ABSTRACT
Evidence-based approaches were used in making recommendations for vaccination against vaccine-preventable diseases for HIV-infected and HIV-exposed individuals but with limited substantiation. We conducted a systematic review and meta-analysis with randomized-controlled trials (RCTs), cohort and case–control studies that have efficacy and effectiveness of vaccines in HIV-infected and HIV-exposed children as outcomes. Web of Science, Cochrane Library, PubMed and Scopus databases were searched for articles. Efficacy of 9-valent pneumococcal conjugate vaccine (PCV9) against total vaccine serotype invasive pneumococcal disease was 32% in HIV-infected children and 78% among HIV-uninfected children. Vaccine effectiveness of Bacillus Calmette–Guérin vaccine in preventing tuberculosis in HIV-infected children was zero compared to 59% protection in HIV-unexposed children. Likewise, HIV-uninfected children have better protection against invasive Haemophilus influenzae type b disease than the HIV-infected children. Effectiveness studies of rotavirus vaccines show that HIV-exposed uninfected children have similar protection against rotavirus gastroenteritis compared to the non-exposed children. Children who are severely immunosuppressed are poorly protected against invasive pneumococcal diseases. HIV-infected children tend to have lesser vaccine protection against vaccine-preventable diseases when compared to unexposed children. HIV-infected children who are immunocompetent are more likely to have better vaccine protection against vaccine-preventable diseases than those who are immunosuppressed. The overall quality of the observational studies was very low with very little confidence in the effect estimate. The overall quality of evidence for the RCT outcomes was mainly high. This study reveals a dearth of efficacy and effectiveness studies among HIV-infected and exposed children.
KEYWORDS: HIV, vaccine-preventable diseases, sub-Saharan Africa, efficacy, effectiveness
Background
Immunization is an essential aspect of preventive medicine and critical in reducing morbidity and mortality attributed to vaccine-preventable diseases in children, adolescents and adults.1 The use of vaccines against various vaccine-preventable diseases is beneficial and an effective measure for protecting different age groups.2,3 The vaccination rates of children remain insufficient for vaccine-preventable diseases in many developing countries with only 86% of infants vaccinated with three doses of diphtheria-tetanus-pertussis containing vaccine in 2016.4 Low vaccination uptake rate results in an increase in unvaccinated and under-vaccinated human immunodeficiency virus (HIV)-infected and HIV-exposed children who are more likely to die of preventable diseases than their immunocompetent age mates.5,6 Several care and treatment guidelines have identified vaccination as a crucial preventive strategy for people living with HIV7,8 but information on the use of certain vaccines in this population are still scanty.9
Experts using evidenced-based approaches on the vaccination of immunocompromised individuals made specific recommendations for vaccination against major vaccine-preventable diseases for these patients but with limited proof.8 Research gaps were also identified by this group for future investigation. One of these gaps was that of understanding the mediators of vaccine protection, adverse effects and basic aspects of the epidemiology of various vaccine-preventable diseases.8
Vaccines stimulate immunity that protects against specific disease-causing organisms. However, the effectiveness of different recommended vaccines in HIV-infected children may be reduced as a result of the decline in vaccine-induced antibodies.10 The changing pattern of some vaccine-preventable diseases is poorly understood, and this changing pattern and epidemiology make it important to better understand these diseases because of apparent resurgence and epidemics in the future.11 The suboptimal uptake of vaccines in sub-Saharan Africa coupled with the high HIV burden are risk factors that may facilitate future epidemics.11,12
Previous reviews on the efficacy and effectiveness of vaccines in HIV-infected and exposed children were not specific on the vaccine efficacy/effectiveness against disease outcomes and were not conducted as systematic reviews.13,14 It is paramount to evaluate the available evidence by identifying high-quality literature and investigating the reliability of key findings as they relate to the pre-licensure efficacy and post-licensure effectiveness of vaccines in HIV-infected and HIV-exposed children compared to HIV unexposed children. The findings will provide the needed evidence to guide health-care policymakers, guideline developers, vaccinologists and health-care workers in developing improved long-term vaccination strategies for HIV-infected children. Current and reliable evidence-based data on the efficacy and effectiveness of vaccines in HIV-infected and HIV-exposed children are also vital to inform a better understanding of the prevention and management of vaccine-preventable diseases in these children.
This systematic review and meta-analysis summarised available data from studies which have efficacy or effectiveness of vaccines in HIV-infected and HIV-exposed children as outcomes.
Results
Description of included studies
The flow diagram in Figure 1 shows the studies identified and selected for this review. We identified 725 publications through databases and clinical trial registry search with 479 studies after removal of duplicates. A total of 14 publications were included in this review. These publications comprise five randomized-controlled trials (RCTs),15–19 six case–control studies,20–25 one cohort study26 and two cross-sectional studies.27,28 Three of the included studies were publications from a particular South African trial that reported different vaccine efficacy outcomes.15,17,18 The included studies were published from 1993 to 2017. All the included studies were conducted in sub-Saharan Africa with 10 publications from South Africa, one each from Malawi, Angola and Zambia, and one multinational study conducted in Mali, Kenya and Ghana.
Figure 1.
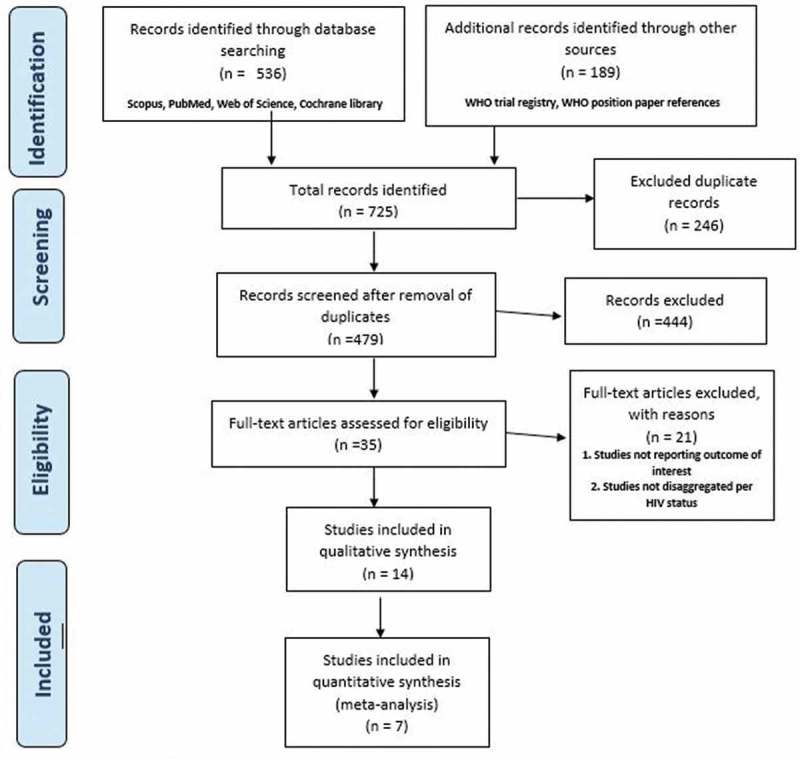
Flow diagram of the search and selection process for this review.
