Abstract
Conventional mice are protected from Campylobacter jejuni infection by the murine host-specific gut microbiota composition. We here addressed whether peroral fecal microbiota transplantation (FMT) might be an antibiotics-independent option to lower even high gastrointestinal C. jejuni loads in the infected vertebrate host. To address this, secondary abiotic mice were generated by broad-spectrum antibiotic treatment and perorally infected with C. jejuni by gavage. One week later, mice were stably colonized with more than 109 C. jejuni and subjected to peroral FMT from murine donors on three consecutive days. Two weeks post-intervention, gastrointestinal C. jejuni loads were up to 7.5 orders of magnitude lower following murine FMT versus mock challenge. Remarkably, FMT reversed C. jejuni induced colonic epithelial apoptosis, but enhanced proliferative and regenerative responses in the colon thereby counteracting pathogenic cell damage. Furthermore, FMT dampened both, innate and adaptive immune cell responses in the large intestines upon C. jejuni infection that were accompanied by less C. jejuni-induced colonic nitric oxide secretion. Our study provides strong evidence that novel probiotic formulations developed as alternative option to FMT in severe intestinal inflammatory morbidities including Clostridoides difficile infection might be effective to treat campylobacteriosis and lower pathogen loads in colonized vertebrates including farm animals.
Subject terms: Bacterial infection, Bacteriology, Pathogens
Introduction
Campylobacter jejuni constitute the most common agents of bacterial gastroenteritis in industrialized nations, causing more than 240,000 cases in the European Union per year1. According to the European Food Safety Authority (EFSA) and the European Center for Disease Prevention and Control (ECDC), campylobacteriosis represents the most reported zoonosis in the European Union, by far outnumbering salmonellosis, yersinosis and diseases caused by pathogenic variants of Escherichia coli2. The Gram-negative bacteria are transmitted to humans via contaminated food products including meat, milk and eggs from livestock animals, particularly poultry, or surface water. Up to 40% of fresh broiler and turkey meat have been tested positive for Campylobacter species recently2. Whereas C. jejuni is equipped with a multi-facetted arsenal of virulence factors, humans can be infected with an infectious dose of 500 to 800 bacterial cells only, regardless of their health conditions3–5. Following predominant colonization of the terminal ileum and colon, the pathogen invades colonic epithelial cells and induces mucosal pro-inflammatory immune responses leading to crypt abscesses and focal ulcerations6–8. After an incubation period of 2 to 5 days, infected patients present with a broad variety of symptoms ranging from only mild malaise to fever, abdominal cramps, myalgia, and watery or even bloody diarrhea7–9. In rare cases, however, post-infectious sequelae such as Guillain-Barré syndrome, Bickerstaff encephalitis, Miller Fisher syndrome, Reiter’s syndrome and chronic intestinal inflammatory morbidities might arise with a latency of weeks to months10,11. Whereas the majority of infections is self-limited and require symptomatic treatment such as fluid replacement only, particularly infected multi-morbid patients with immune-suppressive comorbidities are subjected to antibiotic treatment. This intervention, however, is paid by expense of the potential antibiotics-induced collateral damages leading to a compromised commensal gut microbiota composition subsequently facilitating (opportunistic) pathogenic colonization and infection of the gastrointestinal tract, for instance12–15 besides to unwanted effects to the vertebrate immune system16. It is thus utmost appreciable to search for antibiotics-independent approaches for the prevention and treatment of C. jejuni colonization and infection in farm animals and humans, respectively.
In this context, one needs to take into consideration that it is the host-specific composition of the commensal gut microbiota determining whether the vertebrate host is susceptible towards or resistant against C. jejuni infection. Conventionally colonized wildtype mice, for instance, are protected from stable C. jejuni colonization and infection even upon peroral infection with high pathogenic doses17–19. Following murine microbiota depletion and hence, abrogation of the physiological colonization resistance by broad-spectrum antibiotic treatment, however, secondary abiotic mice, but also secondary abiotic mice that had been reconstituted with a complex human as opposed to a murine gut microbiota following fecal microbiota transplantation (FMT) could be stably colonized with the pathogen at high loads, but did not develop overt pathogen-induced symptoms such as wasting or bloody diarrhea17.
Therapeutic application of FMT has been already reported since the fourth century during the Chinese Dong-jin dynasty20. Lately, FMT has experienced a renaissance as a promising treatment option of recurrent and refractory Clostridoides difficile toxin mediated pseudomembranous colitis in antibiotics-pretreated patients, of inflammatory bowel morbidities such as ulcerative colitis, irritable bowel syndrome and constipation, of obesity and diabetes and of chronic fatigue syndrome21–25.
Based upon these intriguing results in mice and men, we addressed in the present antibiotic-independent intervention/treatment study whether murine FMT in secondary abiotic mice that were harboring the pathogen in their gastrointestinal tract (GIT) at high loads could i.) sufficiently lower pathogenic burdens, ii.) alleviate gut epithelial cell damage and iii.) dampen intestinal pro-inflammatory immune responses upon C. jejuni infection. Our preclinical intervention study provides strong evidence that FMT from distinct donors might represent promising options for the prevention and/or treatment of C. jejuni colonization and/or infections in farm animals and/or humans, respectively.
Results
Gastrointestinal pathogen burdens following murine fecal microbiota transplantation in C. jejuni infected secondary abiotic mice
Secondary abiotic mice were perorally infected with 109 viable C. jejuni strain 81–176 by gavage on days 0 and 1 and harbored median loads of approximately 109 colony forming units (CFU) of the pathogen per g feces in both, the FMT and mock intervention cohorts a week later. On days 7, 8 and 9 post-infection (p.i.), mice were either subjected to murine FMT or received vehicle (D0, D1, D2). Cultural analyses of fecal samples revealed that as early as 24 hours after the latest murine FMT (i.e., 10 days post-infection), fecal C. jejuni loads were significantly lower in the FMT versus the mock cohort (p < 0.01; Fig. 1). Until the end of the observation period (i.e., two weeks after the initial FMT; 21 days post-infection), median intestinal pathogenic burdens had decreased by up to 5 log orders of magnitude following murine FMT (p < 0.001, Fig. 1; Supplementary Information), but remained stable in mock control mice (n.s.; Supplementary Information). Of note, 12.5% of mice from the FMT cohort, but none of the mock controls had completely cleared the pathogen from their intestines (Supplementary Information).
