Abstract
Human adipose-derived stem cells (hASCs) show great potential for healing bone defects. Bone morphogenetic protein-2 (BMP-2) has been reported to stimulate their osteogenic differentiation both in vitro and in vivo. Here, methacrylated gelatin (GelMA) hydrogels were evaluated as a system to deliver BMP-2 to encapsulated hASCs from two different donors, and BMP-2 delivered from the hydrogels was compared to BMP-2 presented exogenously in culture media. GelMA hydrogels were shown to provide sustained, localized presentation of BMP-2 due to electrostatic interactions between the growth factor and biomaterial after an initial burst release. Both donors exhibited similar responses to the loaded and exogenous growth factor; BMP-2 from the hydrogels had a statistically significant effect on hASC osteogenic differentiation compared to exogenous BMP-2. Expression of alkaline phosphatase was accelerated, and cells in hydrogels with loaded BMP-2 deposited more calcium at one, two, and four weeks than cells without BMP-2 or with the growth factor presented in the media. There were no statistically significant differences in calcium content between groups with 25, 50, or 100 μg/mL loaded BMP-2, suggesting that using a lower growth factor dose may be as effective as a higher loading amount in this system. Taken together, these findings suggest that controlled delivery of BMP-2 from the GelMA enhances its osteogenic bioactivity compared to free growth factor presented in the media. Thus, the GelMA system is a promising biomaterial for BMP-2-mediated hASC osteogenesis.
Keywords: bone tissue engineering, drug delivery, growth factor, gelatin methacrylate, biomaterials
INTRODUCTION
Human adipose-derived stem cells (hASCs) are multipotent adult stem cells that are easily isolated from adipose tissue and can differentiate down the osteogenic lineage both in vitro1 and in vivo.2 These cells can be obtained in high number from both young and elderly patients,3 possess immunomodulatory properties,4 and are highly proliferative in vitro allowing for cell expansion.5 Compared with human bone marrow-derived mesenchymal stem cells (hMSCs), hASCs can more easily be obtained in larger numbers6 and are more proliferative in culture.7 Combined with their strong osteogenic capacity, these factors make hASCs a promising cell source for bone tissue engineering strategies.
Bone morphogenetic protein-2 (BMP-2) is a growth factor known for its role in bone tissue formation. With respect to hASCs, exogenous presentation of this growth factor in culture media has also been shown to induce osteogenesis by hASCs both in vitro8–10 and when injected periodically in vivo into defects filled with hASC-laden scaffolds.10 However, delivery of free growth factors, either in cell culture media or to a bone defect site, is problematic as they are typically unstable in physiologic conditions with very short half-lives. When growth factors are injected intravenously their half-life is on the order of minutes,11,12 and, for BMP-2 specifically, it is on the order of hours in serum-containing culture medium at 37°C in vitro.13 As a result, regular replenishment is required to maintain their bioactivity. Such replenishment is not only inconvenient, but the total amount of protein used in all doses may be cost-prohibitive. While extracellular matrix (ECM) proteins can bind BMP-2,14 protecting and stabilizing the growth factor, this slows its diffusion, possibly keeping it from cells in the center of a tissue engineered construct.15 Sustained presentation of the growth factor from a biomaterial directly to encapsulated cells would alleviate these concerns, as the BMP-2 would be available to cells in the interior of the construct as well as those near its edges, while the biomaterial can serve the role of protecting and stabilizing the growth factor, thus maintaining its bioactivity. Such a strategy may readily be translated to the clinic as a construct loaded with cells and growth factor could be injected or implanted into a patient, and sustained release of the bioactive factor could preclude the need for future growth factor injections.
Methacrylated gelatin (GelMA) is a good candidate material for both cell encapsulation and sustained, localized BMP-2 presentation. A prepolymer solution can be crosslinked into a hydrogel in the presence of UV light under conditions that support viability of encapsulated cells,16 and the hydrogel can take the shape and size of a mold in vitro or defect in vivo. Further, since the gelatin is derived from collagen, it contains adhesive peptide sequences that cells can bind to via integrins,17 meaning that the material does not have to be modified for cell adhesion, and matrix metalloproteinase (MMP)-sensitive degradation sites, so that the material can be degraded by cell-secreted enzymes.18 An additional important characteristic of GelMA is its ability to form polyionic complexes with growth factors including BMP-2.19 The isoelectric point of BMP-2 is 8.5, meaning it is positively charged at physiological pH. Gelatin Type B, obtained by processing collagen-containing tissues in an alkaline solution, typically has an isoelectric point near 5.0, meaning it is negatively charged at physiological pH.19 Thus, the BMP-2 can ionically interact with the GelMA, which has been shown to delay its release from gelatin matrices both in vitro and in vivo.20 While delivery of growth factors, including BMP-2, from gelatin matrices has been studied extensively, glutaraldehyde19,20 or genipin21,22 were often used as crosslinking agents, neither of which enables encapsulation of viable cells. Utilizing the capacity to encapsulate growth factor and cells within photopolymerizing GelMA while maintaining high cell viability, transforming growth factor-β1 (TGF-β1) has been incorporated into these hydrogels with chondrocytes and shown to enhance their proliferation.23 Recently, BMP-4 release from GelMA microparticles was characterized,24 but its osteogenic bioactivity was not evaluated, and release profiles of proteins delivered from microparticles are different from those delivered from a bulk hydrogel.25 BMP-2 release from GelMA/alginate coacervate hydrogels has also been characterized, and the growth factor was shown to be osteogenic to encapsulated hMSCs.26 However, BMP-2 release profiles from bulk GelMA hydrogels have not yet been reported.
Here, BMP-2 presented in a sustained, localized fashion from GelMA hydrogels was compared with exogenously delivered BMP-2 in media for its ability to drive in vitro hASC osteogenic differentiation. The exogenously supplied BMP-2 concentrations used, 100 ng/mL and 300 ng/mL, are in the range of what is used in the literature to drive hASC osteogenic differentiation.5,9,10 BMP-2 amounts loaded directly into the hydrogels were similar to the total amounts of exogenous growth factor delivered over 4 weeks. Delivery of BMP-2 from the GelMA was shown to support hASC proliferation and differentiation and enhance the osteogenic bioactivity of the growth factor. To our knowledge, this is the first report comparing the osteogenic response of hASCs encapsulated in gelatin-based hydrogels to BMP-2 exogenously presented and to BMP-2 presented in a sustained manner from the hydrogels. This bioactive factor delivery system with encapsulation of a highly clinically relevant stem cell population may have great utility in treating bone defects.
