ABSTRACT
Background: In South Korea, the one-dose varicella vaccine was included in the National Immunization Program for children aged 12–15 months in 2005, and the vaccine coverage reached >95%. The impact of varicella vaccination on varicella and herpes zoster (HZ) was investigated, accounting for demographic changes over time.
Methods: We calculated the crude and age-sex standardized incidence rates (IRs) and age-specific IRs of varicella and HZ from 2003 to 2015, using the National Health Information Database including approximately 50 million Koreans. The annual incidence rate ratios (IRRs) were calculated using a negative binomial regression analysis, adjusting for age and sex.
Results: The crude varicella IR steadily declined by 67%, from 5.70/1000 to 1.87/1000 person years (IRR per year: 0.91; 95% CI 0.89–0.93), but the adjusted IRs showed a significant decline only during 2010–2015 (adjusted IRR per year: 0.90; 95% CI 0.88–0.93). The greatest decline was found in children ≤4 years of age, whereas the IR increased until 2011 and then declined afterward in children aged 5–9 years, who represented the highest incidence age group in 2013–2015. The crude HZ IR increased from 2.67/1000 to 9.80/1000 person years (IRR per year: 1.12; 95% CI 1.10–1.15), and the adjusted IR also followed the same trend. A similar increasing trend was observed before and after universal vaccination.
Conclusions: One-dose varicella vaccination was moderately effective in preventing varicella, but this strategy was insufficient to interrupt varicella transmission in children. Furthermore, the HZ incidence dramatically increased over this decade. The current vaccination strategy against varicella-zoster disease should be reconsidered.
KEYWORDS: Varicella, herpes zoster, epidemiology, varicella vaccine, South Korea
Background
Varicella-zoster virus (VZV) causes both varicella and herpes zoster (HZ). Primary infection of VZV leads to varicella, which mostly affects children. Once infected, children are considered to have lifelong immunity against varicella. However, VZV becomes latent in dorsal root ganglia, and its reactivation causes HZ later in life.1 The reactivation of VZV is believed to be associated with waning cell-mediated immunity,2 which is postulated to be boosted exogenously via re-exposure to VZV, through contact with varicella-infected individuals.3
Varicella vaccination has proven effective in reducing the disease burden and severity of varicella.4 Thus far, varicella vaccines have been recommended in 33 countries.5 With the introduction of the one-dose universal varicella vaccination, the disease incidence has markedly declined in many countries.6–12 However, a one-dose varicella vaccine schedule was insufficient to effectively interrupt VZV transmission in children, resulting in continued outbreaks among highly vaccinated children. Thus, the necessity of a second-dose vaccine was suggested.13 In several countries, including the US, Canada, and Germany, the two-dose varicella vaccine schedule has been implemented. However, there have been concerns that the universal varicella vaccination may increase the incidence of HZ by reducing the exogenous boosting to natural varicella or cause the disease burden to shift towards older individuals who are at a higher risk of severe varicella.14 Due to such concerns, the vaccination policies against varicella vary from country to country.5
In South Korea, the varicella vaccine was introduced in 1988. In 2005, the one-dose varicella vaccine was included in the National Immunization Program (NIP) for children aged 12–15 months, and varicella became a notifiable disease.15 Since then, the number of varicella cases reported to the Korea Centers for Disease Control and Prevention (KCDC) has increased steadily from 11,207 in 2006 to 46,330 in 2015 (Supplementary Figure S1),16 despite the high coverage of varicella vaccine in more than 95% of eligible children under the NIP.17–19 Thus, the effectiveness of the varicella vaccines that are widely used in South Korea have been critically questioned,20,21 and the current one-dose varicella vaccination strategy has been debated. Also, it has been suggested that the increase in notified varicella cases could be attributable to the increasing awareness among physicians and the strengthened legal liability in reporting notifiable cases.22 Interestingly, as the number of varicella notifications increased, the incidence of HZ was also reported to increase in South Korea,23 which appears to contradict previous studies in other countries that have found an inverse relationship between varicella and HZ incidence.24
In the current epidemiological transition that has used universal one-dose varicella vaccination for more than a decade, we aimed to investigate the temporal trends of varicella and HZ incidence using nationwide population-based data and to evaluate the long-term impact of universal varicella vaccination on varicella and HZ in South Korea.
Results
Trends in varicella incidence following universal varicella vaccination
Before the introduction of the varicella vaccine into the NIP in South Korea, the varicella vaccine uptake was 69–73%. According to national immunization surveys, the coverage level has been sustained at greater than 95% among individuals who were born after the year 2007. As the vaccine uptake increased, the varicella incidence tended to decrease over time (Table 1, Supplementary Figure S2).
Table 1.
