ABSTRACT
Measles is one of the most contagious infectious diseases. Measles vaccine, which has been introduced in Italy in 1979, is highly effective in preventing the disease (two-dose vaccine effectiveness is 99%). In 2017, Italy was the second country of EU for number of cases of measles. A study conducted in the same year showed that 22.3% of measles infection happened in hospital settings and 6.6% of cases occurred in HCWs. This risk group showed low rates of adhesion to the vaccination campaign. For this reason, we hypothesized that workplace vaccination could lead to better vaccination rates in HCWs. Moreover, we focused the vaccination campaign on a specific target group composed of HCWs not serologically immune and previously not vaccinated. We analyzed the clinical records of measles-specific IgG antibodies of 2,940 HCWs, that underwent occupational health surveillance between 1 January 2017 and 31 December 2017. 15.3% (450) was seronegative for measles, especially in the age group under 35 years. We compared the costs related to strategies with and without serological screening. Our study confirmed that immunization strategy with pre-vaccination screening was cost-effective compared to the vaccination without screening. In our sample, in fact, administration of two dose vaccine only susceptible HCWs determine a saving of 146,262 €. The vaccination of HCWs remains a topical issue in preventing the transmission of infectious disease in the hospital setting. Due to the cost-effectiveness evaluation, we recommend extending the pre-vaccination screening to identify the real susceptible workers.
KEYWORDS: Measles, outbreak, vaccination, health care workers, students
Introduction
Measles is a highly contagious, vaccine-preventable infection caused by the measles virus (Parvomyxoviridae family). It is one of the most contagious infectious diseases, with a basic reproduction number (R0) between 12 and 18, which implies that 90% of non-immune persons who get in touch with a sick person develops the disease.1,2 Measles vaccine, which has been introduced in Italy in 1979, is highly effective in preventing the disease (two-dose vaccine effectiveness is 99%). Two doses of measles vaccines are considered to provide long-lasting immunity even if antibody levels decline over time.3
Nowadays, in developed countries, measles outbreaks still occur despite high vaccination coverage highlighting an immune gap in the populations.4 Italy, in the period between 1 January and 31 December 2017, was the second highest Country, after Romania, in the number of cases of measles (4,991). In particular, the highest number of cases (n = 1,699) and the highest incidence rate (28.8 cases/100,000 inhabitants) happened in Lazio Region (the Region including Rome district). As far as the prevalence among health-care workers (HCWs) is concerned, a total of 322 cases were reported in 18 Regions (all except Valle d’Aosta, Molise and Trentino Alto-Adige).5 HCWs infected can be considered as additional risk factor for patients. Clearly, this outbreak can be explained by the low vaccination coverage recorded for Measles, Mumps, and Rubella (MMR).6 This reduction of vaccination coverage below 95% prompted the Italian Government to issue recommendations aimed at increasing vaccination coverage in HCWs exposed to measles.7–9
An Italian study showed that 22.3% of measles infection happened in hospital settings and 6.6% of cases occurred in HCWs in the period from 1 January to 31 August 2017.10 4–10% of all hospital workers lacked specific measles-specific IgG antibodies, according to other studies among HCWs.11 Non-protective measles-specific IgG antibody titers were found in a substantial percentage of our HCWs, especially those younger than 35 years.12 The literature reports that HCWs are at least 2 to 19 times more likely to incur measles infection than the general population.13–15
According to Center for Disease Control (CDC) guidelines, all HCWs should have evidence of immunity to measles. Birth before 1957 is considered acceptable evidence of measles immunity. Unvaccinated HCWs born before 1957 who lack laboratory evidence of disease or immunity to measles should be vaccinated with two doses of MMR vaccine at the appropriate interval. Adults born in 1957 or later can be considered immune to measles only if they documented laboratory confirmation of disease or immunity or appropriate vaccination with two doses of MMR vaccine (at the appropriate interval). It is not recommended to test serologically for immunity HCWs who document two doses of MMR vaccine. If the results of their tests are negative or equivocal for measles, they should be considered to have presumptive evidence of disease immunity and other doses of MMR vaccine are not necessary. CDC does not suggest serologic testing before vaccination unless the health-care facility considers it cost-effective.1,11
In a previous study, we assessed the vaccine compliance and the effectiveness of workplace vaccination against measles of HCWs and medical students.16 Studies conducted on HCWs showed low rates of adhesion to the vaccination campaign among all the categories. Different motivations are related to the vaccine hesitancy. One of these is the necessity to go into other settings to obtain vaccination. For this reason, it was hypothesized that workplace vaccination could lead to better vaccination rates in HCWs.17–19
In this study, we assessed the cost-effectiveness of this vaccination program according to CDC guidelines.1
Results
This study included 2,940 subjects, 964 men and 1,976 women. 1,772 (60.27%) were HCWs and 1,168 (39.73%) were medical students. The mean age is 32.89 years (SD 10.8). The main features and the vaccination coverage of the population are shown in Tables 1 and 2. 450/2,940 (15.31%) were serologically not immune to measles. 77/450 (17.11%) were previously vaccinated with two doses of MMR vaccine and considered immune to the disease, despite the non-protective titer, according to CDC guideline.1,11 The remaining 373/450 (82.89%) were the target of the vaccination campaign (Figure 1). 179/373 (47.99%) subjects were vaccinated with MMR vaccine. In order to evaluate the effectiveness of the vaccination campaign, we related the trend of vaccination coverage in the population to the new measles cases in the period under examination. No new cases of measles occurred for vaccine coverage in the population above 90%.
