ABSTRACT
Background: Traditional measurements of vaccine coverage at specific ages can mask poor vaccine timeliness. However, optimal measurement of timing is unclear due to variations in countries’ recommended vaccination schedules and lack of a commonly accepted standard for “timeliness”. We conducted a systematic review of literature on vaccine timeliness and delay in low- and middle-income countries from 2007 to 2017.
Methods: A search of articles published between January 1 2007 and December 31 2017, was performed in PubMed, EBSCOhost, and Embase.
Results: 67 papers were included, of which 83% used a categorical measure of delay and 41% evaluated continuous delay. The most common age at assessment was 1 month, with earlier age benchmarks typically used with birth doses.
Conclusions: Categorical definitions of vaccination timing vary widely, with benchmarks of delay varying from days to weeks to months. Use of a continuous measure of vaccine delay may be more informative and comparable.
KEYWORDS: Vaccine timeliness, vaccine coverage, expanded program on immunization
Introduction
Vaccines have proven to be one of the most effective preventive interventions and remain one of public health’s most successful means for controlling and eradicating serious and sometimes fatal diseases.1 Since 1974, the World Health Organization (WHO) has promoted vaccines through the Expanded Program on Immunization (EPI), which includes recommendations for countries to publicly fund select vaccines.2 The initial EPI-recommended pediatric vaccines were Bacillus Calmette-Guérin (BCG), oral polio (OPV), diphtheria-tetanus-pertussis (DTP), and measles vaccine, although the list has since expanded to include hepatitis B, Haemophilus influenzae type b (Hib), rubella, pneumococcal conjugate vaccine (PCV), and rotavirus vaccine.2 Most countries have a National Immunization Technical Advisory Group (NITAG) which provides country-specific recommendations, such as which vaccines are offered, whether combination vaccines are used, and when vaccines should be administered.3
Widespread use of these vaccines has had a lasting impact on the occurrence of vaccine-preventable diseases. The incidence of most vaccine-preventable diseases in the US has declined >99% over the twentieth century.4 Worldwide, we are on the verge of polio and measles eradication, due in part to the widespread adoption of EPI programs.
Vaccination program performance has typically been assessed through measurements of vaccine coverage: the proportion of individuals who have received a vaccine by a benchmark age (such as 5 years), regardless of the timing of administration.5 However, vaccination coverage and timeliness of vaccine administration are related but separate issues, and high levels of coverage can sometimes mask low levels of timeliness. For example, an analysis of the Vaccine Safety Datalink, a consortium of 10 healthcare delivery organizations in the United States, shows that although vaccination coverage is high (>90%),6,7 less than half of the children had received all vaccine doses on time.6 Untimely vaccination could relate to access or affordability, or could instead be the result of the parent’s not accepting the recommended vaccine schedule and being vaccine hesitant.8,9
Studies measuring vaccine timeliness have become more frequent in the literature in recent years. In 1998, Bolton et al. explored issues with vaccination coverage estimates within Baltimore, Maryland, and recommended the use of age-appropriate indicators,10 and a 2007 article11 and 2009 editorial12 both encouraged more regular measurement of vaccine timeliness. Subsequently, additional studies have been conducted focusing on low- and middle-income countries (LMICs). LMICs have a disproportionately high burden of vaccine-preventable diseases, which are often more serious in younger infants so that administration of vaccines at an early age (as scheduled) is especially important.13 Additionally, children in LMICs are more likely to be non- and under-vaccinated and encounter significant barriers to vaccination access, magnifying the challenges faced in vaccination receipt and delivery. However, the optimal measure of timeliness remains unclear, resulting from both differences in schedules among different countries, but more so by confusion as to what constitutes “timeliness” relative to vaccination date recommendations. There currently is no agreed-upon definition of timeliness across international bodies.
This systematic review presents a summary of the literature on vaccine timeliness and delay in low- and middle-income countries between the years of 2007 and 2017, with a focus on timeliness definition, age at assessment, countries/setting of research, and analytic approach, among others. It is the first paper, to the author’s knowledge, which seeks to evaluate variable ways of measuring and quantifying vaccine timing in LMICs. This manuscript serves to describe the findings of previously conducted studies and to provide recommendations for future studies measuring vaccination timing.
Methods
This systematic review followed guidelines from the Preferred Reporting Items of Systematic reviews and Meta-Analyses (PRISMA).14
Search strategies
Searches were performed in 3 different electronic databases: PubMed/MEDLINE, EBSCOhost, and Embase. The search terms used were (“vaccination” or “immunization” or “immunisation” or “vaccine” or “EPI”) and (“timely administration” or “timeliness” or “on time”). The searches were limited to papers published between January 1, 2007 and December 31, 2017.
The titles of all papers returned through the use of the search terms were initially screened for relevance. The abstracts of all remaining papers were then assessed with specific inclusion and exclusion criteria in mind. Manuscripts were included if they met the following inclusion criteria: (1) studies were conducted on data from LMICs, as defined by the World Bank;15 (2) studies examined pediatric vaccines that were a part of the WHO EPI2 – studies only featuring adolescent and adult vaccines (e.g. human papillomavirus vaccine (HPV)) or vaccines not on the EPI (e.g. influenza vaccine) were excluded; (3) studies had to calculate some measure of timeliness – broadly defined as a vaccination measure which was age-specific; (4) the study had to provide some analysis about vaccination timeliness – studies presenting only descriptive statistics were excluded; comparisons between groups with or without statistical testing was required and, (5) the study had to be a non-randomized-controlled trial exploring vaccination outcomes, as RCTs test an intervention to change vaccine administration rather than assess vaccination timeliness. Finally, non-English studies were excluded.
Vaccination timing definitions
We use the following definitions: vaccine uptake refers to any vaccine measurement, vaccine coverage is the vaccine uptake measured without regard to any timing measure, and vaccination timing is vaccine uptake with some measurement of time or date. Vaccination timing could refer to one of the three conditions: (1) up-to-date vaccination means an individual received a vaccine by a certain threshold age, (2) vaccination delay is the continuous measurement of vaccination – either in the age of vaccination, or the time elapsed since the vaccine was recommended to be administered, and (3) vaccination timeliness refers to vaccination administered within a certain time since a recommended age of vaccination.
Study selection
NBM removed all duplicates and assessed all titles for relevance. Two reviewers (NBM/ALW) independently assessed all abstracts and full-text publications for eligibility using the eligibility criteria laid out. All disagreements were resolved by discussion between reviewers, and the tie-break of a third reviewer. In addition to assessment for relevance, data were also extracted independently and a quality assessment was conducted by the two reviewers (NBM/ALW). The following data were abstracted: location of study; study population; study design; sample size; study year; vaccines considered; definition of timeliness/vaccine delay; explanatory variables considered. Risk of bias was assessed using an adaptation of the Downs & Black checklist,16 which had previously been used in a systematic review of vaccine uptake.17 Items relating to interventions (Question 4, 8, 13–15, 19, 20, 23, 24, and 26) were dropped as they were not relevant to our inclusion criteria. As a result of eliminating the aforementioned questions, the maximum quality score was an 18. Studies were considered to be good quality if they achieved a score of 14–19, Moderate quality if they achieved a score of 9–13, and low quality if they achieved a score of <9. Further information regarding the quality assessment can be found in the Supplementary Materials.
Study quality and synthesis of findings
The goal of this review was to examine how researchers define and analyze vaccine timeliness. We present a description of the current state of studies on vaccination timeliness, and discuss the temporal and geographical distribution, the study and analytical design, the definition of timeliness, and the inter-group comparisons of timeliness.
Ethical approval
As a systematic review of previously published manuscripts, this study was not under the purview of studies examined by the University of Michigan Health Sciences and Behavioral Sciences Institutional Review Board.
Results
Overview of included papers
The search terms yielded 488 articles for screening from PubMed, 388 papers from EBSCOhost, of which 16 were duplicates from the PubMed search, and 636 papers from Embase, of which 271 were unique non-duplicates with either PubMed or EBSCOhost. After the title, abstract, and full paper screen, 67 papers were included in the systematic review.18–84 Full details regarding paper selection are found in Figure 1.
Figure 1.

Paper selection scheme.
Definition
Studies measuring timing were generally of two types: those using continuous vs. categorical measures, although some papers used both. Only one study75 did not explicitly evaluate vaccine timing on either a continuous or categorical scale, but rather just measured whether children were up-to-date by a threshold date of 14 weeks. Of the 66 remaining studies, 27 (41%) papers used a continuous outcome such as median delay in days or weeks: for example, Hughes et al. graphed cumulative incidence curves of age at vaccination for BCG, pentavalent, and polio vaccine series.39 More simply analyzed, Wagner et al. present the mean age at measles vaccination for a number of different demographic groups.76 The majority of papers (55/66, 83%) used some categorical measure of timeliness. More than half of these papers (30/55, 55%) used variable definitions for vaccine timeliness based on the vaccine in question (i.e. different definitions for measles vs. DTP vs. HepB), whereas (21/55, 38%) used a fixed definition of timeliness for all considered vaccines. Only 7% of papers (4/55) used an interval definition between doses to determine timeliness.