Annual incidence rates of varicella and herpes zoster before and after one-dose varicella vaccination inclusion in the National Immunization Program in 2005 in South Korea, 2003–2015.
| Varicella cases (N) | Crude IR (per 1000 PY) | Standardized IRa (per 1000 PY) | Adjusted IRRa (95% CI) | HZ cases (N) | Crude IR (per 1000 PY) | Standardized IRa (per 1000 PY) | Adjusted IRRa (95% CI) | |
|---|---|---|---|---|---|---|---|---|
| 2003 | 267198 | 5.70 | 4.40 | 1 | 125405 | 2.67 | 3.02 | 1 |
| 2004 | 271495 | 5.76 | 4.80 | 1.07 (0.91–1.26) | 143759 | 3.03 | 3.39 | 1.13 (1.05–1.21) |
| 2005 | 271412 | 5.75 | 5.15 | 1.13 (0.96–1.34) | 168546 | 3.54 | 3.89 | 1.31 (1.22–1.40) |
| 2006 | 239525 | 5.06 | 4.91 | 1.06 (0.90–1.24) | 192448 | 4.02 | 4.34 | 1.49 (1.38–1.60) |
| 2007 | 246241 | 5.21 | 5.35 | 1.11 (0.94–1.30) | 263495 | 5.50 | 5.84 | 2.04 (1.90–2.20) |
| 2008 | 227877 | 4.75 | 5.16 | 1.05 (0.89–1.24) | 329815 | 6.79 | 7.10 | 2.49 (2.32–2.67) |
| 2009 | 211385 | 4.40 | 5.00 | 1.03 (0.88–1.22) | 355992 | 7.33 | 7.55 | 2.68 (2.50–2.88) |
| 2010 | 184411 | 3.83 | 4.52 | 0.91 (0.77–1.07) | 375261 | 7.73 | 7.85 | 2.81 (2.62–3.02) |
| 2011 | 221192 | 4.59 | 5.64 | 1.10 (0.93–1.30) | 404088 | 8.34 | 8.34 | 2.99 (2.79–3.21) |
| 2012 | 137702 | 2.87 | 3.58 | 0.76 (0.64–0.89) | 436516 | 9.02 | 8.91 | 3.18 (2.96–3.42) |
| 2013 | 124807 | 2.58 | 3.26 | 0.71 (0.60–0.83) | 458848 | 9.51 | 9.28 | 3.31 (3.09–3.56) |
| 2014 | 114747 | 2.36 | 2.98 | 0.69 (0.58–0.81) | 467696 | 9.73 | 9.40 | 3.38 (3.14–3.62) |
| 2015 | 90959 | 1.87 | 2.34 | 0.60 (0.51–0.71) | 469268 | 9.80 | 9.37 | 3.34 (3.11–3.58) |
IR, incidence rate per 1000 person years; aIRR, adjusted incidence rate ratio; CI, confidence interval; HZ, herpes zoster.
aIncidence rates were standardized to the census population of the year of 2010.
bThe IRR for each year were adjusted for age and sex and calculated between the respective year and the reference year 2003.
The number of incident varicella cases decreased from 267,198 in 2003 to 90,959 in 2015. The crude annual incidence rates of varicella decreased significantly during the whole study period (IRR per year: 0.91; 95% CI 0.89–0.93), showing a 67% reduction from the pre-NIP period. However, age-sex standardized incidence rates showed a segmented trend despite an overall decrease (adjusted IRR per year: 0.96; 95% CI 0.95–0.97) (Table 1).
Segmented regression analysis, after adjustments for age and sex, showed that the varicella incidence rate did not change during the pre-NIP (2003–2005) (adjusted IRR per year: 1.03; 95% CI 0.98–1.08) and early post-NIP (2006–2009) (adjusted IRR per year: 0.98; 95% CI 0.95–1.01) periods. A significant decline was observed only during the late post-NIP period (2010–2015) (adjusted IRR per year: 0.90; 95% CI 0.88–0.93) (Supplementary Figure S2). When comparing the incidence rates by birth cohort, the age-specific incidence rates began to decline in the 2008–2009 birth cohort, and a substantial decrease was noted in birth cohorts born after 2010 (Figure 1).
Figure 1.
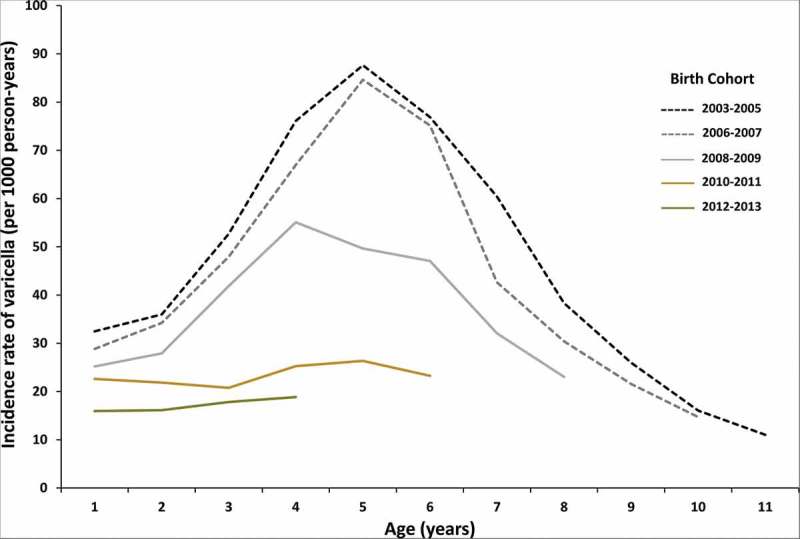
Age-specific varicella incidence rates by birth cohort in South Korea, 2003–2015.