Figure 1.
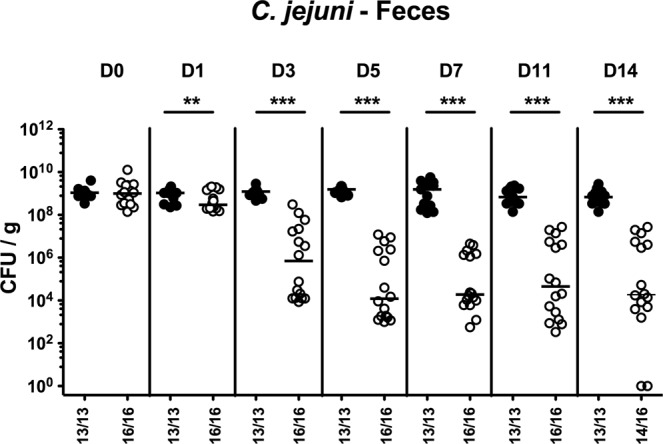
Intestinal pathogenic burdens over time following murine fecal microbiota transplantation of C. jejuni infected secondary abiotic mice. Secondary abiotic mice were infected with C. jejuni on days 0 and 1 by gavage. Starting a week later, infected mice were subjected to peroral fecal microbiota transplantation (FMT; Day (D) 0) from murine donors (open circles) on three consecutive days or received vehicle (mock; closed circles). Before and after FMT, C. jejuni loads were determined in fecal samples taken at indicated time points by culture and expressed as colony forming units per gram (CFU/g). Medians, numbers of culture-positive mice out of the total numbers of analyzed animals (in parentheses) and significance levels (p-values; **p < 0.01; ***p < 0.001) determined by the Mann-Whitney U test are given. Data were pooled from three independent experiments.
Upon necropsy (i.e., 21 days post-infection, 14 days post-FMT), we assessed C. jejuni loads alongside the GIT. Following murine FMT, C. jejuni counts were lower in luminal samples taken from stomach, duodenum, ileum, and colon as compared to mock control mice (p < 0.001; Fig. 2). Our culture-independent analyses further confirmed that the murine microbiota had sufficiently established in the large intestines of mice following FMT given that gene numbers of the main bacterial species and groups abundant in the murine gut microbiota including enterobacteria, enterococci, lactobacilli, bifidobacteria, Bacteroides/Prevotella species, Clostridium coccoides group, Clostridium leptum group, and Mouse Intestinal Bacteroides were comparable in the murine fecal donor transplants and in the gut microbiota following murine FMT (Fig. 3; Supplementary Information).
Figure 2.
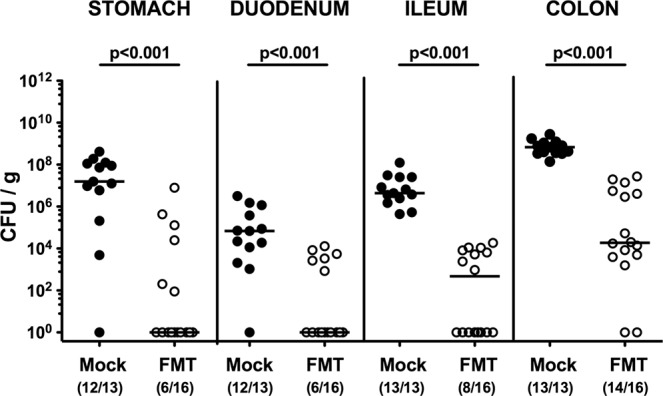
Gastrointestinal pathogenic loads following murine fecal microbiota transplantation of C. jejuni infected secondary abiotic mice. Secondary abiotic mice were infected with C. jejuni on days 0 and 1 by gavage. Starting a week later, infected mice were subjected to peroral fecal microbiota transplantation (FMT) from murine donors (open circles) on three consecutive days or received vehicle (mock; closed circles). On day 21 post-infection (i.e., 14 days post FMT), C. jejuni loads were determined in luminal samples taken from stomach, duodenum, ileum, and colon by culture and expressed as colony forming units per gram (CFU/g). Medians, numbers of pathogen-positive mice out of the total number of analyzed animals (in parentheses) and significance levels (p-values) determined by the Mann-Whitney U test are given. Data were pooled from three independent experiments.
Figure 3.
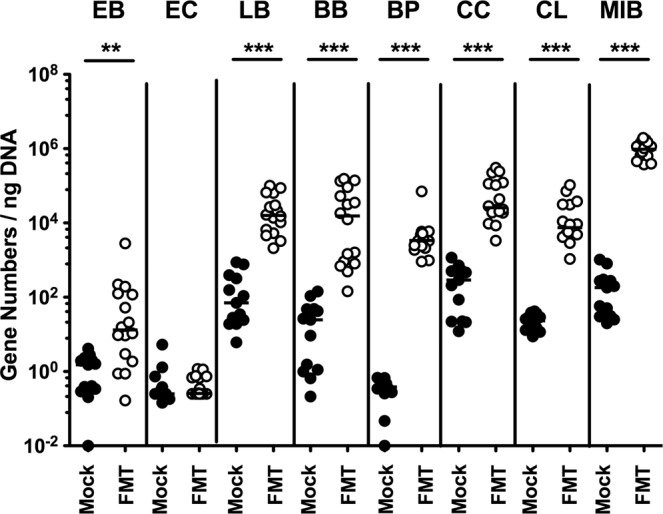
Fecal commensal microbiota composition following fecal microbiota transplantation in C. jejuni infected secondary abiotic mice. Secondary abiotic mice were infected with C. jejuni on days 0 and 1 by gavage. Starting a week later, infected mice were subjected to peroral fecal microbiota transplantation (FMT) from murine donors (open circles; n = 16) on three consecutive days or received vehicle (mock; closed circles; n = 13). On day 21 post-infection (i.e., 14 days post FMT), the fecal commensal microbiota composition was surveyed by culture-independent 16S rRNA methods quantitating the main bacterial groups and species including enterobacteria (EB), enterococci (EC), lactobacilli (LB), bifidobacteria (BB), Bacteroides/Prevotella (BP) species, Clostridium coccoides group (CC), Clostridium leptum group (CL) and Mouse Intestinal Bacteroides (MIB) and expressed as gene numbers per ng DNA. Medians and significance levels (p-values; **p < 0.01; ***p < 0.001) determined by the Mann-Whitney U test are given. Data were pooled from three independent experiments.
