ABSTRACT
Curcumin possesses medicinal properties that are beneficial in various diseases, such as heart disease, cancer, and type 2 diabetes mellitus (T2 DM). It has been proposed that pancreatic beta cell dysfunction in T2 DM is promoted by oxidative stress caused by NADPH oxidase over-activity. The aim of the present study was to evaluate the efficacy of curcumin as a protective agent against high glucose/palmitate (HP)-induced islet cell damage and in streptozotocin (STZ)-induced DM rats. INS-1 cells were exposed to HP with or without curcumin. Cell proliferation, islet cell morphological changes, reactive oxygen species production, superoxide dismutase and catalase activity, insulin levels, NADPH oxidase subunit expression, and the expression of apoptotic factors by INS-1 cells were observed. Our results show that curcumin can effectively inhibit the impairment of cell proliferation and activated oxidative stress, increase insulin levels, and reduce the high expression of NADPH oxidase subunits and apoptotic factors induced by HP in INS-1 cells. The STZ-induced DM rat model was also used to determine whether curcumin can protect islets in vivo. Our results show that curcumin significantly reduced pathological damage and increased insulin levels of islets in STZ-induced DM rats. Curcumin also successfully inhibited the high expression of NADPH oxidase subunits and apoptotic factors in STZ-induced DM rats. These results suggest that curcumin is able to attenuate HP-induced oxidative stress in islet cells and protect these cells from apoptosis by modulating the NADPH pathway. In view of its efficiency, curcumin has potential for translation applications in protecting islets from glucolipotoxicity.
KEYWORDS: Curcumin, islet, glucolipotoxicty, diabetes mellitus, oxidative stress, NADPH oxidases
Introduction
The loss of pancreatic beta cells, and subsequent impairment of compensation for insulin resistance, contribute to the development of type 2 diabetes mellitus (T2 DM).1-5 Increased serum free fatty acids (FFAs), in conjunction with hyperglycemia, are believed to induce a synergistic loss of beta cells in T2 DM; this is termed ‘glucolipotoxicity’.6 In fact, long-term exposure to saturated FFAs induces cell death in insulin-producing beta cells and isolated human islets,7,8 and exposure to an elevated concentration of glucose augments FFA-induced cell death.9 Although the cellular mechanisms involved in high glucose and FFA-induced cell death are not fully understood, glucolipotoxicity is thought to be an augmented form of high glucose-induced glucotoxicity or FFA-induced lipotoxicity.6
Many studies have revealed that high glucose and FFA-induced glucolipotoxicity increases intracellular reactive oxygen species (ROS) in islet cells, and such excessive levels of ROS result in islet cell apoptosis.10 Therefore, inhibiting the rise of intracellular ROS in islet cells may be a viable approach for protecting these cells against glucolipotoxicity. The excessive production and accumulation of ROS is, at least in part, due to hyperactivity of NADPH oxidases.11-15 The NADPH oxidase family consists of seven isoforms that perform normal cellular functions under basal conditions, but when activated persistently, produce harmful levels of ROS.16-20 Based on these facts, it can be hypothesized that glucolipotoxicity to islet cells can be avoided if certain antioxidants are used to suppress the intracellular ROS increase, or activities of the NADPH oxidases are inhibited in a safe and effective manner, thereby attenuating oxidative stress.
Curcumin is a hydrophobic polyphenol derivative with diverse pharmacological properties that is usually extracted from natural herbs.21-31 Curcumin has been used as an anti-inflammatory, antioxidant, and anticancer agent over a long period of time, including for the treatment of varied types of cancers, diabetes, cardiovascular diseases, and autoimmune diseases.32-37 A recent study revealed that curcumin attenuates oxidative stress in islet cells and can play protective and therapeutic roles in protecting these cells.38 With renewed interest in the pharmaceutical potential of natural products with minimal side effects, many studies have been carried out exploring the use of curcumin for medicinal purposes.39 Furthermore, although many aspects of curcumin-induced cytoprotection are studied, its efficacy in protecting islet cells against glucolipotoxicity-induced oxidative stress and NADPH oxidase-mediated damage has not been demonstrated so far. We hypothesized that curcumin may protect islet cells against glucolipotoxicity-induced oxidative stress and NADPH oxidase activation and resulting beta cell damage. Logically, curcumin would impart some protection against oxidative damage to beta cells, but itwould be of interest to investigate the mode of curcumin-induced cytoprotection mechanism. In this study, we have investigated the protective action of curcumin on glucolipotoxicity-induced islet cells damage in vitro and in vivo. We show here for the first time that curcumin treatment prior to high glucose/palmitate or streptozotocin (STZ) exposure rescues islets from damage and dysfunction by virtue of its free radical scavenging activity and the NADPH pathway modulating.
Results
Effect of curcumin on HP-induced islet cell damage
To determine whether curcumin affected high glucose/palmitate (HP)-induced glucolipotoxicity, its effect on the reduction in INS-1 cell viability induced by HP was investigated. Curcumin showed a concentration-dependent protective effect on the HP-induced viability reduction of INS-1 cells (Figure 1). Treatment with 20 μM curcumin increased viability to 89% compared to 59% in cells not treated with curcumin (p < .05). These data confirm that curcumin can protect against HP-induced islet cell proliferation dysfunction.
Figure 1.
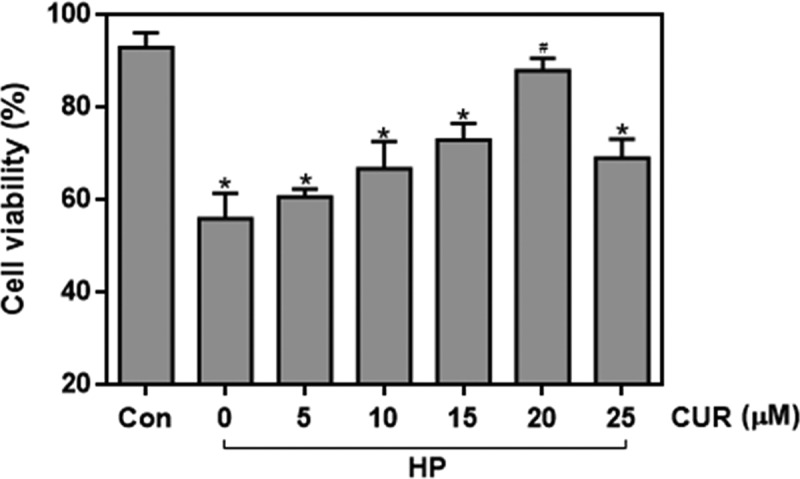
Effect of curcumin (20 μM) on the decreased viability caused by treating INS-1 cells with HP. n = 5 independent experiments. Culture time: 48 h. (*p < .05 vs Con group, #p < .05 vs HP group). CUR, curcumin; HP, 30 mM glucose+0.5 mM palmitate; Con, control.