Table 1.
Description of study population.
| General population | Serologically immune | Serologically not Immune | ||
|---|---|---|---|---|
| Number | 2,940 | 2,490 (84.70%) | 450 (15.30%) | |
| Gender | Men | 964 | 797 (82.68%) | 167 (17.32%) |
| Women | 1,976 | 1,693 (85.68%) | 283 (14.32%) | |
| Mean age | Mean | 32.89 | 34.15 | 25.88 |
| (yy) | Mean male | 32.85 | 34.43 | 25.32 |
| Mean female | 32.91 | 34.02 | 26.20 | |
| age groups | <35 | 1,852 | 1,430 (77.21%) | 422 (22.79%) |
| (yy) | 35–55 | 961 | 934 (97.19%) | 27 (2.81%) |
| >55 | 127 | 126 (99.21%) | 1 (0.79%) | |
| Measles specific antibody serum value (AU/ml) | Mean male | 198.04 | 237.95 | 6.92 |
| Mean female | 212.33 | 234.51 | 7.57 |
Table 2.
Vaccination status of study population vaccination status.
| 2 doses of MMR | 1 of MMR | No doses | |
|---|---|---|---|
| Whole population | 405 (13.78%) | 210 (7.14%) | 2,325 (79.08%) |
| Serologically immune subjects | 329 (13.21%) | 163 (6.55%) | 1,998 (80.24%) |
| Serologically not immune subjects | 77 (17.02%) | 48 (10.64%) | 325 (72.34%) |
Figure 1.
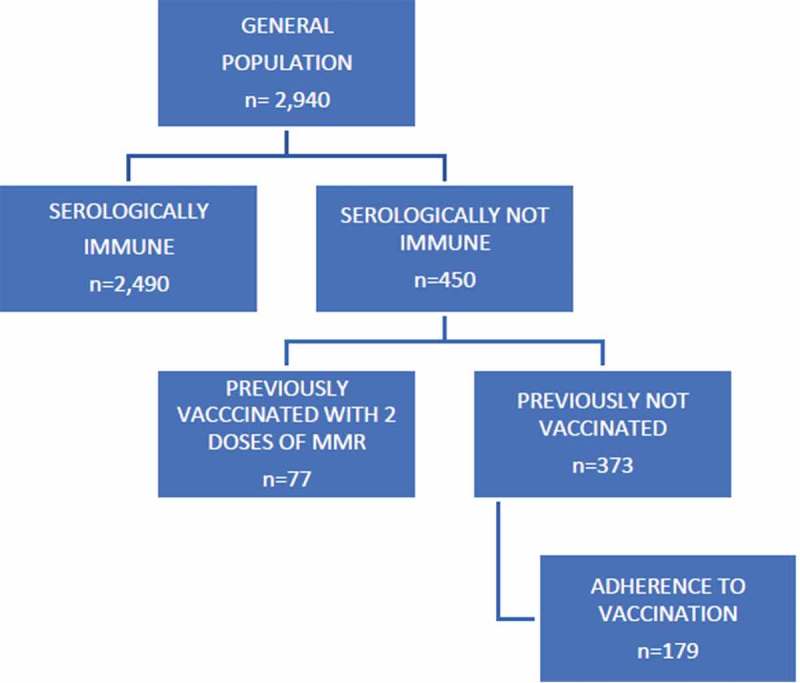
Flow chart of vaccination campaign.
