Abstract
Targeted protein degradation using Proteolysis Targeting Chimeras (PROTACs) has emerged as a novel therapeutic modality in drug discovery. PROTACs mediate the degradation of select proteins of interest (POIs) by hijacking the activity of E3 ubiquitin ligases for POI ubiquitination and subsequent degradation by the 26S proteasome. This hijacking mechanism has been used to degrade various types of disease-relevant POIs. In this review, we aim to highlight the recent advances in targeted protein degradation and describe the challenges that need to be addressed in order to efficiently develop potent PROTACs.
I. Introduction
Protein conjugation with ubiquitin, a small protein modifier, is essential for regulated protein degradation by the 26S proteasome. Despite delineating the ATP-dependent pathway of protein degradation in the late 1970s [1–6], the first application to exploit this system for targeted protein degradation was reported thirty years later [7]. Proteolysis Targeting Chimeras (PROTACs) are heterobifunctional molecules consisting of: (1) a ligand that binds a POI; (2) a ligand for recruiting an E3 ubiquitin ligase (E3 recruiting element; E3RE) to promote POI ubiquitination; and (3) a linker connecting these ligands (Figure 1A) [7–11]. To date, there are over 100 reports describing the use of PROTACs for targeted protein degradation (Web of Science search: February 14, 2018) and their utility in chemical biology and drug development. In this review, we describe recent advances in the targeted protein degradation field and discuss those principles underlying efficient PROTAC design that remain to be elucidated.
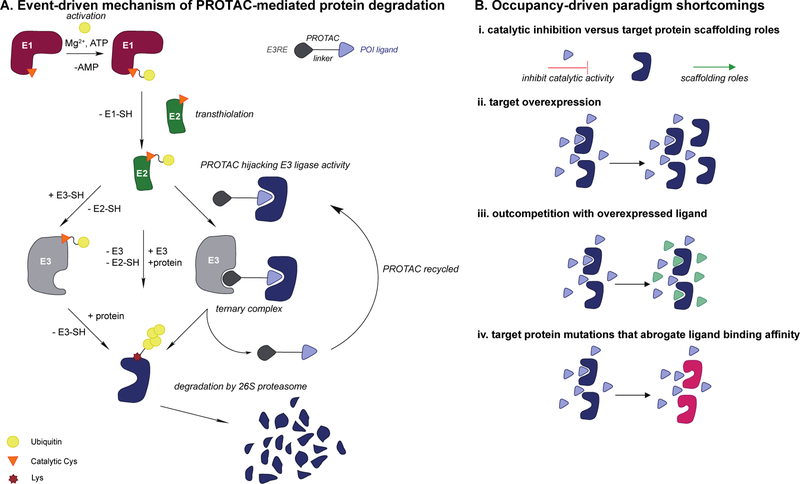
Figure 1A.
Mechanistic overview of PROTAC-mediated POI ubiquitination via the ubiquitination enzymatic cascade, and POI degradation via the 26S proteasome. B. Potential shortcomings of occupancy-driven paradigm of small-molecule/drug-target binding wherein sustained target engagement is limited due to: i. catalytic inhibition offers cannot potentiate non-catalytic and/or scaffolding roles of target-protein, ii. target protein overexpression, iii. competition with overexpressed native ligand for same binding site, and iv. target protein mutations potentiate small-molecule/drug binding.
I.i. Mechanistic Overview of PROTAC-mediated Protein Degradation
Ubiquitin is conjugated to a protein substrate via an enzymatic cascade [5,6,12]. First, an E1 activating enzyme primes ubiquitin via an ATP-dependent mechanism forming an E1~ubiquitin conjugate (~; thioester bond) [5,6,13] followed by formation of an E2~ubiquitin conjugate via a transthiolation reaction with an E2 conjugating enzyme (Figure 1A) [5,6,14]. Finally, one of the ~600 putative E3 ligases mediates the transfer of ubiquitin to a substrate protein [5,6,15].
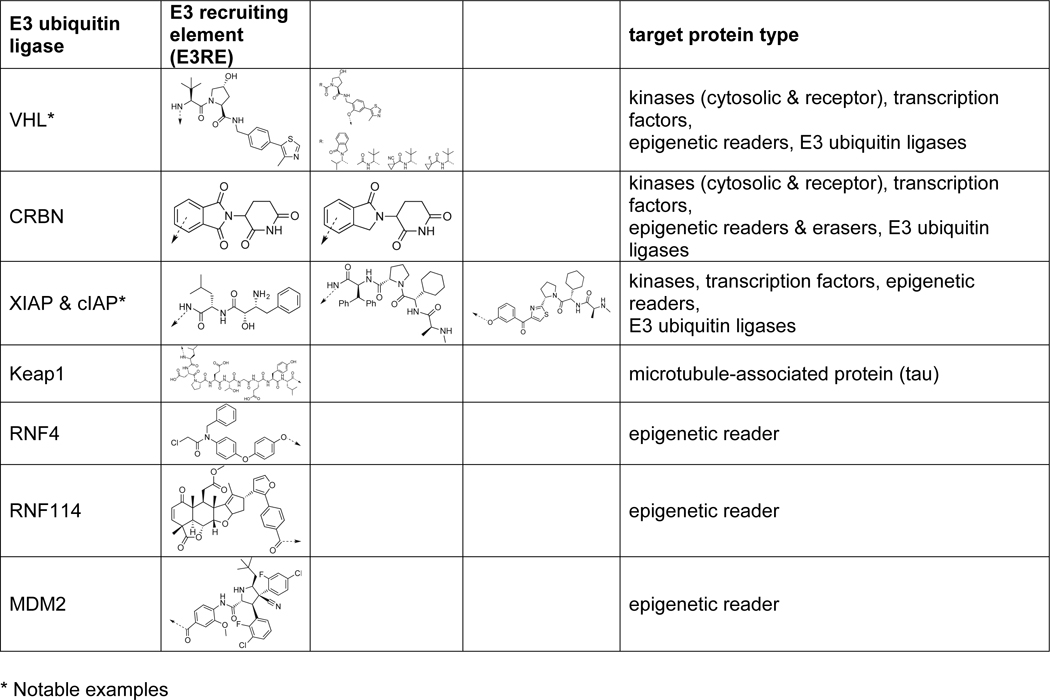
E3 ligases mediate protein substrate specificity and catalyze this final transfer via a non-covalent or covalent mechanism depending on the E3 type [12,15]. The three major families of E3 ligases include the RING/U-box family [16–18] and the active-site cysteine-containing HECT [19,20] and RING-in-Between-RING (RBR) families [21,22]. Some E3 ligases function by recognizing specific degradation motifs, known as degrons [23,24]. For example, UBR E3 ligases function via the N-end rule pathway, wherein a ‘destabilizing’ N-terminal amino acid promotes UBR-mediated ubiquitination [23,25]. Meanwhile, the von Hippel Lindau (VHL) E3 ligase recognizes Hypoxia-Inducible Factor 1 α (HIF1-α) whereby hydroxylation of a key proline residue on the HIF1-α degron motif is essential for VHL -recruitment [26–28]. This degron forms the basis of one of the most widely used E3REs for PROTACs (Table 1) [29–31].
Table 1.
