Abstract
This is a retrospective study examining the efficacy and safety of Gamma Knife radiosurgery (GKS) in treating patients with cerebral cavernous malformations (CCMs). Between 1993 and 2018, 261 patients with 331 symptomatic CCMs were treated by GKS. The median age was 39.9 years and females were predominant (54%). The median volume of CCMs was 3.1 mL. The median margin dose was 11.9 Gy treat to a median isodose level of 59%. Median clinical and imaging follow-up times were 69 and 61 months, respectively. After the initial hemorrhage that led to CCM diagnosis, 136 hemorrhages occurred in the period prior to GKS (annual incidence = 23.6%). After GKS, 15 symptomatic hemorrhages occurred within the first 2 years of follow-up (annual incidence = 3.22%), and 37 symptomatic hemorrhages occurred after the first 2 years of follow-up (annual incidence = 3.16%). Symptomatic radiation-induced complication was encountered in 8 patients (3.1%). Mortality related to GKS occurred in 1 patient (0.4%). In conclusion, GKS decreased the risk of hemorrhage in CCM patients presenting with symptomatic hemorrhage. GKS is a viable alternative treatment option for patients with surgically-inaccessible CCMs or significant medical comorbidities.
Subject terms: Diseases of the nervous system, Cerebrovascular disorders, Cerebrovascular disorders
Introduction
Prevention of recurrent hemorrhage and hemorrhage-associated complications are the primary objectives of cerebral cavernous malformation (CCM) treatment. Surgical resection is the definitive treatment for CCMs. However, stereotactic radiosurgery has played an increasingly important role in deep-seated CCMs and in patient with high surgical risks over the past 20 years. In our previous study published in 20051, Gamma Knife radiosurgery (GKS) decreased the annual incidence of hemorrhage in CCMs from 29.2% to 10.3% (within 2 years) and to 3.3% (beyond 2 years) over a mean follow-up period of 5.4 years (range: 9–123 months). Seizure control was achieved in 53% of patients (Engel Class I and II), and there were no treatment-related deaths1. GKS also decreased the annual incidence of hemorrhage in brainstem CCMs from 31.3% to 4.29% (within 2 years) and to 3.64% (beyond 2 years)2. Therefore, GKS appears to be an alternative treatment for patients with CCMs. In this study, we report the outcomes of 261 CCM patients treated with GKS.
Results
Patient population
A consecutive series of 261 patients presenting 331 CCMs underwent GKS between March 1993 and June 2018. The median age was 39.9 years (range: 7.4–75.3 years) and females were predominant (54%). The location of the 331 CMs varied: 111 lesions were found in the brainstem (33.5%), 47 in the basal ganglia and thalamus (14.2%), 115 in the cortical/subcortical region (34.7%), and 41 in the cerebellum (12.4%).
Among the 261 patients, 149 patients (57.1%) had one symptomatic hemorrhage, 99 patients (37.9%) had two symptomatic hemorrhages, 9 patients (3.5%) had three symptomatic hemorrhages, 2 patients (0.8%) had four symptomatic hemorrhages, 1 patient (0.4%) had five symptomatic hemorrhages, and 1 patient (0.4%) had ten symptomatic hemorrhages prior to GKS (Table 1). All patients had signs and symptoms that corresponded to CCMs, such as hemiparesis (47.5%), headache (30.3%), cranial nerve deficits (28.4%), hemisensory deficits (25.7%), dizziness (23.0%), and seizure (13.8%) (Table 1).
Table 1.
Characteristics of 261 patients with 331 CCMs treated with GKS between 1993 and 2018.