By outcomes, three studies reported rotavirus vaccine outcomes, six studies reported on pneumococcal vaccine, one study reported on Hib vaccine, two studies on Bacillus Calmette–Guérin (BCG) and two studies reported on Hepatitis B virus (HBV) vaccines (Table 1). Two studies compared vaccine strains with placebo among HIV-infected children while three studies compared vaccine strains with placebo among HIV-infected and HIV-unexposed children. Six studies compared HIV-infected children with HIV-unexposed children, while two studies compared HIV-exposed and uninfected children with HIV-unexposed children. In total, 66,220 children in comparative studies were involved in the included studies. The vaccine schedule and doses for the included studies were according to various national programme except for Madhi 200717 participants who were followed up for five years. Antiretroviral therapy (ART) usage varied between 22.5% and 67.0% among the HIV-infected children.
Table 1.
Characteristics of included studies.
| Participants |
Intervention |
Control |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st Author & Year | Study period | Study design | Study country | Sample size (n = ) | Participant age range | HIV status | % on ART | CD4% or count | Age (median or mean) | Vaccine strain | HIV status | % on ART | CD4% or count | Age (median or mean) | Vaccine strain |
| Beghin 201727 | 2014 | Cross- sectional study | South Africa | 291 | 5-15y | HI | NR | NR | 9.1y | HBV | HU | - | - | 9.0y | HBV |
| Cohen 201721 | 2012–2014 | Case- Control | South Africa | 1716 | ≥6w | HI | 58% | - | 48-53w | PCV13 | HU | - | - | 36-37w | PCV13 |
| Bar-Zeev 201625 | 1997–2007 | Case-control | Malawi | 919 | <5y | HEU | - | - | NA | RV | HU | - | - | NA | RV |
| Van-Dunem 201522 | 2005–2006 | Case-control | Angola | 902 | 18m – 13y | HI | 67% | NR | 4.83y | BCG Connaught | HI | 61% | NR | 3.50y | - |
| Cohen 201419 | 2010–2012 | Case- Control | South Africa | 1395 | ≥8w | HI | 46% | NR | 52-54w | PCV7 | HU | - | - | 38-39w | PCV7 |
| Groome 201423 | 2010–2012 | Case-control | South Africa | 1195 | 18w-23m | HEU | - | - | 9m | Monovalent human RV | HU | - | - | 10 m | Monovalent human RV |
| Feikin 201216 | 2007–2009 | RCT | Kenya, Ghana, Mali | 29 | 4-12w | HI | NR | NR | 17.1w | PRV | HI | NR | NR | 17.0 | Placebo |
| Steele 201119 | 2005–2008 | RCT | South Africa | 100 | 6-10w (at dose 1) | HI | 62% | 2074 | 7w | RIX4414 | HI | 52% | 2022 | 7w | Placebo |
| Simani 200828 | 2003–2004 | Cross- sectional study | South Africa | 303 | 5-24m | HI | NR | NR | 8.7m | HBV | HU | - | - | 11.9m | HBV |
| Madhi 200717 | 2001 −2005 | Post RCT | South Africa | 39836 | 5.57–5.80y | HI | 22.5% | 493; 627 | 5.80y; 5.68y | PCV9 | HU | - | - | 5.68y; 5.57y | Placebo |
| Madhi 200518 | 1998–2001 | RCT | South Africa | 39836 | 28-84d | HI | NR | NR | NA | PCV9 | HU | - | - | Placebo | |
| Klugman 200315 | 1998–2001 | RCT | South Africa | 39836 | 28-84d | HI | NR | NR | NA | PCV9 | HU | - | - | 7w | Placebo |
| Madhi 200226 | 1997–2000 | Cohort | South Africa | 19267 | <1y | HI | NR | NR | NR | HibCV | HU | - | - | NR | HibCV |
| Bhat 199324 | 1991 | Case-control | Zambia | 270 | 1m-14y | HI | NR | NR | NR | BCG | HU | NR | NR | NR | BCG |
HI – HIV-infected; HEU – HIV-exposed uninfected; HU – HIV-uninfected; NR – not reported; m – month; w – week; d – day; y – year; RCT – randomized controlled trial; HBV – Hepatitis B vaccine; HibCV- haemophilus influenzae b conjugate vaccine; PCV7 – 7-valent pneumococcal conjugate vaccine; PCV9 – 9-valent pneumococcal conjugate vaccine; PCV13 – 13-valent pneumococcal conjugate vaccine; BCG – Bacillus Calmette–Guérin; PRV – pentavalent rotavirus.
Quality of evidence
Risk of bias assessment of individual studies
Risk of bias assessment of the included studies is summarised separately for RCTs (Figure 2) and observational studies (Figure 3). All the studies except one contained at least one domain classified as high risk of bias or with no clear information.
Figure 2.
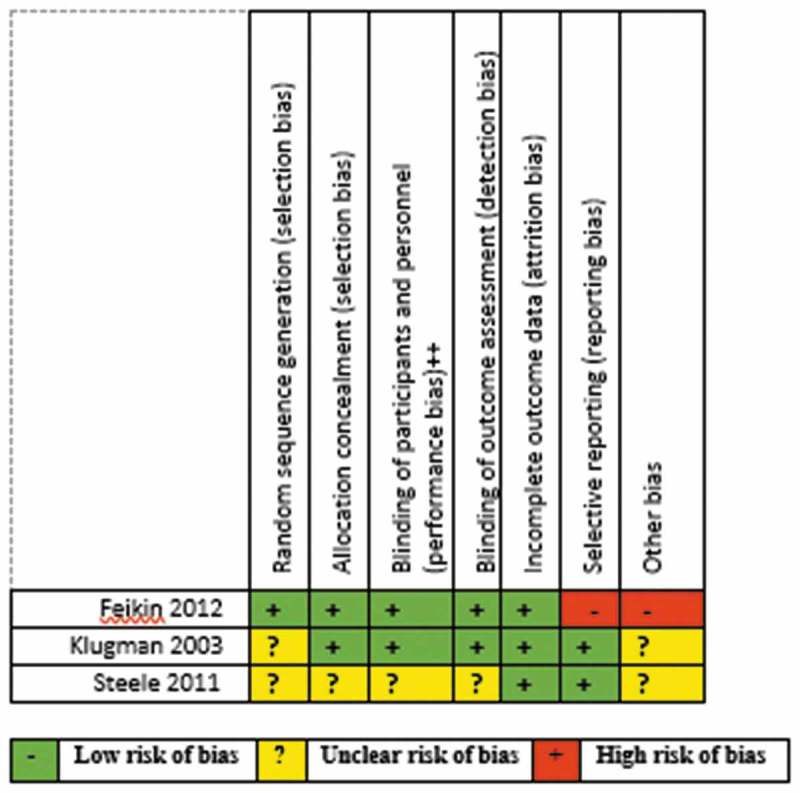
Risk of bias summary for the included randomized-controlled trials.
Figure 3.