Trends in age-specific varicella incidence rates
The age-specific varicella incidence rates were significantly reduced in all age groups, except for the 5–9-year-old children. Among the 5–9-year-old children, the varicella incidence rates began to decline after a steady increase until 2011, with an overall 9.9% reduction (Figure 2). The greatest decline was identified among children <1 and 1–4 years, with 57.1% and 64.0% reductions, respectively. During 2013–2015, the age group with the highest incidence changed from the 1–4-year-olds to the 5–9-year-olds. In adolescents and adults, even though the percent change was small, the varicella incidence rates declined significantly during the late post-NIP period (Figure 2).
Figure 2.
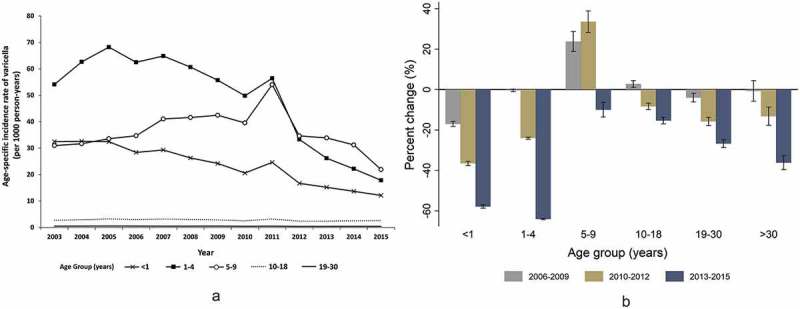
Trends in annual age-specific incidence rates of varicella (a) and percent change in age-specific incidence rates after inclusion of a one-dose varicella vaccine in the National Immunization Program compared to that of the year 2003–2005 (b) in South Korea.
The peak age of varicella infection slowly shifted from 3–4 years of age in 2003–2005 to 5–6 years in 2013–2015, and the median age of varicella increased from 4 to 6 years of age (Supplementary Figure S3).
Trends in herpes zoster incidence following universal varicella vaccination
The crude HZ incidence rate continuously increased during the whole study period, from 2003 to 2015 (2.67/1000 person years to 9.80/1000 person years) (IRR per year: 1.12; 95% CI 1.10–1.15). A similar trend was also observed in age, sex-standardized HZ incidence (adjusted IRR per year: 1.11; 95% CI 1.11–1.12) (Table 1). During the pre-NIP period, the increasing trend was significant (adjusted IRR per year: 1.18; 95% CI 1.15–1.21), and the slope of the trend was similar to that during the early post-NIP period (adjusted IRR per year: 1.15; 95% CI 1.13–1.17). (Supplementary Figure S2)
Discussion
In this population-based longitudinal study over a 13-year span, we observed a steady decline in the crude varicella incidence after implementation of the universal varicella vaccination in 2005. After adjusting for age and sex, a declining trend was noted since 2010. These findings suggest that the impact of universal vaccination only became apparent after population immunity increased in all age populations belonging to the risk age group. As the varicella vaccination program matured, the varicella incidence among infants and adults, who are not the target of varicella vaccination, decreased. This finding indicates the effect of herd immunity during the late post-NIP period.
In our study, the greatest decline in varicella incidence was observed in children aged 1–4 years. Contrastingly, in children aged 5–9 years, the varicella incidence rate increased steadily and reached a peak in 2011, after which it started to decline. During 2003–2011, this age group of 5–9 years comprised children who were born during the period of 1994–2006, and the majority of them (89%, 40/45 birth cohorts) were not included in the universal varicella vaccination program. In South Korea, when one dose varicella vaccine at 12–15 months of age was introduced in the NIP, a catch-up vaccination was not concurrently implemented. Although the vaccine coverage was approximately 70% during the pre-NIP period,25,26 the remaining unvaccinated children could have contributed to the ongoing varicella activity during the early post-NIP period. In addition, suboptimal vaccine effectiveness and rapid waning of immunity with a single-dose varicella vaccine could cause accumulation of susceptible individuals towards older ages. All of these factors may have contributed to the increasing varicella incidence in those aged 5–9 before 2012. In addition, the ongoing VZV activity in this age group could have affected susceptible individuals who accumulated in other age groups, which possibly led to peak varicella incidence rates in all age groups in 2011. However, when the birth cohorts with high vaccination coverage entered the 5–9 years age group during 2012–2015, the varicella incidence rate began to decrease in this age group.
In a systematic review, the overall single-dose varicella vaccine effectiveness was approximately 80% against any varicella infection.4,27 However, a recent matched case–control study in South Korea reported that the overall one-dose varicella vaccine effectiveness was as low as 13%.20 In this matched case–control study, the vaccination rates in cases and controls were 76% and 78%, respectively, which was much lower than the results (>95%) of the nationwide vaccination surveys. Thus, the estimated vaccine effectiveness might have been biased towards the null. Our study also showed that the crude varicella incidence was reduced by 67%, after maintaining the high varicella vaccine coverage at >95%; therefore, the varicella vaccine effectiveness might be higher than previously estimated in South Korea.
