ABSTRACT
Non-replicating parenteral rotavirus (RV) vaccine candidates are in development in an attempt to overcome the lower efficacy and effectiveness of oral RV vaccines in low-income countries. One of the leading candidates is a truncated recombinant VP8* protein, expressed in Escherichia coli from original sequences of the prototype RV genotypes P[8], P[4], or P[6] isolated before 1983. Since VP8* is highly variable, it was considered useful to examine the evolutionary changes of RV strains reported worldwide over time in relation to the three P2-VP8 vaccine strains. Here, we retrieved from the GenBank 6,366 RV VP8* gene sequences of P[8], P[4], or P[6] strains isolated between 1974 and 2017, in 77 countries, and compared them with those of the three P2-VP8 vaccine strains: Wa (USA, 1974, G1P[8]), DS-1 (USA, 1976, G2P[4]), and 1076 (Sweden, 1983, G2P[6]). Phylogenetic analysis showed that 94.9% (4,328/4,560), 99.8% (1,141/1,143), and 100% (663/663) of the P[8], P[4], and P[6] strains, respectively, reported globally between 1974 and 2018 belong to non-vaccine lineages. These P[8], P[4], and P[6] RV strains have a mean of 9%, 5%, and 6% amino acid difference from the corresponding vaccine strains. Additionally, in the USA, the mean percentage difference between all the P[8] RV strains and the original Wa strain increased over time: 4% (during 1974–1980), 5% (1988–1991), and 9% (2005–2013). Our analysis substantiated high evolutionary changes in VP8* of the P[8], P[4], and P[6] major RV strains and their increasing variations from the candidate subunit vaccine strains over time. These findings may have implications for the development of new RV vaccines.
KEYWORDS: Rotavirus, vaccine, VP8* protein, VP4, P type, genotype, evolution, diversity, diarrhea, protection
Introduction
Human species A rotaviruses (RVA) are major cause of severe diarrhea in infants and young children worldwide.1 The genome consists of 11 segments of double-stranded RNA encoding 12 viral proteins, the nonstructural proteins (NSP1–NSP6) and the structural proteins (VP1–VP4, VP6, and VP7).2 The two outer capsid proteins VP7 and VP4 are at the basis of a binary classification system of RVA strains into G and P serotypes or genotypes, respectively. Sequential point mutations and reassortment of the genes encoding VP4 and VP7 have resulted in great genotypic diversity worldwide among the circulating human RVA strains, with 36 G-genotypes and 51 P-genotypes recognized to date.3 The most prevalent circulating VP7 genotypes are G1, G2, G3, G4, G9, and G12 and the most common VP4 genotypes are P[8], P[4], and P[6].4–6 Through proteolysis, VP4 is cleaved into two fragments, the comparatively conserved VP5* and the more variable VP8*.7 VP8*, which forms the head of the VP4 spike, interacts with receptors on host cells and is required for rotavirus (RV) attachment and hence infection.8,9
Oral RV vaccines mimic natural infection and have been shown to be effective in protecting children from severe diarrhea. One pentavalent vaccine, RotaTeq, that contains a mixture of five bovine-human mono-reassortants carrying the human VP7 types G1–G4 and the most common VP4 type P[8] in the genetic background of a bovine RVA (Merck), and a monovalent vaccine, Rotarix, containing a single human G1P[8] (GSK), are recommended by the WHO for worldwide use in young children. These and other vaccines recently licensed in developing countries are all orally administered and have shown lower efficacy in low-income countries.1,10–14 Research to date has failed to fully identify the factors determining lower efficacy and strategies to improve the efficacy of oral RV vaccines.15 In addition, reports of a low-level risk of intussusception, prolonged shedding and severe disease in children with severe combined immunodeficiency disease have led to search for alternative strategies, including inactivated (RV) particles and recombinant subunit RV proteins for parenteral immunization.16–18
One of the leading candidates of non-replicating parenteral RV vaccines is a truncated recombinant VP8* (aa 65-223) protein, based on the sequence of the human RV Wa strain and fused with the P2 epitope from tetanus toxin and expressed in Escherichia coli.19 This monovalent P[8] subunit vaccine induced neutralizing antibody to the homotypic Wa strain, but not to the heterotypic strains, which led to the construction and testing of a trivalent P[8], P[4], and P[6] VP8* vaccine based on sequences of the originally Wa, DS-1, and 1076 strains reported in 1974, 1976, and 1983, respectively. Given the highly variable nature of the VP8* subunit, we conducted a comprehensive sequence analysis to examine the phylogenetic and evolutionary relationships between the original prototype viruses and their corresponding genotype strains reported globally between 1974 and 2017.