Effect of curcumin on HP-induced oxidative stress in islet cells
ROS levels in INS-1 cells were detected by using 2,7-dichlorodihydrofluorescin diacetate (DCFH-DA) as a fluorescent probe. The images in Figure 2A show that cells treated with HP exhibited brighter green fluorescence than control cells, signifying that the HP treatment led to an increase in intracellular ROS levels. In contrast, green fluorescence in cells treated with the combination of HP and curcumin was reduced in intensity when compared with cells treated with HP alone. Data for ROS fluorescence intensity are depicted in Figure 2B and the bar-graphs provide quantitative evidence that curcumin can significantly inhibit the HP-induced increase in ROS levels (P < .05). This demonstrates that curcumin significantly inhibits the HP-induced production of ROS in islet cells. Figure 2C,D demonstrate that the HP-treatment resulted in a big decrease in superoxide dismutase (SOD) and catalase activity (P < .05). The decrease of activity of two antioxidant enzymes is closely related to the increase of intracellular oxidative stress level. In contrast to these observations, curcumin can inhibit the decrease in SOD and catalase activity, allowing these two typical indexes to be back to the normal cellular levels, and ultimately resist the increase of oxidative stress in cells.
Figure 2.
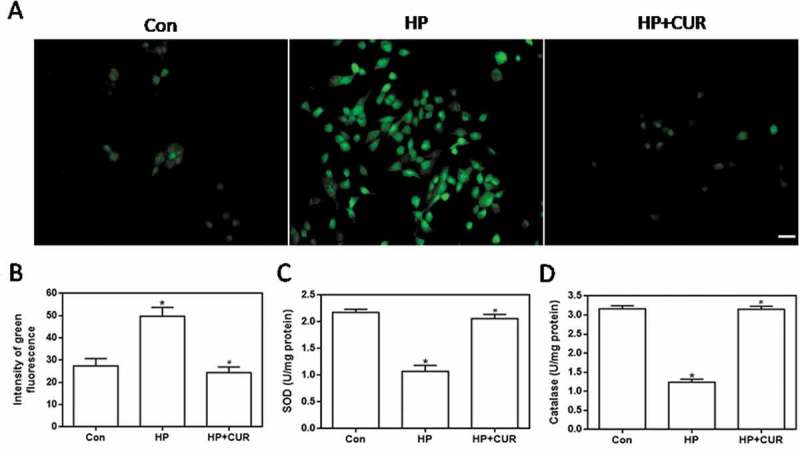
Effect of curcumin (20 μM) on the increased oxidative stress in INS-1 cells treated with HP. ROS levels were determined using DCFH-DA. (A) Representative images of cells stained with DCFH-DA. Scale bar: 20 μm. (B) Fluorescence intensity of DCFH-DA (*p < .05 vs Con group, #p < .05 vs HP group). (C) SOD levels in cells. (D) catalase activity in cells. n = 5 independent experiments. CUR, curcumin; ROS, reactive oxygen species; CUR, curcumin; HP, 30 mM glucose+0.5 mM palmitate; DCFH-DA, 2’,7’-dichlorodihydro fluorescein diacetate; Con, control; SOD, super oxide dismutase.
Effect of curcumin on insulin levels in HP-treated islet cells
HP-induced islet cell damage was detected as green fluorescence by using an anti-insulin antibody. Figure 3A shows that HP-treated cells had less green fluorescence than control cells (see middle column). Importantly, the fluorescence of cells treated with the combination of HP and curcumin was increased. Data for insulin fluorescence intensity are depicted in Figure 3B and the bar-graphs provide quantitative evidence that curcumin can significantly inhibit the HP-induced decrease in insulin levels (P < .05). This indicates that HP can significantly reduce insulin production by islet cells, and this effect can be inhibited by curcumin. Thus, curcumin can effectively protect islet cells from glucolipotoxicity and restore insulin production.
Figure 3.
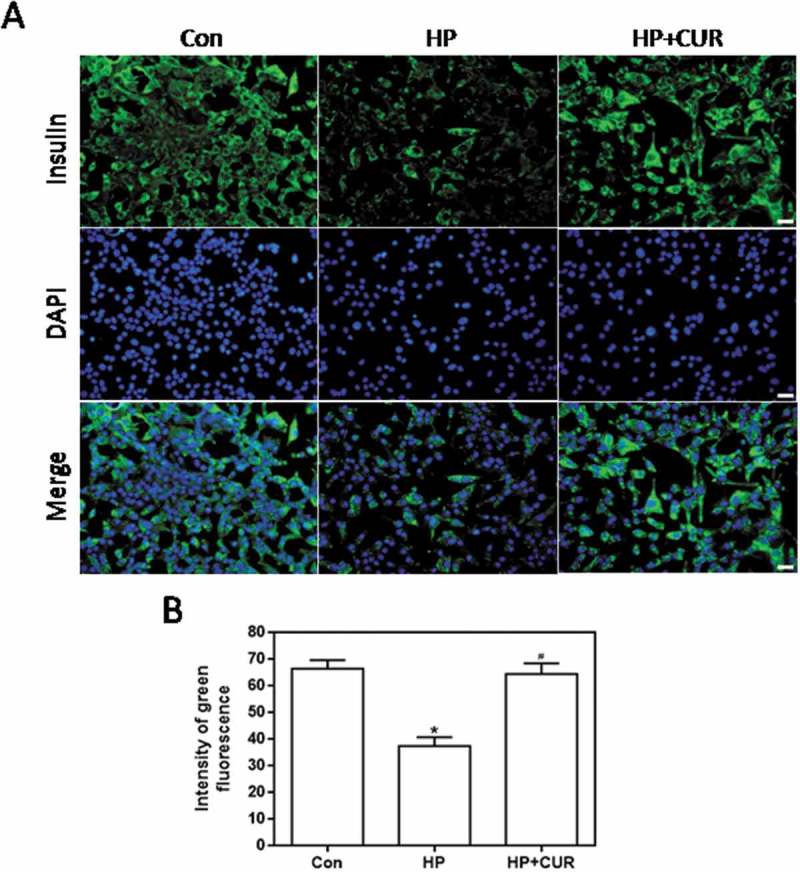
Effect of curcumin (20 μM) on the decreased insulin levels in INS-1 cells treated with HP. Insulin levels were assessed immunofluorescence. Insulin was stained using a FITC-conjugated secondary antibody (green), and nuclei were stained with DAPI (blue). (A) Representative images of cells stained with insulin. Scale bar: 20 μm. (B) Fluorescence intensity of insulin (*p < .05 vs Con group, #p < .05 vs HP group). n = 5 independent experiments. CUR, curcumin; HP, 30 mM glucose+0.5 mM palmitate; DAPI: 4’,6-diamidino-2-phenylindole; Con, control.
Effect of curcumin on islet cell damage in STZ-induced DM rats
Histological analysis using hematoxylin and eosin staining showed pancreatic tissue damage after STZ administration (Figure 4). Control rats showed normal acini and a normal cellular population of islets of Langerhans, while in STZ-induced DM rats there were pancreatic lesions with reduced islet numbers and size (Figure 4). Curcumin treatment rescued pancreatic tissue in STZ-induced DM rats, reducing tissue degeneration as evidenced by the partial restoration of a normal cellular population of islets of Langerhans and the absence of islet damage.
Figure 4.