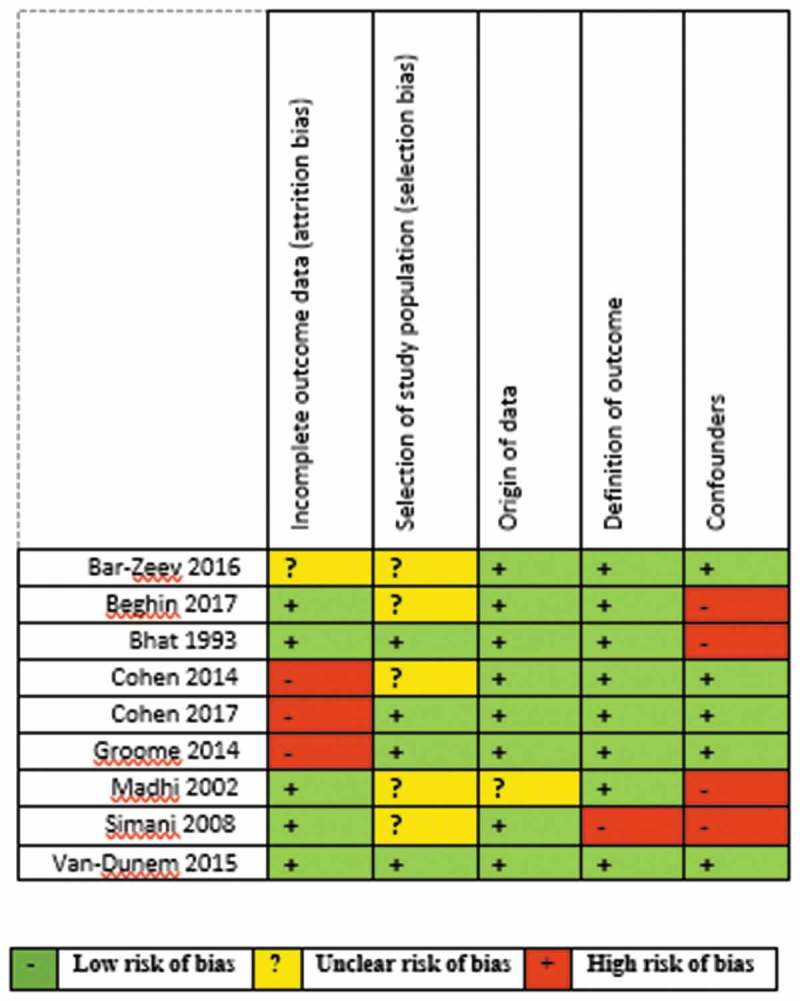
Risk of bias summary for the included observational studies.
Randomized trials
Only three RCTs were assessed.15,16,19 Klugman 200315 was used in assessing two other included studies17,18 since the study participants were the same for all three publications. There was insufficient information on random sequence selection in the majority of the studies as shown in Figure 4. Allocation concealment, performance and detection biases were low for most of the studies. Steele 201119 had an unclear risk of bias for most of the domains. Feikin 201216 had a high risk of bias for reporting and other bias domains for not reporting all the pre-specified primary outcomes and having numerous limitations.
Figure 4.
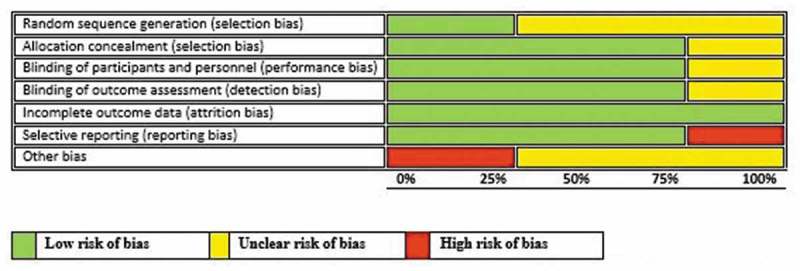
Risk-of-bias graph: review authors‘ judgments about each risk-of-bias item presented as percentages across all included studies.
Observational studies
All the observational studies had one high or unclear risk of bias across different domains except one study.15,20,21,23–28 The reasons for the high risk of bias varied and ranged from the use of hospital control instead of community controls, imbalanced missing participant numbers and unmeasured confounders (Figure 5).
Figure 5.
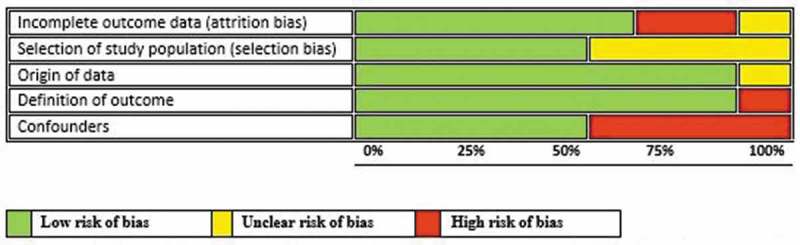
Risk of bias graph: review authors‘ judgments about each risk-of-bias item presented as percentages across all included studies.
The quality of the evidence was also evaluated using the Grades of Recommendations, Assessment, Development and Evaluation (GRADE) approach. Overall quality for the observational studies was very low with very little confidence in the effect estimate. The overall quality of evidence for the RCT outcomes was mainly high. This makes our confidence in the effect estimate to be moderate. With these results, we are confident that the true effect lies close to that of the estimate of the effect and does not require further research. See Summary of findings in Tables 2 and 3.
Table 2.
Summary of findings table for the efficacy of vaccines in HIV-infected, HIV-exposed and HIV-uninfected children (RCTs).
| Patient or population: HIV-infected, HIV-exposed and HIV-uninfected children Intervention: Vaccines Comparison: Placebo | |||||
|---|---|---|---|---|---|
| Anticipated absolute effects* (95% CI) |
|||||
| Outcomes | Risk with placebo | Risk with vaccines | Relative effect (95% CI) |
№ of participants (studies) |
Certainty of the evidence (GRADE) |
| HI/PRV/RVGE | 0 per 1,000 |
0 per 1,000 (0 to 0) |
RR 2.81 (0.12 to 63.83) |
29 (1 RCT) |
⊕⊕◯◯ LOW a |
| HI/RIX4414/RVGE | 80 per 1,000 |
80 per 1,000 (21 to 270) |
RR 1.00 (0.26 to 3.78) |
100 (1 RCT) |
⊕⊕⊕◯ MODERATE a |
| HI/PCV9/severe pneumonia | 280 per 1,000 |
233 per 1,000 (205 to 266) |
RR 0.83 (0.73 to 0.95) |
2577 (1 RCT) |
⊕⊕⊕⊕ HIGH |
| HU/PCV9/severe pneumonia | 36 per 1,000 |
32 per 1,000 (28 to 36) |
RR 0.89 (0.80 to 1.00) |
37259 (1 RCT) |
⊕⊕⊕⊕ HIGH |
| HI/PCV9/Total IPD | 26 per 1,000 |
18 per 1,000 (11 to 30) |
RR 0.68 (0.40 to 1.14) |
2577 (1 RCT) |
⊕⊕⊕⊕ HIGH |
| HU/PCV9/Total IPD | 1 per 1,000 |
0 per 1,000 (0 to 0) |
not estimable | 37259 (1 RCT) |
⊕⊕⊕⊕ HIGH |
*The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI: Confidence interval; RR: Risk ratio; HI: HIV-infected; PRV: Pentavalent rotavirus vaccine; RVGE: Rotavirus gastroenteritis; HU: HIV-uninfected; PCV9: 9-valent pneumococcal conjugate vaccine; IPD: Invasive pneumococcal disease
Explanation: a. A wide confidence interval of the estimate
GRADE Working Group grades of evidence
High certainty: We are very confident that the true effect lies close to that of the estimate of the effect
Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different
Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect
Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
Table 3.