Examples of E3 recruiting elements and respective E3 ubiquitin ligases employed in recent years (~2) for PROTACdevelopment [8–11,35–80]. Dashed arrows represent vectors used for linker attachment in PROTAC synthesis.
 |
By recruiting an E3 to a POI, PROTACs hijack ligase activity for POI ubiquitination and subsequent degradation by the 26S proteasome (Figure 1A) [8–11]. PROTACs induce the ternary complex (POI:PROTAC:E3 ligase) for ubiquitination, after which the POI is committed for destruction. Since the PROTAC is not degraded in this process, it can promote ubiquitination and degradation of multiple POI equivalents, thus operating sub-stoichiometrically [32]. This catalytic, event-driven modality contrasts with the traditional inhibitor paradigm wherein sustained target binding is indispensable for eliciting a desired biological response. In the standard occupancy-driven paradigm of drug development, potency is dependent on binding affinity. For example, POI inhibition likely cannot influence non-catalytic target protein function(s) (Figure 1B). Additionally, sustained target engagement is difficult in cases of target overexpression, the presence of competing native ligand(s), or target protein mutations that result in loss of target engagement and subsequent resistance (Figure 1B) [33,34]. Since PROTACs inhibit protein function via degradation, this event-driven technology can be used to circumvent the common disadvantages of traditional occupancy-driven inhibitors described above.
II. Current Status of the PROTAC Technology
In the past several years, targeted protein degradation has generated excitement in both academic and industrial settings where POIs ranging in protein class, function, and/or subcellular localization have been successfully degraded (Table 1) [8–11,35–80]. In contrast to this wide range of targeted POIs, relatively few RING E3 ligases have been targeted for recruitment by PROTACs [8–11,35–80].
II.i. PROTAC-mediated Degradation of Epigenetic Erasers
Some PROTACs have been developed to degrade nuclear proteins such as histone deacetylases (HDACs) [44,81]. For example, sirtuin2 (Sirt2), an NAD+-dependent class III HDAC was targeted for degradation [44] by appending SirReal3b, a potent Sirt2 inhibitor [82], to thalidomide via a triazole-based linker to yield a Sirt2-selective PROTAC exhibiting micromolar activity in HeLa cells [44]. This PROTAC retained inhibitory activity against Sirt2 and promoted Sirt2 degradation in a CRBN- and proteasome-dependent manner while eliciting downstream α-tubulin hyperacetylation [44]. Similarly, degradation of HDAC6, a class IIB Zn2+-dependent HDAC, was achieved using a PROTAC incorporating a hydroxamic-acid-based HDAC6 inhibitor and pomalidomide as its E3RE [81]. Since most HDAC inhibitors bind the active site and/or chelate Zn2+[83], degradation may help characterize the non-catalytic roles of HDACs in disease development and progression.
II.ii. Challenging Localization, Affinity, and Resistance Mechanisms
Recently, an enzalutamide-based PROTAC, ARCC-4, resulted in androgen receptor (AR) degradation at low nanomolar concentrations in VCaP cells, a model for castration-resistant prostate cancer (CRPC) [57]. Surpassing the potency of enzalutamide itself, ARCC-4 also circumvented resistance mechanisms that emerge in patients due to previous therapeutic regimens [57].
The advantages of degradation over inhibition has also been shown when studying PROTAC-mediated degradation of membrane-bound receptor tyrosine kinases (RTKs). As a major drug target group, 47 small-molecule kinase inhibitors have been approved by the FDA. Some of these inhibitors have proven as useful recruiting elements in PROTACs to degrade both serine/threonine and tyrosine kinases[35,41,43,47,49,50,52,55,58,63,65,69–71,84–89]. Despite the clinical success of inhibiting receptor tyrosine kinases (RTKs) [90,91], ‘kinome rewiring’, i.e., the compensatory feedback activation of alternative kinases, is often observed as a resistance mechanism. As previously shown when targeting other POIs (i.e. AR) [55,57,66], degradation can circumvent resistance mechanisms. Appending RTK inhibitors to the VHL-recruiting E3RE afforded PROTACs that degrade membrane-bound WT EGFR as well as disease-relevant EGFR mutants [35]. Moreover, longer-sustained suppression of RTK-downstream signaling was observed in contrast to the kinome rewiring that results from inhibition alone [35] highlighting both the scope of PROTAC target space, and the advantages of degradation over inhibition, respectively.
Interestingly, the use of promiscuous kinase ligands in PROTACs has provided insights into the basis for PROTAC-mediated POI selectivity [35,49,50]. Using foretinib, a kinase inhibitor that binds over 130 kinases at 10 µM, interesting and non-overlapping degradation profiles were observed depending on the E3RE used (i.e., VHL vs CRBN) [49]. Moreover, degradation was observed even with some weak-binding kinases, such as p38α, likely due to positive cooperativity via protein:protein interactions (PPIs) between p38α and VHL in the ternary complex [49]. Similarly, a CRBN-recruiting PROTAC with a promiscuous kinase ligand exhibited different target degradation profiles unrelated to their binding affinities across different cell lines [50], further corroborating the disconnect between target affinity and degradation efficiency. Interestingly, robust TBK1 degradation (>70%) was observed with PROTACs containing VHL ligands displaying up to 3-fold diminution in affinity relative to the typical VHL E3RE (Table 1) [43], further supporting the idea that POI and/or E3 engagement alone does not determine a PROTAC’s efficacy. This diminishes the need for high affinity ligands and expands the potential PROTAC target space.
II.iii. ‘Ineffectual’ ligands can be used for POI degradation
In addition to not needing high affinity ligands to ensure PROTAC efficacy, ligands which lack the ability to modulate cellular POI function can be utilized in PROTACs for POI degradation, as highlighted by a PROTAC which induced the degradation of a multidomain co-regulator of transcription, tripartite motif 24 protein (TRIM24, originally classified as transcriptional intermediary factor 1α). [80] Potent and selective inhibitors of the TRIM24 bromodomain (BD) showed little to no phenotypic consequences in TRIM24-endogenously expressing cells, suggesting BD-inhibition alone may not be sufficient as an anti-cancer strategy in TRIM24-dependent cancers. [92,93] Interestingly, the ligand exhibiting lower affinity and selectivity towards TRIM24[92] afforded a selective and efficient VHL-recruiting PROTAC (dTRIM24). [80] In parallel with genetic studies, dTRIM24-mediated TRIM24 degradation revealed the functional importance of its RING domain in acute leukemia cellular proliferation while disproving its previously characterized dependency in MCF-7 tumour cells. [80] This application exemplifies “non-functional” selective ligands can be exploited by the PROTAC technology to characterize the functional importance of select POIs in disease development and progression.
II.iv. Current Toolbox for Studying what Determines PROTAC Efficacy
BD and extra-terminal (BET) domain family are attractive therapeutic targets given their role in transcriptional control of key oncogenic driver genes [94–97]. Two BD inhibitors, OTX-015[98] and JQ1[99], have been incorporated into PROTACs to study the biological consequences of BET degradation and to characterize the POI:PROTAC:E3 ternary complex [100]. Crystallographic data of the BRD4:PROTAC:VHL ternary complex revealed the PROTAC promotes protein:protein interactions covering 700 Å2 worth of surface area between BRD4BD2 and VHL [100]. Interestingly, the PEG linker forms additional protein-ligand interactions within the ternary complex [100]. In contrast, efficient POI degradation was observed for a selection of CRBN-based PROTACs having either no or negative cooperativity [56]. Therefore, due to its multi-faceted nature, the importance of ternary complex formation remains empirical.
