Abstract
The problem of food spoilage due to Aspergillus flavus (A. flavus) needs to be resolved. In this study, we found that the minimum inhibitory concentration of cinnamaldehyde (CA) that inhibited A. flavus was 0.065 mg/ml and that corn can be prevented from spoiling at a concentration of 0.13 mg/cm3. In addition to inhibiting spore germination, mycelial growth, and biomass production, CA can also reduce ergosterol synthesis and can cause cytomembrane damage. Our intention was to elucidate the antifungal mechanism of CA. Flow cytometry, fluorescence microscopy, and western blot were used to reveal that different concentrations of CA can cause a series of apoptotic events in A. flavus, including elevated Ca2+ and reactive oxygen species, decrease in mitochondrial membrane potential (Δψm), the release of cytochrome c, the activation of metacaspase, phosphatidylserine (PS) externalization, and DNA damage. Moreover, CA significantly increased the expression levels of apoptosis-related genes (Mst3, Stm1, AMID, Yca1, DAP3, and HtrA2). In summary, our results indicate that CA is a promising antifungal agent for use in food preservation.
Keywords: cinnamaldehyde, Aspergillus flavus, antifungal, apoptosis, food preservation
Introduction
Aspergillus flavus (A. flavus) is one of the most common species among the filamentous fungi. In addition, A. flavus is reported to be the second largest cause of aspergillosis infection in humans (Varga et al., 2011). The notorious A. flavus metabolite aflatoxin B1, which has been recognized by the World Health Organization (WHO) as a primary carcinogen, is absorbed by humans and animals through contaminated agricultural crops and animal feed, such as maize, peanuts, nuts, cottonseed, and edible oil (Yang et al., 2015). Therefore, controlling the food spoilage mediated by A. flavus at its source is critical to limiting the health hazards of aflatoxin and to preventing substantial economic losses. Nevertheless, traditional antifungal drugs have continuously posed problems, which include the increasingly serious problem of drug resistance, the toxicity of the chemical antifungal compounds, drug interactions, and the high costs. Research into new antifungal agents needs to be carried out urgently because of the drug resistance and the toxicity of the compounds currently available (Sarkar et al., 2014; Qu et al., 2019). Consequently, increasing numbers of scientists are exploring novel natural products from medicinal plants such as Geraniol and Citral in an attempt to solve the question of fungal drug resistance and with consideration for the natural low toxicity and high antifungal activity of these products (Atanasov et al., 2015; Tang et al., 2018).
Apoptosis is a form of cell death that plays a vital role in the normal development and maturation cycle. In routine physiological processes, the homeostatic balance between cell proliferation and cell death is critical (Fuchs and Steller, 2011). Some scholars have suggested that phenolics can damage mitochondrial function through targeting antioxidative signal transduction and thereby inhibit pathogenic Aspergillus (Kim et al., 2004, 2006). Our own previous studies have indicated that apoptosis-promoting compounds are a promising direction in the exploration for a novel antimicrobial drug, and many antifungal agents have been investigated through the apoptotic pathway, such as amphotericin B and anacardic acid (Tian et al., 2017; Qu et al., 2019). In addition, our recent research has shown that Nerol possesses an anti-A. flavus ability through apoptosis. Other researchers have indicated that cinnamaldehyde (CA) can decrease the expression of the aflatoxin biosynthetic gene and inhibit the biosynthesis of aflatoxin B1 (Liang et al., 2015; Tian et al., 2018).
Cinnamaldehyde is an α, β-unsaturated aldehyde, abundant in cinnamon and widely used as a food additive in products such as drinks, candies, ice cream, chewing gum, and condiments (Cabello et al., 2009). Furthermore, CA is a traditional Chinese medicine used for gastritis, indigestion, blood circulation disorders, and inflammation (Liao et al., 2012; Chen et al., 2019). CA has been reported to inhibit Geotrichum citri-aurantii in citrus fruits and Phytophthora capsici in peppers, both of which result in food decay (Hu et al., 2013; OuYang et al., 2019). CA is well-tolerated in humans and animals and is considered a safe natural active ingredient. The FDA and the council of Europe have accepted this concept and recommend daily intake of 1.25 mg/kg (Zhu et al., 2017). Furthermore, CA has been reported to remove natural or chemical toxicities such as ochratoxin A and to protect human health (Dorri et al., 2018; Wang et al., 2018a). The antioxidant activity and the anti-cerebral thrombosis ability of CA have been proven in mice (Zhao et al., 2015; Buglak et al., 2018). Some reports have indicated that CA can initiate the production of reactive oxygen species (ROS) and damage the mitochondrial membranes of Penicillium expansum (Wang et al., 2018b, c). The use of CA as a preservative in food storage and transportation is widely recognized to be beneficial. In a recent publication, CA is reported to inhibit A. flavus at lower concentrations, and CA has also been recognized as able to induce apoptosis in cancer cells (Roth-Walter et al., 2014; Li et al., 2015). Another report indicates that CA mediates A. flavus oxidative stress, but it only detected changes in antioxidant enzyme activity, and the follow-on mechanism of ROS in A. flavus is not clear (Sun et al., 2016). Nevertheless, the mechanism by which CA inhibits A. flavus is considered worth exploring. Therefore, this research investigated the apoptotic effects of CA in A. flavus, such as intracellular ROS, calcium concentration, mitochondrial membrane potential, cytochrome c, phosphatidylserine, metacaspase, and DNA damage.
Materials and Methods
Materials and Strain
Cinnamaldehyde (CAS registry no. 104-55-2) was purchased from Shanghai Macklin Biochemical, Co., Ltd. (Shanghai, China) and prepared as a stock solution in 0.1% (v/v) Tween-80. The A. flavus (NRRL 3357) used in this research was purchased from the China General Microbiological Culture Collection Center (CGMCC). It was cultured in potato dextrose agar (PDA: 200 g peeled potato, 20 g dextrose, 15 g agar powder, and 1000 ml distilled water) for 4 days at 28°C and stored at 4°C.
Antifungal Susceptibility Testing
Broth microdilution methods are commonly used for in vitro antifungal assays (Tian et al., 2017). For our experiments, 80 μl of different concentrations of CA, 100 μl of potato dextrose broth (PDB), and 20 μl 5 × 106 spores/ml A. flavus were added to each of 10 wells. The 11th well was used as a blank control without the CA, and the 12th well was used as a negative control, without the fungal suspension. After incubation at 28°C for 48 h, the minimal drug concentration that inhibited the growth of the A. flavus was described as its minimum inhibitory concentration (MIC).
Effect of CA on A. flavus Pathogenicity in Corn
Fungal infection in corn was investigated using a method described previously with minor modifications (Yang et al., 2018). After the tip of each corn kernel was scratched with a knife, it was immersed in 5% sodium hypochlorite and then placed in a shaker for 10 min. The corn was washed twice with sterile water, twice with 70% ethanol, and again twice with sterile water. It was finally shaken with A. flavus for 30 min. Six corn kernels were placed in each Petri dish, and then sterile water and various concentrations of CA were added, and sterile filter paper was put it on the bottom of the plate. For the control, the corn was not treated with CA, and neither was it co-incubated with A. flavus. All samples were incubated for 5 days at 28°C after sealing.
Fungal Culture Conditions
Fungal cells were suspended in phosphate buffered saline (PBS) and adjusted to 5 × 106 spores/ml with a hemocytometer. The PDB and different concentrations of CA (0, 0.033, 0.065, 0.13, 0.26, and 0.52 mg/ml) were mixed and added to the A. flavus spore suspension; they were then cultured in a shaker at 28°C for 6 h. At least 200 spores were observed in each treatment group, confirming the spore germination. Nine mm agar disks were prepared on an A. flavus plate with a puncher and placed in the center of a PDA medium with the CA at 28°C. The colony diameters were measured after 3 days to measure mycelial growth. The cells were cultured at a constant temperature in a shaker at 28°C for 72 h, and then the hyphae were collected. The hyphae were treated in an oven at 60°C for 24 h and then weighed to determine their biomass.
Effect of CA on Biofilm of A. flavus
A. flavus cells were treated with various concentrations of CA for 12 h at 28°C. The morphological changes in the A. flavus were observed and analyzed by the forward-scattered light (FSC) and the side-scattered light (SSC) channels of flow cytometry (BD Biosciences, San Jose, CA, United States). As in the previous method, the content of ergosterol in the A. flavus cell membrane was analyzed by spectral scanning (Tian et al., 2012). Membrane integrity was determined by monitoring the uptake of fluorescent nuclear staining propidium iodide (PI)—a DNA-stained fluorescent probe. Cells were incubated with 5 μg/ml PI for 30 min at room temperature in the dark and detected by an Accuri C6 flow cytometer (BD Biosciences, San Jose, CA, United States). A. flavus cells were centrifuged, and the supernatant was taken out. After dilution, the OD260 nm value was measured for soluble content release by using an ultraviolet-visible spectrophotometer (Thermo Fisher Scientific, Waltham, MA, United States).
ROS, Δψm, and Ca2+ Measurement
The A. flavus cells treated with CA were analyzed by flow cytometry with DCFH-DA (Sigma-Aldrich, St. Louis, MO, United States) to detect the production and accumulation of ROS. JC-1 (Sigma-Aldrich, St. Louis, MO, United States) staining was used to measure Δψm. Fluo-3/AM (Sigma-Aldrich, St. Louis, MO, United States) and Rhod-2/AM (Sigma-Aldrich, St. Louis, MO, United States) are commonly used to detect cytoplasmic and mitochondrial Ca2+ levels (Yun and Lee, 2016). The cells treated with different concentrations of CA (0, 0.033, 0.065, 0.13, 0.26, and 0.52 mg/ml) at 28°C for 12 h were harvested by centrifugation at 5000 × g for 5 min, washed twice, and then resuspended in PBS. Cells were then incubated with 10 μM DCFH-DA, 10 μg/ml JC-1, Fluo-3/AM and Rhod-2/AM at 28°C for 30 min in the dark. Finally, the cells were washed in PBS and then analyzed using an Accuri C6 flow cytometer (BD Biosciences, San Jose, CA, United States).