Costs related to our strategy with screening, as shown in Table 3are the following: 34,986 € (2,940 subjects x 11.9 €) for pre-vaccination serological screening; 25,438.6 € [(373 subjects x 2 doses) x 34.10 €] for vaccination campaign; 7,460 € [(373 subjects x 2 doses) x10 €] for personnel. Total cost that we sustained is 68,064.6 €. Costs of the hypothetical strategy without screening are the following: 165,726 € [(210 subjects that document 1 dose of MMR vaccine x 34.10 €) + (2325 subjects previously not vaccinated x 34.10 €) x 2 doses] for vaccination campaign; 48,600 € [(210 subjects + (2,325 subjects x 2)) x 10 €] for personnel. In total, the hypothetical intervention would have cost 214,326 €.
Table 3.
Comparison between costs of strategy with screening and strategy without screening.
| Costs of strategy with screening | Cost of hypothetical strategy without screening | Cost difference | |
|---|---|---|---|
| Direct cost | 34,986 € serological screening; 25,438.6 € vaccination campaign; 7,460€ personnel cost. |
165,726 € vaccination campaign; 48,600 € personnel cost. |
|
| Total | 68,064.6 € | 214,326 € | 146,262 |
Discussion
Our study confirmed that immunization strategy with pre-vaccination screening was cost-effective compared to the hypothetical vaccination strategy without screening. In our sample, in fact, administration of two dose vaccine only susceptible HCWs determine a saving of 146,262 €.20–23 In a previous study, we found that pre-vaccination screening for mumps of HCWs was similarly cost-effective.24
Other important data from this study are the high percentages of HCWs susceptible to measles (15.30%), especially in the age group under 35 years. They are subjects born between 1982 and 1999 when the vaccination coverage against measles was inadequate in Italy.12,23 In our population, they represent a risk group.
Even if the vaccination coverage reached with this strategy was below 50%, no new cases of measles occurred with the achievement of a percentage of immune subjects equal to 90% in our population.
“Vaccine hesitancy” among HCWs is a phenomenon in continuous expansion, especially in non-medical operators.17 Among the main factors of hesitancy, there are conspiracy theories concerning the existence of conflicts of interest between pharmaceutical companies and the Ministry of Health, doubts about the effectiveness of vaccines and the fear of adverse events following administration.18,19 We contrasted this attitude through the high level of knowledge of the majority of the staff of Occupational Medicine and the ready availability of the vaccine.
Despite the recent update of the national guidelines, Measles vaccination is not mandatory for Italian HCWs and the low levels of coverage represent the main risk factor for the nosocomial transmission of the virus.7,17 Recent studies have shown that in cases of nosocomial outbreaks of measles the unvaccinated or incompletely vaccinated subjects represent the main target of the virus.13,14 In our hospital, the coverage rates were inadequate in 2017. All cases that occurred among the HCWs in the course of 2017 (21 subjects) concerned the age group under 35 years, that is the one in which the levels antibody coverage has been found to be lower than the older age groups for which the largest coverage is derived from having contracted the disease naturally, when measles was endemic in our country.25,26
The main limitations of the study are possible lack in recall of vaccination due to loss of written documentation. Another limit is that we didn’t calculate costs related to absence from work related to common the side effects of vaccination as fever (5–10%), rash (5%) and injection site reaction (17–30%).7 Other costs that we did not calculate are those related to lost work time due to having measles.
The Occupational Medicine has the responsibility to sensitize the hospital staff about the importance of vaccinations, highlighting the risks to which workers are exposed in the absence of them and providing all the information about the efficacy and safety of vaccines.27–29
The vaccination of HCWs remains a topical issue in preventing the transmission of infectious disease in the hospital setting. Due to the cost-effectiveness evaluation, we recommend extending the pre-vaccination screening to identify the real susceptible workers.
Methods
In our study, we investigated the cost-effectiveness of workplace vaccination against measles performed in a teaching hospital of Rome (Foundation PTV Polyclinic Tor Vergata) among HCWs and students of the medical area of University Rome Tor Vergata in the period from 1 January to 31 December 2017.
All HCWs and medical students have been tested serologically for measles during health surveillance at the Occupational Medicine. Measles-specific IgG antibodies had been measured in all subjects using chemiluminescent test (DiaSorin LIASON® Measles IgG essay) on serum. The DiaSorin LIAISONa ® Measles IgG assay uses a chemiluminescence immunoassay (CLIA) technology: sensitivity and specificity are 97% (95% CI: 91.7–99.4) and 93% (95% CI: 82.5–97.7), respectively. The related data were extracted from the computer system (ModuLab®).