Given the complexity of the cellular environment, technologies that monitor ternary complex formation, POI ubiquitination and degradation in cellulo are of great benefit. PROTAC-mediated protein degradation by E3 ligases belonging to other families can be monitored using HaloTag7-E3 ligase fusion proteins and a GFP- FKBPF36V reporter system [101]. Excitingly, GFP- FKBPF36V degradation was observed for select RING, HECT and RING-in-Between-RING (RBR) E3 ligases, revealing the applicability of PROTACs to E3 ligases of other families [101]. Similarly, the dTAG system can be used to evaluate and validate the biological consequences of PROTAC-mediated POI degradation without need for POI ligand development by by monitoring the degradation of FKBPF36V POI fusion proteins [102]. This technique has been used to: (1) selectively induce POI degradation that discriminates the target from similar isoforms [102]; (2) validate preclinical therapeutic targets [103]; and (3) validate previously identified targets in disease progression [104]. However, despite the utility of these systems, E3 ligase efficacy, POI ubiquitination and degradation may be misrepresented using these larger tags [101,102]. Moreover, monitoring the various steps of PROTAC-mediated POI degradation (Figure 2) in real-time remains difficult using these approaches.
Figure 2.
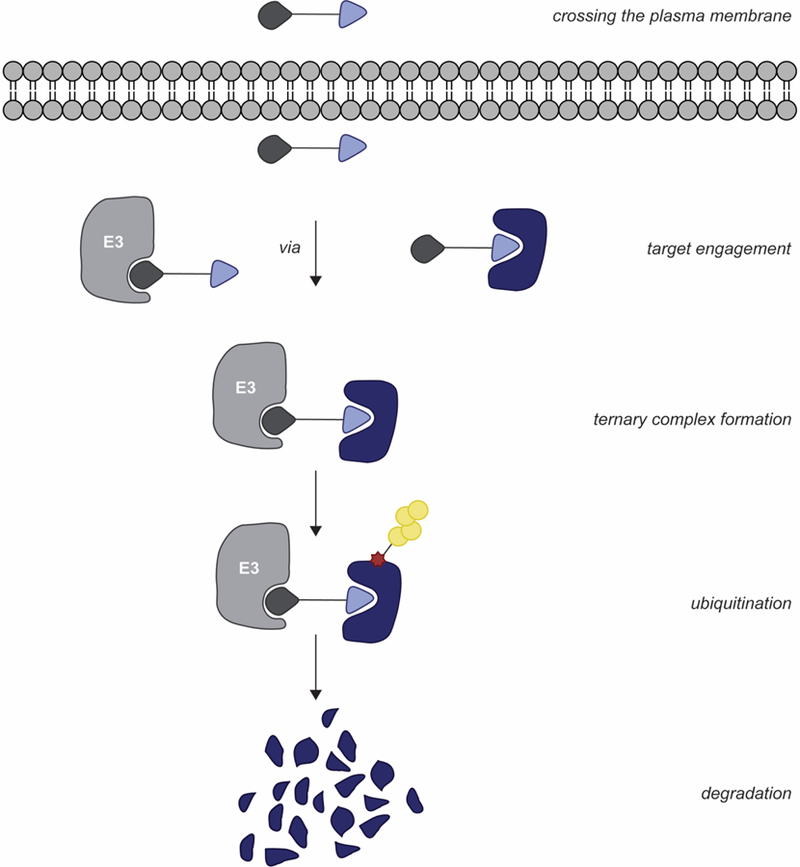
Overview of steps for PROTAC-mediated POI degradation in a cellular context.
An innovative, modular live-cell platform using CRISPR/Cas9 endogenous tagging, luminescence readouts, and NanoBRET technology has been developed to provide functional real-time characterization of PROTAC-mediated POI degradation [105]. This new system allows for monitoring the kinetics of HiBiT-BRD2, BRD3, and BRD4 ternary complex formation, ubiquitination and degradation using CRBN-recruiting and VHL-recruiting PROTACs [105]. For instance, despite all reaching DMAX >86%, maximal degradation levels and recovery rates were observed at different times for each BET protein, even when treated with the same PROTAC [105]. A direct correlation between degradation rates and ubiquitination rates was observed, as opposed to ternary complex formation rate and stability [105]. Interestingly, a significantly different degradation profile of ectopically expressed NLuc-BRD4, as compared to the HiBiT- BRD4 system is observed. Both degradation rates and levels were reduced, while recovery rates appeared more rapid compared to endogenously-tagged HiBiT-BRD4 [105]. Therefore, initially screening for active compounds using methods lacking real-time capabilities, and/or using reporter systems that rely on ectopic expression may overlook: (1) PROTAC-mediated degradation levels, (2) the potential for degrading select POIs, and (3) whether new E3 ligases can be hijacked. Altogether, systems such as NanoBRET can help with PROTAC development and optimization.
III. What does the Future hold for PROTACs?
Irrespective of the mechanistic insight acquired, the full potential of PROTAC technology remains untapped. We have learned a lot from using tool compounds that target kinases and BET proteins, but it remains to be determined how transferable these discoveries are to other protein families and/or classes.
In addition to exploring other protein types, it is imperative that we explore the “PROTACability” of other E3 ligases given observed discrepancies in POI degradation depending on which E3 ligase is recruited. Only <1% of the putative E3s in the human proteome have successfully been hijacked, specifically those belonging to the RING family (Table 1) [8–11,35–80,101]. We have yet not recruited other E3 ligases such as U-box, HECT, and RBR E3 ligases using small-molecule ligands specific for these E3s[101]. Since degradation efficiency is unaffected by attenuating E3 ligase affinity with small changes to the E3RE [43], one could target other E3s, hypothetically even in a tissue-specific manner, potentially without laborious efforts to optimize E3RE affinity. Alternatively, significant contributions are more likely to be made by exploring E3RE vector attachment point(s) (Table 1) and other features important for optimal ternary complex geometry.
Techniques enabling the classification and inhibition of active E2/E3 pairs may afford the identification of novel E3 modulators [106–108]. Meanwhile, some E3 ligases are auto-inhibited via post-translation modification dependent mechanisms[109]. For example, phosphorylation of NEDD4–2, a HECT-type E3 ligase, via GPCR-mediated activation of c-Src kinase activity mediates its activity [110]. Therefore, one could hijack these activation mechanisms to design conditional PROTAC-mediated POI degradation. Given that some E3 ligases bind viral proteins [111], characterizing such binding interactions could inform the design of nature-inspired E3REs. Finally, complimentary to N-terminal degrons, recent reports of C-terminal degrons for the Cullin2-RING ligases suggest there are likely degrons for other E3 ligase families [112,113]. As was done with for VHL[29–31], we could potentially use degrons to develop E3REs [101], increasing the repertoire of ‘hijackable’ E3s in the PROTAC toolbox.