METHODS
Gelatin modification
GelMA was synthesized as previously described.27 Briefly, gelatin Type B (Sigma Aldrich, St. Louis, MO) dissolved in phosphate buffered saline (PBS, HyClone, Logan, UT) at 10% w/v was heated to 50°C. Methacrylic anhydride (Sigma Aldrich) was added dropwise to the stirring solution at 0.8 mL per gram of gelatin. The reaction continued at 50°C with stirring for 1 hour. Then, an equal volume of warmed PBS was added to the reaction mixture, and the solution was dialyzed using a membrane with MWCO 12,000–14,000 Da (Spectrum Laboratories, Rancho Dominguez, CA) against ultrapure deionized water (diH2O) at 37°C for 7 days, filtered through a 0.22 μm membrane, frozen and lyophilized until dry. Modification of lysine residues of the gelatin backbone with methacrylate groups was characterized by 1H NMR (Varian, Palo Alto, CA) using the quantification method described in Ovsianikov et al.28 Percent modification was shown to range from 81.0% to 93.6% depending on the batch.
Growth factor release
Radiolabeled recombinant human BMP-2 (125I labeled BMP-2, Perkin Elmer, Waltham, MA) was used to determine BMP-2 release from the GelMA hydrogels. Lyophilized GelMA was dissolved at 10% w/v in Dulbecco’s Modified Eagle Medium-low glucose (DMEM-LG) containing 0.25% Irgacure 2959 photoinitiator (Sigma Aldrich). Recombinant human BMP-2 (Department of Developmental Biochemistry, University of Würzburg, Germany) was dissolved at 1 μg/mL in a reconstitution buffer of diH2O containing 4 mM HCl and 0.01% w/v bovine serum albumin (BSA, Fisher Scientific). For groups to be loaded with growth factor, BMP-2 in buffer solution was added to achieve final concentrations of 25, 50, or 100 μg/mL. Reconstitution buffer was added to the GelMA solution for groups that received less or no BMP-2 to ensure all groups had the same ratio of GelMA to BMP-2 reconstitution buffer. Each solution also received 20 μL/mL125I BMP-2 in PBS, and 20 μL droplets of GelMA/BMP-2/125I BMP-2 solution were dispensed onto a glass plate and covered with a quartz plate on 0.75 mm spacers. Samples were irradiated with a UV lamp (Lumen Dynamics, Missisagua, ON, Canada) with a 320–500 nm filter at 3.0 mW/cm2 for 45 s to crosslink. Each hydrogel was then placed in a low retention microcentrifuge tube containing 1 mL PBS and kept at 37°C. After 2 h, and then again on days 1, 3, 5, 8, 11, 18, 25, 33, and 51, the PBS was removed and replaced with fresh solution. To quantify the amount of BMP-2 in the release samples, 50 μL of PBS releasate was mixed with 150 μL of Optiphase Hi Safe II liquid scintillation cocktail (Perkin Elmer) and the resulting luminescence was measured on a 1450 MicroBeta Trilux Scintillation Counter (Perkin Elmer). Radiolabeled 125I BMP-2 from the same batch was diluted in PBS and also kept at 37°C and used to create standard curves. Hydrogels were broken down in 1% collagenase Type IA (Sigma Aldrich) to confirm the amount of BMP-2 remaining at the end of the release experiment. Release experiments were conducted with N = 6 replicates per condition.
Stem cell isolation and expansion
hASCs were generously provided by the Stem Cell Biology Laboratory at the Pennington Biomedical Research Center (Baton Rouge, LA). Cells were isolated following an Institutional Review Board-approved protocol as previously described.29 Fresh human subcutaneous lipoaspirate from patients undergoing elective liposuction surgery was processed by digestion in collagenase Type IA (Sigma Aldrich), and then density centrifugation was used to isolate the stromal vascular fraction. These cells were plated in tissue culture flasks at 3500 cells/cm2 in DMEM-F12 with 10% fetal bovine serum (FBS; HyClone), 100 U/mL penicillin and 100 μg/mL streptomycin (BioWhittaker, Suwanee, GA) and cultured in a humidified 37°C, 5% CO2 incubator. The adherent cell population was cryopreserved in medium containing 80% FBS, 10% DMEM, and 10% dimethylsulfoxide (DMSO; Sigma-Aldrich).
On thawing, cells were expanded by plating at 3500 cells/cm2 in DMEM-F12 (HyClone) medium containing 10% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin (MP Biomedicals, Solon, OH), and 1 ng/mL fibroblast growth factor-2 (FGF-2; generously provided by the NCI BRB Preclinical Repository). After 80%–90% confluency was reached, cells were trypsinized and frozen in liquid nitrogen in cryopreservation medium containing 90% FBS and 10% DMSO. Cells were expanded a second time following the procedure described above and used at passage 3 for all experiments. Cells were used from two donors, both females, ages 36 and 44, with body mass indices of 25 and 27.
Hydrogel formation
Ten percent GelMA solutions were prepared as described earlier, and hASCs were uniformly suspended in this solution at 10 million cells/mL. For sample groups with loaded growth factor, BMP-2 was mixed into the prepolymer solution at the concentrations indicated in Table I. BMP-2 reconstitution buffer was added to groups that received less or no BMP-2 solution to ensure all groups had the same ratio of GelMA to BMP-2 buffer. Hydrogels were formed by crosslinking the cell- and BMP-2-laden GelMA solutions as described above and then placed in 24-well culture plates and cultured in DMEM-LG (Sigma Aldrich) containing 10% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin, 10 mM β-glycerophosphate disodium salt (EMD Millipore, Billerica, MA) and 120 nM L-ascorbic acid phosphate magnesium salt (Wako Chemicals, Osaka, Japan) in a humidified 37°C, 5% CO2 incubator. In the conditions receiving exogenous growth factor supplementation, BMP-2 was added to the media at the concentrations presented in Table I. Control hydrogels were made without cells and cultured under the same conditions to measure any calcification that was not cell-mediated. For all groups, culture medium was replaced every 3 days.
TABLE I.
