ABSTRACT
Background: We studied direct effects of human granulocyte-macrophage colony stimulating factor (GM-CSF) on phenotypical characteristics and cytokine-production of non-activated and activated human monocytes/macrophages (Mc/Mphs) and T cells.
Methods: Purified Mc/Mphs were activated by bacterial lipopolysaccharide (LPS, 1 μg/ml) for 24 h, while T cells were activated by particles conjugated and antibodies (Abs) against human CD2, CD3, and CD28 for 48 h.
Results: GM-CSF treatment (0.01–10 ng/ml) was shown to reduce percentages of CD197 (CCR7)-positive cells in non-activated Mph cultures, without affecting significantly CD14+ (LPS co-receptor), CD16+ (FcγRIII, low-affinity Fc-receptor), CD119+ (interferon-gamma receptor 1), and CD124+ (IL4 receptor α-subunit) cells. In addition, GM-CSF reduced relative numbers of CD197+ cells, as well as CD14+, CD16+, and CD119+ cells in activated Mph cultures without affecting CD124+ cell distribution. GM-CSF at the highest dose of 10 ng/ml enhanced TNF-α and IL-6 (but not IL-1β and IL-10) production in activated Mc/Mphs. In activated T cell cultures, GM-CSF at 0.1–1.0 ng/ml augmented CD38+ cell numbers in naïve СD45RA+/СD197+ and central memory СD45RA−/СD197+ cell subsets, with no effect on effector СD45RA−/СD197− and terminally differentiated effector СD45RA+/СD197− cells. GM-CSF at a low dose (0.01 ng/ml) down-regulated INF-γ production, while at a high dosage (10.0 ng/ml) up-regulated IL-2 and IL-4 production.
Conclusion: In general, the results suggest that GM-CSF is able to facilitate the implication of both Mph and T cells in the adaptive immunogenesis.
KEYWORDS: Granulocyte-macrophage colony-stimulating factor, monocyte/macrophage, T-cell subsets, СD expression, cytokines
Introduction
Granulocyte-macrophage colony stimulating factor (GM-CSF) is known to stimulate growth and differentiation of granulocytes and macrophages (Mphs), thus playing an important role in regulation of innate and adaptive immunity. GM-CSF is capable of maintaining differentiation and survival of dendritic cells (DCs), which not only initiate antigen-dependent proliferation and differentiation of T cells,1,2 but also support many mechanisms underlying adaptive immune reactivity.3,4 Importantly, GM-CSF is produced both in central hematopoietic organs and in the periphery, and its production is dramatically increased in tissues upon inflammatory processes implying the active involvement of GM-CSF in regulation of immune processes in the periphery. Mphs, mast cells, fibroblasts, endothelial cells, and T cells are considered to be major GM-CSF producers,5 and various factors such as pro-inflammatory cytokines (IL-1, IL-6, and TNF-α) could up-regulate its production levels.6 GM-CSF receptors (GM-CSFR) consist of α and β chains, with the latter known to be shared by receptors for GM-CSF, IL-3, and IL-5.7 Recognition of GM-CSF by its receptor triggers JAK2- and STAT-5-dependent signaling pathways, which regulate differentiation and functional activity of the cell,2,8 while implication of PI3K-dependent signal transduction pathway contributes to growth and survival of immune cells.9 Low expression levels (20–200 copies per cells) of high-affinity GM-CSFR have been demonstrated on granulocytes, monocytes (Mcs), Mphs, endothelial cells, and lymphocytes.10-12 Membrane-associated GM-CSFR expression on T cells was found to be functionally significant.13
Taken together, major effects of GM-CSF on adaptive immunity are likely to be facilitated via Mc/Mph-T cell axis. Therefore, in this study, we addressed direct effects of GM-CSF on phenotypical characteristics and cytokine production pattern of Mphs and T lymphocytes.
Results
GM-CSF-mediated effects on phenotype and cytokine production pattern of Mc/Mphs
The particular flow cytometry gating strategy employed in this study enabled identification of CD14+, CD16+, CD119+, CD124+, and CD197+ Mc/Mph cells. This strategy was described in detail in our previous reports.14,15 Purity of CD14-positive cells isolated from blood by positive magnetic separation was 98.7% (92.5–99.4), with cell viability of 96.3% (94.0–99.8).14
CD14 is aglycosylphosphatidylinositol (GPI)-linked 55-kDa protein present on the surface of Mphs and polymorphonuclear leukocytes; it constitutes a high-affinity receptor for lipopolysaccharide (LPS) (a conservative gram-negative bacterial component), as well as peptidoglycan (an abundant component of gram-positive bacterial cell wall). Interaction between CD14 and its ligands is known to induce production and release of pro-inflammatory cytokines.16 CD16 molecules are involved in Mph-mediated cytolysis and phagocytosis of Ab-opsonized cells.17 CD119 (IFNG-R1) is a receptor for IFN-ɣ. Upon interaction with its ligand, this receptor triggers classical pro-inflammatory M1-type Mph activation.18 CD124 is a receptor for IL-4. Its ligation by IL-4 facilitates polarization of Mph cells via alternative anti-inflammatory M2-type pathway.19 CD197 (CCR7) is a chemokine receptor for two ligands/chemokines (CCL19 and CCL21) produced by stromal cells in T-zones of lymph nodes. CD197 ligation induces migration of Mc/Mph to T-dependent zones of secondary lymphoid organs, thus effectively enabling participation of these immune cells in adaptive immunogenesis.20 Data presented in Figure 1 and Table 1 suggest that GM-CSF (0.01–10.0 ng/ml) significantly reduced the proportion of CD197+ cells in non-activated Mc/Mph cultures, without exerting a significant impact on the relative numbers of СD14+, СD16+, СD119+, and СD124+ cells (Table 1).
