Abstract
Multiple sclerosis (MS) is an autoimmune demyelinating disease with progressive neurodegeneration and complex etiology likely involving genetic and environmental factors. MS has been associated with Epstein Barr virus (EBV) infection, with patients often showing enhanced responses to EBV antigens. To determine whether abnormal EBV nuclear antigen-1 (EBNA-1) humoral immunity can serve as an initiator of autoimmune responses in MS, we investigated the fine specificities of the humoral immune response against EBNA-1 in MS patients using solid phase epitope mapping. Antibodies from MS patients recognized an EBNA-1 epitope spanning amino acids 411–426, previously unknown to be recognized specifically by untreated MS patients. Antibodies against this epitope cross-reacted to myelin basic protein (MBP). Furthermore, animals immunized with this EBNA-1 polypeptide mounted a response against MBP and developed signs of experimental autoimmune encephalitis (EAE). These data support a link between MS and EBV through antibodies that cross-react between EBV proteins and the MBP autoantigen.
Keywords: Epstein Barr Virus, EBNA-1, multiple sclerosis
1. Introduction
Multiple sclerosis (MS) is an autoimmune demyelinating disease leading to progressive neurologic deficits. Etiology of the disease remains unknown. Genome-wide association studies have discovered many genetic variants that contribute to susceptibility to MS, including genes involved in the regulation of immune responses in MS. The strongest genetic associations are due to variations in major histocompatibility complex (i.e. HLA) genes [1–3]. Additionally, several environmental factors have also been associated with the development of MS. Discordance in monozygotic twins further supports the role of environmental factors. Among infectious agents, Epstein Barr virus (EBV) shows the strongest association [4, 5]. EBV-induced infectious mononucleosis increases the MS risk (OR of 2.3, 95% CI, 1.7–3.0) similar to the strongest genetic risk factor (HLA DRB1* 1501; average OR of 3.08, 95% CI) [3, 6–9].
EBV is a gamma-herpes virus that infects >90% of the adult world population, and has been directly associated with a number of diseases including Hodgkin’s lymphoma, nasopharyngeal carcinoma, and Burkitt’s lymphoma [10–12]. EBV maintains latency in B cells with frequent reactivation, a process that is sufficient to cause a continuous stimulation of the immune system. MS patients have an increased antibody response to EBV, and several studies with both adult and pediatric MS patients suggest that prior EBV infection increases risk for development of MS [13–16]. The combination of high EBV nuclear antigen-1 (EBNA-1) antibodies and HLA-DRB1*1501 positivity is associated with a significantly enhanced risk for MS [17]. Anti-EBNA-1 antibody levels have been reported to be a HLA-DRB15-independent risk factor in both adult and pediatric MS patients [13, 18]
EBNA-1 is ubiquitously expressed in latency and is responsible for EBV DNA localization to the nuclear chromatin in latent infection [19]. Antibodies are often formed against EBNA-1 in previously EBV-infected individuals [20]. Molecular mimicry between EBNA-1 and disease specific autoantigens has been shown in both rheumatoid arthritis and systemic lupus erythematosus [21, 22]. As molecular mimicry has also been considered as a potential partial hypothesis for the onset of MS, the ability of EBNA-1 to trigger cross-reactive responses in susceptible hosts marks it as a potential candidate to drive development of MS in susceptible individuals.
To understand the immunologic role of EBNA-1 in MS, we studied the fine specificity of the anti-EBNA-1 antibody responses and identified qualitative differences between MS patients and healthy controls. We demonstrate that a unique epitope of EBNA-1 that is recognized by MS patients, but not healthy controls, cross-reacts with myelin basic protein (MBP) and exposure to this EBNA-1 sequence can induce clinical signs of experimental autoimmune encephalitis (EAE) in mice.
2. Materials and Methods
2.1. Patients
This study initially used sera from 23 MS patients: 11 MS patients treated with interferon β (IFNβ), 12 untreated MS patients, and 7 healthy human controls enrolled at the Rutgers New Jersey Medical School (RNJMS). For confirmatory studies, sera from 45 patients with relapsing remitting MS (RRMS), 19 with secondary progressive MS (SPMS), 15 with primary progressive MS (PPMS), and 6 with clinically isolated syndrome (CIS) were obtained through the MS repository at the Oklahoma Medical Research Foundation.Written informed consent was obtained for all patients and controls. Study was approved by the Institutional Review Boards of Oklahoma Medical Research Foundation (OMRF) and RNJMS in accordance with the Declaration of Helsinki (IRB# 05–24, IRB# 11–18, IRB#06–12).
2.2. Anti-viral antibody assays
Antibodies against EBV-Viral Capsid Antigen (VCA), Herpes Simplex Virus-1 (HSV-1), Herpes Simplex Virus-2 (HSV-2), Cytomegalovirus (CMV), and EBNA-1 were measured by commercial ELISAs according to manufacturer’s instructions (Wampole Laboratories, Cranberry, NJ). The assay results and analyses are presented as units of the International Standardized Ratio (ISR), which utilizes calibrators to normalize between assays. ISR is a semi-quantitative measure of the relative level of antibody; values ≥1.1 are considered positive.
2.3. Solid-phase peptide synthesis and antibody assays
317 unique decapeptides spanning the EBNA-1 protein were constructed according to the primary amino acid sequence [23] using solid phase peptide chemistry [24]. Each decapeptide overlapped the sequential peptide by eight amino acids to ensure maximal mapping efficiency. Anti-peptide assays were conducted at a 1:100 dilution of patient or control sera utilizing a modified ELISA technique that we have described previously [25]. The 484 unique maximally overlapping octapeptides of the CMV immediate early (CMV IE) antigen [26] and 82 unique decapeptides of MBP (NCBI ref: NP_001020261.10) were synthesized and tested in a similar manner.
