ABSTRACT
There are knowledge gaps regarding evidence-based research on the burden of vaccine-preventable diseases among human immunodeficiency virus (HIV)-infected and HIV-exposed children aged <18 years in sub-Saharan Africa. It is therefore essential to determine the trend and burden of vaccine-preventable diseases. We completed a systematic review and meta-analysis to identify the incidence, prevalence and case-fatality rates (CFR) attributed to various vaccine-preventable diseases among HIV-infected and HIV-exposed children in sub-Saharan Africa. The trends in the prevalence of vaccine-preventable diseases among HIV-infected and HIV-exposed children were also determined. Nine studies on tuberculosis (TB) were pooled to give an overall incidence rate estimate of 60 (95% confidence interval [CI] 30–70) per 1,000 child-years. The incidence of pneumococcal infections varied between 109–1509 per 100,000 while pertussis was between 2.9 and 3.7 per 1000 child-year. Twenty-two TB prevalence studies reported an estimated prevalence of 16%. Fifteen prevalence studies on hepatitis B infection were pooled together with an estimated prevalence of 5%. The pooled prevalence for pneumococcal infections was 2% while rotavirus diarrhoea reported a prevalence of 13%. Twenty-nine studies on TB were pooled to give an overall CFR estimate of 17% while pneumococcal infections in HIV-infected and exposed children were pooled together with a resultant rate of 15%. Some of the vaccine-preventable diseases still have high incidences, prevalence and CFR among HIV-infected and HIV-exposed children. There is also a dearth of research data on the burden of several vaccine-preventable diseases among HIV-infected and exposed children and a need for more studies in this area.
Keywords: HIV, vaccine-preventable diseases, sub-Saharan Africa, burden
Background
Human immunodeficiency virus (HIV) infection remains a leading public-health challenge and a principal cause of the infectious disease burden in low- and middle-income countries especially in sub-Saharan Africa.1 This region accounts for the bulk of HIV infection with about 36.7 million people living with the disease an estimated 75% of the global burden.2,3 It was also estimated that approximately 2.1 million children aged under 15 years were living with HIV with the majority coming from sub-Saharan Africa and about 31% having access to antiretroviral therapy in 2014.4 The incidence of HIV infections among children declined in 2014 but there were still 220,000 new infections that year alone.4 HIV-infected children have an increased risk of developing various vaccine-preventable diseases due to their defective immune systems.5 This makes it crucial to focus on the vaccination of HIV-infected and exposed children. The majority of these children are also residents of low-and-middle-income countries characterised by limited access to HIV diagnosis, treatment and care.2
Vaccination against various vaccine-preventable diseases has been proven to be a beneficial and cost-effective public-health measure for protecting children, adolescents and adults from these diseases, thereby reducing the morbidity and mortality attributable to them.6,7 Coverage of routine vaccinations is still low in some developing countries and not sufficient to meet the Global Vaccine Action Plan (GVAP) targets.8–10 Some African countries have low or decreasing immunisation coverage over the years with some not achieving ≥ 90% national coverage for vaccines included in their national immunisation schedule by the World Health Organization (WHO) in 2016.11 Sub-Saharan African countries account for about 34% of the global vaccine-preventable diseases burden, and are also responsible for the highest proportion of under-five mortality from these diseases.12
Recently, most developing countries have included routine childhood vaccines such as hepatitis B; Bacillus Calmette–Guérin (BCG); diphtheria, tetanus and pertussis (DTP); Haemophilus influenzae type b (Hib); polio; pneumococcal conjugate; measles; rotavirus (RV), rubella and yellow fever vaccines in their national Expanded Programme on Immunisation (EPI).13 These vaccines also protect against diseases such as tuberculosis, poliomyelitis, rotavirus gastroenteritis, diphtheria, tetanus, pertussis, pneumococcal diseases, hepatitis B infection, rubella, measles and yellow fever.
The gap in knowledge, especially in terms of evidence-based research, on the burden of vaccine-preventable diseases among HIV-infected and HIV-exposed children in sub-Saharan Africa, warrants this study.14 This study completed a systematic review of literature and meta-analysis to identify the incidence, prevalence and mortality due to various vaccine-preventable diseases among HIV-infected and HIV-exposed children in sub-Saharan Africa since the advent of HIV in the 1980s. This study is essential in determining the trend and current burden of vaccine-preventable disease epidemiology in sub-Saharan Africa.
Objectives
Primary objectives
To appraise all available published literature on the incidence and prevalence of vaccine-preventable diseases such as tuberculosis, poliomyelitis, hepatitis B virus infection, rotavirus gastroenteritis, diphtheria, tetanus, pertussis, pneumococcal diseases, measles, rubella and yellow fever among HIV-infected and HIV-exposed children in sub-Saharan Africa.
To determine the trend in the incidence and/or prevalence of vaccine-preventable diseases such as tuberculosis, poliomyelitis, hepatitis B virus infection, rotavirus gastroenteritis, diphtheria, tetanus, pertussis, pneumococcal diseases, measles, rubella and yellow fever among HIV-infected and HIV-exposed children in sub-Saharan Africa from 1980 to 2018.
Secondary objective
To describe the case-fatality rate ascribed to vaccine-preventable diseases such as tuberculosis, poliomyelitis, hepatitis B virus infection, rotavirus gastroenteritis, diphtheria, tetanus, pertussis, pneumococcal diseases, measles, rubella and yellow fever among HIV-infected and HIV-exposed children in sub-Saharan Africa.
Results
Literature search and result
Figure 1 shows the study selection process reported in line with PRISMA guidelines. We identified 3430 publications through the search of different databases. We also identified 13 additional articles through the screening of reference lists of various related articles. We screened 188 full-text articles and selected 76 articles for inclusion in the review and 70 articles were suitable for the meta-analysis (Figure 1).
Figure 1.
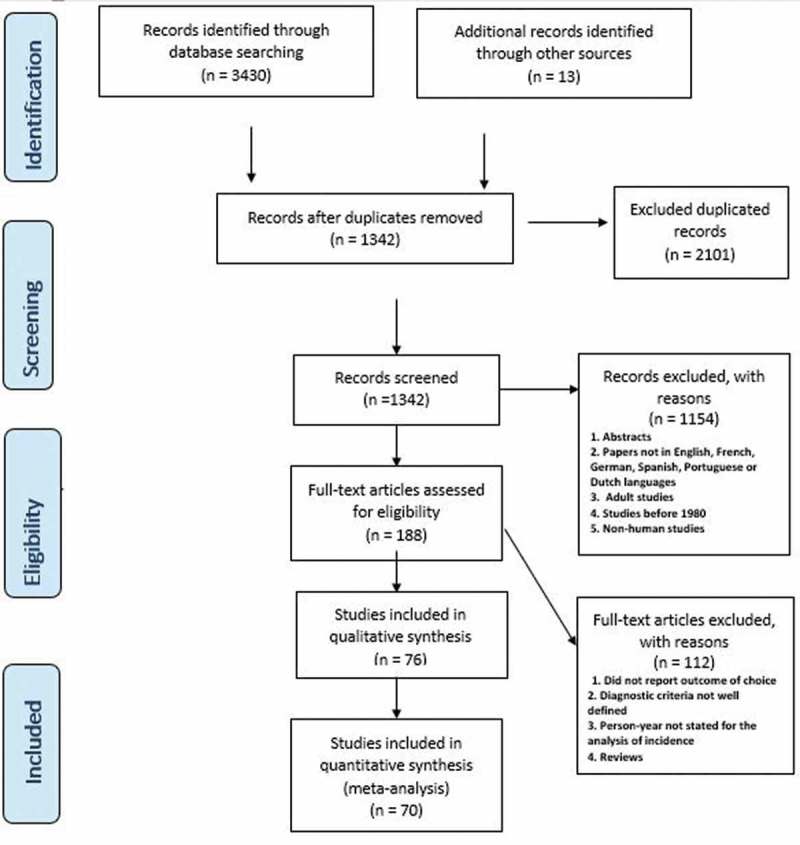
Flow diagram of the selection process.
Study characteristics
Table 1 provides a summary of the included studies and the vaccine-preventable diseases of interest. The table shows that 45 articles reported on tuberculosis, 14 on hepatitis B virus infection, ten studies focused on pneumococcal infections, two on rotavirus gastroenteritis, three on measles and three on pertussis. The included articles consist of 41 cross-sectional studies, 31 cohort studies, four case-control studies and one time-series analysis.
Table 1.
Characteristics of the study population.
