score [Mayo or SES-CD] was not available for most of the IBD patients because scoring is not performed routinely by gastroenterologists at CHUM. Disease remission was assessed on the basis of endoscopic criteria. Non-inflamed and inflamed colonic tissues [from the same patient], non-IBD [control] colonic tissue and mesenteric lymph nodes [mLNs] from UC patients only were acquired from endoscopic biopsies or surgical resections, respectively.
Table 1.
Patient characteristics.
| UC | CD | Non-IBD | |
|---|---|---|---|
| N | 83 | 19 | 6 |
| Females, n [%] | 47 [56.6] | 11 [63.1] | 3 [50] |
| Age, years, median [range] | 42 [18–80] | 37 [21–80] | 60 [36–76] |
| Age at diagnosis, years | |||
| <16 | 6 | 3 | |
| 17–40 | 52 | 11 | |
| >40 | 25 | 5 | |
| Treatment | |||
| None | 15 | 8 | |
| 5-ASA alone | 38 | 1 | |
| Thiopurine or methotrexate | 14 | 6 | |
| TNFα inhibitor | 9 | 4 | |
| Corticosteroid | 21 | 2 | |
| Disease location: UC | |||
| Proctitis | 15 | ||
| Left-sided colitis | 39 | ||
| Pancolitis | 26 | ||
| Proximal colitis | 3 | ||
| Disease location: CD | |||
| Terminal ileum | 0 | ||
| Colon | 15 | ||
| Ileocolonic | 4 | ||
| Upper GI tract | 0 | ||
| Disease behavioor | |||
| Non-stricturing/non-penetrating | 15 | ||
| Stricturing | 3 | ||
| Penetrating | 1 | ||
| Perianal disease | 3 | ||
| Diagnosis: Control | |||
| Screening colonoscopy | 6 |
Abbreviations: UC, ulcerative colitis; CD, Crohn’s disease; IBD, inflammatory bowel disease; 5-ASA, 5-aminosalicylic acid; TNF, tumour necrosis factor; GI, gastrointestinal.
2.2. Cell purification
Intestinal mucosa, from biopsies or surgical samples, was first processed by enzymatic digestion with DNase I and Collagenase D [both Roche] followed by mechanical digestion with gentle magnetically activated cell sorting [Miltenyi Biotec] to isolate LPMCs. MLNs were digested mechanically to obtain cellular suspensions.22
2.3. Cell staining
LPMCs were stained using the monoclonal antibodies listed in Supplementary Table S1, and analyses were performed with FCS Express 6 [de novo software] or FlowJo v10.5.3. Unsupervised analyses were performed using plugins available (t-SNE [t-Distributed Stochastic Neighbor Embedding] and FlowSOM [Flow self-organizing map]),31,32 in FlowJo. The data were manually gated on single viable CD45+HLADR+SIPRα +CD14+CD64+ cells, and all gated cells were subjected to analysis. The cells were assigned to a self-organizing map, automatically segregating cells into five clusters and visualizing data in four ways: [a] cell affiliation in five clusters visualized in t-SNE plot; [b] surface marker expression level visualized by histograms; [c] relative mean intensities depicted according to colour gradient in a heatmap; and [d] assignment of cells to the self-organizing map with a 5 × 5 grid, resulting in 25 nodes, depicted as a minimal spanning tree, built to visualize similar nodes in branches. A concatenated file from four UC patients as well as each individual file were analysed separately with t-SNE and FlowSOM algorithms. The FlowSOM algorithm was run five times to ensure reproducibility of the results.
2.4. Cell sorting
Human CD4+CD8−CD45RA−CD25− mucosal T cells and HLA-DR+SIRPα +CD14+CD64+ MNPs co-expressing or not expressing CD163 were sorted at the same time to perform co-culture experiments. HLA-DR+SIRPα +CD14+CD64+ MNPs co-expressing or not expressing CD163 were also sorted to analyse cell morphology. MLN CD4+CD45RO+CD62LlowCD8−CD45RA−CD25− effector memory T cells [TEM] were stratified into CCR6+CXCR3− [Th17 TEM] and CCR6−CXCR3+ [Th1 TEM] and sorted for culture with either IL-1β or IL-12, and ex vivo phorbol 12-myristate 13-acetate [PMA] ionomycin stimulation. Sorting was performed using a fluorescence activated cell sorting [FACS] Aria II cell sorter and data were analysed using FACS Diva 6 [BD Biosciences].
2.5. In vitro MNP/T cell co-cultures
Total CD4+ T cells, depleted in CD8+ T cells, CD25+ regulatory T cells and CD45RA+ naïve T cells, were purified from inflamed colon. T cells were stimulated with anti-CD3/CD28 coated beads [Miltenyi Biotec], and either [a] cultured with or without IL-1β [10 ng/mL, R&D systems], IL-12 [20 ng/mL, R&D systems] or IL-23 [10 ng/mL, R&D system] for 6 days; or [b] co-cultured with autologous MNP subsets purified from inflamed colonic mucosa, at a 10:1 ratio for 6 days, in the presence of peptidoglycan [10 µg/mL]. For some experiments, anti-IL-1β receptor [10 µg/mL], anti-IL-1β [10 µg/mL] or anti-IL12p70 [10 µg/mL, R&D systems] monoclonal antibodies [mAbs] were added to the co-cultures.
Total CD4+CD8−CD45RA−CD25− T cells, Th17 TEM and Th1 TEM purified from mLNs were co-cultured in the presence of anti-CD3/CD28-coated beads, with or without IL-1β [10 ng/mL] or IL-12 [20 ng/mL] for 6 days.
For all cultures: [a] RPMI 1640 medium with 10% fetal calf serum [FCS] and 1% penicillin/streptomycin was used; [b] for intracytoplasmic staining, cells were re-stimulated after culture, with PMA and ionomycin for 6 h in the presence of brefeldin A for the last 3 h, then fixed and stained with mAbs (CD3, IL-17, IFN-γ, IL-8, IL-22, IL-6, tumour necrosis factor-α [TNF-α], granulocyte-macrophage colony-stimulating factor [GM-CSF], as listed in Supplementary Table S1); and [c] IL-17, IFN-γ, IL-6, TNF-α, GM-CSF and IL-8 release were measured by a multiplex assay [Eve Technologies] in the culture supernatants.
2.6. Cytokine expression
Ex vivo isolated LPMCs were immediately stained for CD45, HLA-DR, CD172α [SIRPα], CD64 and CD163, in the absence of brefeldin A, then fixed/permeabilized and stained for intracytoplasmic cytokine expression [IL-1β, IL-10, IL-12p40 and IL-23].
Freshly isolated LPMCs were cultured with PMA and ionomycin for 4 h, in the presence of brefeldin A, then fixed and stained for CD45, CD3, CD4, CD8 and CD25. Intra-cytoplasmic expression of Foxp3, IL-8, IL-17A, TNF-α, IFN-γ, IL-6 and GM-CSF was evaluated after permeabilization. Co-expression of IL-17A, TNF-α, IFN-γ, IL-6 and GM-CSF was evaluated in CD3+CD4+CD8−CD25−Foxp3−IL-8+ cells.
Freshly purified Th1 TEM and Th17 TEM were stimulated with PMA and ionomycin for 4 h, in the presence of brefeldin A, then stained for intracytoplasmic IFN-γ and IL-17 expression.
2.7. Morphology
For morphological studies, FACS-sorted MNPs were cytospun and stained according to the Wright Stain procedure. A Leica DM4000B microscope, equipped with Leica DFC300FX camera, was used to visualize cells.
2.8. Statistical analysis
Statistical analysis was performed with GraphPad Prism version 6 [GraphPad Software]. Data were checked for normality using a Shapiro–Wilk test and then the appropriate test was applied as indicated in the figure legends. Two-tailed Wilcoxon’s signed rank test [represented by *] and Mann–Whitney test [represented by §] were used. Friedman’s test was used followed by Dunn’s test [represented by Ω]. The threshold for significance was adjusted when indicated to account for test multiplicity. A Kruskal–Wallis test was employed followed by Dunn’s test [represented by #]. Repeated-measures one way ANOVA was employed followed by Bonferroni test [represented by ¤]. For all tests, one symbol indicates p < 0.05, two symbols p < 0.01 and three symbols p < 0.001. Bar graph data are shown as mean ± SEM
3. Results
3.1. IL-1β promotes Th17 and Th17/Th1 responses in CD4+ T cells isolated from inflamed colon of UC patients
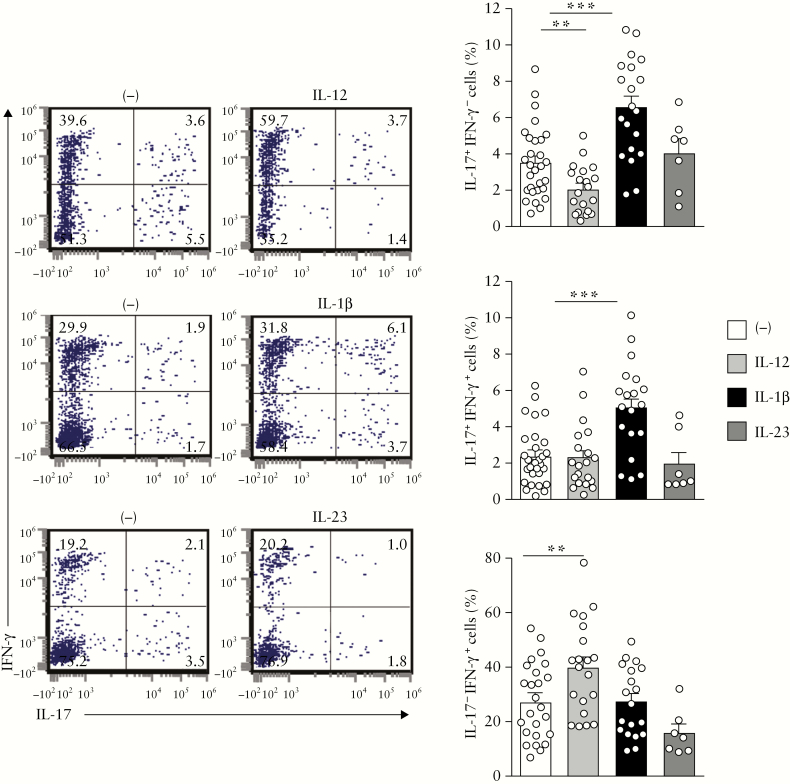
IL-23 and IL-12 are key cytokines in Th17/Th1-associated CD pathogenesis.17–19 Here, we first evaluated how IL-23 and IL-12 regulated the Th17, Th17/Th1 and Th1 profiles of CD4+ T cells isolated from inflamed colon of UC patients. As expected, IL-12 augmented the frequency of single IFN-γ-producing CD4+ T cells [Th1] [p < 0.002] and decreased the frequency of single IL-17-producing CD4+ T cells [Th17] [p < 0.006]. However, the percentages of Th17 and double IL-17/IFN-γ-producing CD4+ T cells [Th17/Th1] were not modulated by IL-23 [Figure 1]. As recently reported in CD,33 IL-1β significantly increased Th17 [p < 0.0002] and Th17/Th1 [p < 0.0002] but not Th1 responses in all UC patients examined [Figure 1].