BMP-2 Presentation Experimental Conditions
| Group | Exogenous BMP-2 (ng BMP/mL culture media) | Loaded BMP-2 (μg BMP/mL GelMA) |
|---|---|---|
| Control | None | None |
| Exo100 | 100 | None |
| Exo300 | 300 | None |
| Load25 | None | 25 |
| Load50 | None | 50 |
| Load100 | None | 100 |
Three-dimensional osteogenesis assays
After 1, 2, or 4 weeks of culture, hydrogels were placed in 1 mL of Cellytic-M cell lysis buffer (Sigma Aldrich) and homogenized for 60 s using an OMNI-TH handheld homogenizer (Omni International, Kennesaw, GA). DNA was quantified with a Picogreen kit (Life Technologies, Carlsbad, CA) according to the manufacturer’s instructions using a Synergy H1 Multi-Mode plate readder (BioTek, Winooski, VT) to measure fluorescence. ALP activity was quantified by mixing equal volumes of buffer containing homogenized hydrogels with a p-nitrophenyl phosphate substrate solution (pNPP, Sigma Aldrich), and after 30 min at 37°C, measuring conversion of pNPP to 4-nitrophenol by reading absorbance at 405 nm on a VersaMax plate readder (Molecular Devices, Sunnyvale, CA). One unit of ALP is defined as the amount that hydrolyzes 1 μmole of pNPP per minute at 37°C.30 Calcium content was measured using a cetylpyridinium chloride assay kit (Pointe Scientific, Canton, MI) according to the manufacturer’s instructions, reading absorbance at 575 nm on a VersaMax plate readder. For all assays, there were N = 4 samples per condition at each time point.
Histology
After 4 weeks of culture, samples were embedded in OCT compound (Fisher Scientific) and frozen in liquid nitrogen. Ten micron thick sections were cut using a Microm HM 505E cryostat (Thermo Scientific, Waltham, MA), placed on Superfrost Plus microscope slides (Fisher Scientific) and kept frozen until staining. Before Alizarin Red S staining, slides were equilibrated to room temperature, fixed in acetone for 20 min and then stained with 2% Alizarin Red S (pH = 4.15, Sigma Aldrich) for 5 min. Slides were rinsed with acetone and mounted with Permount mounting medium (Fisher Scientific). Photomicrographs were acquired with an Olympus BX61VS microscope (Olympus, Center Valley, PA) with a Pike F-505 camera (Allied Vision Technologies, Stadtroda, Germany). N = 4 samples from each condition were sectioned and stained, with representative images shown.
Statistical analysis
All quantitative data are expressed as mean ± standard deviation. Statistical analysis was performed with one-way analysis of variance (ANOVA) with Tukey honestly significant difference post hoc tests using Minitab software (Minitab, State College, PA). A value of p < 0.05 was considered statistically significant.
RESULTS
BMP-2 release
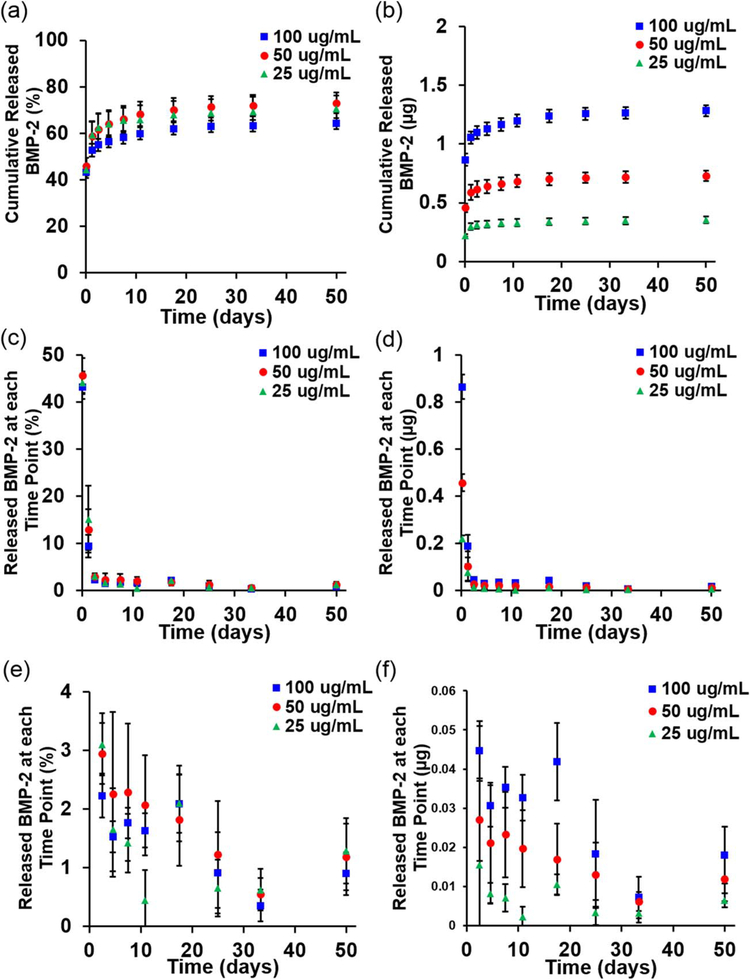
All BMP-2 loading concentrations in the GelMA hydrogels examined exhibited similar cumulative release profiles [Fig. 1(a)]. An initial burst of growth factor diffused out within the first 2 h: 44.2% in the group loaded with 25 μg/mL (load25), 45.7% in the group loaded with 50 μg/mL (load50) and 43.3% of the loaded growth factor in the group loaded with 100 μg/mL (load100). Within the first 24 h, another 15.1% (load25), 13.0% (load50), and 9.4% (load100) was released [Fig. 1(c)]. The release rate then slowed, with between 0.5% and 3.0% of the loaded growth factor released at each time point after 24 h [Fig. 1(e)] as the release profiles approached plateaus of 64.1–73.0% of total BMP-2 released out to 7 weeks [Fig. 1(a)]. Hydrogel degradation with collagenase confirmed that 35.9%, 27.0%, and 29.4% of the BMP-2 remained in the groups loaded with 100, 50, and 25 μg/mL, respectively. While the percentages released were similar across loading groups, the total amount of growth factor released at each time point varied with the amount loaded [Fig. 1(b,d,f)] as expected.
FIGURE 1.
Cumulative release of BMP-2 as a (a) percentage or (b) amount from GelMA hydrogels loaded with 100 μg/mL (2 μg/hydrogel), 50 μg/mL (1 μg/hydrogel) or 25 μg/mL (0.5 μg/hydrogel). Release at each time point as a (c) percentage or (d) amount, and an inset showing release after two days as a (e) percentage or (f) amount.
Cell viability and proliferation.