Thus, FMT could sufficiently lower gastrointestinal pathogenic loads in C. jejuni infected mice, even upon high-dose infection.
Apoptotic and proliferating colonic epithelial cell responses following murine fecal microbiota transplantation in C. jejuni infected secondary abiotic mice
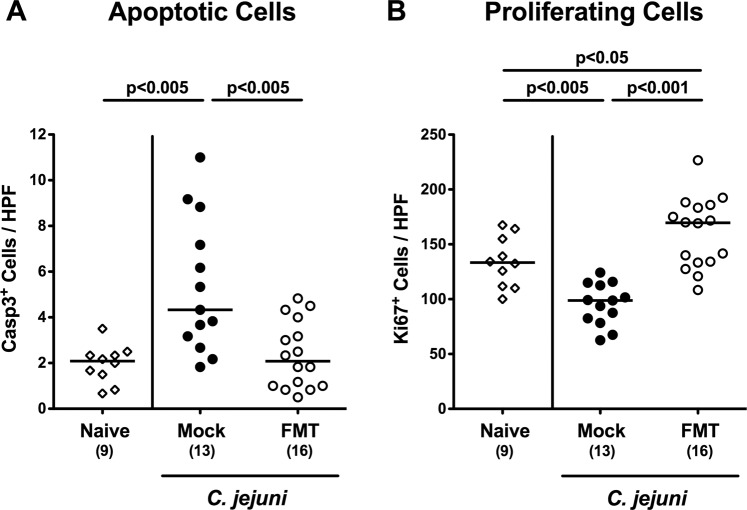
In order to address whether murine FMT resulted in less distinct C. jejuni induced intestinal cell damage, we quantitatively assessed cleaved caspase3+ apoptotic cells in colonic paraffin sections applying in situ immunohistochemistry. Whereas within 21 days C. jejuni had induced marked increases in apoptotic colonic epithelial cell numbers in mock control mice (p < 0.005; Fig. 4A; Supplementary Information), this was not the case following murine FMT (p < 0.005 vs mock; Fig. 4A; Supplementary Information). We further quantitated Ki67+ colonic epithelial cells indicative for cell proliferation and regeneration. Whereas Ki67+ colonic epithelial cells decreased upon C. jejuni infection of mock control animals (p < 0.005) proliferating cell numbers were even higher in infected mice of the FMT cohort as compared to uninfected and untreated (i.e., naive) mice (p < 0.05; Fig. 4B; Supplementary Information). Thus, murine FMT prevented from C. jejuni induced colonic epithelial apoptosis and enhanced proliferative and regenerative cell responses in the large intestines counteracting pathogenic cell damage.
Figure 4.
Apoptotic and proliferating colonic epithelial cell responses upon fecal microbiota transplantation of C. jejuni infected secondary abiotic mice. Secondary abiotic mice were infected with C. jejuni on days 0 and 1 by gavage. Starting a week later, infected mice were subjected to peroral fecal microbiota transplantation (FMT) from murine donors (open circles) on three consecutive days or received vehicle (mock; closed circles). On day 21 post-infection (i.e., 14 days post FMT), the average numbers of (A) apoptotic (caspase3, Casp3+) and (B) proliferating (Ki67+) colonic epithelial cells were determined from six high power fields (HPF, 400 × magnification) per mouse in immunohistochemically stained large intestinal paraffin sections. Naive secondary abiotic mice served as uninfected and untreated controls (open diamonds). Medians, numbers of analyzed animals (in parentheses) and significance levels (p-values) determined by the Mann-Whitney U test are given. Data were pooled from three independent experiments.
Large intestinal inflammatory immune responses upon murine fecal microbiota transplantation of C. jejuni infected mice
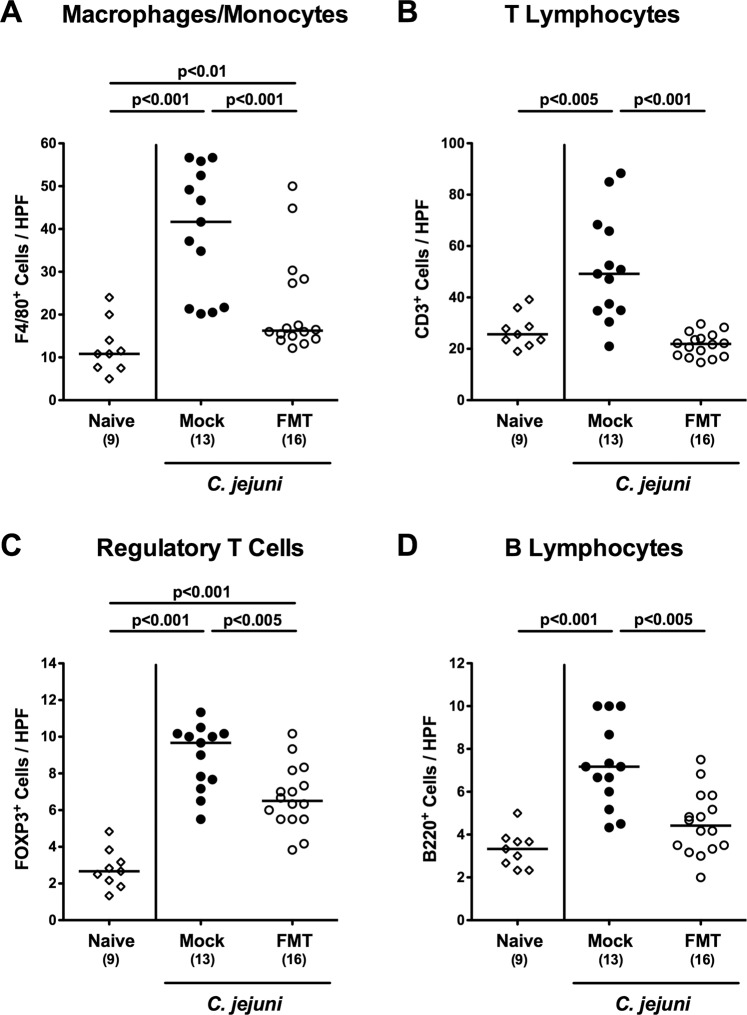
We next surveyed innate and adaptive immune cell responses in the large intestines of C. jejuni infected mice following FMT applying quantitative in situ immunohistochemistry. At day 21 p.i., mice displayed multi-fold increased numbers of innate immune cell subsets such as F4/80+ macrophages and monocytes in their colonic mucosa and lamina propria (p < 0.01–0.001 vs naive), but far less distinctly in mice of the FMT cohort (p < 0.001 vs mock; Fig. 5A; Supplementary Information). Furthermore, adaptive immune cell populations such as CD3+ lymphocytes and B220+ B lymphocytes had increased within 21 days post-infection of mock (p < 0.005 and p < 0.001 vs naive, respectively), but not FMT treated mice (n.s. vs naive; p < 0.005–0.001 vs mock; Fig. 5B,D; Supplementary Information). As for F4/80+ cells, C. jejuni induced increases in FOXP3+ regulatory T cell numbers were less pronounced in the colonic mucosa and lamina propria following FMT versus mock challenge at day 21 p.i. (p < 0.005; Fig. 5C; Supplementary Information).