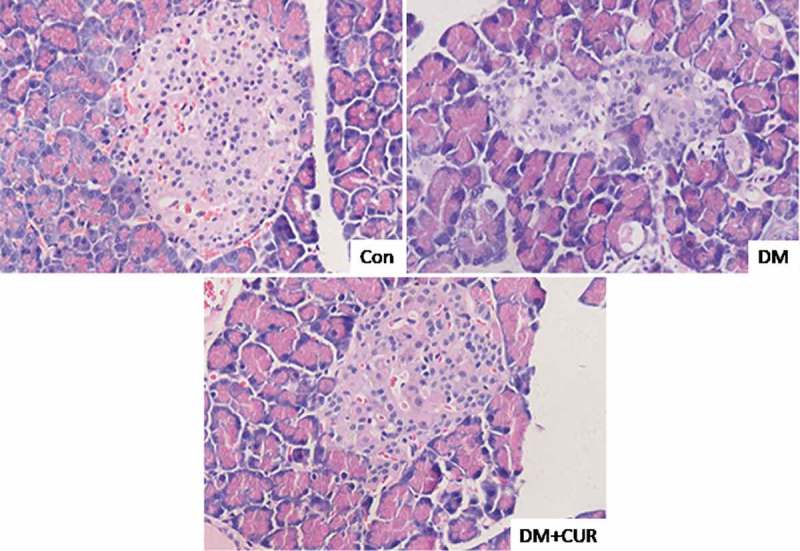
Effect of curcumin on histological abnormalities of islet cells in STZ-induced DM rats. Pancreatic tissue was sectioned at 5 μm and the slides were stained with hematoxylin and eosin. Representative images fromlongitudinal and transverse H&E staining for pancreatic tissue were shown. All images were obtained by microscope with 400× amplification. CUR, curcumin; STZ, streptozotocin; DM, diabetes mellitus; Con, control.
Effect of curcumin on insulin levels in STZ-induced DM rats
The insulin level in islets from STZ-induced DM rats was detected as green fluorescence by using an anti-insulin antibody. Figure 5A shows that the damaged islets in STZ-induced DM rats had less green fluorescence than matching control islets (see middle column). The green fluorescence associated with STZ-induced DM rats treated with curcumin was increased. Data for insulin fluorescence intensity are depicted in Figure 5B and the bar-graphs provide quantitative evidence that curcumin can significantly inhibit the STZ-induced decrease in insulin levels (P < .05). This indicates that STZ significantly reduces insulin levels of pancreatic islet tissue, which can be inhibited by curcumin. Thus, curcumin can effectively protect islets from glucolipotoxicity, which is consistent with the immunofluorescence results with INS-1 cells.
Figure 5.
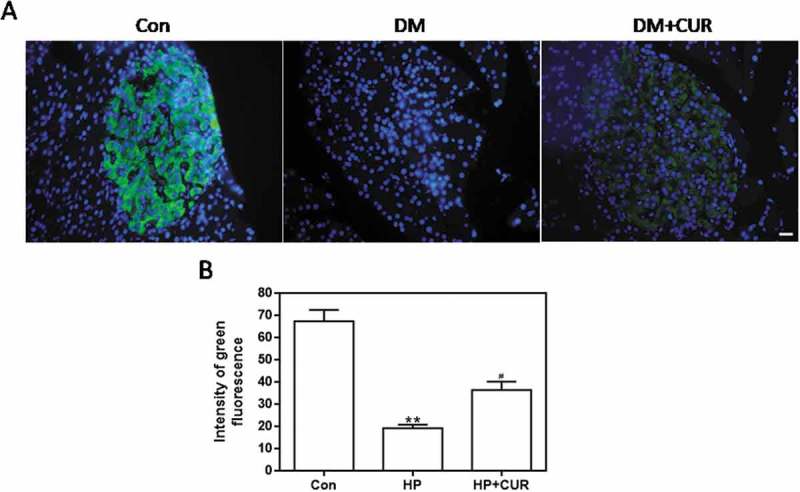
Effect of curcumin on the decreased insulin levels in islet cells from STZ-induced DM rats. Pancreatic tissue was sectioned at 5 μm and the slides were stained immunohistofluorescence. All images were obtained by microscope with 400× amplification. (A) Representative images of cells stained with insulin. Scale bar: 20 μm. (B) Fluorescence intensity of insulin (**p < .01 vs Con group, #p < .05 vs DM group). There were 3 animals in each group, and, for each animal, 5 pathologic slices from the histological specimens were observed. CUR, curcumin; STZ, streptozotocin; DM, diabetes mellitus; Con, control.
Influence of curcumin on the expression of NADPH oxidase and apoptosis- promoting factors in islet cells exposed to HP
The NADPH oxidase pathway plays an important role in regulating cellular survival, oxidative stress, and apoptosis.40 The NADPH oxidase complex, including gp91phox, p67phox, p47phox, and p22phox, is expressed in the pancreas and regulates the production of ROS. We measured the expression of these subunits in islet cells using western blots. The expression of gp91phox, p67phox, and p47phox was significantly higher in cells from the HP group than the control group (Figure 6; p < .05). However, there was no significant difference in the expression of p22phox. Importantly, curcumin markedly reduced gp91phox, p67phox, and p47phox levels in islet cells, which was increased when cells were exposed to HP (Figure 6; p < .05). Apoptosis is regulated by a series of apoptotic-related proteins. Among them, cleaved caspase-3 and Bax are regarded as pro-apoptotic effectors. We investigated whether curcumin suppressed HP-induced apoptosis by measuring levels of these regulatory proteins. Western blotting demonstrated enhanced expression of cleaved caspase-3 and Bax in HP-treated INS-1 cells compared to control cells (Figure 7; p < .05). Remarkably, curcumin significantly down-regulated cleaved caspase-3 and Bax-2 expression in islet cells exposed to HP (Figure 7; p < .05).
Figure 6.
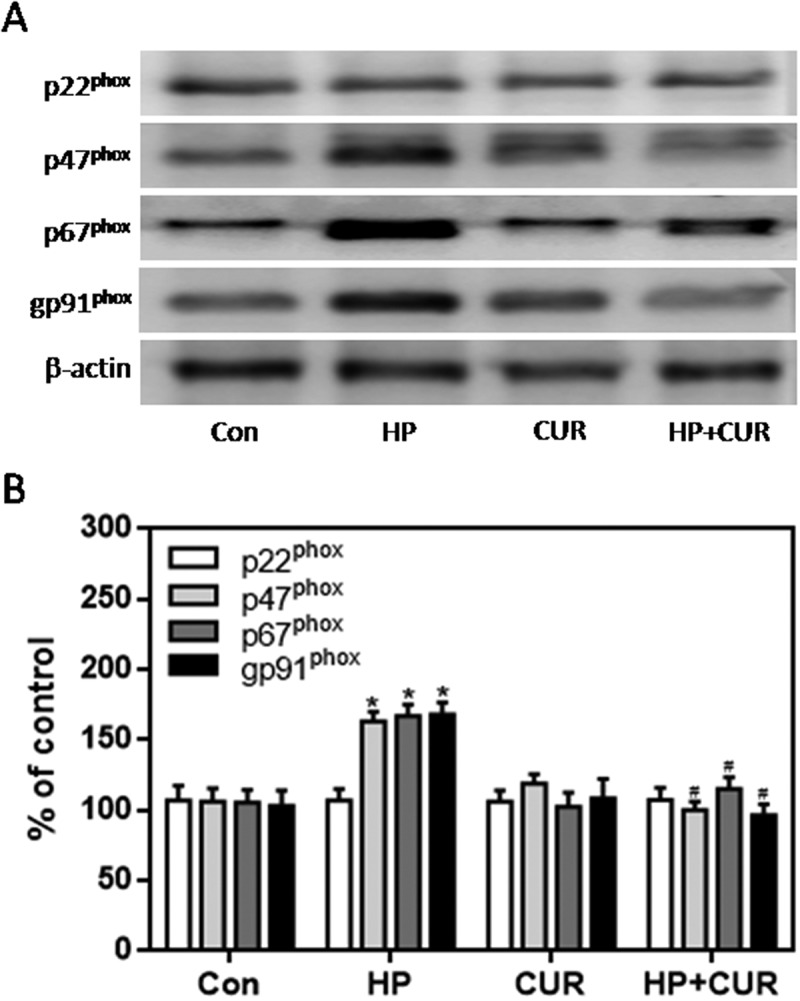
Effect of curcumin (20 μM) on the increased expression of NADPH oxidase subunits in INS-1 cells treated with HP. NADPH oxidase subunits were detected by western blot. A. Representative bands of p22phox, p47phox, p67phox and gp91phox (inner reference: β-actin). B. Quantitative analysis of p22phox, p47phox, p67phox and gp91phox expression (*p < .05 vs Con group, #p < .05 vs HP group). n = 5 independent experiments. CUR, curcumin; HP, 30 mM glucose+0.5 mM palmitate; Con, control.