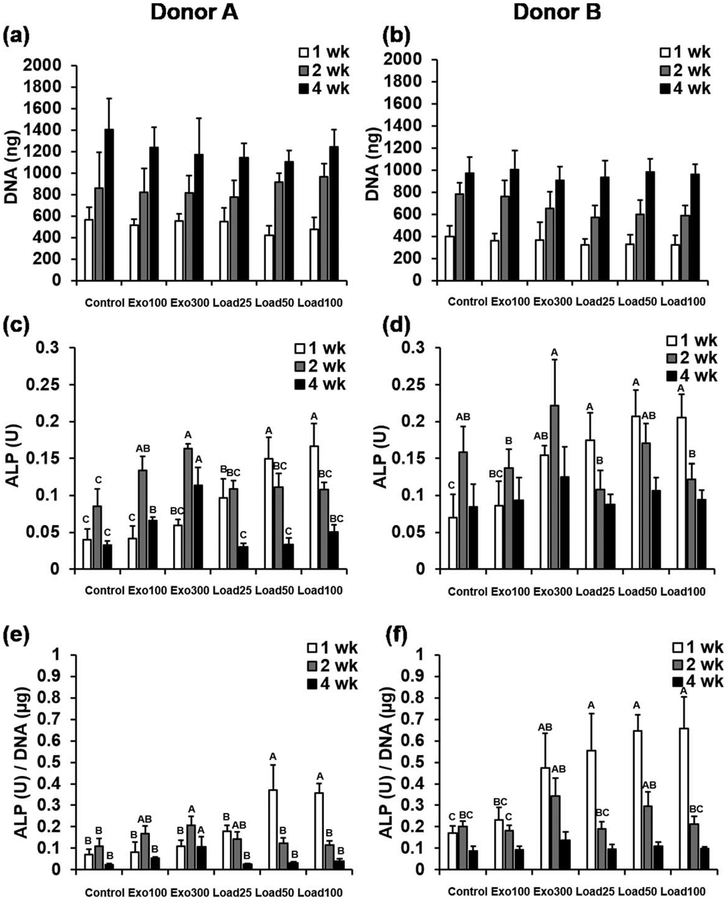
Cells in hydrogels from all groups showed clear proliferation over time, but there were no statistically significant differences in DNA content between groups at any time point for both donors [Fig. 2(a,b)]. Similarly, when the data from the two donors were pooled, there remained no significant differences in average DNA content between groups at any time point, further supporting the trends seen (Supporting Information, Fig. S1a). H&E staining supports the DNA data, also suggesting similar levels of cellularity of the constructs in all groups after 4 weeks (Supporting Information, Fig. S2).
FIGURE 2.
(a,b) DNA content, (c,d) ALP activity and (e,f) ALP normalized to DNA for hydrogels from Donors (a,c,e) A and (b,d,f) B at 1, 2 and 4 weeks. Within each time point, individual comparisons are significant at P < 0.05 for groups that do not share a letter.
Osteogenic differentiation
There were statistically significant differences in ALP activity [Fig. 2(c,d)], as well as ALP activity normalized to DNA content [Fig. 2(e,f)], between hydrogels cultured with exogenous or loaded BMP-2, for both donors. Since there were no statistically significant differences in DNA content, relative differences between groups were similar for both ALP and ALP/DNA. For conditions with no BMP-2 (control) or exogenous BMP-2 (exo100 and exo300), ALP activity increased from 1 to 2 weeks, and then decreased from 2 to 4 weeks. For all conditions with loaded BMP-2 (load25, load50, and load100), except for donor A load25, ALP activity decreased steadily from 1 to 2 to 4 weeks. After 1 week in culture, cells from donor B cultured with 300 ng/mL exogenous BMP-2 (exo300) showed statistically significant increases in ALP and ALP/DNA compared with cells cultured without BMP-2 (control). Donor A showed the same statistically significant increases in ALP/DNA after 2 and 4 weeks in culture, and increases in ALP activity for both 100 ng/mL (exo100) and 300 ng/mL (exo300) exogenous BMP-2 compared with the control group at these time points. However, at the early 1 week time point, the magnitude of increase in ALP activity compared with control was greater for cells in hydrogels with loaded BMP-2. For donor A, conditions with 50 and 100 μg/mL loaded BMP-2 (load50 and load100) showed significantly higher ALP activity and ALP/DNA after 1 week in culture than any of the control or exogenous BMP-2 groups (control, exo100, and exo300). For donor B, at the same 1 week time point all of the conditions with loaded BMP-2 (load25, load50, load100) showed significantly higher ALP and ALP/DNA than the control group or the cells in hydrogels cultured with 100 ng/mL exogenous BMP-2 (exo100). For both donors, these differences were much less dramatic after 2 and 4 weeks, with donor B showing no significant differences in ALP or ALP/DNA between any groups at 4 weeks. Pooling the donor data showed similar trends. Groups load50 and load100 exhibited significantly higher ALP and ALP/DNA activity after 1 week compared with control, and then by 2 and 4 weeks, lower ALP and ALP/DNA activity compared with hydrogels with 300 ng/mL of exogenous BMP-2 (exo300), but this latter trend was not statistically significant (Supporting Information, Fig. S1b and c).
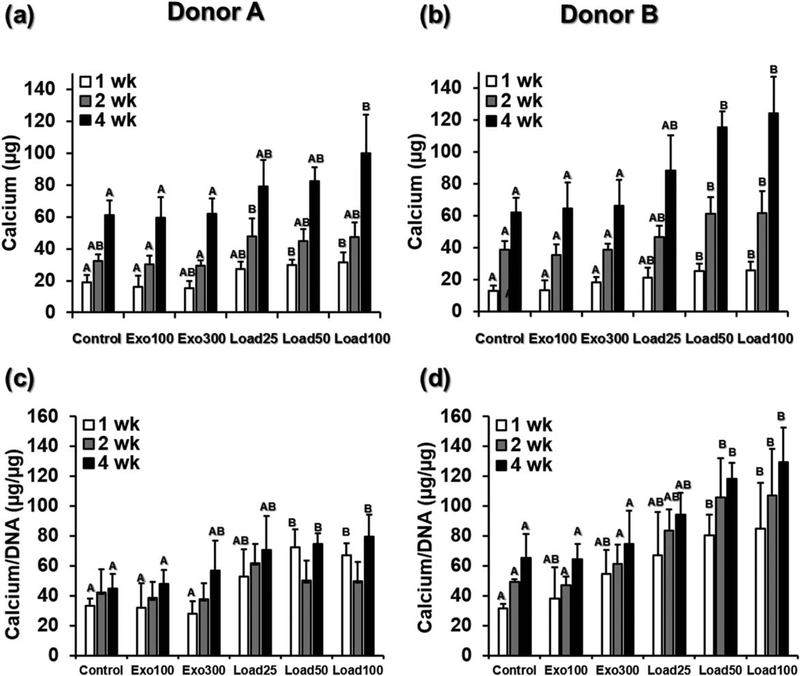
Hydrogels in all groups from both donors showed increasing mineralization over time (Fig. 3). In contrast, hydrogels without cells incubated in DMEM-LG for the same amount of time achieved an average of only 3.8 ± 0.8 μg calcium per sample at 1 week, 4.6 ± 0.6 μg at 2 weeks, and 5.9 ± 1.1 μg at 4 weeks, one to two orders of magnitude lower than the cell-laden hydrogels. At the early 1 week time point, for both donors the conditions with 50 and 100 μg/mL loaded BMP-2 (load50 and load100) had significantly higher calcium content than the control group and the cells in hydrogels cultured with 100 ng/mL exogenous BMP-2 (exo100). This significant difference held at 2 and 4 weeks for donor B, while for donor A only the 100 μg/mL loaded BMP-2 condition (load100) was significantly higher than control and exogenous BMP-2 (control, exo100, and exo300) after 4 weeks. After 4 weeks the differences between the control group and the 100 μg/mL loaded BMP-2 group (load100) were large, with 61.3 μg compared with 100.0 μg calcium for donor A and 61.8 μg compared with 124.3 μg calcium for donor B. These trends were again consistent when the donor data were pooled. After 1, 2, or 4 weeks, the largest amount of calcium was detected in the load100 group, which was significantly higher than the control, exo100 and exo300 (Supporting Information, Fig. S1d).