Figure 1.
IL-1β increases Th17 and Th17/Th1 responses in UC patients. Representative dot plots and percentages of mucosal CD4+ T cells expressing IL-17 and/or IFN-γ after 6 days of culture with either recombinant IL-12 [n = 19], IL-1β [n = 18] or IL-23 [n = 7]. A Wilcoxon signed rank test, p < 0.01 threshold for significance to account for test multiplicity.
Thus, IL-1β, but not IL-23 or IL-12, promotes a Th17 and Th17/Th1 profile in mucosal CD4+ T cells in UC.
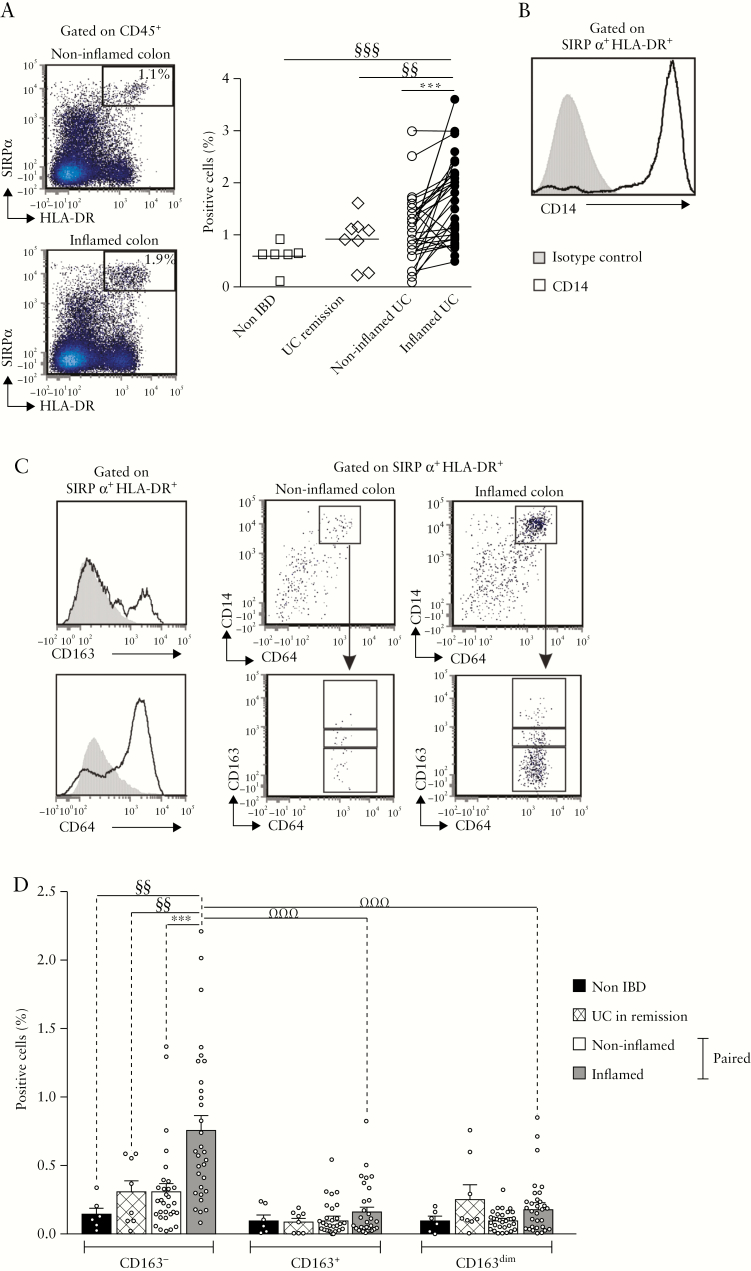
3.2. HLA-DR+SIRPα+CD14+CD64+CD163− cells selectively accumulate in inflamed UC mucosa
CD14+ MNPs are a cellular source of IL-1β, IL-12 and IL-23 in inflamed gut mucosa.21 A previous report showed that HLA-DR+SIRPα + MNPs accumulate in inflamed compared to non-inflamed colonic mucosa of patients with CD22. Here we examined whether MNPs with a similar phenotype infiltrated the UC mucosa. The frequencies of HLA-DR+SIRPα + MNPs were higher in inflamed relative to paired non-inflamed colonic mucosa [n = 31], as well as to colon of UC patients in remission [n = 8] and non-IBD controls [n = 6] [p < 0.0007, p < 0.007 and p < 0.009, respectively; Figure 2a]. In inflamed UC mucosa, more than 95% of HLA-DR+SIRPα + MNPs expressed CD14 [Figure 2b]. To assess the heterogeneity of the HLA-DR+SIRPα +CD14+ population, these cells were further stratified according to expression of CD64 [the Fc-gamma receptor 1] and scavenger receptor CD163, expressed on human gut Mɸ.34,35 CD14+CD64+ cells were subdivided according to the intensity of CD163 expression [Figure 2c]. The data revealed that CD163− cells but not CD163dim or CD163+ cells accumulated in inflamed compared to paired non-inflamed UC colon [p < 0.0001], and were detected in low frequencies in healed mucosa of UC patients in endoscopic remission and control patients [p < 0.01 and p < 0.008, respectively; Figure 2d].
Figure 2.
HLA-DR+SIRPα +CD14+CD64+CD163− MNPs accumulate in inflamed UC mucosa. [a] Percentage of HLA-DR+SIRPα + cells among CD45+ intestinal lamina propria mononuclear cells [LPMCs]: cell distribution in non-IBD control [n = 6], UC in remission [n = 8], and paired non-inflamed and inflamed UC patients [n = 31]. [b] CD14 expression on HLA-DR+SIRPα + cells. [c] HLA-DR+SIRPα +CD14+ MNPs subdivided according to CD64 and CD163 expression. [d] Frequencies of CD163−, CD163+, CD163dim cells among CD45+ LPMCs in non-IBD control [n = 6], UC in remission [n = 8], and paired non-inflamed and inflamed UC patients [n = 31]. [a,d] Wilcoxon signed rank test, Mann–Whitney test and Friedman test with Dunn’s post test, p < 0.01 threshold for significance to account for test multiplicity.
In conclusion, CD163− cells are the predominant HLA-DR+SIRPα +CD14+CD64+ MNP subpopulation that infiltrates inflamed UC mucosa.
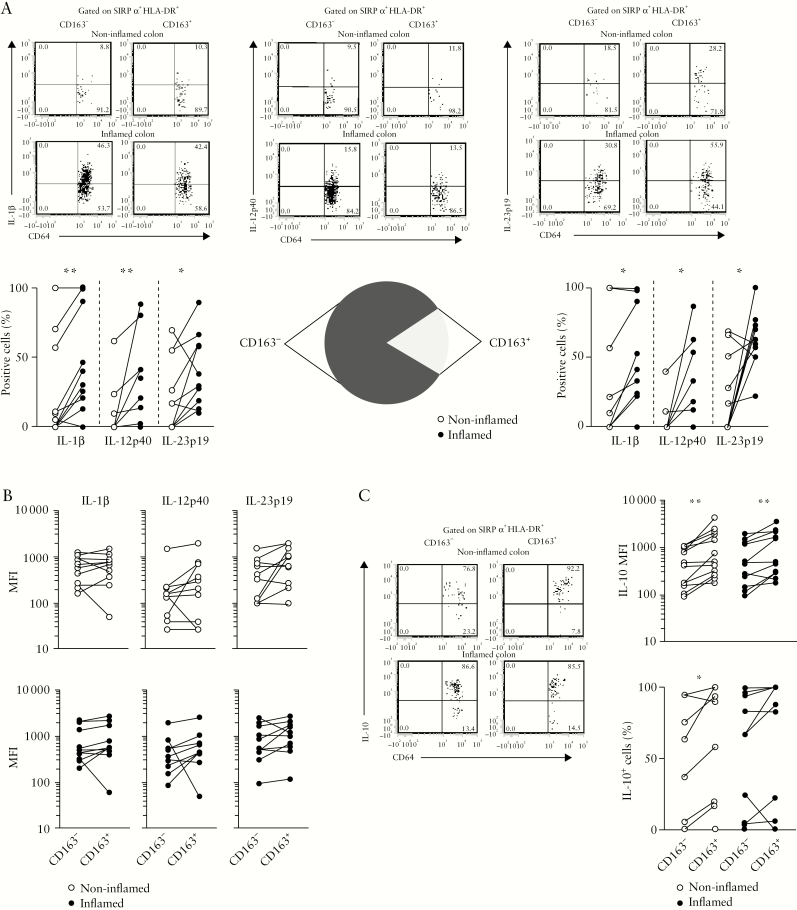
3.3. Mucosal CD163− and CD163+ MNPs express similar amounts of pro-inflammatory cytokines but CD163+ cells produce more IL-10 relative to CD163− cells
Next, we analysed cytokine expression in colonic CD163+ or CD163− subpopulations. The frequencies of IL-1β-, IL-12p40- and IL-23-producing cells were augmented in inflamed relative to paired non-inflamed UC mucosa in CD163− [p < 0.008, p < 0.004 and p < 0.5, respectively] and CD163+ cells [p < 0.04, p < 0.02 and p < 0.03] [Figure 3a]. Furthermore, the amount of pro-inflammatory cytokine expression per cell [MFI] was similar in CD163− and CD163+ cells in both non-inflamed and inflamed tissues [Figure 3b]. Because colonic CD163+ macrophages are known to produce IL-10,36,37 we further examined IL-10 expression in CD163− and CD163+ cells [Figure 3c]. Unlike pro-inflammatory cytokine secretion, the amount of IL-10 per cell was higher in CD163+ relative to CD163− cells in inflamed and non-inflamed mucosa [p < 0.002 and p < 0.005] while the percentage of IL-10-producing cells was higher in CD163+ when compared to CD163− cells in non-inflamed mucosa only [p < 0.04] [Figure 3c].
