Abstract
Study Objectives
Short sleep duration and sleep disturbances have been related to obesity and metabolic disruption. However, the behavioral and physiological mechanisms linking sleep and alterations in energy balance and metabolism are incompletely understood. In rodents, sleep regulation is closely related to appropriate brown adipose tissue (BAT) thermogenic activity, but whether the same is true in humans has remained unknown. The present work examines whether sleep duration and quality are related to BAT volume and activity (measured by 18F-FDG) and BAT radiodensity in humans.
Methods
A total of 118 healthy adults (69% women, 21.9 ± 2.2 years, body mass index: 24.9 ± 4.7 kg/m2) participated in this cross-sectional study. Sleep duration and other sleep variables were measured using a wrist-worn accelerometer for seven consecutive days for 24 hours per day. The Pittsburgh Sleep Quality Index was used to assess sleep quality. All participants then underwent a personalized cold exposure to determine their BAT volume, activity, and radiodensity (a proxy of the intracellular triglyceride content), using static positron emission tomography combined with computed tomography (PET/CI) scan.
Results
Neither sleep duration nor quality was associated with BAT volume or activity (the latter represented by the mean and peak standardized 18F-FDG uptake values) or radiodensity (all p > .1). The lack of association remained after adjusting the analyses for sex, date of PET/CT, and body composition.
Conclusions
Although experiments in rodent models indicate a strong relationship to exist between sleep regulation and BAT function, it seems that sleep duration and quality may not be directly related to the BAT variables examined in the present work.
Clinical Trial Registration
NCT02365129 (ClinicalTrials.gov).
Keywords: brown fat, cold-induced thermogenesis, energy balance, glucose uptake, sleep curtailment, thermoregulation
Statement of Significance.
The behavioral and physiological mechanisms linking sleep and alterations in energy balance and metabolism are not well understood. This study uncovers for the first time that neither sleep duration nor quality is related to brown adipose tissue (BAT) volume, activity, or radiodensity after cold exposure, in a large cohort of young healthy adults. This suggests that the relationship between short sleep duration and poor sleep quality with the obesity pandemic and the increase in cardiometabolic disease is more likely to be influenced by other mechanisms rather than BAT function. Future studies should examine whether BAT function assessed by radio-imaging techniques (or continuously measured through indirect markers) is specifically related to sleep stages using polysomnography records, and whether it is altered after sleep deprivation.
Introduction
Sleep is an active, regulated metabolic state essential for health [1, 2]. An extensive body of epidemiological and experimental evidence has shown that sleep curtailment and disturbances are related to an increased risk of obesity and the disruption of metabolic and endocrine functions [2–4], becoming a new avenue for intervention. However, the behavioral and physiological mechanisms linking sleep and alterations in energy balance and metabolism are not well understood.
Brown adipose tissue (BAT) is a specialized thermogenic organ that dissipates heat, especially during cold exposure, a process mediated by uncoupling protein 1 (UCP1) [5]. In rodents, BAT is characterized by its strong thermogenic capacity, but also by its contribution to metabolic homeostasis via the uptake of nutrients [5, 6] and its role as an endocrine organ [7]. Until a decade ago, it was thought that it is present only in small rodents and neonates, but a number of studies simultaneously confirmed its existence and metabolic activity in adult humans [8–11]. Since its “rediscovery,” manipulating human BAT activity has been contemplated as means of combating obesity and diabetes, although recent evidence calls into question whether its impact on energy balance is as substantial as initially thought [6, 12, 13].
The systems that regulate energy balance and metabolic homeostasis are often linked to the neural circuits that modulate sleep duration and quality [14]. For instance, the dorsomedial nucleus in the hypothalamus, which projects into different nuclei and areas related to energy expenditure, feeding, and sleep regulation [15–18], plays a key role in BAT sympathetic premotor neuron excitement. It is also well known that the sleep and thermoregulatory mechanisms are closely related [19–22]. In adult humans, an increase in the distal skin temperature during the night (which is phased-opposed to the decrease in core temperature) [23] is associated with shortened sleep latency and increases in sleep duration and depth [24, 25]. Therefore, it is biologically plausible that sleep regulation and BAT thermogenic function are related in humans.
The first observations of this potential relationship arose from experiments in sleep-deprived rodents; these animals showed a hyperphagic response and an increase in energy expenditure despite a falling body temperature [26, 27]. This led to the hypothesis that BAT is activated to compensate for the heat loss typically observed in sleep deprivation states [27]. Accordingly, Balzano et al. [26] confirmed the 5′-deiodinase activity of BAT to be prompted when rats were sleep deprived. Later experiments [28, 29] in rodents showed that intact BAT thermogenesis is required for restorative sleep responses after induced sleep loss and that BAT has an important function as a sleep-inducing signaling organ. In fact, sleep deprivation induces a sixfold increase in UCP-1 mRNA expression in the BAT of wild-type mice [28]. Interestingly, the activation of BAT is related to rodent rapid and nonrapid eye movement (REM and NREM, respectively) sleep phases under normal and inflammatory conditions [28, 30–32]. There is, therefore, evidence that supports the idea of crosstalk between sleep regulation and BAT function—at least in these animals.
Whether these observations also apply to healthy humans remains to be seen. The present study examines whether sleep duration and quality are related to BAT volume and activity (both determined via 18F-FDG uptake) and radiodensity (a proxy of the intracellular triglyceride content) [33] after personalized cold exposure in a cohort of young healthy adults. Unfortunately, nearly all the cross-sectional and longitudinal studies [1–4, 14, 34–36] that have examined the evidence for a relationship between sleep curtailment/other sleep variables and obesity have suffered from (1) the lack of an objective assessment of these variables, (2) not simultaneously assessing sleep duration and quality, and (3) only including body mass index (BMI) among the measured body composition variables. A complementary aim of this work was therefore to determine whether sleep duration and quality are associated with obesity and body composition.
Methods
Research design and participants
A total of 137 young healthy adults took part in this cross-sectional study; all were recruited into the ACTIBATE study [37] (ClinicalTrials.gov, ID: NCT02365129) via advertisements in electronic media and leaflets. Supplementary Figure S1 shows a flow-chart explaining how they were enrolled in the present work. The inclusion criteria were as follows: age 18–25 years old, having a sedentary lifestyle (ie, undertaking <20-minute moderate–vigorous physical activity < 3 d/wk at baseline), to not be a smoker or take any medication, having had a stable body weight over the last 3 months (changes <3 kg), to have no cardiometabolic disease (eg, hypertension or diabetes), and to have no first-degree relative history of cancer. Positron emission tomography combined with computed tomography (PET/CT) assessments were completed over eight dates distributed over October, November, and December of 2015 and 2016 (four per year); all assessments were made in Granada (south of Spain). The study was approved by the University of Granada Ethics Committee on Human Research (no. 924) and by that of the Servicio Andaluz de Salud. All work was performed in accordance with the Declaration of Helsinki (2013 revision); all subjects gave their written informed consent to be included.