| Characteristic | No. (percentage or range) |
|---|---|
| Age in yrs (range) | 39.9 (7.4–75.3) |
| Gender (% female) | 141 (54) |
| Total no. of CCMs | 331 |
| No. of CCMs by location | |
| Brainstem | 111 (33.5) |
| Basal ganglion/thalamus | 47 (14.2) |
| Cortical/subcortical | 115 (34.7) |
| Cerebellum/4th ventricle | 41 (12.4) |
| Multiple lesions | 17 (5.1) |
| No. of pre-GKS hemorrhages (% of 261 total) | |
| 1 | 149 (57.1) |
| 2 | 99 (37.9) |
| 3 | 9 (3.5) |
| 4 | 2 (0.8) |
| 5 | 1 (0.4) |
| >5 | 1 (0.4) |
| CCM volume (cm3) | 3.1 (0.03–28.9) |
| Symptoms | |
| Hemisensory deficit | 194 (74.3) |
| Cranial nerve deficits | 187 (71.7) |
| Hemiparesis | 124 (47.5) |
| Headache | 79 (30.3) |
| Dizziness | 60 (23.0) |
| Seizure | 36 (13.8) |
| Clinical follow-up median (months) | 68.9 (6–280) |
| Image follow-up median (months) | 60.7 (6–266) |
| GKS Parameters (mean) | |
| Margin dose (Gy) | 11.9 (8.5–18.0) |
| Max dose (Gy) | 20.3 (10.9–35.0) |
| Isodose level (%) | 59 (50–90) |
Pre-GKS incidence of hemorrhage
We calculated the pre-GKS incidence of hemorrhage among patients that experienced >1 bleeding episode. The pre-GKS observation period extended from the first symptomatic, image-documented hemorrhage to the time of GKS (577.09 patient-years), during which there were 397 hemorrhages. After excluding the initial hemorrhages that led to diagnosis, the calculated annual incidence of hemorrhage was 23.6% (136 hemorrhages/577.09 patient-years).
Post-GKS incidence of hemorrhage
Hemorrhage episodes can be classified as symptomatic or asymptomatic bleeding. In this study, hemorrhage was defined as any new hemorrhage on MRI with or without neurological symptoms. The post-treatment observation period was the period from the time of GKS until any one of the following: the most recent clinical or imaging follow-up, surgical intervention, or death. The median post-GKS image follow-up time was 60.7 months (range: 6–266 months), with an overall observation period of 1635.08 patient-years. During this period, 130 hemorrhages among 112 patients were observed (0.5 hemorrhages per patient). Among these, 42 hemorrhages occurred within 2 years after GKS, whereas 88 episodes occurred >2 years after GKS. The annual incidence of hemorrhage during the first 2 years after GKS was 9.02% (42 hemorrhages/465.40 patient-year). The annual incidence of hemorrhage after the initial 2-year follow-up was 7.52% (88 hemorrhages/1169.68 patient-year).
A total of 52 symptomatic hemorrhages occurred in 43 patients during this period (0.16 symptomatic hemorrhages/patient). Among these, 15 symptomatic hemorrhages occurred within 2 years after GKS, whereas 37 symptomatic hemorrhages occurred >2 years after GKS. The annual incidence of symptomatic hemorrhage during the first 2 years after GKS was 3.22% (15 hemorrhages/465.40 patient-years). The annual incidence of symptomatic hemorrhage after the initial 2-year follow-up was 3.16% (37 hemorrhages/1169.68 patient-years). Figure 1 illustrates the changes in annual incidence of hemorrhage before and after GKS. Figure 2 illustrates a case of a patient with brainstem CCM who presented with symptomatic hemorrhage treated with GKS.
Figure 1.
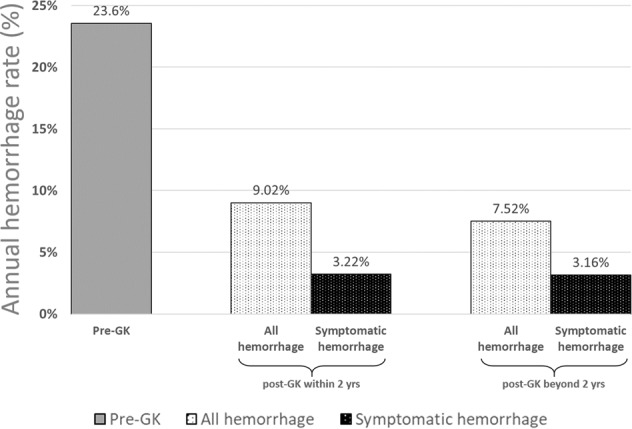
Annual incidence of hemorrhage before and after GKS.