Results
We first determined the profiles of RV VP8* lineages for P[8], P[4], and P[6] strains reported globally between 1974 and 2017 by phylogenetic analysis (Figure 1). We showed that 94.9% (4,328/4,560), 99.8% (1,141/1,143), and 100% (663/663) of the strains P[8], P[4], and P[6], respectively, form separate clades and thus belong to VP8* non-vaccine lineages. Globally, 232 (5.1%) of P[8] sequences clustered with the Wa strain, while 4,002 (87.8%) clustered in the lineage 3 (Figure 1(a)). In P[4], only 2 sequences (0.2%) clustered with the DS-1 strain, though 1,092 (95.7%) strains clustered in the lineage 5 (Figure 1(b)). In P[6], no sequences clustered with the 1076 strain, and 613 (92%) strains clustered in the lineage 2 (Figure 1(c)).
Figure 1.
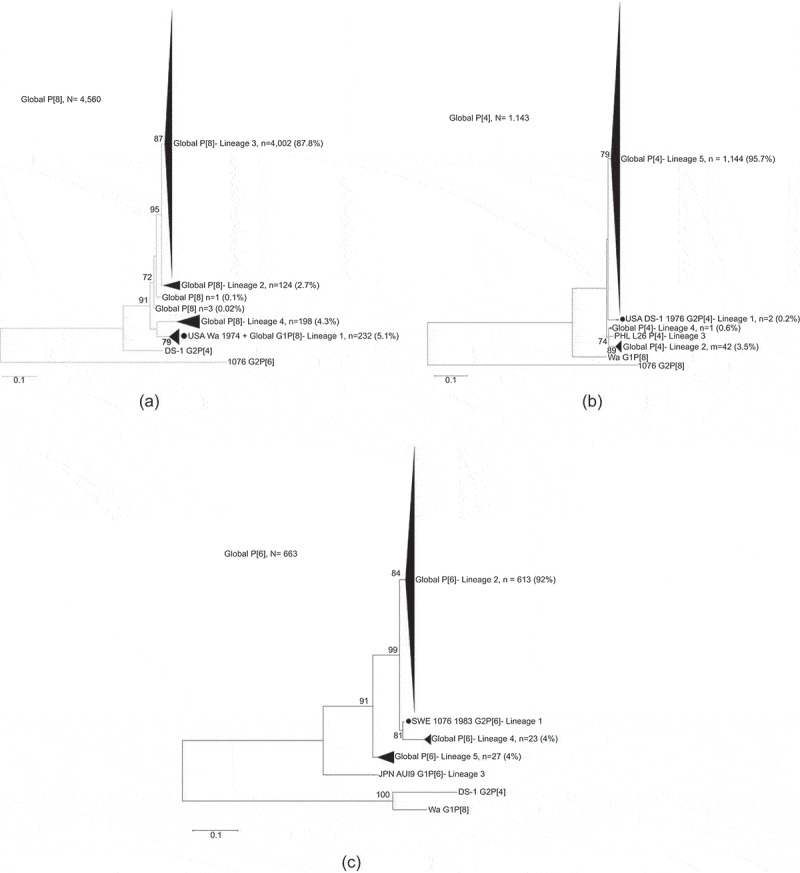
Maximum likelihood phylogenetic trees of VP8* subunit nucleotide sequences (amino acids 64-223 of VP4) of the (a) P[8], (b) P[4], and (c) P[6] global human rotaviruses (1974–2017) and the trivalent P2-VP8 subunit vaccine strains. Vaccine strains Wa, DS-1 and 1076 are indicated by a black circle and bootstrap values greater than 70% are indicated in each node. The scale bar indicates number of substitution per site.