Analysis of Cytochrome c Release
The A. flavus cells were treated with various concentrations of CA for 12 h at 28°C for the detection of cytochrome c. The cells were then harvested, and mitochondrial and cytosolic fractions were prepared with an ultrasonic cell disruptor. Mitochondrial fractions were collected by using a filamentous fungus mitochondrial protein extraction kit (BestiBio, Shanghai, China), and cytoplasmic proteins were collected with a filamentous fungal cytoplasmic protein extraction kit (BestiBio, Shanghai, China). The protein concentration was tested using a microplate reader and a bicinchoninic acid (BCA) Protein Assay Kit (Solarbio, Beijing, China). Sixty micrograms of total cellular proteins were separated by 15% sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis and transferred to the polyvinylidene difluoride (PVDF) membrane (Merck Millipore, Billerica, MA, United States). The PVDF membrane was blocked with 5% non-fat milk (m/v) for 1 h and then washed with 0.1% Tween-20 in Tris saline buffer. It was then incubated with rabbit anti cytochrome c (Proteinsimple, Silicon Valley, CA, United States) and mouse anti-GAPDH (Bioss, Beijing, China) for 12 h at 4°C. The membrane was investigated with western blot chemiluminescence reagents (GE Healthcare, Little Chalfont, United Kingdom), and the reactive density was measured using ImageJ software 1.48 V.
Detection of Metacaspase Activity
Activated metacaspases in A. flavus cells were measured with the CaspACE FITC-VAD-FMK in situ marker (Promega, Madison, WI, United States). A. flavus cells were treated with various concentrations of CA for 12 h at 28°C. The cells were then harvested by centrifugation at 5000 × g for 5 min, washed twice, and then resuspended in PBS. The cells were stained with 10 μM of CaspACE FITC-VAD-FMK for 30 min at room temperature in the dark. Finally, the samples were analyzed by using an Accuri C6 flow cytometer (BD Biosciences, San Jose, CA, United States).
Detection of PS Externalization
PS externalization was detected by fluorescence microscopy using Annexin V-FITC and PI. The method used to prepare the protoplasts has been described in a previous study (Tian et al., 2018). Subsequently, the protoplasts were treated with CA for 12 h at 28°C. Next, the CA-treated protoplasts were stained with 5 μl/ml of PI and FITC-labeled Annexin V, and then analyzed using the Annexin V-FITC apoptosis detection kit (BD Biosciences, San Jose, CA, United States). Finally, the test protoplasts were analyzed by using an Accuri C6 flow cytometer (BD Biosciences, San Jose, CA, United States).
Analysis of DNA and Nuclear Damage
The TUNEL assay and DAPI staining were used to confirm the diagnostic markers of yeast apoptosis, including DNA and nuclear fragmentation. A. flavus cells were treated with various concentrations of CA for 12 h at 28°C. For the DAPI staining, the CA-treated cells were permeabilized and fixed with 70% absolute ethanol at 4°C for 30 min and then treated with 5 μg/ml DAPI (Sigma-Aldrich, St. Louis, MO, United States) for 10 min in the dark. Cells were then harvested and examined under fluorescence microscopy (Leica, Wetzlar, Germany). For the TUNEL assay, 20 μl of the A. flavus suspension was added to the adhering slide, and then 100 μl of 4% paraformaldehyde was added, dropwise. Subsequently, 100 μl of 0.2% Triton X-100 and 50 μl of the reaction system were added according to the instructions with the TUNEL kit (Solarbio, Beijing, China). The DNA breaks were observed under fluorescence microscopy (Leica, Wetzlar, Germany).
Quantitative Real-Time PCR
Total RNA was extracted using the TRI reagent method. RNA was extracted from the mycelium after growth in a liquid culture to ensure that a high yield was obtained with purity of RNA (Canciani et al., 2017). The A. flavus mycelia were treated with 0, 0.065, 0.13, and 0.26 mg/ml CA for 12 h, then collected, washed, and resuspended in sterile PBS. The collected mycelia were then fragmented with liquid nitrogen and transferred to a TRI reagent solution. We used a Micro UV spectrophotometer (Thermo Fisher Scientific, Waltham, MA, United States) to test at 260 and 280 nm, and then the RNA was sequentially reverse transcribed to the first strand of cDNA by using the RevertAid First Strand cDNA Synthesis Kit and following the manufacturer’s instructions (Thermo Fisher Scientific, Waltham, MA, United States). The obtained cDNA was used in the analysis of real-time PCR (RT-PCR). The primers used in this study are presented in Table 1. SYBR Green was used (Takara, Tokyo, Japan), and the procedures for Q-PCR were performed on AB (Applied Biosystems, Thermo Fisher Scientific, Waltham, MA, United States) and consisted of denaturation at 95°C for 30 s and 40 cycles of 95°C for 5 s and 60°C for 40 s. The expression level of the target genes relative to the reference was determined by using 2–Δ Δ Ct (Jahanshiri et al., 2012).
TABLE 1.
Names and nucleotide sequences of primers used for RT-PCR in this study.
| Primer name | Sequence (5′–3′) |
| Mst3-Forward | TGTGCATCTGGCTTGGCTTA |
| Mst3-Reverse | ATGGTGGGTGCTTTGACTGT |
| Stm1-Forward | ATTGCCTGCAACAGCGAATC |
| Stm1-Reverse | CTTCCTGAGTTGCGCCCTAT |
| AMID-Forward | TTGCGAACCGAGGCTGAATA |
| AMID-Reverse | ATTGGGACTCGCAGGTTCTC |
| Yca1-Forward | GTATTCTTGGGGAGCGCCTT |
| Yca1-Reverse | CTGCGCAATAGCCTACCAGA |
| DAP3-Forward | GGAAGACTAGAAGGAGACGCA |
| DAP3-Reverse | TGGTGTCAGAGGGTCAGGAA |
| HtrA2-Forward | GGCATGAAGCTGATTGCGTT |
| HtrA2-Reverse | ATGCCGTCCTTGTGTTTGGA |
| β-Tubulin-Forward | GCTGGAGCGTATGAACGTCT |
| β-Tubulin-Reverse | GGCACGAGGGACATACTTGT |
Statistical Analysis
All experiments were performed in triplicate (n = 3). One-way ANOVAs and Tukey tests were used and data were assessed with GraphPad Prism software, version 8.0.0. The p-values were considered significant at <0.05, <0.01, and <0.001.
Results
CA Reduced Fungal Viability of A. flavus
The spoilage by A. flavus of seed crops and foodstuffs, together with the contamination by aflatoxins produced by this fungus, have caused significant concern to farmers and the food industry. To resolve the problem of food spoilage by A. flavus, CA, an α, β-unsaturated aldehyde that is widely used in food additives, was introduced to treat this fungus. We found that A. flavus treated with CA showed MIC at 0.065 mg/ml when the viability of the CA treatment was assessed after 5 days according to visual observation (Figure 1A).
FIGURE 1.
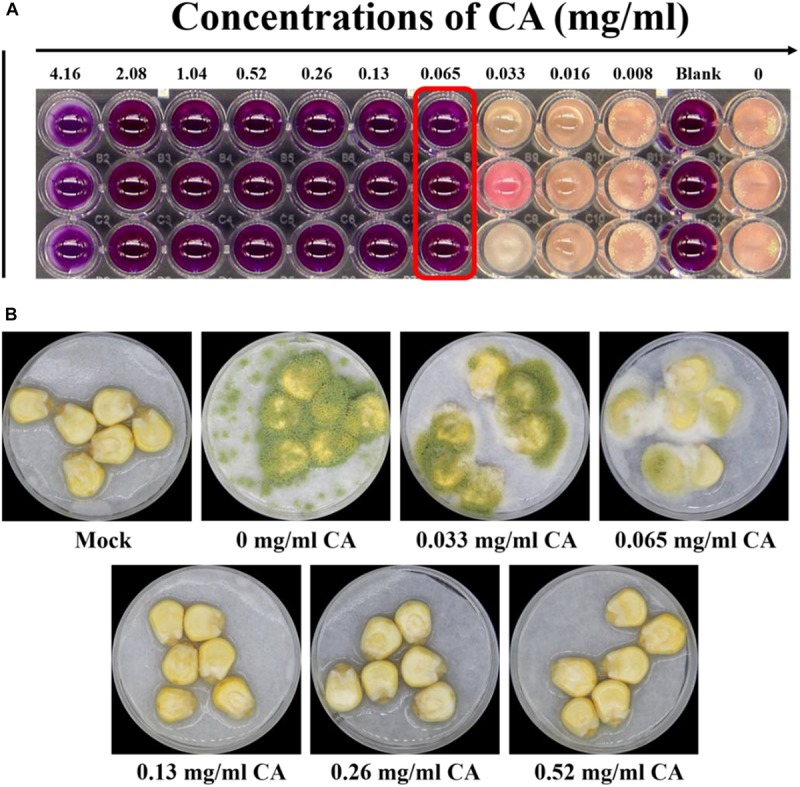
Effects of CA on Aspergillus flavus viability and fungal virulence. (A) The MIC for A. flavus treated with CA was detected by the microdilution method, and the endpoint was observed by using resazurin. (B) The antifungal effect of CA on corn: 5 × 106 spores/ml suspension of spore in 0.01% Tween 20 was inoculated into corn, which was treated with CA volatilization, and the Petri dish was kept in a moist incubator at 28°C with 12 h cycles of light/dark for 5 days. The mock control was treated with sterile water, and spore were not inoculated into the corn.
We then examined the ability of A. flavus to invade maize kernels treated with CA. As shown in Figure 1B, the maize kernels in the control were unspoiled by fungus. In the treatment samples, maize kernels with little CA treatment were seriously invaded by the A. flavus, which produced a large amount of green spore. The spoilage of the maize kernels by A. flavus was significantly inhibited by treatment with greater concentrations of CA (0.13, 0.26, and 0.52 mg/cm3). A. flavus failed to colonize the maize kernels after they were treated with a concentration of 0.13 mg/cm3 CA (Figure 1B). At concentrations of 0.26 and 0.52 mg/cm3, CA prevented all fungal spoilage. Our results indicated that CA is a promising agent for preventing the infection of seed crops by A. flavus.
