ABSTRACT
A 68-year-old woman under maintenance hemodialysis was admitted to our hospital with fever and dyspnea that had developed two days after the second vaccination with the 23-valent pneumococcal polysaccharide vaccine (PPV23). She had received the first vaccination with PPV23 five years earlier without any complications. Chest X-ray and computed tomography (CT) showed bilateral diffuse infiltrative and ground glass opacities. Bronchoalveolar lavage (BAL) revealed an abundance of eosinophils. A positive test result for PPV23 was obtained in the drug lymphocyte stimulation test. The patient was diagnosed as having eosinophilic pneumonia caused by the pneumococcal vaccination and was successfully treated by 3-week’s administration of a steroid. No disease recurrence was observed at the three-month follow-up. Thus, EP is a rare, but life-threating condition following pneumococcal vaccination.
KEYWORDS: Pneumococcal vaccine, eosinophilic pneumonia, respiratory insufficiency
Introduction
The 23-valent-polysaccharide pneumococcal vaccine (PPV23) is currently recommended for patients at a high risk for the development of invasive diseases caused by Streptococcus pneumoniae. PPV23 is generally safe; most adverse events, such as local reactions at the injection site, fever, generalized malaise and headache, are usually mild and self-limiting,1 although local reactions have been reported to occur more frequently following revaccination with PPV23 than following the initial vaccination.2,3 Although reports of serious systemic adverse reactions are few, the Vaccine Adverse Event Reporting System (VAERS) in the US reported 32 cases of interstitial lung disease and one case of interstitial pneumonia that developed after vaccination with the polyvalent pneumococcal vaccine.4 Herein, we report a rare case of acute repiratory failure due to eosinophilic pneumonia (EP) developing following a second vaccination with PPV23.
Patient presentation
A 68-year-old Japanese woman with a 20-year history of being under maintenance dialysis for end-stage renal disease caused by chronic glomerulonephritis was admitted to our hospital with a 5-day’s history of fever and dyspnea that developed two days after she received her second vaccination with PPV23 (PNEUMOVAX® NP) (MSD K.K., Tokyo, Japan). She reported having received vaccination with PPV23 for the first time five years earlier, without any complications. She was a nonsmoker and had no history of respiratory disease, inhalation of dusts or fumes, allergies to foods, or contact with any sick persons. She was receiving multiple medications including amlodipine, carvedilol, enarapril, ethyl icosapentate, esomeprazole, pregabaline, meloxicam, etizolam, ferric citrate hydrate, lanthanum carbonate hydrate, sodium polystyrene sulfonate, sevelamer hydrochloride, but denied having received any new medications. On admission, she had fever (38°C), tachycardia (102/min), tachypnea (24/min) and hypoxemia (SpO2 88% in room air). Chest auscultation revealed coarse crackles in the lung regions bilaterally, and the heart sounds were normal. She did not have any skin rashes, arthralgia, myalgia, or pretibial edema. A plain chest X-ray (Figure 1a) and chest computed tomography (CT) (Figure 1b) revealed diffuse infiltrative and ground glass opacities in both lung fields. Twelve-lead electrocardiography revealed sinus tachycardia, but no other abnormalities. Echocardiography showed normal cardiac function, with an ejection fraction of 67%. Laboratory tests revealed a white blood cell count of 8,800 cells/μL with 11% eosinophils and elevated serum levels of C-reactive protein (11.8 mg/dL) and lactate dehydrogenase (407 IU/L; normal<220 IU/L). The serum creatinine (9.8 mg/dL) and BUN (72.2 mg/dL) levels were also elevated, as the patient was receiving maintenance hemodialysis because of chronic kidney disease. The serum levels of Krebs von den Lungen-6 and immunoglobulin E were normal. Serological tests for anti-nuclear antibody, anti-neutrophil cytoplasmic antibody, and other known autoantibodies against specific antigens were all negative. Testing of a nasopharyngeal swab for influenza was negative. Stool tests for parasites were negative. Fiberoptic bronchoscopy was performed and specimens of bronchoalveolar lavage (BAL) fluid obtained from right middle lobe after infusion of 150 ml of saline solution (recovery rate: 60%) showed an abundance of eosinophils; the findings were as follows: white blood cell count 9.0 × 105 cells/mL, macrophage 10% (normal>90%), lymphocytes 12% (normal<5%), neutrophils 26% (normal<5%), and eosinophils 51% (normal<1%). BAL cultures for bacteria, tuberculosis and fungi were all negative. A drug lymphocyte stimulation test (DLST) conducted on a sample of peripheral blood showed a positive memory T-cell reaction to PPV23 (stimulation index based on 3H-thymidine uptake: 341%; cutoff for positivity: 180%). The DLST was performed with all of the 23 antigens in one assay. Based on these findings, acute respiratory failure due to EP caused by the pneumococcal vaccination was suspected and the patient was started on pulse methylprednisolone (1.0 g/day) therapy. After three days of this treatment, her fever subsided, with marked improvement of the dyspnea. Thereafter, the patient was continued on treatment with oral prednisolone (PSL) (40 mg/day), the dose of which was gradually tapered over a period of 8 weeks. A follow-up chest CT on day 52 revealed marked improvement (Figure 1c). No recurrence was observed at the three-month follow-up.