| First author and year | Study period | Study design | Country | Sample size | VPD | Outcomes | HIV status | Quality scores |
|---|---|---|---|---|---|---|---|---|
| Abuogi 201315 | 2009–2010 | Cohort | Kenya | 689 | Tuberculosis | C, I, P | HI | 7 |
| Adams 201416 | 2006–2012 | Cross-sectional | Tanzania | 1193 | Tuberculosis | C, P | HI | 4 |
| Alemu 201617 | 2009–2014 | Cohort | Ethiopia | 645 | Tuberculosis | I | HI | 6 |
| Anigilaje 201618 | 2010–2013 | Cohort | Nigeria | 368 | Tuberculosis | P | HI | 8 |
| Auld 201419 | 2004–2008 | Cohort | Cote d‘ Ivoire | 2110 | Tuberculosis | I, P | HI | 8 |
| Bakeera 201120 | 2003–2006 | Cohort | Uganda | 1806 | Tuberculosis | I, C | HI | 8 |
| Bonnet 201821 | 2012–2014 | Cohort | Uganda | 113 | Tuberculosis | C | HI | 7 |
| Braitstein 200922 | 2001–2007 | Cohort | Kenya | 6,535 | Tuberculosis | I, P | HI | 8 |
| Buck 201323 | 2010 | Cohort | Malawi | 4874 | Tuberculosis | C, P | HI | 8 |
| Carlucci 201724 | 2012–2014 | Cohort | Multiple | 386 | Tuberculosis | C | HI | 8 |
| Cavanaugh 201225 | 2006–2007 | Cross-sectional | Kenya | 323 | Tuberculosis | C | HI | 6 |
| Chaya 201626 | 2006–2011 | Cross-sectional | South Africa | 47 | Tuberculosis | I | HI | 6 |
| Cruz 201527 | NR | Cohort | Botswana | 100 | Tuberculosis | P | HI | 6 |
| Dangor 201328 | 2005–2009 | Time-series analysis | South Africa | 1985 | Tuberculosis | I | HI | 7 |
| De Maayar 201129 | NR | Cross-sectional | South Africa | 58 | Tuberculosis | P | HI | 7 |
| Ebonyi 201630 | 2005–2013 | Cohort | Nigeria | 260 | Tuberculosis | C | HI | 8 |
| Ebonyi 2016b31 | 2005–2012 | Cohort | Nigeria | 876 | Tuberculosis | P | HI | 8 |
| Elenga 200532 | 2000–2003 | Cohort | Cote d‘ Ivoire | 282 | Tuberculosis | I | HI | 8 |
| Ferrand 201033 | 2007–2008 | Cross-sectional | Zimbabwe | 139 | Tuberculosis | P | HI | 7 |
| Hall 201734 | 2005–2008 | Cohort | South Africa | 224 | Tuberculosis | C | HI | 8 |
| Hesseling 2009a35 | 2004–2006 | Cross-sectional | South Africa | 3321 | Tuberculosis | C | HI | 6 |
| Hesseling 200536 | 1992–2000 | Cohort | South Africa | 93 | Tuberculosis | C | HI | 8 |
| Hesseling 200637 | 2002–2005 | Cohort | South Africa | 108 | Tuberculosis | C, P | HI | 7 |
| Hesseling 2009b38 | 2004–2006 | Cross-sectional | South Africa | 3321 | Tuberculosis | I, P | HI | 7 |
| Hicks 201439 | 2009–2010 | Cohort | South Africa | 64 | Tuberculosis | C | HI | 6 |
| Jeena 200040 | 1995–1998 | Cross-sectional | South Africa | 27 | Tuberculosis | P | HI | 5 |
| Kasambira 201141 | 2006–2009 | Cross-sectional | South Africa | 270 | Tuberculosis | P | HI | 6 |
| Madhi 2000b42 | 1996–1997 | Cross-sectional | South Africa | 67 | Tuberculosis | C | HI | 5 |
| Meyers 200043 | 1996 | Cross-sectional | South Africa | 144 | Tuberculosis | P | HI | 5 |
| Mwangwa 201744 | 2012–2013 | Cohort | Multiple | 17 | Tuberculosis | C | HI | 7 |
| Obiagwu 201345 | 2010 | Cross-sectional | Nigeria | 22 | Tuberculosis, Measles | P | HI | 6 |
| Okechukwu 201146 | 2007–2008 | Cross-sectional | Nigeria | 210 | Tuberculosis | C, P | HI | 6 |
| Osman 201747 | 2005–2012 | Cohort | South Africa | 3143 | Tuberculosis | C | HI | 6 |
| Padayatchi 200648 | 1993–2002 | Cross-sectional | South Africa | 6 | Tuberculosis | C | HI | 5 |
| Palme 200249 | 1995–1997 | Cohort | Ethiopia | 58 | Tuberculosis | C | HI | 6 |
| Patel 201350 | 2007–2009 | Cohort | Congo DRC | 31 | Tuberculosis | C | HI | 7 |
| Robinson 200751 | 1999–2001 | Case-control | South Africa | 47 | Tuberculosis | P | HI | 6 |
| Rose 201252 | 2008–2010 | Cohort | Tanzania | 54 | Tuberculosis | P | HI | 6 |
| Schaaf 200753 | 2003–2005 | Cross-sectional | South Africa | 133 | Tuberculosis | C | HI | 5 |
| Soeters 200554 | 2000–2001 | Cross-sectional | South Africa | 43 | Tuberculosis | C | HI | 4 |
| Walters 201455 | 2003–2010 | Cohort | South Africa | 494 | Tuberculosis | C | HI | 6 |
| Walters 200856 | 2003–2005 | Cross-sectional | South Africa | 137 | Tuberculosis | C | HI | 6 |
| Westerlund 201457 | 2003–2008 | Cohort | Ethiopia | 138 | Tuberculosis | P | HI | 7 |
| Wiseman 201158 | 2004–2006 | Cross-sectional | South Africa | 52 | Tuberculosis | C | HI | 5 |
| Yotebieng 201059 | 2004–2008 | Cohort | South Africa | 573 | Tuberculosis | C | HI | 6 |
| Kouakoussui 200460 | 2003 | Cohort | Cote d’Ivoire | 270 | Tuberculosis | I | HI | 7 |
| Abera 201461 | 2014 | Cross-sectional | Ethiopia | 253 | HBV infection | P | HI | 6 |
| Ashir 200962 | 2007 | Case-control | Nigeria | 284 | HBV infection | P | HI | 5 |
| Beghin 201763 | 2014 | Cross-sectional | South Africa | 183 | HBV infection | P | HI | 6 |
| Chotun 201564 | 2011–2012 | Cross-sectional | South Africa | 1000 | HBV infection | P | HE | 6 |
| Uleanya 201665 | NR | Cross-sectional | Nigeria | 140 | HBV infection | P | HI | 4 |
| Dziuban 201366 | 2009–2011 | Cross-sectional | Swaziland | 500 | HBV infection | P | HI | 3 |
| Ikpeme 201367 | 2010–2011 | Cross-sectional | Nigeria | 166 | HBV infection | P | HI | 4 |
| Jooste 201668 | 2015–2016 | Cohort | South Africa | 625 | HBV infection | P | HI | 7 |
| Muro 201369 | 2006–2008 | Cross-sectional | Tanzania | 157 | HBV infection | P | HI | 5 |
| Mutwa 201370 | 2010 | Cohort | Rwanda | 88 | HBV infection | P | HI | 7 |
| Nwolisa 201371 | 2010 | Cross-sectional | Nigeria | 139 | HBV infection | P | HI | 4 |
| Sadoh 201172 | NR | Cross-sectional | Nigeria | 155 | HBV infection | P | HI | 5 |
| Telatela 200773 | 2006 | Cross-sectional | Tanzania | 167 | HBV infection | P | HI | 4 |
| Varo 201674 | 2008–2010 | Cross-sectional | Malawi | 91 | HBV infection | P | HI | 3 |
| Moss 200275 | 1998–2000 | Cross-sectional | Zambia | 93 | Measles | P | HI | 6 |
| Wirth 201576 | 2009–2010 | Case-control | Botswana | 189 | Measles | C | HI | 5 |
| du Plessis 201814 | 2013–2015 | Cross-sectional | South Africa | 300 | Pertussis | P | HI | 6 |
| Gill 201677 | 2015 | Cohort | Zambia | 347 | Pertussis | I | HI | 7 |
| Soofie 201678 | 2015 | Cross-sectional | South Africa | 599 | Pertussis | C, I, P | HE | 5 |
| Johnson 200079 | 1996–1997 | Cross-sectional | South Africa | 31 | Rotavirus gastroenteritis | P | HI | 6 |
| Moyo 201480 | 2010–2011 | Case-control | Tanzania | 26 | Rotavirus gastroenteritis | P | HI | 5 |
| Asbjörnsdóttir 201381 | 1999–2002 | Cohort | Kenya | 388 | Pneumococcal infection | C,I | HI | 6 |
| Nathoo 199682 | 1993–1994 | Cohort | Zimbabwe | 168 | Pneumococcal infection | P | HI | 7 |
| Zar 200183 | 1998 | Cross-sectional | South Africa | 151 | Pneumococcal infection | P | HI | 6 |
| Jones 199884 | 1996 | Cross-sectional | South Africa | 25 | Pneumococcal infection | C | HI | 5 |
| Roca 201085 | 2004–2006 | Cross-sectional | Mozambique | 54 | Pneumococcal infection | C | HI | 6 |
| Cohen 201686 | 2009–2013 | Cross-sectional | South Africa | 211 | Pneumococcal infection | I | HEU,HI | 4 |
| Nunes 201187 | 2003–2008 | Cross-sectional | South Africa | 938 | Pneumococcal infection | I | HI | 6 |
| von Mollendorf 2017a88 | 2009–2013 | Cross-sectional | South Africa | 495 | Pneumococcal infection | C, I | HI | 5 |
| von Gottberg 201389 | 2003–2008 | Cross-sectional | South Africa | 1749 | Pneumococcal infection | I | HI | 5 |
| Nyasulu 201190 | 2003–2005 | Cross-sectional | South Africa | 1124 | Pneumococcal infection | C | HI | 6 |
NR- Not recorded; C- Case-fatality rate; I – Incidence; P – Prevalence; Hib- Haemophilus influenzae type b; HI- HIV-infected; HE- HIV-exposed; HEU – HIV-exposed uninfected; VPD – vaccine–preventable diseases.
South Africa had the highest number of published articles with 35 articles, Nigeria produced 10 articles, four were from Kenya, four from Ethiopia and two studies were conducted in multiple countries. The other studies were conducted in Rwanda, Tanzania, Cote d‘ Ivoire, Uganda, Malawi, Botswana, Zimbabwe, Zambia, Mozambique and Swaziland (Table 1). A total of 46,882 children were included in this review. HIV-infected children were included in 71 studies while two studies had both HIV-infected and HIV-exposed uninfected children, and one study with only HIV-exposed children. The included studies were conducted between 1992 and 2016.
Using the Newcastle-Ottawa Quality Scale for the quality assessment of the eligible studies, 11 articles scored eight points; 15 articles scored seven points; 27 articles scored six points; 15 articles scored five points; seven articles scored four points and two articles scored three points. The characteristics of the eligible studies are summarised in Table 1.
Incidence rates
Tuberculosis
Nine studies15,17–20,22,32,35 on TB were pooled to give an overall incidence rate estimate of 60 (95% CI 30–70) per 1,000 child-years at risk for tuberculosis based on a random-effects model (I2= 99%; Figure 2). Subgroup analysis established change over time in incidence rates when comparing studies conducted before and after 2011. The pooled incidence rates for tuberculosis in those conducted before 2010 was 70 (95% CI −20–160) per 1,000 child-years32,35 and 40 (95% CI 20–50) per 1,000 child-years in studies conducted between 2011 and 2018.15,17–20,22 The heterogeneity of the TB incidence could not be explained by the subgroup analysis. Kouakoussui et al. reported TB incidence of 0.71 per 100 child/months before initiation of highly active antiretroviral therapy (HAART) and 0.16 per 100 child/months during HAART treatment among Ivorian HIV-infected children.60
Figure 2.
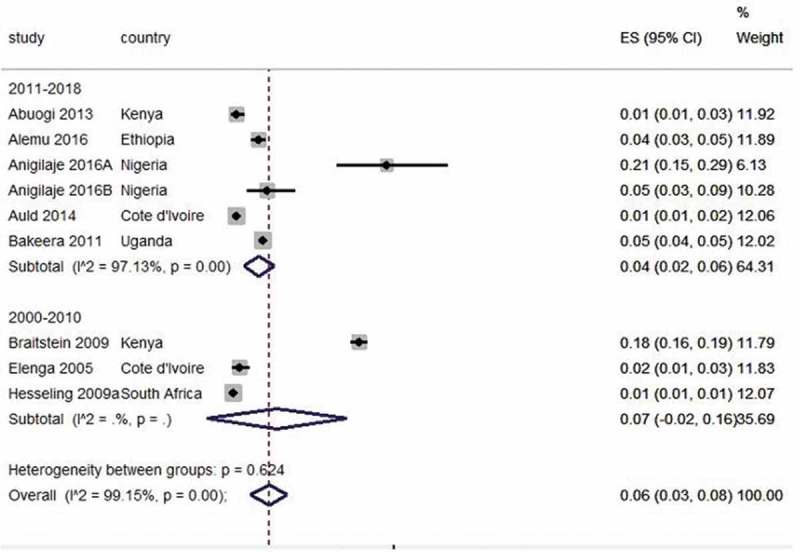
Forest plot of studies with data on incidence rates of tuberculosis in HIV-exposed children.