CA Inhibited the Sporulation and Fungal Development of A. flavus
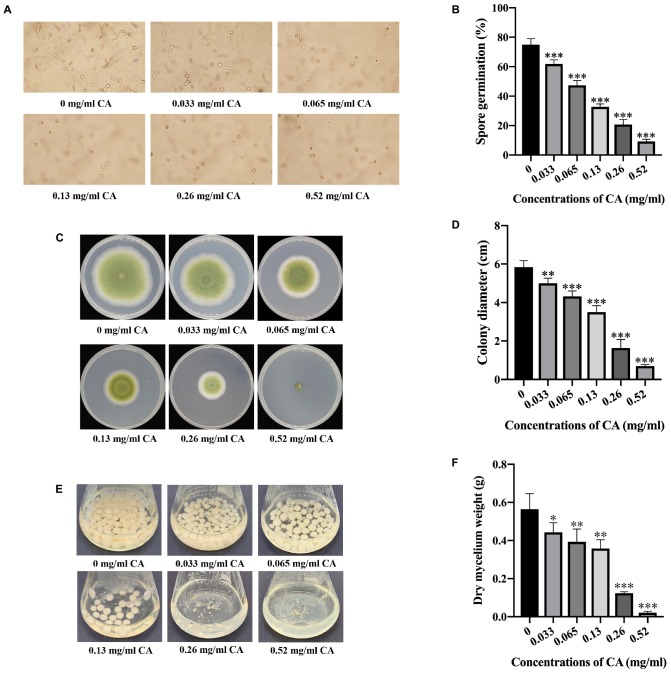
To better understand how it is that CA restricts the growth of A. flavus in seed crops, the effects of CA on the sporulation and development of A. flavus were assayed. As shown in Figures 2A,B, the spore germination of this fungus was significantly inhibited by CA when the concentration was 0.033 mg/ml or more. The results also show that CA had a positive inhibitory effect on the spore germination of A. flavus, indicating that the effect of CA on inhibiting sporulation is dose-dependent. In the assay exploring the effect of CA on A. flavus development, we found that increasing concentrations of CA significantly inhibited the growth of A. flavus (Figures 2C,D). Direct contact with CA significantly inhibited the growth of A. flavus hyphae, and this inhibition was positively correlated with the treatment concentration, such that, when the concentration was at 0.52 mg/ml, the mycelial growth of A. flavus was completely inhibited. The effect of CA on the biomass of A. flavus was also investigated, and this showed that, after treatment with different concentrations of CA, the biomass of A. flavus was significantly decreased (Figures 2E,F). The decrease in biomass was positively correlated with the concentration of CA. These results demonstrate that CA has the potential to significantly inhibit the sporulation and fungal development of A. flavus.
FIGURE 2.
Effects of CA on spore germination and fungal development in A. flavus. (A) Observation by microscope of spore germination of A. flavus treated with different concentrations of CA. (B) Statistical analysis of spore germination percentages. (C) Mycelial growth of A. flavus under various concentrations of CA. (D) Statistical analysis of colony diameters. (E) The biomass production of A. flavus under different concentrations of CA. (F) Statistical analysis of dry mycelium weight. In all statistical analysis, ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001 when compared with the 0 mg/ml CA.
CA Destroyed the Biofilm of A. flavus
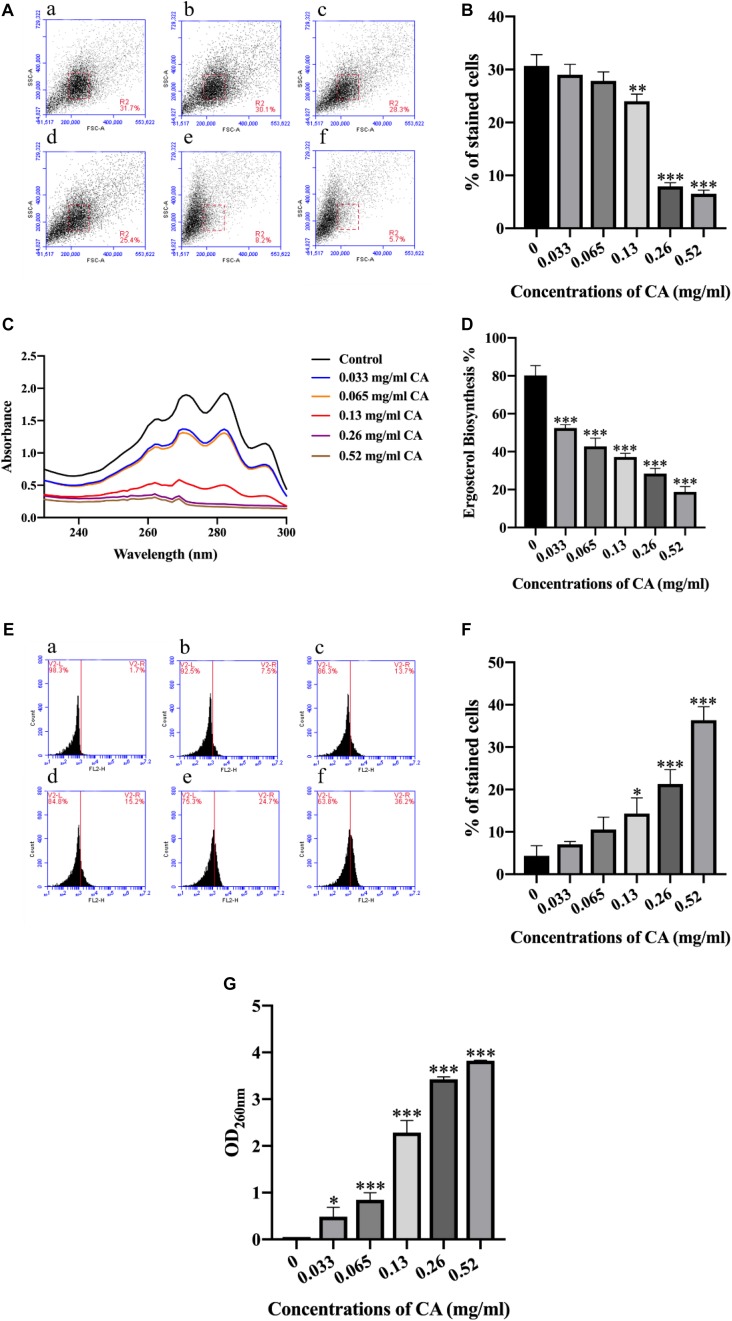
As the sporulation of A. flavus was dramatically inhibited by CA, we wondered whether the integrity of the cell membrane was impaired by the CA treatment. We then examined the cell morphology of A. flavus by using flow cytometry. The FSC (X-axis) indicated the size of the cells, and the SSC (Y-axis) indicated the granularity of the cells. As shown in Figures 3A,B, the morphological characteristics of A. flavus cells were changed following treatment with CA, and the extent of the cell changes differed according to the different concentrations of CA. The results showed that CA significantly changed the cell morphology of A. flavus.
FIGURE 3.
CA destroyed the biofilm of A. flavus. (A) CA destruction of A. flavus cells’ morphology detected by flow cytometry and a histogram analysis of the destruction of cell properties. (a) Fluorescence of cells without CA treatment; (b–f) A. flavus cells exposed to 0.033, 0.065, 0.13, 0.26, and 0.52 mg/ml CA. (B) Statistical analysis of percentage of stained cells. (C,D) CA inhibited the synthesis of ergosterol, which has been considered a classical antifungal target in A. flavus cell membranes. (E) PI staining was used to detect the biofilm damage level after being treated with CA and a statistical analysis of the damage to cells. (a) Fluorescence of cells without CA treatment; (b–f) A. flavus cells as treated with 0.033, 0.065, 0.13, 0.26, and 0.52 mg/ml CA. (F) Statistical analysis of percentage of stained cells. (G) The analysis of A. flavus cellular content after being treated with various concentrations of CA. In all statistical analyses ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001 when compared with the 0 mg/ml CA.
Ergosterol is the principal sterol in filamentous fungi and it is required for fungal cell membrane growth and normal function (de Lira Mota et al., 2012). We detected the synthesis of ergosterol in A. flavus, and the results showed that different concentrations of CA could inhibit the synthesis of ergosterol in the A. flavus cell membrane (Figures 3C,D). Compared with the control group, the ergosterol content of A. flavus cells decreased after treatment with different concentrations of CA, which indicated that CA inhibition of the synthesis of ergosterol in the A. flavus cell membrane was dose-dependent. The cell membrane is an important organelle in cells and plays a key role in material transport and signal transmission. This experiment used PI—a fluorescent dye that can stain nucleic acids—to detect A. flavus cell membrane damage after CA treatment. The A. flavus cells showed a more pronounced fluorescence as the concentration of CA increased. The optical density (OD) value for detecting the release of the content of A. flavus was recorded at a wavelength of 260 nm by an ultraviolet spectrophotometer. As the concentration of CA increased, the OD value obtained from the corresponding experimental group also increased (Figure 3G). The results show that, as the concentration of CA increased, the release of contents also increased. The release of contents after treatment with CA indicates that the cell membrane was destroyed.
The Accumulation of Intracellular ROS Increased With CA
In various physiological and pathological processes, ROS plays a vital role in autophagy and in cell death (Xu et al., 2017). We used the sensitive fluorescent dye DCFH-DA to investigate the production of intracellular ROS by flow cytometer. As shown in Figure 4, the generation of intracellular ROS increased significantly after A. flavus cells were treated with increasing concentrations of CA. The data indicate that CA may be conducive to an accumulation of intracellular ROS.
FIGURE 4.