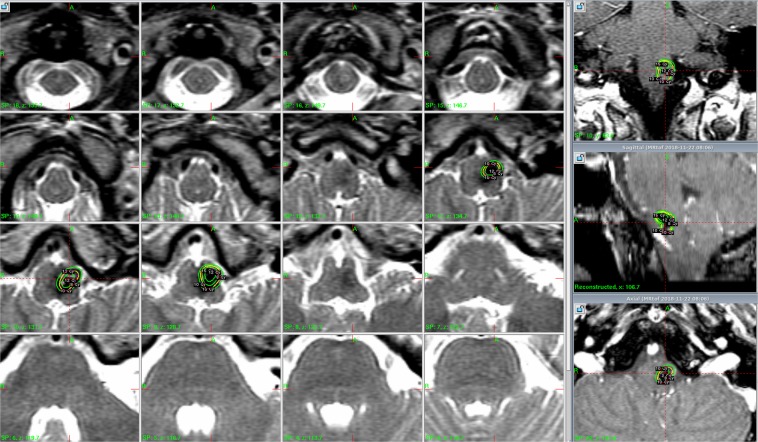
Figure 2.
A 27-year-old female presented with a sudden onset of left limb weakness and numbness, gait disturbance, diplopia, and facial numbness for one week. A CCM with associated hemorrhage that measured 3.5 mL in volume was found in the medulla. T2-weighted imaging on MRI demonstrated a hemosiderin ring around the CCM. The CCM was treated using GKS with a margin dose of 10 Gy at a 55% isodose level. The CCM was controlled for up to 63 months.
Seizure control
Prior to GKS, 36 patients with 39 CCMs presented with seizures, and 29 patients had seizures related to CCM hemorrhage. (Table 2) Among the 27 patients with AED-controlled epilepsy, 22 (82%) patients had improvements in seizure control (Engel class I-III). (Table 3) However, 5 (18%) patients had no improvement in seizure control (Engel class IV). Among the 9 patients with drug-resistant epilepsy, only 2 patients had seizure frequency reduction (Engel class II), while the remaining 7 patients had increased seizures (Engel class IV), and 4 patients eventually underwent craniotomy for CCM resection due to poor seizure control. (Table 3) Overall, a total of 28 (78%) patients had improvement in seizure control, and 8 (22%) patients had worsening of seizure control.
Table 2.
Locations of 39 CCMs in 36 patients who presented with seizures.
| Location | No. | Evidence of associated hemorrhage | AED-controlled epilepsy | Drug-resistant epilepsy |
|---|---|---|---|---|
| Temporal | 9 | 2 | 2 | 7 |
| Parietal | 13 | 12 | 12 | 1 |
| Frontal | 6 | 6 | 5 | 1 |
| Occipital | 0 | 0 | 0 | 0 |
| Insula | 4 | 4 | 4 | 0 |
| Corpus callosum | 2 | 2 | 2 | 0 |
| Corona radiata | 2 | 1 | 2 | 0 |
| Other | 3 | 2 | 3 | 0 |
| Total | 39 | 29 | 30* | 9* |
*30 CCMs in 27 patients without drug-resistant epilepsy, 9 CCMs in 9 patients with drug-resistant epilepsy.
Table 3.
Seizure outcomes in 27 patients without drug-resistant epilepsy and 9 patients with drug-resistant epilepsy after GKS by Engel classification.
| Engel classification | AED-controlled epilepsy (n = 27) | Drug-resistant epilepsy (n = 9) | |
|---|---|---|---|
| GKS | GKS only | GKS + surgical resection | |
| Class I | 4 | 0 | 4 |
| Class II | 11 | 2 | 0 |
| Class III | 7 | 0 | 0 |
| Class IV | 5 | 3 | 0 |
| Total | 27 | 5 | 4 |
Note that most of the patients with drug-resistant epilepsy had temporal CCMs (n = 7/9, 78%), whereas the other CCMs were parietal or frontal in location. All craniotomies were performed for temporal lobe CCMs (n = 4/4). All 4 of these patients achieved seizure-freedom after CCM resection.
Adverse radiation effects
New neurological deterioration after GKS without new hemorrhage were found in 8 patients (3.1%). Among them, one patient had cyst formation, and the others developed permanent neurological deficits due to non-hemorrhagic adverse radiation effects. In 16 other patients (6.1%), new T2 signal abnormalities were observed adjacent to their CCMs; however, these patients were neurologically unchanged. Overall, the neurological status after GKS was stable or improved in 96.9% of the patients.