Vaccination was offered for free to all HCWs and students with measles-specific IgG antibodies less than 16.5 AU/ml. According to CDC guidelines, HCWs and students who documented vaccination with two doses of MMR vaccine were considered protected, independently from their antibody titer.1,11 For each subject, we collected also the following information: age, gender, date of vaccination, vaccination history and profession.
Finally, to assess the cost-effectiveness of the intervention, we analyzed direct costs comparing our vaccination strategy with screening and the hypothetical strategy without screening. As direct cost we considered:
11.9 € for serological test; 34.10 € for MMR vaccine (MMRvaxpro® Sanofi Pasteur MSD); 10 € as personnel cost (considering 15 min to administer a single dose of vaccine).20,21
Analyses were performed using IBM SPSS software (Version 23). Results were considered statistically significant at a p-value threshold of <0.001.
Funding Statement
This study was approved by the Independent Ethics Committee of the University of Rome Tor Vergata (approval number 132/18).
Disclosure of potential conflicts of interest
All other authors declare no conflicts of interest.
Ethical approval
This study was approved by the Independent Ethics Committee of the University of Rome Tor Vergata (approval number 132/18).
References
- 1.Centers for Disease Control and Prevention (CDC) Epidemiology and prevention of vaccine-preventable diseases (The Pink Book) In: Hamborsky J, Kroger A, Wolfe S, editor(s). Chapter 13: measles. 13th ed. Washington (DC): Public Health Foundation; 2015. p. 209–230. [Google Scholar]
- 2.World Health Organization (WHO) Manual for the laboratory diagnosis of measles and rubella virus infection. 2nd ed. Geneva; 2007. [Google Scholar]
- 3.MckeeKEE A, Shea K, Ferrari MJ.. Optimal vaccine schedules to maintain measles elimination with a two-dose routine policy. Epidemiol Infect. 2017;145(2):227–35. doi: 10.1017/S0950268817000668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO Sixth meeting of the Regional Verification Commission for Measles and Rubella Elimination (RVC). 2017. June 5–17; Bucharest (Romania). [Google Scholar]
- 5.Morbillo & Rosolia News – Aggiornamento mensile – Rapporto n°37 – Gennaio 2018. [accessed 2018 December27] http://www.epicentro.iss.it/problemi/morbillo/bollettino/RM_News_2018_37%20def.pdf.
- 6.Ministero della Salute. Le coperture vaccinali dell’età pediatrica e dell’adolescente. I dati 2017 [accessed 2018 December27] http://www.salute.gov.it/portale/news/p3_2_1_1_1.jsp?lingua=italiano&menu=notizie&p=dalministero&id=3348
- 7.Conferenza Stato-Regioni Piano nazionale prevenzione vaccinale 2017–2019. G.U. Serie Generale, n. 41. 2017. February 18.
- 8.Decreto-legge 7 giugno 2017, n. 73 Disposizioni urgenti in materia di prevenzione vaccinale, di malattie infettive e di controversie relative alla somministrazione di farmaci. (17A05515) (GU Serie Generale n.182 del 05- 08-2017)
- 9.Ministero della Salute. Circolare n. 25233. 16 Agosto 2018.
- 10.Filia A, Bella A, Del Manso M, Baggeri M, Magurano F, Rota MC. Ongoing outbreak with well over 4,000 measles cases in Italy from January to end August 2017 − what is making elimination so difficult? Euro Surveill. 2017;22:37. doi: 10.2807/1560-7917.ES.2017.22.37.30614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention (CDC) Immunization of health-care personnel: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011;60(RR–7):1–45. [PubMed] [Google Scholar]
- 12.Coppeta L, Pietroiusti A, Lieto P, Ferraro M, Grelli S, Stillo M, Magrini A. Measles immunity in an Italian teaching hospital. Occup Med (Lond). 2019;69(2):143–145. [DOI] [PubMed] [Google Scholar]
- 13.Fiebelkorn AP, Seward JF, Orenstein WA. A global perpective of vaccination of healthcare personnel against measles: systematic review. Vaccine. 2014;32(38):4823–39. doi: 10.1016/j.vaccine.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maltezou HC, Wiker S. Measles in health-care settings. Am J Infect Control. 2013;41:661–63. [DOI] [PubMed] [Google Scholar]
- 15.Barbadoro P, Marigliano A, Di Tondo E, De Paolis M, Martini E, Prospero E, D’Errico MM. Measles among healthcare workers in a teaching hospital in central Italy. J Occup Health. 2012;54(4):336–39. doi: 10.1539/joh.12-0016-BR. [DOI] [PubMed] [Google Scholar]
- 16.Coppeta L, Pietroiusti A, Morucci L, Neri A, Ferraro M, Magrini A. Workplace vaccination against measles in a teaching hospital of Rome. J Hosp Infect. 2019;101(3):364–65. doi: 10.1016/j.jhin.2018.11.022. [DOI] [PubMed] [Google Scholar]
- 17.Scatigna M, Fabiani L, Micolucci G, Santilli F, Mormile P, Giuliani AR. Attitudinal variables and a possible mediating mechanism for vaccination practice in health care workers of a local hospital in L’Aquila (Italy). Hum Vaccin Immunother. 2017;13(1):198–205. doi: 10.1080/21645515.2016.1225638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karafillakis E, Dinca I, Apfel F, Cecconi S, Wurz A, Takacs J, Suk J, Pastore Celentano L, Kramarz P, Larson HJ. Vaccine hesitancy among healthcare workers in Europe: a qualitative study. Vaccine. 2016;34:5013–20. [DOI] [PubMed] [Google Scholar]
- 19.European Center for Disease Prevention and Control (ECDC) Vaccine hesitancy among healthcare workers and their patient in Europe – a qualitative study. 2015.