However, our study showed a relatively high varicella incidence in children aged ≤ 9 years even after the vaccination program matured. Recent Korean studies have shown that most varicella cases in children were breakthrough infections.20,21 The wide use of the less immunogenic MAV/06 strain vaccine was suggested to be one of the main causes of the high incidence of breakthrough varicella infection in South Korea.20,21,28 The seroconversion rate of the MAV/06 strain vaccine was estimated as 76.7%-83.6%, as measured with a fluorescent antibody to membrane antigen (FAMA) assay.21,28 However, the seroconversion rate of the one-dose Oka strain vaccine was similarly estimated to be 76% in the field study, when measured with FAMA,29 which was comparable to that of the MAV/06 strain vaccine. Thus, primary vaccine failure after one-dose vaccination against varicella, in general, appears to be more concerning rather than the use of the MAV/06 strain vaccine.30 The rapid waning of immunity induced by one-dose vaccination can also explain the high breakthrough infections. It is well known that one-dose varicella vaccination confers only short-lived protection against varicella, and two-dose vaccination is required to reduce breakthrough infections, and control outbreaks.13 Choi et al. also showed a progressive decrease in seropositivity from the age of 1 to 4 years, demonstrating rapidly waning immunity after single-dose varicella vaccination at 12–15 months of age.31 It seems reasonable to provide the second dose vaccine to children before the substantial waning of vaccine-induced immunity. In our study, all children aged ≤9 years were targeted for the universal varicella vaccination program during 2013–2015. Therefore, during 2013-2015, the majority of varicella cases in children were presumed to be breakthrough infections, because vaccine coverage was estimated to be >95%. This demonstrates the limitations of one-dose varicella vaccination for reducing varicella incidence and supports the necessity of the two-dose varicella vaccination in South Korea.
In our study, the demographic change in the Korean population appeared to influence the varicella incidence rates. In South Korea, the birth rate has rapidly declined with the aging population.32 The population size of children under 9 years of age has diminished by 19.1% in 2015, compared to that in 2005 (Supplementary Figure S4),32 which could partly explain the difference between the crude and adjusted varicella incidences during the early post-NIP period.
This study demonstrated a decreasing trend in varicella incidence which contradicted the varicella notification increases to the KCDC. However, the pediatric sentinel surveillance conducted during 2005–2012 showed a gradual decline in the proportion of varicella cases,33–36 which was consistent with our study’s findings (Supplementary Figure S1). Therefore, the rise in varicella notifications to the KCDC appeared to be largely attributable to the increased reporting rate promoted by physicians with heightened awareness and increased legal responsibility to recognize and report notifiable diseases became strengthened under the Infection Control and Prevention Act 2010.22
The incidence of HZ rapidly increased and this trend did not change after adjusting for the demographic change in South Korea. The increasing HZ incidence has become a worldwide phenomenon, and the causes of this epidemiologic change have yet to be clearly proven. Universal varicella vaccination was often postulated as a contributing factor for the rising HZ incidence through reducing varicella incidence and consequently decreasing natural boosting against VZV reactivation. In this study, the relationship between childhood varicella vaccination and adult HZ burden appeared to be less obvious, given that an increasing trend was also observed during the pre-NIP period (2003–2005). However, the pre-NIP period was relatively short in duration and the varicella vaccine coverage reached approximately 70% before its inclusion into the NIP in South Korea. Therefore, the relationship between childhood varicella vaccination and adult HZ is still inconclusive as it was difficult to separately analyze the impact of the universal varicella vaccination program on HZ epidemiology. The aging population and the increased prevalence of comorbid conditions or immunosuppression could be plausible explanations for the increasing HZ incidence. In addition to these factors, the better accessibility to healthcare and increasing public awareness of HZ may contribute to the increased HZ incidence.37,38 In our study, the aging population did not seem to be the main determinant for the increase in HZ cases, as the age-sex standardization did not alter the increasing trend. To understand the drivers of the epidemiologic change in HZ incidence, it is necessary to explore other potential contributing factors, such as the prevalence of immunocompromised status or changes in disease awareness and health behavior. In South Korea, the population over 65 years of age is increasing rapidly and is estimated to be 14% of the total population in 2017 and to rise to 24.3% of the total population in 2030.39 Accordingly, the HZ burden is expected to grow in the future and could become a major public health issue. After the introduction of HZ vaccine in 2012, HZ vaccination has been recommended for adults ≥60 years and 50–59 year-old adults may receive HZ vaccine depending on individual health conditions according to the Korean adult immunization guideline.40 However, the zoster vaccine coverage among adults ≥50 years was estimated as low as 9.4% in 2015 in South Korea,41 and there were barriers to the wide use of HZ vaccine, such as high cost and low perceived risk of HZ.42 To prevent HZ and reduce the disease burden in the adult population, vaccination against HZ should be more actively encouraged.