Discussion
The risk of hemorrhage for a CCM remains undefined3–5. Natural history studies4,6–8 have suggested that the annual risk of hemorrhage ranges between 2.3% and 4.1%, whereas in surgical series3,4,9–13, this risk ranges between 2.7 and 6.8% annually prior to intervention. The risk for recurrent hemorrhage in CCMs is increased after an initial hemorrhage, and this risk can be increased to up to 40%14–17. Although other risk factors have been implicated in CCM hemorrhage, predicting when a CCM will hemorrhage remains challenging. Current treatment options for CCMs include observation, microsurgical resection, and radiosurgery. The selection of treatment modality is based mainly on number of hemorrhages, seizure control, and surgical risks. Although radiosurgery may not provide definitive cure for CCM patients, its minimally-invasive nature may provide a safer alternative for patients with surgically-inaccessible CCMs or those with medical comorbidities that preclude surgery. The radiobiological effects of GKS on CCMs remain unclear. However, gradual endothelial cell proliferation and hyalinization yielding luminal closure are two proposed mechanisms. Histological findings of GKS-treated CCMs demonstrated fibrinoid necrosis, endothelial cell destruction, and marked fibrosis in the stroma of connective tissue18,19. Thus, the observed decrease in the annual incidence of hemorrhage after GKS may be attributed to the delayed luminal closure of vascular channels.
In this study, the annual incidence of symptomatic hemorrhages was approximately 3% after GKS, which was a dramatic decrease from an annual incidence of 24% prior to GKS. When asymptomatic hemorrhages were included, this incidence was 9% within the first 2 years after GKS and 8% with longer follow-up. AREs were encountered in 3% of the patients. In our prior study of CCM patients with high surgical risks, the incidence of hemorrhage was 10% annually within the first 2 years after GKS, and 3% with longer follow-up1. For brainstem CMs, the annual incidence of hemorrhage after GKS was 4% within the first 2 years, and remained approximately 4% thereafter. AREs were observed in 4% of these patients2. Up to 2018, there have been three large studies on the use of GKS (>100 cases with at least 4 years of follow-up) specifically for the treatment of repeated hemorrhagic or symptomatic CCMs, comprising a total of 530 patients (Table 4)1,20,21. The data presented in our study adds to the mounting evidence that GKS decreases the risk of hemorrhage in CCMs. Therefore, GKS may be an option for patients with eloquently located CCMs.
Table 4.
Literature review: Radiosurgical results of CCMs (>100 cases, follow-up duration > 4 years).
| Study, year | Case no. | Margin dose (Gy) | Annual hemorrhage rate (%) | Morbidity (%, radiosurgery-related, AREs) | Mortality (%) | ||
|---|---|---|---|---|---|---|---|
| Pre-GKS | Post-GKS | FU (m) | |||||
| Liscak et al., 2000 | 107 | 16.0 | 2.0 | 1.6 | 48 | 4.5% | 1.9% |
| Liu et al., 2005 | 125 | 12.1 | 29.2 | 10.3 (<2 yr) → 3.3 (>2 yr) | 65 | 2.4% | 0% |
| Kida et al., 2015 | 298 | 14.6 | 21.4 | 7.4 (<2 yr) → 2.8 (>2 yr) | 68 | 6.7% | 2.3% |
| Present study | 261 | 11.9 | 23.6 |
All hemorrhages 9.02% (<2 yr) → 7.52 (>2 yr) Symptomatic hemorrhages 3.22 (<2 yr) → 3.16 (>2 yr) |
61 | 3.1% | 0.4% |
Abbreviation: AREs: adverse radiation effects, CM: cavernous malformations, GKS: gamma-knife radiosurgery, Gy: gray, m: month, N/A: not available, yr: year
Our institutional approach to treating eloquent CCMs (i.e., brainstem) tends to be more aggressive, even for patients with only one hemorrhage. According to a previous study22, the annual incidence of hemorrhage for brainstem CCMs after GKS decreased from 9.5% (within 1 year) to 4.7% (within 2 years). In different study, the annual incidence of hemorrhage after GKS decreased from 15% (within 2 years) to 2.4% (beyond 2 years)23. Nagy et al. stratified brainstem CCM patients into two groups: low-risk (hemorrhage ≤ 1 episode) and high-risk (hemorrhage ≥ 2 episodes). In the high-risk group, the annual incidence of hemorrhage decreased from 15% (within 2 years after GKS) to 2.4% (beyond 2 years after GKS), and the low-risk group exhibited a decrease from 5.1% (within 2 years) to 2.4% (beyond 2 years)24. Radiosurgery-related morbidity ranged from 3.2% to 11.8%. However, Liscak reported transient symptoms in 28% of brainstem CCM patients after GKS21. Mortality has not been reported in recent studies21,22,24. Radiosurgery for eloquently located CCMs appears to provide good hemorrhage control and may be the treatment of choice for select patients (Table 5).