Pneumococcal infections
Incidence of invasive pneumococcal disease among HIV-infected children aged <1 and 1–4 years was 1022 (95% CI 923–1123) per 100,000 and 198 (95% CI 178–220) per 100,000 respectively in 2008.89 The incidence of pneumococcus-associated lower respiratory tract infection among HIV-exposed uninfected children was 109 (95% CI 47–214) per 100,000 and 629 (95% CI 130–1838) per 100,000 among HIV-infected children.86 Ásbjörnsdóttir et al. reported the incidence of pneumonia among Kenyan HIV-exposed uninfected infants to be 900 (95% CI 800–1000) per 1,000 child-years.81 Nunes et al. reported the incidence of invasive pneumococcal disease to be 1509 (95% CI 1350–1680) per 100,000 during early (HAART) and 742 (95% CI 644–851) during established-HAART eras for less than 18-year old South Africans.87
Pertussis
The incidence of pertussis among Zambian HIV-exposed infants was reported to be 3.7 (95% CI 0.9–10.1) per 1000 person-months75 while Soofie et al. reported the incidence to be 2.9 (95% CI 1.8–4.5) per 1,000 child-years.78
Prevalence
Twenty-one TB prevalence studies were pooled together and reported estimated prevalence of 16% (95% CI 12–19, I2 = 99%). For studies conducted within the period 1991–2000, the prevalence was 13% (95% CI 8–18);40,43 lower in 2001–2010 with an estimate of 8% (95% CI 5–11, I2= 96%)22,33,37,38,51 and recorded the highest prevalence in recent years with 15% (95% CI 8–22, I2= 99)15,16,18,19,21,23,27,29,31,41,45,46,52,57 (Figure 3). Fourteen prevalence studies on hepatitis B (HBV) infection in HIV-infected children were pooled together with an estimate prevalence of 5% (95% CI 4–7, I2= 90%). Studies conducted between 2001 and 2010 had a prevalence of 3% (95% CI 2–5)67,68 and 4% (95% CI 3–6) between 2011 and 201861,63–72,74 (Figure 4).
Figure 3.
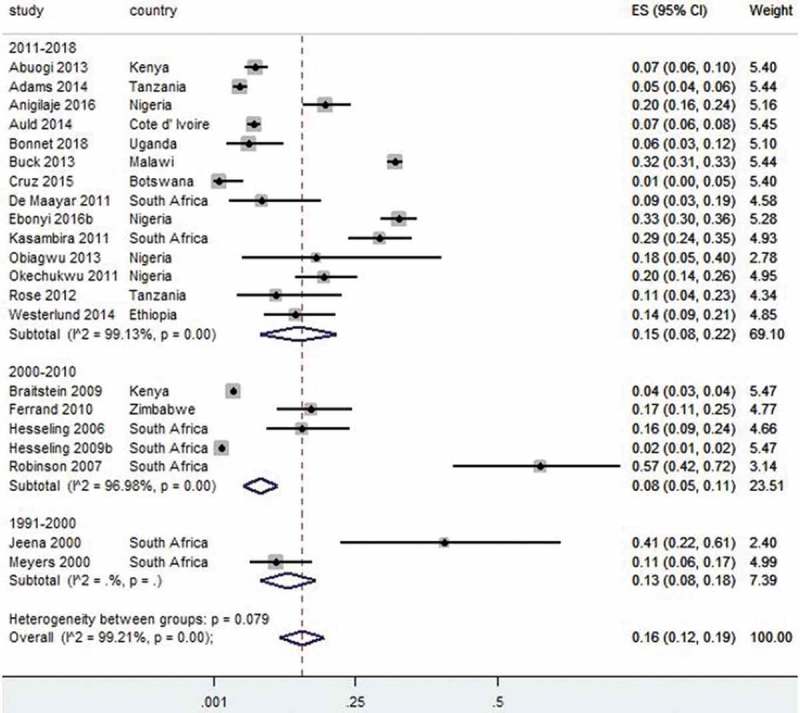
Forest plot of studies with data on the prevalence of tuberculosis in HIV-infected children.
Figure 4.
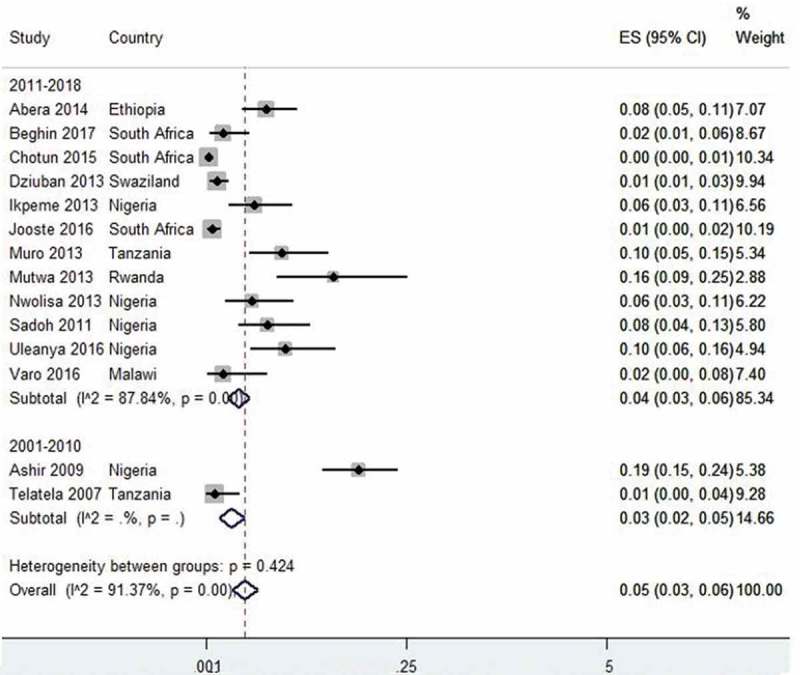
Forest plot of studies with data on the prevalence of hepatitis B virus infection in HIV-infected and HIV-exposed children.
The pooled prevalence for pneumococcal infections was 2% (95% CI 1–4). There has been a reduction in prevalence from 9% (95% CI 5–14)83 in 1996 to 1% (95% CI 0–5)84 in 2001. Pooled prevalence for pertussis was 3% (95% CI 2–4)14,78 while measles was 6% (95% CI 2–10).75,76 Two rotavirus diarrhoea prevalence studies were pooled together and reported an estimated prevalence of 13% (95% CI 8–17, I2= 0%).79,80
Trend in incidence and prevalence
We analysed the trend in TB incidence with respect to publication years. The trend was non-linear with a downtrend from 2000 to 2010 (at −12.5% per year) and a reduced downward trend from 2011 to 2018 (at −1.5 per year) as shown in Figure 5. The trend in HBV prevalence was also analysed. The trend was not linear. There was evidence of a downtrend from 2000 to 2010 (at −4.7% per year) and (at −5.3% per year) from 2011 to 2018 as shown in Figure 6. The TB prevalence trend was also non-linear. There was evidence of initial downtrend from 2000 to 2010 (at −3.2% per year) and upward trend from 2011 to 2018 (at +32.7 per year).
Figure 5.
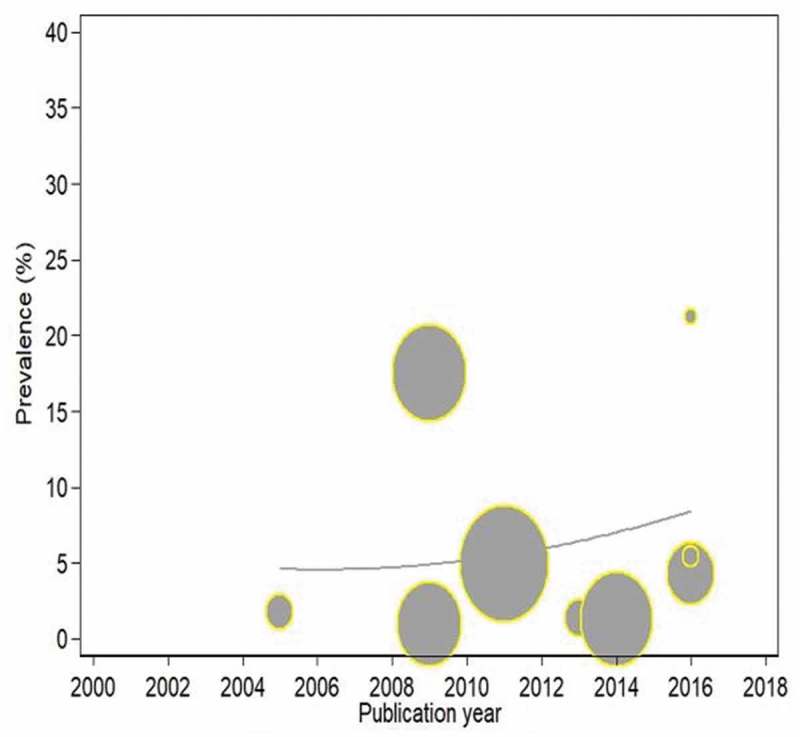
Trends in the incidence of tuberculosis in HIV-infected and exposed children with respect to publication years.
Figure 6.
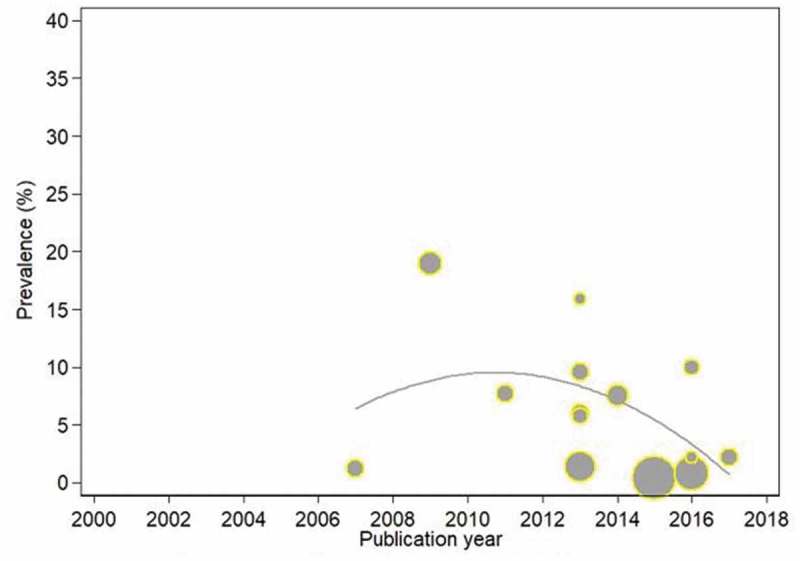
Trends in the prevalence of hepatitis B virus infection in HIV-infected and exposed children with respect to publication years.