Figure 3.
Mucosal CD163− and CD163+ MNPs express similar amounts of pro-inflammatory cytokines but CD163+ cells produce more IL-10 relative to CD163− cells. [a] Frequencies and [b] MFI of IL-1β [n = 10], IL-12p40 [n = 10] and IL-23p19 [n = 10] producing cells among CD163− and CD163+ MNPs in non-inflamed and inflamed UC. Pie displays the relative frequency of CD163− and CD163+ cells among CD14+CD64+ in inflamed UC mucosa. [c] Frequencies and MFI of IL-10 [n = 12] producing cells among CD163− and CD163+ MNPs in non-inflamed and inflamed UC mucosa. [a–c] Wilcoxon signed rank test.
Overall, both CD163− and CD163+ cells produce IL-1β, IL-12p40 and IL-23. However, given the relative distribution of these two cell subpopulations, with an increased proportion of CD163− relative to CD163+ cells within CD14+CD64+ MNPs [Figure 3a, middle panel], the former is the major contributor to pro-inflammatory cytokine production in the inflamed mucosa. In contrast, CD163+ cells produce more IL-10 relative to CD163− cells.
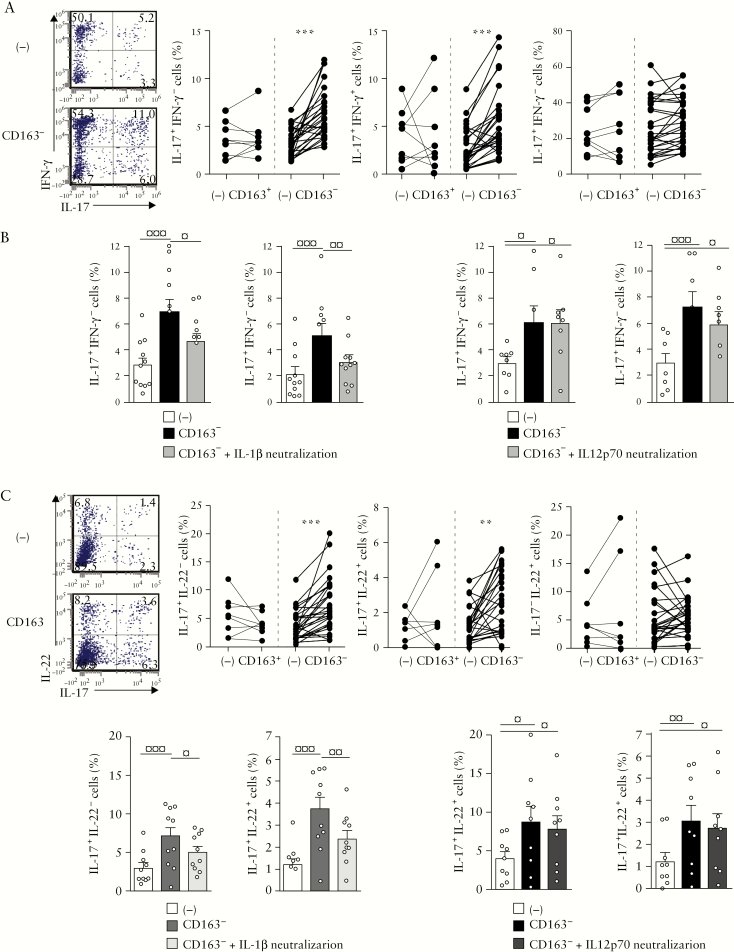
3.4. Mucosal CD163− but not CD163+ MNPs favour autologous Th17/Th1 and Th17/Th22 responses in an IL-1β-dependent manner in inflamed UC colon
We next investigated whether and how CD163− and CD163+ MNPs from inflamed tissue regulate autologous memory Th17, Th17/Th1 and Th1 responses. To this end, we simultaneously purified CD4+ T cells, CD163− and CD163+ MNPs from colonic biopsies, thus excluding the intermediate CD163dim cells, and co-cultured the cells for 6 days [Supplementary Figure S1]. Remarkably, CD163−, but not CD163+, MNPs favoured the emergence of Th17 [p < 0.0001] and Th17/Th1 [p < 0.0001] cells, but not Th1 cells [Figure 4a]. Of note, CD14+CD64+CD163− MNPs were not able to induce naïve T cell proliferation and drive their differentiation into Th effector subsets, while the minor CD14−CD64−CD163− cells, which are enriched in DCs,33 primed naïve T cells and induced polarization towards Th1 effectors [data not shown].
Figure 4.
CD163− but not CD163+ MNPs promote Th17, Th17/Th1 and Th17/Th22 responses in an IL-1β-dependent manner in UC patients. CD4+ T cells isolated from inflamed UC colon were co-cultured with or without autologous mucosal CD163+ [n = 9] or CD163− [n = 27] cells, in the absence or presence of αIL-1R [n = 6], αIL-1β [n = 5] or αIL-12p70 mAbs [n = 8], then stained for intracytoplasmic [a,b] IL-17/IFN-γ, as well as [c,d] IL-17/IL-22 expression. [a,c] Wilcoxon signed rank test; [b,d] Repeated measures ANOVA with Bonferroni’s multiple comparison post test.
We further explored the mechanisms underlying the facilitating activity of colonic CD163− cells on Th17 and Th17/Th1 responses. The frequencies of IL-17+IFN-γ − and IL-17+IFN-γ + T cells were decreased, by adding an mAb that neutralizes IL-1β function to the CD163− plus CD4+ T cell co-cultures [p < 0.05 and p < 0.01, respectively, Figure 4b]. CD163− MNPs and IL-1β appeared to favour the selective expansion of Th17 and Th17/Th1 cells, while proliferation of the Th1 cell population appeared unaltered [data not shown]. However, the Th17 and Th17/Th1 responses were not influenced by the anti-IL-12p70 mAb that selectively blocked IL-12 [Figure 4b]. Similarly, elevated frequencies of IL-17+IL-22− [p < 0.0001] and IL-17+IL-22+ [p < 0.002] cells observed in CD4+ T cells co-cultured with CD163− cells were reduced when neutralizing IL-1β function [p < 0.05 and p < 0.01 respectively; Figure 4c,d].
These data indicate that only CD163− MNPs promote a Th17, Th17/Th1 or Th17/Th22 profile in an IL-1β-dependent manner, corroborating our observations with recombinant IL-1β-stimulated CD4+ T cell cultures [Figure 1].
3.5. Mucosal CD4+ T cells produce IL-8 in UC patients
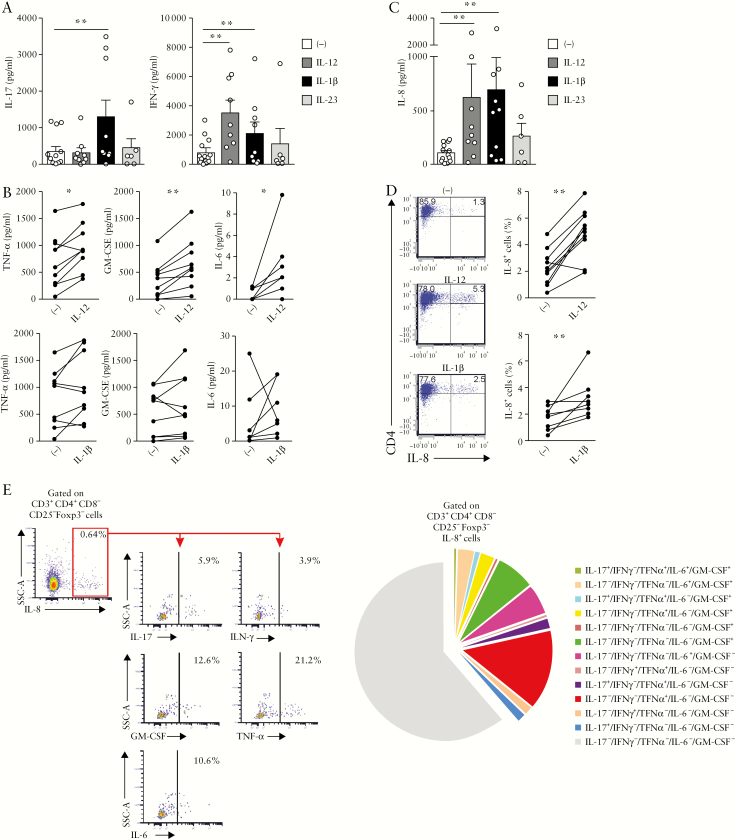
Next, we examined how IL-1β and IL-12 regulate the expression of pro-inflammatory cytokines in the culture supernatant of in vitro activated colonic CD4+ T cells. In agreement with the data regarding intracellular IL-17 and IFN-γ expression, IL-17 production was significantly increased [p < 0.004] by IL-1β, while IL-12 but not IL-23 augmented IFN-γ [p < 0.004] [Figure 5a]. Furthermore, IL-12 but not IL-1β increased TNF-α, GM-CSF and IL-6 secretion [p < 0.04, p < 0.004 and p < 0.02, respectively], suggesting that IL-12 drives a potential pathogenic CD4+ T cell profile in the colon [Figure 5b]. Serendipitously, the multiplex cytokine assay revealed that mucosal CD4+ T cells produced IL-8 in response to IL-12 [p < 0.004] and IL-1β [p < 0.004] but not IL-23 [Figure 5c]. We therefore verified IL-8 expression at the single cell level using intracytoplasmic staining and observed that both IL-12 and IL-1β augmented the frequency of IL-8+CD4+ T cells in UC [p < 0.002 and p < 0.007, respectively] [Figure 5d]. Because IL-8 produced by activated Th17 clones, which were generated from IBD mucosa, promotes neutrophil migration,38 we explored whether IL-8 secreted by IL-1β or IL-12-activated colonic CD4+ T cells attracted neutrophils. Unfortunately, we did not succeed in showing IL-8-mediated chemotactic activity in the culture supernatant of activated primary mucosal CD4+ T cells [data not shown]. Phenotypic analysis of IL-8+CD4+ T cells in UC patients further showed that the majority of these cells expressed α4, while β7 and CD103 expression was barely detectable in all culture conditions [Supplementary Figure S2a]. CCR6, a Th17-associated surface marker, as well as CD69, a surface marker expressed by activated T cells or tissue resident memory T cells, but not the Th1-associated surface marker CXCR3, were expressed on IL-8-producing T cells [Supplementary Figure S2b]. IL-12 significantly decreased the proportion of CCR6- and CD69-positive cells in IL-8+CD4+ T cells [n = 4, p < 0.01 and p < 0.04, respectively] while the percentage of IL-8+ cells expressing CCR6 was augmented in the presence of IL-1β [n = 4, p < 0.04].