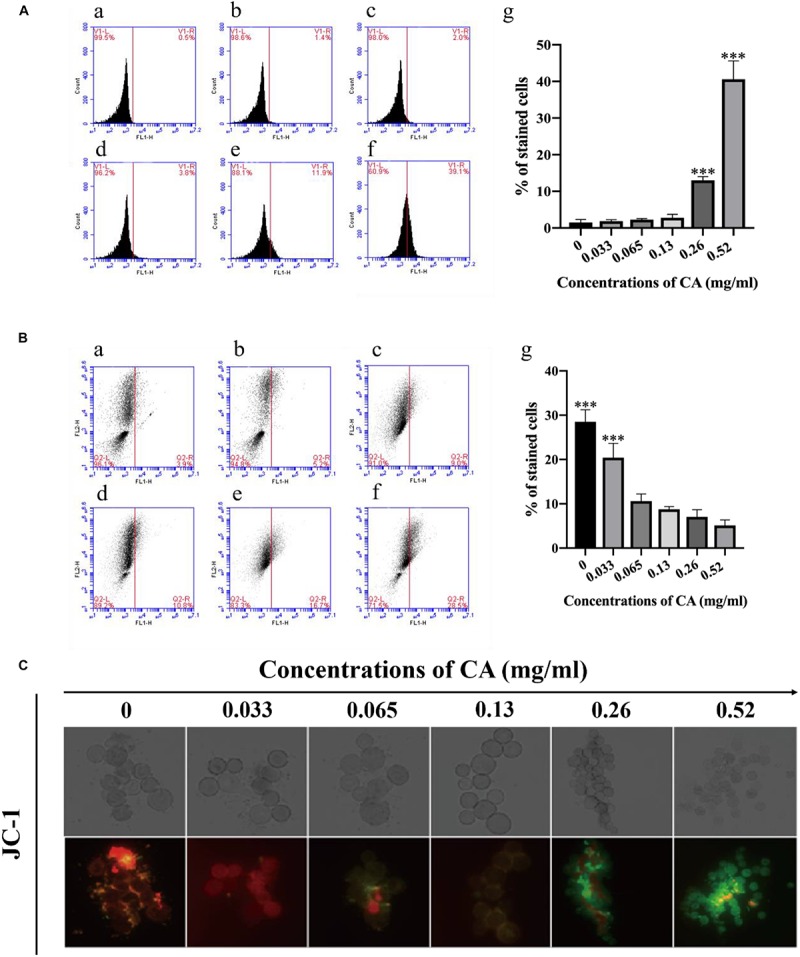
Flow cytometry analysis of ROS content and Δψm in CA-treated A. flavus. A (a) Fluorescence of cells without CA treatment; (b–f) A. flavus cells exposed to 0.033, 0.065, 0.13, 0.26, and 0.52 mg/ml CA. (B) Statistical analysis of percentage of stained cells. (C) CA decreased the extent of mitochondrial damage to cells as detected by flow cytometry and the statistical analysis of the stained cells; (a) Fluorescence of cells without CA treatment; (b–f) A. flavus cells exposed to 0.033, 0.065, 0.13, 0.26, and 0.52 mg/ml CA. A (g) and B (g) Statistical analysis of percentage of stained cells. (C) Fluorescence microscopy analysis of the degree of mitochondrial depolarization. JC-1 generates red fluorescence when the mitochondrial membrane potential is high, and green fluorescence when the mitochondrial membrane potential is low. In all statistical analyses ∗∗∗p < 0.001 when compared with the 0 mg/ml CA.
Effect of CA on Δψm of A. flavus Cells
Δψm is known to promote cell death and to act as a protease in the extracellular matrix (Kimura-Ohba and Yang, 2016). In this study, we used fluorescence microscopy and flow cytometry with JC-1 staining to measure the effect of CA on Δψm in A. flavus cells: a decrease in Δψm was evident in A. flavus cells with increasing concentrations of CA after 12 h of treatment (Figure 4C). As shown in Figure 4, a typical fluorescence distribution of JC-1 was displayed in the non-treated group, and the J-aggregates were red. We found that the cells stained with JC-1 changed to a cytoplasmic formation of J-monomeric (green) forms with increased concentrations of CA. These results indicate that CA may decrease Δψm in A. flavus cells in a concentration-dependent manner.
CA Increased Cytoplasmic and Mitochondrial Ca2+ Levels
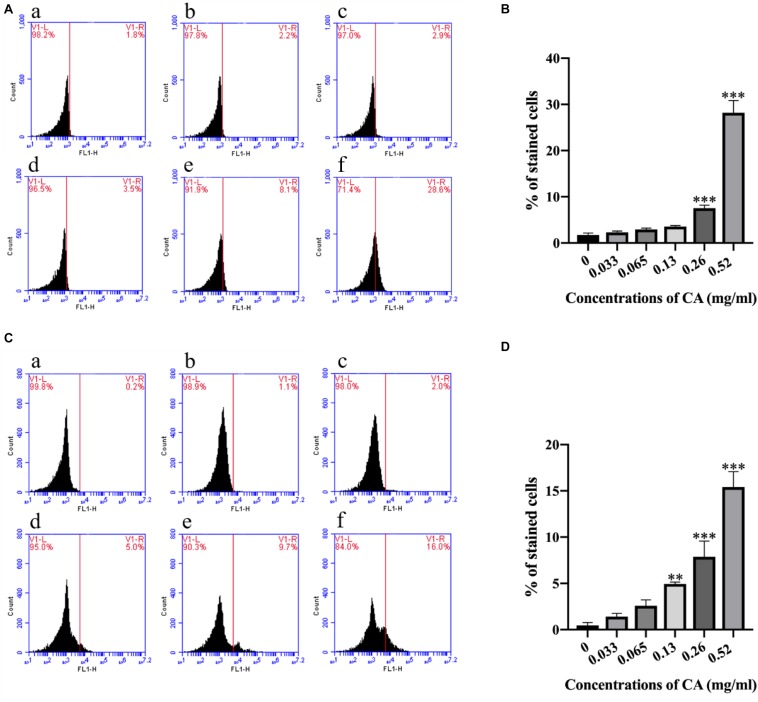
Ca2+ in the mitochondria plays an important role in the regulation of cell survival, apoptosis, and autophagy (Chemaly et al., 2018), and the Ca2+ level in the cytoplasm is always elevated during the process of cell apoptosis (Zhu et al., 2018). In this study, we selected the Fluo-3/AM and Rhod-2/AM stains to detect the levels of Ca2+ in the cytoplasm and mitochondria. Compared with the non-treated cells, the Ca2+ was increased in the mitochondria at different concentrations of CA (Figures 5A,B). Furthermore, the Ca2+ level in the cytoplasm was also elevated with the increasing concentrations of CA (Figures 5C,D). These results suggest that CA may induce an increase in cytoplasmic and mitochondrial Ca2+ levels.
FIGURE 5.
Cinnamaldehyde promoted the Ca2+ accumulation in both the cytoplasm and the mitochondria. (A) Rhod-2/AM fluorescence probe was used to detect the content of calcium ion in the mitochondria after being treated with different concentrations of CA; (a) Fluorescence of cells without CA treatment; (b–f) A. flavus cells exposed to 0.033, 0.065, 0.13, 0.26, and 0.52 mg/ml CA. (B) Statistical analysis of percentage of stained cells. (C) Fluo-3/AM fluorescence probe was used to test the concentrations of Ca2+ in the cytoplasm after co-incubation with CA; (a) Fluorescence of cells without CA treatment; (b–f) A. flavus cells were treated with 0.033, 0.065, 0.13, 0.26, and 0.52 mg/ml CA. (D) Statistical analysis of percentage of stained cells. In all statistical analyses, ∗∗p < 0.01 and ∗∗∗p < 0.001 when compared with the 0 mg/ml CA.
CA Induced the Release of Cytochrome c
Cytochrome c plays an important role in initiating apoptosis, and its release from the mitochondria is a crucial event in the mammalian cell (Liu et al., 2012). The levels of cytochrome c in the mitochondria and cytoplasm were detected by western blot after A. flavus cells were co-incubated with various concentrations of CA. Compared with the non-treated cells, the level of cytochrome c in the mitochondria significantly decreased, while the level in the cytosol increased noticeably (Figures 6A–C). The results demonstrate that CA induced the release of cytochrome c from the mitochondria in A. flavus cells.
FIGURE 6.
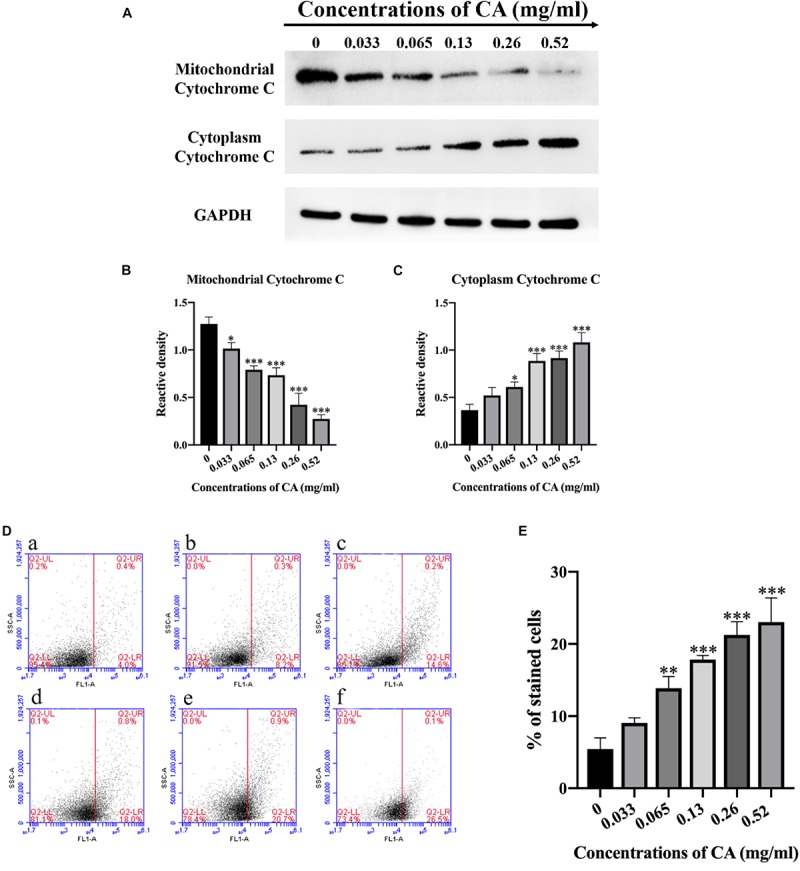
Cinnamaldehyde induced cytochrome c to be released from the mitochondria into the cytoplasm and induced apoptosis of A. flavus through the metacaspase pathway. (A) Western blot analysis of the content of cytochrome c in mitochondria and cytoplasm. (B,C) A gray value analysis of mitochondrial cytochrome c and cytoplasmic cytochrome c. (D) (a) Fluorescence of cells without CA treatment; (b–f) A. flavus cells exposed to 0.033, 0.065, 0.13, 0.26, and 0.52 mg/ml CA. (E) Statistical analysis of percentage of stained cells. In all statistical analyses, ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001 when compared with the 0 mg/ml CA.