Case-fatality rates
Twenty-nine studies on TB were pooled to give an overall CFR estimate of 17% (95% CI: 13–20, I2 = 95%) which translates to 17% of all TB cases dying from the disease. Subgroup analysis shows the CFR was 18% (95% CI 6–24)47 in the 1991–2000 period, 6% (17–38, I2 = 95%)33,35–37,48,49,53,54,56,59 in 2001–2010 and 13% (95% CI 9–17, I2 = 96%)15,16,18,20,23–25,30,34,39,44,46,47,50,55,56 in 2011–2018. Four studies were pooled for pneumococcal infections CFRs in HIV-infected and exposed children with a resultant rate of 15% (95% CI 4–26, I2= 95%).81,84,85,90 One study shows that pertussis has CFRs of 13% (95% CI 2–38)78 and for measles the CFR was 1% (95% CI 0–4).76
Publication bias assessment
Funnel-plot analyses of studies reporting on the prevalence of TB revealed nil significant publication bias, with the P value for the Begg’s test being 0.185 while the studies assessing the prevalence of HBV infection showed significant Begg’s test with P value of 0.001 (Figures 7 and 8). Likewise, studies assessing the CFR of TB demonstrated no significant publication bias Begg’s test P = 0.385 (Figure 9).
Figure 7.
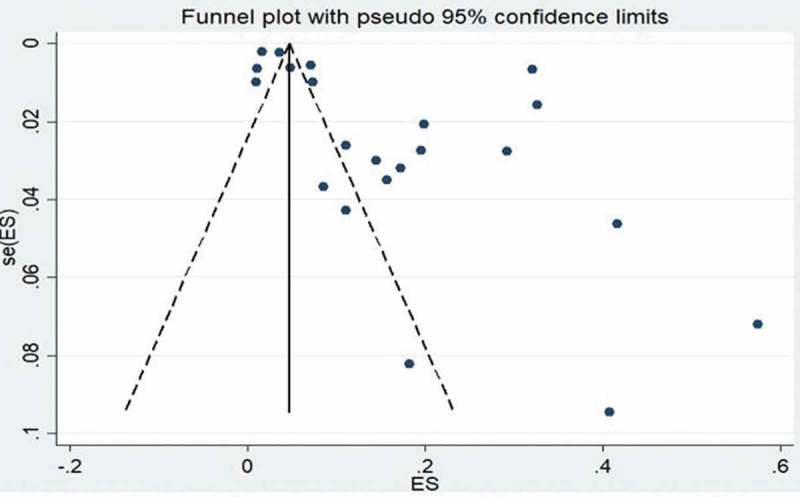
Funnel plot of studies reporting on the prevalence of tuberculosis in HIV-infected children.
Figure 8.
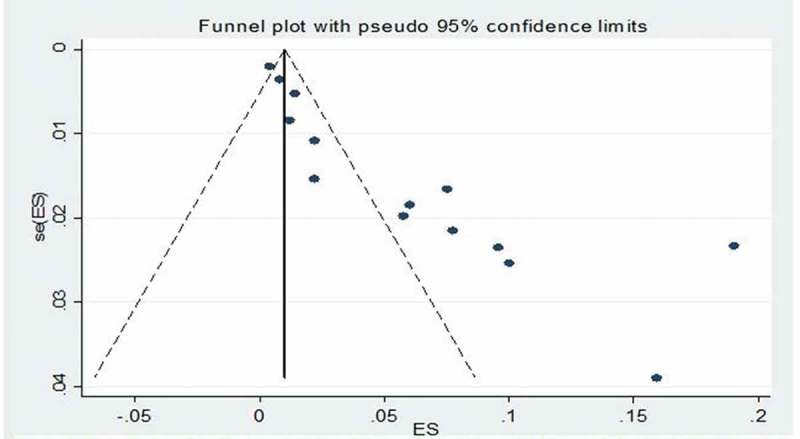
Funnel plot of studies reporting on the prevalence of hepatitis B virus infection in HIV-infected children.
Figure 9.
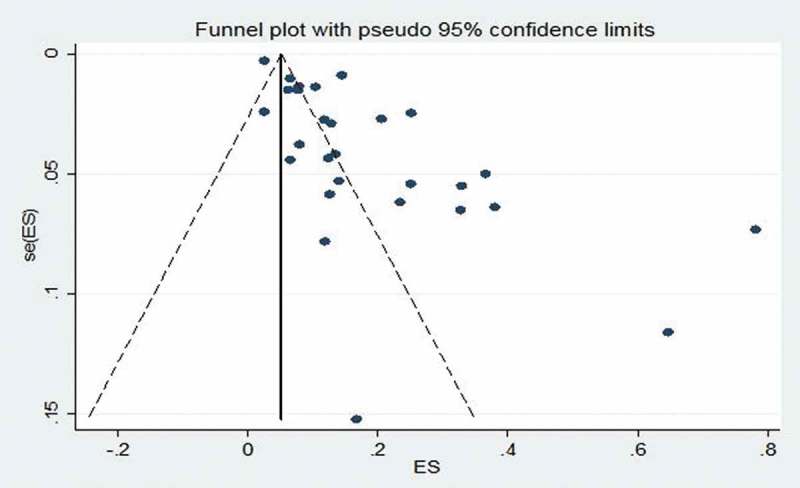
Funnel plot of studies reporting on the case-fatality rate of tuberculosis in HIV-infected children.
Discussion
This study provides a comprehensive overview of the incidence rate, prevalence and case fatality rates of different vaccine-preventable diseases in HIV-infected and HIV-exposed children in sub-Saharan African countries. The review shows that TB is the most researched vaccine-preventable disease in HIV-infected children in various African countries and settings. This is not surprising because of the relationship between TB and HIV infection with respect to the high susceptibility of TB in HIV-infected individuals,11,91 Other vaccine-preventable diseases like HBV infection, pneumococcal infection, measles, rotavirus gastroenteritis, pertussis and Hib infections were also studied in several African countries. Important vaccine-preventable diseases such as poliomyelitis, diphtheria, tetanus and yellow fever had no eligible studies for inclusion revealing the dearth of incidence and prevalence studies on these diseases. The pooled incidence, prevalence and CFRs reveal there are still high burdens of several vaccine-preventable diseases in sub-Saharan Africa.
According to WHO, the global incidence of TB has been reducing at an average of 2 percent per year.11 TB incidence has declined in the African region by 4 percent annually since 2013.11 Southern African countries with the highest prevalence and incidence of HIV such as South Africa, Lesotho, Zimbabwe, Eswatini, Namibia and Zambia had remarkable reductions in TB incidence.11 Our study shows that TB incidence reduced over time, however, the event per child-year is still high when compared with the End TB strategy goals.92 The World Health Assembly adopted the resolution known as “End TB strategy goals” which is about the global strategy and targets for tuberculosis prevention, care and control after 2015.92 In spite of the reduction in TB incidence among children, there are still cases of high incidence in certain countries bearing in mind that many countries in African countries are classified as high-burden.93 A retrospective cohort study in a very high TB/HIV prevalent region in Nigeria showed a high incidence rate of 21.2/100 per year among children within six months of ART enrollment at a period when others were recording much lower incidence.18 TB prevalence has fluctuated over time with about 15% of HIV-infected children having the disease at a given point in time. As of 2017, it was estimated that the global CFR was 16% with many African countries recording more than 20%.11 This rate is also far higher than the End TB Strategy milestone of 10% by 2020.
The pooled HBV infection prevalence among HIV-infected children was 5%, however, a study done in Rwanda in 2010 revealed a seroprevalence of 16%.68 Ott et al. showed that sub-Saharan Africa had the highest HBV burden with West African countries having up to 12% hepatitis B surface antigen prevalence among children and adolescents in the 1990s.94 There has been a reduction within the region largely due to immunisation programmes, however, there is high endemicity in some areas. A systematic review of HBV prevalence in Nigeria from studies conducted from 2000 to 2013 shows that HBV infection ranged from 0.5% to 46.8% with the pooled prevalence estimate for Nigeria being 13.6%.95
Our finding shows that the seroprevalence of rotavirus gastroenteritis among African HIV-infected children was 14% although with a small number of included studies. The incidence and CFR of diarrhoea and pneumonia are much higher in low-income than in high-income countries and this is reflected in many African and southeast Asian countries having the highest burden of the diseases.93 The African region has the highest incidence and total death secondary to diarrhoea and pneumonia with rotavirus and Streptococcus pneumoniae being the commonest culprits.93 Studies have shown that there is still a persistently high incidence of some vaccine-preventable diseases in HIV-infected individuals than non-exposed ones even after the introduction of highly active antiretroviral therapy.96 The incidence of pertussis is also higher in HIV-exposed and infected children, however, this decreases as the number of vaccine doses uptake increases.97
Many African countries with high burdens of HIV are critically lagging in terms of antiretroviral treatment coverage for HIV-infected children.98 Sub-optimal ART coverage in children will lead to viral load increase, immunosuppression, etc. and a subsequent high burden of various vaccine-preventable diseases. Vaccination coverage in many African countries is still below the expected target.99 As of 2017, the average coverage of third-dose pentavalent vaccine was 80% while the first dose of measles vaccine in the Global Alliance for Vaccines and Immunisation (Gavi)-supported countries was 78%.99 The average coverage of Gavi-funded vaccines in supported countries progressed from 37% in 2016 to 41% in 2017.
Use of vaccines has been established to be a beneficial healthcare intervention targeted in protecting children and adolescents from various vaccine-preventable diseases. Low uptake of vaccines by African children exposes them to more diseases than children in other regions. It has also been established that HIV-infected children are more susceptible to vaccine-preventable diseases such as TB, pneumonia, viral hepatitis etc.100,101 Vaccination is therefore essential in HIV-infected patients because of the increased risk of developing various infectious diseases due to their defective immune systems. Studies have also shown that there are poor immune responses to primary vaccination among HIV-infected children in comparison to HIV-unexposed and HIV-exposed children. The poor immune response among HIV-infected children may require booster doses for optimal immunity against vaccine-preventable diseases.102,103
This review revealed research inequalities across the African region regarding studies on the burden of vaccine-preventable diseases among HIV-infected and HIV-exposed children. South Africa contributed about half of the included articles with Nigeria and Kenya following with fewer studies. This finding is not different from an earlier study looking at the distribution of epidemiological studies across Africa.104 Some of the Eastern and Southern African countries with high HIV prevalence had at least an article included in this study, however, West African countries only had publications from Nigeria and Cote d’Ivoire.
To the best of our knowledge, this is the first systematic review that addressed the need for knowing the burden of vaccine-preventable diseases among HIV-infected and HIV-exposed children in sub-Saharan Africa. Knowledge gap concerning the burden of vaccine-preventable diseases will impact negatively on the advocacy endeavours targeted at improving vaccination and vaccine-preventable diseases control efforts in Africa. Healthcare workers and policymakers need to have a good idea of the burden of different diseases to allocate resources and facilitate optimal vaccination coverage.
Recommendations
This review has shown that TB is one of the most important vaccine-preventable diseases in Africa with the BCG vaccine conferring protection against severe forms of the disease. However, the same vaccine is contraindicated in immunocompromised children who ironically are susceptible to the disease.13 The dilemma of BCG use in HIV-exposed children warrants the call for newer and safer vaccines against TB especially in HIV-infected children. African governments and other supporting agencies should ensure that every child has access to routine childhood vaccines. Issues of under-vaccination and vaccine hesitancy should be adequately tackled to ensure better vaccine uptake and reduction in the burden of vaccine-preventable diseases.