Figure 1.
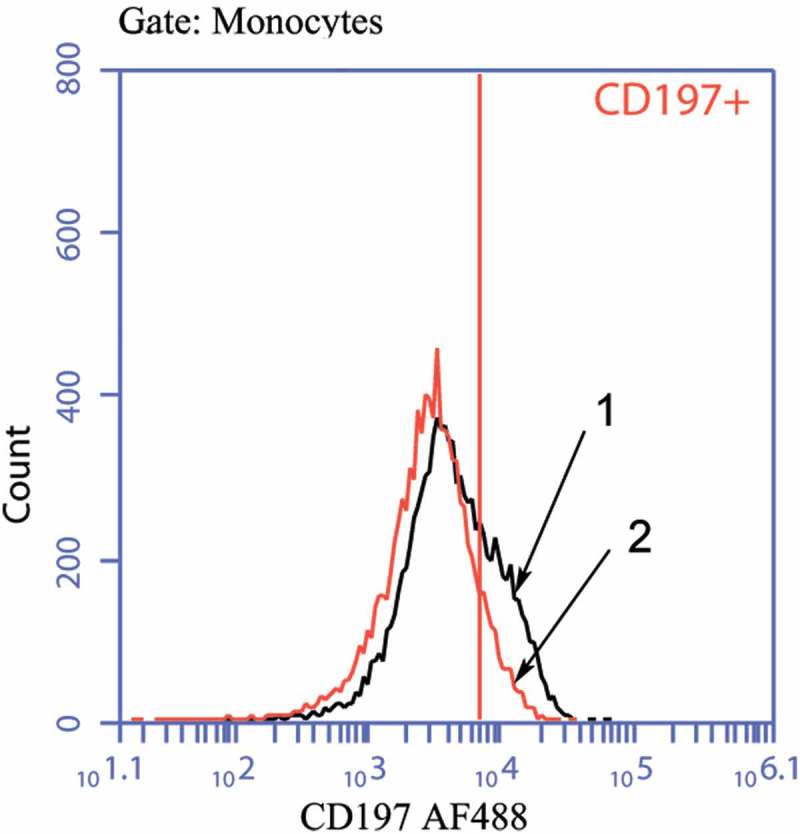
Histograms of staining for CD197+ Mc/Mph.
1 – Mc/Mphs in the absence of GM-CSF;2 – Mc/Mphs in the presence of GM-CSF.
Table 1.
Percentages of CD14+, CD16+, CD119+, CD124+, CD197+ cells in non-activated Mc/Mph cell cultures.
| CD marker | GM-CSF (ng/ml) |
||||
|---|---|---|---|---|---|
| 0.0 | 0.01 | 0.1 | 1.0 | 10.0 | |
| CD14 | 25.05 (23.07–32.16) |
32.30 (11.94–38.25) |
22.76 (11.43–34.53) |
23.82 (9.55–33.49) |
19.72 (11.69–41.79) |
| CD16 | 11.06 (4.87–16.35) |
5.95 (3.82–11.60) |
4.59 (3.52–17.39) |
8.7 (3.32–17.09) |
8.35 (3.32–18.32) |
| CD119 | 83.16 (78.07–88.75) |
74.58 (58.49–82.18) |
80.79 (69.83–84.08) |
81.32 (70.01–88.82) |
82.65 (78.62–89.37) |
| CD124 | 47.01 (14.76–57.57) |
39.60 (11.55–54.93) |
47.43 (11.22–55.10) |
44.45 (11.41–55.48) |
47.05 (9.64–55.13) |
| CD197 | 8.31 (6.65–66.05) |
3.68* (3.58–7.73) |
3.23* (3.08–5.67) |
6.29* (2.51–41.62) |
5.4* (2.69–47.58) |
Notes: Data are presented as Median with the first and third quartiles (Q1-Q3); *p < 0.05 – as compared to Mc/Mph cells cultured in the absence of GM-CSF.
Next, we activated Mc/Mph cultures with LPS in the absence of GM-CSF and found specifically induced CD197+ cell numbers in a statistically significant manner (increase from 8.31 (6.65–66.05) to 20.38 (7.88–50.75); p < .05). When added to Mc/Mphs along with LPS, GM-CSF reduced percentages of CD197+ cells (Figure 2 and Table 2), with additional reductions in СD14+, СD16+, and СD119+ cell numbers and no effect on CD124-positive cells.
Figure 2.
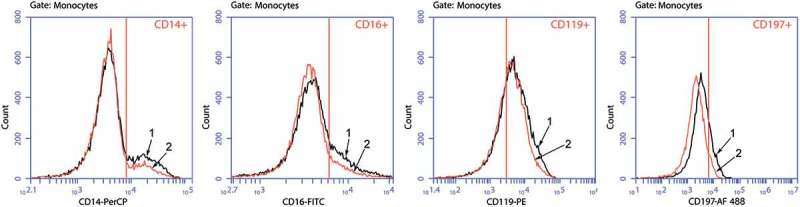
Histograms of staining for CD14+, CD16+, CD119+, and CD197+ Mc/Mphs.