Procedures
Sleep duration and quality
Sleep duration and other sleep variables were objectively measured by triaxial accelerometry. Subjects wore an ActiGraph GT3X+ accelerometer (Actisleep, Pensacola, FL) on the non-dominant wrist for seven consecutive days, 24 hours per day (thus including sleeping and waking hours) [37]. Subjects were allowed to remove it only during bathing or swimming, etc. Raw accelerations were recorded using an epoch length of 5 s at a frequency of 100 Hz [38]. During the measurement period, the subjects were required to make daily notes of their in-bed time (time between going to bed and waking) in a diary. Accelerometer assessments were usually completed within the 7 days before the PET/CT assessment (see below). The raw acceleration data were exported to csv files using ActiLife v.6.13.3 software (ActiGraph, Pensacola, FL) and processed using the GGIR package (v.1.6-0, https://cran.r-project.org/web/packages/GGIR/index.html) [39] in R (v.3.1.2, https://www.cran.r-project.org/). Previously published methods were used to minimize the sensor calibration error (autocalibration of the data based on local gravity) [40], and accelerations were determined by calculating the Euclidean Norm Minus One (ENMO) value as (where 1G ~ 9.8 m/s2) with negative values rounded to zero. The following were then detected and imputed: (1) all nonwear periods, based on the raw acceleration of the three axes, and (2) all sustained, abnormally high accelerations—which are related to the malfunctioning of the accelerometers [39] (see ref. [41] for further information). A previously proposed algorithm (validated via polysomnography) was used to combine data from the accelerometers and the subjects’ diary reports to detect periods of sleep [42, 43]. According to this algorithm, sleep is defined as any period of sustained inactivity in which there is only minimal arm angle change (i.e., <5°) for 5 minutes during a period recorded as sleep in a subject’s diary [42]. Values for the following sleep-related variables were then determined: (1) night onset (time at which the subject fell asleep); (2) wake-up time; (3) in-bed time (time between going to bed and waking up); (4) sleep duration (time between falling asleep and waking up); (5) sleep efficiency (ratio of sleep duration to in-bed time); (6) number and duration of periods spent awake after sleep onset (WASO). Daytime naps were not taken into account. Only data from participants who wore the accelerometers for ≥16 hours per day over at least 4 days (including at least one weekend day) were included in analyses [38].
Sleep quality was determined using the Pittsburgh Sleep Quality Index (PSQI)—a self-rated (via questionnaire), validated, and reliable measurement of this variable that differentiates good from poor sleepers [44]. Subjects responded to 20 items covering seven domains that measure sleep disturbance over the previous month: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction. In the present work, the scoring system was reversed so that higher values indicated better sleep quality (i.e., fewer sleep disturbances). The scores for the seven domains were then summed [44] to obtain an overall PSQI score on an ascending scale from −21 to 0; this eases interpretation and allows comparisons between studies. Good sleepers were deemed to be those with an overall PSQI score of ≥−5, and bad sleepers as those with an overall score of ≤−6 [44].
Sedentary time and physical activity levels
The time spent in sedentary behavior and in light or moderate–vigorous physical activity was determined using a procedure similar to the above, applying age-specific cut-offs for the ENMO value as previously described [45, 46].
Personalized cold exposure and 18F-FDG-PET/CT
The personalized cold-exposure protocol followed, and the quantification of the BAT volume and activity, were as previously reported [41, 47, 48]. Briefly, subjects sat in a cool room (19.5–20°C) wearing a water-perfused cooling vest (Polar Products Inc., Stow, OH). The water temperature was reduced from 16.6°C to ~1.4°C every 10 minutes until they began shivering (visually detected by evaluators or self-reported). After 48–72 hours had elapsed the subjects went to the Hospital Virgen de las Nieves, where they were again placed in a cool room (19.5–20°C) and wore the same cooling vest but with the water temperature set ~4°C above their earlier shivering threshold test result for 2 hours. After the first hour, the subjects received an injection of 18F-FDG (~185 MBq) and the water temperature was increased by 1°C to avoid visually detectable shivering. One hour later, they were subjected to PET/CT using a Siemens Biograph 16 PET/CT scanner (Siemens, Erlangen, Germany). A low-dose CT scan (120 kV) was first performed for attenuation correction and anatomic localization. Immediately thereafter, one static acquisition of 2 PET bed positions (6 minutes each) was performed from the atlas vertebra to the mid-chest region [48]. All personalized cold exposure treatments and 18F-FDG-PET/CT data acquisitions were performed according to current methodological recommendations [49].
The BAT volume and activity, estimated via the 18F-FDG uptake, were then determined using the Beth Israel plug-in for the FIJI program [48]. This required: (1) outlining regions of interest (ROIs) in the supraclavicular, laterocervical, paravertebral, and mediastinal regions from the atlas vertebra to the fourth thoracic vertebra, using a 3D-axial technique; (2) the determination of the number of pixels in the above ROIs with a radiodensity range of −190 to −10 Hounsfield Units; and (3) the calculation of individualized, standardized threshold 18F-FDG uptake values (SUV) [1.2/(lean body mass/body mass)] [49]. BAT volume was determined as the number of pixels in the above range with an SUV value above the SUV threshold. BAT activity was represented as the mean SUV (SUVmean; the mean quantity of 18FDG in the above same pixels) and peak SUV (SUVpeak; the mean of the three highest 18F-FDG contents in three pixels within a volume of <1 cm3). The mean BAT radiodensity was calculated as the mean HU value for the above mentioned ROIs. The SUVpeak for the descending aorta (reference tissue) at the height of the fourth thoracic vertebra was also determined, using a single ROI from one slice (image). For confirmatory analyses, the BAT SUVmean and SUVpeak with respect to lean body mass (SUVLBM) [50] were calculated.
Anthropometry and body composition
Subject height and weight were measured using a SECA scale and stadiometer (model 799, Electronic Column Scale, Hamburg, Germany). Lean mass, fat mass, and visceral adipose tissue (VAT) mass were measured using a Discovery Wi dual-energy x-ray absorptiometer (Hologic, Bedford, MA) [51]. The fat mass index was determined as follows: fat mass (kg)/height squared (m2), and the lean mass index (LMI) as follows: lean mass (kg)/height squared (m2).