Excitingly, technologies such as NanoBiT system [105] allow for the characterization of the dynamic nature of PROTAC-mediated degradation (Figure 2). In the occupancy-driven archetype of drug development, efforts are shifted towards optimizing lead candidate target-residence time (i.e. increasing 1/kOFF) as this has proven efficacious in optimizing for pharmacokinetic and pharmacodynamic drug properties [114]. Using current and new technologies, can we identify which key kinetic steps along the mechanistic coordinate of targeted protein degradation should be optimized? If so, are these target protein- and/or E3 ligase type-dependent?
In addition to characterizing the kinetics of the basic steps required for an effective PROTAC (Figure 2), we should also evaluate what occurs between ubiquitination and degradation. Are there additional cellular systems that can be hijacked or potentiated for the PROTAC technology. For example, p97 prepares protein transport from E3 ligase machinery to the 26S proteasome including with CRBN neosubstrates [115,116]; should this function be further characterized in PROTAC design? Moreover, mechanisms of degradation escape, such as chaperone protein(s) [117,118] and deubiquitinating enzymes [119], should be further characterized for tuning PROTAC efficacy.
IV. Conclusions
Most research efforts highlighted here demonstrate that we have yet to furnish a plug-and-play approach for PROTAC development. However, we can now appreciate that binary target engagement affinities are not indicative of degradation efficiencies for PROTACs [49,50,56]. Meanwhile, the importance of POI ubiquitination versus ternary complex formation and stability for efficient degradation has been uncovered, using well-established target proteins and tool compounds [105]. Given these significant findings, the focus should shift to surveying the broad utility of PROTACs by probing new protein targets and capitalizing on new E3 ligases from other E3 families, including those which are auto-regulated. In parallel, advancing current and new technologies to understand and even predict PROTAC-mediated degradation via computational, biochemical and cellular methods is essential for the field to flourish. These efforts will enable understanding the underlying plastic nature of the PROTAC technology and help truly establish its therapeutic potential.
Acknowlegements
We thank Doris Hellerschmied-Jelinek, Mariell Pettersson and John Hines for helpful discussions and reading of the manuscript.
CMC gratefully acknowledges support from the NIH (R35CA197589) and Arvinas, Inc. CMC is a founder, consultant, and shareholder in Arvinas, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ciehanover A, Hod Y, Hershko A: A heat-stable polypeptide component of an ATP-dependent proteolytic system from reticulocytes. Biochem Bioph Res Co 1978, 81:1100–1105. [DOI] [PubMed] [Google Scholar]
- 2.Ciechanover A, Heller H, Katz-Etzion R, Hershko A: Activation of the heat- stable polypeptide of the ATP-dependent proteolytic system. Proc Natl Acad Sci USA 1981, 78:761–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hershko A, Ciechanover A, Heller H, Haas AL, Rose IA: Proposed role of ATP in protein breakdown: conjugation of protein with multiple chains of the polypeptide of ATP-dependent proteolysis. Proc Natl Acad Sci USA 1980, 77:1783–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hershko A, Ciechanover A, Rose IA: Resolution of the ATP-dependent proteolytic system from reticulocytes: a component that interacts with ATP. Proc Natl Acad Sci USA 1979, 76:3107–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hershko A, Heller H, Elias S, Ciechanover A: Components of ubiquitin-protein ligase system. Resolution, affinity purification, and role in protein breakdown. J Biol Chem 1983, 258:8206–8214. [PubMed] [Google Scholar]
- 6.Hershko A, Ciechanover A: The ubiquitin system for protein degradation. Annu Rev Biochem 1992, 61:761–807. [DOI] [PubMed] [Google Scholar]
- 7.Sakamoto KM, Kim KB, Kumagai A, Mercurio F, Crews CM, Deshaies RJ: Protacs: chimeric molecules that target proteins to the Skp1-Cullin-F box complex for ubiquitination and degradation. Proc Natl Acad Sci USA 2001, 98:8554–8559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burslem GM, Crews CM: Small-Molecule Modulation of Protein Homeostasis. Chem. Rev 2017, 117:11269–11301. [DOI] [PubMed] [Google Scholar]
- 9.Cromm PM, Crews CM: Targeted Protein Degradation: from Chemical Biology to Drug Discovery. Cell Chem Biol 2017, 24:1181–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raina K, Crews CM: Targeted protein knockdown using small molecule degraders. Curr Opin Chem Biol 2017, 39:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher SL, Phillips AJ: Targeted protein degradation and the enzymology of degraders. Curr Opin Chem Biol 2018, 44:47–55. [DOI] [PubMed] [Google Scholar]
- 12.Komander D, Rape M: The ubiquitin code. Annu Rev Biochem 2012, 81:203–229. [DOI] [PubMed] [Google Scholar]
- 13.Schulman BA, Harper JW: Ubiquitin-like protein activation by E1 enzymes: the apex for downstream signalling pathways. Nat Rev Drug Disc 2009, 10:319–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye Y, Rape M: Building ubiquitin chains: E2 enzymes at work. Nat Rev Mol Cell Biol 2009, 10:755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng N, Shabek N: Ubiquitin Ligases: Structure, Function, and Regulation. Annu Rev Biochem 2017, 86:129–157. [DOI] [PubMed] [Google Scholar]
- 16.Deshaies RJ, Joazeiro CAP: RING Domain E3 Ubiquitin Ligases. Annu Rev Biochem 2009, 78:399–434. [DOI] [PubMed] [Google Scholar]
- 17.Lipkowitz S, Weissman AM: RINGs of good and evil: RING finger ubiquitin ligases at the crossroads of tumour suppression and oncogenesis. Nat Rev Cancer 2011, 11:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hatakeyama S, Yada M, Matsumoto M, Ishida N, Nakayama KI: U box proteins as a new family of ubiquitin-protein ligases. J Biol Chem 2001, 276:33111–33120. [DOI] [PubMed] [Google Scholar]
- 19.Metzger MB, Hristova VA, Weissman AM: HECT and RING finger families of E3 ubiquitin ligases at a glance. Journal of Cell Science 2012, 125:531–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rotin D, Kumar S: Physiological functions of the HECT family of ubiquitin ligases. Nat Rev Mol Cell Biol 2009, 10:398–409. [DOI] [PubMed] [Google Scholar]
- 21.Dove KK, Klevit RE: RING‐between‐RINGs—keeping the safety on loaded guns. EMBO J 2012, 31:3792–3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dove KK, Klevit RE: RING-Between-RING E3s ligases: Emerging themes amid the variations. J Mol Biol 2017, 429:1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varshavsky A: The N-end rule: functions, mysteries, uses. Proc Natl Acad Sci USA 1996, 93:12142–12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varshavsky A: N-degron and C-degron pathways of protein degradation. Proc Natl Acad Sci USA 2019, 116:358–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varshavsky A: The N-end rule pathway and regulation by proteolysis. Protein Sci 2011, 20:1298–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ivan M, Kaelin WG: The von Hippel-Lindau tumor suppressor protein. Curr Opin Genet Dev 2001, 11:27–34. [DOI] [PubMed] [Google Scholar]
- 27.Bruick RK, McKnight SL: Building better vasculature. Genes Dev 2001, 15:2497–2502. [DOI] [PubMed] [Google Scholar]
- 28.Min JH: Structure of an HIF-1alpha -pVHL Complex: Hydroxyproline Recognition in Signaling. Science 2002, 296:1886–1889. [DOI] [PubMed] [Google Scholar]
- 29.Buckley DL, Van Molle I, Gareiss PC, Tae HS, Michel J, Noblin DJ, Jorgensen WL, Ciulli A, Crews CM: Targeting the von Hippel-Lindau E3 ubiquitin ligase using small molecules to disrupt the VHL/HIF-1α interaction. J Am Chem Soc 2012, 134:4465–4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buckley DL, Gustafson JL, Van Molle I, Roth AG, Tae HS, Gareiss PC, Jorgensen WL, Ciulli A, Crews CM: Small-molecule inhibitors of the interaction between the E3 ligase VHL and HIF1α. Angew Chem Int Ed 2012, 51:11463–11467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Molle I, Thomann A, Buckley DL, So EC, Lang S, Crews CM, Ciulli A: Dissecting Fragment-Based Lead Discovery at the von Hippel-Lindau Protein:Hypoxia Inducible Factor 1α Protein-Protein Interface. Chem Biol 2012, 19:1300–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bondeson DP, Mares A, Smith IED, Ko E, Campos S, Miah AH, Mulholland KE, Routly N, Buckley DL, Gustafson JL, et al. : Catalytic in vivo protein knockdown by small-molecule PROTACs. Nat Chem Biol 2015, 11:611–617.*First report of catalytic mechanism for PROTAC-mediated protein degradation.