2.4. Affinity purification of antibodies and anti-EBNA411–426 ELISA
EBNA411–426 peptide (EADYFEYHQEGGPDGE) was synthesized as a multiple antigenic peptide (MAP™) by the Oklahoma Molecular Biology Core Facility at the University of Oklahoma Health Science Center. The peptide was bound to cyanogen pre–activated Sepharose 4B [27]. To absorb antibodies, sera were recirculated (2–3 times) and concentrated to volume. Anti-EBNA411–426 antibodies were eluted with 3M sodium thiocyanate.
IgG responses to EBNA411–426 were measured by ELISA. Microliter plates were coated with 10µg/ml peptide in carbonate buffer overnight. Following addition of blocking buffer (0.1% BSA), serum samples diluted 1:100 in diluent (0.1% BSA, 0.05% Tween-20) were added and incubated for 2 hours at room temperature. Alkaline phosphatase conjugated goat anti-human IgG (Jackson Immunoresearch) was used as secondary antibody. The plates were developed using 1mg/ml para-nitrophenyl phosphate substrate (Sigma) in substrate buffer (0.1M glycine, pH 10.4, 1mM MgCL2, 1mM ZnCl2). The plates were read at 405nm on Emax Plus microplate reader (Molecular Devices).
2.5. Murine immunization and characterization
SJL/J and Balb/c female mice, 3–5 weeks of age, were obtained from Jackson Laboratory and housed at the OMRF Laboratory Animal Resource Center for one week prior to experiments. Mice were injected subcutaneously and intraperitoneally [28] with EBNA411–426 or control peptide (100μg) in complete Freund’s adjuvant on day 1. All mice received immunization boosts on days 10 and 34 in incomplete Freund’s adjuvant. Mice were bled weekly via tail vein and serum samples were stored at −20°C. Mice were scored for neurological symptoms in a blinded manner according to the following scoring system: 0- absence of any clinical symptoms, 1- tail weakness, 2- hind leg weakness, 3- front leg weakness, 4- moribund. Mice found dead were given a score of 5. All animal studies were approved by the Institutional Animal Care and Use Committee at the Oklahoma Medical Research Foundation in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
2.6. Murine magnetic resonance imaging (MRI)
Mouse brains were imaged in a cross-coil configuration scheme with actively shielded gradients (diameter 116 mm); with internal diameter 72 mm and 15 mm for transmitter coil and receiving head coil, respectively. Anatomical scans based upon both spin-echo and gradient-echo techniques were obtained. Spin-echo images were based on a technique modified from a Multi-Slice Multi Echo (MSME) protocol using short and long echo times (TE). These T1-weighted and T2-weighted images afford superb contrast and tissue differentiation of brain structures. Fifteen contiguous axial slices of 1 mm thickness were acquired with a repetition time (TR)=1300 ms, TE=11.6 ms, and field of view (FOV)=20 mm x 20 mm, 256 × 256 matrix, yielding an in-plane resolution of 78 µm. T1-weighted images had a short TE=17 ms and T2-weighted images had a long TE=64 ms. Dynamic Contrast Enhanced MRI scan (DCE-MRI) techniques utilized the gradient-echo Fast Imaging with Steady State Precession (FISP) technique for elucidating pre- and post-contrast differences due to uptake and clearance with the contrast agent Magnevist (Gadolinium-Diethylenetriaminepentaacetic acid; Gd-DTPA), administered via tail vein catheter. Thirteen contiguous axial slices of 1 mm were acquired with TR=5 ms, TE=2.5 ms, FOV=25.6 mm x 20 mm, 256 × 200 matrix, and an in-plane resolution of 100 µm. Data were collected using a Bruker 7 Tesla spectrometer.
2.7. ELISPOT assays
Total splenocytes were harvested from immunized and control mice. Cells were plated in various concentrations ranging from 1×105 to 1×106 on 96 well plates coated with 10μg per well of EBNA411–426 peptide or 2μg per well of MBP. ELISPOT kits (BD Pharmingen) were used according to the manufacturer’s recommendations to measure IFN-gamma (IFNγ).
3. Results
3.1. MS patients produce higher levels of antibodies towards EBNA-1
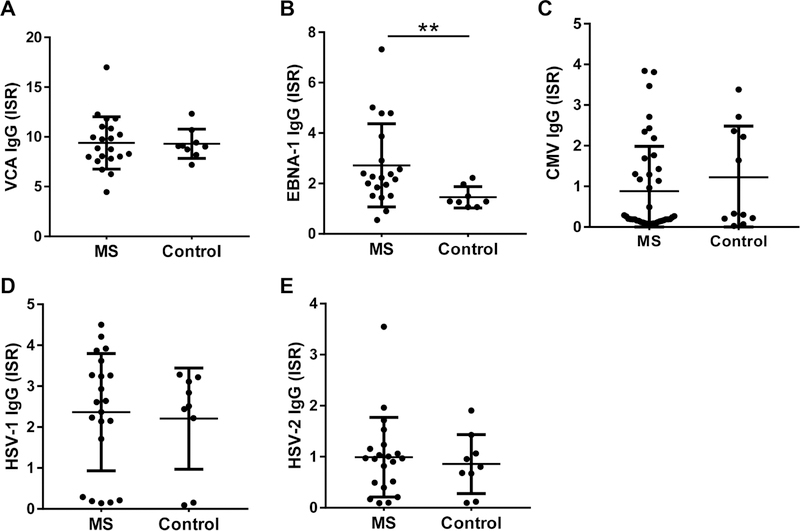
All patients and controls were positive for IgG antibodies towards EBV VCA, indicating that both these groups were exposed to EBV. No differences were observed in anti-VCA levels between MS patients and healthy controls (VCA IgG ISR 9.395±2.655 vs 9.308±1.468, p=0.859, Fig. 1A). However, MS patients had significantly higher levels of anti-EBNA-1 IgG (ISR 2.718±1.652 vs 1.455±0.4243, p=0.0084, Fig. 1B). No significant differences were observed in antibody levels against other herpes viruses (CMV, HSV-1, and HSV-2) between MS patients and healthy controls (Fig. 1C, 1D, and 1E).