Table 5.
Literature review: Radiosurgical results of brainstem CCMs ( >40 cases, follow-up duration > 36 m).
| Study, year | Case no. | Margin dose | Annual hemorrhage rate (%) | Morbidity (%, radiosurgery-related, ARE) | Mortality (%) | ||
|---|---|---|---|---|---|---|---|
| Pre-GKS | Post-GKS | FU (m) | |||||
| Kida et al., 2009 | 63 | 13.4 | — | 9.5 (<1 yr) → 4.7 (1–2 yr) | 55 | 3.2% | — |
| Monaco et al., 2010 | 68 | 15.8 | 32.4 | 8.2 (<2 yr) → 1.4 (>2 yr) | 62 | 11.8% | 0% |
| Nagy et al., 2010 | 79 | 12.0 | 30.0 |
HR:15(<2 yr) → 2.4 (>2 yr) LR: 5.1(<2 yr) → 1.3 (>2 yr) |
48 | 7.3% | 0% |
| Lee et al., 2012 | 49 | 11.0 | 31.3 | 4.3 (<2 yr) → 3.6 (>2 yr) | 41 | 4.1% | 0% |
| Lee et al., 2014 | 49 | 12.0 | 38.4 | 8.3 (<2 yr) → 1.8 (>2 yr) | 64 | 0% | 0% |
| Liu et al., 2016 | 43 | 11.9 | 25.0 | 3.9 (<2 yr) → 1.9 (>2 yr) | 36 | 2.3% | 0% |
| Lopez et al., 2017 | 95 | 11.9 | 3.06 | 1.4 (<3 yr) → 0.2 (>3 yr) | 78 | 7.4% | 0% |
| Kefeli et al., 2018 | 81 | 12.0 | 8.6 | 0.87 | 50 | 4.0% | 0% |
| Park et al., 2018 | 45 | 13.0 | 40.1 | 3.3 (<2 yr) → 1.48 (<5 y) | 112 | 2.2% | 0% |
| Present study | 111 | 12.0 | 31.3 | 3.8 (<2 yr) → 3.1 (>2 yr) | 57 | 5.0% | 0% |
Abbreviation: ARE: adverse radiation effects, CM: cavernous malformations, GKS: gamma-knife radiosurgery, Gy: gray, m: month, HR: high risk group, LR: low risk group, N/A: not available, yr: year
Seizures are common in patients with CCMs, and there appears to be a correlation between hemorrhage and the seizures. New onset seizures and incremental seizures are often accompanied by recent hemorrhage. Many patients also experienced concomitant headaches or dizziness that may be observed with CCM hemorrhages22. Animal studies have suggested that the deposition of blood clot-related metabolites, particularly iron, to be epileptogenic. MRI studies have also suggested that seizures in CCM patients have a temporal relationship to hemorrhages. Other risk factors for seizure development include supratentorial localization, cortical involvement, and archicortical/mesiotemporal localization. Previous studies have reported that approximately 0–18% of patients with infratentorial CCMs suffered from seizures, this was compared to 50–63% of patients with supratentorial CCMs who suffered from seizures5,8,25,26. 57–70% of patients with cortical CCMs had seizures, whereas only 14–20% of patients with exclusively subcortical CCMs had seizures5,8,25. Differentiation between the cortex and the subcortex may be challenging in older studies that utilized computed tomography as the imaging modality. Recent studies using MRI reported that 49 of 81 CCM patients with cortical involvement suffered from seizures, whereas 0 of 17 CCM patients with exclusively subcortical localization suffered from seizures26. CCMs in the temporal lobe are also commonly associated with seizures. One study found seizures in 8 of 9 patients with mesiotemporal CCMs. In contrast, only 41 of 72 patients with other neocortical CCMs suffered from seizures26. This suggests that archicortical/mesiotemporal CCMs are associated with a higher incidence of epilepsy27. Another study reported similar results in the incidence of mesiotemporal CCMs versus other neocortical CCMs (23.8% versus 3.8%). In our experience, surgical resection is preferred over radiosurgery for temporal CCMs, which are associated with recurrent hemorrhages and drug-resistant epilepsy.