Figure 5.
Colonic immune cells responses upon fecal microbiota transplantation of C. jejuni infected secondary abiotic mice. Secondary abiotic mice were infected with C. jejuni on days 0 and 1 by gavage. Starting a week later, infected mice were subjected to peroral fecal microbiota transplantation (FMT) from murine donors (open circles) on three consecutive days or received vehicle (mock; closed circles). On day 21 post-infection (i.e., 14 days post FMT), the average numbers of (A) macrophages and monocytes (F4/80+), (B) T lymphocytes (CD3+), (C) regulatory T cells (FOXP3+), and (D) B lymphocytes (B220+) in the large intestinal mucosa and lamina propria were determined from six high power fields (HPF, 400 x magnification) per mouse in immunohistochemically stained large intestinal paraffin sections. Naive secondary abiotic mice served as uninfected and untreated controls (open diamonds). Medians, numbers of analyzed animals (in parentheses) and significance levels (p-values) determined by the Mann-Whitney U test are given. Data were pooled from three independent experiments.
We further addressed whether less distinct colonic immune cell responses upon FMT of C. jejuni infected mice were accompanied with less intestinal pro-inflammatory mediator secretion. In fact, nitric oxide concentrations were elevated in colonic ex vivo biopsies taken from mock control animals (p < 0.001 vs naive), but not from mice of the FMT cohort at day 21 p.i. (p < 0.001 vs mock; Fig. 6).
Figure 6.
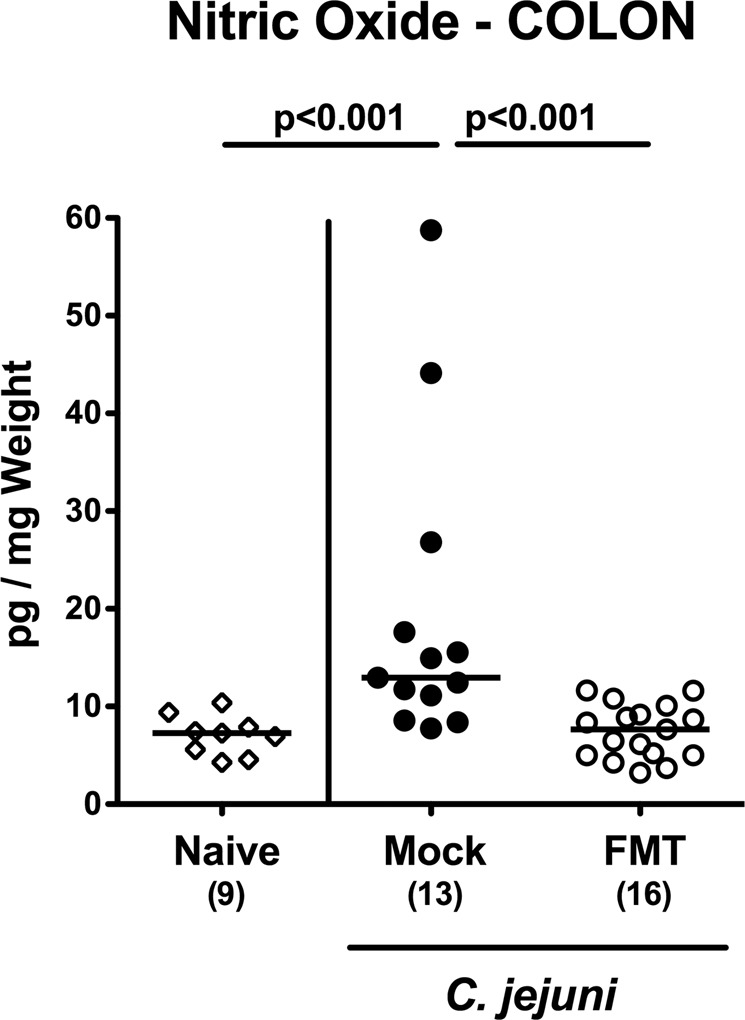
Colonic nitric oxide secretion upon fecal microbiota transplantation of C. jejuni infected secondary abiotic mice. Secondary abiotic mice were infected with C. jejuni on days 0 and 1 by gavage. Starting a week later, infected mice were subjected to peroral fecal microbiota transplantation (FMT) from murine donors (open circles) on three consecutive days or received vehicle (mock; closed circles). On day 21 post-infection (i.e., 14 days post FMT), nitric oxide concentrations were measured in supernatants of colonic ex vivo biopsies. Naive secondary abiotic mice served as uninfected and untreated controls (open diamonds). Medians, numbers of analyzed animals (in parentheses) and significance levels (p-values) determined by the Mann-Whitney U test are given. Data were pooled from three independent experiments.