Summary of findings table for the efficacy of vaccines in HIV-infected, HIV-exposed and HIV-uninfected children (Observational studies).
| Patient or population: HIV-infected, HIV-exposed and HIV-uninfected children Intervention: Vaccine Comparison: Placebo | |||||
|---|---|---|---|---|---|
| Anticipated absolute effects* (95% CI) |
|||||
| Outcomes | Risk with placebo | Risk with vaccines | Relative effect (95% CI) |
№ of participants (studies) |
Certainty of the evidence (GRADE) |
| HBV/Hepatitis B vaccine | 3 per 1,000 |
18 per 1,000 (3 to 103) |
OR 6.02 (0.93 to 38.83) |
594 (2 observational studies) |
⊕◯◯◯ VERY LOW a,b |
| HI/BCG/Tuberculosis | Low |
OR 1.00 (0.22 to 4.56) |
36 cases 18 controls (1 observational study) |
⊕◯◯◯ VERY LOW b,c |
|
| 0 per 1,000 |
0 per 1,000 (0 to 0) |
||||
| HU/BCG/Tuberculosis | Low |
OR 0.41 (0.18 to 0.92) |
60 cases 116 controls (1 observational study) |
⊕◯◯◯ VERY LOW b,c |
|
| 0 per 1,000 |
0 per 1,000 (0 to 0) |
||||
*The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI: Confidence interval; OR: Odds ratio; BCG: Bacillus Calmette–Guérin
Explanations: a. Confounders were not taken into account and unclear about the selection of study participants; b. A wide confidence interval around the estimate of the effects; c. Confounders not taken into account
GRADE Working Group grades of evidence
High certainty: We are very confident that the true effect lies close to that of the estimate of the effect
Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different
Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect
Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect
Vaccine efficacy for vaccine-preventable diseases outcomes
Table 4 shows reported risk ratios and vaccine efficacy for vaccine-preventable diseases outcomes in vaccinated versus non-vaccinated participants in trials for several outcomes. Vaccine efficacy of 9-valent pneumococcal conjugate vaccine (PCV9) vs. placebo in preventing first episodes of invasive pneumococcal disease was 53% (95% CI 21–73) among HIV-infected children and 42% (95% CI −28–75) among HIV-uninfected children. Efficacy of PCV9 against total vaccine serotype invasive pneumococcal disease was 32% (95% CI −14–60) in HIV-infected and 78% (95% CI 34–92) among HIV-uninfected children.
Table 4.
Reported risk ratios and vaccine efficacy for vaccine-preventable diseases outcomes in vaccinated vs. non-vaccinated participants in randomized-controlled trials.
| Study ID (year) | Doses | Experimental recipients/vaccine | Control recipients/vaccine | Vaccine efficacy (%) | Disease of interest |
|---|---|---|---|---|---|
| Klugman 200315 | 3 | HI/PCV9 | HI/placebo | 53 (21 to 73) | First episodes of invasive pneumococcal disease |
| Klugman 200315 | 3 | HU/PCV9 | HU/placebo | 42 (−28 to 75) | |
| Klugman 200315 | 3 | HI/PCV9 | HI/placebo | 13 (−7 to 29) | First episodes of radiologically confirmed pneumonia |
| Klugman 200315 | 3 | HU/PCV9 | HU/placebo | 20 (2 to 35) | |
| Madhi 200517 | 3 | HI/PCV9 | HI/placebo | 17 (5, 27) | WHO-defined severe pneumonia |
| Madhi 200517 | 3 | HU/PCV9 | HU/placebo | 11 (1, 20) | |
| Madhi 200718 | 3 | HI/PCV9 | HI/placebo | 32 (−14, 60) | Total vaccine serotype invasive pneumococcal disease |
| Madhi 200718 | 3 | HU/PCV9 | HU/placebo | 78 (34, 92) | |
| Steele 201119 | 3 | HI/RIX4414 | HI/placebo | 0 (−278, 74) | Acute rotavirus diarrhoea |
| Feikin 201216 | 3 | HI/PRV | HI/placebo | −181 (−6283, 88) |
There was similar response among HIV-infected children who were given RIX4414 vaccine and those given placebo for prevention of acute rotavirus diarrhea (RR = 1.00; 95% CI 0.26–3.78) (Table 5). The subset of HIV-infected children in a particular trial that compared pentavalent rotavirus vaccine (PRV) and placebo showed RR of 2.81 (95% CI 0.12–63.83) (Table 5).
Table 5.
Calculated vaccine efficacy and effectiveness for various vaccine outcomes.
| Outcomes | Number of studies | Experimental group | Control group | Relative effects | Study references |
|---|---|---|---|---|---|
| Hepatitis B virus infection | 2 | HI/HBV | HU/HBV | OR = 6.02 (0.93, 38.83) | 27,28 |
| Rotavirus gastroenteritis | 1 | HI/PRV | HI/Placebo | RR = 2.81 (0.12, 63.83) | 16 |
| Rotavirus gastroenteritis | 1 | HI/RIX4414 | HI/Placebo | RR = 1.00 (0.26, 3.78) | 19 |
| Severe pneumonia | 1 | HI/PCV9 | HI/Placebo | RR = 0.83 (0.73, 0.95) | 17 |
| Severe pneumonia | 1 | HU/PCV9 | HU/Placebo | RR = 0.89 (0.80, 1.00) | 17 |
| Total Invasive Pneumococcal Disease | 1 | HI/PCV9 | HI/Placebo | RR = 0.68 (0.40, 1.14) | 18 |
| Total Invasive Pneumococcal Disease | 1 | HU/PCV9 | HU/Placebo | RR = 0.22 (0.08, 0.66) | 18 |
| Tuberculosis | 1 | HI/BCG | HI/Unvaccinated | OR = 1.00 (0.22, 4.56) | 24 |
| Tuberculosis | 1 | HU/BCG | HU/Unvaccinated | OR = 0.41 (0.18, 0.92) | 24 |
BCG – Bacillus Calmette–Guérin vaccine; HI – HIV-infected; HU – HIV-uninfected; HBV – Hepatitis B virus; PCV – pneumococcal conjugate vaccine; PRV – pentavalent rotavirus;
OR – odds ratio; RR – risk ratio.
Vaccine effectiveness for vaccine-preventable diseases outcomes
Table 6 reports vaccine effectiveness for vaccine-preventable diseases outcomes in vaccinated versus non-vaccinated participants in observational studies for different outcomes. The pooled odds ratio (OR) of two studies on the effectiveness of HBV vaccines between HIV-infected and HIV-uninfected children was OR = 6.02 (95% CI 0.93–38.83; I2 = 0.00%) (Table 5; Figure 6). Vaccine effectiveness of BCG vaccine in preventing tuberculosis in HIV-infected children was zero compared to 59% protection in HIV-unexposed children (Table 5). Likewise, HIV-uninfected children have better protection against invasive Hib disease than HIV-infected children (97% versus 44%). Effectiveness studies of rotavirus vaccines show that HIV-exposed uninfected children have similar protection against rotavirus gastroenteritis comparable to the non-exposed children. The adjusted vaccine effectiveness of PCV13 against invasive pneumococcal disease was 78% (95% CI 46 to 91) in HIV-uninfected children, 17% (95% CI – 304–80) in HIV-infected and – 104% (95% CI – 1433–73) among HIV-infected children who were severely immunosuppressed.