The research capacity of African clinicians, researchers and health administrators should be built up for them to conduct basic epidemiological research such as incidence, prevalence, mortality and CFR among HIV-exposed children in various health facilities and communities. Researchers should be encouraged to disseminate their findings to their immediate communities and Departments of Health, and to publish their findings in peer-reviewed journals. Established research groups such as Global Burden of Diseases Network should include the burden of vaccine-preventable diseases in HIV-exposed and non-exposed children as part of their regular or annual publications. Other African countries should emulate South Africa in increasing their research activities and outputs with respect to HIV-exposed children.
There is a need to advocate for an equitable share of healthcare budgeting and finance at every level of governance in African countries. This will help in ensuring that there is a fair share of resources for preventive and treatment services such as vaccination and antiretroviral therapy for HIV-exposed children. African countries should, as a matter of urgency, complete the introduction of newer and important vaccines such as rotavirus vaccine, Hib vaccine and pneumococcal vaccine. These should be included as part of their current national immunisation programme schedule.95 According to WHO, the global coverage for both pneumococcal vaccine and rotavirus vaccines were as little as 44 percent and 25 percent respectively.99 African countries should be supported in developing vaccine procurement budgets, procurement practices, and capacity development for vaccine planning and advocacy.105
Study limitations
This study was limited by several factors beyond the reviewers’ control. We planned to review all the vaccine-preventable diseases associated with vaccines included in the national immunisation programme schedule in sub-Saharan Africa, however, we could not find articles that met the eligibility criteria for some of the diseases. Secondly, there was high heterogeneity even with sub-group analysis between included studies, which implies the possibility of other contributory factors associated with the diseases. Some of the studies did not include relevant information such as antiretroviral coverage, CD4 count, viral load, vaccination status and other contributory factors. Thirdly, we could not include many studies because the diagnostic criteria for different vaccine-preventable diseases were not specified and clearly defined.
Furthermore, the presence of various limitations did not stop us from making some meaningful conclusions from this study. This review gives a clearer picture of the burden and trend of TB and able to have insights about the burden of other diseases as well despite having a small number of studies included in this review. African investigators should as a matter of priority have proper diagnostic criteria and documentation for diseases for all HIV-infected and HIV-exposed children treated at the health facilities across the region. Key parameters such as CD4 counts, vaccination status etc. should be included in future studies.
Conclusions
This systematic review and meta-analysis provide an all-inclusive analysis of the incidence rates, prevalence and CFR of various vaccine-preventable diseases. This study shows that some vaccine-preventable diseases still have high incidence, prevalence and CFRs in HIV-infected and HIV-exposed children. There was also the dearth of research activities on vaccine-preventable disease studies concerning HIV-infected and HIV-exposed uninfected children in many African countries. The findings are useful in advocating for a more equitable share of healthcare financing especially for preventive services such as vaccination of both HIV-exposed and non-exposed children to reduce the burden of vaccine-preventable diseases.
Methods and design
This systematic review was developed in line with the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) 2015 statement.106 The review was registered with PROSPERO (International prospective register of systematic reviews) (CRD42018095341).
Inclusion criteria
Type of participants
The review included sub-Saharan African children who are HIV-infected or HIV-exposed and aged <18 years old.
Types of outcome
We included studies that reported the incidence, prevalence and case-fatality rates (CFR) as outcomes in HIV-infected and HIV-exposed children.
Primary outcomes
Prevalence was defined as proportions of all individuals suspected of having specific vaccine-preventable diseases with confirmed laboratory diagnosis or proportions of individuals fulfilling clinical case definition for specific vaccine-preventable diseases. Incidence was defined as the number of new cases of different vaccine-preventable diseases that occur during a given period in the defined population.
We also determined the trend in the incidence and/or prevalence of vaccine-preventable diseases among HIV-infected and HIV-exposed children in sub-Saharan Africa from 1980 to 2018.
Secondary outcomes
We included CFRs associated with vaccine-preventable diseases. Case fatality was described as mortality among confirmed or probable cases for a specific vaccine-preventable disease.
Type of studies
The review included cohort studies, case-control studies, cross-sectional studies and other observational studies. We planned to include studies that involved any of the following vaccine-preventable diseases:
Tuberculosis
Poliomyelitis
Hepatitis B virus infection
Rotavirus gastroenteritis
Diphtheria
Tetanus
Pertussis
Pneumococcal diseases
Measles
Rubella
Yellow fever
Exclusion criteria
Intervention studies
Unclear diagnostic criteria
Search strategy methods for the identification of studies
A comprehensive search strategy was developed to identify relevant studies up to August 2018, regardless of publication status or language. Scopus, Web of Science, MEDLINE via PubMed and CINAHL were searched for relevant publications. The search process was complemented by reviewing citations of all identified eligible studies. We also searched relevant World Health Organization position papers and documents on vaccines. (See Appendix for PubMed search strategy).
Selection of eligible studies
Two of the authors, (OOA and AA) screened the search results using the abstract titles. They also independently went through the full text of potential studies to assess whether they met the required inclusion criteria. Non-human studies, reviews, intervention studies, letters, commentaries and editorials were excluded. Studies not written in English, French, German, Spanish, Portuguese or Dutch were excluded. We resolved disagreements by consensus.
Data collection process
The two authors then extracted data from text, tables and figures. The data were recorded on a standardised form. We planned to contact authors of included studies in case of unclear or missing data.
The following data were extracted from selected studies:
Study characteristics including period and design.
Vaccine-preventable diseases patient characteristics such as age and HIV status.
Prevalence or incidence of vaccine-preventable diseases: confirmed cases and cases meeting the clinical definition.
Diagnostic methods: laboratory methods and clinical case definitions.
Death attributed to vaccine-preventable diseases.
Risk of bias in individual studies
The risk of bias and quality of the included studies were assessed with the Newcastle-Ottawa Quality Scale.107 The criteria assessed included the following (1) selection of participants, (2) comparability, (3) exposure, and (4) outcome.
Data synthesis
OOA summarised the incidence and prevalence of various vaccine-preventable diseases. Where possible, incidence and prevalence data from each of the included studies were combined by random effects meta-analysis in accordance with the Mantel-Haenszel method.
Heterogeneity was evaluated using the Chi-squared test of homogeneity (significant for P < 0.1) and quantified using the I-squared statistic (>50% substantial heterogeneity).108 Subgroup analyses were conducted in cases with substantial heterogeneity. Subgroup analysis was conducted using the following variables: period of study (1991–2000, 2001–2010 and 2011–2018). We also used funnel plot regression to assess publication bias. STATA software version 14.0 (STATA Corporation, College Station, TX, USA) was used to do all calculations, the meta-analysis and generate forest plots.109
Additional analyses: trend analysis
We examined time trends in the incidence and prevalence of vaccine-preventable diseases estimates using Poisson regression models with the prevalence estimates as the outcome variable and the calendar year of the publication as the predictor. This method allows for estimation of time trends across individual calendar years to obtain average annual percentage change (AAPC), if the rate of change is at a constant rate of the previous year.110 The Poisson regression procedure fits a model of the following form:
| (1) |
where ‘cases’ equal prevalence estimates reported per year, log is the natural log, b0 is the intercept, b1 is the trend, y is the year – given as 0, 1, 2, … 18 (year 0 is 1970, year 1 is 1971, and so on to 2014), and log of ‘sample size’ was entered as the offset. The AAPC was calculated using the following formula:
| (2) |
Appendix
Search strategy – PubMed
| Search | Add to builder | Query | Items found |
|---|---|---|---|
| #6 | Add | Search ((((#1) AND #2) AND #3) AND #4) AND #5 Sort by: Best Match | 1364 |
| #4 | Add | Search (newborn* OR bab* OR infan* OR child* OR adolescen* OR teen*) Sort by: Best Match | 3843580 |
| #2 | Add | Search (tuberculosis OR TB OR poliomyelitis OR polio OR rotavirus OR diphtheria OR tetanus OR pertussis OR pneumococcal OR pneumonia OR measles OR “yellow fever” OR “Hepatitis B” OR “Haemophilus influenza” OR “Hemophilus influenza” OR influenza) Sort by: Best Match | 707401 |
| #5 | Add | Search (incidence OR prevalence OR mortality) Sort by: Best Match | 3171459 |
| #3 | Add | Search (“HIV infected” OR “HIV exposed” OR “HIV-infected” OR “HIV-exposed” OR “HIV positive” OR “HIV exposed uninfected”) Sort by: Best Match | 74539 |
| #1 | Add | Search (Angola OR Benin OR Botswana OR “Burkina Faso” OR Burundi OR Cameroon OR “Cape Verde” OR “Central African Republic” OR Chad OR Congo OR “Democratic Republic of Congo” OR DRC OR Djibouti OR “Equatorial Guinea” OR Eritrea OR Ethiopia OR Gabon OR Gambia OR Ghana OR “Guinea Bissau” OR Guinea OR “Ivory Coast” OR “Cote d’Ivoire” OR Kenya OR Lesotho OR Liberia OR Madagascar OR Malawi OR Mali OR Mauritania OR Mauritius OR Mozambique OR Namibia OR Niger OR Nigeria OR “Republic of the Congo” OR Reunion OR Rwanda OR Senegal OR Seychelles OR “Sierra Leone” OR “Sao Tome and Principe” OR Somalia OR “South Africa” OR “South Sudan” OR Sudan OR Swaziland OR Tanzania OR Togo OR Tunisia OR Uganda OR Zambia OR Zimbabwe OR Africa OR “sub Saharan Africa”) Sort by: Best Match | 558013 |
Funding Statement
National Research Foundation of South Africa. 106035; South African Medical Research Council. 10.13039/501100001322.
Authors’ contributions
OOA developed the protocol, search strategy, the data analysis and manuscript preparation. OOA and AA did the screening, study selection and data extraction. OAU and CSW guided the development of this study. All authors were involved in the results interpretation, revision and approval of the final manuscript.
Source of funding
OOA, DN and CSW are supported by the National Research Foundation of South Africa (Grant numbers: 106035 and 108571) and the South African Medical Research Council. OAU is supported by the National Institute of Health Research using Official Development Assistance funding. The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, National Institute for Health.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Abbreviations
- BCG
Bacillus Calmette–Guérin
- DTP
Diphtheria, tetanus and pertussis
- EPI
Expanded Programme on Immunisation
- GVAP
Global Vaccine Action Plan
- Hib
Haemophilus influenzae type b
- HIV
Human immunodeficiency virus
- PCV
Pneumococcal conjugate vaccine
- PRISMA
Preferred Reporting Items for Systematic Review and Meta-Analysis
- RV
Rotavirus
- WHO
World Health Organization
References
- 1.Naghavi M, Abajobir AA, Abbafati C, Abbas KM, Abd-Allah F, Abera SF, Aboyans V, Adetokunboh O, Afshin A, et al. Global, regional, and national age-sex-specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet. 2017;390:1151–210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joint United Nations Programme on HIV/AIDS How AIDS changed everything. MDG 6. Geneva, Switzerland: UNAIDS; 2015. [Google Scholar]
- 3.Joint United Nations Programme on HIV/AIDS AIDSInfo. 2017. [accessed 2018 June26]. http://aidsinfo.unaids.org/.