The most common age benchmark used to assess timeliness was around 1 month. All but three (18/21, 86%) of papers which used a fixed definition employed this timeframe, operationalized as 1 month [9 papers], 4 weeks [3 papers], 28 days [3 papers], 4.3 weeks [1 paper] and within the same month as recommended [2 papers]. Additionally, there were a few papers which used an age benchmark for timely vaccination of around 1 month for all vaccines other than Hepatitis B vaccine, which had a timeliness definition of 1 day [2 papers]. Other age benchmarks used were 1 day (4/55, 7%), though 3 of these papers were combined with longer delay definitions for other vaccines, 1 week (3/55, 5%) or 2 weeks (6/55, 11%). Lower benchmarks (e.g. 1 day, 1 week) were typically used with birth doses (HepB, BCG, OPV0). Most papers did not explain why specific vaccination benchmarks were selected; those that did indicated that the recommendations derived from various sources, including manufacturer’s instructions77 and/or used the WHO definition of full vaccination according to scheduled vaccination dates, not definitions of timeliness.52 Finally, 4 papers (7%) evaluated timeliness in terms of the interval which elapsed between doses rather than the date of vaccination itself, which would result in ‘timely’ vaccination calculations if the first vaccine in the series was initiated late but all subsequent vaccines were given within the recommended interval.21,23,73,84
Country under study
The number of relevant studies published by year increased from 1 in 2007 to 9 in 2017, with noticeably more papers examining vaccine timing per year since 2014 (Figure 2). Overall, the largest represented WHO region was the African region (29/67, 43%). The next most represented WHO region was the Western Pacific region (19/67, 28%) with 68% of these papers (13/19) focused on China. Considerably fewer studies were conducted in the Region of the Americas (10/67, 15%), the South East Asian Region (8/67, 12%), and the Eastern Mediterranean Region (8/67, 12%). Finally, only 3 studies (4%) were conducted in the European Region (Table 2). Studies used data from 79 different countries (Table 3), with the most common countries studied being China (13/67, 19%) and Kenya (8/67, 12%). Most studies (53/67, 79%) were not nationally representative and instead involved samples from a particular district or city, 10 (15%) used nationally representative data from a single country, and 4 (6%) studies used data from multiple countries. There was no discernible pattern of specific countries as more common sources of data over the study period from 2007 to 2017.
Figure 2.

Number of papers included by year of publication, 2007–2017 (n = 67).
Table 2.
Number of papers by WHO region.
| WHO Region | Number of Papers |
|---|---|
| African Region | 29 |
| Western Pacific Region | 19 |
| Region of the Americas | 10 |
| South East Asian Region | 8 |
| Eastern Mediterranean Region | 8 |
| European Region | 3 |
Table 3.
Number of included papers by country of study.
| Country | Number of Papers |
|---|---|
| China | 13 |
| Kenya | 8 |
| Burkina Faso, Ghana, India | 5 |
| Gambia, Iran, Nigeria | 4 |
| Bangladesh, Brazil, Cameroon, Malawi, Senegal, Tanzania, Uganda | 3 |
| Colombia, Cote d’Ivoire, Democratic Republic of Congo, El Salvador, Guatemala, Guinea-Bissau, Haiti, Kazakhstan, Kyrgyzstan, Mauritania, Pakistan, Peru, Sierra Leone, Togo, Uzbekistan, Yemen | 2 |
| Albania, Armenia, Belarus, Belize, Benin, Bolivia, Bosnia and Herzegovina, Burundi, Cambodia, Chad, Comoros, Djibouti, Dominican Republic, Egypt, Eritrea, Federated States of Micronesia, Gabon, Guinea, Guyana, Honduras, Iraq, Jamaica, Laos, Lesotho, Macedonia, Madagascar, Mali, Mongolia, Montenegro, Morocco, Mozambique, Namibia, Nepal, Nicaragua, Niger, Papua New Guinea, Philippines, Rwanda, Serbia, South Africa, Syria, Thailand, Trinidad and Tobago, Turkey, Venezuela, Vietnam, Wallis and Futuna, Zambia | 1 |
Antigens
The vaccines assessed are shown in Figure 3. The most common vaccine was BCG (38/67, 57%), followed by the measles-containing vaccine (33/67, 49%), and OPV or IPV (19/67, 28%), though some studies examined multiple vaccines (Table 1). Timeliness of DTP was assessed in 13 papers (19%) and the pentavalent vaccine was explored in 6 papers (9%). However, only 21 papers (31%) examined full vaccination (i.e. receipt of all doses of all recommended EPI vaccines) as a primary or secondary study outcome measure, with most of the surveyed papers instead addressing a specific vaccination or a combination of vaccinations, but less than full vaccination, as their study measures.
Figure 3.

Number of papers assessing the timeliness of different antigens as primary or secondary outcome measures (n = 67).
Table 1.
Summary of included papers, quality assessment, and key details related to vaccine delay.
| Authors & Year | Quality Score | Country (ies) of Study | Nationally representative | Age Range |
Survey Type | Analysis | Antigens | Delay Definition | Predictors |
|---|---|---|---|---|---|---|---|---|---|
| Akmatov, MK, Mikolajczyk RT. 2012.18 | Moderate | Burkina Faso, Burundi, Cameroon, Cote d'Ivoire, Djibouti, Gambia, Ghana, Guinea-Bissau, Malawi, Mauritania, Sierra Leone, Togo, Belarus, Kazakhstan, Albania, Bosnia and Herzegovina, Macedonia, Montenegro, Serbia, Belize, Guyana, Jamaica, Trinidad and Tobago, Bangladesh, Lao People’s Democratic Republic, Thailand, Vietnam, Mongolia, Iraq, Syria, Yemen | Yes | 12-59 months | Cross-sectional | Kaplan-Meier | BCG, Polio, DPT, MCV | 4.3 weeks, continuous delay | SES, Child Gender, Urban/Rural Environment, Household Size |
| Akmatov, MK et al. 2008.19 | Moderate | Armenia, Kazakhstan, Kyrgyzstan, Uzbekistan | Yes | 12-59 months, 0-35 months |
Cross-sectional | Kaplan-Meier, Logistic Regression | DPT, MCV | 1 month | Maternal Education, Paternal Education, Child Gender, Urban/Rural, Household Size |
| Babirye, JN et al. 2012.20 | Good | Uganda | No | 10-23 months | Cross-sectional | Kaplan-Meier, Cox Proportional Hazards | BCG, Polio, PENTA, MCV | variable | Birth Setting, Maternal Education, Number of Children, Paternal Age, Maternal Age, Marital Status, SES |
| Barman, D, Dutta, A. 2013.21 | Moderate | India | No | 12-23 months | Cross-sectional | Logistic Regression | BCG, Polio, DPT, MCV, Full Vaccination | within same month, interval | Religion, Birth Setting, Maternal Education, SES, Child Gender, Birth Order, Village Electrified, Equipment in Health Center, Drugs in Health Center… |
| Barman, M et al. 2015.22 | Good | India | No | 12-36 months | Cross-sectional | Kaplan-Meier, Cox Proportional Hazards | BCG, Polio, DPT, MCV | continuous delay | Maternal Education, Paternal Education, Mother's Age, Caste, Child Gender, Maternal Occupation, Paternal Occupation, SES |
| Bicaba, A et al. 2009.23 | Poor | Burkina Faso | Yes | unclear | Cross-sectional | Cumulative-Age-At-Vaccination Curves | BCG, DTP, MCV, Full Vaccination | interval, continuous delay | Region |
| Calhoun, LM et al. 2014.24 | Good | Kenya | No | 12-23 months | Cross-sectional | Logistic Regression | BCG, Polio, PENTA, MCV, Full Vaccination | within same month | Maternal Education, Paternal Education, Child Gender, Mother's Age, Birth Order, Household Size, Distance to Clinic, Maternal and Paternal Occupation, Mother & Father Alive, Mother with child at interview, Mother looks after Child, Paternal Age, Orphan Status… |
| Chiabi, A et al. 2017.25 | Good | Cameroon | No | 0-11 months | Cross-sectional | Logistic Regression | BCG, Polio, PENTA, MCV, PCV, Rotavirus, Full Vaccination | 2 weeks | Religion, Maternal Education, Mother's Age, Marital Status, Distance to the Clinic, Paternal Education, Maternal Occupation, Paternal Occupation |
| Clark, A, Sanderson C. 2009.26 | Moderate | Bangladesh, Benin, Bolivia, Brazil, Burkina Faso, Cambodia, Cameroon, Chad, Colombia, Comoros, Congo, Côte d’Ivoire, Dominican Rep, Egypt, Eritrea, Gabon, Ghana, Guatemala, Guinea, Haiti, Honduras, India, Kenya, Kyrgyz, Lesotho, Madagascar, Malawi, Mali, Mauritania, Morocco, Mozambique, Namibia, Nicaragua, Niger, Nigeria, Peru, Rwanda, Senegal, Tanzania, Togo, Turkey, Uganda, Uzbekistan, Yemen, Zambia | Yes | 0-5 years | Cross-sectional | Cumulative-Age-At-Vaccination Curves | BCG, DPT, MCV | continuous delay | Birth Setting, Maternal Education, Child Gender, Mother's Age, Child Age, Urban/Rural, Birth Order |
| D’Ardenne, KK et al. 2016.27 | Good | Guatemala and Peru | No | 0-5 years | Cross-sectional | Log Binomial Regression, Cumulative-Age-At-Vaccination Curves | PENTA, MMR, Rotavirus | 28 days | Maternal Education, Child Gender, Mother's Age, Child Age, Household Size, Food Insecurity, Birth Cohort |