Table 6.
Reported vaccine effectiveness against vaccine-preventable diseases in observational studies.
| Study ID (year) | Vaccine type | Doses | HIV status | Vaccine effectiveness (%) | Adjusted vaccine effectiveness (%) | Disease of interest |
|---|---|---|---|---|---|---|
| Bhat (1993)24 | BCG | 1 | HI | 0 (−360 to 78) | Tuberculosis | |
| 1 | HU | 59 (8 to 82) | ||||
| Madhi (2002)26 | HibCV | 3 | HI | 43.9 (76.1 to 82.1) | Invasive Hib disease | |
| 3 | HU | 96.5 (74.4 to 99.5) | ||||
| Groome (2014)23 | Monovalent RV | 2 | HEU | 58% (16 to 79) | Acute rotavirus diarrhea | |
| 2 | HU | 52% (23 to 70) | ||||
| Cohen (2014)20 | PCV7 | ≥3 | HI | 43 (−108 to 85) | 57 (−371 to 96) | Invasive pneumococcal disease |
| ≥3 | HU | 57 (−100 to 91) | 90 (14 to 99) | |||
| Van-Dunem (2015)22 | BCG Connaught | 1 | HI | 8 (−26 to 32) | 30 (−75 to 72) | Tuberculosis |
| Bar-Zeev (2016)25 | Monovalent RV | 2 | HEU | 42.2% (−106.9–83.8) | Acute rotavirus diarrhea | |
| 2 | HU | 60.5% (13.3–82.0) | ||||
| Cohen (2017)21 | PCV13 | ≥2 | HI (overall) | 26% (–98 to 72) | 17% (–304 to 80) | Invasive pneumococcal disease |
| ≥2 | HI with severe immunosuppression | –42% (–723 to 76) | – 104% (–1433 to 73) | |||
| ≥2 | HI with no severe immunosuppression | 75% (–31 to 95) | 66% (–94 to 94) | |||
| ≥2 | HU (overall) | 83% (61 to 92) | 78% (46 to 91) | |||
| ≥2 | HEU | 91% (60 to 98) | 87% (38 to 97) |
Figure 6.
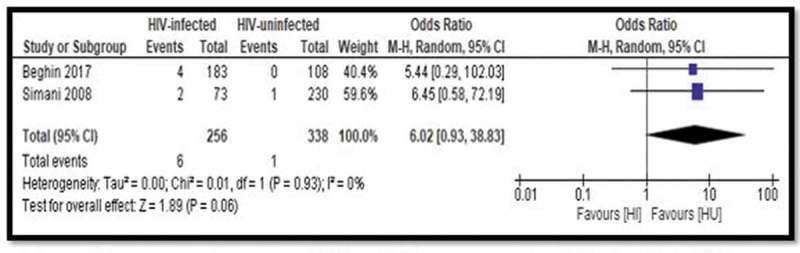
Forest plot of comparison: Vaccine effectiveness comparing HIV-infected and HIV-uninfected – Hepatitis B vaccine, outcome: HBV/Hepatitis B vaccine.
Discussion
The findings of this systematic review show that various routine vaccines have varying levels of protective efficacy and effectiveness against different vaccine-preventable diseases among HIV-infected and HIV-exposed children. This study demonstrates that PCV9 and 13-valent pneumococcal conjugate vaccine (PCV13) vaccines are efficacious in preventing invasive pneumococcal disease, radiologically confirmed pneumonia and severe pneumonia.15 PCV9 also reduced the incidence of antibiotic-resistant invasive and vaccine serotype pneumococcal disease in both HIV-infected and uninfected children.15 However, PCV vaccines are less efficacious in preventing total vaccine serotype invasive pneumococcal disease in HIV-infected children compared to HIV-uninfected children.16 Cohen et al. show that HIV-infected children have less protection against invasive pneumococcal disease when vaccinated with doses of PCV13.21 HIV-infected children with severe immunosuppression are unprotected against invasive pneumococcal disease even at higher vaccine doses.21
Vaccine-efficacy studies show that RIX4414 and PRV do not have protective activities against acute rotavirus diarrhea in HIV-infected children.16,19 The poor efficacy of PRV in children living with HIV may largely be as a result of the small sample size of the HIV-infected children subset in a Kenyan trial.16 However, Feikin et al. show that PRV efficacy against severe rotavirus gastroenteritis was 63.9% (95% CI −5.9–89.8) in a study with a large number of both HIV-infected and uninfected children in the second year of life and 83% in the first year of life. The study on RIX4414 shows that there was no significant difference in the incidence of rotavirus diarrhea in the vaccine and placebo groups thereby deducing that the vaccine did not have any significant protective effect in HIV-infected children.17 Monovalent rotavirus vaccines provided at least 40–60% protection against acute rotavirus gastroenteritis in both HIV-exposed uninfected and HIV-unexposed children, but the effectiveness in HIV-infected children is not yet known.23,26
Vaccine-effectiveness studies show that Hib conjugate vaccine provided more than 50% protection against invasive Hib disease in HIV-uninfected children when compared to HIV-infected children.24 Hib conjugate vaccine has a protective effect of 83% in preventing overall invasive Hib disease in among HIV-infected children and very useful.26 A study among Zambian children shows that BCG has 59% protective effect against tuberculosis in HIV-uninfected children and none in HIV-infected children.24 The findings of a case–control study among Brazilian children also allude to the fact that BCG does not protect against tuberculosis in immunodeficient HIV-infected children.20
Studies have shown that most of the vaccines included in this review are safe for use in all categories of children.1,15,19,29,30 A number of reviews and safety studies on several routine vaccines among HIV-infected/exposed children and HIV-unexposed children show that there was no significant difference in these groups of children with respect to adverse events, serious adverse events and death.26–33 Most of the serious adverse events and deaths were not vaccine related. Reviews also show that immune responses to primary vaccination in HIV-infected children were less likely compared to HIV-unexposed and HIV-exposed children and may require booster doses.31–33
There is a dearth of vaccine efficacy and effectiveness studies against vaccine-preventable diseases among HIV-infected and exposed children. This review shows that some efficacy studies have been done for PCV, BCG, rotavirus vaccines and Hib vaccines in HIV-infected children. There is a need to close the knowledge gap in relation to pre-licensure vaccine efficacy and post-licensure vaccine effectiveness against key vaccine-preventable diseases among these groups of children. Closing the gaps will entail conducting efficacy and effectiveness studies for several routine vaccines in HIV-infected and exposed children.13 Use of BCG vaccines in HIV-infected children can lead to disseminated tuberculosis hence it is contraindicated in immunocompromised children. It is, therefore, not advisable to do a BCG vaccine-efficacy study in these children.34 BCG is safe in immunocompetent infants, however, immunocompromised infants are at high risk of developing disseminated BCG disease.35
It is estimated that 1.8 M children are currently with living with HIV, most of them residing in sub-Saharan Africa.36 This region also has the highest burden for most of the vaccine-preventable diseases such as tuberculosis.37 It is, therefore, essential to have the children living with HIV and those exposed to HIV be protected against vaccine-preventable diseases despite possible lower vaccine efficacy among such populations.