The radiobiological effects of GKS on CMs remains uncertain; however, gradual endothelial cell proliferation and hyalinization yielding luminal closure are two possible mechanisms. Gewirtz et al. and Nyáry et al. reviewed the histology of patients who underwent GKS18,19. Their lesions presented indications of fibrinoid necrosis, endothelial cell destruction, and marked fibrosis in the stroma of connective tissue. This means that the decrease in the annual incidence of hemorrhage after GKS may be due to a delay in the luminal closure of vascular channels.
In the past, the effectiveness of radiosurgery for CCMs was limited by poor neuroimaging (pre-MRI period), excessive radiation doses (>15 Gy), and incomplete or overlarge target coverage. Advancements in neuroimaging, reasonable doses, and better planning software has greatly reduced the risk of complications. Although the definitive treatment for CCM is microsurgical resection, GKS is a viable treatment option for those with surgically-inaccessible CCMs or significant medical comorbidities.
Methods
Patient consent and institutional review
A consecutive series of 261 patients presenting 331 CCMs underwent GKS between March 1993 and June 2018. Patient consent was not required by the institutional review board (IRB) committee due to the retrospective nature of the review and because data had been anonymized. (Taipei Veteran General Hospital IRB number: 2018-09-007BC).
The treatment criteria for the GKRS including: A) patient with a cavernoma or multiple cavernomas, B) hemorrhage at least once, C) the hemorrhage causes the clinical symptoms.
Gamma knife radiosurgery
Radiosurgery was performed using the Leksell Gamma Unit Model C (Elekta Instrument, Inc). The median lesion volume was 3.1 ml (0.03–28.9 ml). Figure 2 presents a typical dose plan for a representative case. The prescription dose was set at an isodose level of 50–90%, and the median margin dose was 11.9 Gy (range 8.5–18 Gy). A higher margin dose (>12 Gy) was avoided due to the benign nature of the lesions and used only in the early part of this study. A higher margin dose (>12 Gy) was avoided due to the benign nature of the lesions and used only in the early part of this stud To achieve a highly conformal dose distribution, multiple small shots were used to maximize the mean dose and minimize the radiation volume outside the target. No identifiable portion of the facial nerve received more than 13 Gy and the trigeminal nerve received no more than 15 Gy. The margin dose administered to tumors that bulged into tissue of the brainstem was reduced to 11 Gy. y. To achieve a highly conformal dose distribution, multiple small shots were used to maximize the mean dose and minimize the radiation volume outside the target. No identifiable portion of the facial nerve received more than 13 Gy and the trigeminal nerve received no more than 15 Gy. The margin dose administered to tumors that bulged into tissue of the brainstem was reduced to 11 Gy.
Follow-up imaging and clinical evaluation
Following GKS, all of the patients underwent MR imaging studies and clinical evaluation at 6-month intervals. The median follow-up time was 60.7 months (range 6–266 months). We divided those patients into two groups based on their follow-up time: 171 patients were followed up regularly for at least 2 years, and another 90 patients had regular follow-up for less than 2 years. The patients were carefully examined for any clues of hemorrhage, including new foci of high signal intensity in T1WI, volume expansion of radiated lesions, or edematous changes in T2WI. Not all hemorrhages were symptomatic; however, we recorded the data for further analysis.
It is not uncommon for anti-epileptic drugs (AEDs) to be taken for at least 2 years after GKS for seizure control in our protocol of treating cavernoma-related epilepsy (CRE). Prior to GKS, 36 patients with 39 CCMs presented with seizure, including 30 patients with AEDs control and 9 patients with drug-resistant epilepsy (Table 2). The definition of drug-resistant epilepsy is the failure of two antiepileptic trials that were tolerated, chosen appropriately, and administered (either individually or together) in order to achieve a seizure-free state28. Engel’s classification was used to evaluate the effectiveness of GKS in seizure control29.