We then grouped VP8*sequences of all RV strains by geographical regions (Supplementary figures 1–3). In Americas, Europe, and West Pacific, 134 (12%), 49 (4%), and 49 (5%) of P[8] strains clustered with the Wa strain, respectively (Supplementary figures 1a, 1c, and 1e). In contrast, no P[8] strains clustered with Wa in Africa, South Asia, and Middle East (Supplementary figures 1b and 1d). In Americas, Europe, Africa, South Asia and Middle East, and West Pacific, 284 (98%), 105 (72%), 190 (98%), 180 (100%), and 333 (99%) of P[4] strains formed lineage 5, respectively, which was distinct from the prototype lineage 1 vaccine strain (Supplementary figures 2a–2e). In Americas, Africa, South Asia and Middle East, and West Pacific, 93 (100%), 315 (100%), 137 (100%), and 43 (46%) of P[6] strains clustered within lineage 2, respectively (Supplementary figures 3a–3d). There were too few P[6] strains from Europe for the analysis.
To gain more detailed insights into how globally reported RVs were closely related to the prototype vaccine strains, the genetic distances of 4,560 P[8], 1,143 P[4], and 663 P[6] strains to trivalent P2-VP8* sequences were determined (Figure 2). In general, the genetic distances to the vaccine strains were similar at nucleotide and amino acid levels. At amino acid level, the maximum genetic distance to the Wa strain was the largest for strains reported in West Pacific (9.4%), and South Asia and Middle East (9.4%). The maximum distance to the DS-1 strain was in Americas (5.5%), and South Asia and Middle East (5.5%). The maximum distance to the 1076 strain was in West Pacific (9.1%) and Europe (8.7%).
Figure 2.
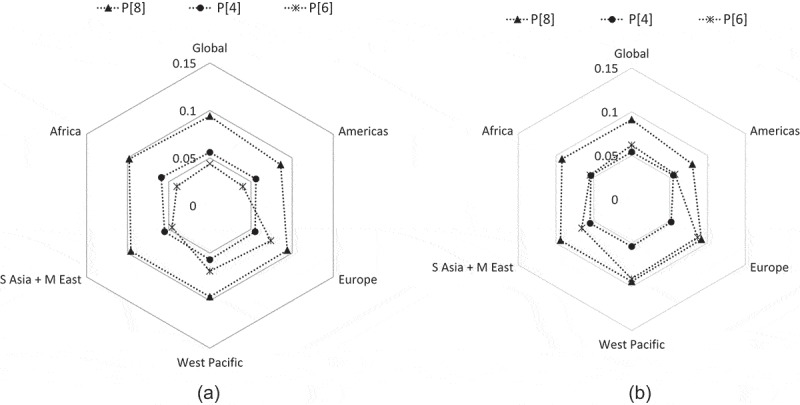
Genetic distances of the VP8* subunit sequences (amino acids 64–223 of VP4) between global P[8], P[4], and P[6] human rotavirus strains and the trivalent P2-VP8 subunit vaccine strains at (a) the nucleotide level and (a) the amino acid level. Vaccine strains are positioned in the center and the mean percentage difference is represented with a filled triangle (P[8]), circle (P[4]), or asterisk (P[6]). A higher genetic distance to the vaccine strains is indicated by a more outward position. Numbers of sequences retrieved per strain and regions are indicated in Supplementary Table 1.
Last, we examined phylogenetic relationship and evolutionary changes of the VP8* sequences available in the Genbank from 1974 to 2013, of all P[8] RV strains reported in the United States (Figure 3). We found that 96% (320/332) of the P[8] strains reported from 2005 to 2013 belonged to non-Wa P[8] lineage 3 (Figure 3(a)). By contrast, most of the strains reported before 1991 clustered in P[8] lineages 1 and 2. When plotting the genetic distances at amino acid level between circulating strains and the prototype Wa strain during three periods of time, we found that the mean percentage difference increased over three periods of time: 4% during 1974–1980, 5% during 1988–1991, and 9% during 2005–2013 (Figure 3(b)).