Effectiveness research is essential and relevant for decision-making by policy makers, treatment guideline researchers, vaccine development researchers and health-care providers.38 Vaccine-efficacy research is essential in making the necessary decisions to achieve the goals of the Global Health 2035 Grand Convergence.39 The World Health Organization (WHO) has already recommended many vaccines for use in immunocompromised children especially those who have had exposure to HIV, however, most of these recommendations were made without specific vaccine-efficacy and effectiveness studies conducted in this population but rather from research findings on immunocompetent children or by using safety and immunogenicity studies.1,34 Advisory Committee on Immunization Practices (ACIP) also recommended various licensed vaccines for HIV-exposed children from birth through adolescence years except for BCG.40 Knowing the vaccine efficacy and effectiveness against specific diseases will help steer guideline development and the need for better vaccines if the level of protection is low.
Strengths of this systematic review and meta-analysis are the comprehensive search conducted in several databases and the inclusion of several routine vaccines. This review also compiled evidence on efficacy and effectiveness of vaccines that could be of use in HIV-infected and HIV-exposed children, especially in sub-Saharan Africa. The outcomes reported and pooled for this review were based on clinical features and diagnostic methods that have not changed significantly over the last two decades and as such not a limitation for this study. Lack of direct comparisons between HIV-infected and unexposed children with respect to various clinical cases of vaccine-preventable diseases limited straightforward grading of the evidence for clinical case outcomes. Only seven studies could be included in the meta-analysis due to lack of data information on some clinical outcomes and reported efficacy and effectiveness as described by the authors. Most of the included papers did not relate the immune status of the children with the efficacy of the administered vaccines except for Cohen et al.21 which shows that lesser efficacy in children with severe immunosuppression. The included studies also did not report on the time interval between vaccination and the onset of the vaccine-preventable diseases.
Conclusions
Efficacy and effectiveness studies on vaccination exhibit possibilities for direct and indirect protection against various vaccine-preventable diseases among HIV-infected and HIV-exposed children. HIV-infected children tend to have less protection against vaccine-preventable diseases when compared to unexposed children. HIV-infected children who are immunocompetent are more likely to have better vaccine protection against vaccine-preventable diseases than the immunosuppressed ones. There is also a need to bridge the knowledge gap on vaccine efficacy and effectiveness of several routine vaccines in HIV-infected and exposed children. The study suggests that only a few vaccine-efficacy and effectiveness studies have been done in HIV-infected and exposed children previously.
Methods
Search strategy and selection criteria
This review followed the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA) guideline.41 The review was registered with PROSPERO (International prospective register of systematic reviews) (CRD42018095334).
Eligibility criteria
Inclusion criteria
We included randomized-controlled trials, cohort and case–control studies that included efficacy or effectiveness of vaccines in HIV-infected in comparison with HIV-exposed or HIV-uninfected children aged ≤18 years. The intervention group included those with standard vaccines or dosages while the comparison groups comprised placebo, non-vaccinated groups, groups that were vaccinated with other control vaccines or other dosages among HIV-infected and HIV-exposed children. For case–control studies, cases were HIV-infected while controls were HIV-exposed uninfected and HIV-uninfected children.
The review planned to include the following licensed vaccines: Bacillus Calmette–Guérin, hepatitis B vaccine, oral polio vaccine, inactivated polio vaccine, diphtheria-tetanus-pertussis containing vaccines, Haemophilus Influenzae type B vaccine (Hib), pneumococcal conjugate vaccine (PCV), rotavirus vaccine (RV), yellow fever vaccine and measles-containing vaccines. These vaccines were chosen because they are the frequently used childhood vaccines in countries most affected by the HIV epidemic.
Exclusion criteria
Studies having population aged ≥18 years old individuals were excluded. We also excluded non-human studies and reviews. Most of the excluded studies reported outcomes such as level of antibodies, duplicates, reviews, studies not involving human, studies not reporting confirmed cases of vaccine-preventable diseases, reported vaccine efficacy and reported vaccine effectiveness.
Outcomes
The following were the outcome measures of interest:
Clinical and/or confirmed cases of vaccine-preventable diseases of interest.
Pooled/reported vaccine efficacy.
Pooled/reported vaccine effectiveness.
Data sources
One of the authors, OOA, searched the Web of Science, Cochrane Library, MEDLINE via PubMed and Scopus databases. Reference lists from identified papers and ClinicalTrials.gov trials registry platform were also checked. Relevant WHO position papers and documents on vaccines were also scrutinized. There was no language or date restriction.
Selection of studies
Two authors, OOA and DN, independently screened the search results using the abstract titles. They also independently went through the full text of potential studies to determine if the studies meet the inclusion criteria. Discrepancies in the selection process were resolved by consensus.
Data extraction
The two reviewers extracted data from selected articles using a pre-specified form. The extracts included information such as author, journal, year of publication, study design, country of study, participants‘ characteristics, intervention, comparator, type of vaccine and outcomes. Efficacy and effectiveness data were separately extracted for each vaccine group, target group (i.e. HIV-infected versus HIV-exposed/HIV-uninfected) and study type (interventional versus observational).
Quality assessment
The review quality assessment was guided by the use of Cochrane Collaboration’s tool for assessing the risk of bias for included trials and the use of adapted Cochrane tool for observational studies.42,43 Two authors, OOA and DN, independently assessed the methodological quality of all included studies that met the eligibility criteria. The researchers compared notes for each item and resolved discrepancies through discussion.
Synthesis of data
Synthesis of data was carried out using meta-analysis where applicable. Where meta-analysis was not possible, a narrative synthesis was used. We reported the dichotomous outcomes as risk ratios or odds ratio with their corresponding 95% confidence intervals (CI) while continuous outcomes were reported as mean differences.44 We reported the vaccine effectiveness with the random-effects odds ratio (OR) using the formula (1 – OR) × 100 while vaccine efficacy was established with risk ratio (RR): (1 – RR) × 100. The efficacy and effectiveness of each vaccine in the intervention arm were compared with that of the control arm. We also planned to use funnel-plot regression to assess publication bias if we had up to 10 studies per vaccine type. RevMan statistical software was used to do all calculations, the meta-analysis and to generate the forest plot.45
Sensitivity analysis
The certainty of the evidence regarding primary outcomes was determined by the use of the Grades of Recommendations, Assessment, Development and Evaluation (GRADE) approach.46 The term relative effect as used in GRADE refers to either Relative risk or Odds Ratio. The risk with placebo is an assumed risk or score in a group of people who do not receive the intervention. The risk with the vaccine is a corresponding risk or score in a group of people who do receive the intervention. We planned to assess substantial heterogeneity if I2 exceeded 50%, and the meta-analysis had up to five studies and to perform subgroup analyses using pre-specified potential sources of heterogeneity such as: type of comparison (i.e. placebo or no vaccine), blinding of patients (only for trials); blinding of outcome assessors; and overall methodological quality.
Funding Statement
OOA, DN and CSW are supported by the National Research Foundation of South Africa (Grant numbers: 106035 and 108571) and the South African Medical Research Council. OAU is supported by the National Institute of Health Research using Official Development Assistance funding. The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, National Institute for Health.
