Abstract
New robotic systems have recently emerged to assist with peripheral lung access, but a robotic system for rigid bronchoscopy has yet to be developed. We describe a new robotic system that can deliver thin robotic manipulators through the ports of standard rigid bronchoscopes. The manipulators bend and elongate to provide maneuverability of surgical tools at the endoscope tip, without endoscope motion. We describe an initial feasibility study on the use of this system to bronchoscopically treat a central airway obstruction (CAO). CAO is prevalent and can be life-threatening in patients with large tumors, and conventional rigid bronchoscopic treatments place patients at risk of complications including broken teeth, neck trauma and damage to oropharyngeal structures due to significant forces induced by bronchoscope tilting and manipulation. In this study, we used an ex vivo ovine airway model to demonstrate the ability of a physician using the robotic system to efficiently remove tissue and restore the airway. Pre- and post-operative CT scans showed that the robot was able to reduce the degree of airway obstruction stenosis from 75% to 14% on average for 5 CAO resections performed in an ex vivo animal model. Using cadaver experiments, we demonstrated the potential of the robotic system to substantially reduce the intraoperative forces applied to the patient’s head and neck (from 80.6N to 4.1N). These preliminary results illustrate that CAO removal is feasible with our new rigid bronchoscopy robot system, and that this approach has the potential to reduce forces applied to the patient due to bronchoscope angulation, and thereby reduce the risk of complications encountered during CAO surgery.
Keywords: Central Airway Obstruction, Bronchoscopy, Robotics
1. Introduction
Central airway obstruction (CAO), or the obstruction of airflow at the level of the trachea or mainstem bronchi, affects more than 80,000 patients per year in the USA5,8. CAO may be caused by a variety of malignant and nonmalignant processes, and the incidence of CAO has steadily increased over the past decade10,2,7,4, a trend that is expected to continue in part due to increasing benign disease and an increasing prevalence of lung cancer10,43. Approximately a third (30%) of advanced-stage lung cancer patients will develop a CAO and 40% of lung cancer deaths can be attributed to loco-regional progression in the regions affected by CAO 14,10,40. Therapeutic treatment of CAO has been shown to improve symptoms and overall quality of life, and enable patients to move forward with oncologic treatments21. However, treatment options available for CAO patients are limited, since pharmacological intervention, chemotherapy, and radiotherapy fail to alleviate the airway obstruction in the vast majority of patients40,18,9.
Furthermore, in emergencies or when other treatments fail, CAO patients are rushed to emergency surgery to attempt to restore the obstructed airway and prevent suffocation. Without this intervention, patients suffering from severe CAO will die10. Open surgery is highly invasive, and in practice has been largely supplanted by bronchoscopic techniques20,45,44, which involve deploying mechanical (microdebriders) and ablative (fiber optic laser, electrosurgery, Argon plasma, cryotherapy) therapeutic tools through the bronchoscope’s port, and using them to resect the tumor and restore the airway21. Despite advances in flexible bronchoscopy, rigid bronchoscopy remains the preferred approach for severe CAO, owing to better airway control, larger-suction catheters, and the ability to simultaneously deploy manipulators such as forceps and electrocautery devices to treat the obstruction in a timely fashion27,41,15,19.
Despite being the preferential course of treatment, CAO resection via rigid bronchoscopy is challenging for the physician. The challenge arises because the only way to aim tools is to tilt the bronchoscope itself, since the tool can only advance straight out of a fixed bronchoscope port. Due to the anatomical constraints imposed by the patient’s mouth and throat, only a small degree of tilting is possible, and even that requires substantial forces to be applied to the patient’s teeth, neck, laryngeal and epiglottic structures39. Achieving complex surgical motions to visualize, manipulate, and resect tissue through bronchoscope tilting is challenging, particularly when several instruments are used simultaneously. Furthermore, the necessary act of tilting/angling the bronchoscope poses substantial risks to the patient, because the physician must physically tilt the head and neck of the patient to bring the laser to bear on the intended target. Thus, it is not surprising that up to 32.3% of patients experience complications including broken teeth, hyperextension and/or hyperflexion of the neck, and (in extreme cases) death10,42,26.