FIGURE 3.
(a) Calcium content and (b) calcium content normalized to DNA for hydrogels from Donors A and B at 1, 2 and 4 weeks. Within each time point, individual comparisons are significant at P < 0.05 for groups that do not share a letter.
When normalized to DNA, hydrogels with loaded BMP-2 continued to show the highest calcium content [Fig. 3(c,d)]. After 2 and 4 weeks, the normalized calcium content of hydrogels with cells from donor B and loaded with 50 or 100μg/mL was significantly higher than the normalized calcium content of the control hydrogels or those with exogenously presented BMP-2 (control, exo100, exo300). For donor A, the trends were similar, with significantly higher normalized calcium content of in groups load50 and load100 compared with control and exo100 after 4 weeks. Analogous results were seen when the normalized calcium data were pooled across both donors: at all time points hydrogels with loaded BMP-2 had higher average normalized calcium content than those with no or exogenously presented BMP-2 (Supporting Information, Fig. S1e). These differences were statistically significant after 4 weeks for hydrogels with the highest amount of loaded BMP-2 (load100), compared with control hydrogels or those with exogenously presented BMP-2. For hydrogels loaded with 50 μg/mL BMP-2 (load50), normalized calcium content was higher than either control hydrogels or those cultured with 100 ng/mL exogenous BMP-2 (exo100) at 4 weeks.
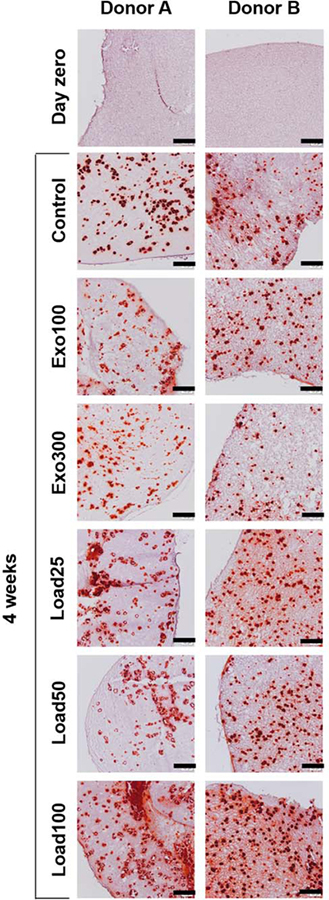
For both donors at all time points, there were no statistically significant differences in calcium content between the groups with BMP-2 loading (load25, load50, and load100), although in all cases load100, which contained the highest concentration of loaded BMP-2, had the highest calcium content. Further, when the donor data were pooled, there was no significant difference between load25, load50, and load100 within each time point, except for load25 being lower than load100 after 4 weeks (Supporting Information, Fig. S1d). Alizarin Red S staining for calcium corroborated these findings (Fig. 4). After 4 weeks, all groups showed visible calcification nodules, indicating bone-like mineralization. Load100, which had significantly more quantified calcium content than the control and exogenous BMP-2 groups at 4 weeks, also showed the strongest Alizarin Red S staining for both donors.
FIGURE 4.
Alizarin Red S staining of hydrogel sections on day zero or after 4 weeks. Scale bars indicate 200 μm.
DISCUSSION
In this work, GelMA hydrogels were used to control BMP-2 presentation to encapsulated hASCs, with the goal of enhancing hASC osteogenic differentiation compared with exogenous delivery of the growth factor in the media. In vitro release curves show that GelMA hydrogels loaded with 25–100 μg/mL of BMP-2 retained growth factor for 7 weeks or longer, providing sustained, localized presentation of the growth factor. The significant burst of growth factor released from the GelMA system after 2 h shows that diffusion dominates the initial release kinetics. However, electrostatic interactions between the growth factor and locally charged regions of the GelMA were sufficient to delay release, retaining the BMP-2 within the hydrogel. While groups with different loading amounts released different absolute amounts of BMP-2, their release kinetics as a percentage of total loaded protein were similar, suggesting that for these concentrations of BMP-2, the number of charged sites on the GelMA far exceeds the number of BMP-2 molecules that can interact with them. The GelMA hydrogel system also offers several simple ways to tune BMP-2 release kinetics, such as changing the concentration or methacrylation level of GelMA to affect the crosslinking density and pore size of the hydrogel. Increasing GelMA concentration or methacrylation level may prolong hydrogel degradation and slow BMP-2 diffusion during the burst phase, while decreasing them may accelerate hydrogel degradation and lead to more BMP-2 released at earlier time points.31 Alternatively, incorporating methacrylated heparin into the hydrogels may endow them with stronger affinity interactions with the BMP-2 and further delay its release.32
The sustained, localized BMP-2 presentation from the GelMA hydrogels led to a statistically significant improvement in hASC osteogenesis in both donors studied. The improvement was seen compared with cells in hydrogels cultured both in the absence of BMP-2 and with exogenously supplemented BMP-2. This was clear from the 1 week ALP activity, which typically increases during the first 1–2 weeks of osteogenic differentiation before decreasing as later stage mineralization occurs.33 Groups with loaded BMP-2 had the highest ALP activity at 1 week, and then exhibited a steady drop to 2 and then to 4 weeks, indicating that ALP activity reached its peak early. In contrast, groups with no BMP-2 or exogenous BMP-2 showed an increase in ALP activity from 1 to 2 weeks, before the protein expression decreased at 4 weeks. This suggests that the sustained, localized BMP-2 presentation from GelMA hydrogels may have accelerated osteogenic differentiation in this system. This is further supported by the calcium content data, as groups with loaded BMP-2 had higher calcium content even at early time points, suggesting that mineralization was also accelerated. Since this enhanced calcium content was maintained at later time points, BMP-2 loading was shown to lead to more overall mineralization.