Statistical analysis
Descriptive statistics for continuous and categorical variables were used to analyze the subjects’ sociodemographic and clinical characteristics. Pearson correlations were performed to examine the association between the studied sleep variables and BAT volume, activity, and radiodensity. Partial correlations were then performed to examine the previous relationship after adjusting for sex, and for sex and PET/CT date. One-way analyses of variance, as well as one-way analyses of covariance adjusting for sex and PET/CT date, were also performed to examine whether there was any difference in the measured BAT variables based on the number of hours that subjects spent sleeping and on whether they were good or poor sleepers. Pairwise comparisons were performed using Bonferroni post hoc tests when applicable. Pearson and partial correlation tests were also performed to examine whether sleep variables were related to the 18F-FDG uptake in the descending aorta (reference tissue). As complementary analyses, Pearson and partial correlations were also used to examine whether sleep variables were associated with body composition, before and after adjusting for sex. The level of significance was set at p ≤ .05. All statistical analyses were performed using the Statistical Package for the Social Sciences v.24 (SPSS, Inc., Chicago, IL).
Results
From the initial sample size (participants with complete sleep, 18F-FDG, and body composition data, n = 137), 19 participants were excluded due to problems with data collection or analysis (see Supplementary Figure S1). Hence, a final sample of 118 participants (69% women) was included in the main analyses. Table 1 shows their descriptive characteristics. The participants wore the accelerometers for 6.8 ± 0.5 days, including almost all the night (~99.4% of in-bed time). They slept 6.34 ± 0.73 hours per day, and ~52% were classified as good sleepers (score ≥ −5). Because the interaction of sex with the determined sleep variables did not have any effect on BAT volume, activity or radiodensity or body composition (p > .05), all analyses were performed pooling the data for women and men together.
Table 1.
Subject characteristics
| All (n = 118)a | Women (n = 81) | Men (n = 37) | ||||
|---|---|---|---|---|---|---|
| Age (y) | 22 | (2) | 22 | (2) | 22 | (2) |
| Professional status, n (%) | ||||||
| Student | 57 | (49) | 39 | (48) | 18 | (50) |
| Unemployed | 40 | (34) | 31 | (38) | 9 | (25) |
| Other professional activities | 20 | (17) | 11 | (14) | 9 | (25) |
| Body composition | ||||||
| BMI (kg/m2) | 24.9 | (4.7) | 23.7 | (3.8) | 27.5 | (5.4) |
| LMI (kg/m2) | 14.5 | (2.4) | 13.3 | (1.4) | 17.2 | (2.0) |
| FMI (kg/m2) | 9.0 | (3.0) | 9.1 | (2.7) | 8.7 | (3.6) |
| Fat mass (%) | 36.2 | (7.3) | 38.4 | (5.9) | 31.3 | (7.6) |
| VAT mass (g) | 333.8 | (177.7) | 284.2 | (157.6) | 442.3 | (172.7) |
| Objective sleep measures | ||||||
| Valid days (d) | 6.8 | (0.5) | 6.8 | (0.5) | 6.7 | (0.5) |
| Nonwear time at night (min/d) | 3 | (6) | 3 | (7) | 2 | (5) |
| Night onset (hh:mm) | 01:16 | (01:11) | 01:12 | (01:11) | 01:24 | (01:12) |
| Wake-up time (hh:mm) | 08:52 | (01:03) | 08:47 | (00:59) | 09:03 | (01:10) |
| In-bed time (min/d) | 440 | (47) | 441 | (43) | 437 | (55) |
| Sleep duration (min/d) | 381 | (44) | 386 | (43) | 369 | (45) |
| Sleep efficiency | 0.87 | (0.05) | 0.88 | (0.05) | 0.85 | (0.05) |
| Time in WASO (min/d) | 59 | (27) | 55 | (22) | 69 | (34) |
| Blocks in WASO (no/d) | 56 | (35) | 52 | (25) | 63 | (51) |
| Subjective sleep measures (PSQI) | ||||||
| Sleep quality | −1.1 | (0.7) | −1.1 | (0.6) | −1.3 | (0.7) |
| Sleep latency | −1.1 | (0.8) | −1.1 | (0.8) | −1.2 | (0.8) |
| Sleep duration | −0.8 | (0.8) | −0.8 | (0.8) | −0.9 | (0.8) |
| Sleep efficiency | −0.5 | (0.8) | −0.5 | (0.8) | −0.6 | (0.8) |
| Sleep disturbances | −1.1 | (0.4) | −1.1 | (0.4) | −1.0 | (0.3) |
| Sleep medication | −0.1 | (0.5) | −0.1 | (0.5) | −0.2 | (0.6) |
| Daytime dysfunction | −0.9 | (0.7) | −0.9 | (0.7) | −0.9 | (0.7) |
| Global PSQI score | −5.8 | (2.6) | −5.6 | (2.6) | −6.1 | (2.7) |
| Sedentary behavior and PA | ||||||
| Sedentary time (min/d) | 794 | (65) | 786 | (55) | 812 | (80) |
| Light PA (min/d) | 118 | (27) | 123 | (25) | 107 | (30) |
| Moderate–vigorous PA (min/d) | 89 | (32) | 92 | (31) | 84 | (34) |
| PET/CT parameters | ||||||
| SUV threshold | 2.06 | (0.23) | 2.13 | (0.21) | 1.90 | (0.21) |
| BAT volume (mL) | 68.11 | (57.89) | 63.72 | (52.79) | 77.70 | (67.53) |
| BAT SUVmean | 3.74 | (1.97) | 3.96 | (2.15) | 3.26 | (1.40) |
| BAT SUVpeak | 11.19 | (8.32) | 11.71 | (8.61) | 10.07 | (7.66) |
| BAT radiodensity (HU) | −59.03 | (11.76) | −60.21 | (11.55) | −56.40 | (11.95) |
| Descending aorta SUVpeak | 0.80 | (0.20) | 0.81 | (0.21) | 0.77 | (0.17) |
Continuous variables are presented as mean (standard deviation) and categorical variables as number (percentage). BAT = brown adipose tissue, BMI = body mass index, FMI = fat mass index, HU = Hounsfield units, LMI = lean mass index, PA = physical activity, PET/CT = positron emission tomography combined with computed tomography, PSQI = Pittsburgh Sleep Quality Index, SUV = standardized uptake value, VAT = visceral adipose tissue, WASO = awake after sleep onset.
aSome data were missing for professional status (remaining cases, n = 117) and BAT radiodensity (remaining cases, n = 116).