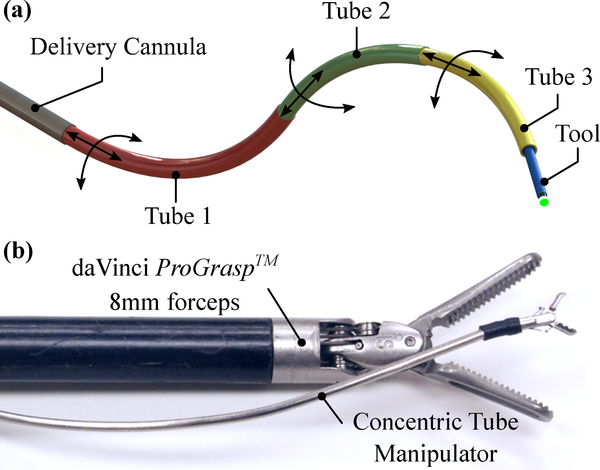
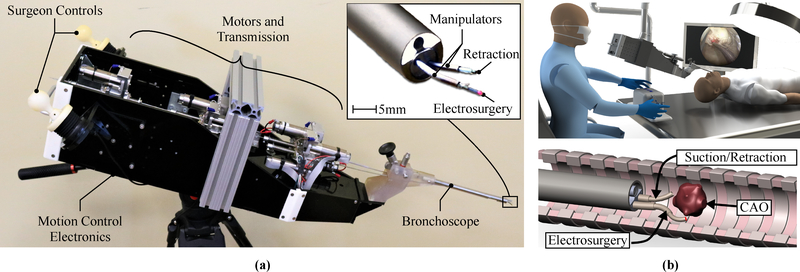
To overcome dexterity limitations inherent in conventional rigid bronchoscopy, we have developed a new robotic system based on concentric-tube robotic technology shown in Fig. 1. The robotic system, shown in Fig. 2, features two dexterous, tentacle-like, needle-sized arms, each with three independent degrees of freedom, that can be passed through commercial bronchoscopes, and used to manipulate a variety of cutting and ablative tools commonly used in airway surgery, such as laser fibers and electrosurgical probes. For a review on the needle-sized arm technology, which has been studied extensively in the engineering literature but has not yet become a commercial product or reached a real-world operating room, and further, has never before been applied to central airway surgery, please see13,22,17. The arms are motor-driven and computer-controlled. The physician controls the position and orientation of the arms (and, accordingly, the tools passed through them) with two joysticks (one for each arm). This platform enables physicians to access the trachea and aim tools without moving or tilting the bronchoscope, as shown in Fig. 2(b), eliminating the loads associated with intraoperative endoscope tilting applied to the patient. This new rigid bronchoscopic robotic system provides an array of robotic tools for the physician, and complements recent advances in thoracic imaging and registration software (Medtronic SuperDimension) and the primarily navigation-based, commercial flexible bronchoscopy robots that have recently been released (Monarch system from Auris, Inc.) or are currently under development (Ion system from Intuitive Surgical, Inc.) that target sites deeper in the lung for sampling and needle-based therapy delivery, rather than resection.
Figure 1:
Needle-sized robotic manipulators enabled by pre-curved, concentric tubes: (a) functional principle, showing the degrees-of-freedom offered by each tube, (b) concentric tube manipulator shown next to daVinci ProGrasp™ laparoscopic forceps for scale.
Figure 2:
Robotic system for CAO resection: (a) an image of the robot, where the inset shows the needle-sized, robotically-controlled tool manipulators extending out from the bronchoscope, (b) conceptual rendering of the operating room for robot-assisted bronchoscopy (top), and a conceptual rendering of a robotic CAO removal (bottom).