There are several limitations to our study. First, this study was based on data from the insurance claims database; thus, patients who did not seek medical care could not be captured, causing the possible under-reporting. However, improved access to healthcare and public awareness could have increased the utilization of healthcare service over the study period. Another limitation of using claims data is that diagnosis verification was difficult; some cases might have been misclassified. Particularly, physicians might have had a tendency to over-diagnose varicella during the post-NIP period as their legal responsibility to recognize and report notifiable diseases increased over time. Even with the possibility of overestimation, the decreasing trend was continuously observed over the years, highlighting the effectiveness of universal varicella vaccination in South Korea. Similarly, HZ cases may have been over diagnosed; identification of HZ cases using diagnostic codes alone has been reported to be inaccurate.43,44 In an effort to detect HZ cases more accurately, we used the previously validated operational definition comprising diagnostic codes and medications.43 Second, although this study demonstrated a decrease in varicella incidence following universal varicella vaccination, the one dose varicella vaccine effectiveness was not accurately estimated since individual-level vaccination information was unavailable from the National Health Information Database (NHID). Despite these limitations, the present study is population-based and includes almost all of the Korean population thereby increasing the generalizability of this study results. Also, this study demonstrated the longitudinal epidemiological change in varicella and HZ incidence before and after universal varicella vaccination over more than a decade in South Korea.
In summary, the varicella incidence was moderately reduced after introducing the one-dose varicella vaccine into the NIP; however, a large number of varicella cases occurred in 2015, despite the high vaccine coverage. The current vaccination strategy against varicella appears to be insufficient for interrupting VZV transmission in children, leading to breakthrough infections. In addition, the HZ incidence has dramatically increased over more than a decade. In this study, the universal varicella vaccination did not appear to be the main determinant of this increase; thus, other contributing factors should be explored. Vaccinating children against varicella can ultimately lead to a decrease in the HZ incidence in the long run.45 Yet, in the meantime, the increasing HZ burden will be a public health issue. Furthermore, children who have experienced breakthrough varicella will be at risk of HZ in the future, and this can contribute to the ongoing HZ disease burden. Therefore, the current varicella vaccination policy should be reconsidered to prevent varicella-zoster diseases in South Korea: a second-dose varicella vaccine should be included in the NIP to reduce breakthrough varicella, and vaccination against HZ should be more actively encouraged. Furthermore, it is necessary to establish an effective VZV disease prevention strategy based on further research on the effects of childhood varicella vaccination on the epidemiology of varicella-zoster disease and the underlying causes of the increasing HZ incidence.
Materials and methods
Data source
This study used the National Health Information Database (NHID), from January 1, 2002 to December 31, 2015. The NHID is provided by the National Health Insurance Service (NHIS), which is the compulsory health insurance scheme that covers the whole Korean population of approximately 50 million Koreans, of whom 97% are under the National Health Insurance and the rest are protected under the Medical Aid Scheme. The data contains sociodemographic variables, primary and secondary diagnoses, the date of hospital visits, detailed medication information that is prescribed during inpatient and outpatient visits, hospital admissions, types of insurance, and medical expenses, along with each patient’s encrypted identification number.46 The diagnoses were coded according to the International Classification of Disease, 10th Revision (ICD-10). Varicella vaccination coverage data was collected from the national vaccination surveys, from 2011–2016,17–19 and previous studies.25,26,47,48 This study was approved by the Institutional Review Board of the Catholic University of Korea, Daejeon St Mary’s Hospital, with a waiver for informed consent (DC16ENSI0065).
Case definitions and data collection
Varicella cases were identified through a database search for subjects with the varicella-related ICD-10 codes (B01) in any diagnostic fields. In order to ascertain HZ cases, we adopted a different approach, because the identification of HZ cases based on the diagnostic code alone has been reported as less optimal since the NHID data was collected for medical service claims and reimbursement.43 HZ cases were operationally defined as patients with the HZ-related ICD-10 codes (B02) in any diagnostic fields, who received either intravenous acyclovir ≥1 day or oral antiviral agents ≥5 days. Use of antiviral therapy was a usual practice during the study period.49,50 An incident case of varicella or HZ was defined as the first ever identified case during the study period. Because the NHID data started to be electronically collected in 2002, we considered the year 2002 as the wash-out period.
Statistical analyses
We estimated the annual crude and age-sex standardized rates for varicella per 1000 person years. The annual incidence rates were calculated by dividing the number of incident varicella cases by person years at risk for each disease. The person years at risk were calculated as the total population enrolled in the NHIS program in the given year, excluding the number of incident cases from the previous year. To account for temporal changes in population age structure, incidence rates were age- and sex-standardized to the census population of the year 2010.34 For trend analyses, a negative binomial regression analysis was used to estimate the crude and age-sex-adjusted annual incidence rate ratios (IRRs) and 95% confidence intervals (CIs) per year as a continuous or categorical variable, with the year 2003 as the reference. We also performed a segmented regression analysis to examine the incidence rates of varicella before and after inclusion of the varicella vaccine in the NIP. For segmented analysis, the study period was divided into three ranges: pre-NIP (2003-2005), early post-NIP (2006–2009), and late post-NIP (2010–2015) periods. The year 2005 was included in the pre-NIP period because the new vaccination program takes time to be properly implemented. The trend analyses were also performed on HZ cases to assess the impact of universal varicella vaccination on HZ incidence.