Figure 7.
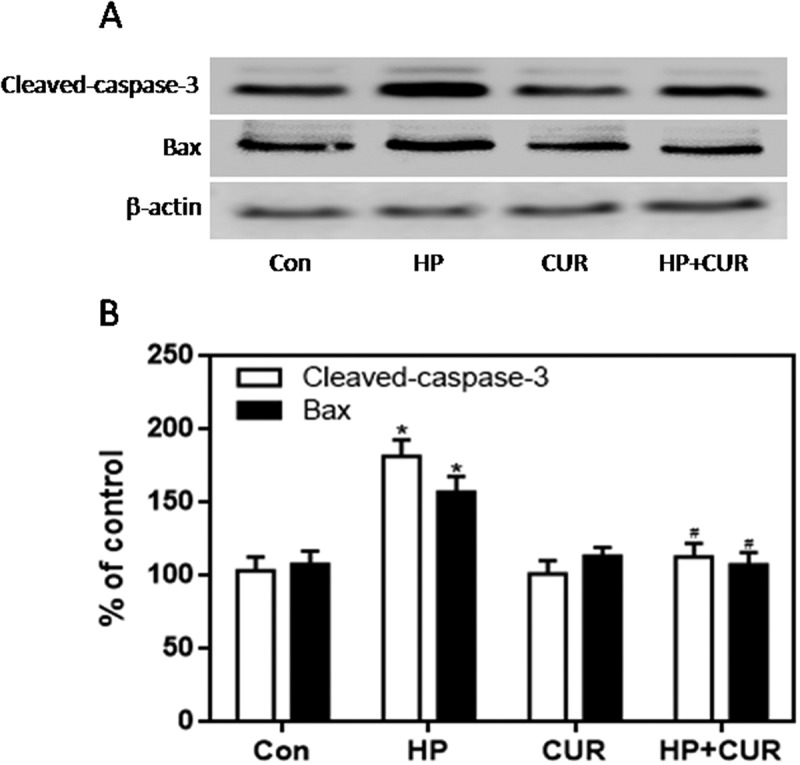
Effect of curcumin (20 μM) on the increased expression of cleaved caspase-3 and Bax in INS-1 cells treated with HP. Cleaved caspase-3 and Bax were detected by western blot. A. Representative bands of cleaved caspase-3 and Bax (inner reference: β-actin). B. Quantitative analysis of cleaved caspase-3 and Bax expression (*p < .05 vs Con group, #p < .05 vs HP group). n = 5 independent experiments. CUR, curcumin; HP, 30 mM glucose+0.5 mM palmitate; Con, control.
Influence of curcumin on the expression of NADPH oxidase and apoptosis- promoting factors in pancreatic tissue of STZ-induced DM rats
The expression of gp91phox, p67phox, and p47phox in pancreatic tissue was much higher in the STZ-induced DM group than control group (Figure 8; p < .05, p < .01). However, there was no significant difference in the expression of p22phox. Moreover, the increased expression of gp91phox, p67phox, and p47phox was markedly inhibited by curcumin in pancreatic tissue of STZ-induced DM rats (Figure 8; p < .05, p < .01). These data suggest that the protective role of curcumin against islet injury in STZ-induced DM rats is largely through inhibition of the NADPH oxidase-mediated pathway. We also examined the anti-apoptotic effect of curcumin in pancreatic tissue of STZ-induced DM rats. Western blotting showed enhanced expression of cleaved caspase-3 and Bax in the STZ-induced DM group compared to control (Figure 9; p < .05). Curcumin significantly down-regulated cleaved caspase-3 and Bax-2 expression in pancreatic tissue of STZ-induced DM rats (Figure 9; p < .05). These findings suggest that curcumin attenuates pancreatic apoptosis in STZ-induced DM rats. This may be partially related to its ability to decrease the activity of the NADPH oxidase pathway and suppress oxidative stress.
Figure 8.
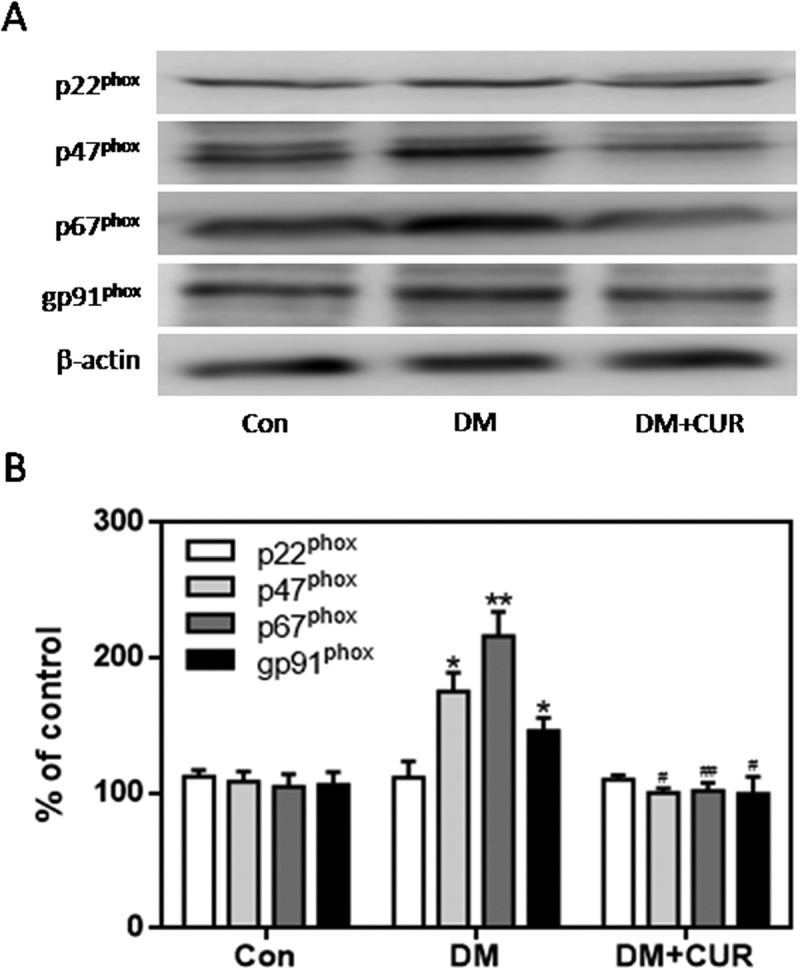
Effect of curcumin on the increased expression of NADPH oxidase subunits in pancreatic tissue from STZ-induced DM rats. NADPH oxidase subunits were detected by western blot. A. Representative bands of p22phox, p47phox, p67phox and gp91phox (inner reference: β-actin). B. Quantitative analysis of p22phox, p47phox, p67phox and gp91phox expression (*p < .05 vs Con group, #p < .05 vs DM group). There were 3 animals in each group. CUR, curcumin; STZ, streptozotocin; DM, diabetes mellitus; Con, control.