In contrast, we target surgical removal of obstructions in the initial bronchi that are accessible via rigid bronchoscopy. This is the first application of robotics to central airway obstruction surgery. The aforementioned Monarch and Ion systems are the only robotic systems developed specifically for transluminal lung surgery, and they are not designed for tumor resection. Other surgical robotic systems available are similarly not suited to attempt this surgery due to their diameter and kinematic requirements. While platforms like Intuitive Surgical’s daVinci S, Si, Si-e, and SP models, and Medrobotics’ FLEX systems have been cleared for trans-oral robotic surgery16,25,37, their use is limited to head and neck interventions (at the level of the tongue base, larynx and pharynx) with no documented use in the trachea or mainstem bronchi. Indeed, due to the use of rigid arms with remote centers of motion, the da Vinci S, Si, and Si-e cannot reach the trachea transluminally. While the daVinci SP does facilitate single-port surgery, its manipulators require more open space in which to move than is available in the trachea, and it is more than twice as large as the standard clinical endoscope used in our system (25mm vs. 10mm in diameter)6. This is due in part to the tools themselves, consisting of discrete linkages driven by pull-wires, which are each 5mm in diameter (compared to our concentric tube manipulators which are less than 2mm in diameter). The SurgiBot system from TransEnterix and Sport system from Titan Medical both employ a similar single-port approach to the daVinci SP, and suffer from the same large diameter (25mm) that would also preclude their use in the trachea3, due to its average diameter of 15–20mm1. The MedRobotics FLEX system features a robotically-controlled flexible endoscope, but its manually-controlled tools are routed along the outside of the endoscope because they are too large (4mm in diameter) to pass through the endoscope’s working channel. This drives up the overall diameter of the system to 28mm, including the ports for arms, at the distal end of the device38. In summary, no surgical robotic system currently on the market is suitable for CAO removal surgery, and no attempts to perform it have been reported with any of the above-mentioned systems, motivating the development of the new robotic system we report in this paper.
The system we report in this paper delivers two needle-sized robotic manipulators through a standard rigid endoscope. In the context of a CAO resection, the most significant value additions to the procedure are: (1) dexterous and intuitive tool control at the endoscope tip for precision resection, (2) minimal endoscope angulation to limit secondary damage to the patient, and (3) minimal endoscope diameter for better airway management. Our system (1) enables dexterous, bi-manual robotic control of two robotic arms inside the patient’s airway, (2) enables tool manipulation and control without requiring endoscope motion, and (3) is deployed through a standard 10mm bronchoscope, which is a clinically proven platform for ensuring sufficient airway control35. Therefore, our system provides minimal disruption to the current clinical workflow, while offering the potential for lower forces on the patient’s mouth and neck, as we demonstrate in this paper.
2. Materials and Methods
We performed two sets of experiments. The first evaluated the feasibility of robot-assisted CAO resection through a series of experiments on ex vivo ovine models, and the second examined the extent to which forces are reduced with the robotic approach in comparison to conventional rigid endoscopy. We note that all experiments were performed by a single interventional pulmonologist (Dr. Fabien Maldonado, a co-author on this paper), with the goal of demonstrating the feasibility of using our system to complete a CAO resection.
2.1. Robot System Hardware
The robotic system shown in Fig. 2 deploys two concentric tube robot arms through a 10mm rigid bronchoscope. Each robotically-controlled arm consists of two pre-curved Nitinol tubes, each with two degrees of freedom (telescopic extension and axial rotation) that are independently actuated through a compact actuation system consisting of two brushless DC motors and a differential drive mechanism. Each motor is equipped with an optical encoder (1024 counts per turn) for closed-loop position/orientation feedback of each tube. Given two arms of two tubes (each tube with 2 degrees of freedom), the total system has 8 degrees of freedom. A custom-designed user interface enables the surgeon to provide control inputs to the robotic system. The resulting position of each arm is controlled in task space based on these surgeon input commands. Maxon EPOS2 motor drivers (Maxon Motor AG, Sachseln, Switzerland) are used for low-level motor control with the desired input positions processed on the main control computer and then sent to the drivers using CAN communication. The input devices each have four analog encoders, one for each of the four independent degrees of freedom (pan, tilt, roll, and translation along roll axis), which are read by a microcontroller and sent to the main control computer using serial communication.
2.2. Ex Vivo Robotic Resection
A series of robotic CAO resections were performed by an experienced physician on an ovine ‘pluck’ model (ex vivo larynx, trachea, lungs and heart), which is an established model for training thoracic surgeons28,12. For each experiment, a model CAO was created by making a horizontal incision on the anterior side of the trachea, between two cartilagenous ridges, and suturing raw chicken breast into the tracheal wall such that a significant proportion of the airway was obstructed. The plucks, with the obstructions in place, were scanned in a CT scanner to verify the degree of pre-operative stenosis.