Fig. 1.
Multiple sclerosis patients show increased humoral responses to EBV EBNA-1 antigen. (A-E) IgG responses against EBV VCA (A), EBV EBNA-1 (B), CMV (C), HSV-1 (D), and HSV-2 (E) were measured by ELISAs, and were calculated as International Standard Ratio (ISR). MS patients showed significantly higher levels of anti-EBNA-1 IgG. The percent positivity for VCA IgG (100%) and EBNA-1 IgG, defined as percent of subjects with ISR>1.1, was not significantly different between MS patients and controls by Chi-square test. The data are represented as mean±SD; ** p<0.01 by Mann-Whitney test.
3.2. MS patients recognize unique epitopes in EBNA-1
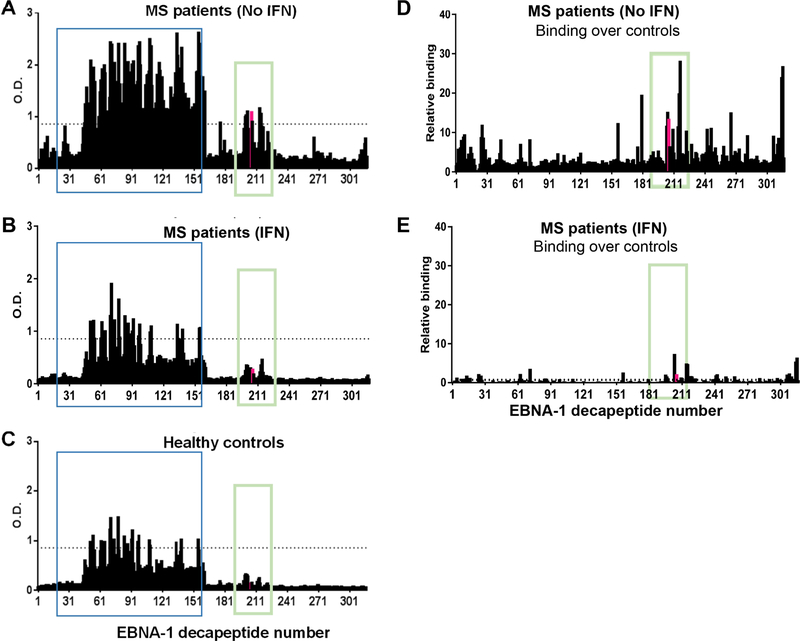
To further understand whether and how the response to EBNA-1 was qualitatively different between MS patients and healthy controls, we performed solid phase humoral epitope mapping using decapeptides spanning the EBNA-1 protein, as described previously [22]. As expected, MS patients showed an overall higher binding to peptides from EBNA-1 compared to controls (Fig. 2A and 2C). Interestingly, MS patients exhibited a distinctive binding profile that includes peptides recognized by healthy controls, as well as unique peptides that were recognized only by MS patient samples (Fig. 2A, 2C, and 2D, supplementary table 1, supplementary figure S1). Of particular interest was the region spanning amino acids 411–426 (EBNA411–426) that was recognized by MS patients, while none of the controls showed binding to these peptides. In agreement with previous reports [17, 29, 30], we observed increased binding to peptides spanning aa 52–91, as well as aa 89–330 that span the glycine-alanine rich of EBNA-1 sequence (Figure 2 and Supplementary figure S2). To determine whether this response is restricted to specific clinical type of MS, we tested EBNA411–426 responses in 85 additional MS patients by ELISA. Patients with relapsing remitting MS and secondary progressive MS tend to have higher EBNA411–426 IgG responses (Supplementary Figure S3) compared to primary progressive MS and CIS.
Fig. 2.
Multiple sclerosis patients exhibit different humoral specificities to EBNA-1. (A-C) Average binding profiles of the untreated MS patients (A), MS patients treated with interferon β (B), and healthy controls (C) against unique overlapping decapeptides of EBNA-1 were determined using solid-phase ELISAs. Positive cutoff (dotted line) was defined as mean plus twice the standard deviation (SD) of healthy controls. The overlapping decapeptides are numbered from 1–317. (D, E) The changes in epitope recognition over control for each peptide was calculated as [(Mean OD of the peptide for MS patient samples) – (Mean OD of the peptide for controls)] / Standard Deviation of peptide OD for controls]; (D) SD over controls in untreated MS patients; (E) SD over control in interferon β-treated MS patients. Unique epitopes recognized by untreated MS patients are outlined by green boxes. The EBNA411–420 peptide is highlighted in pink. The regions previously reported to be recognized by MS patients are outlined by blue boxes. The reactivity to unique epitopes observed in untreated patients was lost following treatment with IFNβ.
Interferon beta (IFNβ) mediates a suppressor effect on neuronal cells, thereby limiting MS relapses and is, therefore, a widely used therapy for MS. We observed that patients receiving IFNβ therapy showed reduced binding to unique epitopes recognized by untreated MS patients (Fig. 2A, 2B, 2D and 2E, supplementary figure S2) resulting in the binding pattern similar to that in healthy controls (Fig. 2B, 2C, and 2E).