Thus, murine FMT could dampen both, innate and adaptive immune responses in the large intestines upon C. jejuni infection that was accompanied by less C. jejuni induced colonic nitric oxide secretion. Furthermore, murine FMT promoted Ki67 positive cell regenerative properties also in the colon of C. jejuni infected mice.
Discussion
The physiological colonization resistance protecting the vertebrate host from unwanted pathogenic colonization and infection provides us promising opportunities to identify distinct molecules and gut bacterial populations as potential novel options for the prevention and treatment of campylobacterisis and its post-infectious sequelae. We are currently, however, still at the beginning of understanding the underlying mechanisms. In our actual antibiotics-independent intervention study applying a murine high dose C. jejuni infection model, we were able to show that murine FMT treatment of secondary abiotic mice harboring very high pathogenic loads of approximately 109 viable bacteria per g intestinal luminal sample lowered gastrointestinal C. jejuni loads up to 7.5 orders of magnitude within two weeks post-intervention. This pronounced pathogen-lowering effect is even more intriguing when considering the low infectious doses of a few hundred bacterial cells needed to induce campylobacteriosis in humans5. Besides the substantial pathogen-lowering effect, murine FMT did also affect the murine C. jejuni carrier rates given that 12.5% of high-dose infected mice had even completely expelled the pathogen from their GIT. Remarkably, FMT could effectively reverse C. jejuni induced colonic epithelial apoptosis, and enhance proliferative and regenerative cell responses in the large intestines thereby counteracting pathogenic cell damage. Furthermore, FMT dampened both, innate and adaptive immune cell responses in the large intestines upon C. jejuni infection that was accompanied by less secretion of nitric oxide exerting oxidative stress to the intestinal epithelia26,27. In support, we were able to show very recently that murine FMT could decrease intestinal loads of the Gram-negative opportunistic pathogen Pseudomonas aeruginosa in murine carriers28. The immune cell function restoring properties of murine FMT in secondary abiotic mice was convincingly shown by Ekmekciu and coworkers previously. Whereas gut microbiota depletion by broad-spectrum antibiotic treatment resulted in profound changes of the immune cell repertoire in local intestinal and systemic compartments, reintroduction of the complex murine gut microbiota by peroral FMT could reverse the collateral damages of antibiotic treatment16.
A key question is whether the entire complex gut microbiota, defined bacterial species alone or in combination, and/or distinct molecules shaping a (for the pathogen) hostile intraluminal milieu within the GIT are needed to expel the pathogen from already taken ecological niches. Our recent study revealed that application of a single Lactobacillus johnsonii strain that had been isolated from a healthy conventional C57BL/6j mouse resulted in ameliorated intestinal, extra-intestinal and even systemic immune responses upon high-dose C. jejuni infection of secondary abiotic mice in a time-of-treatment dependent fashion29, but without having a pathogen-lowering effect. Ekmekciu et al. further addressed the capabilities of defined Gram-positive and Gram-negative intestinal commensals such as Lactobacillus johnsonii and Escherichia coli, respectively, to restore cellular immune functions as compared to complex microbiota in mice that had been subjected to broad-spectrum antibiotic treatment with subsequent immune-suppressive sequelae30. In fact, FMT appeared to be most potent to reverse antibiotics-induced immune dysfunction, whereas respective commensal species could effectively restore individual functions of both, intestinal and systemic immunity. Furthermore, recolonization of secondary abiotic mice with the probiotic formulation VSL#3 consisting of eight bacterial strains (i.e., four different Lactobacillus species, three Bifidobacterium species and Streptococcus thermophilus), has been shown to reverse antibiotics-induced immune cellular dysfunction and to provide anti-inflammatory responses in intestinal mucosal and systemic compartments31. Notably, probiotic VSL#3 treatment of C. jejuni infected secondary abiotic mice could alleviate intestinal and extra-intestinal including systemic sequelae of infection32.
Nevertheless, it appears to be rather a search for the needle in the hay-stack when trying to identify effective pathogen-lowering and immune-modulatory (probiotic) bacterial strains. It is rather more likely, that literally, the orchestrated interplay of commensals with the immune system as the conductor constitutes the most promising approach to tackle intestinal pathogenic colonization/infection of vertebrates.
From disease-preventive strategies of human campylobacteriosis, however, it is of even more importance to lower the intestinal C. jejuni loads in poultry and other livestock animals for subsequent risk reduction of pathogenic transmission to human via the food chain. In support, both, prophylactic and therapeutic application of a commercial bacterial formulation (Aviguard®) has been successfully shown to compete with intestinal colonization and expansion, respectively, of chicken with unwanted bacteria such as Clostridium perfringens33,34 and Salmonella typhimurium35 additionally resulting in less distinct intestinal inflammatory sequelae of colonization/infection. We are currently evaluating potential intestinal pathogenic burden lowering and anti-inflammatory effects of prophylactic and therapeutic application in high-dose C. jejuni infected mice.
In conclusion
Our intervention study provides strong evidence that active modulation of the microbiota composition by murine FMT might be considered an effective measure to lower even high intestinal C. jejuni loads and dampen pathogen-induced inflammatory immune responses in colonized/infected vertebrates including farm animals. Subsequent application-oriented efforts to develop pharmaceutical probiotic formulations that might even replace FMT in clinical practice36 would provide novel options for a safe, easy-to-handle and (hopefully) cost-efficient treatment of C. jejuni infections in humans. From the prophylactic perspective, efficient reduction of C. jejuni colonization in farm animals would minimize the risk of pathogenic transmission via the food chain, of human infection and of post-infectious sequelae.
Materials and Methods
Ethical statement
Mouse experiments were conducted in accordance with the European Guidelines for animal welfare (2010/63/EU) after approval by the commission for animal experiments headed by the “Landesamt für Gesundheit und Soziales” (LaGeSo, Berlin, registration numbers G0097/12 and G0039/15. One a day clinical conditions of mice were monitored.