| Delrieu, I et al. 2015.28 | Moderate | Burkina Faso, Ghana, Kenya, Senegal, Tanzania | Yes | 0-5 years | Cross-sectional | Kaplan-Meier | DPT, MCV | continuous delay | N/A |
| Ettarh, RR et al. 2012.29 | Moderate | Kenya | No | 9–59 months | Cross-sectional | Kaplan-Meier, Cox Proportional Hazards | MCV | 1 month | Ethnicity, Maternal Education, SES, Child Gender, Village |
| Fadnes, LT et al. 2011.30 | Good | South Africa | No | 0-2 years | Cluster RCT (non-vaccination outcome) | Kaplan-Meier, Cox Proportional Hazards | BCG, Polio, PENTA, Full Vaccination | variable | Birth Setting, Maternal Education, SES, Household Size, Breastfeeding, Peer Counseling, Mother's BMI, Marital Status |
| Fadnes, LT et al. 2011.31 | Good | Uganda | No | 0-2 years | Cluster RCT (non-vaccination outcome) | Kaplan Meier, Cox Proportional Hazards | BCG, Polio, PENTA, MCV | variable | Birth Setting, Maternal Education, SES, Child Gender, Mother's Age, Urban/Rural, Household Size, Mother's BMI, Health Counseling |
| Fisker, AB et al. 2014.32 | Good | Guinea-Bissau | No | 12-23 months | Cohort | Rank Sum Test | BCG, PENTA, Polio, MCV, Full Vaccination | continuous delay | Ethnicity, Maternal Education, Child Gender, Mother's Age, Child Age, Introduction of new Vaccine, Mid-Upper Arm Circumference |
| Flannery, B et al. 2013.33 | Moderate | Brazil | Yes | unclear | Cross-sectional | N/A | DTP-Hib, Rotavirus | variable | N/A |
| Gibson, DG et al. 2015.34 | Moderate | Kenya | No | 12-23 months | Cross-sectional | Log Binomial Regression | PENTA, MCV, Full Vaccination |
1 month | Maternal Education, SES, Child Gender, Mother's Age, Child Age, Marital Status, Previous Child Died, Distance to Clinic,Maternal Literacy, Number of Children <5, Mother's Phone Ownership |
| Gram, L. et al. 2014.35 | Good | Ghana | No | 0-1 year | Cross-sectional | Cox Proportional Hazards | BCG, Polio, PENTA, MCV, Full Vaccination |
Variable, continuous delay | Religion, Birth Setting, Maternal Education, SES, Child Gender, Mother's Age, Household Size, Distance to Clinic |
| Hu, Y et al. 2013.36 | Moderate | China | No | 8-48 months | Cohort | Kaplan-Meier, Logistic Regression | MCV | 1 month | Maternal Education, SES, Mother's Age, Household Size, Mother's Occupation |
| Hu, Y et al. 2014.37 | Good | China | No | 2-3 years | Cross-sectional | Kaplan-Meier, Logistic Regression | HepB, Polio, DPT, MCV | Variable, continuous delay |
Birth Setting, Maternal Education, SES, Child Gender, Mother's Age, Household Size, Distance to Clinic, Mother's occupation, Resident (migrant) Status, Caregiver Attitude towards Vaccination, Awareness of Immunization, Satisfaction with Immunization Service |
| Hu, Y et al. 2017.38 | Good | China | No | 24-35 months | Cross-sectional | Kaplan Meier, Cox Proportional Hazards | BCG, HepB, Polio, DPT, MCV, Full Vaccination | variable | Birth Setting, Maternal Education, SES, Household Size, Maternal Age, Urban/Rural, Immigration Status |
| Hughes, MM et al. 2016.39 | Good | Nepal | No | 0-6 months | RCT (non-vaccination outcome) | Kaplan Meier, Cox Proportional Hazards | BCG, Polio, PENTA | continuous delay | Ethnicity, SES, Child Gender, Child Age, Birth Order, Household Size, Gestational Age, Birthweight, Breastfeeding, Literacy |
| Iqbal, W et al. 2015.40 | Moderate | Pakistan | No | 0-2 years | Cross-sectional | Chi-Square (unadjusted) | BCG | variable | ANC, Birth Setting, Maternal Education, Paternal Education, SES, Awareness of EPI Schedule, Place of Vaccination |
| Jadidi, R et al. 2015.41 | Moderate | Iran | No | 24-47 months | Cohort | Chi-Square (unadjusted) | MMR | 1 week | Maternal Education, Paternal Education, Child Gender, Urban/Rural, Birth Order, Iranian/Non Iranian |
| Laryea, D et al. 2014.42 | Moderate | Ghana | No | unclear | Cross-sectional | N/A | BCG, Polio, PENTA, MCV | 4 weeks | ANC, Birth Setting, Child Gender, Mother's Age, Child Age, Mode of Delivery |
| Le Polain de Waroux, O et al. 2013.43 | Good | Tanzania | No | 12-23 months | Cross-sectional | Log Binomial Regression; Kaplan-Meier | BCG, DPT, MCV | 1 month | Ethnicity, Maternal Education, SES, Child Gender, Mother's Age, Child Age, Household Size, Distance to the Clinic, Mother's Occupation, Seasonality |
| Li, Q et al. 2014.44 | Poor | China | No | 1-7 years | Cohort | N/A | BCG, HepB, Polio, DPT, MCV, Full Vaccination | 1 month | Immigration Status, Municipality, Birth Cohort |
| Lin, W et al. 2014.45 | Moderate | China | No | 9-24 months | Case-Control | Logistic Regression | MCV | 1 month | Child Gender, Household Size, Distance to Clinic, Residential Status, Health Status of Child, Maternal and Paternal Occupation, History of Vaccine Delay, Awareness of Necessity of Timely Vaccination, Waiting Time in EPI Clinic… |
| Mbengue, MA et al. 2017.46 | Good | Senegal | Yes | 12-23 months | Cross-sectional | Kaplan-Meier, Cox Proportional Hazards | BCG, Polio, PENTA, MCV, PCV, Rotavirus, Full Vaccination | 4 weeks | Maternal Education, SES, Child Gender, Urban/Rural, Marital Status, Birth Order, ANC |
| Miyahara, R et al. 2016.47 | Good | Gambia | No | unclear | Cross-sectional | Logistic Regression | BCG, HepB, Polio | variable (1 day, 1 week) | Ethnicity, Maternal Education, SES, Child Gender, Mother's Age, Child Age, Birth Order, Birth Year, Birth Spacing, Birth Season |
| Moïsi, JC et al. 2010.48 | Moderate | Kenya | No | unclear | Cross-sectional | Kaplan-Meier, Cox Proportional Hazards | BCG, Polio, PENTA, MCV | continuous delay | Ethnicity, Maternal Education, Child Gender, Distance to Clinic, Migration Status |
| Mokhtari, M 2015.49 | Poor | Iran | No | 24-47 months | Cohort | Kaplan-Meier | DPT | continuous delay | Ethnicity, Maternal Education, Paternal Education, Child Gender, Urban/Rural, Birth Order, City Location, Maternal and Paternal Occupation |
| Montgomery, JP et al. 2015.50 | Good | China | No | 8 months - 8 years | Cross-sectional | Logistic Regression | MCV | variable | Child Gender, Urban/Rural, Birth Year |
| Mutua, MK et al. 2015.51 | Good | Kenya | No | unclear | Cohort | Kaplan-Meier, Cox Proportional Hazards | BCG | continuous delay | Ethnicity, Birth Setting, Maternal Education, Child Gender, Low Birth Weight, Pregnancy Intention, Type of Health Facility, Settlement Area |
| Mutua, MK et al. 2016.52 | Good | Kenya | No | 12-23 months | Cohort | Kaplan-Meier | Full Vaccination | Variable, continuous delay |
Ethnicity, Birth Setting, Maternal Education, SES, Child Gender, Study Location |
| Mvula, H et al. 2016.53 | Good | Malawi | No | >1 year | Cohort | Poisson Regression, Cox Proportional Hazards | MCV, PCV, Rotavirus | variable, continuous delay | Maternal Education, Child Gender, Mother's Age, Child Age, Marital Status, Household Size, Mother's Occupation, Orphanhood, Place of Birth, Distance to Clinic, Vaccination due in Rainy Season |
| Narváez, J et al. 2017.54 | Good | Colombia | No | 0-6 years | Cross-sectional | Mixed Models | BCG, HepB, Polio, DPT, PENTA, MMR, PCV, Rotavirus, Full Vaccination | continuous delay | Ethnicity, Maternal Education, SES, Child Gender, Mother's Age, Child Age, Household Size, Insurance, Displaced by Armed Conflict |
| Odutola, A et al. 2015.55 | Good | Gambia | No | 12-59 months | Cross-sectional | Logistic Regression | BCG, DPT, Polio, MCV | variable, continuous delay | Ethnicity, Birth Setting, Child Gender, Child Age, Marital Status, Birth Order, Family Type: Monogamous/Polygamous/Single Parent |
| Olusanya BO 2010.56 | Moderate | Nigeria | No | 0-3 months | Cross-sectional | Kaplan-Meier, Logistic Regression | BCG | variable | Religion, Ethnicity, ANC, Birth Setting, Maternal Education, SES, Child Gender, Mother's Age, Child Age, Marital Status, Birth Order, Maternal Occupation, Child has Jaundice |
| Pezzoli, L et al. 2017.57 | Moderate | Wallis and Futuna | No | 9-11 years | Cross-sectional | Kaplan-Meier | HepB | variable | N/A |
| Poorolajal, J. et al. 2012.58 | Moderate | Iran | No | 12-24 months | Cross-sectional | Kaplan-Meier | BCG, HepB, Polio, DPT, MMR | variable | ANC, Maternal Education, Child Gender, Urban/Rural, Distance to Clinic, Vaccinator's Education Level |
| Rainey, JJ. et al. 2012.59 | Poor | Haiti | Yes | 12-23 months | Cross-sectional | Rao-Scott (unadjusted) | BCG, Polio, DPT, MR, Full Vaccination | Variable, continuous delay |
Vaccine Hesitancy Questions |
| Rejali, M et al. 2015.60 | Moderate | Iran | No | 24-47 months | Cross-sectional | Logistic Regression | BCG, HepB, Polio, DPT, MMR | variable | Maternal Education, Paternal Education, Child Gender, Urban/Rural, Birth Order, City, Nationality, Maternal and Paternal Occupation |
| Sadoh, EA, Eregie OC. 2009.61 | Moderate | Nigeria | No | unclear | Cross-sectional | N/A | Full Vaccination | 4 weeks, continuous delay | Child Gender, Mother's Age, Paternal Age |