Neither sleep duration nor sleep quality was associated with BAT volume, activity, or radiodensity
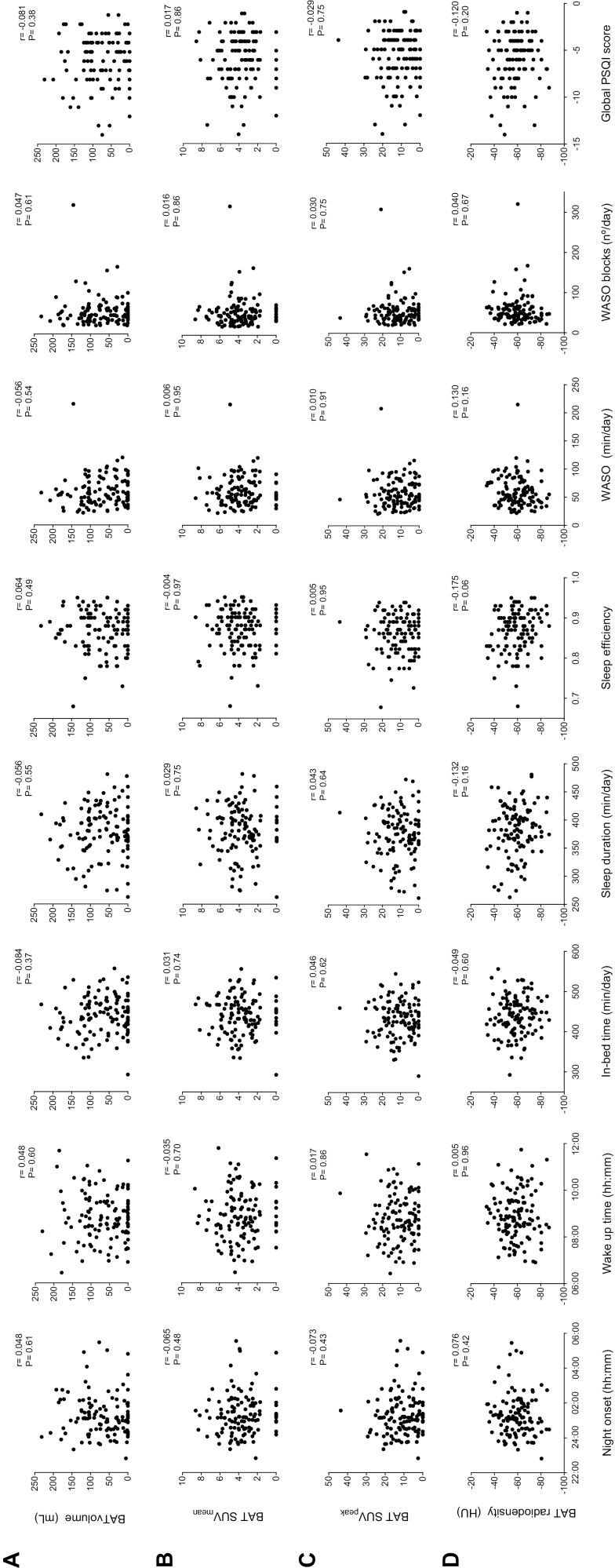
Figure 1 shows that no objective or subjective sleep variable was associated with BAT volume, activity (SUVmean, SUVpeak), or BAT radiodensity (all p > .05). Similarly, partial correlations, after adjusting for sex, and for sex and PET/CT date, revealed no sleep variable to be associated with any measured BAT variable (all p > .05; data not shown). These results remained similar when the analyses were repeated including only those participants with detectable BAT (data not shown). Neither did the results change following additional adjustment for in-bed time, sleep efficiency, nonwear time of the accelerometer during the night, sedentary time, physical activity levels, or any of the body composition variables examined (all p > .05; data not shown). No changes were appreciated when SUV was normalized to lean body mass (SUVLBM), instead of total body mass (SUVBM) for calculating BAT SUVmean and SUVpeak (data not shown).
Figure 1.
Association between sleep variables and brown adipose tissue (BAT) volume and activity (determined via 18F-FDG uptake) (n = 118) and radiodensity (n = 116). Pearson correlations were performed to examine the association between sleep variables and BAT volume (A), mean standardized uptake value (SUVmean) (B), SUVpeak (C), and radiodensity (D). No significant associations were found (p > .05). Higher global Pittsburgh Sleep Quality Index (PSQI) scores are indicative of better sleep quality.
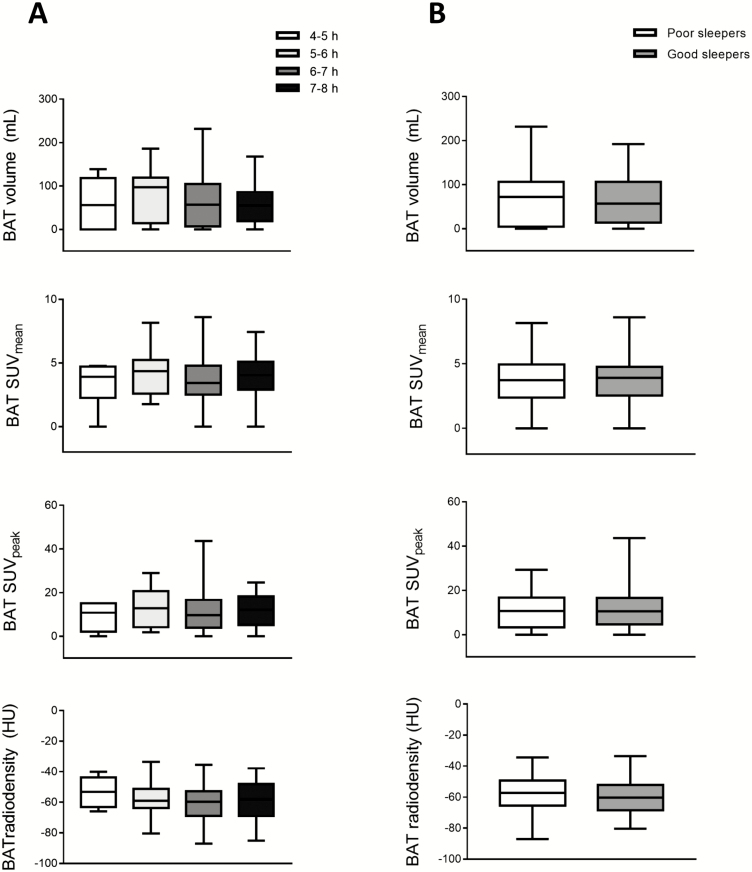
No differences were found in BAT volume, activity, or radiodensity among subjects who slept 4–5 hours (n = 7), 5–6 hours (n = 23), 6–7 hours (n = 67), 7–8 hours (n = 21) or between good sleepers (n = 61) and poor sleepers (n = 57) (Figure 2) (all p > .05). Neither did any appear following adjustment for sex or for sex and PET/CT date (all p > .05). It is noteworthy that the subjects in these previous categories had similar body composition and cardiometabolic profile and undertook similar levels of physical activity (all p > .05; data not shown). However, the participants who slept for 4–5 hours spent considerably longer in sedentary behavior than those who slept 6–7 hours (879 vs 785 min/d; p < .001) and 7–8 hours (879 vs 746 min/d; p < .001). These results remained after grouping subjects as sleeping for 4–5 or 5–6 hours (data not shown).