Figure 3.
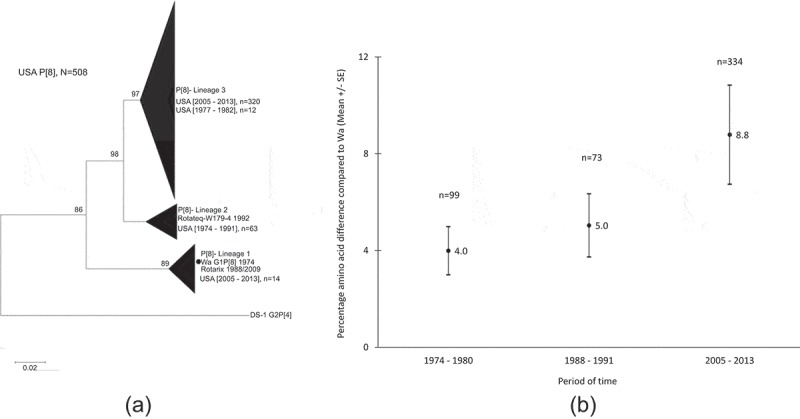
Phylogenetic relationship and changes of VP8* subunit sequences of P[8] RV strains reported in the USA from 1974 to 2013. (a): Phylogenetic tree of the VP8* subunit sequences of the 506 P[8] rotaviruses and the strain Wa (1974), which is indicated by a black circle. Bootstrap values greater than 70% are indicated in each node. The scale bar indicates number of substitution per site. (b): Genetic distance at amino acid level between circulating strains and the prototype Wa strain by three periods of time.
Discussion
Accumulation of point mutations and reassortment events between gene segments are major mechanisms driving the evolution and lineage replacement of RV during the course of adaptation to different immunological environments.20–23 When compared with the candidate subunit vaccine strains: Wa (USA, 1974, G1P[8]), DS-1 (USA, 1976, G2P[4]), and 1076 (Sweden, 1983, G2P[6]), our analysis found high mutational changes and variations in VP8* of the three major RV P[8], P[4], and P[6] strains over time. We found that only a low proportion of globally reported strains belonged to the lineages of the trivalent P2-VP8* subunit vaccine. We have shown that the genetic distance between the Wa strain and the P[8] viruses reported in the USA increased over time from 1974 to 2013, and accumulation of point mutations was accelerated in the last period of time (2005–2013). Of note, the United States introduced RotaTeq (P[8]-lineage 2) in 2006 and Rotarix (P[8]-lineage 1) in 2008; whether these RV vaccine introductions resulted in higher rate of point mutations and contributed to P[8] lineage and strain shifts is not known. A recent study reported substantial amino acid sequence differences between the VP4 and VP7 antigenic epitopes of the vaccine viruses and the circulating strains in Belgium.20 The majority of Belgian P[8] strains belonged to lineage 3, which had 6–9 amino acid differences per strain when compared to the P[8] lineage 1 epitopes of Rotarix and 4–6 differences per strain when compared to the P[8] lineage 2 epitopes of RotaTeq. A much larger number of amino acid differences, mostly in VP8* epitopes, were observed between circulating Belgian P[4] and P[6] strains and the two vaccine viruses. Similar results were found in India24 and Russia.25 Despite these differences, the two vaccines have showed equal efficacy against broad range of vaccine and non-vaccine strains in many countries.26
A monovalent truncated P2-VP8-P[8] subunit vaccine was immunogenic against the target VP8* protein in phases I and II clinical trials in South Africa.27 However, its inability to induce significant heterotypic immunity led to the subsequent testing of a trivalent P2-VP8-P[8]/P[6]/P[4] vaccine in South African adults, children, and infants. Three dose levels (15, 30, and 90 µg) of the vaccine were assessed in infants.28 Anti-P2-VP8* IgG and neutralizing antibody responses to the three corresponding vaccine P-types were high. However, the proportion of infants with anti-P2-VP8 IgA seroresponse to each individual antigen was between 20% and 34% across all three dose groups. Whether the trivalent P2-VP8 subunit vaccine will provide protection against infection and diarrhea from increasingly variable homologous and heterologous RV strains in children remains to be determined.