| Sadoh, EA et al. 2016.62 | Moderate | Nigeria | No | unclear | Cohort | Kaplan-Meier, Cox Proportional Hazards | BCG, DPT, PENTA | continuous delay | Birth Setting, Maternal Education, Child Gender, Mother's Age, Introduction of New Vaccine, Paternal Age |
| Sanchez, D et al. 2015.63 | Moderate | Venezuela | No | 0-6 years | Cross-sectional | Hazard-based estimator (unadjusted) | BCG, HepB, Polio, PENTA, MMR, Rotavirus, Full Vaccination | continuous delay | N/A |
| Sartori, AL et al. 2017.64 | Good | Brazil | No | 0-23 months | Cohort | Log Binomial Regression | PCV | 28 days, continuous delay | ANC, Maternal Education, Mother's Age, Child Birthweight |
| Schoeps, A. et al. 2013.65 | Good | Burkina Faso | No | 12-23 months | Cross-sectional | Logistic Regression | BCG, PENTA, MCV, Full Vaccination | 28 days | Religion, Ethnicity, Maternal Education, SES, Child Gender, Mother's Age, Urban/Rural, Household Size, Season of Birth |
| Scott, S et al. 2014.66 | Moderate | Gambia | Yes | 12-23 months; 9-60 months | Cross-sectional | Kaplan Meier, Cox Proportional Hazards | BCG, DPT, MCV, Full Vaccination | variable, continuous delay |
Ethnicity, Region, Previous Vaccine Delay |
| Senessie, C et al. 2007.67 | Moderate | Sierra Leone | No | 0-35 months | Cross-sectional | Chi-Square (unadjusted) | Full Vaccination | variable | Child Age, Time Relative to Civil Conflicts |
| Shrivastwa, N. et al. 2016.68 | Moderate | India | Yes | 0-60 months | Cross-sectional | Turnbull Estimator | BCG, DPT, MCV | continuous delay | N/A |
| Suárez-Castaneda, E et al. 2014.69 | Good | El Salvador | Yes | 23-59 months | Cross-sectional | Logistic Regression, Cox Proportional Hazards | BCG, Polio, PENTA, MMR, Rotavirus, Full Vaccination | variable | Ethnicity, Maternal Education, Paternal Education, Child Gender, Urban/Rural, Marital Status, Household Size, Distance to Clinic, Parental Occupation, Region, Birth Year |
| Suárez-Castaneda, E et al. 2015.70 | Good | El Salvador | Yes | 24-59 months | Cross-sectional | Logistic Regression, Cumulative-Age-At-Vaccination Curves | PENTA, Rotavirus | continuous delay | Parental Education, Child Gender, Urban/Rural, Marital Status, Household Size, Distance to Clinic, Parental Occupation, Primary Mode of Transportation, Presence of Organized Crime, Region |
| Tang, X et al. 2016.71 | Good | China | No | 18-54 months | Cross-sectional | Kaplan-Meier, Logistic Regression | MCV | variable | Ethnicity, Maternal Education, SES, Child Gender, Mother's Age, Child Age, Household Registration, Guardian Occupation, Average Duration of Time Father (and Mother) is Away from Home, Residential Status of Mother, Measles Vaccination Status of Mother |
| Tang, X et al. 2017.72 | Good | China | No | 18-54 months | Cross-sectional | Kaplan Meier, Cox Proportional Hazards | MCV | continuous delay | Ethnicity, Child Gender, Child Age |
| Tippins, A et al. 2017.73 | Moderate | Federated States of Micronesia | No | 24-35 months | Cross-sectional | Kaplan Meier, TSEIR model | TDaP, MMR | 1 month, interval | Household Size |
| Toikilik, S et al. 2010.74 | Moderate | Papua New Guinea | Yes | 12-23 months | Cross-sectional | N/A | BCG, HepB, Polio, DPT, MCV | variable | Birth Setting, Urban/Rural, Vaccine Hesitancy Questions |
| Vasudevan, L et al. 2014.75 | Moderate | Bangladesh | No | 11-18 weeks | Cohort | Logistic Regression | Full Vaccination | Fixed time point | ANC, Maternal Education, SES, Child Gender, Mother's Age, Urban/Rural, Size of Infant at Birth, Infant Sickness |
| Wagner, AL et al. 2014.76 | Moderate | China | No | 8 months - 6 years | Cross-sectional | Wilcoxon/Kruskall-Wallis (unadjusted) | MCV | variable, continuous delay | Child Gender, Resident District, Birth Year |
| Wagner, AL et al. 2014.77 | Moderate | China | No | 2-7 years | Cohort | Binomial Regression, Cumulative Age-At-Vaccination Curve | Hib, PCV | variable | Child Gender, Urban/Rural, Birth Year |
| Wagner, AL et al. 2016.78 | Good | China | No | 9 months - 14 years | Cross-sectional | Logistic Regression | MCV, PCV | variable | Child Gender, Child Age, Urban/Rural, Birth Year, Township factors |
| Wallace, AS et al. 2012.79 | Good | Philippines | No | 5-7 months | Cross-sectional | Chi-Square (unadjusted) | HepB | variable | Child Gender, Introduction of New Vaccine Schedule, Provider Size, Provider Type |
| Wu, JN et al. 2015.80 | Good | China | No | 1-14 years | Cross-sectional | Logistic Regression | HepB | variable | Ethnicity, Birth Setting, Child Gender, Urban/Rural, HBC Serum Biomarker |
| Yadav, K et al. 2012.81 | Moderate | India | No | unclear | Cross-sectional | Chi-Square (unadjusted) | BCG, Polio, DPT, MCV | variable, continuous delay | Hard to reach areas, Lower priority, Family Refusal, Lack of Knowledge |
| Zaidi, SM et al. 2014.82 | Moderate | Pakistan | Yes | 0-5 years | Cross-sectional | Logistic Regression | BCG, Polio, DPT, MCV | 1 month | Ethnicity, ANC, Birth Setting, Maternal Education, SES, Child Gender, Child Age, Urban/Rural, Household Size, Telephone Connection, Male Household Head, Breastfeeding Duration, Maternal Tetanus Vaccine |
| Zhou, Y et al. 2009.83 | Moderate | China | No | unclear | Cross-sectional | Logistic Regression | HepB | 1 day | Ethnicity, Birth Setting, Maternal Education, Paternal Education, Child Gender, Urban/Rural, Possession of EPI Card, Knowledge of Birth Dose of HepB, Migrant Status |
| Zivich, PN et al. 2017.84 | Moderate | Democratic Republic of Congo | No | 0-6 months | Cohort | Logistic Regression | BCG, Polio, DPT, PCV | variable, interval | ANC, Maternal Education, SES, Mother's Age, Marital Status, Birth Order, Previous Child Died, Pregnancy Intention, Clinic |
Predictors
Most of the included papers (62/67, 93%) adjusted for some variables in multivariate regression in an attempt to identify predictors of untimely vaccination with the other 5 papers only assessed crude measures of vaccine timeliness. In their analysis of vaccine timeliness, a majority of studies considered child’s gender (46/62, 74%) and maternal education (40/62, 65%). Maternal age (26/62, 42%), wealth/socio-economic status (25/62, 40%), urban/rural environment (20/62, 32%), household size (20/62, 32%), and birth setting (19/62, 31%–32%) were also common predictors used in multivariate regression (Figure 4), whereas very few papers examined religion (5/62, 8%) or the introduction of a new vaccine (1/62, 2%) in the context of vaccination timeliness.
Figure 4.
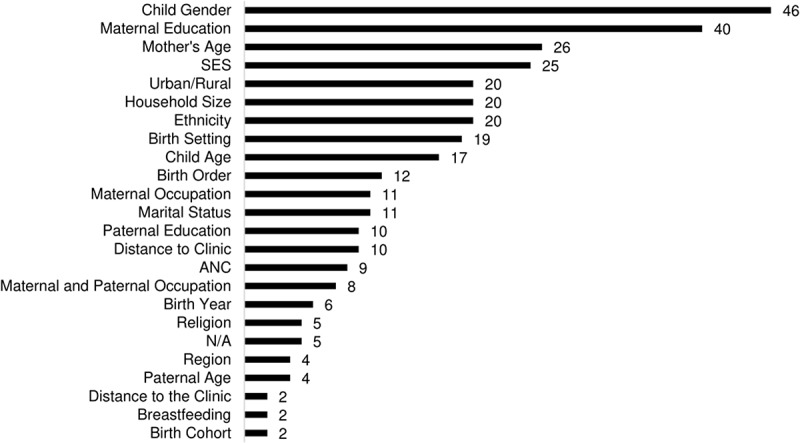
Number of papers reporting different variables used as predictors of vaccine timeliness in multivariable regression (n = 67).
Study design
The studies varied in design, with most using cross-sectional surveys (50/67, 75%) including the Demographic Health Survey or DHS (6/67, 9%), Multiple Indicator Cluster Survey or MICS (1/67, 2%), with the remainder using other types of non-nationally representative cross-sectional surveys (43/67, 64%). A smaller number, 13/67 (19%) of the studies were cohort studies (including data from surveillance systems and immunization information systems), and 3/67 (5%) were randomized controlled trials of which two were cluster-randomized, although none of these used vaccination as either a randomized intervention or an outcome, just as secondary analysis. There was also 1 included case-control study.
Date source(s)
A total of 62 studies (93%) reported the source of information on vaccination dates used to calculate vaccine timeliness within their study. Most studies (49/62, 79%) used data from vaccination cards (or other types of health cards, social welfare cards, or vaccination certificates based upon the country in which the study was conducted); 13% (8/62) used data from immunization information systems, 5% (3/62) used data abstracted from clinic records, and two (3%) based the date of vaccine receipt off the date of study, i.e. when study or data collection occurred on the date of vaccination.
Age
There was a wide diversity of ages in the study population across the 67 papers. In the 57 studies with a clearly stated age range, 35 different age ranges were used. The most common age range were children in the second year of life (12–23 months), with 21% of studies (12/57) including only this age range (see Figure 5 for details). Otherwise, 49% of studies (28/57) included children under 1 year of age, 51% (29/57) included children 1-2 years of age, 53% (30/57) included children 2-3 years of age, 44% (25/57) included children 3-4 years of age, 39% (22/57) included children 4-5 years of age, and 14% (8/57) included children aged 5 and older. There were 10 studies which had an unclearly defined age range.
Figure 5.

Number of papers reporting different age-ranges (n = 67).