CA Caused Activation of Metacaspase
In fungi, plants, and in some bacteria, the metacaspases have been implicated in programmed cell death (PCD) (Asplund-Samuelsson et al., 2012). We stained the cells with CaspACE FITC-VAD-FMK, and the cells were incubated with CA. As shown in Figure 6D, the percentage of A. flavus cells that were significantly stained increased in a dose-dependent manner. This result indicates that CA induced the activation of the metacaspases to initiate apoptosis in A. flavus.
CA Caused PS Externalization
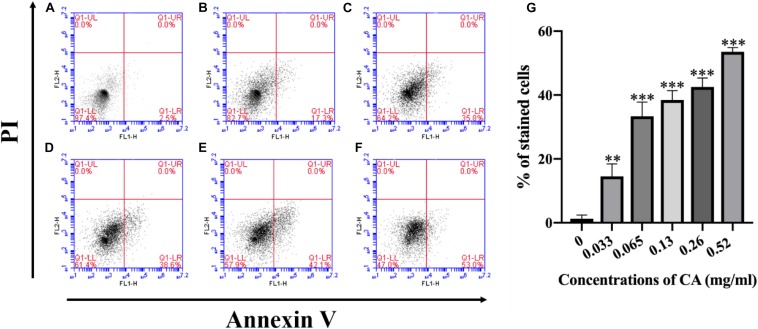
Phosphatidylserine (PS) is expressed in the outer layers of the cell membrane and is “flipped out” from the inner layers in early apoptosis (Chowdhury et al., 2014). In this assay, A. flavus cells were double-stained with Annexin V-FITC and PI at various concentrations of CA treatment, and apoptotic cells were identified by flow cytometry. Figure 7 depicts the percentages of early apoptotic cells (Annexin V-positive and PI-negative) in the lower right quadrant and this increases in a time-concentration-dependent manner. The results conclusively indicate that CA can lead to apoptosis through the externalization of PS.
FIGURE 7.
Flow cytometry measures PS externalization in A. flavus after being treated with CA. (A) Fluorescence of cells without CA treatment. (B–F) A. flavus cells treated with 0.033, 0.065, 0.13, 0.26, and 0.52 mg/ml CA. (G) Statistical analysis of percentage of stained cells. In all statistical analyses, ∗∗p < 0.01 and ∗∗∗p < 0.001 when compared with the 0 mg/ml CA.
Effect of CA on DNA Damage and Nuclear Fragmentation
The degree of DNA damage is related to the degree of apoptosis, with DNA damage preceding apoptosis, which is consistent with the time of execution of apoptosis (Rai et al., 2015). We used DAPI and TUNEL staining to detect DNA damage and nuclear fragmentation, which are hallmarks of late apoptosis. In the microscopic analysis, the cells treated for 12 h with various concentrations of CA showed an increasing fluorescence intensity, which indicated CA induced DNA damage (Figure 8B). Similarly, we found that when A. flavus cells were exposed to CA they had a DAPI-positive phenotype and showed chromatin condensation, which suggested that the CA induced nuclear fragmentation (Figure 8A). Our results show that CA caused DNA damage and nuclear fragmentation in A. flavus cells. In recent years, the literature has demonstrated that Mst3, Stm1, AMID, Yca1, DAP3, and HtrA2 are all genes associated with apoptosis (Fedorova et al., 2005). As Figure 8C shows, after 12 h of treatment with CA, the expression levels of Mst3, Stm1, AMID, Yca1, DAP3, and HtrA2 were significantly increased. β-tubulin was selected as the reference gene as it displayed the same expression level in different samples. We found that the relative expression levels of the apoptotic genes were significantly changed after A. flavus cells were treated with 0.26 mg/ml CA. The results showed that CA can affect the expression of Mst3, Stm1, AMID, Yca1, DAP3, and HtrA2, which then activate related pathways to induce apoptosis in A. flavus cells.
FIGURE 8.
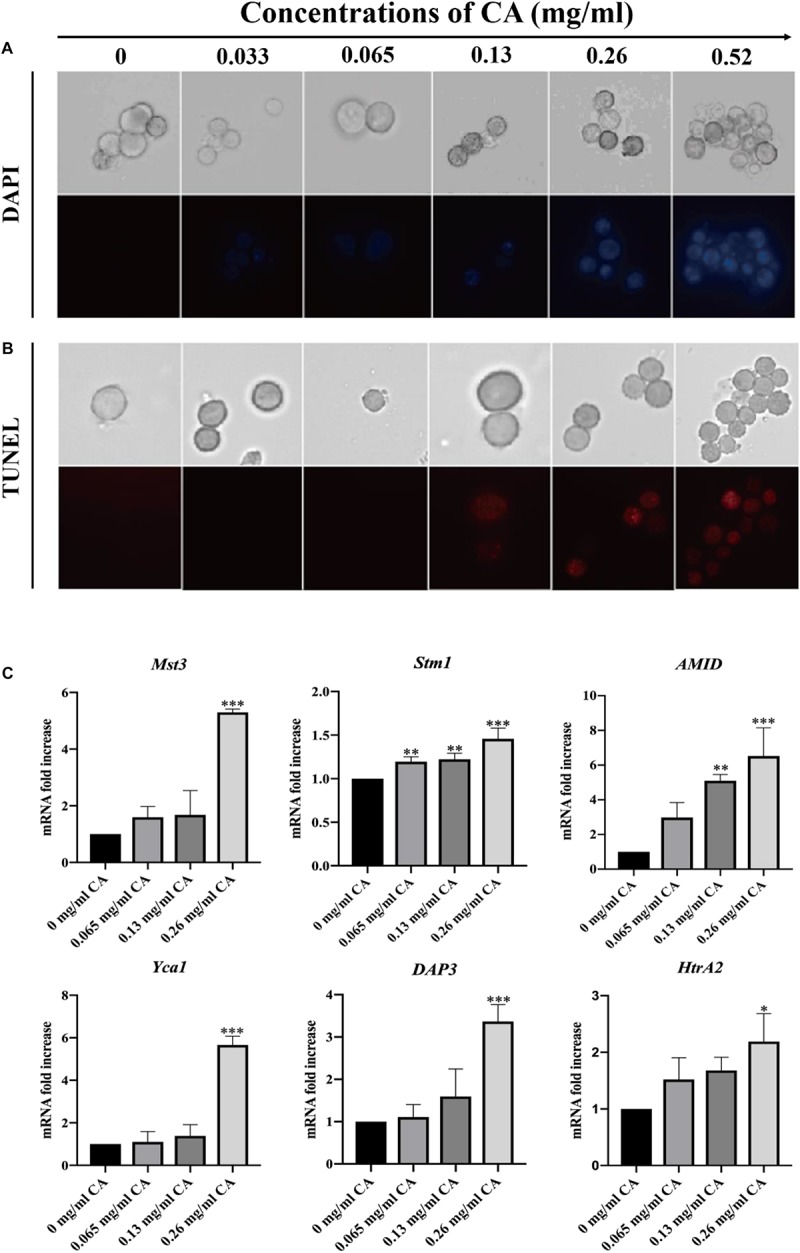
DNA damage and nuclear fragmentation by CA were visualized with fluorescence microscopy using TUNEL and DAPI staining. (A) Blue fluorescence indicates a nuclear signal after staining by DAPI. (B) Red fluorescence means a positive signal in TUNEL staining. (C) The expression levels of apoptosis-related genes (Mst3, Stm1, AMID, Yca1, DAP3, and HtrA2) at various concentrations of CA (0, 0.065, 0.13, and 0.26 mg/ml) were examined by Real-Time PCR. In all statistical analyses, ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001 when compared with the 0 mg/ml CA.
Discussion
Aromatic and medicinal plants have been used as pharmaceutical and food preservatives for decades. Many plants, such as cloves, thyme, and cinnamon, have been used to treat infectious diseases and to protect foods because they have been shown to have antimicrobial activity against spoilage by fungi and bacteria (Liu et al., 2017). Aromatic and medicinal plants produce essential oils in the form of secondary metabolites (Pandey et al., 2016). Essential oils have been reported to have a wide range of antifungal activities (Tian et al., 2011). CA is the main component of the cinnamon essential oil, and this has been developed as a food antimicrobial agent due to its activity against bacteria, yeast, and filamentous mold (Hu et al., 2013). According to the report, during corn storage, a complex essential oil rich in CA reduced the content of aflatoxin B1, zearalenone, and deoxycaprolol. Furthermore, this complex essential oil also showed the capability to reduce the contamination by Fusarium, Wallemia, Sarocladium, and Penicillium in the process of maize kernels storage (Wang et al., 2019). CA was reported to be highly safe, 20 times of effective dose (20 mg/kg) of this compound does not cause abnormal behavioral signs and serum chemical damage throughout the study (Subash Babu et al., 2007; Anand et al., 2010). Therefore, the development of CA into a new type of natural preservative has a certain safety and theoretical basis.
This study showed that CA has potential antifungal activity against A. flavus and may be a source of natural antifungal compounds that negatively affect the growth of A. flavus. The MIC of CA against A. flavus is 0.065 mg/ml (Figure 1A). When we applied CA to corn preservation we found that CA inhibited corn spoilage at a concentration of 0.13 mg/cm3 (Figure 1B). CA inhibited spore germination and mycelial growth and reduced biomass production (Figure 2). In addition, the morphology of A. flavus cells changed after CA treatment (Figures 3A,B). These results are consistent with previous research that reported that A. flavus seemed to be shriveled and wrinkled after treatment by CA as shown by scanning electron microscopy (Sun et al., 2016). The ability of CA to inhibit spore germination, mycelium growth, and biomass production in A. flavus is consistent with the results of previous studies (Tian et al., 2015; Sun et al., 2016).