- 4.United Nations Children's Fund (UNICEF) The State of the World's Children 2016: A Fair Chance for Every Child. New York, USA. UNICEF; 2016.
- 5.Tejiokem MC, Gouandjika I, Béniguel L, Zanga M-CE, Tene G, Gody JC, Njamkepo E, Kfutwah A, Penda I, Bilong C, et al. HIV-infected children living in Central Africa have low persistence of antibodies to vaccines used in the expanded program on immunization. PLoS One. 2007;2:e1260. doi: 10.1371/journal.pone.0001260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller MA, Hinman AR. Chapter 72. Economic analyses of vaccine policies. In: Miller MA, Hinman AR, Plotkin SA, Orenstein WA, editors. Vaccines, 1413–1426. Philadelphia, USA: Elsevier Inc; 2004. 10.1016/b978-1-4557-0090-5.00072-0 [DOI] [Google Scholar]
- 7.Hadler SC, Cochi SL, Bilous J, Cutts FT Chapter 55. Vaccination programs in developing countries. In: Plotkin SA, Orenstein WA, editors. Vaccines, 1407–1442. Philadelphia, USA: Saunders. 2003.
- 8.Tao W, Petzold M, Forsberg BC. Routine vaccination coverage in low- and middle-income countries: further arguments for accelerating support to child vaccination services. Glob Health Action. 2013;6:0–8. doi: 10.3402/gha.v6i0.20343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tchidjou HK, Vescio MF, Sanou Sobze M, Souleyman A, Stefanelli P, Mbabia A, Moussa I, Gentile B, Colizzi V, Rezza G. Low vaccine coverage among children born to HIV infected women in Niamey, Niger. Human Vacc Immunoth. 2016;12:540–44. doi: 10.1080/21645515.2015.1069451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization Global Vaccine Action Plan 2011-2020. Geneva, Switzerland: WHO; 2013. [Google Scholar]
- 11.World Health Organization 2015 Assessment report of the global vaccine action plan. Geneva, Switzerland: WHO; 2016. [Google Scholar]
- 12.World Health Organization Estimates of disease burden and cost effectiveness. Geneva, Switzerland: WHO; 2017. [Google Scholar]
- 13.World Health Organization Summary of WHO position papers-recommendations for routine immunization. 2018. [accessed 2018 June26]. http://www.who.int/immunization/policy/Immunization_routine_table1.pdf?ua=1.
- 14.Du Plessis NM, Ntshoe G, Reubenson G, Kularatne R, Blumberg L, Thomas J, Avenant T. Risk factors for pertussis among hospitalized children in a high HIV prevalence setting, South Africa. Int J Infect Dis. 2018;68:54–60. doi: 10.1016/j.ijid.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Abuogi LL, Mwachari C, Leslie HH, Shade SB, Otieno J, Yienya N, Sanguli L, Amukoye E, Cohen CR. Impact of expanded antiretroviral use on incidence and prevalence of tuberculosis in children with HIV in Kenya. Int J Tuberc Lung Dis. 2017;17:1291–97. doi: 10.5588/ijtld.12.0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.L V A, Iii TM, Naburi H, Lyatuu G, Ippolito M, Saunders A, Kiravu A, Palumbo P. Diagnosis and treatment of tuberculosis among children at an HIV care program in Dar es Salaam, Tanzania. Pediatr Inf Dis J. 2015;33:1234–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alemu YM, Andargie G, Gebeye E, Paraskevis D. High incidence of tuberculosis in the absence of isoniazid and cotrimoxazole preventive therapy in children living with HIV in Northern Ethiopia: a retrospective follow-up study. PLoS One. 2016;11:e0152941. doi: 10.1371/journal.pone.0152941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anígilájé EA, Aderibigbe SA, Adeoti AO, Nweke NO, Adam RD. Tuberculosis before and after antiretroviral therapy among HIV-Infected children in Nigeria: what are the risk factors? PLoS One. 2016;11:e0156177. doi: 10.1371/journal.pone.0156177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Auld AF, Tuho MZ, Ekra KA, Kouakou J, Shiraishi RW. Tuberculosis in human immunodeficiency virus-infected d’ Ivoire children starting antiretroviral therapy in Cote D’Ivoire. Int J Tuberc Lung Dis. 2014;18:381–87. doi: 10.5588/ijtld.13.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bakeera-Kitaka S, Conesa-Botella A, Dhabangi A, Maganda A, Kekitiinwa A, Colebunders R. Tuberculosis in HIV-infected Ugandan children starting on antiretroviral therapy. Int J Tuberc Lung Dis. 2012;15:1082–86. doi: 10.5588/ijtld.10.0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonnet M, Kyomugasho N, Kiwanuka J, Kumbakumba E. Outcome of children with presumptive tuberculosis in Mbarara, Rural Uganda. Pediatr Infect Dis J. 2018;37:147–52. doi: 10.1097/INF.0000000000001727. [DOI] [PubMed] [Google Scholar]
- 22.Braitstein P, Wools-Kaloustian K, Sidle J, Ayaya S, Carter EJ. The clinical burden of tuberculosis among Human Immunodeficiency Virus-infected children in Western Kenya. Pediatr Infect Dis J. 2009;28:626–32. doi: 10.1097/INF.0b013e31819665c5. [DOI] [PubMed] [Google Scholar]
- 23.Buck WC, Olson D, Kabue MM, Ahmed S, Nchama LK, Munthali A, Kazembe PN. Risk factors for mortality in Malawian children with human immunodeficiency virus and tuberculosis co-infection. Int J Tuberc Lung Dis. 2017;17:1389–95. doi: 10.5588/ijtld.13.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carlucci JG, Peratikos MB, Kipp AM, Lindegren ML, Du QT, Renner L, Reubenson G, Ssali J, Yotebieng M, Mandalakas AM, et al. Tuberculosis treatment outcomes among HIV/TB-coinfected children in the International Epidemiology Databases to Evaluate AIDS (IeDEA) Network. JAIDS J Acquir Immune Defic Syndr. 2017;75:156–63. doi: 10.1097/QAI.0000000000001335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cavanaugh J, Genga K, Marigu I, Laserson K, Ackers M, Cain K. Tuberculosis among children in Kenya: epidemiology and impact of HIV in two Provinces. J Trop Pediatr. 2012;58:292–96. doi: 10.1093/tropej/fmr098. [DOI] [PubMed] [Google Scholar]
- 26.Chaya S, Dangor Z, Solomon F, Nzenze SA, Izu A, Madhi SA. Incidence of tuberculosis meningitis in a high HIV prevalence setting: time-series analysis from 2006 to 2011. Int J Tuberc Lung Dis. 2016;20:1457–62. doi: 10.5588/ijtld.15.0845. [DOI] [PubMed] [Google Scholar]
- 27.Cruz AT, Marape M, Graviss EA, Starke JR. Performance of the QuantiFERON-TB Gold Interferon Gamma Release Assay among HIV-infected children in Botswana. J Int Assoc Provid AIDS Care. 2015;14:4–7. doi: 10.1177/2325957414547432. [DOI] [PubMed] [Google Scholar]
- 28.Dangor Z, Izu A, Hillier K, Solomon F, Beylis N, Moore DP, Nunes MC, Madhi SA. Impact of the antiretroviral treatment program on the burden of hospitalization for culture-confirmed tuberculosis in South African children. Pediatr Infect Dis J. 2013;32:972–77. doi: 10.1097/INF.0b013e31828d9aa4. [DOI] [PubMed] [Google Scholar]
- 29.De MT, Saloojee H. Clinical outcomes of severe malnutrition in a high tuberculosis and HIV setting. Arch Dis Child. 2011;96:560–64. doi: 10.1136/adc.2010.205039. [DOI] [PubMed] [Google Scholar]
- 30.Ebonyi AO, Oguche S, Agbaji OO, Sagay AS, Okonkwo PI, Idoko JA, Kanki PJ. Mortality among pulmonary tuberculosis and HIV-1 co-infected Nigerian children being treated for pulmonary tuberculosis and on antiretroviral therapy: a retrospective cohort study. GERMS. 2016;6:139–50. doi: 10.11599/issn.2248-2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ebonyi AO, Oguche S, Ejeliogu EU, Agbaji OO, Shehu NY, Abah IO. Prevalence of and risk factors for pulmonary tuberculosis among newly diagnosed HIV-1 infected Nigerian children. GERMS. 2016;6:21–28. doi: 10.11599/germs.2016.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elenga N, Kouakoussui KA, Bonard D, Fassinou P, Wemin M, Dick-Amon-Tanoh F, Msellati P. Diagnosed tuberculosis during the follow-up of a cohort of Human Immunodeficiency Virus-infected children in Abidjan, Cote d’Ivoire. Pediatr Infect Dis J. 2005;24:1077–82. doi: 10.1097/01.inf.0000190008.91534.b7. [DOI] [PubMed] [Google Scholar]
- 33.Ferrand RA, Bandason T, Musvaire P, Larke N, Nathoo K, Mujuru H, Ndhlovu CE, Munyati S, Cowan FM, Gibb DM, et al. Causes of acute hospitalization in adolescence: burden and spectrum of HIV-related morbidity in a country with an early-onset and severe HIV epidemic: a prospective survey. PLoS Med. 2010;7:e1000178. doi: 10.1371/journal.pmed.1000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hall EW, Morris SB, Moore BK, Erasmus L. Treatment outcomes of children with HIV infection and drug-resistant TB in three provinces. Pediatr Infect Dis J. 2017;36:2005–08. doi: 10.1097/INF.0000000000001691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hesseling AC, Johnson LF, Jaspan H, Cotton MF, Whitelaw A, Schaaf HS, Fine PEM, Eley BS, Marais BJ. Disseminated bacille Calmette – guérin disease in HIV-infected South African infants. Bull World Health Organ. 2009;87:505–11. doi: 10.2471/BLT.08.055657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hesseling AC, Westra AE, Werschkull H, Donald PR, Beyers N, Hussey GD, El-Sadr W, Schaaf HS. Outcome of HIV infected children with culture confirmed tuberculosis. Arch Dis Child. 2005;90:1171–74. doi: 10.1136/adc.2004.070466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hesseling AC, Rabie H, Marais BJ, Manders M, Lips M, Schaaf HS, Gie RP, Cotton MF, van Helden PD, Warren RM, et al. Bacille Calmette-Guerin vaccine-induced disease in HIV-infected and HIV-uninfected children. Clin Inf Dis. 2006;42:548–58. doi: 10.1086/499953. [DOI] [PubMed] [Google Scholar]
- 38.Hesseling AC, Cotton MF, Jennings T, Whitelaw A, Johnson LF, Eley B, Roux P, Schaaf HS. High incidence of tuberculosis among HIV-infected infants: evidence from a South African population- based study highlights the need for improved tuberculosis control strategies. Clin Infect Dis. 2009;48:108–14. doi: 10.1086/595012. [DOI] [PubMed] [Google Scholar]