Figure 2.
Differences in brown adipose tissue (BAT) volume and activity (determined via 18F-FDG uptake) (n = 118) and radiodensity (n = 116), based on the number of hours spent sleeping and on whether subjects were good or poor sleepers. (A) BAT volume, mean standardized uptake value (SUVmean), SUVpeak, and radiodensity were compared by one-way analysis of variance (ANOVA) based on the average number of hours per night subjects spent sleeping (measured via accelerometry). Subjects were divided into four categories: those who had 4- to 5-h sleep (n = 7), 5- to 6-h sleep (n = 23), 6- to 7-h sleep (n = 67), 7- to 8-h sleep (n = 21). (B) BAT volume, SUVmean, SUVpeak, and radiodensity were compared by ANOVA based on whether subjects were good or bad sleepers. Good sleepers (n = 61) were defined as those who had an overall Pittsburgh Sleep Quality Index (PSQI) score of ≥−5, and bad sleepers (n = 57) as those with a score of ≤−6. Measurements of BAT radiodensity were missing for two subjects (one in the 5- to 6-h sleep time group and one in the 6- to 7-h sleep time group; and one good sleeper and one poor sleeper). HU, Hounsfield units.
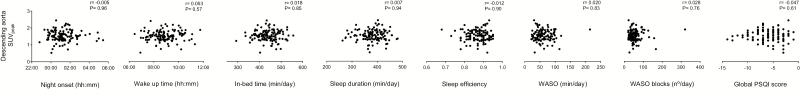
No association was found between any sleep variable and the descending aorta SUVpeak value, even after adjustment for sex, and for sex and PET/CT date (all p > .05; Figure 3).
Figure 3.
Association between sleep variables and the descending aorta peak standardized uptake value (SUVpeak) (n = 118). Pearson’s correlations were performed. Higher values in the global Pittsburgh Sleep Quality Index (PSQI) score are indicative of better sleep quality.
Association between sleep duration and quality and body composition
In-bed time was inversely associated with BMI and VAT mass (r = −.188, p = .04 and r = −.18, p = .05, respectively), and sleep duration was inversely associated with LMI and VAT mass (r = −.226, p = .014 and r = −.190, p = .04; Table 2). In addition, sleep efficiency and time in WASO were significantly associated with fat mass (r = .229, p = .01 and r = −.269, p = .003). After adjustment for sex, only in-bed time remained significantly associated with BMI and VAT mass, along with time in WASO with body fat mass (r = −.189, p = .041; r = −.185, p = .046 and r = −.186, p = .045; Table 2).
Table 2.
Association between sleep variables and body composition (n = 118)
| Night onset (hh:mm) | Wake-up time (hh:mm) | In-bed time (min/d) | Sleep duration (min/d) | Sleep efficiency | Time in WASO (min/d) | Blocks in WASO (no/d) | Global PSQI score | |
|---|---|---|---|---|---|---|---|---|
| BMI (kg/m2) | 0.068 | 0.086 | −0.188a | −0.173 | 0.003 | −0.049 | 0.070 | −0.054 |
| LMI (kg/m2) | 0.070 | 0.095 | −0.133 | −0.226 | −0.156 | 0.137 | 0.192 | −0.120 |
| FMI (kg/m2) | 0.043 | 0.055 | −0.165 | −0.067 | 0.129 | −0.180 | −0.045 | 0.029 |
| Fat mass (%) | 0.024 | 0.012 | −0.129 | 0.026 | 0.229 | −0.269a | −0.151 | 0.080 |
| VAT mass (g) | 0.093 | 0.067 | −0.183a | −0.190 | −0.050 | −0.011 | 0.060 | -0.117 |
Pearson’s correlation coefficients are shown. Statistically significant values are shown in bold (p ≤ .05). BMI = body mass index, FMI = fat mass index, LMI = lean mass index, PSQI = Pittsburgh sleep quality index, VAT = visceral adipose tissue, WASO = awake after sleep onset.
aAssociations that remained significant (p ≤ .05) after adjusting for sex.
Supplementary Table S1 shows the relationships among sleep variables measured objectively (by accelerometry) and subjectively (PSQI); the results agree with those of other studies [52].
Discussion
The present results show that sleep duration and quality are not associated with BAT volume or activity (both estimated via 18F-FDG uptake) or BAT radiodensity following cold exposure in young, sedentary adults. These findings persisted after adjusting for sex, PET/CT date, and body composition.
Although experiments with rodents indicate sleep homeostatic mechanisms to be closely related to BAT function, evidence for the same in adult humans is scarce. In the single study that exists, Enevoldsen et al. [53] examined whether BAT function was similar in seven patients with narcolepsy type I compared with seven matched healthy controls. Narcolepsy type I is a neurological disorder characterized by the loss of orexinergic neurons; this leads to excessive daytime sleepiness, dysregulated REM sleep, cataplexy, fragmented light sleep, and a higher frequency of sleeping-awake transitions [53, 54]. Thus, it is plausible that patients with narcolepsy, who have largely altered sleep patterns, might also show altered BAT function. However, the latter study found that BAT 18F-FDG uptake and sympathetic outflow upon cold exposure were similar in narcoleptic and control participants, calling into question whether sleep duration and quality have any influence over human BAT recruitment and activation. Given the limited sample size, the latter results cannot be generalized to the healthy population, especially because patients with narcolepsy normally have autonomic dysfunction, including changes in their cardiovascular, sympathetic, and temperature regulation [55].
No previous studies have examined the relationship between sleep and BAT in healthy adults, precluding any comparison with other results. The present results do not concur, however, with observations made in rodent models revealing an intimate relationship between sleep regulation and BAT thermogenic activity. This disagreement might be explained in several ways. First, there are vast differences between species in terms of their morphology and physiology [56]. Rodents have a smaller body volume to surface area ratio and their thermoregulation system is designed to conserve heat, whereas humans have a larger body volume to surface area ratio and thus dissipate more heat. Hence, rodents have a higher reliance on BAT thermogenic activity during cold exposure than humans [57]. Because the systems that regulate energy balance and metabolic homeostasis are often linked to the neural circuit that regulates sleep duration and quality [14–18], it would seem coherent that in rodents, in which BAT thermogenic activity makes important contributions to energy balance and metabolic homeostasis, brown adipocyte function should be intimately related to sleep regulation. The same may not hold in humans, however, because recent evidence indicates that the relative contribution of BAT to energy expenditure is rather low and might be insufficient to affect energy balance [12, 13, 58]. Second, to be translatable, experiments in rodents must be performed under conditions that can accurately reflect the physiology and pathophysiology of the humans (e.g., housing mice within their thermoneutral temperature range) [56]. Third, previous studies in rodent models that examined the relationship between sleep regulation and BAT function were performed under different conditions (e.g., following sleep deprivation, the pharmacological or agonist activation of BAT, or in a scenario of systemic inflammation) to those of the present work.