Analysis
There were a wide variety of methodological approaches used to assess vaccination timing. Most studies included some sort of significance testing (62/67, 93%). About one-third of studies (22/62, 36%) used logistic regression, and 5/62 (8%) used log-binomial or binomial regression; 6/62 (10%) only used unadjusted chi-square tests (mostly Pearson’s test, although one used survey procedures with a Rao–Scott test). A large portion of studies (48/62, 77%) used some sort of survival analysis, like hazards estimation (16/62, 26%) or Kaplan-Meier analyses (26/62, 42%). One study used Turnbull estimators to account for some participants having information about vaccination dates, but other participants only having a recollection of having received the shot.
Discussion
Measures of age-specific aggregate coverage can obscure or conceal untimely vaccination. The measurement of vaccination timeliness is increasingly perceived as essential to an assessment of immunization system performance and estimates of vaccination coverage, and inclusion of timeliness measures should progressively become the standard in assessment of vaccine coverage in the future. In this systematic review of studies on vaccination timing in LMICs, we found a lack of agreement about what constituted a “timely” vaccination, which was compounded by different vaccination schedules in different countries. Altogether, there was significant disagreement about the definition of timely and delayed vaccinations, with some papers using continuous delay and some categorical timeliness measures. The included papers spanned broad geographic reach but were heavily skewed to the African region and China. The most commonly studied antigens were BCG, measles, and OPV/IPV. Predictors were highly variable, but maternal sociodemographic factors were commonly used. The vast majority of studies included were cross-sectional in study design, most of which used data from vaccination cards, though there was very little consensus among the age range of children to assess for timing. The diversity in operationalization of variables limits comparisons among studies.
The significant variability in defining timing found in this review presents problems for comparing timely vaccinations across countries. Categorizing vaccination timeliness requires a consensus on what constitutes a timely vs. untimely vaccine. Unfortunately, there is often not clear guidance on this. For example, the manufacturer’s instructions for PCV list a four-dose schedule which can start as early as 6 weeks. WHO, however, recommends a three-dose schedule, with several interpretations by region (6, 10, 14 weeks; 2, 4, 6 months; or the first two doses, followed by a booster between 9 and 15 months)85. Clearly, any country-specific evaluation of timeliness should follow guidelines from that country’s Ministry of Health, but the definition of timeliness (e.g. 1 week vs 1 month after recommended vaccination date) has different implications when doses within a series are spaced by 4 weeks vs. 2 months.
This variability and the difficulty in comparing findings across studies is compounded by the uneven geographic distribution represented in this review. Almost half of the included papers were focused on the African WHO Region, and another third were from the Western Pacific, specifically China. These papers thus do not paint a representative picture of vaccination timing among LMICs in the European, Eastern Mediterranean, South East Asian, and American Region. In particular, timeliness studies in South and Southeast Asia are needed given the population concentration there and a large burden of vaccine-preventable diseases.
Overall, the most commonly investigated vaccines (measles, BCG, DTP, and polio vaccine) seemed to be relative to international concern about a given disease. For example, measles and polio are both subject to global eradication plans,86,87 perhaps a reason for their high representation in timeliness studies. Vaccination of measles in a timely manner is particularly important given that the disease is highly contagious and earlier vaccination can reduce the number of susceptible persons in the population,88 although vaccination at 8–11 months of age is also associated with reduced effectiveness89. DTP dose 3 is often used in international assessments of vaccination programs,90 not only because the vaccine protects against three serious diseases, but also because the vaccine has been widely available for decades, and the third dose represents an immunization system’s ability to revaccinate a child on multiple occasions. A large number of studies investigated BCG, even though the vaccine has seen declining use; more countries have stopped routine BCG vaccination programs since 1981, though most of these are high-income countries in western Europe.91 Newer vaccines, like rotavirus, PCV, and Hib vaccine, understandably were less likely to be included; these vaccines were added to the WHO EPI in 2013, 2012, and 2006, respectively2. However, with the increasing complexity of the EPI schedule and new vaccines being regularly added in different countries, there is also a need for future timeliness studies to address a broader array of antigens, particularly those that are strictly age-limited like Rotavirus and Hib.
Beyond the antigens assessed, there was also considerable variability in the predictors explored as correlates of untimely or delayed vaccination. Gender, education, socio-economic status, urban/rural residence, maternal age, maternal occupation, and birth setting can be situation-specific and highly informative. Interestingly, religion, caste, tribe, and distance to the clinic are potentially important predictors which were very rarely represented in this review but should be considered for inclusion in timing studies depending on the countries involved. Future studies should examine the contribution of vaccine hesitancy to vaccination timeliness and other vaccination metrics.
Vaccination date sources were also surprisingly variable. While most studies used vaccination cards, restricting a study population to those who have vaccination cards can reduce the generalizability of findings as those who possess vaccination cards might not be representative of the general population as a whole. Additionally, vaccination cards can be prone to data recording errors. Immunization information systems (IIS) are preferred in terms of providing an objective and accurate source of vaccination data without depending on retention of a vaccination card, and also allows for following a child longitudinally. Finally, IISs can assist with timeliness determinations while making vaccination data available to the family and clinicians. However, most LMICs do not have functional IISs and large, nationally representative surveys like DHS will continue to be a critical source of information about vaccination coverage and timeliness and researchers using these data sources should include timeliness measures in their analysis, though most included studies did not use nationally representative datasets like DHS.
Finally, the age benchmarks used to include children in timeliness studies were exceedingly variable, which is another, albeit very important, reason why a continuous measure is preferred in addition to improving comparability across studies. Certain historically used age groups (e.g. 23 months) will continue to be important seminal markers of ‘on-time’ vaccination coverage, although the use of continuous measure allows for uninterrupted use of those while also permitting much greater flexibility in looking at vaccination coverage and timeliness over the childhood life span. While analytic approaches also varied, the inclusion of survival analysis techniques in a majority of papers points to the utility, again, of continuous measures of vaccination delay as an interpretable and comparable metric.
Weaknesses in current studies and potential solutions
Any study on vaccination timing will require information about birth and vaccination dates. These are common data fields in electronic registries, such as immunization information systems, and should be included on all vaccination cards or booklets held by families. However, the retention of these cards can be low. In one study from the Southern Nations, Nationalities, and Peoples’ region of Ethiopia, 51% of respondents did not have a vaccination card; however, of those without a vaccination card, 62% previously had a card – but were unable to find it for the interview. Accordingly, only 20% never had a vaccination card given to them.92 Relying on parental recall of vaccination dates is also likely fraught with problems. A systematic review of the validity of vaccination cards vs parental recall found that coverage (not including any timing or date information) from recall was an underestimate of 7 percentage points vs medical records, and recall had a median sensitivity of 90% and specificity of 50%.93 Therefore, in some situations, analyses of immunization timing may be biased towards individuals who are more likely to retain a vaccination card. For example, according to the 2011 Ethiopia Demographic and Health Survey (DHS), 57.0% of children with a vaccination card were fully vaccinated, compared to 11.2% who did not have a card.94
Future studies on vaccination timing in LMICs will likely continue to rely heavily on recall of vaccines administered which means that some participants will have vaccination date information, and others will not. The use of statistics that take into account both left and right censoring – such as nonparametric Turnbull estimators or parametric-accelerated failure time models – would permit greater use of vaccination data from those both with and without vaccination cards. Turnbull estimation has been used in recent papers from India.68,95 The Turnbull estimator allows for both right censoring (e.g. the child is not vaccinated at the time of the survey) and left censoring (the child has been vaccinated, but with an unknown date that is at the very latest, the day of the interview). These statistics can be brought up in SAS using the lifereg procedure (SAS Institute, Inc., Cary, NC, USA) or in R using the survival and SurvRegCensCov packages (R Foundation for Statistical Computing, Vienna, Austria). These procedures can be thought of as an extension of typical methods of survival analysis (e.g. Cox regression), which only consider right censoring.
We also recommend that future studies employ precise methods to estimate months. Months can vary between 28 and 31 days, according to the Western calendar, and some countries use different systems of measurement – for example, the Ethiopian calendar consists of 30-day months, in addition to an intercalated month of 5 or 6 days. As a shortcut, many studies employ 30 days as an average. In one study, where “month” and “year” of vaccination, but not “day,” was collected, vaccinations were considered timely if it was given in the month it was due based on the child’s birth date.24 However, this would underestimate timely vaccinations (relative to a 1-month benchmark). A better method is to identify the actual date that a vaccine administration would be untimely. For instance, a child born on February 14 2007, who should receive a certain vaccine within one month for it to be timely would need to be vaccinated by March 14. These dates can be identified through certain coding steps (for instance, using an intnx statement in a data step in SAS).
Vaccination timeliness can be considered as both continuous (e.g. mean number of days delayed, or average age at vaccination) and categorical measures (e.g. % with a timely vaccination). Due to differences between countries in vaccination schedules and a lack of concordance in the literature on what constitutes a “timely” dose, a continuous measure may be more appropriate. Cumulative incidence curves (or reverse Kaplan-Meier plots) can graphically depict this information in an easy-to-understand manner. If a categorization is desired, multiple groupings (e.g. both timeliness based on 1 week and timeliness based on 1 month) is preferable, in the absence of any country-specific guidance on what constitutes a timely dose.
Finally, certain statistical basics should always be followed. Studies with clustering of the sample should specify survey procedures in the regression analysis (for instance, using Taylor series estimation of standard errors). In cross-sectional or cohort studies, multivariable regression with an outcome of the “prevalence” or “likelihood” of a timely vaccine can utilize a binomial or log-binomial regression, which would output a “risk ratio” or “prevalence ratio” which is more easily understood than an odds ratio.96
Strengths and limitations
This study has several limitations. We limited ourselves to studies published in PubMed, EBSCOhost, and Embase (in addition to studies found through an abstract search), however, manuscripts on timeliness could have also been submitted to regional journals not indexed in international databases. We also limited our search to LMICs, as these are countries where timely administration of vaccines can be an especially important mechanism to reduce morbidity from infectious diseases, and it was beyond the purview of this review to examine differences in timeliness between high-income countries and LMICs.