Generation of secondary abiotic mice
Conventional female and male C57BL/6j mice were bred and reared under specific pathogen free (SPF) conditions in the same unit of the Forschungseinrichtungen für Experimentelle Medizin (FEM, Charité - University Medicine Berlin). In order to counteract physiological colonization resistance, secondary abiotic mice with a depleted gut microbiota were generated as described previously17,26. In brief, upon weaning 3-week old mice were placed into autoclaved cages and subjected to broad-spectrum antibiotic treatment for 8 weeks (ampicillin plus sulbactam (1 g/L; Ratiopharm, Germany), vancomycin (500 mg/L; Cell Pharm, Germany), ciprofloxacin (200 mg/L; Bayer Vital, Germany), imipenem (250 mg/L; MSD, Germany) and metronidazole (1 g/L; Fresenius, Germany)) added to autoclaved tap water. To control the intestinal colonization status and absence of cultivable bacteria in the murine intestinal tract, individual fecal samples were derived once a week as well as immediately before the first C. jejuni infection and incubated in thioglycolate broths (Oxoid) for one week at 37 °C. Bacterial growth was monitored daily by turbidity assessment. Aliquots from turbid broths as well as from broths remaining clear after one week of incubation were cultivated on solid media under aerobic, microaerobic and obligate anaerobic conditions and grown bacteria identified microscopically and biochemically as described26. Virtual absence of fastidious and uncultivable bacteria in intestinal tract of secondary abiotic mice has been confirmed in our previous study by quantitative 16S rRNA based PCR analyses showing that bacterial gene numbers in fecal samples following quintuple antibiotic treatment were comparable to those detected in autoclaved food pellets16. Three days before start of the infection experiments the antibiotic cocktail was replaced by autoclaved tap water (ad libitum).
C. jejuni infection and colonization properties
At days 0 and 1, sex and age matched secondary abiotic mice were perorally infected with 109 colony forming units (CFU) of the C. jejuni 81–176 strain by gavage (in a total volume of 0.3 mL phosphate buffered saline (PBS, Gibco, Life Technologies, UK)). Animals remained in a sterile environment (with autoclaved food and drinking water or sterile antibiotic cocktail) and handled under strictly aseptic conditions to avoid contaminations.
C. jejuni loads were quantitatively assessed in fecal samples over time post-infection and upon necropsy (i.e., at day 21 p.i.) in luminal samples derived from the stomach, duodenum, ileum, and colon by culture as reported earlier17,37. The detection limit of viable pathogens was 100 CFU per g.
Fecal microbiota transplantation
On days 7, 8 and 9 p.i., mice were subjected to peroral FMT from murine donors on three consecutive days. Fresh murine fecal samples were collected from 10 age and sex matched SPF control mice, pooled, dissolved in 10 mL sterile PBS and the supernatant taken as murine donor suspension. Aliquots from each murine fecal donor solutions were collected for quantitative molecular analyses of main intestinal bacterial communities as reported previously17,26,38 (Supplementary Information).
Molecular analysis of gut microbiota composition
DNA was extracted from fecal and colonic luminal samples as well as from fecal docor suspensions as described previously26,39. In brief, DNA was quantified by using Quant-iT PicoGreen reagent (Invitrogen, UK) and adjusted to 1 ng per µL. Then, the main bacterial species and groups abundant in the murine gut microbiota including enterobacteria, enterococci, lactobacilli, bifidobacteria, Bacteroides/Prevotella spp., Clostridium coccoides group, Clostridium leptum group, and Mouse Intestinal Bacteroides were assessed by quantitative real-time polymerase chain reaction (qRT-PCR) with species-, genera- or group-specific 16S rRNA gene primers (Tib MolBiol, Germany) as stated elsewhere17,38,40 and expressed as numbers of 16S rRNA gene copies per ng DNA.
Sampling procedures
Mice were sacrificed at day 21 p.i. (i.e., 14 days post-FMT) by isofluran inhalation (Abbott, Germany). Luminal samples were derived from distinct parts of the GIT (i.e., from stomach, duodenum ileum, and colon) and ex vivo biopsies from the large intestines were taken under sterile conditions. From each mouse colonic samples were collected in parallel for microbiological, immunohistopathological and immunological analyses.
Immunohistochemistry
Colonic ex vivo biopsies were immediately fixed in 5% formalin, embedded in paraffin and subjected to in situ immunohistochemical analyses as stated elsewhere38,41–43. In brief, apoptotic epithelial cells, proliferating epithelial cells, macrophages/monocytes, T lymphocytes, regulatory T cells, and B lymphocytes were quantitated in paraffin sections (5 μm) that had been stained with primary antibodies against cleaved caspase 3 (Asp175, Cell Signaling, Beverly, MA, USA, 1:200), Ki67 (TEC3, Dako, Denmark, 1:100), F4/80 (# 14–4801, clone BM8, eBioscience, San Diego, CA, USA, 1:50), CD3 (#N1580, Dako, 1:10), FOXP3 (FJK-16s, eBioscience, 1:100), and B220 (No. 14-0452-81, eBioscience; 1:200), respectively. Positively stained cells were counted by a blinded independent investigator (light microscopy, magnification 100 x and 400 x), and the average number of respective positively stained cells for each mouse determined within at least six high power fields (HPF, 0.287 mm2, 400 x magnification).
Nitric oxide measurement
Colonic ex vivo biopsies were cut longitudinally, washed in PBS, and strips of approximately 1 cm2 tissues were placed in 24-flat-bottom well culture plates (Nunc, Germany) containing 500 μL serum-free RPMI 1640 medium (Gibco, life technologies, UK) supplemented with penicillin (100 U/mL) and streptomycin (100 µg/mL; PAA Laboratories, Germany). After 18 h at 37 °C, culture supernatants were subjected to nitric oxide concentration measurements applying the Griess reaction as stated elsewhere26,27.
Statistical analysis
Medians and levels of significance were determined using Mann-Whitney test (GraphPad Prism v7, USA) as indicated. Two-sided probability (p) values ≤ 0.05 were considered significant. Experiments were reproduced twice.