Evidence collected in rodents has shown that their thermoregulatory and sleep mechanisms are closely related [19–21]. Wild-type mice exposed to warm temperatures (35°C) show a robust increase in NREM sleep [28]. In addition, sleep-promoting mechanisms and BAT thermogenesis are both stimulated by sleep loss, being positively correlated [22, 28]. Similarly, in adult humans, an increase in the distal skin temperature during the night (which is phased-opposed to the decrease in core temperature) [23], is associated with shortened sleep latency and increases in sleep duration and depth [24, 25], demonstrating a link between the thermoregulatory and sleep centers. Accordingly, we previously determined in a subcohort of the present subjects (n = 77) that the time at which this increase occurs is weakly related to BAT activity (reflected as SUVmean) (B = −0.2, p = .04; paper submitted) [59]. These findings, together with the present results, suggest that sleep duration and quality might not be directly related to BAT activity following cold exposure. In addition, rodent experiments have shown that BAT may act as a sleep-promoting signaling organ; there are extensive afferent projections running from the BAT into the hypothalamic area, and a population of these is thermosensitive, raising the possibility that BAT thermogenesis may induce sleep independent of changes in core temperature [14, 22, 28, 29]. This hypothesis agrees with the fact that there seems to be a significant time lag between the somnogenic and delayed body temperature effects following pharmacological activation of BAT in rodents [28]. Whether BAT might act as a sleep-prompting signaling organ in humans remains to be seen. Nor can it be ruled out that human BAT might exert its function on sleep regulation via endocrine mechanisms. For instance, BAT secretes adenosine [60], an endogenous factor that promotes sleep by blocking inhibitory inputs to the ventrolateral preoptic area’s sleep-active neurons [61], and can express factors such as interleukin-6, interleukin-1, and tumor necrosis factor, all of which have somnogenic effects [7, 28].
A complementary aim of the present work was to examine whether sleep duration and quality are associated with obesity and body composition. The results show a weak inverse relationship between in-bed time and both BMI and VAT mass (after controlling for sex). Interestingly, in-bed time and sleep duration were moderately and inversely associated, whereas the moment of entering sleep was positively associated with the time spent in sedentary behavior (Supplementary Table S2). This suggests that those subjects who slept less, and who went to sleep later, where those who spent more time in sedentary behavior. This may be explained in that sleep curtailment, or a late chronotype (which is related to misalignment between social rhythms and the circadian clock), may be related to greater drowsiness during the day, and consequently to more sedentary behavior and an increased risk of obesity [62]. Therefore, the in-bed time may be related to increased risk of obesity through indirect mechanisms. Taking everything into account, it is tempting to speculate that the relationship between sleep curtailment and risk of obesity might not be influenced by BAT volume or activity. Other behavioral (e.g., sedentary time) and physiological mechanisms related to homeostatic (e.g., sleep pressure), circadian (e.g., sleeping-awake cycle schedule), and metabolic control (e.g., dysregulated secretion of gastrointestinal peptides, alterations to the appetite regulation centers of the brain) may explain this relationship better.
The present results should be interpreted with caution; the study has a cross-sectional design that precludes the establishment of causal relationships. For instance, it might be possible that habitual short sleep and poor sleep quality could alter the function of the BAT metabolism, as it has been previously shown with other metabolic functions (e.g., glucose metabolism) [2]. In contrast, it could be hypothesized that BAT exerts its influence on sleep duration and quality. Anyhow, sleep is a complex phenomenon, which is influenced not only by behavioral, but also by physiological mechanisms related to homeostatic, circadian, and metabolic control under the participant’s natural sleep environment. Therefore, it exits the possibility that these factors could be influencing the relationship between sleep parameters and 18F-FDG uptake and radiodensity. Furthermore, the sample was composed of young adults, most of whom had a healthy cardiometabolic profile (data not shown); this could have masked or weakened the associations between sleep variables and BAT 18F-FDG uptake, BAT radiodensity, or obesity risk. It should also be remembered that the use of the shivering threshold (subjectively assessed) as the end point of the personalized cooling protocol may have introduced variation into the cooling stimulation, which would be reflected in the subjects’ BAT activation [63]. Despite being the most used technique to assess BAT, a single static 18F-FDG-PET/CT scan has several limitations that might not allow for the accurate estimation of cold-induced BAT metabolic activity [64]. Whether the present findings will be replicated when using other radiotracers such as 15O-oxygen [11], C-acetate, or 18F-fluoro-6-thia-heptadecanoic acid to quantify BAT metabolism remains to be seen. It is also necessary to consider that (1) napping time was not included in the analyses, since we do not have information that allows an accurate quantification of it. The timing and duration of napping could have a profound effect on night sleep, and might partially mask the relationship between sleep parameters and BAT 18F-FDG uptake and radiodensity. However, based on the acceleration records and participant reports, it seems that most of our participants did not nap during the day (also probably because many of them were university students and had to attend classes); (2) although accelerometer records (combined with sleep diaries and subjective measures) are a valid and extensively used measure of sleep duration and quality under free-living conditions [43, 65], they are not able to differentiate between REM and NREM sleep, and thus they may provide a limited insight into the real architecture of sleep wake-activity; (3) although we followed the most updated international recommendations [49] to quantify and analyze BAT 18F-FDG uptake, we performed an unique temporal measure after a personalized cold exposure. Therefore, future studies should examine how continuously measured BAT activity is specifically related to REM and NREM sleep using polysomnography records (since these phases are metabolically different [34]), to get a deeper insight into the interaction between BAT function and sleep regulation. This fact will be conditioned by the advance of the current technologies to assess BAT metabolic activity in a noninvasive and nonionizing manner, or by the validation of indirect markers that accurately reflect its activity. Furthermore, experimental studies should manipulate sleep (e.g., sleep deprivation) and/or BAT function (e.g., use of beta-3 adrenergic agonists available) under well-controlled lab conditions to establish a causal relationship.
In conclusion, sleep duration and quality appear not to be related to BAT volume or activity (both estimated by 18F-FDG uptake) or BAT radiodensity following cold exposure, in young healthy, sedentary adults. Further studies are needed to fully understand the underlying mechanisms of sleep regulation, and how short sleep duration and poor sleep quality are related to the obesity pandemic and the increase in cardiometabolic disease.