- 33.Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG: Cancer drug resistance: an evolving paradigm. Nat Rev Drug Disc 2013, 13:714–726. [DOI] [PubMed] [Google Scholar]
- 34.Longley DB, Johnston PG: Molecular mechanisms of drug resistance. J Pathol 2005, 205:275–292. [DOI] [PubMed] [Google Scholar]
- 35.Burslem GM, Smith BE, Lai AC, Jaime Figueroa S, McQuaid DC, Bondeson DP, Toure M, Dong H, Qian Y, Wang J, et al. : The Advantages of Targeted Protein Degradation Over Inhibition: An RTK Case Study. Cell Chem Biol 2018, 25:67–77.e3.*First report of PROTAC-mediated degradation of a membrane-bound protein.
- 36.Saenz DT, Fiskus W, Qian Y, Manshouri T, Rajapakshe K, Raina K, Coleman KG, Crew AP, Shen A, Mill CP, et al. : Novel BET protein proteolysis-targeting chimera exerts superior lethal activity than bromodomain inhibitor (BETi) against post-myeloproliferative neoplasm secondary (s) AML cells. Leukemia 2017, 31:1951–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collins I, Wang H, Caldwell JJ, Chopra R: Chemical approaches to targeted protein degradation through modulation of the ubiquitin–proteasome pathway. Biochem J 2017, 474:1127–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohoka N, Morita Y, Nagai K, Shimokawa K, Ujikawa O, Fujimori I, Ito M, Hayase Y, Okuhira K, Shibata N, et al. : Derivatization of inhibitor of apoptosis protein (IAP) ligands yields improved inducers of estrogen receptor α degradation. J Biol Chem 2018, 293:6776–6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohoka N, Misawa T, Kurihara M, Demizu Y, Naito M: Development of a peptide-based inducer of protein degradation targeting NOTCH1. Bioorg Med Chem Lett 2017, 27:4985–4988. [DOI] [PubMed] [Google Scholar]
- 40.Ohoka N, Nagai K, Shibata N, Hattori T, Nara H, Cho N, Naito M: SNIPER(TACC3) induces cytoplasmic vacuolization and sensitizes cancer cells to Bortezomib. Cancer Sci 2017, 108:1032–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Demizu Y, Shibata N, Hattori T, Ohoka N, Motoi H, Misawa T, Shoda T, Naito M, Kurihara M: Development of BCR-ABL degradation inducers via the conjugation of an imatinib derivative and a cIAP1 ligand. Bioorg Med Chem Lett 2016, 26:4865–4869. [DOI] [PubMed] [Google Scholar]
- 42.Okitsu K, Hattori T, Misawa T, Shoda T, Kurihara M, Naito M, Demizu Y: Development of a Small Hybrid Molecule That Mediates Degradation of His-Tag Fused Proteins. J Med Chem 2017, 61:576–582. [DOI] [PubMed] [Google Scholar]
- 43.Crew AP, Raina K, Dong H, Qian Y, Wang J, Vigil D, Serebrenik YV, Hamman BD, Morgan A, Ferraro C, et al. : Identification and Characterization of Von Hippel-Lindau-Recruiting Proteolysis Targeting Chimeras (PROTACs) of TANK-Binding Kinase 1. J Med Chem 2017, 61:583–598.**This results in this report highlight that a high E3RE binding affinity is not necessary for efficient PROTAC-mediated degradation.
- 44.Schiedel M, Herp D, Hammelmann S, Swyter S, Lehotzky A, Robaa D, Oláh J, Ovádi J, Sippl W, Jung M: Chemically Induced Degradation of Sirtuin 2 (Sirt2) by a Proteolysis Targeting Chimera (PROTAC) Based on Sirtuin Rearranging igands (SirReals). J Med Chem 2017, 61:482–491. [DOI] [PubMed] [Google Scholar]
- 45.Robb CM, Contreras JI, Kour S, Taylor MA, Abid M, Sonawane YA, Zahid M, Murry DJ, Natarajan A, Rana S: Chemically induced degradation of CDK9 by a proteolysis targeting chimera (PROTAC). Chem Commun 2017, 53:7577–7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shibata N, Nagai K, Morita Y, Ujikawa O, Ohoka N, Hattori T, Koyama R, Sano O, Imaeda Y, Nara H, et al. : Development of Protein Degradation Inducers of Androgen Receptor by Conjugation of Androgen Receptor Ligands and Inhibitor of Apoptosis Protein Ligands. J Med Chem 2017, 61:543–575. [DOI] [PubMed] [Google Scholar]
- 47.Shibata N, Shimokawa K, Nagai K, Ohoka N, Hattori T, Miyamoto N, Ujikawa O, Sameshima T, Nara H, Cho N, et al. : Pharmacological difference between degrader and inhibitor against oncogenic BCR-ABL kinase. Sci Rep 2018, 8:13549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ward CC, Kleinman JI, Chung CYS, Kim K, Petri Y, Lee PS, Thomas JR, Tallarico JA, McKenna JM, Schirle M, et al. : Covalent Ligand Screening Uncovers a RNF4 E3 Ligase Recruiter for Targeted Protein Degradation Applications. bioRxiv 2018, doi: 10.1101/439125.**The authors here identify a recruiting ligand for RNF4 using activity-based protein profiling (ABPP)-based covalent ligand screening and apply this to the PROTAC technology.
- 49.Bondeson DP, Smith BE, Burslem GM, Buhimschi AD, Hines J, Jaime Figueroa S, Wang J, Hamman BD, Ishchenko A, Crews CM: Lessons in PROTAC Design from Selective Degradation with a Promiscuous Warhead. Cell Chem Biol 2018, 25:78–87.e5.**The authors here highlight that a relatively promiscuous ligand afforded selective degradation when incorporated in PROTACs and selectivity profiles were different depending on the E3RE used.