3.3. MS patients and controls produce a similar antibody response to CMV-IE
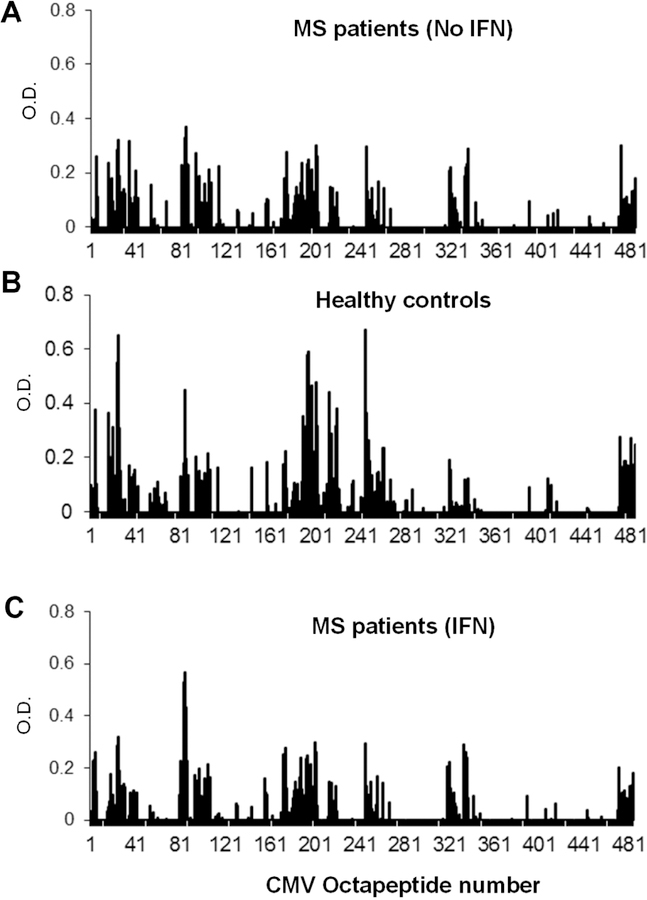
To determine whether the increased or altered response seen towards EBNA-1 was a generalized dysregulated anti-viral response in MS patients, we tested the antibody binding of patient and healthy control samples to octapeptides from CMV IE antigen. The regions of CMV-IE recognized by MS patients were similar to those recognized by healthy controls (Fig. 3A and 3B). Furthermore, there were no significant differences observed between the CMV IE epitope profiles of MS patients with or without IFNβ therapy (Fig. 3A, and 3C). These data suggest that MS patients show an altered humoral response specifically to EBNA-1.
Fig. 3.
Multiple sclerosis patients and healthy controls recognize similar epitopes in CMV IE. (A-C) Binding profiles of untreated MS patients (A), MS patients treated with interferon β (B), and healthy controls (C) against overlapping octapeptides from CMV IE were determined by solid phase ELISAs. No significant differences in epitope recognition were observed.
3.4. Anti-EBNA-1 antibodies exhibit cross-reactivity to myelin basic protein
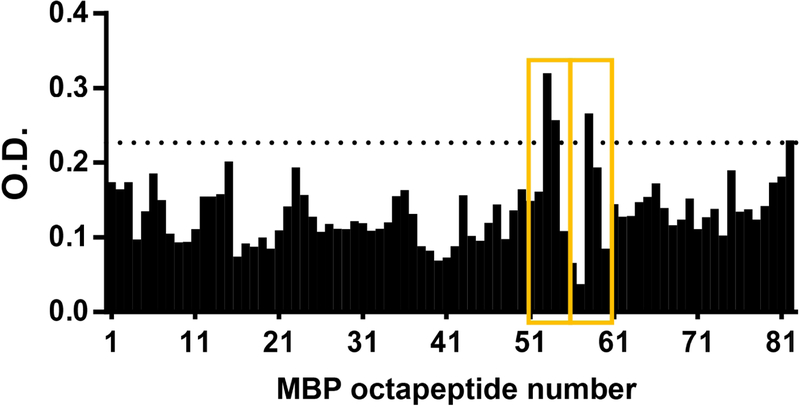
To test whether the unique viral epitopes recognized by MS patients may contribute to cross-reactivity to neuronal antigens, sera from 12 MS patients were tested for reactivity to MBP by western blot. Six patients were positive for anti-MBP antibodies, which is consistent with the frequency of anti-MBP responses observed in MS patients. Of those, 4 patients also produced antibodies directed against the EBNA411–426 peptide, which is recognized specifically by samples from untreated MS patients (Figure 2A), but not by those from IFNβ-treated MS patients or healthy controls (Fig. 2B, 2C). To further determine whether anti-EBNA411–426 antibodies cross-react with MBP, we affinity purified EBNA411–426 antibodies from five EBNA411–426 positive MS patients and tested the ability of these antibodies to bind to decapeptides derived from MBP. Figure 4 shows that purified EBNA-specific antibodies from MS patients exhibit binding to specific epitopes of MBP, with the greatest reactivity targeting peptides 53 (GKGRGLSLSR), 54 (GRGLSLSRFS), and 58 (FSWGAEQQRP) that span amino acid region 205–224 of MBP.
Fig. 4.
Anti-EBNA-1 antibodies cross-react with MBP. Anti-EBNA411–426 antibodies from MS patients were affinity purified, and their ability to bind octapeptides from MBP was determined. The overlapping octapeptides are numbered from 1–484. Positive cutoff (dotted line) was defined as mean+2xSD of all fractions from all patients. Average binding of antibodies from 5 patients is shown. The anti-MBP epitopes that anti-EBNA411–426 antibodies cross-react with are indicated by yellow boxes.