It is surprising that exogenous BMP-2 had no effect on hASC mineralization in this work. The ability of loaded BMP-2 from the same batch to drive hASC osteogenic differentiation, and, in other work from our lab, to drive hMSC osteogenesis,26,34 demonstrates the bioactivity of the growth factor used. Since exogenous BMP-2 presentation did lead to increases in hASC ALP activity at 2 or 4 week time points compared with hASCs cultured without BMP-2, it is possible that mineralization would have been increased compared with the control group after a longer culture period. Additionally, while many published studies suggest that exogenously presented BMP-2 does drive hASC osteogenic differentiation,8–10,35,36 there are other reports that contradict this finding.37–39 The data presented here suggest that BMP-2 stability in the culture conditions used in each experiment may play a key role in moderating its effect. For example, more frequent changing of culture medium to replace the degraded growth factor or culturing in a defined medium without the addition of serum to decrease the presence of enzymes that may break down BMP-2 could enhance the osteogenic bioactivity of this growth factor.
Notably, despite the four-fold increase in loaded BMP-2 concentration from load25 to load100, there were no statistically significant differences in calcium content at any time point between any of the groups with loaded BMP-2. This suggests that for the range studied, the effects of BMP-2 may not be concentration-dependent, and it may be possible to use the lower amounts of growth factor. Over 4 weeks of culture, the amount of growth factor used in the culture media with 100 ng/mL of exogenous BMP-2 is approximately equivalent to the amount loaded in a hydrogel with 25 μg/mL BMP-2. Therefore, while 300 ng/mL of exogenous BMP-2 did not lead to enhanced osteogenic differentiation compared with the control group, smaller amounts loaded in the hydrogels (load50) did show significant increases in ALP activity and/or calcium content compared with both the control and groups with exogenous BMP-2 presentation. Future work could examine the response of multiple donors to a wider range of BMP-2 loading amounts to identify a range where concentration dependence may occur, or a minimum threshold necessary to stimulate osteogenesis.
BMP-2 that is electrostatically immobilized in the GelMA may exhibit enhanced bioactivity because it mimics in vivo growth factor sequestration by affinity interactions in the ECM.40 Here, the scaffold may protect the BMP-2 from degradation prior to its being ultimately released and acting as soluble growth factor. BMP-2 has exhibited instability in serum-containing culture medium. For instance, when BMP-2 (100ng/mL) was placed in DMEM with 5% FBS, after only 2 h 50% of the growth factor could not be detected by an ELISA assay, indicating enzymatic degradation or inactivation by BMP inhibitor molecules such as noggin.13 This could also provide a potential mechanism for improved BMP-2 osteogenic effects when delivered from a GelMA hydrogel that can shield the growth factor. This additional benefit to the scaffold-based delivery system is especially valuable in vivo, where biomaterial scaffolds can protect a loaded growth factor from enzymes in the body, preserving its bioactivity until it is presented to cells.41 Future work could examine the bioactivity of covalently bound BMP-2 to the GelMA hydrogel, and compare it to the BMP-2 sequestered in the hydrogel by affinity interactions in this work. Such tethering in other systems has been reported to increase its bioactivity compared with the same amount of free BMP-2; the growth factor is not internalized and instead can continue to activate its receptor.42,43
Importantly, in addition to the increases in osteogenic differentiation seen by encapsulated cells in this system in vitro, sustained, localized BMP-2 presentation from the GelMA hydrogel can also deliver this growth factor to host cells. Therefore, the growth factor can act not only on the incorporated hASCs, but also on osteoprogenitor cells that are present at or recruited to the site of a bone defect during the normal healing process.44 These cells are known to be BMP-2 responsive,45 and delivery of BMP-2 without transplantation of cells has shown promise in a variety of systems by targeting host cells for repairing in vivo bone defects.20,32,46 However, when a mineral-coated poly(lactic-co-glycolic acid) scaffold delivering BMP-2 was compared with a scaffold seeded with hASCs for its ability to repair mouse critical size cranial defects, the scaffold with hASCs performed dramatically better, demonstrating the benefits of cell transplantation in addition to growth factor delivery in vivo.10
While not explored here, the modularity of this GelMA system allows for exploration of the role of a number of interesting microenvironmental variables, in addition to growth factor delivery, on osteogenesis. For example, it is possible to examine the combined signaling interactions between hydrogel stiffness, a variable that has been extensively shown to affect cell behaviors including osteogenic differentiation,47,48 and the effects of growth factors on cell function examined here. The hydrogels used here have a shear storage modulus, G’ ≈ 20 kPa (measured without encapsulated cells, data not shown), which is in a range of stiffnesses shown previously to be conducive to osteogenesis of encapsulated stem cells or preosteoblasts.49 However, GelMA offers a number of simple ways to tune hydrogel stiffness as well as nanoscale porosity independent of changing gelatin density, and thereby insoluble biochemical signals such as adhesion ligand density. This can be accomplished, for example, by varying the concentration of photoinitiator,50 or changing the level of gelatin methacrylation27,50,51 to achieve a range of stiffnesses from less than 1 to greater than 30 kPa.50 Further, the addition of sacrificial porogens52,53 could allow for investigation of the impact of changes in hydrogel porosity along with growth factor presentation on osteogenesis. Because these hydrogel modifications may also change BMP-2 release rate, future work could also use the method described earlier, incorporating methacrylated heparin to limit the growth factor burst release independent of hydrogel stiffness or porosity, to study the potential interacting effects of substrate stiffness and BMP-2 release kinetics on hASC osteogenesis.
CONCLUSIONS
In this work, GelMA hydrogels were shown to provide sustained, localized presentation of BMP-2 loaded at concentrations of 25–100 μg/mL; 27.0%–35.9% of loaded growth factor was retained in the hydrogels due to electrostatic interactions for at least 7 weeks. This loaded BMP-2 was shown to have a statistically significant effect on osteogenic differentiation of encapsulated hASCs, as compared with cells cultured in the absence of growth factor or with exogenous BMP-2. ALP activity was accelerated and there were significant increases in calcium content after 1, 2, and 4 weeks of culture in the growth factor loaded groups compared with the control group or groups with BMP-2 presented exogenously, with little concentration dependence shown. This suggests that the GelMA system is protecting or enhancing the bioactivity of BMP-2 as compared with growth factor delivered in media. This system for sustained BMP-2 presentation also has the potential to deliver the growth factor to host osteoprogenitor cells in in vivo bone defects, further increasing its utility for bone tissue engineering.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr. Jeffrey Gimble for generously providing the hASCs, Amad Awadallah for assistance with histology and Colin Morlock for technical assistance.