Figure 9.
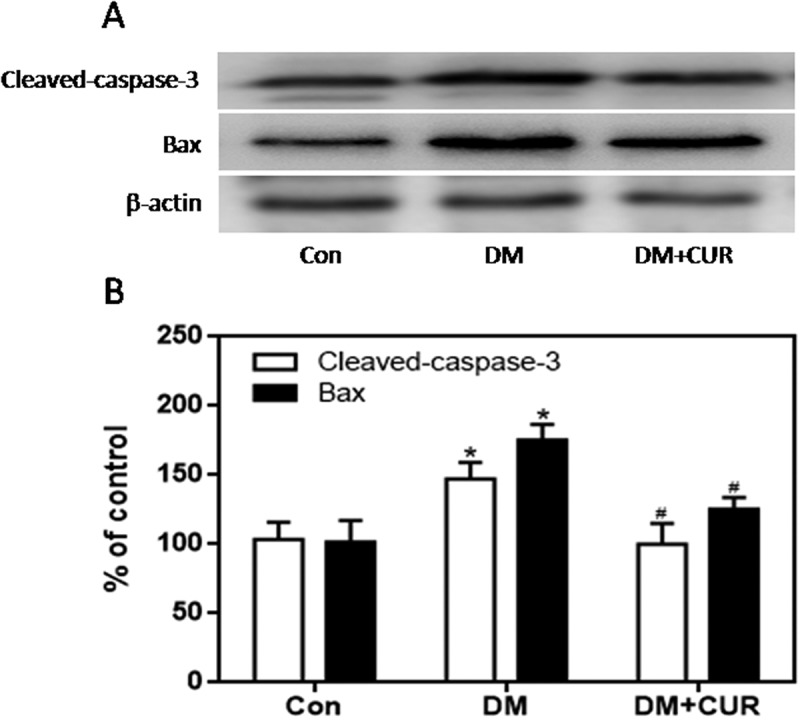
Effect of curcumin on the increased expression of cleaved caspase-3 and Bax in pancreatic tissue from STZ-induced DM rats. Cleaved caspase-3 and Bax were detected by western blot. A. Representative bands of cleaved caspase-3 and Bax (inner reference: β-actin). B. Quantitative analysis of cleaved caspase-3 and Bax expression (*p < .05 vs Con group, #p < .05 vs DM group). There were 3 animals in each group. CUR, curcumin; STZ, streptozotocin; DM, diabetes mellitus; Con, control.
Discussion
Chronic increases in blood glucose and FFAs in T2 DM impair or damage to beta cells.41 Furthermore, increased FFAs, in conjunction with hyperglycemia, are believed to induce a synergistic loss of beta cells in T2 DM; this is termed ‘glucolipotoxicity’.6 Oxidative stress is one causal factor of glucolipotoxicity.42 ROS are generated in response to mitochondrial dysfunction, altering the production of intracellular energy.43 Normally, the generation of ROS in cells is a natural process, and the majority of normal cells can remove ROS generated in this manner using enzymatic and non-enzymatic antioxidants.44-47 However, the expression levels of antioxidant enzymes in beta cells are lower than those in other cells. Thus, these cells tend to be more sensitive to damage from ROS.48,49 In this study, we observed an increase in ROS levels and a significant decrease in proliferation after exposure of INS-1 cells to HP. Therefore, we hypothesized that ROS induced by glucolipotoxicity may cause beta cell dysfunction.
Because elevated ROS levels cause strong injurious effects on diabetic patients, various types of antioxidant agents have been investigated for their potential to treat T2 DM.50 Curcumin, a natural antioxidant, has been used to scavenge excessive amounts of ROS and enhance antioxidant defenses.51 The current results confirm that curcumin can alleviate the oxidative stress of INS-1 cells induced by HP, and completely reverse the decreased proliferation caused by this treatment. These results suggest that curcumin has a protective effect on islet cell injury induced by glucolipotoxicity. Furthermore, we found that curcumin treatment significantly reduced the pancreatic damage in STZ-induced DM rats and restore insulin to normal levels, compared with STZ-induced DM rats not treated with curcumin. These in vivo results are in accordance with the in vitro experiments suggesting that curcumin pretreatment can significantly protect insulin producing cells from HP-induced damage. Overall, it can be concluded that curcumin significantly diminishes the islet damage caused by glucolipotoxicity, and the mechanism is related to the effects on the oxidative stress pathway.
The membrane bound enzyme complex, NADPH oxidase, is a major source of ROS in islet cells.11 It has been proposed that pancreatic beta cell dysfunction in T2 DM is promoted by oxidative stress caused by over-activity of NADPH oxidase.52 Based on these reported results, we investigated whether curcumin could inhibit the NADPH oxidase pathway, thereby protecting islet cells from glucolipotoxicity. In the present study, elevated expression of the NADPH oxidase subunits p47phox, p67phox, and gp91phox was induced by HP in islet cells. Curcumin is a potent free radical scavenger that can quench ROS such as hydrogen peroxide, superoxide, and peroxynitrite.53,54 Under our experimental conditions, curcumin was able to return the increased expression of p47phox, p67phox, and gp91phox induced by HP back to normal and, hence, protect islet cells from HP-induced glucolipotoxicity. These results were confirmed in vivo by showing that the expression of p47phox, p67phox, and gp91phox in pancreatic tissue of STZ-induced DM rats was significantly increased, and curcumin treatment could significantly reduce these changes.
Cleaved caspase-3 and Bax proteins play crucial roles in mitochondrial-mediated apoptosis.55 In our study, when curcumin was applied to cells exposed to HP, cell injury and apoptotic death were greatly reduced. Because apoptosis is tightly controlled by a series of regulatory proteins, we determined the expression of the pro-apoptotic proteins, cleaved caspase-3 and Bax. As expected, the increased expression of cleaved caspase-3 and Bax in islet cells exposed to HP was inhibited by the addition of curcumin. Interestingly, we found that the expression of cleaved caspase-3 and Bax in pancreatic tissue of STZ-induced DM rats was significantly higher than that of normal rats. It is noteworthy that these increases were inhibited after curcumin treatment. On the basis of all western blot observations mentioned in this study, it can be deduced that curcumin may inhibit NADPH oxidase activity and prevent glucolipotoxicity-induced islet injury.