Supplementary information
Acknowledgements
We thank Alexandra Bittroff-Leben, Ines Puschendorf, Ulrike Fiebiger, Ulrike Escher, Sarah Schimmelpfennig, Sigri Kløve, Gernot Reifenberger, and the staff of the animal research facility at Charité - University Medicine Berlin for excellent technical assistance and animal breeding. We further thank Dr. Anja A. Kühl (Department of Medicine I for Gastroenterology, Infectious Diseases and Rheumatology/Research Center ImmunoSciences (RCIS), Charité – Universitätsmedizin Berlin) for taking representative photomicropgraphs of immunhistochmically stained paraffin sections. We acknowledge support from the German Research Foundation (DFG) and the Open Access Publication Fund of Charité – Universitätsmedizin Berlin. This work was supported by grants to MMH and SB from the German Federal Ministries of Education and Research (BMBF) in frame of the zoonoses research consortium PAC-Campylobacter (IP7/01KI1725D) and from the Federal Ministry for Economic Affairs and Energy following a resolution of the German National Parliament, Deutscher Bundestag (ZIM, ZF4117908 AJ8) to SB and MMH. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author contributions
M.M.H.: Designed and performed experiments, analyzed data, wrote paper. K.M.: Analyzed data, co-edited paper. S.B.: Provided advice in experimental design, critically discussed results, co-edited paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-019-56442-7.
References
- 1.Dicksved J, Ellstrom P, Engstrand L, Rautelin H. Susceptibility to Campylobacter infection is associated with the species composition of the human fecal microbiota. MBio. 2014;5:e01212–01214. doi: 10.1128/mBio.01212-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.European Food Safety, A., European Centre for Disease, P. & Control. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA Journal, 16, e05500, doi:10.2903/j.efsa.2018.5500 (2018). [DOI] [PMC free article] [PubMed]
- 3.Jagannathan A, Constantinidou C, Penn CW. Roles of rpoN, fliA, and flgR in expression of flagella in Campylobacter jejuni. J Bacteriol. 2001;183:2937–2942. doi: 10.1128/JB.183.9.2937-2942.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reuter M, Mallett A, Pearson BM, van Vliet AH. Biofilm formation by Campylobacter jejuni is increased under aerobic conditions. Appl Environ Microbiol. 2010;76:2122–2128. doi: 10.1128/AEM.01878-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Vliet AH, Ketley JM, Park SF, Penn CW. The role of iron in Campylobacter gene regulation, metabolism and oxidative stress defense. FEMS Microbiol Rev. 2002;26:173–186. doi: 10.1016/s0168-6445(02)00095-5. [DOI] [PubMed] [Google Scholar]
- 6.van Spreeuwel JP, et al. Campylobacter colitis: histological immunohistochemical and ultrastructural findings. Gut. 1985;26:945–951. doi: 10.1136/gut.26.9.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker RI, et al. Pathophysiology of Campylobacter enteritis. Microbiol Rev. 1986;50:81–94. doi: 10.1128/mr.50.1.81-94.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ketley JM. Pathogenesis of enteric infection by Campylobacter. Microbiology (Reading, England) 1997;143(Pt 1):5–21. doi: 10.1099/00221287-143-1-5. [DOI] [PubMed] [Google Scholar]
- 9.Kist M, Bereswill S. Campylobacter jejuni. Contrib Microbiol. 2001;8:150–165. doi: 10.1159/000060405. [DOI] [PubMed] [Google Scholar]
- 10.Wakerley BR, Uncini A, Yuki N, Group GBSC, Group GBSC. Guillain-Barre and Miller Fisher syndromes–new diagnostic classification. Nat Rev Neurol. 2014;10:537–544. doi: 10.1038/nrneurol.2014.138. [DOI] [PubMed] [Google Scholar]
- 11.Backert, S., Tegtmeyer, N., Cróinín, T., Boehm, M. & Heimesaat, M. 1–25 (2017).
- 12.von Klitzing E, Ekmekciu I, Kuhl AA, Bereswill S, Heimesaat MM. Multidrug-resistant Pseudomonas aeruginosa aggravates inflammatory responses in murine chronic colitis. Scientific reports. 2018;8:6685. doi: 10.1038/s41598-018-25034-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olsan EE, et al. Colonization resistance: The deconvolution of a complex trait. J Biol Chem. 2017;292:8577–8581. doi: 10.1074/jbc.R116.752295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vogt SL, Finlay BB. Gut microbiota-mediated protection against diarrheal infections. Journal of travel medicine. 2017;24:S39–s43. doi: 10.1093/jtm/taw086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Klitzing E, Ekmekciu I, Bereswill S, Heimesaat MM. Intestinal and Systemic Immune Responses upon Multi-drug Resistant Pseudomonas aeruginosa Colonization of Mice Harboring a Human Gut Microbiota. Front Microbiol. 2017;8:2590. doi: 10.3389/fmicb.2017.02590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ekmekciu I, et al. Immune Responses to Broad-Spectrum Antibiotic Treatment and Fecal Microbiota Transplantation in Mice. Front Immunol. 2017;8:397. doi: 10.3389/fimmu.2017.00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bereswill S, et al. Novel murine infection models provide deep insights into the “menage a trois” of Campylobacter jejuni, microbiota and host innate immunity. PloS one. 2011;6:e20953. doi: 10.1371/journal.pone.0020953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masanta WO, et al. Modification of intestinal microbiota and its consequences for innate immune response in the pathogenesis of campylobacteriosis. Clin Dev Immunol. 2013;2013:526860. doi: 10.1155/2013/526860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fiebiger U, Bereswill S, Heimesaat MM. Dissecting the Interplay Between Intestinal Microbiota and Host Immunity in Health and Disease: Lessons Learned from Germfree and Gnotobiotic Animal Models. Eur J Microbiol Immunol (Bp) 2016;6:253–271. doi: 10.1556/1886.2016.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Faming, Luo Wensheng, Shi Yan, Fan Zhining, Ji Guozhong. Should We Standardize the 1,700-Year-Old Fecal Microbiota Transplantation? American Journal of Gastroenterology. 2012;107(11):1755. doi: 10.1038/ajg.2012.251. [DOI] [PubMed] [Google Scholar]