Figure 1.
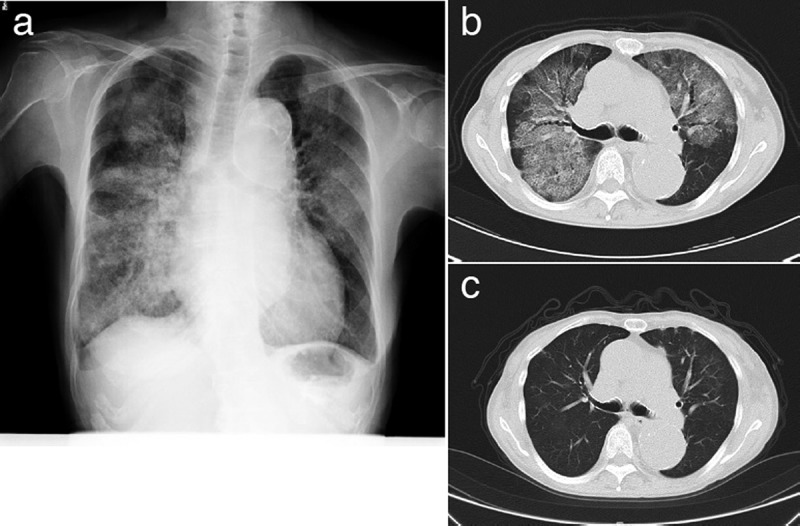
(a) Chest X-ray and (b) CT on admission showing diffuse infiltrative and ground glass opacities in both lungs. (c) A CT mage obtained after 52 days of steroid treatment.
Discussion
EP can arise from diverse etiologies; however, EP occurring after vaccination has rarely been reported; only one case of EP following influenza vaccination has been reported until date.5 To the best of our knowledge, this is the first report of EP developing after pneumococcal vaccination. We speculate that the pneumococcal vaccination was causally related to the development of EP in our patient, because it developed soon after the PPV23 vaccination, and no other potential causes of EP could be identified, such as exposure to drugs, dust and toxic substances. The positive result of the DLST for PPV23 further lent support to our notion of a causal association of PPV23 with the EP, although DLST has been reported to yield false-positive results in some cases.6 Vaccination-associated eosinophilia has been described in the literature. For example, aluminum adjuvant-containing vaccines have been reported to induce a Th2-type cell-mediated immune response leading to eosinophilia.7 Vaccination with formalin-inactivated respiratory syncytial virus (RSV) has also been reported to induce pulmonary eosinophilia in patients subsequently infected with live RSV.8 However, the PPV23 is adjuvant-free and not formalin-treated, suggesting that the vaccination-associated eosinophilia in our patient was not caused by adjuvants or formalinized vaccine immunogens. EP is a hypersensitivity syndrome associated with eosinophilic infiltration of the lung tissue. In the present case, the patient developed EP a few days after receiving her second anti-pneumococcal vaccination with PPV23. She denied having developed any complications following the initial vaccination that she had received five years earlier. Although revaccination with PPV23 is generally safe,2 it is suspected that the second time vaccination can cause hypersensitivity reactions, such as type III hypersensitivity reactions due to the formation of antigen-antibody complexes.3 Thus, the development of EP after revaccination with the pneumococcal vaccine in our patient was probably caused by a severe acute hypersensitivity reaction. Although large populations have been vaccinated with PPV23, few cases of severe hypersensitivity reactions have been reported previously. We speculate that re-vaccination, hemodialysis and multiple drug medications could have served as risk factors for the development of EP in our patient.