1 – Mc/Mphs activated by LPS in the absence of GM-CSF;2 – Mc/Mphs activated by LPS in the presence of GM-CSF.
Table 2.
Percentages of CD14+, CD16+, CD119+, CD124+, CD197+ cells in LPS-activated Mc/Mph cell cultures.
| CD marker | GM-CSF (ng/ml) |
||||
|---|---|---|---|---|---|
| 0.0 | 0.01 | 0.1 | 1.0 | 10.0 | |
| CD14 | 25.01 (11.37–33.46) |
18.42* (7.56–30.70) |
20.99* (7.98–28.95) |
20.91* (7.99–31.94) |
20.35* (23.07–32.16) |
| CD16 | 12.05 (4.94–24.10) |
9.11* (3.27–14.29) |
8.13* (3.60–16.96) |
7.75* (3.55–13.47) |
8.13* (3.77–12.70) |
| CD119 | 84.55 (65.02–86.62) |
77.92 (74.54–79.66) |
77.15* (65.45–80.28) |
78.31* (68.12–80.31) |
79.29* (67.94–79.68) |
| CD124 | 45.12 (15.91–56.74) |
45.70 (14.31–53.22) |
43.79 (16.83–54.3) |
42.62 (16.92–53.26) |
44.92 (16.11–53.05) |
| CD197 | 20.38 (7.88–50.75) |
12.34* (6.59–46.22) |
16.55* (5.44–39.62) |
11.60* (5.55–38.97) |
11.52* (5.46–41.86) |
Notes: Data are presented as Median with the first and third quartiles (Q1-Q3); *p < 0.05 – as compared to Mc/Mph cells activated by LPS in the absence of GM-CSF.
As expected, LPS activation resulted in pronounced up-regulation of TNF-α, IL-1β, IL-6, and IL-10 production. The data of Table 3 demonstrate that GM-CSF at the highest dose of 10 ng/ml caused further significant up-regulation of TNF-α and IL-6 production by activated Mc/Mphs, with no effect on IL-1β and IL-10 production.
Table 3.
Concentrations of cytokines (pg/ml) in Mc/Mph cell culture supernatants.
| Cytokine | Without activation | Activation + GM-CSF (ng/ml) |
||||
|---|---|---|---|---|---|---|
| 0.0 | 0.01 | 0.1 | 1.0 | 10.0 | ||
| TNF-α | 320.8 (74.2–421.6) |
1198.2 (745.1–1553.6) |
1284 (533.8–1638) |
1166 (496.3–1516) |
1282.2 (551.1–1599.8) |
1418.6* (1002.4–1849.9) |
| IL-1β | 5.7 (0–157.6) |
459.0 (265.8–507.6) |
300.6 (118.2–621.6) |
372.6 (183.6–683.4) |
369.0 (337.8–754.8) |
399 (288–757.8) |
| IL-6 | 124.0 (36.0–733.5) |
8339 (4734–14577) |
10881 (6328–13633) |
9336 (5711–13269) |
9192 (5103–13315) |
12216* (8921–17225) |
| IL-10 | 0.8 (0–31.6) |
118.98 (95.87–127.54) |
124.95 (86.25–161.03) |
139.2 (92.93–165.3) |
93.6 (78.75–140.1) |
89.85 (75.68–139.65) |
Notes: Data are presented as Median with the first and third quartiles (Q1-Q3); * p < 0.05 – as compared to LPS-activated Mc/Mph cells cultured in the absence of GM-CSF.
Effects of GM-CSF on phenotype and cytokine production patterns of various T cell subpopulations
In this part of the study, we applied a multi-color flow cytometry protocol established in our laboratory and described in detail in our previous reports.21-24 Briefly, our algorithm allowed for identification of human CD4-positive and CD4-negative T cells, with their further sub-classification into naïve СD45RA+/СD197+, central memory СD45RA−/СD197+, effector memory СD45RA−/СD197−, and terminally differentiated effector СD45RA+/СD197− T cells. We showed previously that the overwhelming majority of CD4-negative T cells belonged to a CD8-positive cell population. Here, purity of T cells isolated from PBMC by magnetic separation was 98.6% (94.7–99.2), with the baseline viability of 95.4% (94.1–99.4).23,24
First, we addressed direct effects of GM-CSF on membrane expression of a T cell activation marker CD38, which is a multifunctional receptor/ectoenzyme participating for instance in synthesis of adenosine diphosphate–ribose (ADPR) and cyclic ADPR, which is a potent Ca2+ mobilizer from intracellular stores. Data presented in Table 4 suggest that T cell activation resulted in significant and pronounced increases in CD38+ T cell numbers in all T cell subpopulations studied. Adding GM-CSF (0.01–1.0 ng/ml) further augmented the numbers of CD38+ cells in СD4-negative and СD4-positive naïve СD45RA+/СD197+ and central memory СD45RA−/СD197+ T cell compartments. No GM-CSF-mediated effect on CD38 expression was detected in effector СD45RA−/СD197− and terminally differentiated effector СD45RA+/СD197 T cell subsets, altogether suggesting that direct effects of GM-CSF on T cells was dependent on the particular T cell population.
Table 4.