Ergosterol is a unique component in the fungal cell membrane and plays a vital role in the activity of fungal cells, where it serves to stabilize the membrane structure, regulate membrane fluidity, and ensure material transportation (de Lira Mota et al., 2012). Most antifungal drugs used clinically target ergosterol or its biosynthesis (Shapiro et al., 2011). When ergosterol synthesis is reduced, the physiological activity of the cell membrane is affected, which is likely to cause fungal cell membrane damage and cell breakage (Georgopapadakou and Walsh, 1994). Some researchers have shown that the lipophilic nature of essential oils allows them to pass easily through the cell membrane to induce biological responses (Tian et al., 2015). We examined the synthesis of ergosterol and detected cell membrane damage by monitoring the uptake of the fluorescent nuclear stain PI. The results showed that when A. flavus was treated with different concentrations of CA (0, 0.033, 0.065, 0.13, 0.26, and 0.52 mg/ml) it reduced the synthesis of ergosterol and caused cell membrane damage (Figures 3C–F). This result is consistent with previously reported results that tested products such as citral, octanal, and alpha-terpineol, which can all damage cell membranes with consequent bacteriostatic action (Zhou et al., 2014). We also detected a release of the contents of A. flavus and found that CA can cause this release of contents. This result indicated that the cell membrane was damaged (Figure 3G), verifying the previous results.
Apoptosis is a unique form of PCD in which cells activate an intrinsic suicide program for self-destruction (Perez-Garijo et al., 2013). Currently, clinically used antifungal drugs such as peptaibols, anacardic acid, and amphotericin B, which are cytotoxic to pathogenic fungi through activation of an apoptotic pathway (Muzaffar et al., 2016) are considered to offer a promising approach to the prevention of fungi and food contamination. In the light of previous results, we determined that CA can effectively inhibit A. flavus. We therefore focused on its mechanism of apoptosis.
The redox state of cells plays a crucial role in cell fate. A slight imbalance between the rate of production and the breakdown of reactive oxygen and nitrogen species (ROS and RNS) may lead to activation of the cell death pathway (Hirpara et al., 2001). It is worth noting that mitochondria are the primary intracellular source of ROS, mainly superoxide () and hydrogen peroxide (H2O2), as electrons are promoted leading to oxygen leakage through high-throughput electron transport chains (ETCs) (Finkel and Holbrook, 2000). The accumulation of ROS is considered to be one of the earliest changes associated with PCD (Tian et al., 2016). With this in mind, a DCFH-DA assay was applied to detect changes in the levels of ROS in CA-treated A. flavus cells. Compared with the non-CA-treated A. flavus cells, our results indicated that intracellular ROS levels increased significantly in CA-treated A. flavus (Figures 4A,B). The emergence of high levels of ROS can lead to mitochondrial damage, cell membrane damage, and even DNA breaks (Phaniendra et al., 2015). It has also been reported that an increase in Δψm is associated with high intracellular ROS accumulation (Sukumar et al., 2016).
Mitochondria are essential regulators of cellular bioenergetics, and mitochondria that are damaged by ROS tend to produce more ROS, thereby activating mitochondria-mediated apoptosis or necrosis pathways. The opening of mitochondrial permeability transition pores (MPT pores) leads to a loss of the mitochondrial inner membrane integrity. MPT pores allow flux of small molecules, <1500 Da, and protons, leading to mitochondrial swelling, loss of Δψm, rupture of the outer membrane, and death through apoptosis or necrosis. The formation of these pores can occur in response to several stimuli, including Ca2+ overload and oxidative stress (Handy and Loscalzo, 2012). Therefore, we measured changes in Δψm in CA-treated A. flavus cells by using a JC-1 probe. As shown in Figure 4C, Δψm significantly decreased after incubation with different concentrations of CA (0, 0.033, 0.065, 0.13, 0.26, and 0.52 mg/ml), and this result is consistent with the results of previous research (Yun et al., 2017). We speculated that the mitochondrial homeostasis is disrupted, causing its dysfunction and leading to changes in membrane potential.
The role of ROS and Ca2+ channels may potentially modulate mitochondrial dysfunction, form MPT pores, and induce apoptosis. Ca2+ overload can lead to cell death. Calcium, as a major second messenger in cells, is well-known for its important role in mediating PCD (Handy and Loscalzo, 2012). Increased intracellular calcium is a sign of early apoptosis in cells. When the balance of intracellular calcium is disrupted, it leads to the release of cytochrome c and other pro-apoptotic factors (Garcia-Prieto et al., 2013). The results of this study illustrated a concentration-dependent increase in cytoplasmic Ca2+ in CA-treated A. flavus as well as significant increases in mitochondrial Ca2+ levels (Figure 5), which echo the results of previous reports. Overloading of mitochondrial Ca2+ disrupts mitochondrial function and depolarization of Δψm. This result confirms the pathological events leading to apoptosis (Yun and Lee, 2016).
Given the above-mentioned disruption of the intracellular calcium balance leading to the release of cytochrome c, the collapse of Δψm is closely related to a series of events, including the release of cytochrome c, the activation of metacaspase, DNA damage, and nuclear fragmentation. Cytochrome c is a pro-apoptotic protein, and the opening of the MPT pores causes the mitochondrial membrane rupture to release cytochrome c (Mallick et al., 2015). In our study, we measured the release of cytochrome c from the mitochondria into the cytoplasm in CA-treated A. flavus cells by western blot (Figures 6A–C). Release of cytochrome c from mitochondria is a key event initiating apoptosis. It induces the assembly of apoptotic bodies and activates downstream caspase. Further, apoptosis and caspase were initially thought to be crucial markers of apoptosis (Yuan et al., 2016).
In yeast apoptosis, there are caspase-dependent and caspase-independent cell death pathways. The yeast caspase-like protease, known as metacaspase, is encoded by YCA1. ROS is a major factor in inducing apoptosis in yeast cells, and it regulates cell death pathways by activating yeast metacaspase (Kim et al., 2016). Therefore, we examined the metacaspase activity of A. flavus cells following CA treatment and found that the activity of metacaspase increased in tandem with increased CA concentration, indicating that CA activated metacaspase (Figures 6D,E).
Changes in the phospholipid bilayer in the cell membrane usually occur in the early stages of apoptosis. When apoptosis occurs, the PS component of the phospholipid bilayer will move from the inner membrane to the outer membrane (Tian et al., 2016). We examined the apoptotic characteristics of CA-treated cells, and the results showed that CA caused the externalization of PS on the outer surface of the plasma membrane (Figure 7). DNA damage and nuclear fragmentation are typical morphological features of apoptotic cells in the late stage of apoptosis (Tian et al., 2017). It is well-known that DAPI fluorescent probes are used to detect chromatin condensation, and TUNEL staining is one of the most reliable strategies for identifying the amount of DNA fragmentation visible. Our fluorescence results indicated that CA can significantly affect DNA damage and chromatin condensation in A. flavus (Figures 8A,B).
In summary, our study demonstrates that Ca2+ and ROS-mediated apoptosis can occur in A. flavus treated with CA. We propose a model of apoptosis mechanism, as shown in Figure 9. CA causes an increase in Ca2+ and ROS. Ca2+ overload and oxidative stress disrupt mitochondrial function and cause the loss of Δψm, which in turn promote the release of cytochrome c from the mitochondria into the cytoplasm. PS externalization can be observed in the early stages of apoptosis, and the increase in ROS activates metacaspase, which further induces apoptosis. Finally, typical morphological features of late apoptosis, DNA fragmentation, and chromatin condensation can be observed.
FIGURE 9.
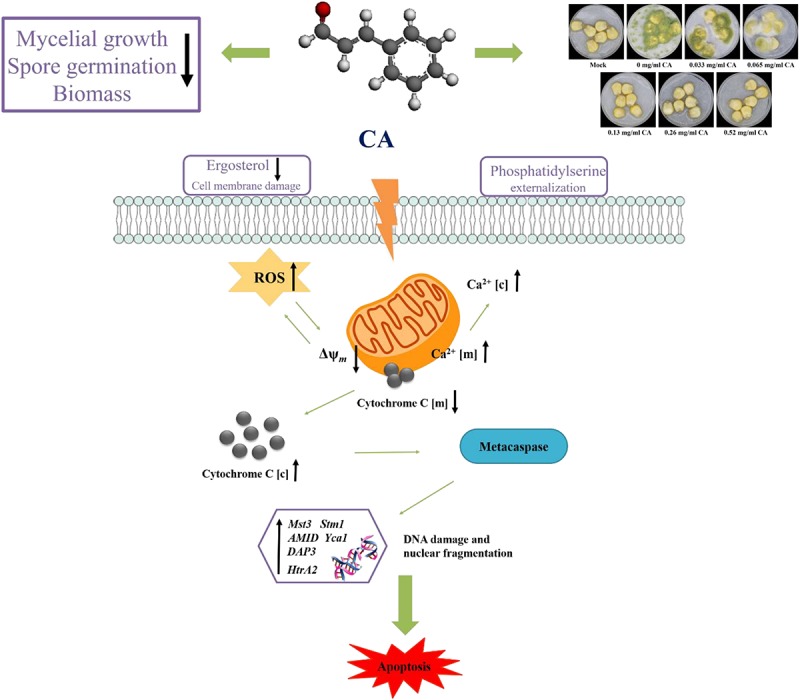
A schematic illustration of the potential inhibition mechanism on A. flavus by CA.