3.5. EBNA411–426 immunization induces MS-like autoimmunity
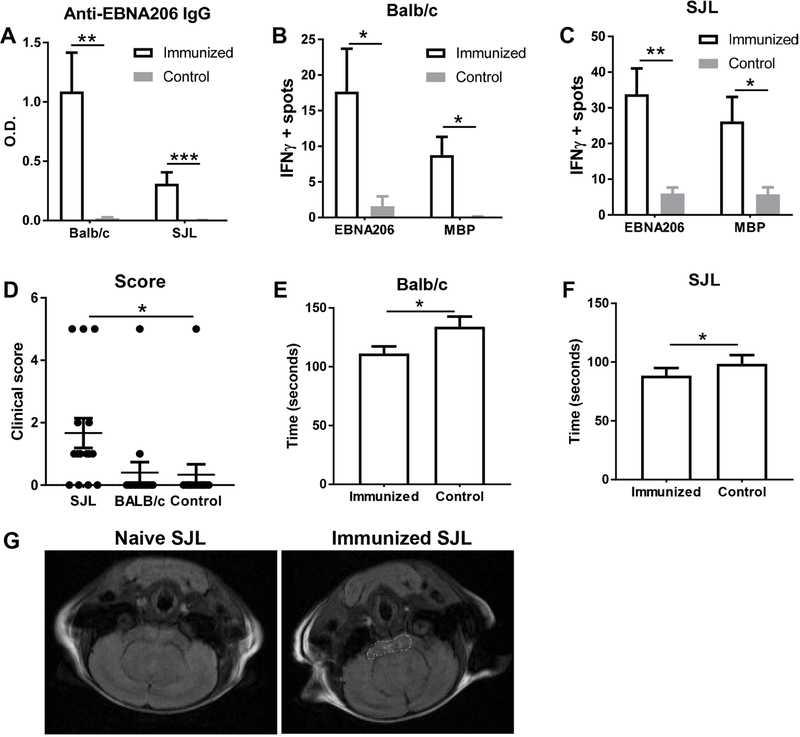
To further determine whether a response to EBNA-1 can lead to anti-MBP response, we immunized mice with EBNA411–426 peptide. Both SJL and Balb/c strains of mice developed an anti-EBNA411–426 response (Fig. 5A). To test whether these mice are able to mount a humoral immune response against MBP, we tested the ability of splenocytes from immunized mice to secrete IFNγ after in vitro stimulation with MBP. Immunized mice had higher numbers of cells secreting IFNγ in response to both EBNA411–426 and MBP stimulation (Fig. 5B and 5C). We further monitored the immunized mice for clinical symptoms of EAE. SJL and Balb/c strains developed varying levels clinical symptoms typical of EAE after immunization, although only SJL mice demonstrated significant increases in clinical scores compared to controls (Fig. 5D). Both strains, however, show a modest but statistically significant impairment in rotarod performance (Fig. 5E and 5F).
Fig. 5.
Immunization with EBNA411–426 peptide induce MS-like disease in mice. SJL and Balb/c mice were immunized with EBNA411–426 peptide. (A) IgG antibody responses to EBNA-1 were measured by ELISAs. (B, C) Isolated splenocytes from immunized Balb/c mice (B) and immunized SJL (C) mice were stimulated with EBNA411–426 or MBP, and the number of IFNγ producing cells were determined by ELISPOTs. (D) Mice were observed for clinical signs of MS and scored as in section 2.5 90 days post first immunization. (E, F) Rotarod latency was monitored in immunized Balb/c (E) and SJL (F) mice. Data are represented as mean±SEM of 3–15 mice per group; * p<0.05; ** p<0.01; *** p<0.001 by Mann-Whitney test (A-F). (G) Representative image of an MRI scan of the brains from naive or immunized SJL mice. Lesions in the cortical areas of the brain are marked by dotted shapes.
As another objective measure of clinical MS, MRI was performed on immunized SJL mice with high antibody titers to EBNA411–426 (90 days post-immunization). Five of 10 immunized SJL mice showed lesions in the cortical areas of the brain (Fig. 5G), consistent with anatomic locations commonly affected in human MS, while control mice showed no lesions. The 5 mice with white matter lesions had clinical signs of neurological deficits, whereas the mice without lesions did not show any clinical symptoms.
4. Discussion
Our data show that the relationship between EBV and MS involves a fundamentally altered humoral response to EBNA-1 in MS patients compared to healthy controls. For the first time we have demonstrated that MS patient antibodies recognized unique epitopes in EBNA-1, and that immune responses to these antigens are capable of triggering some MS-like clinical manifestations in an animal model. These findings further suggest that the observed cross-reactivity between EBNA-1 viral antigens and MBP autoantigens may, in part, contribute to MS development.
We saw increased titers of antibodies directed against the EBNA-1 protein as a whole, as well as against unique antigenic regions of EBNA-1 in MS patients compared to controls. These aberrant responses were not a consequence of immunosuppressive medication and they do not suggest a broadly dysregulated anti-viral immune response in MS patients since, at the epitope level, MS patients produced an essentially identical antibody profile to that of healthy controls against the CMV-IE antigen (another major latent protein from a herpes virus). In addition, there was no difference in antibody levels against EBV VCA. These data suggest that altered humoral responses to EBV in MS patients are directed primarily against EBNA-1, in agreement with previous reports [31].