Conclusions
Ensuring children have access to timely vaccinations is a critically important and cost-effective way to decrease the incidence of disease and reduce morbidity within a population. In this systematic review of 67 papers from LMICs, analysis of vaccination timing has become more common over time, and papers represented all 6 WHO regions. However, there is a lack of concordance in the literature on what constitutes a “timely” vaccination – with studies variously using benchmarks of 1 day, 1 week, 2 weeks, 4 weeks, 1 month, etc. As such, providing information on the continuous spread of date of vaccination administration (e.g. graphing the cumulative incidence of vaccination by increasing age), or characterizing median delay in vaccine receipt from the suggested schedule might provide more valuable information than a categorization (e.g. stating the proportion vaccinated by a certain benchmark), although both can be presented within one study.
Funding Statement
ALW’s salary was supported by the National Institute Of Allergy And Infectious Diseases of the National Institutes of Health under Award Number K01AI137123. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health;National Institute of Allergy and Infectious Diseases [K01AI137123];
Abbreviations
- ANC
Antenatal Care
- BCG
Bacillus Calmette-Guérin
- DHS
Demographic Health Survey
- DTP
Diphtheria-tetanus-pertussis vaccine
- EPI
Expanded Program on Immunization
- HepB
Hepatitis B
- HPV
Human papillomavirus
- MCV
Measles-containing vaccine
- MICS
Multiple Indicator Cluster Survey or MICS
- Hib
Haemophilus influenzae type b
- IIS
Immunization Information System
- IPV
Inactivated polio vaccine
- LMICs
Low- or middle-income country
- OPV
Oral polio vaccine
- PCV
Pneumococcal conjugate vaccine
- WHO
World Health Organization
Acknowledgments
We appreciate the work of all the researchers who contributed to the literature on vaccination timeliness.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was reported by the authors.
Authors’ contributions
ALW contributed to study design, helped review articles, and wrote the first draft of the manuscript. NBM reviewed articles and performed analysis. MLB contributed to study design. All authors revised the manuscript for important intellectual content and gave approval for this version to be published.
References
- 1.Centers for Disease Control and Prevention Ten great public health achievements, 1900– 1999: impact of vaccines universally recommended for children. Morb Mortal Wkly Rep 1999;48:243–48. [Google Scholar]
- 2.World Health Organization Summary of WHO position papers - recommendations for routine immunization [Internet]. 2017. [accessed 2017 September7]. http://www.who.int/immunization/policy/Immunization_routine_table1.pdf
- 3.Howard N, Walls H, Bell S, Mounier-Jack S.. The role of National Immunisation Technical Advisory Groups (NITAGs) in strengthening national vaccine decision-making: A comparative case study of Armenia, Ghana, Indonesia, Nigeria, Senegal and Uganda. Vaccine. 2018;36:5536–43. doi: 10.1016/j.vaccine.2018.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roush SW, Murphy TV. Historical comparisons of morbidity and mortality for vaccine-preventable diseases in the United States. Jama. 2007;298:2155–63. doi: 10.1001/jama.298.18.2155. [DOI] [PubMed] [Google Scholar]
- 5.Luman ET, Barker LE, Shaw KM, McCauley MM, Buehler JW, Pickering LK. Timeliness of childhood vaccinations in the United States: days undervaccinated and number of vaccines delayed. Jama. 2005;293:1204–11. doi: 10.1001/jama.293.10.1204. [DOI] [PubMed] [Google Scholar]
- 6.Glanz JM, Newcomer SR, Narwaney KJ, Hambidge SJ, Daley MF, Wagner NM, McClure DL, Xu S, Rowhani-Rahbar A, Lee GM, et al. A population-based cohort study of undervaccination in 8 managed care organizations across the United States. JAMA Pediatr. 2013;167:274–81. doi: 10.1001/jamapediatrics.2013.502. [DOI] [PubMed] [Google Scholar]
- 7.McCarthy NL, Irving S, Donahue JG, Weintraub E, Gee J, Belongia E, Baggs J. Vaccination coverage levels among children enrolled in the vaccine safety datalink. Vaccine. 2013;31:5822–26. doi: 10.1016/j.vaccine.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Thomson A, Robinson K, Vallée-Tourangeau G. The 5As: A practical taxonomy for the determinants of vaccine uptake. Vaccine. 2016;34:1018–24. doi: 10.1016/j.vaccine.2015.11.065. [DOI] [PubMed] [Google Scholar]
- 9.The Strategic Advisory Group of Experts (SAGE) Report of the SAGE working group on vaccine hesitancy [Internet]. 2014. [accessed 2018 June14]. http://www.who.int/immunization/sage/meetings/2014/october/SAGE_working_group_revised_report_vaccine_hesitancy.pdf
- 10.Bolton P, Hussain A, Hadpawat A, Holt E, Hughart N, Guyer B. Deficiencies in current childhood immunization indicators. Public Health Rep. 1998;113:527–32. [PMC free article] [PubMed] [Google Scholar]
- 11.Guerra FA. Delays in immunization have potentially serious health consequences. Pediatr Drugs. 2007;9:143–48. doi: 10.2165/00148581-200709030-00002. [DOI] [PubMed] [Google Scholar]
- 12.Buttery JP, Graham SM. Immunisation timing: the protective layer in vaccine coverage. Lancet. 2009;373:1499–500. doi: 10.1016/S0140-6736(09)60340-8. [DOI] [PubMed] [Google Scholar]
- 13.Wahl B, O’Brien K, Greenbaum A, Liu L, Chu Y, Majumder A, Lukšić I, Nair H, McAllister D, Campbell H, et al. Global, regional, and national burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: updated estimates from 2000-2015. Lancet Glob Health. 2018;6:e744–57. doi: 10.1016/S2214-109X(18)30247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group . Guidelines and guidance preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Bank World Bank Country and Lending Groups [Internet]. 2017. [accessed 2017 August16]; https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups
- 16.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–84. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith LE, Amlôt R, Weinman J, Yiend J, Rubin GJ. A systematic review of factors affecting vaccine uptake in young children. Vaccine. 2017;35:6059–69. doi: 10.1016/j.vaccine.2017.09.046. [DOI] [PubMed] [Google Scholar]
- 18.Akmatov MK, Mikolajczyk RT. Timeliness of childhood vaccinations in 31 low and middle-income countries. J Epidemiol Community Health. 2012;66:e14. doi: 10.1136/jech.2010.124651. [DOI] [PubMed] [Google Scholar]
- 19.Akmatov MK, Kretzschmar M, Krämer A, Mikolajczyk RT. Timeliness of vaccination and its effects on fraction of vaccinated population. Vaccine. 2008;26:3805–11. doi: 10.1016/j.vaccine.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 20.Babirye JN, Engebretsen IMS, Makumbi F, Fadnes LT, Wamani H, Tylleskar T, Nuwaha F. Timeliness of childhood vaccinations in kampala uganda: A community-based cross-sectional study. PLoS One. 2012;7:1–6. doi: 10.1371/journal.pone.0035432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barman D, Dutta A. Access and barriers to immunization in West Bengal, India: quality matters. J Health Popul Nutr. 2013;31:510–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barman MP, Nath K, Hazarika J. Factors affecting timeliness of immunization coverage among children of Assam, India. J Health Manag. 2015;17:274–84. doi: 10.1177/0972063415589243. [DOI] [Google Scholar]
- 23.Bicaba A, Haddad S, Kabore M, Taminy E, Feletto M, Fournier P. Monitoring the performance of the expanded program on immunization: the case of Burkina Faso. BMC Int Health Hum Rights. 2009;9(Suppl 1):S12. doi: 10.1186/1472-698X-9-S1-S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calhoun LM, Van Eijk AM, Lindblade KA, Odhiambo FO, Wilson ML, Winterbauer E, Slutsker L, Hamel MJ. Determinants and coverage of vaccination in children in western Kenya from a 2003 cross-sectional survey. Am J Trop Med Hyg. 2014;90:234–41. doi: 10.4269/ajtmh.13-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiabi A, Nguefack FD, Njapndounke F, Kobela M, Kenfack K, Nguefack S, Mah E, Nguefack-Tsague G, Angwafo F. Vaccination of infants aged 0 to 11 months at the Yaounde Gynaeco-obstetric and pediatric hospital in Cameroon: how complete and how timely? BMC Pediatr. 2017;17:206. doi: 10.1186/s12887-017-0954-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clark A, Sanderson C. Timing of children’s vaccinations in 45 low-income and middle-income countries: an analysis of survey data. Lancet. 2009;373:1543–49. doi: 10.1016/S0140-6736(09)60317-2. [DOI] [PubMed] [Google Scholar]
- 27.D’Ardenne KK, Darrow J, Furniss A, Chavez C, Hernandez H, Berman S, Asturias EJ. Use of rapid needs assessment as a tool to identify vaccination delays in Guatemala and Peru. Vaccine. 2016;34:1719–25. doi: 10.1016/j.vaccine.2016.01.060. [DOI] [PubMed] [Google Scholar]
- 28.Delrieu I, Gessner BD, Baril L, Roset Bahmanyar E. From current vaccine recommendations to everyday practices: an analysis in five sub-Saharan African countries. Vaccine. 2015;33:7290–98. doi: 10.1016/j.vaccine.2015.10.107. [DOI] [PubMed] [Google Scholar]