The age-specific incidence rates of varicella were calculated, and the age-specific percent changes were compared between the pre-NIP (2003-2005) and post-NIP periods (2006–2009, 2010–2012, and 2013–2015). To evaluate the effect of vaccine coverage by birth cohort, the age-specific incidence rates by birth cohort were estimated. The shift in age distribution was assessed by comparing the age-specific incidences between 2003–2005 and 2013–2015.
All analyses were performed with SAS software, version 9.3 (SAS institute Inc.) and Stata, version 13 (Stata Corp, College Station, TX).
Funding Statement
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2016R1A2B1008828) .
Disclosure of potential conflicts of interest
None of the authors declare any conflicts of interest.
Supplementary materials
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Widgren K, Giesecke J, Lindquist L, Tegnell A.. The burden of chickenpox disease in Sweden. BMC Infect Dis. 2016;16(1):666. doi: 10.1186/s12879-016-1957-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marinelli I, van Lier A, de Melker H, Pugliese A, van Boven M.. Estimation of age-specific rates of reactivation and immune boosting of the varicella zoster virus. Epidemics. 2017;19:1–12. doi: 10.1016/j.epidem.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Hope-Simpson RE. The nature of herpes zoster: A long-term study and a new hypothesis. Proc R Soc Med. 1965;58:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marin M, Marti M, Kambhampati A, Jeram SM, Seward JF. Global varicella vaccine effectiveness: A meta-analysis. Pediatrics. 2016;137(3):e20153741. doi: 10.1542/peds.2015-3741. [DOI] [PubMed] [Google Scholar]
- 5.Wutzler P, Bonanni P, Burgess M, Gershon A, Safadi MA, Casabona G. Varicella vaccination - the global experience. Expert Rev Vaccines. 2017;16(8):833–43. doi: 10.1080/14760584.2017.1343669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seward JF, Marin M, Vazquez M. Varicella vaccine effectiveness in the US vaccination program: a review. J Infect Dis. 2008;197(2):S82–9. doi: 10.1086/522145. [DOI] [PubMed] [Google Scholar]
- 7.Bechini A, Boccalini S, Baldo V, Cocchio S, Castiglia P, Gallo T, Giuffrida S, Locuratolo F, Tafuri S, Martinelli D, et al. Impact of universal vaccination against varicella in Italy. Hum Vaccin Immunother. 2015;11(1):63–71. doi: 10.4161/hv.34311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Latasa P, Gil de Miguel A, Barranco Ordonez MD, Rodero Garduno I, Sanz Moreno JC, Ordobas Gavin M, Esteban Vasallo M, Garrido-Estepa M, Garcia-Comas L. Effectiveness and impact of a single-dose vaccine against chickenpox in the community of Madrid between 2001 and 2015. Hum Vaccin Immunother. 2018;14(9):2274–80. doi: 10.1080/21645515.2018.1475813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang LY, Huang LM, Chang IS, Tsai FY. Epidemiological characteristics of varicella from 2000 to 2008 and the impact of nationwide immunization in Taiwan. BMC Infect Dis. 2011;11:352. doi: 10.1186/1471-2334-11-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wormsbecker AE, Wang J, Rosella LC, Kwong JC, Seo CY, Crowcroft NS, Deeks SL. Twenty years of medically-attended pediatric varicella and herpes zoster in Ontario, Canada: A population-based study. PLoS One. 2015;10(7):e0129483. doi: 10.1371/journal.pone.0129483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heywood AE, Wang H, Macartney KK, McIntyre P. Varicella and herpes zoster hospitalizations before and after implementation of one-dose varicella vaccination in Australia: an ecological study. Bull World Health Organ. 2014;92(8):593–604. doi: 10.2471/BLT.13.132142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siedler A, Dettmann M. Hospitalization with varicella and shingles before and after introduction of childhood varicella vaccination in Germany. Hum Vaccin Immunother. 2014;10(12):3594–600. doi: 10.4161/hv.34426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaves SS, Gargiullo P, Zhang JX, Civen R, Guris D, Mascola L, Seward JF. Loss of vaccine-induced immunity to varicella over time. N Engl J Med. 2007;356(11):1121–29. doi: 10.1056/NEJMoa064040. [DOI] [PubMed] [Google Scholar]
- 14.Ogunjimi B, Van Damme P, Beutels P. Herpes zoster risk reduction through exposure to chickenpox patients: A systematic multidisciplinary review. PLoS One. 2013;8(6):e66485. doi: 10.1371/journal.pone.0066485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sadzot-Delvaux C, Rentier B, Wutzler P, Asano Y, Suga S, Yoshikawa T, Plotkin SA. Varicella vaccination in Japan, South Korea, and Europe. J Infect Dis. 2008;197(2):S185–90. doi: 10.1086/522163. [DOI] [PubMed] [Google Scholar]