Acknowledgements
This work was financially supported in part by the Ministry of Science and Technology, Taiwan, under the project MOST 107-2314-B-075 -059 and MOST108-2321-B-010-012-MY2. The work was also supported from Veterans General Hospitals and University System of Taiwan Joint Research Program (VGHUST108-G1-1-3).
Author contributions
Conception and design: Lee, Wang, Yang. Acquisition of data: Lee, Yang, C.J. Lin, Y.Y. Lin, Wu, Hu, Chung, Shiau, Guo, Pan, Hsu. Analysis and interpretation of data: C.J. Chen, Chou. Drafting the article: Lee, Hsu. Reviewed submitted version of manuscript: Lee, Hsu. Statistical analysis: Y.W. Chen. Administrative/technical/material support: Chou, Liu, Shiau, Chung, Pan, Guo, Hsu. Study supervision: Hsu, Chung, Guo, Pan, Shiau, Wu.
Data availability
All data generated or analysed during this study are included in this published article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Liu K-D, et al. Gamma knife surgery for cavernous hemangiomas: an analysis of 125 patients. J. neurosurgery. 2005;102:81–86. doi: 10.3171/sup.2005.102.s_supplement.0081. [DOI] [PubMed] [Google Scholar]
- 2.Lee C-C, et al. Brainstem cavernous malformations: the role of gamma knife surgery. J. neurosurgery. 2012;117:164–169. doi: 10.3171/2012.8.GKS121066. [DOI] [PubMed] [Google Scholar]
- 3.Frischi J. Cavernous malformations of the brain stem. a review of 139 cases. Acta Neurochir (Wien) 1994;130:35–46. doi: 10.1007/BF01405501. [DOI] [PubMed] [Google Scholar]
- 4.Porter RW, et al. Cavernous malformations of the brainstem: experience with 100 patients. J. neurosurgery. 1999;90:50–58. doi: 10.3171/jns.1999.90.1.0050. [DOI] [PubMed] [Google Scholar]
- 5.Robinson JR, Jr, Awad IA, Magdinec M, Paranandi L. Factors predisposing to clinical disability in patients with cavernous malformations of the brain. Neurosurgery. 1993;32:730–736. doi: 10.1227/00006123-199305000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Cantu C, et al. Predictive factors for intracerebral hemorrhage in patients with cavernous angiomas. Neurol. research. 2005;27:314–318. doi: 10.1179/016164105X39914. [DOI] [PubMed] [Google Scholar]
- 7.Kondziolka D, Lunsford LD, Kestle JR. The natural history of cerebral cavernous malformations. J. neurosurgery. 1995;83:820–824. doi: 10.3171/jns.1995.83.5.0820. [DOI] [PubMed] [Google Scholar]
- 8.Moriarity JL, et al. The natural history of cavernous malformations: a prospective study of 68 patients. Neurosurgery. 1999;44:1166–1173. [PubMed] [Google Scholar]
- 9.Ferroli P, et al. Brainstem cavernomas: long-term results of microsurgical resection in 52 patients. Neurosurgery. 2005;56:1203–1214. doi: 10.1227/01.NEU.0000159644.04757.45. [DOI] [PubMed] [Google Scholar]
- 10.Isamat F, Conesa G. Cavernous angiomas of the brain stem. Neurosurg. Clin. 1993;4:507–518. doi: 10.1016/S1042-3680(18)30574-6. [DOI] [PubMed] [Google Scholar]
- 11.Nataf F, et al. Brainstem cavernomas: surgical experience at the ch sainte-anne general hospital. Neuro-Chirurgie. 2007;53:192–201. doi: 10.1016/j.neuchi.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Sandalcioglu I, Wiedemayer H, Secer S, Asgari S, Stolke D. Surgical removal of brain stem cavernous malfor- mations: surgical indications, technical considerations, and results. J. Neurol. Neurosurg. & Psychiatry. 2002;72:351–355. doi: 10.1136/jnnp.72.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang C-c, Liu A, Zhang J-t, Sun B, Zhao Y-l. Surgical management of brain-stem cavernous malformations: report of 137 cases. Surg. neurology. 2003;59:444–454. doi: 10.1016/S0090-3019(03)00187-3. [DOI] [PubMed] [Google Scholar]