- 20.Kang JH, Park YS, Park SY, Kim SB, Ko KP, Seo YH. Varicella seroprevalence among health care workers in Korea: validity of self-reported history and cost-effectiveness of prevaccination screening. Am J Infect Control. 2014;42(8):885–87. doi: 10.1016/j.ajic.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Alp E, Cevahir F, Gokahmetoglu S, Demiraslan H, Doganay M. Prevaccination screening of health-care workers for immunity to measles, rubella, mumps, and varicella in a developing country: what do we save? J Infect Public Health. 2012;5(2):127–32. doi: 10.1016/j.jiph.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Barracco GJ, Eisert S, Saavedra S, Hirsch P, Marin M, Ortega-Sanchez IR. Clinical and economic impact of various strategies for varicella immunity screening and vaccination of health care personnel. Am J Infect Control. 2015;43(10):1053–60. doi: 10.1016/j.ajic.2015.05.027. [DOI] [PubMed] [Google Scholar]
- 23.Celikbas A, Ergonul O, Aksaray S, Tuygun N, Esener H, Tanir G, Eren S, Baykam N, Guvener E, Dokuzoguz B. Measles, rubella, mumps, and varicella seroprevalence among health care workers in Turkey: is prevaccination screening cost-effective? Am J Infect Control. 2006;34(9):583–87. doi: 10.1016/j.ajic.2006.04.213. [DOI] [PubMed] [Google Scholar]
- 24.Coppeta L, Balbi O, Baldi S, Pietroiusti A, Magrini A. Pre-vaccination IgG screening for mumps is the most cost-effectiveness immunization strategy among health care workers. Hum Vaccin Immunother. 2019;15:1135–38. doi: 10.1080/21645515.2018.1564442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Epicentro Morbillo: aspetti [accessed 2018 December27] http://www.epicentro.iss.it/problemi/morbillo/epidItalia.asp.
- 26.Shakoor S, Mir F, Zaidi AKM, Zafar A. Hospital preparedness in community measles outbreaks - challenges and recommendations for low-resource settings. Emerging Health Treats J. 2015;8:24173. doi: 10.3402/ehtj.v8.24173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferraro M, Morucci L, Coppeta L, De Carolis G, Pietroiusti A, Franco E, Magrini A. Managing the risk of bacterial meningitis among health care workers. Occup Med (London). 2019;69(2):113–117. [DOI] [PubMed] [Google Scholar]
- 28.Riccò M, Cattani S, Casagranda F, Gualerzi G, Signorelli C. Knowledge, attitudes, beliefs and practices of occupational physicians towards vaccinations of health care workers: a cross sectional pilot study in North-Eastern Italy. Int J Occup Med Environ Health. 2017;30(5):775–90. doi: 10.13075/ijomeh.1896.00895. [DOI] [PubMed] [Google Scholar]
- 29.Kestenbaum LA, Feemster KA. Identifying and addressing vaccine hesitancy. Pediatr Annu. 2015;44(4):e71–e75. doi: 10.3928/00904481-20150410-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Morbillo & Rosolia News – Aggiornamento mensile – Rapporto n°37 – Gennaio 2018. [accessed 2018 December27] http://www.epicentro.iss.it/problemi/morbillo/bollettino/RM_News_2018_37%20def.pdf.