Percentages of CD38+ T cells in T cell subpopulations studied.
| T cell subpopulation | Without activation | Activation + GM-CSF (ng/ml) |
|||||
|---|---|---|---|---|---|---|---|
| 0 | 0.01 | 0.10 | 1.00 | 10.00 | |||
| CD4+ | Naïve cells | 23.4 (15.5–30.5) |
47.1 (29.1 − 62.7) |
49.5 (39.8–66.4) |
51.0 (36.0–67.5) |
50.8* (41.5–63.3) |
49.9 (35.7–61.6) |
| Central memory cells | 9.1 (7.1–12.8) |
28.6 (24.3–29.9) |
29.1 (24.6–32.6) |
31.3* (27.6–32.3) |
29.1 (25.8–33.4) |
28.9* (25.7–30.0) |
|
| Effector memory cells | 3.7 (2.2–5.3) |
14.2 (10.3–19.0) |
15.5 (9.8–20.0) |
15.7 (9.7–21.1) |
16.8 (11.0–20.9) |
15.1 (11.1–19.5) |
|
| Terminally-differentiated effector cells | 8.3 (5.7–15.2) |
40.3 (29.8–51.0) |
45.7 (37.6–54.6) |
47.1 (38.6–55.5) |
44.2 (38.1–53.4) |
42.3 (36.9–54.6) |
|
| CD4− | Naïve cells | 9.5 (5.3–24.4) |
25.7 (19.2–37.6) |
28.6 (22.6–38.1) |
29.3* (22.5–40.6) |
26.5 (21.0–38.8) |
24.6 (21.8–37.5) |
| Central memory cells | 12.6 (9.6–18.4) |
25.4 (17.7–33.3) |
26.0 (19.8–34.3) |
26.9* (21.7–34.0) |
26.3 (19.1–33.9) |
23.8 (19.6–29.2) |
|
| Effector memory cells | 2.8 (1.7–4.9) |
8.0 (4.9–10.2) |
6.3 (5.4–10.0) |
8.1 (5.5–11.8) |
8.0 (5.0–10.0) |
7.3 (4.6–10.6) |
|
| Terminally-differentiated effector cells | 6.7 (2.4–8.9) |
15.4 (11.7–18.9) |
15.5 (12.7–22.3) |
16.8 (14.9 − 22.3) |
17.1 (15.1–19.5) |
15.4 (13.8–21.5) |
|
Notes: T cell activation was performed in the presence of MACSiBead particles conjugated with Abs against human CD2, CD3, and CD28. Data are presented as Median with the first and third quartiles (Q1-Q3); * p < 0.05 – as compared to T cells activated in the absence of GM-CSF.
Table 5 summarizes data on cytokine levels in activated T cell cultures in the presence and absence of GM-CSF. T cell activation alone stimulated IL-2 (major T cell growth factor), INF-γ (major pro-inflammatory Th1-type cytokine), IL-4 (anti-inflammatory Th2-type cytokine), and IL-10 (immunosuppressive cytokine). In these experimental settings, GM-CSF at the highest concentration of 10.0 ng/ml significantly up-regulated IL-2 and IL-4 production by activated T cells, with concomitant down-regulation of IL-10 secretion. Interestingly, treatment with low GM-CSF concentrations (0.01 ng/ml) reduced INF-γ production. Taken together, GM-CSF affected immunoregulatory cytokine production pattern by activated T cells polarizing it to pro- or anti-inflammatory direction, which could be subject to the particular GM-CSF concentrations present in cell microenvironment.
Table 5.
Cytokine concentrations in T cell culture supernatants (pg/ml).
| Cytokine | Without activation | Activation + GM-CSF (ng/ml) |
||||
|---|---|---|---|---|---|---|
| 0 | 0.01 | 0.10 | 1.00 | 10.00 | ||
| IL-2 | <10 | 590.0 (121.0–869.5) |
580.0 (96.0–987.5) |
540.0 (106.0–1034.5) |
468.0* (244.0–1120.0) |
614.0* (272.0–1142.0) |
| INF-γ | <10 | 5094.0 (4039.8–9688.3) |
2652.0* (2290.0–5530.0) |
3312.0 (2524.0–7669.5) |
5764.0 (3941.5–9071.5) |
6276.0 (3716.0–9346.0) |
| IL-4 | <2 | 11.6 (5.4–29.5) |
7.1 (0.5–30.6) |
7.4 (2.4–31.5) |
8.8 (4.6–31.6) |
13.9* (10.8–30.8) |
| IL-10 | <5 | 620.6 (484.8–870.9) |
528.9 (452.7–668.4) |
509.4 (430.2–732.3) |
512.1 (366.9–934.8) |
452.4* (322.2–769.5) |
Notes: T cell activation was performed in the presence of MACSiBead particles conjugated with Abs against human CD2, CD3, and CD28. Data are presented as Median with the first and third quartiles (Q1-Q3); * p < 0.05 – as compared to cell culture supernatants derived from activated T cells cultured in the absence of GM-CSF.