Funding
This study was supported by the Spanish Ministry of Economy and Competitiveness via the Fondo de Investigación Sanitaria del Instituto de Salud Carlos III (PI13/01393) and PTA-12264, Retos de la Sociedad (DEP2016-79512-R) and European Regional Development Funds (ERDF), the Spanish Ministry of Education (FPU13/04365 and FPU 15/04059), the Fundación Iberoamericana de Nutrición (FINUT), the Redes Temáticas de Investigación Cooperativa RETIC (Red SAMID RD16/0022), the AstraZeneca HealthCare Foundation, the University of Granada Plan Propio de Investigación 2016—Excellence actions: Unit of Excellence on Exercise and Health (UCEES)—and Plan Propio de Investigación 2018—Programa Contratos-Puente, and the Junta de Andalucía, Consejería de Conocimiento, Investigación y Universidades (ERDF: SOMM17/6107/UGR). This study is part of a PhD thesis defended in the Biomedicine Doctoral Studies Programme of the University of Granada, Spain.
Supplementary Material
Acknowledgments
F.A.M., G.S.D., B.M.T., J.M.L.L.E., and J.R.R. designed the study; F.A.M., G.S.D., B.M.T., and F.J.A.G. conducted the experiments; F.A.M., G.S.D., B.M.T., and J.H.M. analyzed the data; F.A.M. wrote the manuscript; F.A.M., G.S.D., B.M.T., J.H.M., F.J.A.G., P.C.N.R., J.M.L.L.E., D.P.B., and J.R.R. critically reviewed of the manuscript; J.R.R. was primarily responsible for the article’s final content.
References
- 1. Carley DW, et al. Physiology of sleep. Diabetes Spectr. 2016;29(1):5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Spiegel K, et al. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol. 2009;5(5):253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ogilvie RP, et al. The epidemiology of sleep and obesity. Sleep Health. 2017;3(5):383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Capers PL, et al. A systematic review and meta-analysis of randomized controlled trials of the impact of sleep duration on adiposity and components of energy balance. Obes Rev. 2015;16(9):771–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cannon B, et al. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84(1):277–359. [DOI] [PubMed] [Google Scholar]
- 6. Ruiz JR, et al. Role of human brown fat in obesity, metabolism and cardiovascular disease: strategies to turn up the heat. Prog Cardiovasc Dis. 2018;61(2):232–245. [DOI] [PubMed] [Google Scholar]
- 7. Villarroya F, et al. Brown adipose tissue as a secretory organ. Nat Rev Endocrinol. 2017;13(1):26–35. [DOI] [PubMed] [Google Scholar]
- 8. Virtanen KAK, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360(15):1518–1525. doi: 10.1056/NEJMoa0808949 [DOI] [PubMed] [Google Scholar]
- 9. van Marken Lichtenbelt WD, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360(15):1500–1508. [DOI] [PubMed] [Google Scholar]
- 10. Cypess AM, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360(15):1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Saito M, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58(7):1526–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. U Din M, et al. Human brown adipose tissue [15O]O2 PET imaging in the presence and absence of cold stimulus. Eur J Nucl Med Mol Imaging. 2016;43(10):1878–1886. doi: 10.1007/s00259-016-3364-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. U Din M, et al. Postprandial oxidative metabolism of human brown fat indicates thermogenesis. Cell Metab. 2018;28(2):207–216. doi: 10.1016/j.cmet.2018.05.020 [DOI] [PubMed] [Google Scholar]
- 14. Shukla C, et al. Metabolic signals in sleep regulation: recent insights. Nat Sci Sleep. 2016;8:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Orozco-Solis R, et al. The circadian clock in the ventromedial hypothalamus controls cyclic energy expenditure. Cell Metab. 2016;23(3):467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morrison SF, et al. Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metab. 2014;19(5):741–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morrison SF. Central pathways controlling brown adipose tissue thermogenesis. News Physiol Sci. 2004;19:67–74. [DOI] [PubMed] [Google Scholar]
- 18. Chou TC, et al. Afferents to the ventrolateral preoptic nucleus. J Neurosci. 2002;22(3):977–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kumar VM, Kumar VM.. Body Temperature and Sleep: Are They Controlled by the Same Mechanism? Vol. 2; 2004. https://link.springer.com/content/pdf/10.1111/j.1479-8425.2004.00136.x.pdf. Accessed November 6, 2018. [Google Scholar]
- 20. Glotzbach SF, et al. Central nervous regulation of body temperature during sleep. Science. 1976;194(4264):537–539. [DOI] [PubMed] [Google Scholar]
- 21. Szymusiak R, et al. Hypothalamic regulation of sleep and arousal. Ann N Y Acad Sci. 2008;1129:275–286. [DOI] [PubMed] [Google Scholar]
- 22. Kapás L, et al. Brown adipose tissue at the intersection of sleep and temperature regulation. Temperature. 2014;1(1):16–17. doi: 10.4161/temp.29120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kräuchi K, et al. Circadian rhythm of heat production, heart rate, and skin and core temperature under unmasking conditions in men. Am J Physiol. 1994;267(3):R819–R829. [DOI] [PubMed] [Google Scholar]
- 24. Raymann RJEM, et al. Skin temperature and sleep-onset latency: changes with age and insomnia. Physiol Behav. 2007;90(2–3):257–266. doi: 10.1016/J.PHYSBEH.2006.09.008 [DOI] [PubMed] [Google Scholar]
- 25. Kräuchi K, et al. Warm feet promote the rapid onset of sleep. Nature. 1999;401(6748):36–37. [DOI] [PubMed] [Google Scholar]
- 26. Balzano S, Bergmann BM, Gilliland MA, Silva JE, Rechtschaffen A, Refetoff S.. Effect of Total Sleep Deprivation on 5’-Deiodinase Activity of Rat Brown Adipose Tissue. Endocrinology. 1990;127(2):882–890. doi: 10.1210/endo-127-2-882 [DOI] [PubMed] [Google Scholar]
- 27. WEBB WB, et al. Sleep deprivation, age, and exhaustion time in the rat. Science. 1962;136(3522):1122. [DOI] [PubMed] [Google Scholar]
- 28. Szentirmai É, et al. Intact brown adipose tissue thermogenesis is required for restorative sleep responses after sleep loss. Eur J Neurosci. 2014;39(6):984–998. [DOI] [PubMed] [Google Scholar]
- 29. Szentirmai É, et al. The role of the brown adipose tissue in β3-adrenergic receptor activation-induced sleep, metabolic and feeding responses. Sci Rep. 2017;7(1):958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dewasmes G, et al. Activation of brown adipose tissue thermogenesis increases slow wave sleep in rat. Neurosci Lett. 2003;339(3):207–210. [DOI] [PubMed] [Google Scholar]
- 31. Koban M, et al. Chronic REM-sleep deprivation of rats elevates metabolic rate and increases UCP1 gene expression in brown adipose tissue. Am J Physiol Endocrinol Metab. 2005;289(1):E68–E74. [DOI] [PubMed] [Google Scholar]