Figure 5.
Mucosal CD4+ T cells produce IL-8 in UC patients. UC mucosal CD4+ T cells were cultured with recombinant IL-12 [n = 9], IL-1β [n = 9] or IL-23 [n = 4 to 6] for 6 days. [a] IL-17 and IFN-γ, [b] TNF-α, GM-CSF, IL-6 and[ c] IL-8 secretion were measured in the culture supernatant. [d] Percentage of IL-8+CD4+ T cells after culture of UC mucosal CD4+ T cells with recombinant IL-12 [n = 10] and IL-1β [n = 8] for 6 days. [e] Ex vivo stimulation of LPMCs with PMA-ionomycin in the presence of brefeldin A for 4 h [n = 6]. Left panel: percentage of IL-17, IFN-γ, GM-CSF, TNF-α, IL-6 positive cells among CD4+CD25−Foxp3−IL-8+ T cells; right panel: pie chart depicting the co-expression of IL-17, IFN-γ, GM-CSF, TNF-α and IL-6 in CD4+CD25−Foxp3−IL-8+ T cells. [a–d] Wilcoxon signed rank test; for a and c, p < 0.01 threshold for significance to account for test multiplicity.
We further examined ex vivo IL-8 expression in colonic CD4+ T cells using freshly isolated cells from inflamed mucosa [Figure 5e]. A significant proportion [40%] of the IL-8-producing cells co-expressed pro-inflammatory cytokines. Specifically, IL-8+ cells co-produced GM-CSF [14%], TNF-α [20.2%], IL-6 [8.6%], IFN-γ [2.1%] and IL-17 [4.7%]. Of note, IL-8 could not be detected in significant proportions in CD4+CD25+Foxp3+ regulatory T cells [Supplementary Figure S3].
Taken together, IL-8+CD4+ T cells co-producing GM-CSF, TNF-α, IL-6 and IFN-γ are detected ex vivo in inflamed UC colon and this IL-8 pathogenic profile is further augmented by IL-12 but not IL-1β in vitro.
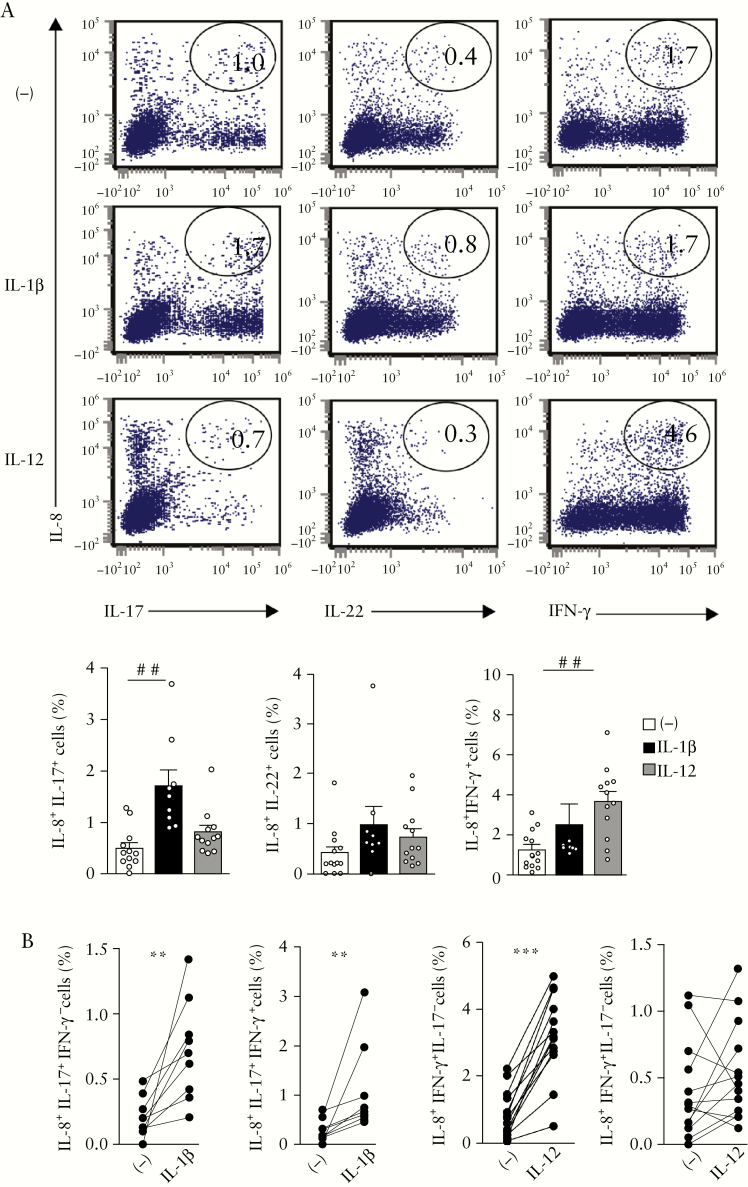
3.6. IL-12 promotes IL-8 and IFN-γ expression whereas IL-1β favours IL-17 and IL-8 in colon of UC patients
We further showed that frequencies of IL-8+IL-17+ [p < 0.002] but not IL-8+IL-22+ CD4+ T cells were increased by IL-1β while IL-12 augmented IL-8+IFN-γ + CD4+ T cells [p < 0.003] [Figure 6a]. More precisely, IL-1β augmented the proportion of IL-8+IL-17+IFN-γ − and IL-8+IL-17+IFN-γ + cells [p < 0.004 and p < 0.009, respectively; Figure 6b]. The frequency of IL-8+IFN-γ +IL-17− cells was increased by IL-12 [p < 0.0002; Figure 6b].
Figure 6.
IL-12 promotes IL-8 and IFN-γ expression whereas IL-1β favours IL-17 and IL-8 in colon of UC patients. UC mucosal CD4+ T cells were cultured for 6 days with recombinant IL-1β [n = 9] or IL-12 [n = 12]. [a] Representative dot plots showing percentage of IL-8+IL17+, IL-8+IL-22+, IL-8+IFN-γ + T cells, and [b] percentages of IL-8+/−IFN-γ +/−IL17+/−T cells. [a] Kruskal–Wallis test with Dunn’s post test; [b] Wilcoxon signed rank test.
Taken together, IL-1β favoured the emergence of IL-8+IL-17+IFN-γ −/+CD4+ T cells whereas IL-12 promoted IL-8+IFN-γ +IL-17−/+CD4+ T cells in UC colon.
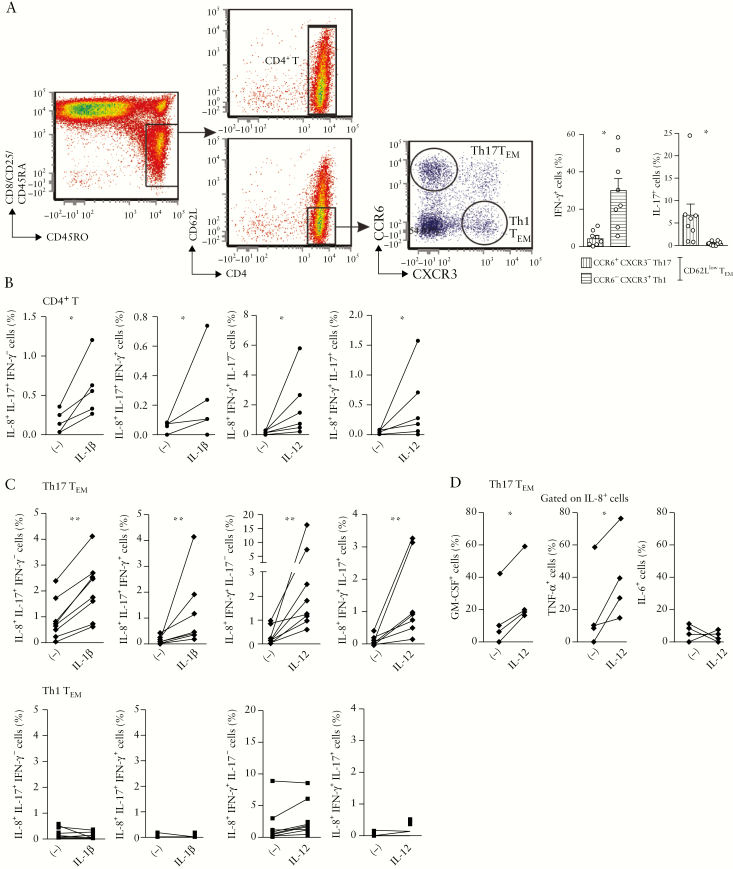
3.7. IL-12 promotes IL-8 expression in effector memory Th17 cells from mesenteric lymph nodes of UC patients
Because mucosal CD4+ T cells emigrate from mLNs to gut tissue, we also examined the ability of IL-12 and IL-1β to regulate IL-8 expression in mLNs before their recruitment to colon. Similar to mucosal CD4+ T cells, IL-1β augmented the frequencies of IL-8+IL-17+IFN-γ − [p < 0.05] and IL-8+IL-17+IFN-γ +CD4+ T cells [p < 0.05], while IL-12 increased IL-8+IFN-γ +IL-17− [p < 0.03] and IL-8+IL-17+IFN-γ +CD4+ T cells [p < 0.05] in mLNs [Figure 7a,b]. To further examine the contribution of Th17 and Th1 cells to the increased IL-8 production in response to IL-1β or IL-12, we purified effector memory [CD62Llow] CD4+ T cells according to CCR6+CXCR3− and CCR6−CXCR3+ expression, which displayed a Th17 TEM and Th1 TEM cytokine profile, respectively [Figure 7a]. In cultures with only Th17 TEM, but not Th1 TEM, increased IL-8 expression was observed under the influence of IL-1β or IL-12 [p < 0.007 and p < 0.008, respectively] [Figure 7c]. Finally, similar to its effect on mucosal CD4+ T cells [Figure 5], IL-12 favoured a pathogenic IL-8 profile in mLN Th17 TEM as shown by co-expression of pro-inflammatory cytokines [p < 0.05; Figure 7d].