The findings in this analysis are subject to some limitations. First, correlates of protection, in regard to RV infection and disease in humans, remain incompletely understood.29 Epidemiological and clinical studies have showed that infection or immunization with a single RV strain generates cross-reactive protective immunity to heterotypic strains in children.30–32 Children who have one RV infection usually develop less severe disease in subsequent exposure.33,34 For example, in a 2-y prospective cohort study, children in Mexico were protected from illness of both homotypic and heterotypic RV infection, though with a somewhat stronger homotypic response to the first infection.35 Similar findings were observed among children with RV infection in India.36 In addition, the comparable efficacy of the monovalent Rotarix and the pentavalent RotaTeq vaccines reinforces this cross-reactive immunity and protection.26 These studies have demonstrated that RV protective immunity is not entirely dependent on RV serotypes, and might involve multiple viral antigens. Of note, a recent study showed that monoclonal antibodies specific for VP5* had a strong heterotypic neutralizing activity, suggesting that recombinant VP5* might be a target for the development of a broadly effective RV vaccine.37 Second, the majority of sequences in this analysis belong to strains isolated in recent years, which did not allow us to perform a suitable analysis of genetic distances, or antigenic epitopes changes over the time. Third, this study was a fully in-silico analysis. Further studies will be required to examine whether antibodies to the VP8* can neutralize RV strains of various lineages in the same or different P genotypes from diverse geographic locations over time.
Our findings may have implications for the development of new RV vaccines, given the high mutation rate and diversity in VP8* of the currently most prevalent three RV P genotypes around the world. To broaden immunity and enhance protection against increasingly diverse RV P types among children throughout the world, alternate approaches, such as the development of parenteral vaccine candidates based on whole inactivated RV, should be considered.
Methods
We retrieved VP4 gene sequences of human RVA strains from the NCBI gene database (http://www.ncbi.nlm.nih.gov/), with the associated sampling date and location information available as of March 30, 2018. We aligned truncated VP8* gene sequences of P[8], P[4], or P[6] strains using the MUSCLE algorithm in MEGA 7.0 with manual adjustment and removed partial sequences from the dataset. Lineages were formed by including a wide selection of different representative sequences of the VP8* of the P[8], P[4], and P[6] RV strains available in the GenBank database.38–40 We constructed phylogenetic trees using the Maximum likelihood method and the Kimura 2-parameter substitution model supported by bootstrap analysis with 1000 replicates. The deduced amino acid sequences were obtained using MEGA 7.0. After removal of short/fragment sequences, a total of 6,366 VP8* gene sequences of strains P[8] (n = 4,560), P[4] (n = 1,143), and P[6] (n = 663) isolated between 1974 and 2017 in 77 countries were used for analysis (Supplementary Table 1). Countries were grouped by five geographical regions: Americas (n = 11 countries), Africa (n = 28), Europe (n = 16), South Asia and Middle East (n = 13), and Western Pacific (n = 9) (Supplementary Table 2).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental data
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Tate JE, Burton AH, Boschi-Pinto C, Parashar UD.. Global, regional, and national estimates of rotavirus mortality in children <5 years of age, 2000–2013. Clin Infect Dis. 2016;62(Suppl 2):S96–s105. doi: 10.1093/cid/civ1013. [DOI] [PubMed] [Google Scholar]