To further explore the molecular mechanism of CA-induced apoptosis in A. flavus, we examined apoptosis-related genes by RT-PCR. As shown in Figure 8C, the expression levels of Mst3, Stm1, AMID, and Yca1 increased in tandem with an increase in CA concentration. Previous studies have shown that these genes are all coding for the caspase family in fungi (Fedorova et al., 2005), which is consistent with our finding that CA activates metacaspase. Over-expression of these genes may be a potential mechanism by which CA activates metacaspase to induce apoptosis. The expression levels of DAP3 and HtrA2 exhibited the same trend, showing concentration-dependence. These two genes have been reported to be involved in mitochondrial homeostasis and injury (Fedorova et al., 2005). Over-expression of these two genes may cause CA to disrupt mitochondrial homeostasis, lead to mitochondrial damage, promote the release of apoptotic factors, and ultimately result in apoptosis.
Conclusion
Cinnamaldehyde can inhibit mycelial growth, buccal germination, and biomass production. It can alter cell morphology, cause cell membrane damage, and cause mitochondrial dysfunction through the interaction between Ca2+ and ROS, leading to apoptosis of A. flavus. CA is also effective in preventing corn spoilage. The results of this study indicate that CA is a potential candidate for use as a antifungal agent in food preservation.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Author Contributions
JT, KY, and YGL designed the experiments. SQ, LC, QG, and XH performed the experiments. KY, ML, and YXL analyzed the data. SQ and LC drafted the manuscript. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This study was funded by the National Natural Science Foundation of China (31972171 and 31671944), Six Talent Peaks Project of Jiangsu Province (SWYY-026), Qing Lan Project of Jiangsu Province, Natural Science Foundation by Xuzhou City (KC17053), and the PAPD of Jiangsu Higher Education Institutions.
References
- Anand P., Murali K. Y., Tandon V., Murthy P. S., Chandra R. (2010). Insulinotropic effect of cinnamaldehyde on transcriptional regulation of pyruvate kinase, phosphoenolpyruvate carboxykinase, and GLUT4 translocation in experimental diabetic rats. Chem. Biol. Interact. 186 72–81. 10.1016/j.cbi.2010.03.044 [DOI] [PubMed] [Google Scholar]
- Asplund-Samuelsson J., Bergman B., Larsson J. (2012). Prokaryotic caspase homologs: phylogenetic patterns and functional characteristics reveal considerable diversity. PLoS One 7:e49888. 10.1371/journal.pone.0049888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atanasov A. G., Waltenberger B., Pferschy-Wenzig E. M., Linder T., Wawrosch C., Uhrin P., et al. (2015). Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotechnol. Adv. 33 1582–1614. 10.1016/j.biotechadv.2015.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buglak N. E., Jiang W., Bahnson E. S. M. (2018). Cinnamic aldehyde inhibits vascular smooth muscle cell proliferation and neointimal hyperplasia in Zucker Diabetic Fatty rats. Redox Biol. 19 166–178. 10.1016/j.redox.2018.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabello C. M., Bair W. B. I. I. I., Lamore S. D., Ley S., Bause A. S., Azimian S., et al. (2009). The cinnamon-derived Michael acceptor cinnamic aldehyde impairs melanoma cell proliferation, invasiveness, and tumor growth. Free Radic. Biol. Med. 46 220–231. 10.1016/j.freeradbiomed.2008.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canciani E., Dellavia C., Marazzi M. G., Augusti D., Carmagnola D., Vianello E., et al. (2017). RNA isolation from alveolar bone and gene expression analysis of RANK, RANKL and OPG: a new tool to monitor bone remodeling and healing in different bone substitutes used for prosthetic rehabilitation. Arch. Oral. Biol. 80 56–61. 10.1016/j.archoralbio.2017.03.011 [DOI] [PubMed] [Google Scholar]
- Chemaly E. R., Troncone L., Lebeche D. (2018). SERCA control of cell death and survival. Cell Calcium 69 46–61. 10.1016/j.ceca.2017.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Wang Z., Liu L., Qu S., Mao Y., Peng X., et al. (2019). Cinnamaldehyde inhibits Candida albicans growth by causing apoptosis and its treatment on vulvovaginal candidiasis and oropharyngeal candidiasis. Appl. Microbiol. Biotechnol. 103 9037–9055. 10.1007/s00253-019-10119-3 [DOI] [PubMed] [Google Scholar]
- Chowdhury S., Mukherjee T., Chowdhury S. R., Sengupta S., Mukhopadhyay S., Jaisankar P., et al. (2014). Disuccinyl betulin triggers metacaspase-dependent endonuclease G-mediated cell death in unicellular protozoan parasite Leishmania donovani. Antimicrob. Agents Chemother. 58 2186–2201. 10.1128/AAC.02193-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lira Mota K. S., De Oliveira Pereira F., De Oliveira W. A., Lima I. O., De Oliveira Lima E. (2012). Antifungal activity of Thymus vulgaris L. essential oil and its constituent phytochemicals against Rhizopus oryzae: interaction with ergosterol. Molecules 17 14418–14433. 10.3390/molecules171214418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorri M., Hashemitabar S., Hosseinzadeh H. (2018). Cinnamon (Cinnamomum zeylanicum) as an antidote or a protective agent against natural or chemical toxicities: a review. Drug Chem. Toxicol. 41 338–351. 10.1080/01480545.2017.1417995 [DOI] [PubMed] [Google Scholar]
- Fedorova N. D., Badger J. H., Robson G. D., Wortman J. R., Nierman W. C. (2005). Comparative analysis of programmed cell death pathways in filamentous fungi. BMC Genomics 6:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T., Holbrook N. J. (2000). Oxidants, oxidative stress and the biology of ageing. Nature 408 239–247. 10.1038/35041687 [DOI] [PubMed] [Google Scholar]
- Fuchs Y., Steller H. (2011). Programmed cell death in animal development and disease. Cell 147 742–758. 10.1016/j.cell.2011.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Prieto C., Riaz Ahmed K. B., Chen Z., Zhou Y., Hammoudi N., Kang Y., et al. (2013). Effective killing of leukemia cells by the natural product OSW-1 through disruption of cellular calcium homeostasis. J. Biol. Chem. 288 3240–3250. 10.1074/jbc.M112.384776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopapadakou N. H., Walsh T. J. (1994). Human mycoses: drugs and targets for emerging pathogens. Science 264 371–373. 10.1126/science.8153622 [DOI] [PubMed] [Google Scholar]
- Handy D. E., Loscalzo J. (2012). Redox regulation of mitochondrial function. Antioxid Redox Signal 16 1323–1367. 10.1089/ars.2011.4123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirpara J. L., Clement M. V., Pervaiz S. (2001). Intracellular acidification triggered by mitochondrial-derived hydrogen peroxide is an effector mechanism for drug-induced apoptosis in tumor cells. J. Biol. Chem. 276 514–521. 10.1074/jbc.m004687200 [DOI] [PubMed] [Google Scholar]
- Hu L., Wang D., Liu L., Chen J., Xue Y., Shi Z. (2013). Ca(2+) efflux is involved in cinnamaldehyde-induced growth inhibition of Phytophthora capsici. PLoS One 8:e76264. 10.1371/journal.pone.0076264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshiri Z., Shams-Ghahfarokhi M., Allameh A., Razzaghi-Abyaneh M. (2012). Effect of curcumin on aspergillus parasiticus growth and expression of major genes involved in the early and late stages of aflatoxin biosynthesis. Iran J. Public Health 41 72–79. [PMC free article] [PubMed] [Google Scholar]
- Kim H. J., Park C., Han M. H., Hong S. H., Kim G. Y., Hong S. H., et al. (2016). Baicalein induces caspase-dependent apoptosis associated with the generation of ROS and the activation of AMPK in human lung carcinoma A549 cells. Drug Dev. Res. 77 73–86. 10.1002/ddr.21298 [DOI] [PubMed] [Google Scholar]
- Kim J. H., Campbell B. C., Mahoney N., Chan K. L., May G. S. (2006). Targeting antioxidative signal transduction and stress response system: control of pathogenic Aspergillus with phenolics that inhibit mitochondrial function. J. Appl. Microbiol. 101 181–189. 10.1111/j.1365-2672.2006.02882.x [DOI] [PubMed] [Google Scholar]
- Kim J. H., Campbell B. C., Mahoney N. E., Chan K. L., Molyneux R. J. (2004). Identification of phenolics for control of Aspergillus flavus using Saccharomyces cerevisiae in a model target-gene bioassay. J. Agric. Food Chem. 52 7814–7821. [DOI] [PubMed] [Google Scholar]
- Kimura-Ohba S., Yang Y. (2016). Oxidative DNA damage mediated by intranuclear MMP activity is associated with neuronal apoptosis in ischemic stroke. Oxid Med. Cell Longev. 2016:6927328. 10.1155/2016/6927328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Shen Q., Zhou W., Mo H., Pan D., Hu L. (2015). Nanocapsular dispersion of cinnamaldehyde for enhanced inhibitory activity against aflatoxin production by Aspergillus flavus. Molecules 20 6022–6032. 10.3390/molecules20046022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang D., Xing F., Selvaraj J. N., Liu X., Wang L., Hua H., et al. (2015). Inhibitory effect of cinnamaldehyde, citral, and eugenol on aflatoxin biosynthetic gene expression and aflatoxin B1 biosynthesis in Aspergillus flavus. J. Food Sci. 80 M2917–M2924. 10.1111/1750-3841.13144 [DOI] [PubMed] [Google Scholar]
- Liao J. C., Deng J. S., Chiu C. S., Hou W. C., Huang S. S., Shie P. H., et al. (2012). Anti-inflammatory activities of cinnamomum cassia constituents In Vitro and In Vivo. Evid. Based Comp. Alternat. Med. 2012:429320. 10.1155/2012/429320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Shu D., Song N., Gai Z., Yuan Y., Li J., et al. (2012). The role of cytochrome c on apoptosis induced by Anagrapha falcifera multiple nuclear polyhedrosis virus in insect Spodoptera litura cells. PLoS One 7:e40877. 10.1371/journal.pone.0040877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Meng X., Li Y., Zhao C. N., Tang G. Y., Li H. B. (2017). Antibacterial and antifungal activities of spices. Int. J. Mol. Sci. 18:E1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallick S., Dey S., Mandal S., Dutta A., Mukherjee D., Biswas G., et al. (2015). A novel triterpene from Astraeus hygrometricus induces reactive oxygen species leading to death in Leishmania donovani. Future Microbiol. 10 763–789. 10.2217/fmb.14.149 [DOI] [PubMed] [Google Scholar]