Figure 7.
IL-1β and IL-12 favour IL-8 responses in Th17 cells isolated from mLNs of UC patients. [a] Gating strategy for sorting CD4+ T cells, Th17 TEM [CD62LlowCCR6+CXCR3−] and Th1 TEM [CD62LlowCCR6−CXCR3+] cells from mLNs of UC patients. Ex vivo intracytoplasmic staining for IFN-γ and IL-17 expression after PMA-ionomycin stimulation in the presence of brefeldin A for 4 h. [b,c] Percentages of IL-8+IL-17+/−IFN-γ +/− after 6 days of culture of [b] total CD4+ T cells with recombinant IL-1β [n = 5] or IL-12 [n = 6], and [c] Th17 or Th1 TEM with recombinant IL-1β [n = 8] or IL-12 [n = 8]. [d] Percentages of GM-CSF+, TNF-α + and IL-6+ T cells among IL-8+ T cells after 6 days of culture of Th17 TEM with recombinant IL-12 [n = 4]. [a–d] Wilcoxon signed rank test.
In conclusion, these data suggest that IL-12 induces a shift of Th17 TEM towards pathogenic IL-8 and IFN-γ co-expressing cells while IL-1β promoted IL-8 and IL-17 co-expressing cells in UC mLNs.
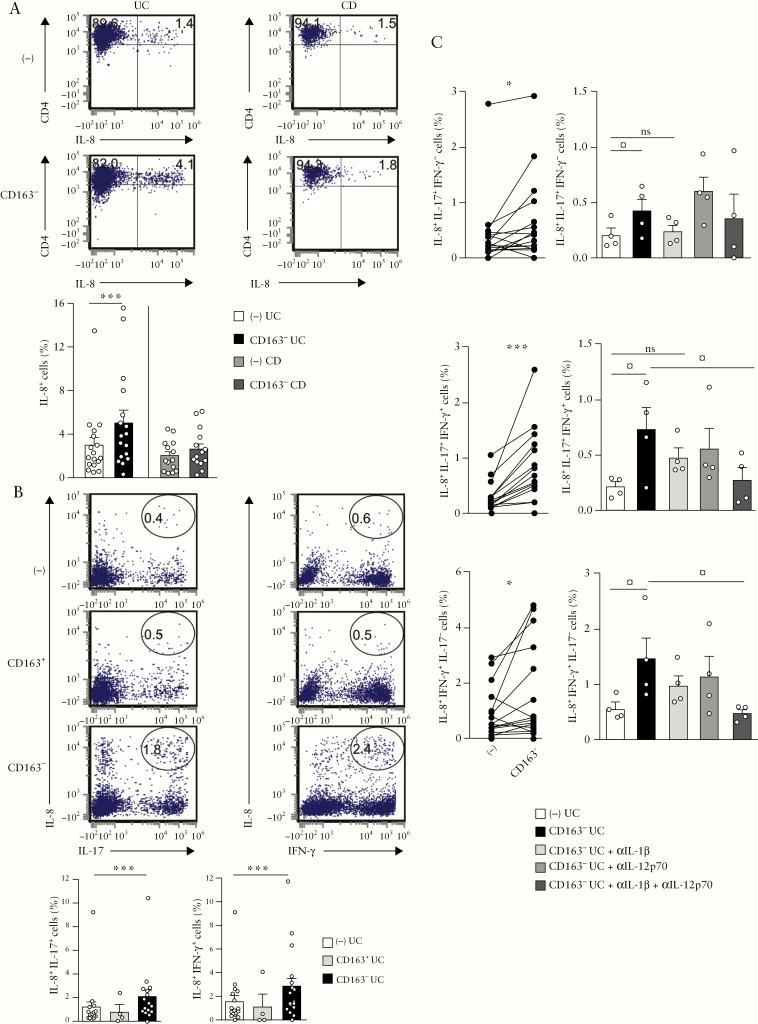
3.8. Mucosal CD163−, but not CD163+, MNPs augment IL-8 expression in colonic CD4+ T cells of UC but not CD patients
Finally, we examined the ability of CD163− cells to regulate IL-8 expression. CD163− cells increased the frequency of IL-8-producing CD4+ T cells [p < 0.0009; Figure 8a]. Interestingly, the enhanced IL-8 expression appeared to be restricted to UC because it was not observed in co-cultures of autologous colonic CD163− and CD4+ T cells isolated from CD patients [Figure 8a]. Notably, IL-8 expression was not detected ex vivo in colonic CD4+ T cells isolated from CD patients nor was it increased in co-cultures with autologous colonic CD163− MNPs, or in response to either IL-1β or IL-12 [Supplementary Figure S4a, b, c]. Furthermore, CD163− but not CD163+ cells increased the frequency of IL-8+IL-17+ and IL-8+IFN-γ + CD4+ T cells in UC patients [p < 0.0004 and p < 0.0009, respectively; Figure 8b]. Also, the frequencies of IL-8+IL-17+IFN-γ − [p < 0.02], IL-8+IL-17+IFN-γ + [p < 0.0007] and IL-8+IFN-γ +IL-17−[p < 0.02] CD4+ T cells were augmented by CD163− cells [Figure 8c, left panels]. Finally, we explored some of the mechanisms that governed the ability of CD163− cells to increase IL-8 expression in mucosal CD4+ T cells in UC. When IL-1β function was neutralized, the proportions of IL-8+IL-17+IFN-γ − and IL-8+IL-17+IFN-γ + CD4+ T cells were not augmented by CD163− cells [Figure 8c, right panels]. Combined IL-1β and IL-12 blockade significantly decreased the frequencies of IL-8+IFN-γ +IL-17−[p < 0.02] and IL-8+IL-17+IFN-γ + CD4+ T cells [p < 0.009], which were not reduced by adding anti-IL-12p70 mAb alone [Figure 8c, right panels].
Figure 8.
CD163− MNPs increase IL-8 expression in colonic CD4+ T cells in UC but not CD patients. CD4+ T cells isolated from inflamed UC or CD colons were co-cultured with or without: [a] autologous mucosal CD163− cells [n = 17 UC, n = 14 CD] and stained for IL-8 intracytoplasmic expression; [b] autologous mucosal CD163+ [n = 4] or CD163− cells [n = 17] and stained for IL-8, IL-17 and IFN-γ intracytoplasmic expression; and [c] autologous CD163− cells [n = 17], in the absence or presence of αIL-1β [n = 4] and/or αIL-12p70 mAbs [n = 4], and stained for IL-8, IL-17 and IFN-γ intracytoplasmic expression. [a–c, left panel] Wilcoxon signed rank test; for b, p < 0.01 threshold for significance to account for test multiplicity. [c, right panel] Repeated measures ANOVA with Bonferroni’s multiple comparison post test.
Taken collectively, mucosal CD163− MNPs augment IL-8 expression by colonic CD4+ T cells in UC but not CD mucosa, and further promote IL-8 and IL-17 co-expression through their secretion of IL-1β, and IL-8 and IFN-γ co-expression via IL-1β and IL-12 production.