- 50.Huang H-T, Dobrovolsky D, Paulk J, Yang G, Weisberg EL, Doctor ZM, Buckley DL, Cho J-H, Ko E, Jang J, et al. : A Chemoproteomic Approach to Query the Degradable Kinome Using a Multi-kinase Degrader. Cell Chem Biol 2018, 25:88–99.e6.**Complimentary to reference 48, a promiscuous ligand for kinases afforded some selectivity for kinase degradation when incorporated in PROTACs.
- 51.Jiang Y, Deng Q, Zhao H, Xie M, Chen L, Yin F, Qin X, Zheng W, Zhao Y, Li Z: Development of Stabilized Peptide-Based PROTACs against Estrogen Receptor α. ACS Chem Biol 2018, 13:628–635. [DOI] [PubMed] [Google Scholar]
- 52.Bian J, Ren J, Li Y, Wang J, Xu X, Feng Y, Tang H, Wang Y, Li Z: Discovery of Wogonin-based PROTACs against CDK9 and capable of achieving antitumor activity. Bioorg Chem 2018, 81:373–381. [DOI] [PubMed] [Google Scholar]
- 53.Lu M, Liu T, Jiao Q, Ji J, Tao M, Liu Y, You Q, Jiang Z: Discovery of a Keap1-dependent peptide PROTAC to knockdown Tau by ubiquitination-proteasome degradation pathway. Eur J Med Chem 2018, 146:251–259. [DOI] [PubMed] [Google Scholar]
- 54.Zhang X, Crowley VM, Wucherpfennig TG, Dix MM, Cravatt BF: Electrophilic PROTACs that degrade nuclear proteins by engaging DCAF16. bioRxiv 2018, doi: 10.1101/443804.**The authors here identify use cysteine-directed heterobifunctional electrophilic compounds to demonstrate that DCAF16 can be used to promote the degradation of nuclear-restricted proteins.
- 55.Buhimschi AD, Armstrong HA, Toure M, Jaime Figueroa S, Chen TL, Lehman AM, Woyach JA, Johnson AJ, Byrd JC, Crews CM: Targeting the C481S Ibrutinib-Resistance Mutation in Bruton’s Tyrosine Kinase Using PROTAC-Mediated Degradation. Biochemistry 2018, 57:3564–3575. [DOI] [PubMed] [Google Scholar]
- 56.Nowak RP, DeAngelo SL, Buckley D, He Z, Donovan KA, An J, Safaee N, Jedrychowski MP, Ponthier CM, Ishoey M, et al. : Plasticity in binding confers selectivity in ligand- induced protein degradation. Nat Chem Biol 2018, doi: 10.1038/s41589-018-0055-y. [DOI] [PMC free article] [PubMed]
- 57.Salami J, Alabi S, Willard RR, Vitale NJ, Wang J, Dong H, Jin M, McDonnell DP, Crew AP, Neklesa TK, et al. : Androgen receptor degradation by the proteolysis- targeting chimera ARCC-4 outperforms enzalutamide in cellular models of prostate cancer drug resistance. Commun Biol 2018, doi: 10.1038/s42003-018-0105-8. [DOI] [PMC free article] [PubMed]
- 58.Zorba A, Nguyen C, Xu Y, Starr J, Borzilleri K, Smith J, Zhu H, Farley KA, Ding W, Schiemer J, et al. : Delineating the role of cooperativity in the design of potent PROTACs for BTK. Proc Natl Acad Sci USA 2018, 115:E7285–E7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang K, Song Y, Xie H, Wu H, Wu Y-T, Leisten ED, Tang W: Development of the first small molecule histone deacetylase 6 (HDAC6) degraders. Bioorg Med Chem Lett 2018, 28:2493–2497. [DOI] [PubMed] [Google Scholar]
- 60.Chen H, Chen F, Liu N, Wang X, Gou S: Chemically induced degradation of CK2 by proteolysis targeting chimeras based on a ubiquitin– proteasome pathway. Bioorg Chem 2018, 81:536–544. [DOI] [PubMed] [Google Scholar]
- 61.Bassi ZI, Fillmore MC, Miah AH, Chapman TD, Maller C, Roberts EJ, Davis LC, Lewis DE, Galwey NW, Waddington KE, et al. : Modulating PCAF/GCN5 Immune Cell Function through a PROTAC Approach. ACS Chem Biol 2018, 13:2862–2867. [DOI] [PubMed] [Google Scholar]
- 62.McCoull W, Cheung T, Anderson E, Barton P, Burgess J, Byth K, Cao Q, Castaldi MP, Chen H, Chiarparin E, et al. : Development of a Novel B-Cell Lymphoma 6 (BCL6) PROTAC To Provide Insight into Small Molecule Targeting of BCL6. ACS Chem Biol 2018, doi: 10.1021/acschembio.8b00698. [DOI] [PubMed]
- 63.Kang CH, Lee DH, Lee CO, Ha Du J, Park CH, Hwang JY: Induced protein degradation of anaplastic lymphoma kinase (ALK) by proteolysis targeting chimera (PROTAC). Biochem Bioph Res Co 2018, doi: 10.1016/j.bbrc.2018.09.169. [DOI] [PubMed]
- 64.Powell CE, Gao Y, Tan L, Donovan KA, Nowak RP, Loehr A, Bahcall M, Fischer ES, Jänne PA, George RE, et al. : Chemically Induced Degradation of Anaplastic Lymphoma Kinase (ALK). J Med Chem 2018, 61:4249–4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Olson CM, Jiang B, Erb MA, Liang Y, Doctor ZM, Zhang Z, Zhang T, Kwiatkowski N, Boukhali M, Green JL, et al. : Pharmacological perturbation of CDK9 using selective CDK9 inhibition or degradation. Nat Chem Biol 2018, 14:163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Neklesa T, Snyder LB, Willard RR, Vitale N, Raina K, Pizzano J, Gordon DA, Bookbinder M, Macaluso J, Dong H, et al. : An oral androgen receptor PROTAC degrader for prostate cancer. J Clin Oncol 2018, 36:381–381. [Google Scholar]
- 67.Maniaci C, Hughes SJ, Testa A, Chen W, Lamont DJ, Rocha S, Alessi DR, Romeo R, Ciulli A: Homo-PROTACs: bivalent small-molecule dimerizers of the VHL E3 ubiquitin ligase to induce self-degradation. Nat Commun 2017, doi: 10.1038/s41467-017-00954-1. [DOI] [PMC free article] [PubMed]
- 68.Steinebach C, Lindner S, Udeshi ND, Mani DC, Kehm H, Köpff S, Carr SA, Gütschow M, Krönke J: Homo-PROTACs for the Chemical Knockdown of Cereblon. ACS Chem Biol 2018, 13:2771–2782. [DOI] [PubMed] [Google Scholar]
- 69.Burslem GM, Song J, Chen X, Hines J, Crews CM: Enhancing Antiproliferative Activity and Selectivity of a FLT-3 Inhibitor by Proteolysis Targeting Chimera Conversion. J Am Chem Soc 2018, 140:16428–16432. [DOI] [PubMed] [Google Scholar]
- 70.Brand M, Jiang B, Bauer S, Donovan KA, Liang Y, Wang ES, Nowak RP, Yuan JC, Zhang T, Kwiatkowski N, et al. : Homolog-Selective Degradation as a Strategy to Probe the Function of CDK6 in AML. Cell Chem Biol 2018, doi: 10.1016/j.chembiol.2018.11.006. [DOI] [PMC free article] [PubMed]
- 71.Cromm PM, Samarasinghe KTG, Hines J, Crews CM: Addressing Kinase- Independent Functions of Fak via PROTAC-Mediated Degradation. J Am Chem Soc 2018, 140:17019–17026. [DOI] [PubMed] [Google Scholar]