- 32. Szentirmai É, et al. Brown adipose tissue plays a central role in systemic inflammation-induced sleep responses. PLoS One. 2018;13(5):e0197409. doi: 10.1371/journal.pone.0197409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. U Din M, et al. Human brown fat radiodensity indicates underlying tissue composition and systemic metabolic health. J Clin Endocrinol Metab. 2017;102(7):2258–2267. [DOI] [PubMed] [Google Scholar]
- 34. Copinschi G, et al. The important role of sleep in metabolism. In: E. Ghigo ed., How Gut and Brain Control Metabolism. 2014;42:59–72. doi: 10.1159/000358858 [DOI] [PubMed] [Google Scholar]
- 35. Spiegel K, et al. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354(9188):1435–1439. [DOI] [PubMed] [Google Scholar]
- 36. Knutson KL, et al. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11(3):163–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sanchez-Delgado G, et al. Activating brown adipose tissue through exercise (ACTIBATE) in young adults: rationale, design and methodology. Contemp Clin Trials. 2015;45(Pt B):416–425. [DOI] [PubMed] [Google Scholar]
- 38. Migueles JH, Cadenas-Sanchez C, Ekelund U, et al. Accelerometer Data Collection and Processing Criteria to Assess Physical Activity and Other Outcomes: A Systematic Review and Practical Considerations. Sports Med. 2017;47(9):1821–1845. doi: 10.1007/s40279-017-0716-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van Hees VT, et al. Separating movement and gravity components in an acceleration signal and implications for the assessment of human daily physical activity. PLoS One. 2013;8(4):e61691. doi: 10.1371/journal.pone.0061691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van Hees VT, et al. Autocalibration of accelerometer data for free-living physical activity assessment using local gravity and temperature: an evaluation on four continents. J Appl Physiol. 2014;117(7):738–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Acosta FM, et al. Association of objectively measured physical activity with brown adipose tissue volume and activity in young adults. J Clin Endocrinol Metab. 2019;104(2):223–233. doi: 10.1210/jc.2018-01312 [DOI] [PubMed] [Google Scholar]
- 42. van Hees VT, et al. A novel, open access method to assess sleep duration using a wrist-worn accelerometer. PLoS One. 2015;10(11):e0142533. doi: 10.1371/journal.pone.0142533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sadeh A. The role and validity of actigraphy in sleep medicine: an update. Sleep Med Rev. 2011;15(4):259–267. doi: 10.1016/j.smrv.2010.10.001 [DOI] [PubMed] [Google Scholar]
- 44. Buysse DJ, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 45. Hildebrand M, et al. Age group comparability of raw accelerometer output from wrist- and hip-worn monitors. Med Sci Sports Exerc. 2014;46(9):1816–1824. [DOI] [PubMed] [Google Scholar]
- 46. Hildebrand M, et al. Evaluation of raw acceleration sedentary thresholds in children and adults. Scand J Med Sci Sports. 2017;27(12):1814–1823. [DOI] [PubMed] [Google Scholar]
- 47. Martinez-Tellez B, et al. The impact of using BARCIST 1.0 criteria on quantification of BAT volume and activity in three independent cohorts of adults. Sci Rep. 2018;8(1):8567. doi: 10.1038/s41598-018-26878-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Martinez-Tellez B, et al. A new personalized cooling protocol to activate brown adipose tissue in young adults. Front Physiol. 2017;8:863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chen KY, et al. Brown Adipose Reporting Criteria in Imaging STudies (BARCIST 1.0): recommendations for Standardized FDG-PET/CT experiments in humans. Cell Metab. 2016;24(2):210–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Leitner BP, et al. Mapping of human brown adipose tissue in lean and obese young men. Proc Natl Acad Sci USA. 2017;114(32):8649–8654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sanchez-Delgado G, et al. Association between brown adipose tissue and bone mineral density in humans. Int J Obes. 2019;43:1516–1525. doi: 10.1038/s41366-018-0261-4. [DOI] [PubMed] [Google Scholar]
- 52. Grandner MA, et al. Criterion validity of the Pittsburgh Sleep Quality Index: investigation in a non-clinical sample. Sleep Biol Rhythms. 2006;4(2):129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Enevoldsen LH, et al. Functional brown adipose tissue and sympathetic activity after cold exposure in humans with type 1 narcolepsy. Sleep. 2018. doi: 10.1093/sleep/zsy092. [DOI] [PubMed] [Google Scholar]
- 54. Thannickal TC, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27(3):469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Plazzi G, et al. Autonomic disturbances in narcolepsy. Sleep Med Rev. 2011;15(3):187–196. [DOI] [PubMed] [Google Scholar]
- 56. Maloney SK, et al. Translating animal model research: does it matter that our rodents are cold? Physiology (Bethesda). 2014;29(6):413–420. [DOI] [PubMed] [Google Scholar]
- 57. Tan CL, et al. Regulation of body temperature by the nervous system. Neuron. 2018;98(1):31–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Marlatt KL, et al. Is activation of human brown adipose tissue a viable target for weight management? Am J Physiol Regul Integr Comp Physiol. 2018;315(3):R479–R483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Acosta FM, et al. Relationship between the Daily Rhythm of Distal Skin Temperature and Brown Adipose Tissue 18F-FDG Uptake in Young Sedentary Adults. J Biol Rhyt. 2019Aug 7; 0748730419865400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gnad T, et al. Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature. 2014;516(7531):395–399. [DOI] [PubMed] [Google Scholar]
- 61. Morairty S, et al. Disinhibition of ventrolateral preoptic area sleep-active neurons by adenosine: a new mechanism for sleep promotion. Neuroscience. 2004;123(2):451–457. [DOI] [PubMed] [Google Scholar]
- 62. Reutrakul S, et al. Interactions between sleep, circadian function, and glucose metabolism: implications for risk and severity of diabetes. Ann N Y Acad Sci. 2014;1311(1):151–173. [DOI] [PubMed] [Google Scholar]
- 63. Acosta FM, et al. Correction: physiological responses to acute cold exposure in young lean men. PLoS One. 2018;13(7):e0200865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Schilperoort M, et al. Relevance of lipid metabolism for brown fat visualization and quantification. Curr Opin Lipidol. 2016;27(3):242–248. [DOI] [PubMed] [Google Scholar]
- 65. Martin JL, et al. Wrist actigraphy. Chest. 2011;139(6):1514–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.