- 39.Hicks RM, Padayatchi N, Shah NS, Wolf A, Werner L, Sunkari VB, O’Donnell MR. Malnutrition associated with unfavorable outcome and death among South African MDR-TB and HIV co-infected children. Int J Tuberc Lung Dis. 2014;18:1074–83. doi: 10.5588/ijtld.14.0231. [DOI] [PubMed] [Google Scholar]
- 40.Jeena PM, Coovadia HM, Hadley LG, Wiersma R, Grant H, Chrystal V. Lymph node biopsies in HIV-infected and non-infected children with persistent lung disease. Int J Tuberc Lung Dis. 2000;4:139–46. [PubMed] [Google Scholar]
- 41.Kasambira TS, Shah M, Adrian PV, Holshouser M, Madhi SA, Chaisson RE, Martinson NA, Dorman SE. QuantiFERON-TB Gold In-Tube for the detection of Mycobacterium tuberculosis infection in children with household tuberculosis contact. Int J Tuberc Lung Dis. 2011;15:628–34. doi: 10.5588/ijtld.10.0555. [DOI] [PubMed] [Google Scholar]
- 42.Madhi SA, Huebner RE, Doedens L, Aduc T, Wesley D, Cooper PA. HIV-1 co-infection in children hospitalized with tuberculosis in South Africa. Int J Tuberc Lung Dis. 2000;4:448–54. [PubMed] [Google Scholar]
- 43.Meyers TM, Pettifor JM, Gray GE, Crewe-Brown H, Galpin JS. Pediatric admissions with human immunodeficiency virus infection at a regional hospital in Soweto, South Africa. J Trop Pediatr. 2000;46:224–30. doi: 10.1093/tropej/46.4.224. [DOI] [PubMed] [Google Scholar]
- 44.Mwangwa F, Chamie G, Kwarisiima D, Ayieko J, Owaraganise A, Ruel TD, Plenty A, Tram KH, Clark TD, Cohen CR, et al. Gaps in the child tuberculosis care cascade in 32 rural communities in Uganda and Kenya. J Clin Tuberc Mycobact Dis. 2017;9:24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Obiagwu PN, Hassan-Hanga F, Ibrahim M. Pediatric HIV in Kano, Nigeria. Niger J Clin Pract. 2013;16:521–25. doi: 10.4103/1119-3077.116905. [DOI] [PubMed] [Google Scholar]
- 46.Okechukwu AA. Discharge against medical advice in children at the University of Abuja Teaching Hospital, Gwagwalada, Nigeria. Niger J Clin Pract. 2011;2:49–54. [DOI] [PubMed] [Google Scholar]
- 47.Osman M, Lee K, Du Preez K, Dunbar R, Hesseling AC, Seddon JA. Excellent treatment outcomes in children treated for tuberculosis under routine operational conditions in Cape Town, South Africa. Clin Inf Dis. 2017;65:1444–52. doi: 10.1093/cid/cix602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Padayatchi N, Bamber S, Dawood H, Bobat R. Multidrug-resistant tuberculous meningitis in children in Durban, South Africa. Pediatr Inf Dis J. 2006;25:147–50. doi: 10.1097/01.inf.0000199314.88063.4c. [DOI] [PubMed] [Google Scholar]
- 49.Palme IB, Gudetta B, Bruchfeld J, Muhe L, Giesecke J. Impact of human immunodeficiency virus 1 infection on clinical presentation, treatment outcome and survival in a cohort of Ethiopian children with tuberculosis. Pediatr Inf Dis J. 2002;21:1053–61. doi: 10.1097/00006454-200211000-00016. [DOI] [PubMed] [Google Scholar]
- 50.Patel MR, Yotebieng M, Behets F, Vanden Driessche K, Nana M, Van Rie A. Outcomes of integrated treatment for tuberculosis and HIV in children at the primary health care level. Int J Tuberc Lung Dis. 2013;17:1206–11. doi: 10.5588/ijtld.12.0074-e. [DOI] [PubMed] [Google Scholar]
- 51.Robinson A, Donald PR, Schaaf HS. Nosocomial infections in HIV-infected and HIV-uninfected children hospitalised for tuberculosis. S Afr Fam Pract. 2007;49:14. [Google Scholar]
- 52.Rose MV, Kimaro G, Nissen TN, Kroidl I, Hoelscher M, Bygbjerg IC, Mfinanga SG, Ravn P. Quantiferon®-TB gold in-tube performance for diagnosing active tuberculosis in children and adults in a high burden setting. PLoS One. 2012;7:1–10. doi: 10.1371/journal.pone.0037851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simon HS, Marais BJ, Whitelaw A, Hesseling AC, Eley B, Hussey GD, Donald PR. Culture-confirmed childhood tuberculosis in Cape Town, South Africa: a review of 596 cases. BMC Inf Dis. 2007;7:2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Soeters M, de Vries AM, Kimpen JL, Donald PR, Schaaf HS. Clinical features and outcome in children admitted to a TB hospital in the Western Cape – the influence of HIV infection and drug resistance. SAMJ. 2005;95:602–06. [PubMed] [Google Scholar]
- 55.Walters E, Duvenhage J, Draper HR, Hesseling AC, Van Wyk SS, Cotton MF, Rabie H. Severe manifestations of extrapulmonary tuberculosis in HIV-infected children initiating antiretroviral therapy before 2 years of age. Archives Dis Childhood. 2014;99:998–1003. doi: 10.1136/archdischild-2013-305509. [DOI] [PubMed] [Google Scholar]
- 56.Walters E, Cotton MF, Rabie H, Schaaf HS, Walters LO, Marais BJ. Clinical presentation and outcome of tuberculosis in Human Immunodeficiency Virus infected children on anti-retroviral therapy. BMC Pediatr. 2008;8:1. doi: 10.1186/1471-2431-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Westerlund E, Jerene D, Mulissa Z, Hallstrã¶M I, Lindtjã¸Rn B. Pre-ART retention in care and prevalence of tuberculosis among HIV-infected children at a district hospital in southern Ethiopia. BMC Pediatr. 2014;14:1–9. doi: 10.1186/1471-2431-14-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wiseman CA, Schaaf HS, Cotton MF, Gie RP, Jennings T, Whitelaw A, Roux P, Hesseling AC. Bacteriologically confirmed tuberculosis in HIV-infected infants: disease spectrum and survival. Int J Tuberc Lung Dis. 2011;15:770–75. doi: 10.5588/ijtld.10.0501. [DOI] [PubMed] [Google Scholar]
- 59.Yotebieng M, Van Rie A, Moultrie H, Cole SR, Adimora A, Behets F, Meyers T. Effect on mortality and virological response of delaying antiretroviral therapy initiation in children receiving tuberculosis treatment. AIDS. 2010;24:1341–49. doi: 10.1097/QAD.0b013e328339e576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kouakoussui A, Fassinou P, Anaky MF, Elenga N, Laguide R, Wemin ML, Toure R, Menan H, Rouet F, Msellati P. Respiratory manifestations in HIV-infected children pre- and post-HAART in Abidjan, the Ivory Coast. Paediatr Respir Rev. 2004;5:311–15. doi: 10.1016/j.prrv.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 61.Abera B, Zenebe Y, Mulu W, Kibret M, Kahsu G. Seroprevalence of hepatitis B and C viruses and risk factors in HIV infected children at the Felgehiwot Referral Hospital, Ethiopia. BMC Res Notes. 2014;7:838. doi: 10.1186/1756-0500-7-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ashir GM, Rabasa AI, Gofama MM, Bukbuk D, Abubakar H, Farouk GA. Study of hepatic functions and prevalence of hepatitis B surface antigenaemia in Nigerian children with human immunodeficiency virus infection. Nigerian J Med. 2009;18:260–62. [DOI] [PubMed] [Google Scholar]
- 63.Beghin J-C, Ruelle J, Sokal E, Bachy A, Krishna M, Hall L, Goubau P, Van der Linden D. Effectiveness of the South African expanded program of immunization against hepatitis B in children infected with human immunodeficiency virus-1 living in a resource-limited setting of Kwazulu-Natal. J Med Virol. 2017;89:182–85. doi: 10.1002/jmv.v89.1. [DOI] [PubMed] [Google Scholar]
- 64.Chotun N, Nel E, Cotton MF, Preiser W, Andersson MI. Hepatitis B virus infection in HIV-exposed infants in the Western Cape, South Africa. Vaccine. 2015;33:4618–22. doi: 10.1016/j.vaccine.2015.06.076. [DOI] [PubMed] [Google Scholar]
- 65.Davidson UN, Chidiebele NI, Josephine EI, Olakunle E, Nnaemeka IA, Chijioke EJ, Kingsley NI. The prevalence of liver function and immunologic status of children with HIV and hepatitis B virus coinfection in Enugu, Nigeria. Afr J Infect Dis. 2016;10:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dziuban EJ, Marton SA, Hughey AB, Mbingo TL, Draper HR, Schutze GE. Seroprevalence of hepatitis B in a cohort of HIV-infected children and adults in Swaziland. Int J STDs AIDS. 2013;24:561–65. doi: 10.1177/0956462413476274. [DOI] [PubMed] [Google Scholar]
- 67.Ikpeme EE, Etukudo OM. Seroprevalence of HBV and HIV co-infection in children and outcomes following highly active antiretroviral therapy (HAART) in Uyo, South-South Nigeria. Afr Health Sci. 2013;13:955–61. doi: 10.4314/ahs.v13i4.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jooste P, Van Zyl A, Adland E, Daniels S, Hattingh L, Brits A, Wareing S, Goedhals D, Jeffery K, Andersson M, et al. Screening, characterisation and prevention of hepatitis B virus (HBV) co-infection in HIV-positive children in South Africa. J Clin Virol. 2016;85:71–74. doi: 10.1016/j.jcv.2016.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Muro FJ, Fiorillo SP, Sakasaka P, Odhiambo C, Reddy EA, Cunningham CK, Buchanan AM. Seroprevalence of hepatitis B and C viruses among children in Kilimanjaro Region, Tanzania. J Pediatric Inf Dis Soc. 2013;2:320–26. doi: 10.1093/jpids/pit018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mutwa PR, Boer KR, Rusine JB, Muganga N, Tuyishimire D, Reiss P, Lange JM, Geelen SPM. Hepatitis B virus prevalence and vaccine response in HIV-infected children and adolescents on combination antiretroviral therapy in Kigali, Rwanda. Pediatr Inf Dis J. 2013;32:246–51. [DOI] [PubMed] [Google Scholar]
- 71.Nwolisa E, Mbanefo F, Ezeogu J, Amadi P. Prevalence of hepatitis B co-infection amongst HIV infected children attending a care and treatment centre in Owerri, South-Eastern Nigeria. Pan Afr Med J. 2013;14:2–6. doi: 10.11604/pamj.2013.14.89.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sadoh AE, Sadoh WE. HIV co-infection with hepatitis B and C viruses among Nigerian children in an antiretroviral treatment programme. S Afr J Child Health. 2011;5:7–10. [Google Scholar]