- 72.Li Y, Yang J, Aguilar A, McEachern D, Przybranowski S, Liu L, Yang C-Y, Wang M, Han X, Wang S: Discovery of MD-224 as a First-in-Class, Highly Potent, and Efficacious Proteolysis Targeting Chimera Murine Double Minute 2 Degrader Capable of Achieving Complete and Durable Tumor Regression. J Med Chem 2018, 62:448–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zoppi V, Hughes SJ, Maniaci C, Testa A, Gmaschitz T, Wieshofer C, Koegl M, Riching KM, Daniels DL, Spallarossa A, et al. : Iterative Design and Optimization of Initially Inactive Proteolysis Targeting Chimeras (PROTACs) Identify VZ185 as a Potent, Fast, and Selective von Hippel– Lindau (VHL) Based Dual Degrader Probe of BRD9 and BRD7. J Med Chem 2018, 62:699–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wurz RP, Cee VJ: Targeted Degradation of MDM2 as a New Approach to Improve the Efficacy of MDM2-p53 Inhibitors. J Med Chem 2018, 62:445–447. [DOI] [PubMed] [Google Scholar]
- 75.Zhao Q, Lan T, Su S, Rao Y: Induction of apoptosis in MDA-MB-231 breast cancer cells by a PARP1-targeting PROTAC small molecule. Chem Commun 2019, 55:369–372. [DOI] [PubMed] [Google Scholar]
- 76.Smith BE, Wang SL, Jaime Figueroa S, Harbin A, Wang J, Hamman BD, Crews CM: Differential PROTAC substrate specificity dictated by orientation of recruited E3 ligase. Nat Commun 2019, doi: 10.1038/s41467-018-08027-7. [DOI] [PMC free article] [PubMed]
- 77.Han X, Wang C, Qin C, Xiang W, Fernandez-Salas E, Yang C-Y, Wang M, Zhao L, Xu T, Chinnaswamy K, et al. : Discovery of ARD-69 as a Highly Potent Proteolysis Targeting Chimera (PROTAC) Degrader of Androgen Receptor (AR) for the Treatment of Prostate Cancer. J Med Chem 2019, 62:941–964. [DOI] [PubMed] [Google Scholar]
- 78.Roy M, Winkler S, Hughes SJ, Whitworth C, Galant M, Farnaby W, Rumpel K, Ciulli A: SPR-measured dissociation kinetics of PROTAC ternary complexes influence target degradation rate. ACS Chem Biol 2019, doi: 10.1021/acschembio.9b00092. [DOI] [PMC free article] [PubMed]
- 79.Steinebach C, Kehm H, Lindner S, Vu LP, Köpff S, López Mármol Á, Weiler C, Wahner KG, Reichenzeller M, Krönke J, et al. : PROTAC-mediated crosstalk between E3 ligases. Chem Commun 2019, doi: 10.1039/C8CC09541H. [DOI] [PubMed]
- 80.Gechijian LN, Buckley DL, Lawlor MA, Reyes JM, Paulk J, Ott CJ, Winter GE, Erb MA, Scott TG, Xu M, et al. : Functional TRIM24 degrader via conjugation of ineffectual bromodomain and VHL ligands. Nat Chem Biol 2018, doi: 10.1038/s41589-018-0010-y.**A “non-functional” ligand can be used as the POI-recruiting element in PROTAC design, highlighting the broad scope of potential targets that can be explored using the PROTAC technology.
- 81.Yang K, Song Y, Xie H, Wu H, Wu Y-T, Leisten ED, Tang W: Development of the first small molecule histone deacetylase 6 (HDAC6) degraders. Bioorg Med Chem Lett 2018, 28:2493–2497. [DOI] [PubMed] [Google Scholar]
- 82.Rumpf T, Schiedel M, Karaman B, Roessler C, North BJ, Lehotzky A, h JOA, Ladwein KI, Schmidtkunz K, Gajer M, et al. : Selective Sirt2 inhibition by ligand-induced rearrangement of the active site. Nat Commun 2015, doi: 10.1038/ncomms7263. [DOI] [PMC free article] [PubMed]
- 83.Falkenberg KJ, Johnstone RW: Histone deacetylases and their inhibitors in cancer, neurological diseases and immune disorders. Nat Rev Drug Disc 2014, 13:673–691. [DOI] [PubMed] [Google Scholar]
- 84.Bian J, Ren J, Li Y, Wang J, Xu X, Feng Y, Tang H, Wang Y, Li Z: Discovery of Wogonin-based PROTACs against CDK9 and Capable of Achieving Antitumor Activity. Bioorg Chem 2018, doi: 10.1016/j.bioorg.2018.08.028. [DOI] [PubMed]
- 85.Shibata N, Miyamoto N, Nagai K, Shimokawa K, Sameshima T, Ohoka N, Hattori T, Imaeda Y, Nara H, Cho N, et al. : Development of protein degradation inducers of oncogenic BCR-ABL protein by conjugation of ABL kinase inhibitors and IAP ligands. Cancer Sci 2017, 108:1657–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang C, Han X-R, Yang X, Jiang B, Liu J, Xiong Y, Jin J: Proteolysis Targeting Chimeras (PROTACs) of Anaplastic Lymphoma Kinase (ALK). Eur J Med Chem 2018, 151:304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen H, Chen F, Liu N, Wang X, Gou S: Chemically induced degradation of CK2 by proteolysis targeting chimeras based on a ubiquitin proteasome pathway. Bioorg Chem 2018, doi: 10.1016/j.bioorg.2018.09.005. [DOI] [PubMed]
- 88.Powell CE, Gao Y, Tan L, Donovan KA, Nowak RP, Loehr A, Bahcall M, Fischer ES, Jänne PA, George RE, et al. : Chemically Induced Degradation of Anaplastic Lymphoma Kinase (ALK). J Med Chem 2018, 61:4249–4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Smith BE, Wang SL, Jaime Figueroa S, Harbin A, Wang J, Hamman BD, Crews CM: Differential PROTAC substrate specificity dictated by orientation of recruited E3 ligase. Nat Commun 2019, doi: 10.1038/s41467-018-08027-7. [DOI] [PMC free article] [PubMed]
- 90.Cohen P, Alessi DR: Kinase drug discovery--what’s next in the field? ACS Chem Biol 2013, 8:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Klaeger S, Heinzlmeir S, Wilhelm M, Polzer H, Vick B, Koenig P-A, Reinecke M, Ruprecht B, Petzoldt S, Meng C, et al. : The target landscape of clinical kinase drugs. Science 2017, 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Palmer WS, Poncet-Montange G, Liu G, Petrocchi A, Reyna N, Subramanian G, Theroff J, Yau A, Kost-Alimova M, Bardenhagen JP, et al. : Structure-Guided Design of IACS-9571, a Selective High-Affinity Dual TRIM24-BRPF1 Bromodomain Inhibitor. J Med Chem 2015, 59:1440–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bennett J, Fedorov O, Tallant C, Monteiro O, Meier J, Gamble V, Savitsky P, Nunez-Alonso GA, Haendler B, Rogers C, et al. : Discovery of a Chemical Tool Inhibitor Targeting the Bromodomains of TRIM24 and BRPF. J Med Chem 2015, 59:1642–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Belkina AC, Denis GV: BET domain co-regulators in obesity, inflammation and cancer. Nat Rev Drug Disc 2012, 12:465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dawson MA, Kouzarides T, Huntly BJP: Targeting Epigenetic Readers in Cancer. N Engl J Med 2012, 367:647–657. [DOI] [PubMed] [Google Scholar]