- 21.Borody TJ, et al. Recurrence of duodenal ulcer and Campylobacter pylori infection after eradication. Med J Aust. 1989;151:431–435. doi: 10.5694/j.1326-5377.1989.tb101251.x. [DOI] [PubMed] [Google Scholar]
- 22.Borody TJ, Khoruts A. Fecal microbiota transplantation and emerging applications. Nat Rev Gastroenterol Hepatol. 2011;9:88–96. doi: 10.1038/nrgastro.2011.244. [DOI] [PubMed] [Google Scholar]
- 23.van Nood, E., Speelman, P., Kuijper, E. J. & Keller, J. J. Struggling with recurrent Clostridium difficile infections: is donor faeces the solution? Euro Surveill, 14, 10.2807/ese.14.34.19316-en (2009). [DOI] [PubMed]
- 24.Vrieze A, et al. The environment within: how gut microbiota may influence metabolism and body composition. Diabetologia. 2010;53:606–613. doi: 10.1007/s00125-010-1662-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manges AR, Steiner TS, Wright AJ. Fecal microbiota transplantation for the intestinal decolonization of extensively antimicrobial-resistant opportunistic pathogens: a review. Infect Dis (Lond) 2016;48:587–592. doi: 10.1080/23744235.2016.1177199. [DOI] [PubMed] [Google Scholar]
- 26.Heimesaat, M. M. et al. Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. Journal of immunology, 177, 8785–8795, doi:177/12/8785 [pii] (2006). [DOI] [PubMed]
- 27.Bryan NS, Grisham MB. Methods to detect nitric oxide and its metabolites in biological samples. Free Radic Biol Med. 2007;43:645–657. doi: 10.1016/j.freeradbiomed.2007.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mrazek K, Bereswill S, Heimesaat MM. Fecal Microbiota Transplantation Decreases Intestinal Loads of Multi-Drug Resistant Pseudomonas aeruginosa in Murine Carriers. Eur J Microbiol Immunol (Bp) 2019;9:14–22. doi: 10.1556/1886.2019.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bereswill S, et al. Lactobacillus johnsonii ameliorates intestinal, extra-intestinal and systemic pro-inflammatory immune responses following murine Campylobacter jejuni infection. Scientific reports. 2017;7:2138. doi: 10.1038/s41598-017-02436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ekmekciu I, et al. Fecal Microbiota Transplantation, Commensal Escherichia coli and Lactobacillus johnsonii Strains Differentially Restore Intestinal and Systemic Adaptive Immune Cell Populations Following Broad-spectrum Antibiotic Treatment. Front Microbiol. 2017;8:2430. doi: 10.3389/fmicb.2017.02430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ekmekciu I, et al. The Probiotic Compound VSL#3 Modulates Mucosal, Peripheral, and Systemic Immunity Following Murine Broad-Spectrum Antibiotic Treatment. Front Cell Infect Microbiol. 2017;7:167. doi: 10.3389/fcimb.2017.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ekmekciu I, Fiebiger U, Stingl K, Bereswill S, Heimesaat MM. Amelioration of intestinal and systemic sequelae of murine Campylobacter jejuni infection by probiotic VSL#3 treatment. Gut Pathog. 2017;9:17. doi: 10.1186/s13099-017-0168-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hofacre CL, et al. Use of Aviguard and other intestinal bioproducts in experimental Clostridium perfringens-associated necrotizing enteritis in broiler chickens. Avian Dis. 1998;42:579–584. doi: 10.2307/1592685. [DOI] [PubMed] [Google Scholar]
- 34.Abudabos, A. Use of a Competitive Exclusion Product (Aviguard®) to Prevent Clostridium perfringens Colonization in Broiler Chicken under Induced Challenge, 45 (2013).
- 35.Nakamura, A. et al. Evaluation of aviguard, a commercial competitive exclusion product for efficacy and after-effect on the antibody response of chicks to Salmonella, 81 (2002). [DOI] [PubMed]
- 36.Suez J, et al. Post-Antibiotic Gut Mucosal Microbiome Reconstitution Is Impaired by Probiotics and Improved by Autologous FMT. Cell. 2018;174:1406–1423.e1416. doi: 10.1016/j.cell.2018.08.047. [DOI] [PubMed] [Google Scholar]
- 37.Heimesaat MM, Plickert R, Fischer A, Gobel UB, Bereswill S. Can microbiota transplantation abrogate murine colonization resistance against Campylobacter jejuni? Eur J Microbiol Immunol (Bp) 2013;3:36–43. doi: 10.1556/EuJMI.3.2013.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heimesaat MM, et al. MyD88/TLR9 mediated immunopathology and gut microbiota dynamics in a novel murine model of intestinal graft-versus-host disease. Gut. 2010;59:1079–1087. doi: 10.1136/gut.2009.197434. [DOI] [PubMed] [Google Scholar]
- 39.Bereswill S, et al. The impact of Toll-like-receptor-9 on intestinal microbiota composition and extra-intestinal sequelae in experimental Toxoplasma gondii induced ileitis. Gut Pathog. 2014;6:19. doi: 10.1186/1757-4749-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rausch S, et al. Small intestinal nematode infection of mice is associated with increased enterobacterial loads alongside the intestinal tract. PLoS One. 2013;8:e74026. doi: 10.1371/journal.pone.0074026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alutis ME, et al. The Role of Gelatinases in Campylobacter Jejuni Infection of Gnotobiotic Mice. Eur J Microbiol Immunol (Bp) 2015;5:256–267. doi: 10.1556/1886.2015.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alutis ME, et al. Matrix Metalloproteinase-2 Mediates Intestinal Immunopathogenesis in Campylobacter Jejuni-Infected Infant Mice. Eur J Microbiol Immunol (Bp) 2015;5:188–198. doi: 10.1556/1886.2015.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heimesaat MM, et al. Impact of Campylobacter jejuni cj0268c knockout mutation on intestinal colonization, translocation, and induction of immunopathology in gnotobiotic IL-10 deficient mice. PloS one. 2014;9:e90148. doi: 10.1371/journal.pone.0090148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.