Previous studies have reported several epitopes in EBNA-1 that are recognized by MS patients [29, 30]. Many of these epitopes were within the glycine-alanine rich region that spans aa 90–328 in EBNA-1 sequence. In agreement with those reports, we observed increased binding to peptides that spanned aa 89–330 (Supplementary Figure 2), with binding being higher in MS patients than controls. We also observed increased binding to peptides spanning aa 1–90 as shown previously [30]. Seemingly in contrast with our studies Mechelli et al. did not observe significant differences between binding to EBNA411–426 between Italian MS patients and controls by ELISA [32]. As shown in Supplementary Figure S1, there is sharp decrease in binding for all patients and controls at region corresponding to aa 415–424, which may decrease the overall binding to 411–426 in this US-based cohort. Differences in the regions reported to be recognized by patients could be attributed to HLA/genetic differences, modifications in techniques used to generate epitopes and the length of the epitopes used. Using longer peptides generated by a recombinant fusion protein approach, increased binding of MS patients to peptides spanning aa 1–90, 402–502, 478–578, and 553–641 was reported [17]. These support the increased binding we see at EBNA411–426. Furthermore, the authors showed that binding to EBNA-1 was inhibited by 10 aa peptides corresponding to aa 401–420 and to aa 421–440, but not by 411–430, which includes the 415–424 region we observed to have reduced binding. Our approach with overlapping peptides helps narrow the epitopes to smaller more defined regions.
Cross-reactivity triggered by EBNA-1 is seen in other autoimmune diseases such as systemic lupus erythematosus, Sjogren’s syndrome, and rheumatoid arthritis [21, 22, 33–35]. In this study, we show that affinity purified antibodies targeted against a unique epitope of EBNA-1, EBNA411–426, can bind MBP. Immunization of mice with EBNA411–426, a peptide recognized only by antibodies from MS patients, resulted in the mice mounting an anti-MBP response with clinical signs of MS, thus further substantiating the role of cross-reactivity between EBNA-1 and MBP antibodies in the development of MS. Interestingly, the cross-reactive peptides discovered share minimal similarity at the primary amino acid level, however, previous work has suggested roles for other mechanisms of viral protein cross-reactivity and autoantigens in MS. Cross-reactivity of EBNA-1-reactive T cell clones from MS patients to peptides from MBP has been attributed to structural similarities in the binding regions rather than similarities in primary sequence [36]. Further studies may delineate whether such processes are at play in the cross-reactivity between antibody responses towards EBNA-1 and MBP. Computer based analyses of decapeptides from extramembrane segments of myelin proteolipid showed significant sequence similarities between viral decapeptides derived from EBV proteins, although not EBNA-1 [37]. EBNA-1 IgG did not correlate with native myelin oligodendrocyte glycoprotein (nMOG) in pediatric MS patients [38]. However, whether response towards EBNA-1 leads to autoimmune response towards other MS associated autoantigens by epitope spreading needs further evaluation as well as longitudinal analyses of antibody specificities in preclinical samples.
Patients with severe clinical MS symptoms developed unique patterns of high-titer antibodies to EBNA-1. Interestingly, following interferon therapy, these complex anti-EBNA-1 antibody binding patterns were simplified, and closely resembled those of healthy controls. As IFNβ therapy typically reduces new clinical MS manifestations [39], these findings suggest a direct relationship between MS disease activity and the aspects of the aberrant immune response that drive production of anti-EBNA-1 antibodies.
Although MS is traditionally considered a primarily T cell mediated disease, the importance of B cells in MS pathogenesis has gained attention with the identification of ectopic B cell follicles in the central nervous system (CNS) of progressive MS patients [40] and the clinical and radiological benefit of anti-B cell therapies [41]. The presence of these ectopic follicles correlates with severity of cortical pathology [42]. These reports suggest that establishment of lymphoid structures in the brain may serve as a microenvironment for local immunoglobulin production and release of pathogenic antibodies by activated B cells. EBV infected B cells are in close proximity to CD8 T cells [43] and class-switched memory B cells are observed in the CNS [44], although the target antigens for these B cell responses have not been determined. In addition, presence of EBNA-1 reactive antibodies has been demonstrated in cerebrospinal fluid from MS patients [45], all of which further suggest that humoral immune responses may contribute to CNS damage.
5. Conclusions
In this study, we demonstrate fundamental differences between the humoral responses of MS patients and healthy individuals to EBV. These aberrant anti-EBNA-1 responses are related to some anti-myelin autoantibodies in MS patients. Whether the recognition of unique EBNA-1 epitopes in MS patients is related to their underlying ability to break tolerance to antigenic viral regions, which presumably mimic neural autoantigenic sequences, remains unclear. Further studies into the immunologic relationship between EBV and MS, as well as the role of molecular mimicry in the development of autoimmune neurologic disease, will provide valuable additional insight into the onset and potential etiologic triggers of this devastating disease.
Supplementary Material
Highlights:
Adult MS patient antibodies recognize EBNA epitope spanning aa411–426
Anti-EBNA411–426 responses are diminished by interferon treatment
Anti-EBNA411–426 cross-reacts with myelin basic protein (MBP)
EBNA411–426 immunized mice mount immune responses toward MBP
EBNA411–426 immunization causes clinical signs of neurologic disease in mice
Acknowledgements.
The authors thank Dr. Andrew R. Pachner for providing MS patient samples; Erin Rapp, Kaylan Lawson, Cassie Pierce, Anthony Birchfield, and Jourdan Anderson, for their technical assistance, and Kandice L. Tessneer, PhD for editorial assistance.
Funding. This study was supported in part by the National Institute of Allergy, and Infectious Diseases [U19AI082714, U01AI130830, R01AI024717], the National Institute of Arthritis, Musculoskeletal and Skin Diseases [R01AR045451], and an Institutional Development Award from the National Institute of General Medical Sciences [U54GM104938], and the US Department of Veterans Affairs [I01BX001834]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the US Government.