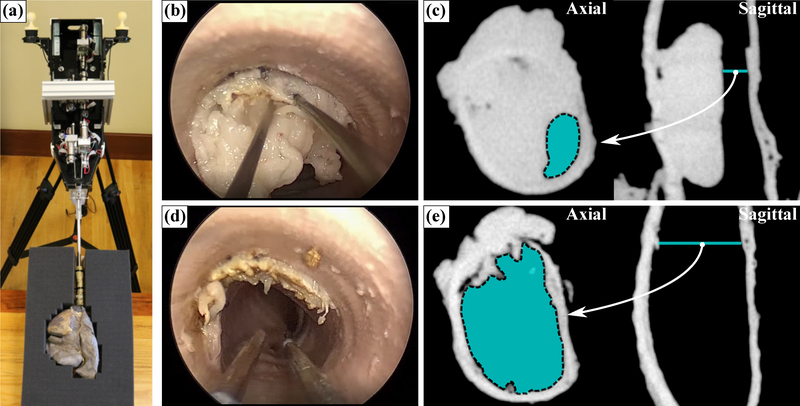
Prior to each experiment, the ovine pluck was mounted to a foam mounting block to prevent motion, as shown in Fig. 4(a). The robot was attached to a medical-grade commercial robotic arm (KUKA LBR Med) configured in impedance control mode to bear the weight of the robot and allow the surgeon to passively and effortlessly maneuver the robot for insertion into the trachea. The robot was inserted into the tracheal opening of the pluck and positioned proximally to the obstruction such that the entire obstruction was within the bronchoscope’s field of view. The KUKA holding arm was then locked into place to prevent inadvertant motion of the robotic system during the procedure. The two robot-controlled manipulators were then deployed through the bronchoscope, and a monopolar electrosurgical probe was inserted through one of them. A return electrode pad was placed under the pluck to complete the electrical circuit. The second robotic manipulator (not carrying the electrosurgical probe) was used for retraction and tissue manipulation. Prior to the first trial, the physician was given five minutes to familiarize himself with the system and the robotic controls. Once the physician was suitably acclimated to the system, he was instructed to use the robot to resect the obstruction until he was satisfied with the degree of airway restoration. Five total resection experiments were performed. The experiments were each video-recorded using an external camera (Nikon DSLR) and endoscopic camera (Karl Storz 22202011 Pure 1 HD).
Figure 4:
Robotic CAO resection: (a) robotic system deployed into an ovine ‘pluck’ model, (b) endoscopic view during the surgery, (c) axial and sagittal CT scan of the obstruction before surgery (where cyan indicates the obstructed airway, Aobstructed), (d) endoscopic view post-surgery, (e) axial and sagittal CT scan post-surgery, showing full restoration of the airway. Please refer to the accompanying video.
2.3. Pre- and Post-processing
The pluck trachea and CAO were scanned both pre- and post-surgery in a Xoran xCAT ENT CT Scanner (Xoran Technologies LLC). In the context of CAO, the degree of obstruction is clinically characterized by a stenosis index (SI), which is the percentage reduction of the airway based on the cross-sectional area of the obstructed airway (Aobstructed) and unobstructed airway measured at a location immediately distal to the obstruction (A)30,31:
| (1) |
Open-source medical image segmentation software (3DSlicer11) was used to calculate the pre- and post-operative SI by identifying the CT slice in the pre-operative scan where the endoluminal diameter was minimized (indicating the highest degree of stenosis), segmenting the airway cross-section in this image, and computing the area of this cross-section (Aobstructed). This process was repeated to calculate the area of the unobstructed airway (A) at a location distal to the obstruction30. Equation 1 was then used to calculate a pre- and post-operative SI based on these measurements to assess the overall efficacy of the resection procedure.