- Muzaffar S., Bose C., Banerji A., Nair B. G., Chattoo B. B. (2016). Anacardic acid induces apoptosis-like cell death in the rice blast fungus Magnaporthe oryzae. Appl. Microbiol. Biotechnol. 100 323–335. 10.1007/s00253-015-6915-4 [DOI] [PubMed] [Google Scholar]
- OuYang Q., Duan X., Li L., Tao N. (2019). Cinnamaldehyde exerts its antifungal activity by disrupting the cell wall integrity of geotrichum citri-aurantii. Front. Microbiol. 10:55. 10.3389/fmicb.2019.00055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey A. K., Kumar P., Singh P., Tripathi N. N., Bajpai V. K. (2016). Essential oils: sources of antimicrobials and food preservatives. Front. Microbiol. 7:2161. 10.3389/fmicb.2016.02161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Garijo A., Fuchs Y., Steller H. (2013). Apoptotic cells can induce non-autonomous apoptosis through the TNF pathway. eLife 2:e01004. 10.7554/eLife.01004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phaniendra A., Jestadi D. B., Periyasamy L. (2015). Free radicals: properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 30 11–26. 10.1007/s12291-014-0446-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu S., Chen L., Tian H., Wang Z., Wang F., Wang L., et al. (2019). Effect of perillaldehyde on prophylaxis and treatment of vaginal candidiasis in a murine model. Front. Microbiol. 10:1466. 10.3389/fmicb.2019.01466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai P., Parrish M., Tay I. J., Li N., Ackerman S., He F., et al. (2015). Streptococcus pneumoniae secretes hydrogen peroxide leading to DNA damage and apoptosis in lung cells. Proc. Natl. Acad. Sci. U.S.A. 112 E3421–E3430. 10.1073/pnas.1424144112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth-Walter F., Moskovskich A., Gomez-Casado C., Diaz-Perales A., Oida K., Singer J., et al. (2014). Immune suppressive effect of cinnamaldehyde due to inhibition of proliferation and induction of apoptosis in immune cells: implications in cancer. PLoS One 9:e108402. 10.1371/journal.pone.0108402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S., Uppuluri P., Pierce C. G., Lopez-Ribot J. L. (2014). In vitro study of sequential fluconazole and caspofungin treatment against Candida albicans biofilms. Antimicrob. Agents Chemother. 58 1183–1186. 10.1128/AAC.01745-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro R. S., Robbins N., Cowen L. E. (2011). Regulatory circuitry governing fungal development, drug resistance, and disease. Microbiol. Mol. Biol. Rev. 75 213–267. 10.1128/MMBR.00045-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subash Babu P., Prabuseenivasan S., Ignacimuthu S. (2007). Cinnamaldehyde–a potential antidiabetic agent. Phytomedicine 14 15–22. 10.1016/j.phymed.2006.11.005 [DOI] [PubMed] [Google Scholar]
- Sukumar M., Liu J., Mehta G. U., Patel S. J., Roychoudhuri R., Crompton J. G., et al. (2016). Mitochondrial membrane potential identifies cells with enhanced stemness for cellular therapy. Cell Metab. 23 63–76. 10.1016/j.cmet.2015.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q., Shang B., Wang L., Lu Z., Liu Y. (2016). Cinnamaldehyde inhibits fungal growth and aflatoxin B1 biosynthesis by modulating the oxidative stress response of Aspergillus flavus. Appl. Microbiol. Biotechnol. 100 1355–1364. 10.1007/s00253-015-7159-z [DOI] [PubMed] [Google Scholar]
- Tang X., Shao Y. L., Tang Y. J., Zhou W. W. (2018). Antifungal activity of essential oil compounds (Geraniol and Citral) and inhibitory mechanisms on grain Pathogens (Aspergillus flavus and Aspergillus ochraceus). Molecules 23:2108. 10.3390/molecules23092108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H., Qu S., Wang Y., Lu Z., Zhang M., Gan Y., et al. (2017). Calcium and oxidative stress mediate perillaldehyde-induced apoptosis in Candida albicans. Appl. Microbiol. Biotechnol. 101 3335–3345. 10.1007/s00253-017-8146-3 [DOI] [PubMed] [Google Scholar]
- Tian J., Ban X., Zeng H., He J., Chen Y., Wang Y. (2012). The mechanism of antifungal action of essential oil from dill (Anethum graveolens L.) on Aspergillus flavus. PLoS One 7:e30147. 10.1371/journal.pone.0030147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J., Ban X., Zeng H., He J., Huang B., Wang Y. (2011). Chemical composition and antifungal activity of essential oil from Cicuta virosa L. var. latisecta Celak. Int. J. Food Microbiol. 145 464–470. 10.1016/j.ijfoodmicro.2011.01.023 [DOI] [PubMed] [Google Scholar]
- Tian J., Gan Y., Pan C., Zhang M., Wang X., Tang X., et al. (2018). Nerol-induced apoptosis associated with the generation of ROS and Ca2+ overload in saprotrophic fungus Aspergillus flavus. Appl. Microbiol. Biotechnol. 102 6659–6672. 10.1007/s00253-018-9125-z [DOI] [PubMed] [Google Scholar]
- Tian J., Wang Y., Lu Z., Sun C., Zhang M., Zhu A., et al. (2016). Perillaldehyde, a promising antifungal agent used in food preservation, triggers apoptosis through a metacaspase-dependent pathway in Aspergillus flavus. J. Agric. Food Chem. 64 7404–7413. [DOI] [PubMed] [Google Scholar]
- Tian J., Wang Y., Zeng H., Li Z., Zhang P., Tessema A., et al. (2015). Efficacy and possible mechanisms of perillaldehyde in control of Aspergillus niger causing grape decay. Int. J. Food Microbiol. 202 27–34. 10.1016/j.ijfoodmicro.2015.02.022 [DOI] [PubMed] [Google Scholar]
- Varga J., Frisvad J. C., Samson R. A. (2011). Two new aflatoxin producing species, and an overview of Aspergillus section Flavi. Stud. Mycol. 69 57–80. 10.3114/sim.2011.69.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Jin J., Liu X., Wang Y., Liu Y., Zhao Y., et al. (2018a). Effect of Cinnamaldehyde on morphological alterations of Aspergillus ochraceus and expression of key genes involved in ochratoxin A biosynthesis. Toxins 10:E340. 10.3390/toxins10090340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Feng K., Yang H., Yuan Y., Yue T. (2018b). Antifungal mechanism of cinnamaldehyde and citral combination against Penicillium expansum based on FT-IR fingerprint, plasma membrane, oxidative stress and volatile profile. RSC Adv. 8 5806–5815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Feng K., Yang H., Zhang Z., Yuan Y., Yue T. (2018c). Effect of Cinnamaldehyde and Citral combination on transcriptional profile, growth, oxidative damage and patulin biosynthesis of Penicillium expansum. Front. Microbiol. 9:597. 10.3389/fmicb.2018.00597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Liu B., Jin J., Ma L., Dai X., Pan L., et al. (2019). The complex essential oils highly control the toxigenic fungal microbiome and major mycotoxins during storage of maize. Front. Microbiol. 10:1643. 10.3389/fmicb.2019.01643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Wu Y., Lu G., Xie S., Ma Z., Chen Z., et al. (2017). Importance of ROS-mediated autophagy in determining apoptotic cell death induced by physapubescin B. Redox Biol. 12 198–207. 10.1016/j.redox.2017.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K., Shadkchan Y., Tannous J., Landero Figueroa J. A., Wiemann P., Osherov N., et al. (2018). Contribution of ATPase copper transporters in animal but not plant virulence of the crossover pathogen Aspergillus flavus. Virulence 9 1273–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K., Zhuang Z., Zhang F., Song F., Zhong H., Ran F., et al. (2015). Inhibition of aflatoxin metabolism and growth of Aspergillus flavus in liquid culture by a DNA methylation inhibitor. Food Addit. Contam. Part A Chem. Anal. Control Exp. Risk Assess. 32 554–563. 10.1080/19440049.2014.972992 [DOI] [PubMed] [Google Scholar]
- Yuan J., Najafov A., Py B. F. (2016). Roles of caspases in necrotic cell death. Cell 167 1693–1704. 10.1016/j.cell.2016.11.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun D. G., Lee D. G. (2016). Silibinin triggers yeast apoptosis related to mitochondrial Ca(2+) influx in Candida albicans. Int. J. Biochem. Cell Biol. 80 1–9. 10.1016/j.biocel.2016.09.008 [DOI] [PubMed] [Google Scholar]
- Yun X., Rao W., Xiao C., Huang Q. (2017). Apoptosis of leukemia K562 and Molt-4 cells induced by emamectin benzoate involving mitochondrial membrane potential loss and intracellular Ca(2+) modulation. Environ. Toxicol. Pharmacol. 52 280–287. 10.1016/j.etap.2017.04.013 [DOI] [PubMed] [Google Scholar]
- Zhao J., Zhang X., Dong L., Wen Y., Zheng X., Zhang C., et al. (2015). Cinnamaldehyde inhibits inflammation and brain damage in a mouse model of permanent cerebral ischaemia. Br. J. Pharmacol. 172 5009–5023. 10.1111/bph.13270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H. E., Tao N. G., Jia L. (2014). Antifungal activity of citral, octanal and alpha-terpineol against Geotrichum citri-aurantii. Food Control 37 277–283. [Google Scholar]
- Zhu J., Jin M., Wang J., Zhang H., Wu Y., Li D., et al. (2018). TNFalpha induces Ca(2+) influx to accelerate extrinsic apoptosis in hepatocellular carcinoma cells. J. Exp. Clin. Cancer Res. 37:43. 10.1186/s13046-018-0714-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu R., Liu H., Liu C., Wang L., Ma R., Chen B., et al. (2017). Cinnamaldehyde in diabetes: a review of pharmacology, pharmacokinetics and safety. Pharmacol. Res. 122 78–89. 10.1016/j.phrs.2017.05.019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.