- 14.Abla AA, Turner JD, Mitha AP, Lekovic G, Spetzler RF. Surgical approaches to brainstem cavernous malformations. Neurosurg. focus. 2010;29:E8. doi: 10.3171/2010.6.FOCUS10128. [DOI] [PubMed] [Google Scholar]
- 15.Kondziolka, D., Flickinger, J. C. & Lunsford, L. D. Radiosurgery for cavernous malformations. In Radiosurgery and Pathological Fundamentals, 20, 220–230 (Karger Publishers, 2007). [DOI] [PubMed]
- 16.Kondziolka D, Lunsford LD, Flickinger JC, Kestle JR. Reduction of hemorrhage risk after stereotactic radio- surgery for cavernous malformations. J. neurosurgery. 1995;83:825–831. doi: 10.3171/jns.1995.83.5.0825. [DOI] [PubMed] [Google Scholar]
- 17.Kupersmith MJ, et al. Natural history of brainstem cavernous malformations. Neurosurgery. 2001;48:47–54. doi: 10.1097/00006123-200101000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Gewirtz RJ, Steinberg GK, Crowley R, Levy RP. Pathological changes in surgically resected angiographically occult vascular malformations after radiation. Neurosurgery. 1998;42:738–741. doi: 10.1097/00006123-199804000-00031. [DOI] [PubMed] [Google Scholar]
- 19.Nyáry I, Major O, Hanzély Z, Szeifert GT. Histopathological findings in a surgically resected thalamic cavernous hemangioma 1 year after 40-gy irradiation. J. neurosurgery. 2005;102:56–58. doi: 10.3171/sup.2005.102.s_supplement.0056. [DOI] [PubMed] [Google Scholar]
- 20.Kida Y, et al. Radiosurgery for symptomatic cavernous malformations: A multi-institutional retrospective study in japan. Surg. neurology international. 2015;6:S249. doi: 10.4103/2152-7806.157071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liák R, et al. Gammakniferadiosurgeryofthebrainstemcavernomas. min-MinimallyInvasive Neurosurg. 2000;43:201–207. doi: 10.1055/s-2000-11378. [DOI] [Google Scholar]
- 22.Kida, Y. Radiosurgery for cavernous malformations in basal ganglia, thalamus and brainstem. In Japanese Experience with Gamma Knife Radiosurgery, 22, 31–37 (Karger Publishers, 2009). [DOI] [PubMed]
- 23.Monaco EA, et al. Stereotactic radiosurgery for the treatment of symptomatic brainstem cavernous malformations. Neurosurg. focus. 2010;29:E11. doi: 10.3171/2010.7.FOCUS10151. [DOI] [PubMed] [Google Scholar]
- 24.Nagy G, et al. Stereotactic radiosurgery for deep-seated cavernous malformations: a move toward more active, early intervention. J. neurosurgery. 2010;113:691–699. doi: 10.3171/2010.3.JNS091156. [DOI] [PubMed] [Google Scholar]
- 25.Kim D-S, Park Y-G, Choi J-U, Chung S-S, Lee K-C. An analysis of the natural history of cavernous malfor- mations. Surg. neurology. 1997;48:9–17. doi: 10.1016/S0090-3019(96)00425-9. [DOI] [PubMed] [Google Scholar]
- 26.Menzler K, et al. Epileptogenicity of cavernomas depends on (archi-) cortical localization. Neurosurgery. 2010;67:918–924. doi: 10.1227/NEU.0b013e3181eb5032. [DOI] [PubMed] [Google Scholar]
- 27.Casazza M, et al. Supratentorial cavernous angiomas and epileptic seizures: preoperative course and postoperative outcome. Neurosurgery. 1996;39:26–34. doi: 10.1097/00006123-199607000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Kwan P, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc task force of the ilae commission on therapeutic strategies. Epilepsia. 2010;51:1069–1077. doi: 10.1111/j.1528-1167.2009.02397.x. [DOI] [PubMed] [Google Scholar]
- 29.Engel J, Jr, Levesque M, Shields W. Surgical treatment of the epilepsies: presurgical evaluation. Clin. neurosurgery. 1992;38:514. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.