2.4. Force-Sensing Overtube
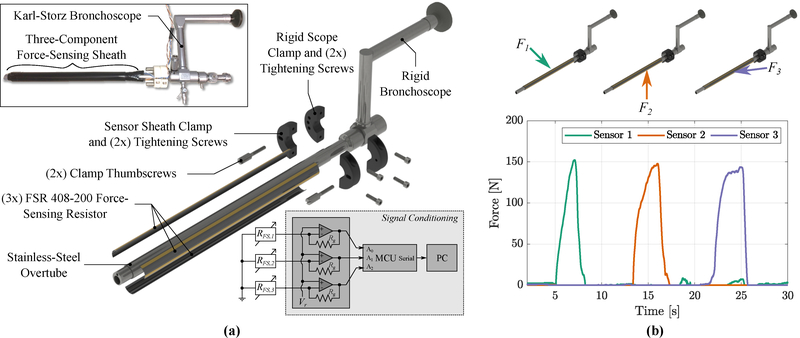
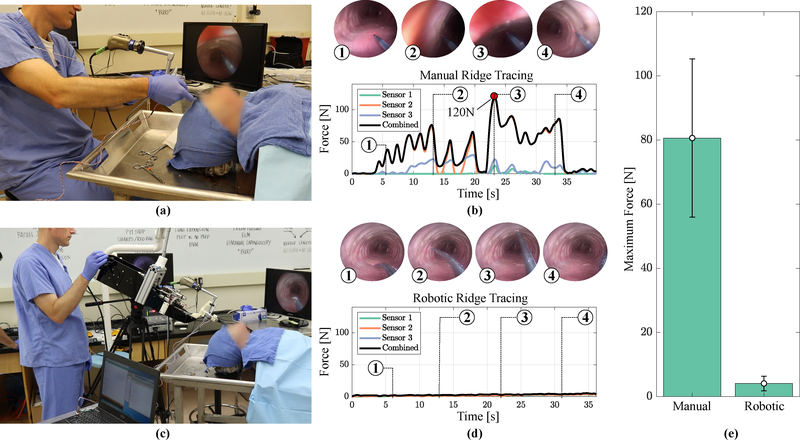
A force-sensing overtube was created to measure intraoperative forces exerted onto the patient by the bronchoscope as it passes through the mouth and throat, as shown in Fig. 3. The overtube consists of a thin-walled stainless-steel tube outfitted with three force-sensing resistors (FSR 408–200, Interlink Electronics) which extend along the length of the overtube, as shown in Fig. 3(a). The overtube is clamped onto a commercial rigid bronchoscope (Karl-Storz 26075AAS, 6° viewing angle) by 3D-printed, screw-tightened C-clamps (printed from PA-12 Nylon-based resin via multi-jet fusion process by Protolabs, Maple Plain, MN), such that forces applied to the patient by the bronchoscope are directly transmitted through the force-sensing overtube. The sensor strips are oriented 120° radially from each-other, enabling the measurement of forces at three radial locations around the tube and along the entire length of the tube, as shown in Fig. 3(a). A non-inverting operational amplifier configuration is used to convert the instantaneous resistance of the FSR strips into a voltage for subsequent data acquisition. After amplification, the calibrated sensitivity is 115 N/V, the range is 0–200N, and the resolution is 1.3N. The three force components are shown in Fig. 3(b), demonstrating high signal-to-noise and negligible cross-axis coupling. This force-sensing overtube enables the measurement of the force applied to the patient’s anatomy by the endoscope.
Figure 3:
Force-sensing overtube developed for intraoperative loading experiments: (a) exploded rendering of the overtube, where insets show an image of the fabricated device (top left) and sensor signal conditioning (bottom right), (b) example loading data showing multi-component force sensing with high signal-to-noise and negligible cross-talk.
2.5. Cadaveric Force Study: Conventional
Intraoperative force studies were performed by an experienced interventional pulmonologist (Dr. Maldonado) on a human cadaver (obtained through Vanderbilt University Medical Center’s S.R. Light Surgical Research and Training Laboratory). The physician inserted the bronchoscope, outfitted with the force-sensing overtube, into the trachea of the cadaver (Fig. 6(a)). The physician then selected a tracheal ridge and touched several evenly-spaced points along the circumference of the tracheal ridge with a Holmium YAG (Ho:YAG) laser fiber using the rigid bronchoscope (shown in Fig. 6(b),(1)–(4)). During the experiment, the forces measured by the force-sensing overtube were sampled at a rate of 100 samples per second. The experiment was repeated three times. Each experiment was video-recorded using an external camera (Nikon DSLR) and endoscopic camera (Karl Storz 22202011 Pure 1 HD). Force data were post-processed and statistically analyzed in mathematical software (MATLAB, Natick, Massachusetts).