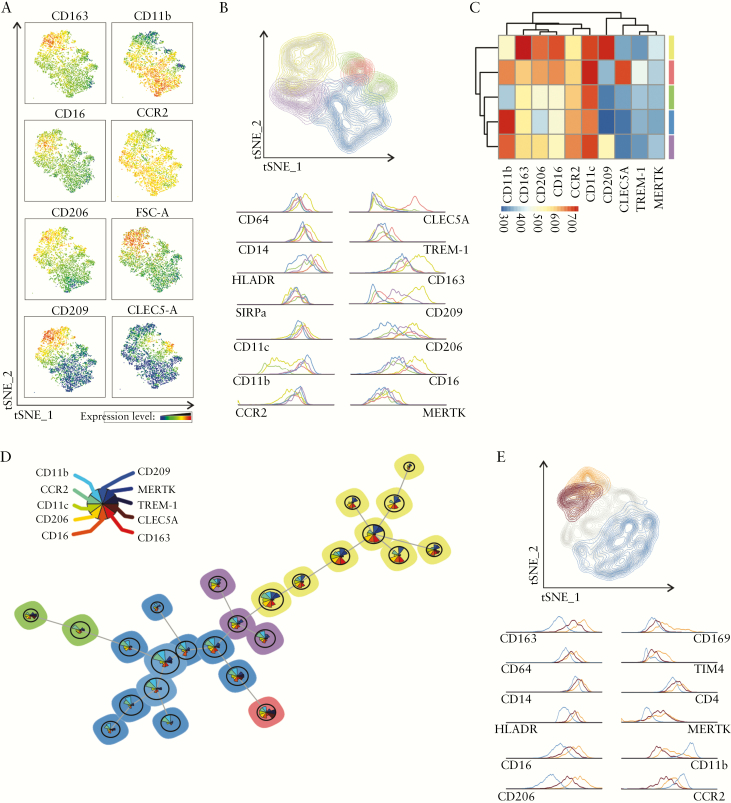
3.9. Unsupervised multi-colour flow cytometry analysis reveals that CD163− and CD163+ MNPs form distinct clusters related to monocyte-like and macrophage cell populations, respectively
The heterogeneity of CD14+CD64+ MNPs was further assessed using multi-colour FACS analysis [inflamed mucosa n = 4 UC patients] [Figure 9a]. Feature t-SNE plots of CD163, CD16, CD206 and CD209 expression identified a cluster, which was distinct from the CD163−/dim cluster best defined by CD11b, CCR2 expression and low Forward Scatter-cell size. Of note, a feature plot of CLEC5-A expression showed that it clustered with a minor fraction of CD11b-expressing cells. To evaluate which markers were driving the CD163− and CD163+ cell-specific signature, the flow cytometry data were next analysed using an unsupervised self-organizing map [FlowSOM] method [Figure 9b–e]. The five clusters identified using FlowSOM were overlaid in the t-SNE plot of concatenated HLA-DR+SIRPα +CD14+CD64+ cells [Figure 9b]. Two CD163− clusters were best identified using CD11b and CD206 expression markers, with the major one [blue: 47.7%] expressing CD11b at the highest and CD206 at the lowest intensity, and vice versa for the minor one [green: 6%], relative to the other three clusters. The CD163dim clusters were defined as CD209dimCD206dim [purple: 11.1%]] or CLEC5Abright TREM-1+ [red: 3.5%] cells. Elevated relative expression of CD14, CD64, MERTK, CD209, CD206 and CD16 but low CD11b expression identified the CD163+ cluster [yellow: 31.6%]. A heatmap and FlowSOM minimal spanning tree, with star charts displaying different intensities of co-expressed surface markers, further characterized the CD163+ population [yellow] that clustered apart from CD163−/dim populations [Figure 9c, d]. Of note, analysis of a second panel of surface markers that included three additional markers [CD169, TIM4 and CD4] and 12 common markers revealed that CD163+ cells were further subdivided into CD11bdimCD169+TIM4+CD4+ and CD11b−CD169−TIM4−CD4+cells [Figure 9e].
Figure 9.
Unsupervised analysis of the phenotype of HLA-DR+SIRPα +CD14+CD64+ MNPs in inflamed UC mucosa. LPMCs from four UC patients were stained with a panel of 15 surface markers. A concatenated file from the four patients was subjected to unsupervised clustering analysis. [a] Expression feature plot of the depicted surface markers in HLADR+SIRPα +CD14+CD64+ cells, using the t-SNE algorithm. [b–d] FlowSOM analysis of HLADR+SIRPα +CD14+CD64+ cells based on expression data: [b] affiliation of cells to five clusters identified in FlowSOM, indicated by colour coding and visualized in t-SNE plot; surface marker expression levels are depicted by histograms; [c] heatmap of mean surface marker expression of the five individual clusters; [d] cells were clustered into 25 nodes and depicted as a minimal spanning tree, with colour coding indicating the five identified clusters. Circle sizes are proportional to the number of cells represented in each node and triangle size in star charts depicts the mean intensities of each marker for all cells assigned to the node [colour legend on the left]. [e] LPMCs from the same four UC patients were stained with another panel of 15 surface markers, only differing for CD169, TIM4 and CD4. A concatenated file of HLA-DR+SIRPa+CD14+CD64+ cells from the four UC patients was subjected to FlowSOM analysis. Affiliation of cells to three out of five clusters identified in FlowSOM is indicated by colour coding and visualized in t-SNE plot; surface marker expression levels are depicted by histograms.
The CD14+CD64+ MNPs were next purified at the extreme ends of the spectrum of CD163 expression, according to the gating strategy that was originally selected to quantify CD163− and CD163+ subsets in the UC mucosa in Figure 2(d), to assess their morphology [Supplementary Figure S5]. CD163− cells displayed a kidney-shaped nucleus while CD163+ cells resembled typical Mϕ with vacuoles and a large cell size, corroborating our multi-colour FACS analysis.
Collectively, the CD14+CD64+ MNP subpopulation that predominates in inflamed UC mucosa is best defined as CD163−CD206−CD209−MERTK−CLEC5-A−TREM-1dimHLA-DRdimCCR2+CD11bbright monocyte-like pro-inflammatory cells, while the minor CD163+ cells are CD209+CD206+MERTK+Mϕ.
4. Discussion
The novel and unexpected finding of the present study is that IL-8-expressing T cells represented a minor CD4+ T population, which was detected in inflamed colon of UC but not CD patients. As such, these cells might be implicated in the development and/or perpetuation of UC. UC shares several genetic, clinical, histological and immunological features with CD,1 both of which are T cell-mediated diseases.15,16 Nonetheless, these two IBDs represent distinct entities,5,39,40 as highlighted in our present study. First, mucosal CD4+ T cells isolated from UC colon did not increase their IL-17 or IFN-γ secretion in response to IL-23, while we and others have reported that IL-23 increased mucosal Th17 and Th17/Th1 responses in CD.17,33 The absence of an IL-23 response in UC could not be attributed to the loss of IL-23 receptor [IL-23R] on the surface of mucosal CD4+T cells, as Kobayashi et al. have demonstrated that colonic CD4+ T cells express IL23R mRNA in both UC and CD.10 Second, IL-8 expression was detected ex vivo in colonic CD4+ T cells, and was augmented by IL-12, IL-1β and CD163− but not CD163+ MNPs in UC only. Third, IL-12 and IL-1β differentially regulated TNF-α, GM-CSF and IL-6 production by colonic CD4+ T cells in CD33 and, as shown here, in UC. These three cytokines were augmented by IL-12 but not IL-1β in UC only and, by IL-1β but not IL-12 in CD only. We therefore propose that the distinct IL-8 responses observed between UC and CD probably result from disease-specific differences in T cells rather than a difference intrinsic to CD163− cell function, which might be clinically relevant with regard to the pathogenesis of UC and CD.
MNPs play a critical role in the maintenance of gut homeostasis, orchestrating the dialogue between innate and adaptive immunity.41 Morphology and phenotypic studies of CD163− and CD163+ cells have attempted to relate the nature of these two functionally distinct CD14+CD64+ subpopulations to intestinal CD14+ MNPs and their murine counterparts previously identified under inflammatory or homeostatic conditions.42 Human CD163− cells displayed a monocyte-like shape, and thus could not be considered as Mϕ. These cells resemble tissue Ly6C+CD64+ inflammatory monocytes in murine colon.43 In colitic mice and ileal CD, extravasated inflammatory monocyte-derived cells [P1], best defined as CD11c−/dim CD11bdim/+ CD14+CD64low SIRPα +MHC class II− cells, progressively develop into mature CD11c++CD11b+CD14++CD64++, SIRPα +MHC class II+++ [P4] Mϕ, unless the maturation process is interrupted under inflammatory conditions.42
We hypothesize that recruited HLA-DRdimCD14+ monocytes [CD206−CD209−MERTK−TREM-1 dimCCR2+CD11bbrightCD163− cells] progressively acquired CD163, MERTK, CD209 and CD206 and down-regulated CD11b and CCR2. In that regard, in inflamed CD colon, we recently characterized two CD14+ populations using single cell RNA profiling and demonstrated that inflammatory monocyte-like Mϕ [TREM-1+CD206−CD209−CD163−] are distinct from TREM-1−CD206+CD209+MERTK+CD163+ Mϕ.33 As in UC, CD163− but not CD163+ cells accumulate in inflamed CD colon.Furthermore, CD163− cells might be related but still distinct from CD14+CLEC5-A+CD209−CD11b+CD11c+ cells, which are potential drivers of the chronic intestinal inflammatory response.36 In fact, CD163− cells clustered apart from CLEC5-AbrightTREM-1+ cells, which together with CD209dimCD206dim cells might represent transitioning CD163dim cells. The latter were excluded for our functional studies. Regarding CD163+ cells, they displayed an Mϕ morphology and thus resemble Mϕ3 or Mϕ4 subsets that are also derived from recruited monocytes in human jejunum at homeostasis.44 As opposed to Mϕ1 or Mϕ2 precursors that expressed CD11c in healthy small intestine, Mϕ3 or Mϕ4 are not CD11c+.44 However, CD11c expression was not a discriminatory surface marker between CD163− and CD163+ cells in inflamed UC mucosa because it was expressed at high intensity in both subsets, corroborating the phenotype of CD14+ MNPs in human inflamed colon.36,45 The CD16+CD163+ Mϕ did not accumulate in inflamed UC colon or regulate IL-8 expression, Th17 and Th17/Th1 responses in colonic CD4+ T cells. In fact, inflammatory CD16+ Mϕ do not regulate memory T cell responses in ascites of cancer patients.46 Finally, CD163+ Mϕ also included a subset of TIM4+CD4+ cells, which have not yet been reported in humans but have recently been defined as tissue-resident Mϕ in mice.47,48 The TIM4+CD4+CD163+ Mϕ subpopulation co-expressed CD169; the CD169+ Mϕ phenotype contributes to monocyte recruitment in mice.49
Overall, we propose to refer to CD163− inflammatory monocyte-like cells as ‘monocyte-derived effector cells [MDECs]’. Conversely, the CD163+ cells might be considered anti-inflammatory, regulatory Mϕ and/or ‘post-inflammatory’ Mϕ because these cells produced more IL-10 while expressing a similar amount of pro-inflammatory cytokines as compared to MDECs [CD163− cells]. Although the interpretation of these data should be taken cautiously, human colonic CD163− cells that accumulate in inflamed UC mucosa could represent a functionally distinct CD14+ monocyte-like subpopulation that has plastic capacities.