Abbreviations:
- CMV
Cytomegalovirus
- CMV IE
CMV immediate early antigen
- DCE-MRI
dynamic contrast enhanced MRI
- EBV
Epstein Barr virus
- EBNA-1
EBV nuclear antigen-1
- EBNA411–426
EBV nuclear antigen epitope spanning amino acids 411–426
- EAE
experimental autoimmune encephalitis
- FISP
fast imaging with steady state precession
- FOV
field of view
- HSV
Herpes Simplex virus
- IFN
interferon
- ISR
International standard ratio
- MAP
multiple antigenic peptide
- MBP
myelin basic protein
- MRI
magnetic resonance imaging
- MS
multiple sclerosis
- MSME
multi-slice multi echo
- TE
MRI echo time
- TR
MRI repetition time
- VCA
viral capsid antigen
Footnotes
Declaration of Interest. The authors have no conflicts of interests to disclose.
References
- [1].Baranzini SE Revealing the genetic basis of multiple sclerosis: are we there yet? Current opinion in genetics & development, 2011;21:317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Nylander A, Hafler DA Multiple sclerosis. The Journal of clinical investigation, 2012;122:1180–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hollenbach JA, Oksenberg JR The immunogenetics of multiple sclerosis: A comprehensive review. Journal of autoimmunity, 2015;64:13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ascherio A, Munger KL Epstein-barr virus infection and multiple sclerosis: a review. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology, 2010;5:271–7. [DOI] [PubMed] [Google Scholar]
- [5].Pender MP The essential role of Epstein-Barr virus in the pathogenesis of multiple sclerosis. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry, 2011;17:351–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tselis A Evidence for viral etiology of multiple sclerosis. Seminars in neurology, 2011;31:307–16. [DOI] [PubMed] [Google Scholar]
- [7].Nielsen TR, Pedersen M, Rostgaard K, Frisch M, Hjalgrim H Correlations between Epstein-Barr virus antibody levels and risk factors for multiple sclerosis in healthy individuals. Multiple sclerosis, 2007;13:420–3. [DOI] [PubMed] [Google Scholar]
- [8].C. International Multiple Sclerosis Genetics, C. Wellcome Trust Case Control, Sawcer S, Hellenthal G, Pirinen M, Spencer CC et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature, 2011;476:214–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Thacker EL, Mirzaei F, Ascherio A Infectious mononucleosis and risk for multiple sclerosis: a meta-analysis. Ann Neurol, 2006;59:499–503. [DOI] [PubMed] [Google Scholar]
- [10].Carbone A, Gloghini A Epstein Barr Virus-Associated Hodgkin Lymphoma. Cancers, 2018;10. [DOI] [PMC free article] [PubMed]
- [11].Tsang CM, Tsao SW The role of Epstein-Barr virus infection in the pathogenesis of nasopharyngeal carcinoma. Virologica Sinica, 2015;30:107–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rochford R, Moormann AM Burkitt’s Lymphoma. Current topics in microbiology and immunology, 2015;390:267–85. [DOI] [PubMed] [Google Scholar]
- [13].Waubant E, Mowry EM, Krupp L, Chitnis T, Yeh EA, Kuntz N et al. Common viruses associated with lower pediatric multiple sclerosis risk. Neurology, 2011;76:1989–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ascherio A, Munger KL, Lennette ET, Spiegelman D, Hernan MA, Olek MJ et al. Epstein-Barr virus antibodies and risk of multiple sclerosis: a prospective study. Jama, 2001;286:3083–8. [DOI] [PubMed] [Google Scholar]
- [15].Munger KL, Levin LI, O’Reilly EJ, Falk KI, Ascherio A Anti-Epstein-Barr virus antibodies as serological markers of multiple sclerosis: a prospective study among United States military personnel. Multiple sclerosis, 2011;17:1185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Nourbakhsh B, Rutatangwa A, Waltz M, Rensel M, Moodley M, Graves J et al. Heterogeneity in association of remote herpesvirus infections and pediatric MS. Annals of clinical and translational neurology, 2018;5:1222–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sundstrom P, Nystrom M, Ruuth K, Lundgren E Antibodies to specific EBNA-1 domains and HLA DRB1*1501 interact as risk factors for multiple sclerosis. Journal of neuroimmunology, 2009;215:102–7. [DOI] [PubMed] [Google Scholar]
- [18].De Jager PL, Simon KC, Munger KL, Rioux JD, Hafler DA, Ascherio A Integrating risk factors: HLA-DRB1*1501 and Epstein-Barr virus in multiple sclerosis. Neurology, 2008;70:1113–8. [DOI] [PubMed] [Google Scholar]
- [19].Petti L, Sample C, Kieff E Subnuclear localization and phosphorylation of Epstein-Barr virus latent infection nuclear proteins. Virology, 1990;176:563–74. [DOI] [PubMed] [Google Scholar]
- [20].Rhodes G, Carson DA, Valbracht J, Houghten R, Vaughan JH Human immune responses to synthetic peptides from the Epstein-Barr nuclear antigen. Journal of immunology, 1985;134:211–6. [PubMed] [Google Scholar]
- [21].Fox R, Sportsman R, Rhodes G, Luka J, Pearson G, Vaughan J Rheumatoid arthritis synovial membrane contains a 62,000-molecular-weight protein that shares an antigenic epitope with the Epstein-Barr virus-encoded associated nuclear antigen. The Journal of clinical investigation, 1986;77:1539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].McClain MT, Heinlen LD, Dennis GJ, Roebuck J, Harley JB, James JA Early events in lupus humoral autoimmunity suggest initiation through molecular mimicry. Nature medicine, 2005;11:85–9. [DOI] [PubMed] [Google Scholar]