Figure 6:
Intraoperative loading experiments: (a) image of the experimental setup for conventional bronchoscopy, (b) exemplary force measurements during one conventional ridge tracing experiment, where numbered insets show the endoscopic view that corresponds with the numbered force landmarks in the graph, (c) image of the experimental setup for robotic bronchoscopy, (d) exemplary force measurements during one robotic ridge tracing experiment, and (e) average maximum forces generated during each experiment showing a substantial reduction in the applied force. Please refer to the accompanying video.
2.6. Cadaveric Force Study: Robotic
The force experiment described in the previous section was repeated with the robot. The robot was supported by a counter-balance arm and tripod (shown in Fig. 6(c)), which bore the weight of the robot but allowed the physician to adjust the robot’s orientation to place the bronchoscope (outfitted with the force-sensing overtube) into the cadaver trachea. A Ho:YAG laser fiber was inserted into one of the robot arms, and as before, the physician touched at several points along the circumference of a cartilaginous ridge with the tip of the laser fiber, which passed through the robotic manipulator, which was itself passed through the endoscope port (see Fig. 6(d),(1)–(4)). The experiment was repeated three times, with the same data acquisition and video recording conditions as were used in the experiments with the conventional endoscope (described in the previous subsection).
3. Results
3.1. Ex Vivo Robotic Resection
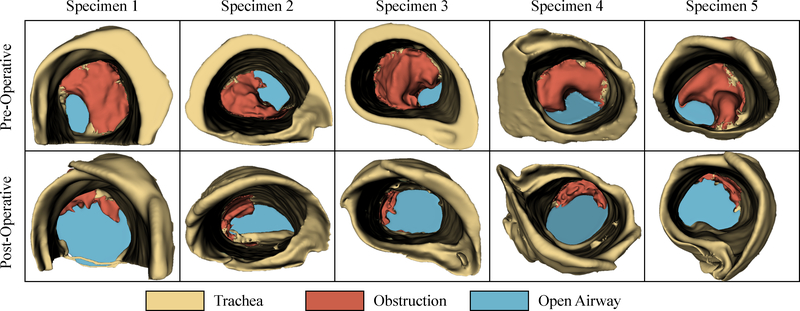
Results from the robotic resection experiments are shown in Figs. 4 and 5, and summarized in Table 1. A total of n=5 ovine plucks were prepared with the model obstructions for robotic resection, and the average pre-operative stenosis index as measured from the CT images was 75±6.9%. We note that obstructions with pre-operative SI of >70% are clinically categorized as ‘severe’30, so these represent significant obstructions that would likely require surgical intervention in clinical practice.
Figure 5:
Reconstructed CT volume renderings of the central airway obstructions for all specimens, both pre- and post-surgery, where the degree of airway restoration is apparent in the bottom row.
Table 1:
Robotic resection results over all trials.
| Specimen | A [mm2] | Aobstructed [mm2] | Pre-Op SI | Post-Op SI | Time [sec] |
|---|---|---|---|---|---|
| 1 | 297 | 57.7 | 80.6% | 15.6% | 876 |
| 2 | 295 | 98.4 | 66.7% | 17.4% | 253 |
| 3 | 291 | 48.5 | 83.3% | 10.5% | 265 |
| 4 | 316 | 93.8 | 70.3% | 12.8% | 203 |
| 5 | 271 | 63.9 | 76.5% | 14.2% | 349 |
| μ ± σ | 294±15.8 | 72.4±22.3 | 75.0±6.94% | 14.1±2.67% | 389±277 |
To resect the obstruction, the physician adopted a two handed approach (Fig. 4(b) and (d)) by using the secondary robotic arm to manipulate the obstruction and apply counter-traction while the primary arm cut through the obstruction with a monopolar electrosurgical probe. The airway cross-section was segmented in pre- and post-operative CT scan slices, shown in cyan in Fig. 4(c) and (e), respectively. Volumetric CT renderings of the pre- and post-operative obstructions are shown in Fig. 5 (with the obstruction shown in red and the airway shown in cyan), illustrating the degree of restoration. In every case, the surgeon was able to successfully restore the airway, achieving an average post-operative stenosis index of 14.1±2.67%. Further, the surgeon was able to complete all resections expeditiously, with an average procedure time of 389±277 seconds. Notably, there were no adverse events (tracheal perforation, equipment failure, or premature termination) recorded during any of the experiments. These results indicate that robotic CAO resection is an effective alternative to conventional rigid bronchoscopic approaches.