- 96.Arrowsmith CH, Bountra C, Fish PV, Lee K, Schapira M: Epigenetic protein families: a new frontier for drug discovery 2012, doi: 10.1038/nrd3674. [DOI] [PubMed]
- 97.Müller S, Filippakopoulos P, Knapp S: Bromodomains as therapeutic targets. Expert Rev Mol Med 2011, 13:e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Boi M, Gaudio E, Bonetti P, Kwee I, Bernasconi E, Tarantelli C, Rinaldi A, Testoni M, Cascione L, Ponzoni M, et al. : The BET Bromodomain Inhibitor OTX015 Affects Pathogenetic Pathways in Preclinical B-cell Tumor Models and Synergizes with Targeted Drugs. Clinical Cancer Research 2015, 21:1628–1638. [DOI] [PubMed] [Google Scholar]
- 99.Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, Morse EM, Keates T, Hickman TT, Felletar I, et al. : Selective inhibition of BET bromodomains. Nature 2010, 468:1067–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gadd MS, Testa A, Lucas X, Chan K-H, Chen W, Lamont DJ, Zengerle M, Ciulli A: Structural basis of PROTAC cooperative recognition for selective protein degradation. Nat Chem Biol 2017, 13:514–521.**The authors demonstrate that PROTACs exhibit potent and cooperative binding in the ternary complex.
- 101.Ottis P, Toure M, Cromm PM, Ko E, Gustafson JL, Crews CM: Assessing Different E3 Ligases for Small Molecule Induced Protein Ubiquitination and Degradation. ACS Chem Biol 2017, 12:2570–2578.**The authors describe the applicability of PROTAC-mediated degradation using E3 ligases from different E3 protein families.
- 102.Nabet B, Roberts JM, Buckley DL, Paulk J, Dastjerdi S, Yang A, Leggett AL, Erb MA, Lawlor MA, Souza A, et al. : The dTAG system for immediate and target-specific protein degradation. Nat Chem Biol 2018, 348:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Erb MA, Scott TG, Li BE, Xie H, Paulk J, Seo H-S, Souza A, Roberts JM, Dastjerdi S, Buckley DL, et al. : Transcription control by the ENL YEATS domain in acute leukaemia. Nature 2017, 543:270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Huang HT, Seo HS, Zhang T, Wang Y, Jiang B, Elife QL, 2017: MELK is not necessary for the proliferation of basal-like breast cancer cells. eLife, doi: 10.7554/elife.26693.001. [DOI] [PMC free article] [PubMed]
- 105.Riching KM, Mahan S, Corona CR, McDougall M, Vasta JD, Robers MB, Urh M, Daniels DL: Quantitative Live-Cell Kinetic Degradation and Mechanistic Profiling of PROTAC Mode of Action. ACS Chem Biol 2018, doi: 10.1021/acschembio.8b00692.**Real-time monitoring for each of the steps involved in PROTAC-mediated degradation in cellulo from cell permeability, ternary complex formation, ubiquitination and degradation.
- 106.De Cesare V, Johnson C, Barlow V, Hastie J, Knebel A, Trost M: The MALDI-TOF E2/E3 Ligase Assay as Universal Tool for Drug Discovery in the Ubiquitin Pathway. Cell Chem Biol 2018, 25:1117–1127.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gabrielsen M, Buetow L, Nakasone MA, Ahmed SF, Sibbet GJ, Smith BO, Zhang W, Sidhu SS, Huang DT: A General Strategy for Discovery of Inhibitors and Activators of RING and U-box E3 Ligases with Ubiquitin Variants. Mol Cell 2017, 68:456–470.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Landré V, Rotblat B, Melino S, Bernassola F, Melino G: Screening for E3- ubiquitin ligase inhibitors: challenges and opportunities. Oncotarget 2014, 5:7988–8013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Berndsen CE, Wolberger C: New insights into ubiquitin E3 ligase mechanism. Nat Struct Mol Biol 2014, 21:301–307. [DOI] [PubMed] [Google Scholar]
- 110.Grimsey NJ, Narala R, Rada CC, Mehta S, Stephens BS, Kufareva I, Lapek J, Gonzalez DJ, Handel TM, Zhang J, et al. : A Tyrosine Switch on NEDD4– 2 E3 Ligase Transmits GPCR Inflammatory Signaling. Cell Rep 2018, 24:3312–3323.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Odon V, Georgana I, Holley J, Morata J, Maluquer de Motes C: A novel class of viral Ankyrin proteins targeting the host E3 ubiquitin ligase Cullin-2. J. Virol 2018, doi: 10.1128/JVI.01374-18. [DOI] [PMC free article] [PubMed]
- 112.Koren I, Timms RT, Kula T, Xu Q, Li MZ, Elledge SJ: The Eukaryotic Proteome Is Shaped by E3 Ubiquitin Ligases Targeting C-Terminal Degrons. Cell 2018, 173:1622–1635.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lin H-C, Yeh C-W, Chen Y-F, Lee T-T, Hsieh P-Y, Rusnac DV, Lin S-Y, Elledge SJ, Zheng N, Yen H-CS: C-Terminal End-Directed Protein Elimination by CRL2 Ubiquitin Ligases. Mol Cell 2018, 70:602–613.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tonge PJ: Drug–Target Kinetics in Drug Discovery. ACS Chem Neurosci 2017, 9:29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.van den Boom J, Meyer H: VCP/p97-Mediated Unfolding as a Principle in Protein Homeostasis and Signaling. Mol Cell 2018, 69:182–194. [DOI] [PubMed] [Google Scholar]
- 116.Nguyen TV, Li J, Lu C-CJ, Mamrosh JL, Lu G, Cathers BE, Deshaies RJ: p97/VCP promotes degradation of CRBN substrate glutamine synthetase and neosubstrates. Proc Natl Acad Sci USA 2017, 114:3565–3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wickner S, Maurizi MR, Gottesman S: Posttranslational quality control: folding, refolding, and degrading proteins. Science 1999, 286:1888–1893. [DOI] [PubMed] [Google Scholar]
- 118.Dobson CM: Protein folding and misfolding. Nature 2003, 426:884–890. [DOI] [PubMed] [Google Scholar]
- 119.Wilkinson KD: Regulation of ubiquitin-dependent processes by deubiquitinating enzymes. FASEB J 1997, 11:1245–1256. [DOI] [PubMed] [Google Scholar]