Contract grant sponsor: National Defense Science and Engineering Graduate Fellowship and National Science Foundation Graduate Fellowship (to JES); contract grant numbers: R01AR066193 and R01AR063194 (to EA)
Footnotes
Additional Supporting Information may be found in the online version of this article.
Conflict of interest: No benefit of any kind will be received either directly or indirectly by the author(s).
Disclosure statement: The author(s) declare that they have no competing interests
REFERENCES
- 1.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng 2001;7: 211–228. [DOI] [PubMed] [Google Scholar]
- 2.Zanetti AS, Sabliov C, Gimble JM, Hayes DJ. Human adipose-derived stem cells and three-dimensional scaffold constructs: A review of the biomaterials and models currently used for bone regeneration. J Biomed Mater Res Part B: Appl Biomaterials 2013; 101:187–199. [DOI] [PubMed] [Google Scholar]
- 3.Zhu M, Kohan E, Bradley J, Hedrick M, Benhaim P, Zuk P. The effect of age on osteogenic, adipogenic and proliferative potential of female adipose-derived stem cells. J Tissue Eng Regener Med 2009;3:290–301. [DOI] [PubMed] [Google Scholar]
- 4.Puissant B, Barreau C, Bourin P, Clavel C, Corre J, Bousquet C, Taureau C, Cousin B, Abbal M, Laharrague P. Immunomodulatory effect of human adipose tissue-derived adult stem cells: Comparison with bone marrow mesenchymal stem cells. Br J Haematol 2005;129:118–129. [DOI] [PubMed] [Google Scholar]
- 5.Bunnell BA, Flaat M, Gagliardi C, Patel B, Ripoll C. Adipose-derived stem cells: Isolation, expansion and differentiation. Methods 2008;45:115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bieback K, Kern S, Kocaömer A, Ferlik K, Bugert P. Comparing mesenchymal stromal cells from different human tissues: Bone marrow, adipose tissue and umbilical cord blood. Bio-medical materials and engineering 2007;18(1 Suppl):S71–6. [PubMed] [Google Scholar]
- 7.Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006;24:1294–1301. [DOI] [PubMed] [Google Scholar]
- 8.Panetta NJ, Gupta DM, Lee JK, Wan DC, Commons GW, Longaker MT. Human adipose-derived stromal cells respond to and elaborate bone morphogenetic protein-2 during in vitro osteogenic differentiation. Plast Reconstr Surg 2010;125:483–493. [DOI] [PubMed] [Google Scholar]
- 9.Overman JR, Farré-Guasch E, Helder MN, ten Bruggenkate CM, Schulten EA, Klein-Nulend J. Short (15 minutes) bone morphogenetic protein-2 treatment stimulates osteogenic differentiation of human adipose stem cells seeded on calcium phosphate scaffolds in vitro. Tissue Eng Part A 2012;19(3–4):571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levi B, James AW, Nelson ER, Vistnes D, Wu B, Lee M, Gupta A, Longaker MT. Human adipose derived stromal cells heal critical size mouse calvarial defects. PloS One 2010;5:e11177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bowen-Pope DF, Malpass TW, Foster DM, Ross R. Platelet-derived growth factor in vivo: Levels, activity, and rate of clearance. Blood 1984;64:458–469. [PubMed] [Google Scholar]
- 12.Edelman ER, Nugent MA, Karnovsky MJ. Perivascular and intravenous administration of basic fibroblast growth factor: Vascular and solid organ deposition. Proc Natl Acad Sci USA 1993;90: 1513–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bramono DS, Murali S, Rai B, Ling L, Poh WT, Lim ZX, Stein GS, Nurcombe V, Van Wijnen AJ, Cool SM. Bone marrow-derived heparan sulfate potentiates the osteogenic activity of bone morphogenetic protein-2 (BMP-2). Bone 2012;50:954–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hynes RO. The extracellular matrix: Not just pretty fibrils. Science 2009;326:1216–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tayalia P, Mooney DJ. Controlled growth factor delivery for tissue engineering. Adv Mater 2009;21:3269–3285. [DOI] [PubMed] [Google Scholar]
- 16.Benton JA, DeForest CA, Vivekanandan V, Anseth KS. Photocrosslinking of gelatin macromers to synthesize porous hydrogels that promote valvular interstitial cell function. Tissue Eng Part A 2009;15:3221–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruoslahti E RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol 1996;12:697–715. [DOI] [PubMed] [Google Scholar]
- 18.Van den Steen PE, Dubois B, Nelissen I, Rudd PM, Dwek RA, Opdenakker G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9). Crit Rev Biochem Mol Biol 2002;37:375–536. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto M, Ikada Y, Tabata Y. Controlled release of growth factors based on biodegradation of gelatin hydrogel. J Biomater Sci Polym Ed 2001;12:77–88. [DOI] [PubMed] [Google Scholar]
- 20.Patel ZS, Yamamoto M, Ueda H, Tabata Y, Mikos AG. Biodegradable gelatin microparticles as delivery systems for the controlled release of bone morphogenetic protein-2. Acta Biomater 2008;4: 1126–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solorio L, Zwolinski C, Lund AW, Farrell MJ, Stegemann JP. Gelatin microspheres crosslinked with genipin for local delivery of growth factors. J Tissue Eng Regener Med 2010;4:514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solorio LD, Dhami CD, Dang PN, Vieregge EL, Alsberg E. Spatio-temporal regulation of chondrogenic differentiation with controlled delivery of transforming growth factor-β1 from gelatin microspheres in mesenchymal stem cell aggregates. Stem Cells Transl Med 2012;1:632–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu X, Ma L, Wang C, Gao C. Gelatin hydrogel prepared by photo-initiated polymerization and loaded with TGF-β1 for cartilage tissue engineering. Macromol Biosci 2009;9:1194–1201. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen AH, McKinney J, Miller T, Bongiorno T, McDevitt TC. Gelatin methacrylate microspheres for controlled growth factor release. Acta Biomater 2015;13:101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hou Q, Chau D, Pratoomsoot C, Tighe PJ, Dua HS, Shakesheff KM, Rose FR. In situ gelling hydrogels incorporating microparticles as drug delivery carriers for regenerative medicine. J Pharm Sci 2008;97:3972–3980. [DOI] [PubMed] [Google Scholar]