- 2.Zeller M, Donato C, Trovao NS, Cowley D, Heylen E, Donker NC, McAllen JK, Akopov A, Kirkness EF, Lemey P, et al. Genome-wide evolutionary analyses of g1P[8] strains isolated before and after rotavirus vaccine introduction. Genome Biol Evol. 2015;7:2473–83. doi: 10.1093/gbe/evv157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matthijnssens J, Ciarlet M, McDonald SM, Attoui H, Banyai K, Brister JR, Buesa J Esona MD, Estes MK, Gentsch JR, et al. Uniformity of rotavirus strain nomenclature proposed by the Rotavirus Classification Working Group (RCWG). Arch Virol. 2011;156:1397–413. doi: 10.1007/s00705-011-1006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowen MD, Mijatovic-Rustempasic S, Esona MD, Teel EN, Gautam R, Sturgeon M, Azimi PH, Baker CJ, Bernstein DI, Boom JA, et al. Rotavirus strain trends during the postlicensure vaccine era: United States, 2008–2013. J Infect Dis. 2016;214:732–38. doi: 10.1093/infdis/jiw233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leshem E, Lopman B, Glass R, Gentsch J, Banyai K, Parashar U, Patel M. Distribution of rotavirus strains and strain-specific effectiveness of the rotavirus vaccine after its introduction: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14:847–56. doi: 10.1016/S1473-3099(14)70832-1. [DOI] [PubMed] [Google Scholar]
- 6.Heylen E, Zeller M, Ciarlet M, Lawrence J, Steele D, Van Ranst M, Matthijnssens J, Human P, et al. Human P[6] rotaviruses from Sub-Saharan Africa and Southeast Asia are closely related to those of human P[4] and P[8] rotaviruses circulating worldwide. J Infect Dis. 2016;214:1039–49. doi: 10.1093/infdis/jiw247. [DOI] [PubMed] [Google Scholar]
- 7.Dormitzer PR, Nason EB, Prasad BV, Harrison SC. Structural rearrangements in the membrane penetration protein of a non-enveloped virus. Nature. 2004;430:1053–58. doi: 10.1038/nature02836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Venkataram Prasad BV, Shanker S, Hu L, Choi JM, Crawford SE, Ramani S, Czako R, Atmar RL, Estes MK. Structural basis of glycan interaction in gastroenteric viral pathogens. Curr Opin Virol. 2014;7:119–27. doi: 10.1016/j.coviro.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Settembre EC, Chen JZ, Dormitzer PR, Grigorieff N, Harrison SC. Atomic model of an infectious rotavirus particle. Embo J. 2011;30:408–16. doi: 10.1038/emboj.2010.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parashar UD, Johnson H, Steele AD, Tate JE. Health impact of rotavirus vaccination in developing countries: progress and way forward. Clin Infect Dis. 2016;62(Suppl 2):S91–5. doi: 10.1093/cid/civ1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD, Feikin DR, Binka FN, Steele AD, Laserson KF, Ansah NA, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376:606–14. doi: 10.1016/S0140-6736(10)60889-6. [DOI] [PubMed] [Google Scholar]
- 12.Madhi SA, Cunliffe NA, Steele D, Witte D, Kirsten M, Louw C, Ngwira B, Victor JC, Gillard PH, Cheuvart BB, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med. 2010;362:289–98. doi: 10.1056/NEJMoa0904797. [DOI] [PubMed] [Google Scholar]
- 13.Zaman K, Dang DA, Victor JC, Shin S, Yunus M, Dallas MJ, Podder G, Thiem VD, Luby SP, Coia ML, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376:615–23. doi: 10.1016/S0140-6736(10)60755-6. [DOI] [PubMed] [Google Scholar]