- 29.Ettarh RR, Mutua MK, Kyobutungi C. Ethnicity and delay in measles vaccination in a Nairobi slum. Trop Med Health. 2012;40:59–62. doi: 10.2149/tmh.2012-09s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fadnes LT, Jackson D, Engebretsen IMS, Zembe W, Sanders D, Sommerfelt H, Tylleskär T. Vaccination coverage and timeliness in three South African areas: a prospective study. BMC Public Health. 2011;11:404. doi: 10.1186/1471-2458-11-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fadnes LT, Nankabirwa V, Sommerfelt H, Tylleskär T, Tumwine JK, Engebretsen IMS. Is vaccination coverage a good indicator of age-appropriate vaccination? A prospective study from Uganda. Vaccine. 2011;29:3564–70. doi: 10.1016/j.vaccine.2011.02.093. [DOI] [PubMed] [Google Scholar]
- 32.Fisker AB, Hornshøj L, Rodrigues A, Balde I, Fernandes M, Benn CS, Aaby P. Effects of the introduction of new vaccines in Guinea-Bissau on vaccine coverage, vaccine timeliness, and child survival: an observational study. Lancet Glob Health. 2014;2:478–87. doi: 10.1016/S2214-109X(14)70274-8. [DOI] [PubMed] [Google Scholar]
- 33.Flannery B, Samad S, Tate JE, Danovaro-Holliday C, Rainey JJ, States U, Program I, States U, Cássio J, Moraes D, et al. Uptake of oral rotavirus vaccine and timeliness of routine immunization in Brazil’s national immunization program. Vaccine. 2013;31:1523–28. doi: 10.1016/j.vaccine.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gibson DG, Ochieng B, Kagucia EW, Obor D, Odhiambo F, O’Brien KL, Feikin DR. Individual level determinants for not receiving immunization, receiving immunization with delay, and being severely underimmunized among rural western Kenyan children. Vaccine. 2015;33:6778–85. doi: 10.1016/j.vaccine.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 35.Gram L, Soremekun S, Ten Asbroek A, Manu A, O’Leary M, Hill Z, Danso S, Amenga-Etego S, Owusu-Agyei S, Kirkwood BR. Socio-economic determinants and inequities in coverage and timeliness of early childhood immunisation in rural Ghana. Trop Med Int Health. 2014;19:802–11. doi: 10.1111/tmi.12324. [DOI] [PubMed] [Google Scholar]
- 36.Hu Y, Li Q, Luo S, Lou L, Qi X, Xie S. Timeliness vaccination of measles containing vaccine and barriers to vaccination among migrant children in east China. PLoS One. 2013;8:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu Y, Chen Y, Guo J, Tang X, Shen L. Completeness and timeliness of vaccination and determinants for low and late uptake among young children in eastern China. Hum Vaccin Immunother. 2014;10:1408–15. doi: 10.4161/hv.28054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu Y, Li Q, Chen Y. Timeliness of childhood primary immunization and risk factors related with delays: evidence from the 2014 Zhejiang Provincial vaccination coverage survey. Int J Environ Res Public Health. 2017;14:1086. doi: 10.3390/ijerph14091086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hughes MM, Katz J, Englund JA, Khatry SK, Shrestha L, LeClerq SC, Steinhoff M, Tielsch JM. Infant vaccination timing: beyond traditional coverage metrics for maximizing impact of vaccine programs, an example from southern Nepal. Vaccine. 2016;34:933–41. doi: 10.1016/j.vaccine.2015.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iqbal W, Danish SH, Ahmad F. Factors responsible for non-compliance of timely administration of BCG vaccine in district Jhelum Pakistan. Pak J Public Health. 2015;5:22–27. [Google Scholar]
- 41.Jadidi R, Mohammadbeigi A, Mohammadsalehi N, Ansari H, Ghaderi E. Inequity in timeliness of MMR vaccination in children living in the suburbs of Iranian cities. Int J Biomed Sci. 2015;11:93–98. [PMC free article] [PubMed] [Google Scholar]
- 42.Laryea DO, Abbeyquaye Parbie E, Frimpong E. Timeliness of childhood vaccine uptake among children attending a tertiary health service facility-based immunisation clinic in Ghana. BMC Public Health. 2014;14:90. doi: 10.1186/1471-2458-14-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Le Polain de Waroux O, Schellenberg JRA, Manzi F, Mrisho M, Shirima K, Mshinda H, Alonso P, Tanner M, Schellenberg DM. Timeliness and completeness of vaccination and risk factors for low and late vaccine uptake in young children living in rural southern Tanzania. Int Health. 2013;5:139–47. doi: 10.1093/inthealth/iht006. [DOI] [PubMed] [Google Scholar]
- 44.Li Q, Hu Y, Zhong Y, Chen Y, Tang X, Guo J, Shen L. Using the immunization information system to determine vaccination coverage rates among children aged 1–7 years: A report from Zhejiang province, China. Int J Environ Res Public Health. 2014;11:2713–28. doi: 10.3390/ijerph110302713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin W, Xiong Y, Tang H, Chen B, Ni J. Factors associated with delayed measles vaccination among children in Shenzhen, China: a case-control study. Hum Vaccin Immunother. 2014;10:3601–06. doi: 10.4161/21645515.2014.979687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mbengue MAS, Mboup A, Ly ID, Faye A, Niang FB, Thiam M, Ndiaye BP, Dieye TN, Mboup S. Vaccination coverage and immunization timeliness among children aged 12–23 months in Senegal: a Kaplan-Meier and Cox regression analysis approach. Pan Afr Med J. 2017;27:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miyahara R, Jasseh M, Gomez P, Shimakawa Y, Greenwood B, Keita K, Ceesay S, D’Alessandro U, Roca A. Barriers to timely administration of birth dose vaccines in The Gambia, West Africa. Vaccine. 2016;34:3335–41. doi: 10.1016/j.vaccine.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moïsi JC, Kabuka J, Mitingi D, Levine OS, Scott JAG. Spatial and socio-demographic predictors of time-to-immunization in a rural area in Kenya: is equity attainable? Vaccine. 2010;28:5725–30. doi: 10.1016/j.vaccine.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mokhtari M, Rezaeimanesh M, Mohammadbeigi A, Zahraei SM, Mohammadsalehi N, Ansari H. Risk factors of delay proportional probability in Diphtheria?Tetanus? Pertussis vaccination of Iranian children; life table approach analysis. J Glob Infect Dis. 2015;7:165–69. doi: 10.4103/0974-777X.170503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Montgomery JP, Zhang Y, Carlson B, Ewing S, Wang X, Boulton ML. Measles vaccine coverage and series completion among children 0–8 years of age in Tianjin, China. Pediatr Infect Dis J. 2015;34:289–95. doi: 10.1097/INF.0000000000000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mutua MK, Ochako R, Ettarh R, Ravn H, Echoka E, Mwaniki P. Effects of low birth weight on time to BCG vaccination in an urban poor settlement in Nairobi, Kenya: an observational cohort study. BMC Pediatr. 2015;15:45. doi: 10.1186/s12887-015-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mutua MK, Kimani-Murage E, Ngomi N, Ravn H, Mwaniki P, Echoka E. Fully immunized child: coverage, timing and sequencing of routine immunization in an urban poor settlement in Nairobi, Kenya. Trop Med Health. 2016;44:13. doi: 10.1186/s41182-016-0013-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mvula H, Heinsbroek E, Chihana M, Crampin AC, Kabuluzi S, Chirwa G, Mwansambo C, Costello A, Cunliffe NA, Heyderman RS, et al. Predictors of uptake and timeliness of newly introduced pneumococcal and rotavirus vaccines, and of measles vaccine in rural Malawi: A population cohort study. PLoS One. 2016;11:1–15. doi: 10.1371/journal.pone.0154997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Narváez J, Osorio MB, Castañeda-Orjuela C, Alvis Zakzuk N, Cediel N, Chocontá-Piraquive LÁ, de La Hoz-Restrepo F. Is Colombia reaching the goals on infant immunization coverage? A quantitative survey from 80 municipalities. Vaccine. 2017;35:1501–08. doi: 10.1016/j.vaccine.2017.01.073. [DOI] [PubMed] [Google Scholar]
- 55.Odutola A, Afolabi MO, Ogundare EO, Lowe-Jallow YN, Worwui A, Okebe J, Ota MO, Heiniger U, Clark A, Sanderson C, et al. Risk factors for delay in age-appropriate vaccinations among Gambian children. BMC Health Serv Res. 2015;15:346. doi: 10.1186/s12913-015-1015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Olusanya BO. Pattern and determinants of BCG immunisation delays in a sub-Saharan African community. Health Res Policy Syst. 2010;8:1. doi: 10.1186/1478-4505-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pezzoli L, Mathelin JP, Hennessey K, Eswara-Aratchige P, Valiakolleri J, Kim SH. Low level of hepatitis B virus infection in children 20 years after initiation of infant vaccination program in Wallis and Futuna. Am J Trop Med Hyg. 2017;16–0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Poorolajal J, Khazaei S, Kousehlou Z, Bathaei SJ, Zahiri A. Delayed vaccination and related predictors among infants. Iran J Public Health. 2012;41:65–71. [PMC free article] [PubMed] [Google Scholar]
- 59.Rainey JJ, Lacapère F, Danovaro-Holliday MC, Mung K, Magloire R, Kananda G, Cadet JR, Lee CE, Chamouillet H, Luman ET. Vaccination coverage in Haiti: results from the 2009 national survey. Vaccine. 2012;30:1746–51. doi: 10.1016/j.vaccine.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 60.Rejali M, Mohammadbeigi A, Mokhtari M, Zahraei SM, Eshrati B. Timing and delay in children vaccination; evaluation of expanded program of immunization in outskirt of Iranian cities. J Res Health Sci. 2015;15:54–58. [PubMed] [Google Scholar]
- 61.Sadoh AE, Eregie CO Timeliness and Completion Rate of Immunization among Nigerian Children Attending a Clinic-based Immunization Service on JSTOR. J Heal Popul Nutr 2009; 27:391–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sadoh AE, Nwaneri DU, Ogboghodo BC, Sadoh WE. Effect of introduction of pentavalent vaccine as replacement for diphtheria-tetanus-pertussis and hepatitis B vaccines on vaccination uptake in a health facility in Nigeria. Vaccine. 2016;34:2722–28. doi: 10.1016/j.vaccine.2016.04.026. [DOI] [PubMed] [Google Scholar]