IL-8 is a potent chemoattractant for neutrophils,29,50,51 and plays an important role in the pathophysiology of UC. Increased IL8 mRNA expression has been detected in the inflamed mucosa of IBD patients and levels of IL-8 expression correlate with endoscopic severity in UC.29 After multiple rounds of expansion and activation, mucosal Th17 clones which were generated from IBD or colorectal cancer secrete IL-8 that attracts neutrophils.38,52 Both studies highlight the biological relevance of IL-8 produced by mucosal Th17 cells.38,52 However, the limitation of the present study was the inability to demonstrate IL-8-induced neutrophil chemotaxis using primary colonic CD4+ T cells of UC patients. Furthermore, IL-8 is abundantly expressed by a variety of cells in the gut mucosa, notably by neutrophils, endothelial and epithelial cells as well as monocytes, Mɸ, fibroblasts and possibly T cells.30,53 We showed here that a minor colonic CD4+ T population expressed IL-8 in UC but not CD mucosa. Some studies have demonstrated IL-8 production by circulating T cells isolated from healthy adults and cord blood, suggesting that T cell priming has occurred in utero.25–27 However, polarizing conditions to differentiate naïve T cells into single IL-8-producing cells [‘Th8’] remain unknown. IL-8 itself might induce IL-8 in human CD4+ T cells.54 Furthermore, the addition of flagellin, which is abundantly detected in the colon, increases the percentage of IL-8+ cells in circulating T cell receptor-stimulated CD4+ T cells.25 In a rat model of colitis, IL-8 levels increase before the influx of neutrophils,55 supporting the concept that IL-8-producing T cells could be implicated at the early phase of disease. However, IL-8 is also detected in CD3+ cells in chronic lesions using an immunofluorescence technique, arguing for a role for T cell-derived-IL-8 in perpetuation of the disease.53
It is unclear whether IL-8-producing T cells play a protective or pro-inflammatory role in UC mucosa. In that regard, circulating IL-8+Foxp3+CD25+ T cells, with a dual suppressive and inflammatory phenotype, that promote pro-inflammatory cytokine production and neutrophil attraction are also reported in UC inflamed mucosa.56 Because we could not detect ex vivo IL-8 expression in Foxp3+CD25+ T cells in inflamed UC mucosa, these T cells were excluded from our gating strategy that exclusively analysed CD4+CD25− T cells. A protective function might be attributed to the colonic IL-8+CD4+ T cell population, due to their GM-CSF production. GM-CSF ameliorates colitis in mice via its effect on monocytes that led to bacterial clearance and epithelial healing.57 Furthermore, human circulating activated Th17 clones directly attract neutrophils through IL-8 release while Th17/Th1 clones increase neutrophil activity via GM-CSF.38 In contrast, both innate lymphoid cells type 3 and T cell-derived GM-CSF contribute to intestinal inflammation in experimental colitis.58 IL-8-producing T cells that co-expressed GM-CSF, IL-6, TNF-α and IFN-γ ex vivo might therefore lead to early destruction of the epithelial barrier. Our data further revealed that IL-12 biased Th17 TEM towards IL-8+IFN-γ + CD4+ T cells that expressed TNF-α and GM-CSF in mLNs, highlighting the potential pathogenicity of IL-8+ T cells in disease tissue. Of note, IL-12 but not IL-1β significantly augmented IL-8+IFN-γ −IL-17−IL-22− CD4+ T cells in UC mucosa, and concomitantly reduced the proportion of IL-22+IL-8−CD4+ T cells, which might further contribute to epithelial cell destruction [Supplementary Figure S6a]. Furthermore, IL-12, IL-1β and CD163− MNPs augmented the percentages of IL-8+IFN-γ +IL-17− or IL-8+L-17+IFNγ + T cells, irrespective of IL-22 co-expression, while the frequencies of IL-8+IL-17+IFN-γ − T cells not co-expressing IL-22 were augmented only by IL-1β or CD163− MNPs [Supplementary Figure S6b]. CD163− MNPs, through IL-1β production, amplified colonic inflammatory Th17 and Th17/Th1 responses in both UC and CD,33 while an IL-1β-dependent increased frequency of IL-8+IL-17+ CD4+ T cells was observed in UC only. In that regard, IL-1β correlates with IL-8 levels in UC mucosa29 and promotes the survival of Th17 cells in murine IBD.59
Currently approved therapeutic approaches using anti-TNF-α or anti-IL-12p40 mAbs and ongoing clinical trials using anti-α4β7 or αEβ7 integrin mAbs in UC patients are aimed toward impairment of cell recruitment, function and/or retention to inflamed mucosa.60,61 Unlike Th17, Th17/Th1 and Th9 cells,61 colonic IL-8+ T cells appeared to be α4β7- or αEβ7-negative as these cells expressed α4 but not β7 integrin, suggesting that intestinal inflammatory IL-8+ T cells might utilize α1 for their tissue recruitment.62 Expression of CD69, but not CD103, by colonic IL-8+ CD4 T cells suggested that they are not related to recently described tissue resident memory CD4+ T cells in CD mucosa.63,64
In conclusion, colonic CD14+CD163− MDECs producing IL-1β and IL-12p40, and their propensity to augment IL-8 expression in tissue T cells, might all be implicated in the regulation of gut inflammation in UC. Nevertheless, the potential role of colonic effector T cells producing IL-8 in vivo and its clinical relevance in UC but not CD warrants further investigations to better understand potentially distinct disease pathogenesis.
Supplementary Material
Acknowledgments
We are grateful to Dr H. Mehta for critical reading and comments on the manuscript. We thank the subjects for donating the samples used in our study; the physicians who provided the samples: E.-J. Bernard, M. Boivin, S. Bouchard, J. Coté-Daigneault, L. Daoust, E. Deslandres, J. Dorais, B. Faulques, R. Lahaie, R. Leduc, M. Lemoyne, B. Panzini, P. Poitras, S. Sidani and D. Von Renteln from the gastroenterology unit; E. Debroux, R. Lougnarath, R. Ratelle, C. Richard, F. Swchenter and R. Wassef from the digestive tract surgery unit; B. Nguyen and G. Soucy from the pathology department, at CHUM, Montreal; and C. Deslandres from the gastroenterology unit at CHU Sainte-Justine, Montreal. We also thank the haematology department and the nurse C. Bergeron for their help.
Funding
Canadian Institutes of Health Research [MOP#130533] [M.S.], Fonds de Recherche en Santé du Québec [L.C., M.Bs.].
Conflict of Interest
The authors have no conflicts of interest to declare.
Author Contributions
Conceptualization: L.C., M.Bs., M.R., S.S., A.C.V. and M.S. Methodology and investigation: L.C., M.Bs., M.R., G.S., S.S., A.C.V. Resources: M.B., K.O., A.W., L.C. Writing: L.C., M.Bs., M.S. 5. Supervision: M.S. 6. Funding acquisition: A.C.V. and M.S.
References
- 1. Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet 2017;389:1756–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fuss IJ, Heller F, Boirivant M, et al. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. J Clin Invest 2004;113:1490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heller F, Florian P, Bojarski C, et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology 2005;129:550–64. [DOI] [PubMed] [Google Scholar]
- 4. Neurath MF, Finotto S, Glimcher LH. The role of Th1/Th2 polarization in mucosal immunity. Nat Med 2002;8:567–73. [DOI] [PubMed] [Google Scholar]
- 5. Christophi GP, Rong R, Holtzapple PG, Massa PT, Landas SK. Immune markers and differential signaling networks in ulcerative colitis and Crohn’s disease. Inflamm Bowel Dis 2012;18:2342–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Biancheri P, Di Sabatino A, Ammoscato F, et al. Absence of a role for interleukin-13 in inflammatory bowel disease. Eur J Immunol 2014;44:370–85. [DOI] [PubMed] [Google Scholar]
- 7. Rosen MJ, Karns R, Vallance JE, et al. Mucosal expression of Type 2 and Type 17 immune response genes distinguishes ulcerative colitis from colon-only Crohn’s disease in treatment-naive pediatric patients. Gastroenterology 2017;152:1345–57.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Danese S, Rudziński J, Brandt W, et al. Tralokinumab for moderate-to-severe UC: a randomised, double-blind, placebo-controlled, phase IIa study. Gut 2015;64:243–9. [DOI] [PubMed] [Google Scholar]
- 9. Reinisch W, Panés J, Khurana S, et al. Anrukinzumab, an anti-interleukin 13 monoclonal antibody, in active UC: efficacy and safety from a phase IIa randomised multicentre study. Gut 2015;64:894–900. [DOI] [PubMed] [Google Scholar]
- 10. Kobayashi T, Okamoto S, Hisamatsu T, et al. IL23 differentially regulates the Th1/Th17 balance in ulcerative colitis and Crohn’s disease. Gut 2008;57:1682–9. [DOI] [PubMed] [Google Scholar]
- 11. Bogaert S, Laukens D, Peeters H, et al. Differential mucosal expression of Th17-related genes between the inflamed colon and ileum of patients with inflammatory bowel disease. BMC Immunol 2010;11:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Granlund Av, Flatberg A, Østvik AE, et al. Whole genome gene expression meta-analysis of inflammatory bowel disease colon mucosa demonstrates lack of major differences between Crohn’s disease and ulcerative colitis. PLoS One 2013;8:e56818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kryczek I, Zhao E, Liu Y, et al. Human TH17 cells are long-lived effector memory cells. Sci Transl Med 2011;3:104ra100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rovedatti L, Kudo T, Biancheri P, et al. Differential regulation of interleukin 17 and interferon gamma production in inflammatory bowel disease. Gut 2009;58:1629–36. [DOI] [PubMed] [Google Scholar]
- 15. Globig AM, Hennecke N, Martin B, et al. Comprehensive intestinal T helper cell profiling reveals specific accumulation of IFN-γ+IL-17+ coproducing CD4+ T cells in active inflammatory bowel disease. Inflamm Bowel Dis 2014;20:2321–9. [DOI] [PubMed] [Google Scholar]
- 16. Li J, Ueno A, Fort Gasia M, et al. Profiles of lamina propria T helper cell subsets discriminate between ulcerative colitis and Crohn’s disease. Inflamm Bowel Dis 2016;22:1779–92. [DOI] [PubMed] [Google Scholar]