Discussion
Mph cells possess effector qualities, as well as antigen-presenting and immunoregulatory properties playing pivotal role in mechanisms facilitating efficient interplay between innate and adaptive immunity. Activation of Mph cells leads to drastic enhancement of their functional activity, which generally occurs via two major polarization pathways. LPS and Th1-type cytokines (IFN-γ) have been shown to skew macrophage polarization toward classical pro-inflammatory (M1-type) pathway manifesting itself in the induction of: (i) several markers, such as CD40 co-stimulatory molecule (belongs to TNF-receptor superfamily) and CD64 (high-affinity Fc-receptor for monomeric IgG) expression, (ii) TNF-α, IL-1β, IL-6, IL-12, and IL-23 cytokine production, and (iii) reactive nitrogen species (NO).25,26 From the functional perspective, M1-type Mphs have been shown: (i) to be able to process and present antigens to T cells, (ii) to possess cytotoxic properties, and (iii) to initiate immune responses and protect against intracellular pathogens.27 On the other hand, glucocorticoids, Th2-type cytokines (IL-4, IL-10, and IL-13), fungal, and helminth infection drive Mph polarization via an alternative (M2-type) pathway, which describes Mphs with enhanced: (i) expression of such markers, as СD163 (an endocytic receptor for hemoglobin-haptoglobin complexes), CD206 (mannose receptor), galactose E-type, and C-type receptors, (ii) anti-inflammatory cytokine production, such as transforming growth factor-β (TNF-β) and IL-10. M2-type Mphs are devoid of antigen presenting functions, but they do play an important role in protection against extracellular pathogens,26 wound healing, and tissue repair.27
GM-CSF is generally regarded as a pro-inflammatory cytokine, which facilitates Mph migration to the periphery and supports classical M1-type Mph activation pathway. Functional balance between migrating M1-type Mph cells and resident M2-type Mphs is believed to determine in part, if not completely, the course of inflammatory process.5 However, direct inhalation exposure of GM-CSF was recently reported to confer anti-inflammatory activity on lung Mphs.28 In agreement with this notion, GM-CSF produced in tumor foci in situ has been shown to facilitate alternative M2-type Mph polarization, thus providing immunosuppressive tumor-supportive milieu.29 Furthermore, GM-CSF-treated Mphs have been shown to lack dominant expression of pro-inflammatory genes,5 altogether suggesting that GM-CSF does not appear to be a cytokine that unidirectionally polarizes Mph cells toward M1 (or indeed M2) phenotype.
Indeed, data presented in this report are consistent with a dual role for GM-CSF in an inflammatory process. On the one hand, GM-CSF could stimulate migration of Mc/Mphs and lymphocytes to inflammatory foci, and support their survival in the periphery. This is achieved via: (i) stimulation of pro-inflammatory cytokine production (TNF-α and IL-6) in Mphs,30 and (ii) reduction in the proportion of the cells expressing CD197+ (CCR7 chemokine receptor), which is likely to be instrumental in promoting Mph migration in lymph tissues. Accordingly, down-regulation of ССR7 expression with reciprocal ССR5 expression has been shown previously to promote Mph migration in the periphery to sites of inflammation.20,31 It is tempting to speculate that from the immunological standpoint, the accumulation of Mc/Mphs in inflammatory sites nurtures conditions for antigen uptake with subsequent antigen processing and presentation, as well as for further differentiation of Mphs into DCs, could promote inductive immunogenesis in the periphery. Such Mph differentiation is accompanied by increase in CCR7 expression on DCs, thus effectively permitting their migration to lymph nodes for developing adaptive immunogenesis.32 On the other hand, by down-regulating CD14, СD16, and CD119 expression (and possibly other Mph-related molecular receptors for inflammatory ligands), GM-CSF could prevent (or restrain) the development of excessive inflammatory activity, which otherwise could hinder the development of a well-balanced adaptive immunogenesis and lead to a severe tissue damage.
Clearly, the effect of GM-CSF on adaptive immunogenesis is not restricted to just Mph cells, but also involves T cells. Here, we showed that GM-CSF treatment was facilitative of direct CD38-dependent activation of relatively low-differentiated T cells, which could migrate to lymphoid tissues and proliferate if appropriate antigenic stimuli are available. Interestingly, in our experiments, GM-CSF did not enhance CD25 expression, and neither it up-regulated T cell sensitivity to IL-2 (data not shown). We speculate that positive GM-CSF-mediated effects on the generation of immunological memory are likely to be associated with its ability to stimulate immunogenic antigen-presentation, rather than with a direct growth effect on T cells. Indeed, CD38 has been shown to concentrate in the contact area of T cell-antigen-presenting cell immunologic synapse,33 suggesting that GM-CSF could affect antigen presentation by fine-tuning immunological synapse configuration via a putative CD38-dependent mechanism.
Taken together, phenotypical data obtained here suggest that local GM-CSF production would stimulate influx of Mc/Mphs to the periphery and their subsequent differentiation into DCs. Furthermore, through favoring T-cell-mediated immunogenesis in regional lymph nodes, GM-CSF could promote the development of the antigen-driven immune memory. In this case, anti-inflammatory activity of GM-CSF would protect tissues per se from excessive cytodestructive inflammation and provide a supportive environment for immune memory formation.
We stress that our experimental system is not without some limitations. Thus, we studied the effects of GM-CSF on Mphs and T cells upon their relatively brief exposure to this cytokine in the absence of co-stimulatory signals in order to minimize secondary effects of mediators produced by immunocompetent cells. This particular situation could to some extent underlie relatively minor functional GM-CSF-mediated changes observed in immunocompetent cells studied. We argue that under in vivo conditions those immunocompetent cells are subject to a more robust multicomponent cell- matrix- and cytokine-dependent stimulation, such that GM-CSF-mediated effects on initiation and maintenance of the adaptive immunogenesis could be much more pronounced, thus playing more substantial role in it.