In conclusion, curcumin was found to significantly inhibit the HP-induced increase of intracellular ROS levels, and significantly diminish glucolipotoxicity- induced apoptosis of islet cells. A possible mechanism for curcumin to protect islets involved inhibition of the NADPH oxidase pathway by diminishing the expression of several specific subunit proteins to their normal levels. Overall, the results presented here suggest that curcumin has the potential to prevent islet cells from glucolipotoxicity both in vitro and in vivo.
Materials and methods
Materials
RPMI 1640, fetal bovine serum (FBS) and type-II collagenase were purchased from Gibco (USA). ROS assay kit was obtained from Applygen Inc. (China). Rabbit polyclonal antibodies against cleaved caspase-3, Bax, p22phox, p47phox p67phox, gp91phox and anti-insulin antibody were purchased from Abcam (USA). β-actin was purchased from Santa Cruz Biotechnology (USA). Hybond C membranes and ECL western blot detection kit were purchased from Pierce Company (USA). 3-(4,5)-dimethylthiahiazo (-z-y1)-3,5-di-phenytetrazoliumro mide (MTT) assay kit were purchased from Sigma-Aldrich (USA). Palmitate, STZ and all other reagents were of analytical grade and used as received.
INS-1 cell culture
INS-1 cells were cultured in RPMI 1640 containing 11.1 mM glucose, supplemented with 10% FBS, 100 U/mL penicillin and 100 mg/mL streptomycin, at 37°C in humidified air containing 5% CO2. The medium was changed every 2 days.
MTT assay
The expended INS-1 cells were suspended in phosphate-buffered saline (PBS) for further use. INS-1 cells were seeded in 96-well plates at a density of 1 × 105 cells/well. After incubation overnight, cells were co-cultured with varied groups of drugs for a period up to 48 h. At the end of the prescribed incubation periods, cells in each well were thoroughly rinsed with PBS, and additionally incubated with an MTT agent (0.5 mg/mL) for 4 h at 37°C. After that, the media were fully discarded and DMSO was added to each well prior to spectrophotometric measurements (570 nm).
Measurement of ROS levels
The fluorescence probe DCFH-DA (Beyotime, Haimen, China) was used to detect intracellular ROS. Briefly, INS-1 cells were seeded in 6-well plates (2 × 104 cells/well) and cultured with RPMI-1640 medium (containing 10% FBS+0.05 mM 2-mercaptoethanol) for 24 h. Cells were then divided into different groups and exposed to HP (30 mM glucose+0.5 mM palmitate) or HP+curcumin (20 μM) for 24 h at 37°C. Cells were then washed with cold PBS and further incubated with fresh medium containing 10 mM of DCFH-DA at 37°C in the dark for 20 min. Fluorescence images of cells were captured using an electronic camera (Olympus, Tokyo, Japan).
Biochemical analysis
Antioxidant enzymes such as SOD and catalase were measured using an SOD activity kit and a catalase activity kit, respectively. SOD assay was determined based on its ability to inhibit the oxidation of oxymine by O2−· produced from the xanthine/xanthine oxidase system. Catalase activity was measured by employing hydrogen peroxide to generate H2O and O2. Protein concentration was determined by the coomassie blue staining. The detailed procedures followed the instructions in the corresponding assay kits.
Animal experiments
Male Sprague-Dawley rats (8 weeks of age) were obtained from the Laboratory Animal Center of Wuhan University (SCXY(E) 2010–0009). The rats were raise under a sonsistent daily light cycle with 12 h of light and 12 h of darkness, and given free access to water and food. After adaptive feeding for one week, the diabete group were given the high glucose (10%) and high-fat (20%) diet till the end of experiment, whereas control group was fed normal diet as before. After 4 weeks of high glucose and high fat diet, the diabete group were induced by intraperitoneal injection of STZ (35 mg/Kg) and control group injected with freshly prepared citrate buffer (pH = 4.5). After 2 weeks, rats with blood glucose levels over 16.7 mM were considered diabetic. Diabetic rats were randomly divided into two groups, including diabetic group (DM group) and curcumin group (DM+CUR). Each group consisting of 9 rats. Every three rats were randomly selected for histology, immunofluorescence staining and western blot analysis. In DM+CUR group, curcumin (about 300 mg/day) was administered intragastrically for 16 weeks and the following tests were performed. In order to successfully complete the intragastric administration, we prepared curcumin suspension with 1% CMCNa solution and suspended it sufficiently before administration until no sediment was found. In control group and DM group, we replaced curcumin with saline of equal volume. All animal experiments were permitted by the Committee of Experimental Animals of Hubei University of Science and Technology.
Histology
Pancreatic samples from STZ-treated and control rats were paraffin-embedded and cut into 3 μm serial sections. Paraffin was then removed by a xylene substitute (Hemo-De; Thermo Fisher Scientific, Darmstadt, Germany). The histology procedure was performed with a BenchMark Automated IHC/ISH slide staining system, according to the manufacturer’s instructions (BenchMark Ventana, Tucson, AZ, USA). Briefly, tissue sections were dehydrated sequentially with ethanol gradient washes, pre-heated, and stained with hematoxylin and eosin. Four distinct preparations for each pancreatic sample were done, and 20 fields of view were analyzed in each preparation for a total area of 1.43012e±0.001 mm2 at 400× magnification.
Immunofluorescence staining
INS-1 cells were seeded into 6-well plates and incubated with complete medium for 24 h. They were then divided into different groups and treated with HP or HP+curcumin (20 μM) for 24 h at 37°C. The washed cells were subjected to immunofluorescence staining for insulin following the methods provided by anti-insulin mouse antibody (WuHan servicebio, GB13121). Pancreatic tissue paraffin sections (3 μm) from STZ-treated and control rats were deparaffinized and rehydrated, then subjected to antigen retrieval in 0.01 M citrate buffer (pH = 6.0) by microwave heating. After blocking with 5% bovine serum albumin, the sections were incubated with an anti-insulin antibody (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4°C, followed by the secondary antibody (1:300; Santa Cruz Biotechnology). 4’,6-Diamidino-2-phenylindole (DAPI) was used to stain the nuclei. Sections were viewed under a fluorescence microscope (Nikon, Japan; 400 × magnification) and images were taken using an electronic camera. Fluorescence intensity (green, insulin; blue, DAPI) measurements and calculations were done using Leica IM500 and Leica QWin image analysis software (Leica, Wetzlar, Germany). In quantification, we first calculated the average value of five slices of each animal, and then calculated the average value of three animals in each group.
Western blot analysis
Cells or tissue homogenates were lysed and total proteins were quantified by the Bio-Rad protein assay (Bio-Rad Laboratories, Milan, Italy). Each protein sample was loaded and electrophoresed in an 8% polyacrylamide gel, and electrotransferred onto a polyvinylidene difluoride membrane. Blots were blocked with 5% nonfat dry milk in PBS-T (PBS-0.05% Tween 20) for 1 h at room temperature, and then incubated overnight at 4°C with the following primary antibodies in blocking solution: p22phox, p47phox, p67phox, gp91phox, cleaved caspase-3, Bax, and β-actin. Immunoreactive signals were detected following a 1 h incubation at room temperature with a goat anti-rabbit (sc-2004) horseradish peroxidase-conjugated secondary antibody (1:2000 in PBS-T; Santa Cruz Biotechnology). The signal was visualized using an enhanced chemiluminescence system (Amersham Pharmacia, Uppsala, Sweden). A semi-quantitative analysis of protein levels was performed by the ChemiDoc-It 5000 system using VisionWorks Life Science Image Acquisition and Analysis software (UVP, Upland, CA, USA). Signals are expressed as densitometric units. Protein levels were normalized with respect to the signal obtained after incubation overnight at 4°C with the anti-β-actin antibody (1:200 in blocking solution; sc-8432, Santa Cruz Biotechnology).