- 16.Infectious diseases surveillance yearbook, 2015. Osong (Republic of Korea): Korea Centers for Disease Control and Prevention; 2016. [accessed 1October 2018]. http://www.cdc.go.kr/npt/biz/npp/portal/nppPblctDtaView.do. [Google Scholar]
- 17.Lee SG. Korean national immunization survey, 2011. Osong (Republic of Korea): Korea Centers for Disease Control and Prevention; 2011. [accessed 1October 2018]. https://www.kdevelopedia.org. [Google Scholar]
- 18.Lee SG. Korean national immunization survey, 2013. Osong (Republic of Korea): Korea Centers for Disease Control and Prevention; 2013. [accessed 1October 2018]. http://www.cdc.go.kr/CDC/cms/cmsFileDownload.jsp?fid=31&cid=26689&fieldName=attach1&index=1. [Google Scholar]
- 19.National childhood vaccination coverage among children aged 3 years in Korea, 2015. Osong (Republic of Korea): Korea Centers for Disease Prevention and Control; 2016. [accessed 1October 2018]. http://www.cdc.go.kr/CDC/info/CdcKrinfo0726.jsp?menuIds=HOME001-MNU1132-MNU2430-MNU2689&fid=9955&q_type=&q_value=&cid=127096&pageNum=. [Google Scholar]
- 20.Lee YH, Choe YJ, Cho SI, Kang CR, Bang JH, M- D O, J- K L. Effectiveness of varicella vaccination program in preventing laboratory-confirmed cases in children in Seoul, Korea. J Korean Med Sci. 2016;31(12):1897–901. doi: 10.3346/jkms.2016.31.12.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oh SH, Choi EH, Shin SH, Kim YK, Chang JK, Choi KM, Hur JK, Kim KH, Kim JY, Chung EH, et al. Varicella and varicella vaccination in South Korea. Clin Vaccine Immunol. 2014;21(5):762–68. doi: 10.1128/CVI.00645-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chun BC. Laws on infectious disease control in Korea: past, present and prospective. Infect Chemother. 2011;43(6):474–84. doi: 10.3947/ic.2011.43.6.474. [DOI] [Google Scholar]
- 23.Choi WS, Noh JY, Huh JY, Jo YM, Lee J, Song JY, Kim WJ, Cheong HJ. Disease burden of herpes zoster in Korea. J Clin Virol. 2010;47(4):325–29. doi: 10.1016/j.jcv.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Schmid DS, Jumaan AO. Impact of varicella vaccine on varicella-zoster virus dynamics. Clin Microbiol Rev. 2010;23(1):202–17. doi: 10.1128/CMR.00031-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee HR, Cha SH, Kim KH. Survey on efficacy and safety of varicella vaccine as a regular vaccine in Korea. Osong (Republic of Korea): Korea Centers for Disease Control and Prevention; 2005. [accessed 1October 2018]. http://www.cdc.go.kr/CDC/cms/content/mobile/44/1144_view.html. [Google Scholar]
- 26.Shin YC, Lee MS, Kown SS, Ki M, Na B, Nam H, Lee SW, Choi BJ, Kim SI, Park YM. Development of vaccination coverage estimation methods and evaluation indicators of national immunization program in Korea. Osong (Republic of Korea): Korea Centers for Disease Control and Prevention; 2005. [accessed 1August 2018]. https://nip.cdc.go.kr/irgd/reference.do?service=getResearchView&strNum=44&GUISEQNUM=14. [Google Scholar]
- 27.World Health Organization.Systematic review of available evidence on effectiveness and duration of protection of varicella vaccines. Geneva (Switzerland): World Health Organization; 2014. [accessed 1September 2018]. https://www.who.int/immunization/sage/meetings/2014/april/4_Systematic_review_on_effectiveness_and_duration_of_protection_of_varicella_vaccines.pdf. [Google Scholar]
- 28.Kim SH, Lee HJ, Park SE, Oh SH, Lee SY, Choi EH. Seroprevalence rate after one dose of varicella vaccine in infants. J Infect. 2010;61(1):66–72. doi: 10.1016/j.jinf.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Michalik DE, Steinberg SP, Larussa PS, Edwards KM, Wright PF, Arvin AM, Gans HA, Gershon AA. Primary vaccine failure after 1 dose of varicella vaccine in healthy children. J Infect Dis. 2008;197(7):944–49. doi: 10.1086/529043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonanni P, Gershon A, Gershon M, Kulcsar A, Papaevangelou V, Rentier B, Sadzot-Delvaux C, Usonis V, Vesikari T, Weil-Olivier C, et al. Primary versus secondary failure after varicella vaccination: implications for interval between 2 doses. Pediatr Infect Dis J. 2013;32(7):e305–13. doi: 10.1097/INF.0b013e31828b7def. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi UY, Huh DH, Kim JH, Kang JH. Seropositivity of Varicella zoster virus in vaccinated Korean children and MAV vaccine group. Hum Vaccin Immunother. 2016;12(10):2560–64. doi: 10.1080/21645515.2016.1190056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Population by census, 2005–2015 27August 2018 Korean statistical information service; [accessed 27 Aug 2018]. http://kosis.kr/statisticsList/statisticsListIndex.do?menuId=M_01_01&vwcd=MT_ZTITLE&parmTabId=M_01_01&statId=1962001&themaId=A#A1.2.