- 73.Telatela SP, Matee MI, Munubhi EK. Seroprevalence of hepatitis B and C viral co-infections among children infected with human immunodeficiency virus attending the paediatric HIV care and treatment center at Muhimbili National Hospital in Dar-es-Salaam, Tanzania. BMC Pub Health. 2007;7:1–6. doi: 10.1186/1471-2458-7-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Varo R, Chris Buck W, Kazembe PN, Phiri S, Andrianarimanana D, Weigel R. Seroprevalence of CMV, HSV-2 and HBV among HIV-infected Malawian children: a cross-sectional survey. J Trop Pediatr. 2016;62:220–26. doi: 10.1093/tropej/fmv105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moss WJ, Monze M, Ryon JJ, Quinn TC, Griffin DE, Cutts F. Prospective study of measles in hospitalized, Human Immunodeficiency Virus (HIV)-infected and HIV-uninfected children in Zambia. Clin Inf Dis. 2002;35:189–96. doi: 10.1086/341248. [DOI] [PubMed] [Google Scholar]
- 76.Wirth KE, Wolf ER, Goldfarb DM, Ho-Foster A, Tolle M, Jacovides C, Kirk B, Chise M, Steenhoff AP. Risk factors for measles in HIV-infected children and adolescents in Botswana. Pediatr Inf Dis J. 2015;34:1093–95. doi: 10.1097/INF.0000000000000816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gill CJ, Mwananyanda L, Macleod W, Kwenda G, Mwale M, Williams AL, Siazeele K, Yang Z, Mwansa J, Thea DM. Incidence of severe and nonsevere pertussis among HIV-exposed and -unexposed Zambian infants through 14 weeks of age: results from the Southern Africa Mother Infant Pertussis Study (SAMIPS), a longitudinal birth cohort study. Clin Infect Dis. 2016;63:S154–S164. doi: 10.1093/cid/ciw526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Soofie N, Nunes MC, Kgagudi P, Van Niekerk N, Makgobo T, Agosti Y, Hwinya C, Pathirana J, Madhi SA. The burden of pertussis hospitalization in HIV-exposed and HIV-unexposed South African infants. Clin Infect Dis. 2016;63:S165–73. doi: 10.1093/cid/ciw545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Johnson S, Hendson W, Crewe-Brown H, Dini L, Frean J, Perovic O, Vardas E. Effect of human immunodeficiency virus infection on episodes of diarrhea among children in South Africa. Pediatr Inf Dis J. 2000;19:972–79. doi: 10.1097/00006454-200010000-00007. [DOI] [PubMed] [Google Scholar]
- 80.Moyo SJ, Blomberg B, Hanevik K, Kommedal O, Vainio K, Maselle SY, Langeland N. Genetic diversity of circulating rotavirus strains in Tanzania prior to the introduction of vaccination. PLoS One. 2014;9:1–10. doi: 10.1371/journal.pone.0097562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ásbjörnsdóttir KH, Slyker JA, Maleche-Obimbo E, Wamalwa D, Otieno P, Gichuhi CM, John-Stewart G. Breastfeeding is associated with decreased risk of hospitalization among HIV-exposed, uninfected Kenyan infants. J Hum Lact. 2016;32:NP61–6. doi: 10.1177/0890334415607854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nathoo KJ, Chigonde S, Nhembe M, Ali MH, Mason PR. Community-acquired bacteremia in human immunodeficiency virus-infected children in Harare, Zimbabwe. Pediatr Inf Dis J. 1996;15:1092–97. doi: 10.1097/00006454-199612000-00007. [DOI] [PubMed] [Google Scholar]
- 83.Zar HJ, Hanslo D, Tannenbaum E, Klein M, Argent A, Eley B, Burgess J, Magnus K, Bateman E, Hussey G. The etiology and outcome of pneumonia in human immunodeficiency virus-infected children admitted to intensive care in a developing country. Acta Paediatr. 2001;90:108–12. [PubMed] [Google Scholar]
- 84.Jones N, Huebner R, Khoosal M, Crewe-Brown H, Klugman K. The impact of HIV on Streptococcus pneumoniae bacteraemia in a South African population. AIDS. 1998;12:2177–88. doi: 10.1097/00002030-199816000-00013. [DOI] [PubMed] [Google Scholar]
- 85.Roca A, Sigaúque B, Quintó L, Morais L, Berenguera A, Corachan M, Ribó JL, Naniche D, Bassat Q, Sacoor C, et al. Estimating the vaccine-preventable burden of hospitalized pneumonia among young Mozambican children. Vaccine. 2010;28:4851–57. doi: 10.1016/j.vaccine.2010.03.060. [DOI] [PubMed] [Google Scholar]
- 86.Cohen C, Moyes J, Tempia S, Groome M, Walaza S, Pretorius M, Naby F, Mekgoe O, Kahn K, von Gottberg A, et al. Epidemiology of acute lower respiratory tract infection in HIV-exposed uninfected infants. Pediatrics. 2016;137:e20153272. doi: 10.1542/peds.2015-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nunes MC, Von Gottberg A, De Gouveia L, Cohen C, Moore DP, Klugman KP, Madhi SA. The impact of antiretroviral treatment on the burden of invasive pneumococcal disease in South African children: a time series analysis. AIDS. 2011;25:453–62. doi: 10.1097/QAD.0b013e328343443b. [DOI] [PubMed] [Google Scholar]
- 88.von Mollendorf C, Tempia S, Von Gottberg A, Meiring S, Quan V, Feldman C, Cloete J, Madhi SA, O’Brien KL, Klugman KP, et al. Estimated severe pneumococcal disease cases and deaths before and after pneumococcal conjugate vaccine introduction in children younger than 5 years of age in South Africa. PLoS One. 2017;12:e0179905. doi: 10.1371/journal.pone.0179905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.von Gottberg A, Cohen C, de Gouveia L, Meiring S, Quan V, Whitelaw A, Crowther-Gibson P, Madhi SA, Whitney CG, Klugman KP. Epidemiology of invasive pneumococcal disease in the pre-conjugate vaccine era: South Africa, 2003-2008. Vaccine. 2013;31:4200–08. doi: 10.1016/j.vaccine.2013.04.077. [DOI] [PubMed] [Google Scholar]
- 90.Nyasulu P, Cohen C, De Gouveia L, Feldman C, Klugman KP, von Gottberg A. Increased risk of death in Human Immunodeficiency Virus-infected children with pneumococcal meningitis in South Africa, 2003–2005. Pediatr Inf Dis J. 2011;30:1075–80. doi: 10.1097/INF.0b013e31822cca05. [DOI] [PubMed] [Google Scholar]
- 91.Rekha Banu VV, Poorana Ganga Devi NP, Swaminathan S. Childhood TB: global epidemiology and impact of HIV. Paediatr Respir Rev. 2007;2:99–106. doi: 10.1016/j.prrv.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 92.World Health Organization WHO end TB strategy. Geneva, Switzerland: WHO; 2015. [Google Scholar]
- 93.Fischer Walker CL, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, O’Brien KL, Campbell H, Black RE. Global burden of childhood pneumonia and diarrhoea. The Lancet. 2013;381:1405–16. doi: 10.1016/S0140-6736(13)60222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30:2212–19. doi: 10.1016/j.vaccine.2011.12.116. [DOI] [PubMed] [Google Scholar]
- 95.Musa BM, Bussell S, Borodo MM, Samaila AA, Femi OL. Prevalence of hepatitis B virus infection in Nigeria, 2000-2013: a systematic review and meta-analysis. Nigerian J Clin Pract. 2015;18:163–72. doi: 10.4103/1119-3077.151035. [DOI] [PubMed] [Google Scholar]
- 96.Nunes MC, von Gottberg A, de Gouveia L, Cohen C, Kuwanda L, Karstaedt AS, Klugman KP, Madhi SA. Persistent high burden of invasive pneumococcal disease in South African HIV-infected adults in the era of an antiretroviral treatment program. PLoS One. 2011;6:e27929. doi: 10.1371/journal.pone.0027929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Muloiwa R, Dube FS, Nicol MP, Zar HJ, Hussey GD. Incidence and diagnosis of pertussis in South African children hospitalized with lower respiratory tract infection. Pediatr Inf Dis J. 2016;35:611–16. doi: 10.1097/INF.0000000000001132. [DOI] [PubMed] [Google Scholar]
- 98.UNAIDS UNAIDS data 2017. Geneva, Switzerland: UNAIDS; 2017. [Google Scholar]
- 99.GAVI Annual progress report 2017. Geneva, Switzerland: GAVI; 2018. [Google Scholar]
- 100.Gona P, Van Dyke RB, Williams PL, Dankner WM, Chernoff MC, Nachman SA, Seage GR. Incidence of opportunistic and other infections in HIV-infected children in the HAART era. JAMA. 2006;296:292–300. doi: 10.1001/jama.296.3.292. [DOI] [PubMed] [Google Scholar]
- 101.Marais BJ, Rabie H, Schaaf SH, Cotton M. Common opportunistic infections in HIV infected infants and children Part 1 – respiratory infections. S Afr Fam Pract. 2006;48:52–56. [Google Scholar]
- 102.Mutsaerts EAML, Nunes MC, van Rijswijk MN, Klipstein-Grobusch K, Grobbee DE, Madhi SA. Safety and immunogenicity of measles vaccination in HIV-infected and HIV-exposed uninfected children: a systematic review and meta-analysis. E Clin Med. 2018;1:28–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Scott P, Moss WJ, Gilani Z, Low N. Measles vaccination in HIV-infected children: systematic review and meta-analysis of safety and immunogenicity. J Inf Dis. 2011;204:S164–78. doi: 10.1093/infdis/jir071. [DOI] [PubMed] [Google Scholar]
- 104.Nachega JB, Uthman OA, Ho Y-S, Lo M, Anude C, Kayembe P, Wabwire-Mangen F, Gomo E, Sow PS, Obike U, et al. Current status and future prospects of epidemiology and public health training and research in the WHO African region. Int J Epidemiol. 2012;41:1829–46. doi: 10.1093/ije/dys189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Saxenian H, Hecht R, Kaddar M, Schmitt S, Ryckman T, Cornejo S. Overcoming challenges to sustainable immunization financing: early experiences from GAVI graduating countries. Health Policy Plan. 2015;30:197–205. doi: 10.1093/heapol/czu003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D, Antes G, Atkins D, Barbour V, Barrowman N, Berlin JA, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–69. [DOI] [PubMed] [Google Scholar]
- 107.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomized studies in meta-analyses. Ottawa, Canada: Ottawa Hospital Research Institute; 2013. [Google Scholar]
- 108.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011] UK: The Cochrane Collaboration; 2011. [Google Scholar]
- 109.StataCorp Stata Statistical Software: release 14. College Station (TX): StataCorp LP; 2015. [Google Scholar]
- 110.Clegg LX, Hankey BF, Tiwari R, Feuer EJ, Edwards BK. Estimating average annual percent change in trend analysis. Stat Med. 2009;28:3670–82. doi: 10.1002/sim.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Joint United Nations Programme on HIV/AIDS AIDSInfo. 2017. [accessed 2018 June26]. http://aidsinfo.unaids.org/.