Abbreviations
- BCG
Bacillus Calmette–Guérin
- CI
Confidence intervals
- DTP
Diphtheria, tetanus and pertussis
- HI
HIV-infected
- HU
HIV-uninfected
- Hib
Haemophilus influenzae type b
- HIV
Human immunodeficiency virus
- PCV9
9-valent Pneumococcal conjugate vaccine
- PCV13
13-valent pneumococcal conjugate vaccine
- PRISMA
Preferred Reporting Items for Systematic Review and Meta-Analysis
- PRV
Pentavalent rotavirus
- RCT
Randomised controlled trials
- RV
Rotavirus
- WHO
World Health Organization
Authors’ contributions
OOA developed the protocol, search strategy, data analysis and manuscript preparation. OOA and DN did the screening, study selection and data extraction. OAU and CSW guided the development of the study. All authors were involved in the interpretation of results, revision and approval of the final review manuscript.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.World Health Organization Position papers - summary of WHO position papers-recommendations for routine immunization; 2018. [accessed 2018 June20]. http://www.who.int/immunization/policy/Immunization_routine_table1.pdf?ua=1.
- 2.Bloom DE, Fan VY, Sevilla JP.. The broad socioeconomic benefits of vaccination. Sci Trans Med. 2018;10(441):eaaj2345. doi: 10.1126/scitranslmed.aaj2345. [DOI] [PubMed] [Google Scholar]
- 3.Ozawa S, Clark S, Portnoy A, Grewal S, Brenzel L, Walker DG. Return on investment from childhood immunization in low-and middle-income countries, 2011–20. Health Affairs. 2016;35::199–207. doi: 10.1377/hlthaff.2015.1086. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization 2015 assessment report of the Global Vaccine Action Plan. Geneva: WHO; 2016. [Google Scholar]
- 5.Heffernan RT, Barrett NL, Gallagher KM, Hadler JL, Harrison LH, Reingold AL, Khoshnood K, Holford TR, Schuchat A. Declining incidence of invasive Streptococcus pneumoniae infections among persons with AIDS in an era of highly active antiretroviral therapy, 1995–2000. J Infect Dis. 2005;191:2038–45. doi: 10.1086/430356. [DOI] [PubMed] [Google Scholar]
- 6.Ormsby CE, de la Rosa-Zamboni D, Vázquez-Pérez J, Ablanedo-Terrazas Y, Vega-Barrientos R, Gómez-Palacio M, Murakami-Ogasawara A, Ibarra-Ávalos JA, Romero-Rodríguez D, Ávila-Ríos S, et al. Severe 2009 pandemic influenza A (H1N1) infection and increased mortality in patients with late and advanced HIV disease. AIDS. 2011;25::435–9. doi: 10.1097/QAD.0b013e328343443b. [DOI] [PubMed] [Google Scholar]
- 7.National Department of Health National consolidated guidelines for the prevention of mother-to-child transmission of HIV (PMTCT) and the management of HIV in children, adolescents and adults. Pretoria: NDOH; 2015. p. 128. [Google Scholar]
- 8.Rubin LG, Levin MJ, Ljungman P, Davies EG, Avery R, Tomblyn M, Bousvaros A, Dhanireddy S, Sung L, Keyserling H, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Inf Dis. 2014;58:e44–100. [DOI] [PubMed] [Google Scholar]
- 9.Valour F, Cotte L, Voirin N, Godinot M, Ader F, Ferry T, Vanhems P, Chidiac C. Vaccination coverage against hepatitis A and B viruses, Streptococcus pneumoniae, seasonal flu, and A (H1N1) 2009 pandemic influenza in HIV-infected patients. Vaccine. 2014;32:4558–64. doi: 10.1016/j.vaccine.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 10.Tejiokem MC, Gouandjika I, Béniguel L, Zanga M-CE, Tene G, Gody JC, Njamkepo E, Kfutwah A, Penda I, Bilong C, et al. HIV-infected children living in Central Africa have low persistence of antibodies to vaccines used in the expanded program on immunization. PLoS ONE. 2007;2:e1260. doi: 10.1371/journal.pone.0001260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark TA. Changing pertussis epidemiology: everything old is new again. J Inf Dis. 2014;209:978–81. doi: 10.1093/infdis/jiu001. [DOI] [PubMed] [Google Scholar]
- 12.Machingaidze S, Wiysonge CS, Hussey GD. Strengthening the expanded programme on immunization in Africa: looking beyond 2015. PLoS Med. 2013;10::1–5. doi: 10.1371/journal.pmed.1001405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mangtani P, Mulholland K, Madhi SA, Edmond K, O’Loughlin R, Hajjeh R. Haemophilus influenzae type b disease in HIV-infected children: A review of the disease epidemiology and effectiveness of Hib conjugate vaccines. Vaccine. 2010;28:1677–83. doi: 10.1016/j.vaccine.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 14.Jallow S, Madhi SA, Madimabe R, Sipambo N, Violari A, Kala U, Petersen K, Naidoo S, Verwey C, Moore DP, et al. Immunogenicity of 13-valent pneumococcal conjugate vaccine among children with underlying medical conditions. Vaccine. 2017;35:4321–29. doi: 10.1016/j.vaccine.2017.06.081. [DOI] [PubMed] [Google Scholar]
- 15.Klugman KP, Madhi SA, Huebner RE, Kohberger R, Mbelle N, Pierce N. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. New England J Med. 2003;349:1341–48. doi: 10.1056/NEJMoa035060. [DOI] [PubMed] [Google Scholar]
- 16.Feikin DR, Laserson KF, Ojwando J, Nyambane G, Ssempijja V, Audi A, Nyakundi D, Oyieko J, Dallas MJ, Ciarlet M, et al. Efficacy of pentavalent rotavirus vaccine in a high HIV prevalence population in Kenya (Special Issue: rotavirus vaccines for children in Developing Countries.). Vaccine. 2012;64(4):407–15. [DOI] [PubMed] [Google Scholar]
- 17.Madhi SA, Adrian P, Kuwanda L, Jassat W, Jones S, Little T, Soininen A, Cutland C, Klugman KP. Long-term immunogenicity and efficacy of a 9-valent conjugate pneumococcal vaccine in human immunodeficient virus infected and non-infected children in the absence of a booster dose of vaccine. Vaccine. 2007;25:2451–57. doi: 10.1016/j.vaccine.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 18.Madhi SA, Kuwanda L, Cutland C, Klugman KP. The impact of a 9-valent pneumococcal conjugate vaccine on the public health burden of pneumonia in HIV-infected and -uninfected children. Clin Inf Dis. 2005;40:1511–18. doi: 10.1086/429828. [DOI] [PubMed] [Google Scholar]
- 19.Steele AD, Madhi SA, Louw CE, Bos P, Tumbo JM, Werner CM, Bicer C, De Vos B, Delem A, Han HH. Safety, reactogenicity, and immunogenicity of human rotavirus vaccine RIX4414 in human immunodeficiency virus-positive infants in South Africa. Pediatr Inf Dis J. 2011;30:125–30. doi: 10.1097/INF.0b013e3181f42db9. [DOI] [PubMed] [Google Scholar]
- 20.Cohen C, Von Mollendorf C, De Gouveia L, Naidoo N, Meiring S, Quan V, Nokeri V, Fortuin-de Smit M, Malope-Kgokong B, Moore D, et al. Effectiveness of 7-valent pneumococcal conjugate vaccine against invasive pneumococcal disease in HIV-infected and -uninfected children in south Africa: a matched case-control study. Clin Inf Dis. 2014;59:808–18. doi: 10.1093/cid/ciu431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen C, von Mollendorf C, de Gouveia L, Lengana S, Meiring S, Quan V, Nguweneza A, Moore DP, Reubenson G, Moshe M, et al. Effectiveness of the 13-valent pneumococcal conjugate vaccine against invasive pneumococcal disease in South African children: a case-control study. Lancet Glob Health. 2017;5:e359–69. doi: 10.1016/S2214-109X(17)30043-8. [DOI] [PubMed] [Google Scholar]