Statistical analysis
The mean±SEM is used to represent the data obtained from the experiment. The Tukey test following one-way ANOVA analysis was used for statistical analysis. Differences were considered to be significant at p < .05.
Funding Statement
This work was supported by grants from National Natural Science Foundation of China [81870173], New Century Excellent Talents Project of the Ministry of Education [NCET-13-0781], and Hubei Major Projects of Technical Innovation [2016ACA148].
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Donath MY, Storling J, Maedler K, Mandrup-Poulsen T.. Inflammatory mediators and islet beta-cell failure: a link between type 1 and type 2 diabetes. J Mol Med. 2003;81:455–470. [DOI] [PubMed] [Google Scholar]
- 2.Sudhahar V, Okur MN, Bagi Z, O’Bryan JP, Hay N, Makino A, Patel VS, Phillips SA, Stepp D, Ushio-Fukai M, et al. Akt2 stabilizes ATP7A, a Cu transporter for SOD3, in vascular smooth muscles: novel mechanism to limit endothelial dysfunction in type2 diabetes. Arterioscler Thromb Vasc Biol. 2018;38:529–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gizaw M, Anandakumar P, Debela T.. A review on the role of irisin in insulin resistance and type 2 diabetes mellitus. J Pharmacopuncture. 2017;20:235–242. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Li YY, Wang H, Yang XX, Wu JJ, Geng HY, Kim HJ, Yang ZJ, Wang LS. LEPR gene Gln223Arg polymorphism and type 2 diabetes mellitus: a meta-analysis of 3,367 subjects. Oncotarget. 2017;8:61927–61934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Momin AA, Bankar MP, Bhoite GM. Association of single nucleotide polymorphisms of adiponectin gene with type 2 diabetes mellitus, and their influence on cardiovascular risk markers. Indian J Clin Biochem. 2017;32:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocrinol Rev. 2008;29:351–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Assaad W, Joly E, Barbeau A, Sladek R, Buteau J, Maestre I, Pepin E, Zhao SG, Iglesias J, Roche E, et al. Glucolipotoxicity alters lipid partitioning and causes mitochondrial dysfunction, cholesterol, and ceramide deposition and reactive oxygen species production in INS832/13 b-cells. Endocrinology. 2010;151:3061–3073. [DOI] [PubMed] [Google Scholar]
- 8.Lupi R, Dotta F, Marselli L, DelGuerra S, Masini M, Santangelo C, Patane G, Boggi U, Piro S, Anello M, et al. Prolonged exposure to free fatty acids has cytostatic and pro-apoptotic effects on human pancreatic islets: evidence that b-cell dea this caspase mediated, partially dependent on ceramide pathway and Bcl-2 regulated. Diabetes. 2002;51:1437–1442. [DOI] [PubMed] [Google Scholar]
- 9.El-Assaad W, Buteau J, Peyot ML, Nolan C, Roduit R, Hardy S, Joly E, Dbaibo G, Rosenberg L, Prentki M. Saturated fatty acids synergize with elevated glucose to cause pancreatic beta-cell death. Endocrinology. 2003;144:4154–4163. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Elksnis A, Wikström P, Walum E, Welsh N, PO Carlsson. The novel NADPH oxidase 4 selective inhibitor GLX7013114 counteracts human islet cell death in vitro. PLoS One. 2018;13:e0204271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daiber A, Di Lisa F, Oelze M, Kröller‐Schön S, Steven S, Schulz E, Münzel T. Crosstalk of mitochondria with NADPH oxidase via reactive oxygen and nitrogen species signalling and its role for vascular function. Br J Pharmacol. 2017;174:1670–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parascandolo A, Laukkanen MO. Carcinogenesis and reactive oxygen species signaling: interaction of the NADPH oxidase NOX1-5 and superoxide dismutase 1-3 signal transduction pathways. Antioxid Redox Sign. 2019;30:443–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skonieczna M, Hejmo T, Poterala-Hejmo A, Cieslar-Pobuda A, Buldak RJ. NADPH oxidases: insights into selected functions and mechanisms of action in cancer and stem cells. Oxid Med Cell Longev. 2017;2017:9420539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zielonka J, Hardy M, Michalski R, Sikora A, Zielonka M, Cheng G, Ouari O, Podsiadły R, Kalyanaraman B. Recent developments in the probes and assays for measurement of the activity of NADPH oxidases. Cell Biochem Biophys. 2017;75:335–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salazar G. NADPH oxidases and mitochondria in vascular senescence. Int J Mol Sci. 2018;19:1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li WY, Chen BX, Chen ZJ, Gao YT, Chen Z, Liu J. Reactive oxygen species generated by NADPH oxidases promote radicle protrusion and root elongation during rice seed germination. Int J Mol Sci. 2017;18:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun QA, Runge MS, Madamanchi NR. Oxidative stress, NADPH oxidases, and arteries. Hamostaseologie. 2016;36:77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi DH, Lee J. A mini-review of the NADPH oxidases in vascular dementia: correlation with NOXs and risk factors for VaD. Int J Mol Sci. 2017;18:2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Pagano PJ. Microvascular NADPH oxidase in health and disease. Free Radic Biol Med. 2017;109:33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyata Y, Matsuo T, Sagara Y, Ohba K, Ohyama K, Sakai H. A mini-review of reactive oxygen species in urological cancer: correlation with NADPH oxidases, angiogenesis, and apoptosis. Int J Mol Sci. 2017;18:2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang GH, Chen SQ, Huang QY, Chen LW, Liao DS. Curcumin suppresses cardiac fibroblasts activities by regulating the proliferation and cell cycle via the inhibition of the p38 MAPK/ERK signaling pathway. Mol Med Rep. 2018;18:1433–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao NJ, Liao MJ, Wu JJ, Chu KX. Curcumin suppresses Notch-1 signaling: improvements in fatty liver and insulin resistance in rats. Mol Med Rep. 2018;17:819–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang C, He LJ, Ye HZ, Liu DF, Zhu YB, Miao DD, Zhang SP, Chen YY, Jia YW, Shen J, et al. Nrf2 is a key factor in the reversal effect of curcumin on multidrug resistance in the HCT-8/5-Fu human colorectal cancer cell line. Mol Med Rep. 2018;18:5409–5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shang W, Zhao LJ, Dong XL, Zhao ZM, Li J, Zhang BB, Cai H. Curcumin inhibits osteoclastogenic potential in PBMCs from rheumatoid arthritis patients via the suppression of MAPK/RANK/c-Fos/NFATc1 signaling pathways. Mol Med Rep. 2016;14:3620–3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang LZ, Xue H, Zhao G, Qiao CX, Sun XM, Pang CJ, Zhang DL. Curcumin and resveratrol suppress dextran sulfate sodium-induced colitis in mice. Mol Med Rep. 2019;19:3053–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He Y, Yue Y, Zheng X, Zhang K, Chen SH, Du ZY. Curcumin, inflammation, and chronic diseases: how are they linked? Molecules. 