- 33.Pediatric sentinel surveillance weekly report. Osong (Republic of Korea): Korea Centers for Disease Control and Prevention; 2012. [accessed 1October 2018]. http://www.cdc.go.kr/CDC/info/CdcKrInfo0301.jsp?menuIds=HOME006-MNU3003-MNU2950-MNU2951&cid=12780. [Google Scholar]
- 34.Korea Centers for Disease Control and Prevention The status of varicella in Korea. Public Health Weekly Rep. 2012;5:590–92. [Google Scholar]
- 35.Korea Centers for Disease Control and Prevention Results of pediatric infectious disease sentinel surveillance in Korea, 2011. Public Health Weekly Rep. 2012;5:362–65. [Google Scholar]
- 36.Korea Centers for Disease Control and Prevention The status of varicella reported through pediatric sentinel surveillance. Public Health Weekly Rep. 2008;1:69–73. [Google Scholar]
- 37.Roh NK, Park YM, Kang H, Choi GS, Kim BJ, Lee YW, Lew BL, Sim WY. Awareness, knowledge, and vaccine acceptability of herpes zoster in Korea: A multicenter survey of 607 patients. Ann Dermatol. 2015;27(5):531–38. doi: 10.5021/ad.2015.27.5.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang TU, Cheong HJ, Song JY, Noh JY, Kim WJ. Survey on public awareness, attitudes, and barriers for herpes zoster vaccination in South Korea. Hum Vaccin Immunother. 2015;11(3):719–26. doi: 10.1080/21645515.2015.1008885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Population projections for Korea (2015–2065) Edited by Statistics Korea. 2016. Government Complex-Daejeon, Republic of Korea: Statistics Korea; [accessed 1May, 2018]. http://kostat.go.kr/portal/eng/pressReleases/8/8/index.board.
- 40.Choi WS, Choi JH, Kwon KT, Seo K, Kim MA, Lee SO, Hong YJ, Lee JS, Song JY, Bang JH, et al. Revised adult immunization guideline recommended by the Korean Society of Infectious Diseases, 2014. Infect Chemother. 2015;47(1):68–79. doi: 10.3947/ic.2015.47.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bresnitz EA. Merck vaccines for older adults. Proceedings of WHO Meeting on Immunization in the Elderly; 2017; Geneva (Switzerland) WHO. [Google Scholar]
- 42.Yang TU, Cheong HJ, Choi WS, Song JY, Noh JY, Kim WJ. Physician attitudes toward the herpes zoster vaccination in South Korea. Infect Chemother. 2014;46(3):194–98. doi: 10.3947/ic.2014.46.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim YS, Seo HM, Bang CH, Lee JH, Park YG, Kim YJ, Kim GM, Park CJ, Park HJ, Yu DS, et al. Validation of herpes zoster diagnosis code in the medical record: A retrospective, multicenter study. Ann Dermatol. 2018;30(2):253–55. doi: 10.5021/ad.2018.30.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klompas M, Kulldorff M, Vilk Y, Bialek SR, Harpaz R. Herpes zoster and postherpetic neuralgia surveillance using structured data. Mayo Clin Proc. 2011;86(12):1146–53. doi: 10.4065/mcp.2011.0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brisson M, Melkonyan G, Drolet M, De Serres G, Thibeault R, De Wals P. Modeling the impact of one- and two-dose varicella vaccination on the epidemiology of varicella and zoster. Vaccine. 2010;28(19):3385–97. doi: 10.1016/j.vaccine.2010.02.079. [DOI] [PubMed] [Google Scholar]
- 46.Song SO, Jung CH, Song YD, Park CY, Kwon HS, Cha BS, Park JY, Lee KU, Ko KS, Lee BW. Background and data configuration process of a nationwide population-based study using the Korean National Health Insurance System. Diabetes Metab J. 2014;38(5):395–403. doi: 10.4093/dmj.2014.38.5.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oh JK. 2002. A cost-benefit analysis of varicella vaccination in Korea [dissertation]. Seoul (Rep of Korea): Seoul National University [Google Scholar]
- 48.Park SK. Nationwide vaccination coverage level: conceptual methodology and survey. Osong (Republic of Korea): Korea Centers for Disease Control and Prevention; 2009. [accessed 1October 2018]. https://nip.cdc.go.kr/irgd/reference.do?service=getResearchView&strNum=97&GUISEQNUM=22. [Google Scholar]
- 49.Song YG. Herpes zoster. Korean J Med. 2001;60:266–68. [Google Scholar]
- 50.Choi WS, Kwon SS, Lee J, Choi SM, Lee JS, Eom JS, Sohn JW, Choeng HJ. Immunity and the burden of herpes zoster. J Med Virol. 2014;86(3):525–30. doi: 10.1002/jmv.23830. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Population by census, 2005–2015 27August 2018 Korean statistical information service; [accessed 27 Aug 2018]. http://kosis.kr/statisticsList/statisticsListIndex.do?menuId=M_01_01&vwcd=MT_ZTITLE&parmTabId=M_01_01&statId=1962001&themaId=A#A1.2.
- Population projections for Korea (2015–2065) Edited by Statistics Korea. 2016. Government Complex-Daejeon, Republic of Korea: Statistics Korea; [accessed 1May, 2018]. http://kostat.go.kr/portal/eng/pressReleases/8/8/index.board.