- 22.Van-Dunem JCVD, Rodrigues LC, Alencar LCA, Militão-Albuquerque MDFP, Ximenes RADA. Effectiveness of the first dose of BCG against tuberculosis among HIV-infected, predominantly immunodeficient children. BioMed Res Int. 2015;2015:275029. doi: 10.1155/2015/275029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Groome MJ, Page N, Cortese MM, Moyes J, Zar HJ, Kapongo CN, Mulligan C, Diedericks R, Cohen C, Fleming JA, et al. Effectiveness of monovalent human rotavirus vaccine against admission to hospital for acute rotavirus diarrhoea in South African children: A case-control study. Lancet Inf Dis. 2014;14:1096–104. doi: 10.1016/S1473-3099(14)70940-5. [DOI] [PubMed] [Google Scholar]
- 24.Bhat GJ, Diwan VK, Chintu C, Kabika M, Masona J. HIV, BCG and TB in children: A case control study in Lusaka, Zambia. J Trop Pediatr. 1993;39(4):219–23. doi: 10.1093/tropej/39.4.219. [DOI] [PubMed] [Google Scholar]
- 25.Bar-Zeev N, Jere KC, Bennett A, Pollock L, Tate JE, Nakagomi O, Iturriza-Gomara M, Costello A, Mwansambo C, Parashar UD, et al. Population impact and effectiveness of monovalent rotavirus vaccination in Urban Malawian children 3 years after vaccine introduction: ecological and case-control analyses. Clin Inf Dis. 2016;62:S213–9. doi: 10.1093/cid/civ1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madhi SA, Petersen K, Khoosal M, Huebner RE, Mbelle N, Mothupi R, Saloojee H, Crewe-Brown H, Klugman KP. Reduced effectiveness of Haemophilus influenzae type b conjugate vaccine in children with a high prevalence of human immunodeficiency virus type 1 infection. Pediatr Inf Dis J. 2002;21:315–21. doi: 10.1097/00006454-200204000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Beghin J-C, Ruelle J, Sokal E, Bachy A, Krishna M, Hall L, Goubau P, Van der Linden D. Effectiveness of the South African expanded program of immunization against hepatitis B in children infected with human immunodeficiency virus-1 living in a resource-limited setting of Kwazulu-Natal. J Med Virol. 2017;89:182–85. doi: 10.1002/jmv.v89.1. [DOI] [PubMed] [Google Scholar]
- 28.Simani OE, Leroux-Roels G, François G, Burnett RJ, Meheus A, Mphahlele MJ. Reduced detection and levels of protective antibodies to hepatitis B vaccine in under 2-year-old HIV positive South African children at a paediatric outpatient clinic. Vaccine. 2009;27:146–51. doi: 10.1016/j.vaccine.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Laserson KF, Nyakundi D, Feikin DR, Nyambane G, Cook E, Oyieko J, Ojwando J, Rivers SB, Ciarlet M, Neuzil KM, et al. Safety of the pentavalent rotavirus vaccine (PRV), RotaTeq®, in Kenya, including among HIV-infected and HIV-exposed infants. Vaccine. 2012;30:A61–70. doi: 10.1016/j.vaccine.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization Pneumococcal vaccines WHO position paper - 2012. Weekly Epidemiol Record [Internet]. 2012;21:421–28. [Google Scholar]
- 31.Mutsaerts EAML, Nunes MC, van Rijswijk MN, Klipstein-Grobusch K, Grobbee DE, Madhi SA. Safety and immunogenicity of measles vaccination in HIV-infected and HIV-exposed uninfected children: a systematic review and meta-analysis. EClinMed. 2018;1:28–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott P, Moss WJ, Gilani Z, Low N. Measles vaccination in HIV-infected children: systematic review and meta-analysis of safety and immunogenicity. J Inf Dis. 2011;204:S164–78. doi: 10.1093/infdis/jir071. [DOI] [PubMed] [Google Scholar]
- 33.Nunes MC, Madhi SA. Safety, immunogenicity and efficacy of pneumococcal conjugate vaccine in HIV-infected individuals. Human Vacc Immunother. 2012;8::161–73. doi: 10.4161/hv.18432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joint United Nations Programme on HIV/AIDS UNAIDS data 2017. Geneva: UNAIDS; 2017. [PubMed] [Google Scholar]
- 35.Hesseling AC, Cotton MF, Fordham von Reyn C, Graham SM, Gie RP, Hussey GD. Consensus statement on the revised World Health Organization recommendations for BCG vaccination in HIV-infected infants: submitted on behalf of the BCG Working Group, Child Lung Health Section, International Union Against Tuberculosis and Lung Disease, 38th Union World Conference on Lung Health, Cape Town, 8–12 November 2007 [Official statement]. Int J Tuberc Lung Dis. 2008;12:1376–79. [PubMed] [Google Scholar]
- 36.UNAIDS Aidsinfo [accessed 2019 January25]. http://aidsinfo.unaids.org/.
- 37.Abajobir AA, Abbafati C, Abbas KM, Abbas KM, Abd-Allah F, Abera SF, Aboyans V, Adetokunboh O, Afshin A, Agrawal A, et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet. 2017;390:1151–210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dlamini SK, Madhi SA, Muloiwa R, Von Gottberg A, Moosa M-YS, Meiring ST, Wiysonge CS, Hefer E, Mulaudzi MB, Nuttall J, et al. Guidelines for the vaccination of HIV-infected adolescents and adults in South Africa. S Afr J HIV Med. 2018;19(1):1–8. doi: 10.4102/sajhivmed.v19i1.839. [DOI] [Google Scholar]
- 39.World Health Organization BCG vaccines: wHOposition paper-February 2018. Weekly Epidemiol Record. 2018;8::73–96. [Google Scholar]
- 40.Centers for Disease Control and Prevention Immunization schedule for infants and children (Birth through 6 Years) [accessed 2019 January25]. https://www.cdc.gov/vaccines/schedules/easy-to-read/child.html.
- 41.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;151:264–69. [PMC free article] [PubMed] [Google Scholar]
- 42.Higgins J, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0. [updated March 2011]. Chichester, UK: Cochrane Collaboration; 2011. [Google Scholar]
- 43.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled Clin Trials. 1996;17:1–2. [DOI] [PubMed] [Google Scholar]
- 45.Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014. [Google Scholar]
- 46.Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, Montori V, Akl EA, Djulbegovic B, Falck-Ytter Y, et al. GRADE guidelines: 4. Rating the quality of evidence - Study limitations (risk of bias). J Clin Epidemiol. 2011;64:407–15. doi: 10.1016/j.jclinepi.2010.07.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- World Health Organization Position papers - summary of WHO position papers-recommendations for routine immunization; 2018. [accessed 2018 June20]. http://www.who.int/immunization/policy/Immunization_routine_table1.pdf?ua=1.