2015;20:9183–9213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarkar A, De R, Mukhopadhyay AK. Curcumin as a potential therapeutic candidate for helicobacter pylori associated diseases. World J Gastroenterol. 2016;22:2736–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swatson WS, Katoh-Kurasawa M, Shaulsky G, Alexander S. Curcumin affects gene expression and reactive oxygen species via a PKA dependent mechanism in dictyostelium discoideum. PLoS One. 2017;12:e0187562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Furlan V, Konc J, Bren U. Inverse molecular docking as a novel approach to study anticarcinogenic and anti-neuroinflammatory effects of curcumin. Molecules. 2018;23:3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang LH, Chen XW, Du ZY, Li GF, Chen MY, Chen X, Liang G, Chen TK. Curcumin suppresses gastric tumor cell growth via ROS-mediated DNA polymerase γ depletion disrupting cellular bioenergetics. J Exp Clin Cancer Res. 2017;36:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maiti P, Dunbar GL. Use of curcumin, a natural polyphenol for targeting molecular pathways in treating age-related neurodegenerative diseases. Int J Mol Sci. 2018;19:1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohajeri M, Sahebkar A. Protective effects of curcumin against doxorubicin-induced toxicity and resistance: a review. Crit Rev Oncol Hematol. 2018;122:30–51. [DOI] [PubMed] [Google Scholar]
- 33.Santezi C, Reina BD, Dovigo LN. Curcumin-mediated photodynamic therapy for the treatment of oral infections-a review. Photodiagnosis Photodyn Ther. 2018;21:409–415. [DOI] [PubMed] [Google Scholar]
- 34.Hosseini A, Hosseinzadeh H. Antidotal or protective effects of curcuma longa (turmeric) and its active ingredient, curcumin, against natural and chemical toxicities: a review. Biomed Pharmacother. 2018;99:411–421. [DOI] [PubMed] [Google Scholar]
- 35.Gawde KA, Sau S, Tatiparti K, Kashaw SK, Mehrmohammadi M, Azmi AS, Iyer AK. Paclitaxel and di-fluorinated curcumin loaded in albumin nanoparticles for targeted synergistic combination therapy of ovarian and cervical cancers. Colloid Surface B. 2018;167:8–19. [DOI] [PubMed] [Google Scholar]
- 36.Jiang S, Han J, Li T, Xin Z, Ma Z, Di W, Hu W, Gong B, Di SY, Wang DJ, et al. Curcumin as a potential protective compound against cardiac diseases. Pharmacol Res. 2017;119:373–383. [DOI] [PubMed] [Google Scholar]
- 37.Zhao G, Liu Y, Yi X, Wang Y, Qiao S, Li Z, Ni J, Song ZQ. Curcumin inhibiting Th17 cell differentiation by regulating the metabotropic glutamate receptor-4 expression on dendritic cells. Int Immunopharmacol. 2017;46:80–86. [DOI] [PubMed] [Google Scholar]
- 38.Assis RP, Arcaro CA, Gutierres VO, Oliveira JO, Costa PI, Baviera AM, Brunetti IL. Combined effects of curcumin and lycopene or bixin in yoghurt on inhibition of LDL oxidation and increases in HDL and paraoxonase levels in streptozotocin-diabetic rats. Int J Mol Sci. 2017;18:332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prasad S, Gupta SC, Tyagi AK, Aggarwal BB. Curcumin, a component of golden spice: from bedside to bench and back. Biotechnol Adv. 2014;32:1053–1064. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen GT, Green ER, Mecsas J. Neutrophils to the ROScue: mechanisms of NADPH oxidase activation and bacterial resistance. Front Cell Infect Microbiol. 2017;7:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oh YS. Mechanistic insights into pancreatic beta-cell mass regulation by glucose and free fatty acids. Anat Cell Biol. 2015;48:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kornelius E, Li HH, Peng CH, Yang YS, Chen WJ, Chang YZ, Bai YC, Liu S, Huang CN, Lin CL. Liraglutide protects against glucolipotoxicity‐induced RIN‐m5F β‐cell apoptosis through restoration of PDX1 expression. J Cell Mol Med. 2019;23:619–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhatti JS, Bhatti GK, Reddy PH. Mitochondrial dysfunction and oxidative stress in metabolic disorders-a step towards mitochondria based therapeutic strategies. Biochim Biophys Acta. 2017;1863:1066–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J Clin Invest. 2006;116:1802–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robertson RP. Oxidative stress and impaired insulin secretionin type 2 diabetes. Curr Opin Pharmacol. 2006;6:615–619. [DOI] [PubMed] [Google Scholar]
- 46.Robertson RP, Harmon J, Tran PO, Tanaka Y, Takahashi H. Glucose toxicity in beta-cells: type 2 diabetes, good radicals gone bad, and the glutathione connection. Diabetes. 2003;52:581–587. [DOI] [PubMed] [Google Scholar]
- 47.Poitout V, Robertson RP. Mini review: secondary beta-cell failure in type 2 diabetes-a convergence of glucotoxicity and lipotoxicity. Endocrinology. 2002;143:339–342. [DOI] [PubMed] [Google Scholar]
- 48.Lenzen S, Drinkgern J, Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med. 1996;20:463–466. [DOI] [PubMed] [Google Scholar]
- 49.Tiedge M, Lortz S, Drinkgern J, Lenzen S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes. 1997;46:1733–1742. [DOI] [PubMed] [Google Scholar]
- 50.Pickering RJ, Rosado CJ, Sharma A, Buksh S, Tate M, de Haan JB. Recent novel approaches to limit oxidative stress and inflammation in diabetic complications. Clin Transl Immunology. 2018;7:e1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hadzi-Petrushev N, Bogdanov J, Krajoska J, Ilievska J, Bogdanova-Popov B, Gjorgievska E, Mitrokhin V, Sopi R, Gagov H, Kamkin A, et al. Comparative study of the antioxidant properties of monocarbonyl curcumin analogues C66 and B2BrBC in isoproteranol induced cardiac damage. Life Sci. 2018;197:10–18. [DOI] [PubMed] [Google Scholar]
- 52.Hayashi M, Tojo A, Shimosawa T, Fujita T. The role of adrenomedullin in the renal NADPH oxidase and (pro)renin in diabetic mice. J Diabetes Res. 2013;2013:134395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holthoff JH, Woodling KA, Doerge DR, Burns ST, Hinson JA, Mayeux PR. Resveratrol, a dietary polyphenolic phytoalexin, is a functional scavenger of peroxynitrite. Biochem Pharmacol. 2010;80:1260–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kovacic P, Somanathan R. Multifaceted approach to resveratrol bioactivity: focus on antioxidant action, cell signaling and safety. Oxid Med Cell Longev. 2010;3:86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ko HM, Joo SH, Jo JH, Park WS, Jung WY, Shin JH, Ahn HJ. Liver-wrapping, nitric oxide-releasing nanofiber downregulates cleaved caspase-3 and Bax expression on rat hepatic ischemia-reperfusion injury. Transplant Proc. 2017;49:1170–1174. [DOI] [PubMed] [Google Scholar]