Interestingly, the hematopoietic system of GM-CSF-deficient mice appears to be normal, with the most significant changes observed in some individual T cell responses.34 This data denote that GM-CSF could be a more significant player in positive regulation of the adaptive immunogenesis rather than of the innate immunity. This conclusion is indeed in agreement with the results of this study, as well as with the current paradigm on the role of GМ-CSF as an immunostimulatory cytokine. On the other hand, GM-CSF plays a key role in the generation of myeloid-derived suppressor cells known to exert negative effects on the development of adaptive T cell immune reactivity. Therefore, multi-directional effects of GM-CSF on innate and adaptive immune reactivity require caution as far as therapeutic applications of this cytokine are concerned.
Conclusions
In general, major effects of GM-CSF on immunity could be directed toward establishing a favorable migration pattern, antigen processing and presentation conditions for implementing adaptive immune reactivity, with concomitant protection against excessive pro-inflammatory activation of the immune system.
Materials and methods
The study protocol has been approved by the committee on human research at the Immanuel Kant Baltic Federal University (№7/10.03.2015). Heparinized blood samples were taken from median cubital vein of 22 healthy donors (both men and women aged between 21 and 40 years) according to a standard clinical procedure. Signed informed consent forms were obtained from all donors.
Isolation of cells and assessment of cell viability
Peripheral blood mononuclear cells (PBMCs) were isolated from blood using Ficoll-Paque (Ficoll-Paque™ PREMIUM, 1.077 ± 0.001 g/mL, GE Healthcare, USA) gradient centrifugation. CD14-positive cells were isolated from PBMCs by magnetic column separation (MS columns, Miltenyi Biotec, Bergisch Gladbach, Germany) using CD14 MicroBeads (CD14 Micro Beads human, Miltenyi Biotec). CD3 + T lymphocytes were also isolated by magnetic column separation using CD3 MicroBeads (CD3 Micro Beads human, Miltenyi Biotech). All procedures were performed exactly as specified in the manufacturer’s instruction. Cells were counted using a Z2 Cell and Particle counter (Beckman Coulter Inc., Fullerton, USA).
Cell cultures
Isolated CD14-positive cells were cultured at 1.0–1.5 × 106/ml in 24-well plates in the presence or absence of bacterial LPS (1.0 μg/ml, Salmonella typhi, Pyrogenalum, Medgamal, Scientific Research Institute of Epidemiology and Microbiology named after N. F. Gamalei, Russian Academy of Russia) in a serum-free cell culture medium TexMACS™ (Miltenyi Biotec) supplemented with 5.0 × 10−5 2-mercaptoethanol (Acros Organics/Thermo Fisher Scientific, NJ, USA) in a humidified CO2 incubator at 37ºC for 24 h. Isolated T cells were cultured at 1.0–1.5 × 106/ml in the presence or absence of MACSiBead particles conjugated with antibodies (Abs) against human CD2, CD3, and CD28 (T Cell Activation/Expansion Kit, human, MACS Miltenyi Biotec) in the same cell culture medium for 48 h. Both Mc/Mph and T cell cultures were incubated in the presence (test cultures) or absence (negative controls) of a tenfold dilution series of recombinant human GM-CSF (0.01; 0.1; 1.0, and 10.0 ng/ml).
Flow cytometry
Surface characteristics of Mc/Mph cells were studied by staining with a cocktail of the following specific monoclonal antibody (Ab)-based reagents: peridinin chlorophyll (PerCP)-labeled anti-CD14 (eBioscience, USA), fluorescein isothiocyanate (FITC)-labeled anti-CD16, phycoerythrin (PE)-labeled anti-CD119, allophycocyanin (APC)-labeled anti-CD124, and PE/Alexa Fluor® 488-labeled anti-CD197 (BioLegend, San Diego, CA). Phenotype of various T cell subsets was analyzed using the following Ab reagents: СD4-PerCP, CD115-FITC (eBioscience), CD197-PE, CD45RA-APC (BD Pharmingen, San Jose, CA), and CD25-FITC (BioLegend). Cell viability was assessed by staining with a membrane impermeable dye propidium iodide (PI) (eBioscience) followed by flow cytometry. Single-stained samples were used to determine the levels of compensation for spectral overlaps. Positive/negative boundaries and non-specific binding were established by using unstained controls and fluorescence minus one controls (FMO). To account for the nonspecific antibody binding, we used isotype controls (Iso IgG2a for APC, PE, AF488; Iso IgG1 for FITC, PE) (BioLegend). Flow cytometry was performed on a BD Accuri™ C6 flow cytometer (BD Biosciences, San Jose, CA, USA), and data analysis was done using BD Accuri™ C6 Software package (BD Biosciences).
ELISA
Concentrations of interleukin-1β (IL-1β), IL-2, IL-4, IL-10, IL-6, interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) in cell culture supernatants were measured using commercially available ELISA kits (Vector-Best, Novosibirsk, Russian Federation), according to the manufacturer’s instructions, using an Automated EIA and Chemistry Analyzer (ChemWell 2910, Awareness Technology, Inc., Palm City, FL).
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics for Windows, version 20.0 (Armonk, NY: IBM Corp). None of the quantitative variables in the comparison groups were normally distributed according to the Kolmogorov–Smirnov test. Therefore, comparison between independent groups was performed using the Mann–Whitney U test. Median (Me) with the first and third quartiles [Ме (Q1-Q3)] were calculated for the variables in the comparison groups. P < .05 was considered statistically significant.