- 26.Jeon O, Wolfson DW, Alsberg E. In situ formation of growth-factor-loaded coacervate microparticle-embedded hydrogels for directing encapsulated stem cell fate. Adv Mater 2015;27:2216–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Den Bulcke AI, Bogdanov B, De Rooze N, Schacht EH, Cornelissen M, Berghmans H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules 2000;1:31–38. [DOI] [PubMed] [Google Scholar]
- 28.Ovsianikov A, Deiwick A, Van Vlierberghe S, Dubruel P, Möller L, Dräger G, Chichkov B. Laser fabrication of three-dimensional CAD scaffolds from photosensitive gelatin for applications in tissue engineering. Biomacromolecules 2011;12:851–858. [DOI] [PubMed] [Google Scholar]
- 29.Dubois SG, Floyd EZ, Zvonic S, Kilroy G, Wu X, Carling S, Halvorsen YDC, Ravussin E, Gimble JM. Isolation of human adipose-derived stem cells from biopsies and liposuction specimens Mesenchymal Stem Cells. Springer; New York, NY: 2008, p. 69–79. [DOI] [PubMed] [Google Scholar]
- 30.Mossner E, Boll M, Pfleiderer G. Purification of human and bovine alkaline phosphatases by affinity chromatography. Hoppe-Seyleŕ S Zeitschr Für Physiol Chem 1980;361:543–550. [DOI] [PubMed] [Google Scholar]
- 31.Hoffman AS. Hydrogels for biomedical applications. Adv Drug Deliv Rev 2002;54:3–12. [DOI] [PubMed] [Google Scholar]
- 32.Jeon O, Powell C, Solorio LD, Krebs MD, Alsberg E. Affinity-based growth factor delivery using biodegradable, photocrosslinked heparin-alginate hydrogels. J Control Release 2011;154:258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lian JB, Stein GS. Concepts of osteoblast growth and differentiation: Basis for modulation of bone cell development and tissue formation. Crit Rev Oral Biol Med 1992;3:269–305. [DOI] [PubMed] [Google Scholar]
- 34.Dang PN, Dwivedi N, Yu X, Bowerman C, Murphy WL, Alsberg E. Guiding Chondrogenesis and Osteogenesis with Mineral-Coated Hydroxyapatite and BMP-2 Incorporated within High-Density hMSC Aggregates for Bone Regeneration. ACS Biomaterials Science & Engineering. [DOI] [PubMed] [Google Scholar]
- 35.Hutton DL, Moore EM, Gimble JM, Grayson WL. Platelet-derived growth factor and spatiotemporal cues induce development of vascularized bone tissue by adipose-derived stem cells. Tissue Eng Part A 2013;19:2076–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knippenberg M, Helder M, Doulabi BZ, Wuisman P, Klein-Nulend J. Osteogenesis versus chondrogenesis by BMP-2 and BMP-7 in adipose stem cells. Biochem Biophys Res Commun 2006;342:902–908. [DOI] [PubMed] [Google Scholar]
- 37.Kyllönen L, Haimi S, Säkkinen J, Kuokkanen H, Mannerström B, Sándor GK, Miettinen S. Exogenously added BMP-6, BMP-7 and VEGF may not enhance the osteogenic differentiation of human adipose stem cells. Growth Factors 2013;31:141–153. [DOI] [PubMed] [Google Scholar]
- 38.Tirkkonen L, Haimi S, Huttunen S, Wolff J, Pirhonen E, Sándor G, Miettinen S. Osteogenic medium is superior to growth factors in differentiation of human adipose stem cells towards bone-forming cells in 3D culture. Eur Cell Mater 2013;25:144–58. [DOI] [PubMed] [Google Scholar]
- 39.Zuk P, Chou Y-F, Mussano F, Benhaim P, Wu BM. Adipose-derived stem cells and BMP2: Part 2. BMP2 may not influence the osteogenic fate of human adipose-derived stem cells. Connective Tissue Res. 2011;52:119–132. [DOI] [PubMed] [Google Scholar]
- 40.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regener 2008;16:585–601. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen MK, Alsberg E. Bioactive factor delivery strategies from engineered polymer hydrogels for therapeutic medicine. Prog Polym Sci 2014;39:1235–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karageorgiou V, Meinel L, Hofmann S, Malhotra A, Volloch V, Kaplan D. Bone morphogenetic protein-2 decorated silk fibroin films induce osteogenic differentiation of human bone marrow stromal cells. J Biomed Mater Res Part A 2004;71A:528–537. [DOI] [PubMed] [Google Scholar]
- 43.Yamachika E, Tsujigiwa H, Shirasu N, Ueno T, Sakata Y, Fukunaga J, Mizukawa N, Yamada M, Sugahara T. Immobilized recombinant human bone morphogenetic protein-2 enhances the phosphorylation of receptor-activated Smads. J Biomed Mater Res Part A 2009;88:599–607. [DOI] [PubMed] [Google Scholar]
- 44.Raisz LG. Physiology and pathophysiology of bone remodeling. Clin Chem 1999;45:1885–1885. [PubMed] [Google Scholar]
- 45.Barnes GL, Kostenuik PJ, Gerstenfeld LC, Einhorn TA. Growth factor regulation of fracture repair. J Bone Miner Res 1999;14:1805–1815. [DOI] [PubMed] [Google Scholar]
- 46.Yasko AW, Lane J, Fellinger E, Rosen V, Wozney J, Wang E. The healing of segmental bone defects, induced by recombinant human bone morphogenetic protein (rhBMP-2). A radiographic, histological, and biomechanical study in rats. J Bone Joint Surg 1992;74:659–670. [PubMed] [Google Scholar]
- 47.Chatterjee K, Lin-Gibson S, Wallace WE, Parekh SH, Lee YJ, Cicerone MT, Young MF, Simon CG. The effect of 3D hydrogel scaffold modulus on osteoblast differentiation and mineralization revealed by combinatorial screening. Biomaterials 2010;31:5051–5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burdick JA, Vunjak-Novakovic G. Engineered microenvironments for controlled stem cell differentiation. Tissue Eng Part A 2008;15: 205–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, Bencherif SA, Rivera-Feliciano J, Mooney DJ. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater 2010;9:518–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sutter M, Siepmann J, Hennink WE, Jiskoot W. Recombinant gelatin hydrogels for the sustained release of proteins. J Control Release 2007;119:301–312. [DOI] [PubMed] [Google Scholar]
- 51.Chen YC, Lin RZ, Qi H, Yang Y, Bae H, Melero -, Martin JM Khademhosseini A. Functional human vascular network generated in photocrosslinkable gelatin methacrylate hydrogels. Adv Funct Mater 2012;22:2027–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim J, Yaszemski MJ, Lu L. Three-dimensional porous biodegradable polymeric scaffolds fabricated with biodegradable hydrogel porogens. Tissue Eng Part C: Methods 2009;15:583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hwang CM, Sant S, Masaeli M, Kachouie NN, Zamanian B, Lee S-H, Khademhosseini A. Fabrication of three-dimensional porous cell-laden hydrogel for tissue engineering. Biofabrication 2010;2:035003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.