- 17. Ramesh R, Kozhaya L, McKevitt K, et al. Pro-inflammatory human Th17 cells selectively express P-glycoprotein and are refractory to glucocorticoids. J Exp Med 2014;211:89–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Annunziato F, Cosmi L, Santarlasci V, et al. Phenotypic and functional features of human Th17 cells. J Exp Med 2007;204:1849–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kleinschek MA, Boniface K, Sadekova S, et al. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. J Exp Med 2009;206:525–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guilliams M, van de Laar L. A hitchhiker’s guide to myeloid cell subsets: practical implementation of a novel mononuclear phagocyte classification system. Front Immunol 2015;6:406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kamada N, Hisamatsu T, Okamoto S, et al. Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-gamma axis. J Clin Invest 2008;118:2269–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baba N, Van VQ, Wakahara K, et al. CD47 fusion protein targets CD172a+ cells in Crohn’s disease and dampens the production of IL-1β and TNF. J Exp Med 2013;210:1251–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thiesen S, Janciauskiene S, Uronen-Hansson H, et al. CD14(hi)HLA-DR(dim) macrophages, with a resemblance to classical blood monocytes, dominate inflamed mucosa in Crohn’s disease. J Leukoc Biol 2014;95:531–41. [DOI] [PubMed] [Google Scholar]
- 24. Magnusson MK, Brynjólfsson SF, Dige A, et al. Macrophage and dendritic cell subsets in IBD: ALDH+ cells are reduced in colon tissue of patients with ulcerative colitis regardless of inflammation. Mucosal Immunol 2016;9:171–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gibbons D, Fleming P, Virasami A, et al. Interleukin-8 (CXCL8) production is a signatory T cell effector function of human newborn infants. Nat Med 2014;20:1206–10. [DOI] [PubMed] [Google Scholar]
- 26. Gasch M, Goroll T, Bauer M, et al. Generation of IL-8 and IL-9 producing CD4⁺ T cells is affected by Th17 polarizing conditions and AHR ligands. Mediators Inflamm 2014;2014:182549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Akhade AS, Qadri A. T-cell receptor activation of human CD4(+) T cells shifts the innate TLR response from CXCL8(hi) IFN-γ(null) to CXCL8(lo) IFN-γ(hi). Eur J Immunol 2015;45:2628–37. [DOI] [PubMed] [Google Scholar]
- 28. Bennike TB, Carlsen TG, Ellingsen T, et al. Neutrophil extracellular traps in ulcerative colitis: a proteome analysis of intestinal biopsies. Inflamm Bowel Dis 2015;21:2052–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mitsuyama K, Toyonaga A, Sasaki E, et al. IL-8 as an important chemoattractant for neutrophils in ulcerative colitis and Crohn’s disease. Clin Exp Immunol 1994;96:432–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Beck PL, Cotton JA, Platnich JM, et al. Interleukin-8 in gastrointestinal inflammation and malignancy: induction and clinical consequences. Int J Interferon Cytokine Mediat Res 2016:13. [Google Scholar]
- 31. Van Gassen S, Callebaut B, Van Helden MJ, et al. FlowSOM: Using self-organizing maps for visualization and interpretation of cytometry data. Cytometry A 2015;87:636–45. [DOI] [PubMed] [Google Scholar]
- 32. R Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2016. [Google Scholar]
- 33. Chapuy L, Bsat M, Sarkizova S, et al. Two distinct colonic CD14+ subsets characterized by single-cell RNA profiling in Crohn’s disease. Mucosal Immunol 2019;12:703–19 34. [DOI] [PubMed] [Google Scholar]
- 34. Jakubzick CV, Randolph GJ, Henson PM. Monocyte differentiation and antigen-presenting functions. Nat Rev Immunol 2017;17:349–62. [DOI] [PubMed] [Google Scholar]
- 35. Segura E, Amigorena S. Inflammatory dendritic cells in mice and humans. Trends Immunol 2013;34:440–5. [DOI] [PubMed] [Google Scholar]
- 36. González-Domínguez É, Samaniego R, Flores-Sevilla JL, et al. CD163L1 and CLEC5A discriminate subsets of human resident and inflammatory macrophages in vivo. J Leukoc Biol 2015;98:453–66. [DOI] [PubMed] [Google Scholar]
- 37. Ogino T, Nishimura J, Barman S, et al. Increased Th17-inducing activity of CD14+ CD163 low myeloid cells in intestinal lamina propria of patients with Crohn’s disease. Gastroenterology 2013;145:1380–91.e1. [DOI] [PubMed] [Google Scholar]
- 38. Pelletier M, Maggi L, Micheletti A, et al. Evidence for a cross-talk between human neutrophils and Th17 cells. Blood 2010;115:335–43. [DOI] [PubMed] [Google Scholar]
- 39. Iboshi Y, Nakamura K, Ihara E, et al. Multigene analysis unveils distinctive expression profiles of helper T-cell-related genes in the intestinal mucosa that discriminate between ulcerative colitis and Crohn’s disease. Inflamm Bowel Dis 2014;20:967–77. [DOI] [PubMed] [Google Scholar]
- 40. Haberman Y, Tickle TL, Dexheimer PJ, et al. Pediatric Crohn disease patients exhibit specific ileal transcriptome and microbiome signature. J Clin Invest 2014;124:3617–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Grainger JR, Konkel JE, Zangerle-Murray T, Shaw TN. Macrophages in gastrointestinal homeostasis and inflammation. Pflugers Arch 2017;469:527–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bain CC, Scott CL, Uronen-Hansson H, et al. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol 2013;6:498–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Grainger JR, Wohlfert EA, Fuss IJ, et al. Inflammatory monocytes regulate pathologic responses to commensals during acute gastrointestinal infection. Nat Med 2013;19:713–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bujko A, Atlasy N, Landsverk OJB, et al. Transcriptional and functional profiling defines human small intestinal macrophage subsets. J Exp Med 2018;215:441–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bernardo D, Marin AC, Fernández-Tomé S, et al. Human intestinal pro-inflammatory CD11chighCCR2+CX3CR1+ macrophages, but not their tolerogenic CD11c-CCR2-CX3CR1- counterparts, are expanded in inflammatory bowel disease. Mucosal Immunol 2018;11:1114–26. [DOI] [PubMed] [Google Scholar]
- 46. Segura E, Touzot M, Bohineust A, et al. Human inflammatory dendritic cells induce Th17 cell differentiation. Immunity 2013;38:336–48. [DOI] [PubMed] [Google Scholar]
- 47. De Schepper S, Verheijden S, Aguilera-Lizarraga J, et al. Self-maintaining gut macrophages are essential for intestinal homeostasis. Cell 2018;175:400–415.e13. [DOI] [PubMed] [Google Scholar]
- 48. Shaw TN, Houston SA, Wemyss K, et al. Tissue-resident macrophages in the intestine are long lived and defined by Tim-4 and CD4 expression. J Exp Med 2018;215:1507–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Asano K, Takahashi N, Ushiki M, et al. Intestinal CD169(+) macrophages initiate mucosal inflammation by secreting CCL8 that recruits inflammatory monocytes. Nat Commun 2015;6:7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bruno ME, Rogier EW, Arsenescu RI, et al. Correlation of biomarker expression in colonic mucosa with disease phenotype in Crohn’s disease and ulcerative colitis. Dig Dis Sci 2015;60:2976–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fonseca-Camarillo G, Yamamoto-Furusho JK. High gene expression of CXCL8 is associated with the presence of extraintestinal manifestations and long-term disease in patients with ulcerative colitis. Inflamm Bowel Dis 2013;19:E22–3. [DOI] [PubMed] [Google Scholar]
- 52. Amicarella F, Muraro MG, Hirt C, et al. Dual role of tumour-infiltrating T helper 17 cells in human colorectal cancer. Gut 2017;66:692–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Brandt E, Colombel JF, Ectors N, et al. Enhanced production of IL-8 in chronic but not in early ileal lesions of Crohn’s disease (CD). Clin Exp Immunol 2000;122:180–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gesser B, Deleuran B, Lund M, et al. Interleukin-8 induces its own production in CD4+ T lymphocytes: a process regulated by interleukin 10. Biochem Biophys Res Commun 1995;210:660–9. [DOI] [PubMed] [Google Scholar]
- 55. Harada K, Toyonaga A, Mitsuyama K, Sasaki E, Tanikawa K. Role of cytokine-induced neutrophil chemoattractant, a member of the interleukin-8 family, in rat experimental colitis. Digestion 1994;55:179–84. [DOI] [PubMed] [Google Scholar]
- 56. Kryczek I, Wang L, Wu K, et al. Inflammatory regulatory T cells in the microenvironments of ulcerative colitis and colon carcinoma. Oncoimmunology 2016;5:e1105430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Däbritz J, Weinhage T, Varga G, et al. Reprogramming of monocytes by GM-CSF contributes to regulatory immune functions during intestinal inflammation. J Immunol 2015;194:2424–38. [DOI] [PubMed] [Google Scholar]
- 58. Pearson C, Thornton EE, McKenzie B, et al. ILC3 GM-CSF production and mobilisation orchestrate acute intestinal inflammation. Elife 2016;5:e10066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Coccia M, Harrison OJ, Schiering C, et al. IL-1β mediates chronic intestinal inflammation by promoting the accumulation of IL-17A secreting innate lymphoid cells and CD4(+) Th17 cells. J Exp Med 2012;209:1595–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Feagan BG, Rutgeerts P, Sands BE, et al. ; GEMINI 1 Study Group. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013;369:699–710. [DOI] [PubMed] [Google Scholar]
- 61. Zundler S, Schillinger D, Fischer A, et al. Blockade of αEβ7 integrin suppresses accumulation of CD8+ and Th9 lymphocytes from patients with IBD in the inflamed gut in vivo. Gut 2017;66:1936–48. [DOI] [PubMed] [Google Scholar]
- 62. Rivera-Nieves J, Olson T, Bamias G, et al. L-selectin, alpha 4 beta 1, and alpha 4 beta 7 integrins participate in CD4+ T cell recruitment to chronically inflamed small intestine. J Immunol 2005;174:2343–52. [DOI] [PubMed] [Google Scholar]
- 63. Zundler S, Becker E, Spocinska M, et al. Hobit- and Blimp-1-driven CD4+ tissue-resident memory T cells control chronic intestinal inflammation. Nat Immunol 2019;20:288–300. [DOI] [PubMed] [Google Scholar]
- 64. Bishu S, El Zaatari M, Hayashi A, et al. Cd4+ tissue-resident memory t-cells expand and are a major source of mucosal tumor necrosis factor alpha in active Crohn’s disease. J Crohns Colitis 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.