Abbreviations
| Ab | antibody |
| DC | dendritic cell |
| GM-CSF | granulocyte-macrophage colony stimulating factor |
| IFN | interferon |
| IL | interleukin |
| GM-CSFR | receptor for GM-CSF |
| Мс | monocyte |
| Mph | macrophage |
| PBMC | peripheral blood mononuclear cell |
| TNF | tumor-necrosis factor. |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Mach N, Gillessen S, Wilson SB, Sheehan C, Mihm M, Dranoff G.. Differences in dendritic cells stimulated in vivo by tumors engineered to secrete granulocyte-macrophage colony-stimulating factor or Flt3-ligand. Cancer Res. 2000;60(12):3239–46. [PubMed] [Google Scholar]
- 2.Hercus TR, Thomas D, Guthridge MA, Ekert PG, King-Scott J, Parker MW, Lopez AF. The granulocyte-macrophage colony-stimulating factor receptor: linking its structure to cell signaling and its role in disease. Blood. 2009;114(7):1289–98. doi: 10.1182/blood-2008-12-164004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Codarri L, Gyulveszi G, Tosevsld V, Hesske L, Fontana A, Magnenat L, Suter T, Becher B. ROR gamma t drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011;12(6):560–67. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 4.El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, Zhang GX, Dittel BN, Rostami A. The encephalitogenicity of T(H)17 cells is dependent on IL-l- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol. 2011;12(6):568–75. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamilton T, Zhao C, Pavicic P, Datta S. Myeloid colony-stimulating factors as regulators of macrophage polarization. Front Immunol. 2014;5:554. doi: 10.3389/fimmu.2014.00554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griffin JD, Cannistra SA, Sullivan R, Demetri GD, Ernst TJ, Kanakura Y. The biology of GM-CSF: regulation of production and interaction with its receptor. Int J Cell Cloning. 1990;8(1):35–45. doi: 10.1002/stem.5530080705. [DOI] [PubMed] [Google Scholar]
- 7.Miyajima A. Molecular structure of the IL-3, GM-CSF and IL-5 receptors. Int J Cell Cloning. 1992;10(3):126–34. doi: 10.1002/stem.5530100302. [DOI] [PubMed] [Google Scholar]
- 8.Croxford AL, Spath S, Becher B. GM-CSF in neuroinflammation: licensing myeloid cells for tissue damage. Trends Immunol. 2015;36(10):651–62. doi: 10.1016/j.it.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 9.van de Laar L, Coffer PJ, Woltman AM. Regulation of dendritic cell development by GM-CSF: molecular control and implications for immune homeostasis and therapy. Blood. 2012;119(15):3383–93. doi: 10.1182/blood-2011-11-370130. [DOI] [PubMed] [Google Scholar]
- 10.Elliott MJ, Vadas MA, Eglinton JM, Park LS, To LB, Cleland LG, Clark SC, Lopez AF. Recombinant human interleukin-3 and granulocyte-macrophage colony-stimulating factor show common biological effects and binding characteristics on human monocytes. Blood. 1989;74(7):2349–59. [PubMed] [Google Scholar]
- 11.Onetto-Pothier N, Aumont N, Haman A, Bigras C, Wong GG, Clark SC, De Léan A, Hoang T. Characterization of granulocyte-macrophage colony-stimulating factor receptor on the blast cells of acute myeloblastic leukemia. Blood. 1990;75(1):59–66. [PubMed] [Google Scholar]
- 12.Becher B, Tugues S, Greter M. GM-CSF: from growth factor to central mediator of tissue inflammation. Immunity. 2016;45(5):963–73. doi: 10.1016/j.immuni.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 13.Wada H, Noguchi Y, Marino MW, Dunn AR, Old LJ. T cell functions in granulocyte/macrophage colony-stimulating factor deficient mice. Proc Natl Acad Sci USA. 1997;94(23):12557–61. doi: 10.1073/pnas.94.23.12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meniailo ME, Malashchenko VV, Shmarov VA, Gazatova ND, Melashchenko OB, Goncharov AG, Seledtsova GV, Seledtsov VI. Interleukin-8 favors pro-inflammatory activity of human monocytes/macrophages. Int Immunopharmacol. 2018;56:217–21. doi: 10.1016/j.intimp.2018.01.036. [DOI] [PubMed] [Google Scholar]
- 15.Melashchenko OV, Meniailo ME, Malashchenko VV, Gazatova ND, Goncharov ND, Seledtsova GV, Seledtsov VI. Erythropoietin-mediated modulation of human macrophage functionality. Curr Pharm Biotechnol. 2018;19(11):902–09. doi: 10.2174/1389201019666181031164520. [DOI] [PubMed] [Google Scholar]