- [23].Arrand JR, Rymo L, Walsh JE, Bjorck E, Lindahl T, Griffin BE Molecular cloning of the complete Epstein-Barr virus genome as a set of overlapping restriction endonuclease fragments. Nucleic acids research, 1981;9:2999–3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].James JA, Harley JB Peptide Autoantigenicity of the Small Nuclear Ribonucleoprotein-C. Clin Exp Rheumatol, 1995;13:299–305. [PubMed] [Google Scholar]
- [25].James JA, Scofield RH, Harley JB Basic-Amino-Acids Predominate in the Sequential Autoantigenic Determinants of the Small Nuclear 70k Ribonucleoprotein. Scand J Immunol, 1994;39:557–66. [DOI] [PubMed] [Google Scholar]
- [26].Bankier AT, Beck S, Bohni R, Brown CM, Cerny R, Chee MS et al. The DNA sequence of the human cytomegalovirus genome. DNA sequence : the journal of DNA sequencing and mapping, 1991;2:1–12. [DOI] [PubMed] [Google Scholar]
- [27].McClain MT, Lutz CS, Kaufman KM, Faig OZ, Gross TF, James JA Structural availability influences the capacity of autoantigenic epitopes to induce a widespread lupus-like autoimmune response. Proceedings of the National Academy of Sciences of the United States of America, 2004;101:3551–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].McClain MT, Scofield RH, Kurien BT, Gross TF, James JA Selective small antigenic structures are capable of inducing widespread autoimmunity which closely mimics the humoral fine specificity of human SLE. Scandinavian journal of immunology, 2002;56:399–407. [DOI] [PubMed] [Google Scholar]
- [29].Ruprecht K, Wunderlich B, Giess R, Meyer P, Loebel M, Lenz K et al. Multiple sclerosis: the elevated antibody response to Epstein-Barr virus primarily targets, but is not confined to, the glycine-alanine repeat of Epstein-Barr nuclear antigen-1. Journal of neuroimmunology, 2014;272:56–61. [DOI] [PubMed] [Google Scholar]
- [30].Hecker M, Fitzner B, Wendt M, Lorenz P, Flechtner K, Steinbeck F et al. High-Density Peptide Microarray Analysis of IgG Autoantibody Reactivities in Serum and Cerebrospinal Fluid of Multiple Sclerosis Patients. Molecular & cellular proteomics : MCP, 2016;15:1360–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Dooley MM, de Gannes SL, Fu KA, Lindsey JW The increased antibody response to Epstein-Barr virus in multiple sclerosis is restricted to selected virus proteins. Journal of neuroimmunology, 2016;299:147–51. [DOI] [PubMed] [Google Scholar]
- [32].Mechelli R, Anderson J, Vittori D, Coarelli G, Annibali V, Cannoni S et al. Epstein-Barr virus nuclear antigen-1 B-cell epitopes in multiple sclerosis twins. Multiple sclerosis, 2011;17:1290–4. [DOI] [PubMed] [Google Scholar]
- [33].Arbuckle MR, Schilling AR, Harley JB, James JA A limited lupus anti-spliceosomal response targets a cross-reactive, proline-rich motif. Journal of autoimmunity, 1998;11:431–8. [DOI] [PubMed] [Google Scholar]
- [34].Arbuckle MR, Gross T, Scofield RH, Hinshaw LB, Chang AC, Taylor FB Jr. et al. Lupus humoral autoimmunity induced in a primate model by short peptide immunization. Journal of investigative medicine : the official publication of the American Federation for Clinical Research, 1998;46:58–65. [PubMed] [Google Scholar]
- [35].Vaughan JH, Nguyen MD, Valbracht JR, Patrick K, Rhodes GH Epstein-Barr virus-induced autoimmune responses. II. Immunoglobulin G autoantibodies to mimicking and nonmimicking epitopes. Presence in autoimmune disease. The Journal of clinical investigation, 1995;95:1316–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lang HL, Jacobsen H, Ikemizu S, Andersson C, Harlos K, Madsen L et al. A functional and structural basis for TCR cross-reactivity in multiple sclerosis. Nature immunology, 2002;3:940–3. [DOI] [PubMed] [Google Scholar]
- [37].Shaw SY, Laursen RA, Lees MB Analogous amino acid sequences in myelin proteolipid and viral proteins. FEBS letters, 1986;207:266–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Selter RC, Brilot F, Grummel V, Kraus V, Cepok S, Dale RC et al. Antibody responses to EBV and native MOG in pediatric inflammatory demyelinating CNS diseases. Neurology, 2010;74:1711–5. [DOI] [PubMed] [Google Scholar]
- [39].Madsen C The innovative development in interferon beta treatments of relapsing-remitting multiple sclerosis. Brain and behavior, 2017;7:e00696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Serafini B, Rosicarelli B, Magliozzi R, Stigliano E, Aloisi F Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain pathology, 2004;14:164–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hauser SL, Bar-Or A, Comi G, Giovannoni G, Hartung HP, Hemmer B et al. Ocrelizumab versus Interferon Beta-1a in Relapsing Multiple Sclerosis. The New England journal of medicine, 2017;376:221–34. [DOI] [PubMed] [Google Scholar]
- [42].Magliozzi R, Howell O, Vora A, Serafini B, Nicholas R, Puopolo M et al. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain : a journal of neurology, 2007;130:1089–104. [DOI] [PubMed] [Google Scholar]
- [43].Serafini B, Rosicarelli B, Franciotta D, Magliozzi R, Reynolds R, Cinque P et al. Dysregulated Epstein-Barr virus infection in the multiple sclerosis brain. The Journal of experimental medicine, 2007;204:2899–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Cepok S, Rosche B, Grummel V, Vogel F, Zhou D, Sayn J et al. Short-lived plasma blasts are the main B cell effector subset during the course of multiple sclerosis. Brain : a journal of neurology, 2005;128:1667–76. [DOI] [PubMed] [Google Scholar]
- [45].Cepok S, Zhou D, Srivastava R, Nessler S, Stei S, Bussow K et al. Identification of Epstein-Barr virus proteins as putative targets of the immune response in multiple sclerosis. The Journal of clinical investigation, 2005;115:1352–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.