- 63.Sanchez D, Sodha SV, Kurtis HJ, Ghisays G, Wannemuehler KA, Danovaro-Holliday MC, Ropero-Alvarez AM. Vaccination week in the Americas, 2011: an opportunity to assess the routine vaccination program in the Bolivarian Republic of Venezuela. BMC Public Health. 2015;15:395. doi: 10.1186/s12889-015-1723-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sartori AL, Minamisava R, Afonso ET, Policena GM, Pessoni GC, Bierrenbach AL, Andrade AL. Timeliness and risk factors associated with delay for pneumococcal conjugate 10-valent routine immunization in Brazilian children. Vaccine. 2017;35:1030–36. doi: 10.1016/j.vaccine.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 65.Schoeps A, Ouédraogo N, Kagoné M, Sié A, Müller O, Becher H. Socio-demographic determinants of timely adherence to BCG, Penta3, measles, and complete vaccination schedule in Burkina Faso. Vaccine. 2013;32:96–102. doi: 10.1016/j.vaccine.2013.10.063. [DOI] [PubMed] [Google Scholar]
- 66.Scott S, Odutola A, Mackenzie G, Fulford T, Afolabi MO, Jallow YL, Jasseh M, Jeffries D, Dondeh BL, Howie SRC, et al. Coverage and timing of children’s vaccination: an evaluation of the expanded programme on immunisation in the Gambia. PLoS One. 2014;9:1–9. doi: 10.1371/journal.pone.0107280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Senessie C, Gage GN, von Elm E. Delays in childhood immunization in a conflict area : a study from Sierra Leone during civil war. Confl Health. 2007;1:14. doi: 10.1186/1752-1505-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shrivastwa N, Gillespie BW, Lepkowski JM, Boulton ML. Vaccination timeliness in children under India’s universal immunization program. Pediatr Infect Dis J. 2016;35:955–60. doi: 10.1097/INF.0000000000001223. [DOI] [PubMed] [Google Scholar]
- 69.Suárez-Castaneda E, Pezzoli L, Elas M, Baltrons R, Crespin-Elías EO, Pleitez OAR, de Campos MIQ, Danovaro-Holliday MC. Routine childhood vaccination programme coverage, El Salvador, 2011-In search of timeliness. Vaccine. 2014;32:437–44. doi: 10.1016/j.vaccine.2013.11.072. [DOI] [PubMed] [Google Scholar]
- 70.Suarez-Castaneda E, Burnett E, Elas M, Baltrons R, Flannery B, Kleinbaum D, Oliveira LH D, Danovaro-Holliday MC. Catching-up with pentavalent vaccine: exploring reasons behind lower rotavirus vaccine coverage in El Salvador. Vaccine. 2015;33:6865–70. doi: 10.1016/j.vaccine.2015.07.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tang X, Geater A, McNeil E, Zhou H, Deng Q, Dong A, Li Q. Parental migration and children’s timely measles vaccination in rural China: a cross-sectional study. Trop Med Int Health. 2016;21:886–94. doi: 10.1111/tmi.12719. [DOI] [PubMed] [Google Scholar]
- 72.Tang X, Geater A, McNeil E, Zhou H, Deng Q, Dong A. Timeliness and completeness of measles vaccination among children in rural areas of Guangxi, China: A stratified three-stage cluster survey. J Epidemiol. 2017;27:317–24. doi: 10.1016/j.je.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tippins A, Leidner AJ, Meghani M, Griffin A, Nyaku M, Underwood JM. Timeliness of childhood vaccination in the federated states of micronesia. Vaccine. 2017;35:6404–11. doi: 10.1016/j.vaccine.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Toikilik S, Tuges G, Lagani J, Wafiware E, Posanai E, Coghlan B, Morgan C, Sweeney R, Miller N, Abramov A, et al. Are hard-to-reach populations being reached with immunization services? Findings from the 2005 Papua New Guinea national immunization coverage survey. Vaccine. 2010;28:4673–79. doi: 10.1016/j.vaccine.2010.04.063. [DOI] [PubMed] [Google Scholar]
- 75.Vasudevan L, Labrique AB, Mehra S, Wu L, Levine O, Feikin D, Klemm R, Christian P, West KP. Maternal determinants of timely vaccination coverage among infants in rural Bangladesh. Vaccine. 2014;32:5514–19. doi: 10.1016/j.vaccine.2014.06.092. [DOI] [PubMed] [Google Scholar]
- 76.Wagner AL, Zhang Y, Montgomery JP, Ding Y, Carlson BF, Boulton ML. Timely measles vaccination in Tianjin, China: a cross-sectional study of immunization records and mothers. BMC Public Health. 2014;14:888. doi: 10.1186/1471-2458-14-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wagner AL, Sun X, Montgomery JP, Huang Z, Boulton ML. The impact of residency and urbanicity on Haemophilus influenzae type b and pneumococcal immunization in Shanghai children: A retrospective cohort study. PLoS One. 2014;9:1–7. doi: 10.1371/journal.pone.0097800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wagner AL, Sun X, Huang Z, Ren J, Mukherjee B, Wells EV, Boulton ML, On-Time M. Pneumococcal vaccination of shanghai children: the impact of individual- and neighborhood-level factors. Pediatr Infect Dis J. 2016;35:1–34. doi: 10.1097/INF.0000000000001267. [DOI] [PubMed] [Google Scholar]
- 79.Wallace AS, Sobel H, Ryman TK, Mantaring JB, Silvestre M, Thorley M, Ducusin J, Nyunt-U S. Timing of hepatitis B vaccination and impact of non-simultaneous vaccination with DTP vaccine following introduction of a hepatitis B birth dose in the Philippines. J Public Health Policy. 2012;33:368–81. doi: 10.1111/jvh.12359. [DOI] [PubMed] [Google Scholar]
- 80.Wu JN, Wen XZ, Zhou Y, Lin D, Zhang SY, Yan YS. Impact of the free-vaccine policy on timely initiation and completion of hepatitis B vaccination in Fujian, China. J Viral Hepat. 2015;22:551–60. doi: 10.1111/jvh.12359. [DOI] [PubMed] [Google Scholar]
- 81.Yadav K, Srivastava R, Kumar R, Chinnakal P, Rai SK, Krishnan A. Significant vaccination delay can occur even in a community with very high vaccination coverage: evidence from Ballabgarh, India. J Trop Pediatr. 2012;58:133–38. doi: 10.1093/tropej/fmr059. [DOI] [PubMed] [Google Scholar]
- 82.Zaidi SMA, Khowaja S, Dharma VK, Khan AJ, Chandir S. Coverage, timeliness, and determinants of immunization completion in Pakistan: evidence from the demographic and health survey (2006–07). Hum Vaccin Immunother. 2014;10:1712–20. doi: 10.4161/hv.28621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhou Y, Wang H, Zheng J, Zhu X, Xia W, Hipgrave DB. Coverage of and influences on timely administration of hepatitis B vaccine birth dose in remote rural areas of the People’s Republic of China. Am J Trop Med Hyg. 2009;81:869–74. doi: 10.4269/ajtmh.2009.09-0238. [DOI] [PubMed] [Google Scholar]
- 84.Zivich PN, Kiketa L, Kawende B, Lapika B, Yotebieng M. Vaccination coverage and timelines among children 0–6 months in kinshasa, the democratic Republic of Congo: a prospective cohort study. Matern Child Health J. 2017; 5:1055–1064. [DOI] [PubMed] [Google Scholar]
- 85.World Health Organization Pneumococcal vaccines: WHO position paper - 2012. Wkly Epidemiol Rec 2012;87:129–44. [PubMed] [Google Scholar]
- 86.Minor PD. Polio eradication, cessation of vaccination and re-emergence of disease. Nat Rev Microbiol. 2004;2:473–82. doi: 10.1038/nrmicro906. [DOI] [PubMed] [Google Scholar]
- 87.World Health Organization Measles deaths decline, but elimination progress stalls in some regions. 2013: 1–4.
- 88.Gay NJ. The theory of measles elimination: implications for the design of elimination strategies. J Infect Dis. 2004;189:S27–35. doi: 10.1086/381592. [DOI] [PubMed] [Google Scholar]
- 89.Uzicanin A, Zimmerman L. Field effectiveness of live attenuated measles-containing vaccines: a review of published literature. J Infect Dis. 2011;204:S133–48. doi: 10.1093/infdis/jir102. [DOI] [PubMed] [Google Scholar]
- 90.Brown DW, Burton A, Gacic-Dobo M, Karimov RI, Vandelaer J, Okwo-Bele JM. A mid-term assessment of progress towards the immunization coverage goal of the Global Immunization Vision and Strategy (GIVS). BMC Public Health. 2011;11:806. doi: 10.1186/1471-2458-11-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zwerling A, Behr MA, Verma A, Brewer TF, Menzies D. The BCG world atlas : a database of global BCG vaccination policies and practices. PLoS Med. 2011;8:e1001012. doi: 10.1371/journal.pmed.1001012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tefera YA, Wagner AL, Berhane EB, Carlson BF, Boulton ML. Predictors and barriers to full vaccination among children in Ethiopia. Vaccines (Basel). 2018;6:22. doi: 10.3390/vaccines6020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Miles M, Ryman TK, Dietz V, Zell E, Luman ET. Validity of vaccination cards and parental recall to estimate vaccination coverage: A systematic review of the literature. Vaccine. 2013;31:1560–68. doi: 10.1016/j.vaccine.2012.10.089. [DOI] [PubMed] [Google Scholar]
- 94.Lakew Y, Bekele A, Biadgilign S. Factors influencing full immunization coverage among 12–23 months of age children in Ethiopia: evidence from the national demographic and health survey in 2011. BMC Public Health. 2015;15:728. doi: 10.1186/s12889-015-2078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wagner AL, Shenton LM, Gillespie BW, Mathew JL, Boulton ML. Assessing the timeliness of vaccine administration in children under five years in India, 2013. Vaccine. 2019;37:558–64. doi: 10.1016/j.vaccine.2018.12.035. [DOI] [PubMed] [Google Scholar]
- 96.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- World Health Organization Summary of WHO position papers - recommendations for routine immunization [Internet]. 2017. [accessed 2017 September7]. http://www.who.int/immunization/policy/Immunization_routine_table1.pdf