- 14.Desselberger U. Differences of rotavirus vaccine effectiveness by country: likely causes and contributing factors. Pathogens. 2017;6:pii: E65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Velasquez DE, Parashar U, Jiang B. Decreased performance of live attenuated, oral rotavirus vaccines in low-income settings: causes and contributing factors. Expert Rev Vaccines. 2018;17:145–61. doi: 10.1080/14760584.2018.1418665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azevedo MS, Gonzalez AM, Yuan L, Jeong KI, Iosef C, Van Nguyen T, Lovgren-Bengtsson K, Morein B, Saif LJ. An oral versus intranasal prime/boost regimen using attenuated human rotavirus or VP2 and VP6 virus-like particles with immunostimulating complexes influences protection and antibody-secreting cell responses to rotavirus in a neonatal gnotobiotic pig model. Clin Vaccine Immunol. 2010;17:420–28. doi: 10.1128/CVI.00395-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blazevic V, Lappalainen S, Nurminen K, Huhti L, Vesikari T. Norovirus VLPs and rotavirus VP6 protein as combined vaccine for childhood gastroenteritis. Vaccine. 2011;29:8126–33. doi: 10.1016/j.vaccine.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 18.Li T, Lin H, Zhang Y, Li M, Wang D, Che Y, Zhu Y, Li S, Zhang J, Ge S, et al. Improved characteristics and protective efficacy in an animal model of E. coli-derived recombinant double-layered rotavirus virus-like particles. Vaccine. 2014;32:1921–31. doi: 10.1016/j.vaccine.2014.01.093. [DOI] [PubMed] [Google Scholar]
- 19.Wen X, Cao D, Jones RW, Hoshino Y, Yuan L. Tandem truncated rotavirus VP8* subunit protein with T cell epitope as non-replicating parenteral vaccine is highly immunogenic. Hum Vaccin Immunother. 2015;11:2483–89. doi: 10.1080/21645515.2015.1054583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeller M, Patton JT, Heylen E, De Coster S, Ciarlet M, Van Ranst M, Matthijnssens J. Genetic analyses reveal differences in the VP7 and VP4 antigenic epitopes between human rotaviruses circulating in Belgium and rotaviruses in Rotarix and RotaTeq. J Clin Microbiol. 2012;50:966–76. doi: 10.1128/JCM.05590-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dennis AF, McDonald SM, Payne DC, Mijatovic-Rustempasic S, Esona MD, Edwards KM, Chappell JD, Patton JT. Molecular epidemiology of contemporary G2P[4] human rotaviruses cocirculating in a single U.S. community: footprints of a globally transitioning genotype. J Virol. 2014;88:3789–801. doi: 10.1128/JVI.03516-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang S, McDonald PW, Thompson TA, Dennis AF, Akopov A, Kirkness EF, Seheri ML, Steele AD, Wentworth DE, Mphahlele MJ. Analysis of human rotaviruses from a single location over an 18-year time span suggests that protein coadaption influences gene constellations. J Virol. 2014;88:9842–63. doi: 10.1128/JVI.01562-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magagula NB, Esona MD, Nyaga MM, Stucker KM, Halpin RA, Stockwell TB, et al. Whole genome analyses of G1P[8] rotavirus strains from vaccinated and non-vaccinated South African children presenting with diarrhea. J Med Virol. 2015;87:79–101. doi: 10.1002/jmv.23971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kulkarni R, Arora R, Arora R, Chitambar SD. Sequence analysis of VP7 and VP4 genes of G1P[8] rotaviruses circulating among diarrhoeic children in Pune, India: a comparison with Rotarix and RotaTeq vaccine strains. Vaccine. 2014;32(Suppl 1):A75–83. doi: 10.1016/j.vaccine.2014.03.080. [DOI] [PubMed] [Google Scholar]
- 25.Morozova OV, Sashina TA, Fomina SG, Novikova NA. Comparative characteristics of the VP7 and VP4 antigenic epitopes of the rotaviruses circulating in Russia (Nizhny Novgorod) and the Rotarix and RotaTeq vaccines. Arch Virol. 2015;160:1693–703. doi: 10.1007/s00705-015-2439-6. [DOI] [PubMed] [Google Scholar]