A limitation of this study is that the DLST often yields a false-positive result.6 For example, the positive result of the DLST for PPV23 may have been the result of effective immunization with PPV23, because immunization elicits constructive immune responses. However, the DLST is a T-cell dependent reaction, whereas the reactions elicited by PPV23 immunization are theoretically T-cell independent. From the latter point of view, the positive result of the DLST is unlikely to be the consequence of previous immunization with PPV23. Comparisons of the DLST for PPV23 in individuals previously immunized with PPV23 and never immunized with PPV23 would be helpful to better understand the causal relationship between a positive result of DLST and previous immunization with PPV23. The second limitation of our study is that the BAL sample was not tested by PCR for Streptococcus pneumoniae, even though the BAL cultures for bacteria were negative. It is possible that EP was triggered by the presence of antigen in the lung at the time or very soon following vaccination with PPV23.
In conclusion, pneumococcal vaccination can be complicated by serious respiratory failure. EP should be considered in all patients who present with dyspnea after receiving the pneumococcal vaccine.
Disclosure of potential conflicts of interest
The authors have no conflict of interest to declare.
References
- 1.Vadlamudi NK, Parhar K, Altre Malana KL, Kang A, Marra F.. Immunogenicity and safety of the 13-valent pneumococcal conjugate vaccine compared to 23-valent pneumococcal polysaccharide in immunocompetent adults: A systematic review and meta-analysis. Vaccine. 2019;37:1021–29. doi: 10.1016/j.vaccine.2019.01.014 PMID: 30685252. [DOI] [PubMed] [Google Scholar]
- 2.Jackson LA, Benson P, Sneller VP, Butler JC, Thompson RS, Chen RT, Lewis LS, Carlone G, DeStefano F, Holder P, et al. Safety of revaccination with pneumococcal polysaccharide vaccine. JAMA. 1999;281:243–48. PMID:9918479. [DOI] [PubMed] [Google Scholar]
- 3.Hilleman MR, Carlson AJ Jr, McLean AA, Vella PP, Weibel RE, Woodhour AF. Streptococcus pneumoniae polysaccharide vaccine: age and dose responses, safety, persistence of antibody, revaccination, and simultaneous administration of pneumococcal and influenza vaccines. Rev Infect Dis. 1981;3 Suppl:S31–42. PMID: 7025159. [DOI] [PubMed] [Google Scholar]
- 4.The Centers for Disease Control and Prevention (CDC) and the U.S. Food and Drug Administration (FDA). Vaccine Adverese Event Reporting System [accessed 2019. May 5]. https://vaers.hhs.gov/
- 5.Pornsuriyasak P, Suwatanapongched T, Klaewsongkram J, Buranapraditkun S, Rotjanapan P. Acute respiratory failure secondary to eosinophilic pneumonia following influenza vaccination in an elderly man with chronic obstructive pulmonary disease. Int J Infect Dis. 2014;26:14–16. doi: 10.1016/j.ijid.2014.04.019 PMID: 24981428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mantani N, Kogure T, Tamura J, Shimada Y, Terasawa K. Lymphocyte transformation test for medicinal herbs yields false-positive results for first-visit patients. Clin Diagn Lab Immunol. 2003;10:479–80. PMID: 12738653. doi: 10.1128/cdli.10.3.479-480.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terhune TD, Deth RC. Aluminum adjuvant-containing vaccines in the context of the hygiene hypothesis: A risk factor for eosinophilia and allergy in a genetically susceptible subpopulation? Int J Environ Res Public Health. 2018;15(5). doi: 10.3390/ijerph15050901 PMID: 29751492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haynes LM, Jones LP, Barskey A, Anderson LJ, Tripp RA. Enhanced disease and pulmonary eosinophilia associated with formalin-inactivated respiratory syncytial virus vaccination are linked to G glycoprotein CX3C-CX3CR1 interaction and expression of substance P. J Virol. 2003;77:9831–44. PMID: 12941892. doi: 10.1128/jvi.77.18.9831-9844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- The Centers for Disease Control and Prevention (CDC) and the U.S. Food and Drug Administration (FDA). Vaccine Adverese Event Reporting System [accessed 2019. May 5]. https://vaers.hhs.gov/