- 16.Lau MY, Dharmage SC, Burgess JA, Win AK, Lowe AJ, Lodge C, Perret J, Hui J, Thomas PS, Morrison S, et al. The interaction between farming/rural environment and TLR2, TLR4, TLR6 and CD14 genetic polymorphisms in relation to early-and late-onset asthma. Sci Rep. 2017;7:43681. doi: 10.1038/srep43681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukherjee R, Kanti Barman P, Kumar Thatoi P, Tripathy R, Kumar Das B, Ravindran B. Non-classical monocytes display inflammatory features: validation in sepsis and systemic lupus erythematous. Sci Rep. 2015;5:13886. doi: 10.1038/srep13886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eshleman EM, Delgado C, Kearney SJ, Friedman RS, Lenz LL. Down regulation of macrophage IFNGR1 exacerbates systemic L. monocytogenes infection. PLoS Path. 2017;13(5):e1006388. doi: 10.1371/journal.ppat.1006388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hurdayal R, Brombacher F. Interleukin-4 receptor alpha: from innate to adaptive immunity in murine models of gutaneous leishmaniasis. Front Immunol. 2017;8:1354. doi: 10.3389/fimmu.2017.01354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kudryavtsev IV. Memory T cells: major populations and stages of differentiation. Russ J Immunol. 2014;8(4):947–64. [Google Scholar]
- 21.Todosenko NM, Shmarov VA, Malashchenko VV, Meniailo ME, Melashchenko OB, Gazatova ND, Goncharov AG, Seledtsov VI. Erythropoietin exerts direct immunomodulatory effects on the cytokine production by activated human T-lymphocytes. Int Immunopharmacol. 2016;36:277–81. doi: 10.1016/j.intimp.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Shmarov VA, Malashchenko VV, Meniailo ME, Gazatova ND, Todosenko NM, Melashchenko OB, Goncharov AG, Seledtsov VI. Direct effects of interleukin-7 on the function of human T cells in vitro. Eur Cytokine Netw. 2016;27(4):102–07. doi: 10.1684/ecn.2016.0385. [DOI] [PubMed] [Google Scholar]
- 23.Malashchenko VV, Meniailo ME, Shmarov VA, Gazatova ND, Melashchenko OB, Goncharov AG, Seledtsova GV, Seledtsov VI. Direct anti-inflammatory effects of granulocyte colony-stimulating factor (G-CSF) on activation and functional properties of human T cell subpopulations in vitro. Cell Immunol. 2018;325:23–32. doi: 10.1016/j.cellimm.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Meniailo ME, Malashchenko VV, Shmarov VA, Gazatova ND, Melashchenko OB, Goncharov AG, Seledtsova GV, Seledtsov VI. Direct effects of interleukin-8 on growth and functional activity of T lymphocytes. Int Immunopharmacol. 2017;50:178–85. doi: 10.1016/j.intimp.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 25.Vogel DY, Glim JE, Stavenuiter AW, Breur M, Heijnen P, Amor S, Dijkstra CD, Beelen RH. Human macrophage polarization in vitro: maturation and activation methods compared. Immunobiology. 2014;219(9):695–703. doi: 10.1016/j.imbio.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 26.Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, Mohammadi A, Afshari JT, Sahebkar A. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233(9):6425–40. doi: 10.1002/jcp.26429. [DOI] [PubMed] [Google Scholar]
- 27.Seledtsov VI, Seledtsova GV. A balance between tissue-destructive and tissue-protective immunities: a role of toll-like receptors in regulation of adaptive immunity. Immunobiology. 2012;217(4):430–35. doi: 10.1016/j.imbio.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 28.Halstead ES, Umstead TM, Davies ML, Kawasawa YI, Silveyra P, Howyrlak J, Yang L, Guo W, Hu S, Hewage EK, et al. GM-CSF overexpression after influenza a virus infection prevents mortality and moderates M1-like airway monocyte/macrophage polarization. Respir Res. 2018;19(1):3. doi: 10.1186/s12931-017-0708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sielska M, Przanowski P, Wylot B, Gabrusiewicz K, Maleszewska M, Kijewska M, Zawadzka M, Kucharska J, Vinnakota K, Kettenmann H, et al. Distinct roles of CSF family cytokines in macrophage infiltration and activation in glioma progression and injury response. J Pathol. 2013;230(3):310–21. doi: 10.1002/path.4192. [DOI] [PubMed] [Google Scholar]
- 30.Darrieutort-Laffite C, Boutet MA, Chatelais M, Brion R, Blanchard F, Heymann D, Le Goff B. IL-1ß and TNFa promote monocyte viability through the induction of GM-CSF expression by rheumatoid arthritis synovial fibroblasts. Mediators Inflamm. 2014;2014:241840. doi: 10.1155/2014/241840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geginat J, Sallusto F, Lanzavecchia A. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4+ T cells. J Exp Med. 2001;194(12):1711–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Son Y, Kim BY, Park YC, Kim K. Diclofenac inhibits 27-hydroxycholesterol-induced differentiation of monocytic cells into mature dendritic cells. Immune Netw. 2017;17(3):179–85. doi: 10.4110/in.2017.17.3.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muñoz P, Mittelbrunn M, de la Fuente H, Pérez-Martínez M, García-Pérez A, Ariza-Veguillas A, Malavasi F, Zubiaur M, Sánchez-Madrid F, Sancho J. Antigen-induced clustering of surface CD38 and recruitment of intracellular CD38 to the immunologic synapse. Blood. 2008;111(7):3653–64. doi: 10.1182/blood-2007-07-101600. [DOI] [PubMed] [Google Scholar]
- 34.Shi Y, Liu CH, Roberts AI, Das J, Xu G, Ren G, Zhang Y, Yuan ZR, Tan HS, Das G, et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: what we do and don’t know. Cell Res. 2006;16:126–33. doi: 10.1038/sj.cr.7310017. [DOI] [PubMed] [Google Scholar]