- 26.Jiang V, Jiang B, Tate J, Parashar UD, Patel MM. Performance of rotavirus vaccines in developed and developing countries. Hum Vaccin. 2010;6:532–42. doi: 10.4161/hv.6.7.11278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Groome MJ, Koen A, Fix A, Page N, Jose L, Madhi SA, McNeal M, Dally L, Cho I, Power M, et al. Safety and immunogenicity of a parenteral P2-VP8-P[8] subunit rotavirus vaccine in toddlers and infants in South Africa: a randomised, double-blind, placebo-controlled trial. Lancet Infect Dis. 2017;17:843–53. doi: 10.1016/S1473-3099(17)30242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fix A, Groome M, Fairlie L, Morrison J, Koen A, Masenya M, Page N, Jose L, Madhi S, McNeal M, et al. Safety and immunogenicity of a parenteral trivalent P2-VP8 subunit rotavirus vaccine. 13th International Double-Stranded RNA Virus Symposium Abstract book 2018:p157; Houffalize, Belgium. [Google Scholar]
- 29.Clarke E, Desselberger U. Correlates of protection against human rotavirus disease and the factors influencing protection in low-income settings. Mucosal Immunol. 2015;8:1–17. doi: 10.1038/mi.2014.114. [DOI] [PubMed] [Google Scholar]
- 30.Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, Abate H, Breuer T, Clemens SC, Cheuvart B, Espinoza F, Gillard P, Innis BL, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- 31.Bhandari N, Rongsen-Chandola T, Bavdekar A, John J, Antony K, Taneja S, Goyal N, Kawade A, Kang G, Rathore SS, et al. Efficacy of a monovalent human-bovine (116E) rotavirus vaccine in Indian children in the second year of life. Vaccine. 2014;32(Suppl 1):A110–6. doi: 10.1016/j.vaccine.2014.04.079. [DOI] [PubMed] [Google Scholar]
- 32.Velasquez DE, Parashar UD, Jiang B. Strain diversity plays no major role in the varying efficacy of rotavirus vaccines: an overview. Infect Genet Evol. 2014;28:561–71. doi: 10.1016/j.meegid.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 33.Desselberger U, Huppertz HI. Immune responses to rotavirus infection and vaccination and associated correlates of protection. J Infect Dis. 2011;203:188–95. doi: 10.1093/infdis/jiq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arias CF, Lopez S, Mascarenhas JD, Romero P, Cano P, Gabbay YB, De Freitas RB, Linhares AC. Neutralizing antibody immune response in children with primary and secondary rotavirus infections. Clin Diagn Lab Immunol. 1994;1:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Velazquez FR, Matson DO, Calva JJ, Guerrero L, Morrow AL, Carter-Campbell S, Glass RI, Estes MK, Pickering LK, Ruiz-Palacios GM, et al. Rotavirus infection in infants as protection against subsequent infections. N Engl J Med. 1996;335:1022–28. doi: 10.1056/NEJM199610033351404. [DOI] [PubMed] [Google Scholar]
- 36.Gladstone BP, Ramani S, Mukhopadhya I, Muliyil J, Sarkar R, Rehman AM, et al. Protective effect of natural rotavirus infection in an Indian birth cohort. N Engl J Med. 2011;365:337–46. doi: 10.1056/NEJMoa1006261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nair N, Feng N, Blum LK, Sanyal M, Ding S, Jiang B, Sen A, Morton JM, He XS, Robinson WH, Greenberg HB. VP4- and VP7-specific antibodies mediate heterotypic immunity to rotavirus in humans. Sci Transl Med. 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Felix-Valenzuela L, Cooley-Garcia DP, Cano-Rangel MA, Durazo-Arvizu ML, Mata-Haro V. Predominance of G9P[4] rotavirus from children with acute gastroenteritis in Northwestern Mexico. Intervirology. 2016;59:228–33. doi: 10.1159/000464132. [DOI] [PubMed] [Google Scholar]
- 39.Seheri LM, Magagula NB, Peenze I, Rakau K, Ndadza A, Mwenda JM, Weldegebriel G, Steele AD, Mphahlele MJ. Rotavirus strain diversity in Eastern and Southern African countries before and after vaccine introduction. Vaccine. 2018;36:7222–30. doi: 10.1016/j.vaccine.2017.11.068. [DOI] [PubMed] [Google Scholar]
- 40.Xu C, Fu J, Ai J, Zhang J, Liu C, Huo X, Bao C, Zhu Y. Phylogenetic analysis of human G9P[8] rotavirus strains circulating in Jiangsu, China between 2010 and 2016. J Med Virol. 2018;90:1461–70. doi: 10.1002/jmv.25214. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