3.2. Cadaveric Force Study
Example force profiles representative of all experiments during both conventional and robotic force experiments are shown in Fig. 6. We controlled for the initial forces associated with rigid endoscope insertion, so the force values reported are those associated with intraoperative manipulation of the bronchoscope. Using the bronchoscope only, the cadaver experienced a maximum intraoperative force of 80.6±24.6N for n=3 (range: 52N - 120N). Using the robotic system, the cadaver experienced a maximum intraoperative force of 4.10±3.03N for n=3 (range: 2.72N - 6.74N). These results, summarized in Fig. 6(e), show that the use of a robotic system can decrease intraoperative forces by 95% compared to the conventional bronchoscopic technique, indicating the potential for associated reductions in complications due to mechanical trauma from excessive bronchoscope tilting.
4. Discussion
Current technological limitations make endoscopic CAO resection challenging for the physician to accomplish, and even in the best of cases place patients at risk for morbidity secondary to the instruments themselves (i.e. from endoscope tilting). We propose a novel robot for rigid bronchoscopy in the upper airway with the potential to address both these issues. We performed an initial feasibility study to determine whether the robot could be used by a physician to remove central airway obstructions in an animal model. Previously, a post-operative stenosis index of <50% has been clinically suggested as a benchmark for a successful surgery34,29. Our series of ex vivo robotic resections produced an average post-operative SI of 14%, substantially exceeding clinically acceptable thresholds. Further, the surgeon was able to complete the procedures in an average of 6 minutes, indicating that robotic resection of even severe obstructions can be performed rapidly.
The availability of two robotic arms also enables bimanual manipulation for simultaneous countertraction and electrosurgical resection, which is not possible in conventional bronchoscopy. However, further work will be needed to quantitatively evaluate the effects of the availability of two manipulators on overall procedure efficiency, although the short procedure durations seen in our study provides some anecdotal evidence that the robot may make the procedure easier to perform. The same is true of learning curves and whether performance improvements generalize to physicians not involved in the development of the robotic system. We also note that our system will be most useful in procedures that require a resection or other complex manipulations, and that for simple single-arm diagnostic or interventional procedures (e.g. those currently performed effectively via a flexible bronchoscope), it may not provide an advantage.
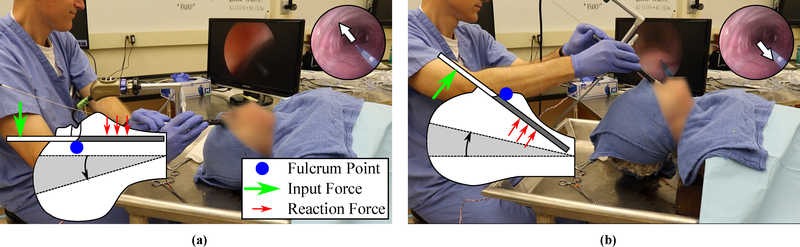
We also showed that the forces associated with intraoperative bronchoscope angulation can be reduced significantly with the new robotic approach. This reduction in forces is notable because bronchoscope angulation during central airway obstruction removal has been linked to mechanical trauma to teeth, oropharynx, vocal cords and other glottic structures, and in extreme cases, pneumothorax, hemorrhage and death39. Our experiments also provided insight into the mechanism of mechanical injury experienced during conventional rigid bronchoscopy. The results revealed that the maximum force is encountered while accessing the anterior side of the trachea, where the patient’s neck is hyperextended. In this configuration, the bronchoscope is putting substantial pressure on sensitive oral and glottic structures, where the palate and upper teeth act as fulcrums to flatten the tongue and epiglottis, in an effort to align the laryngeal and oral axes in an unnatural position (the intubation position32). This is shown in Fig. 7(a). Accessing the lateral sides results in lower (but still substantial) forces, as the glottis is still compressed. The lowest forces are experienced while accessing the posterior side, where the fulcrum shifts to the tongue and the neck is able to adopt a more neutral configuration, as shown in Fig. 7(b).
Figure 7:
Neck hyperextension during conventional bronchoscopy, where inset cartoon shows neck/scope angulation and red arrows illustrate loading: (a) anterior access, (b) posterior access.
During robotic bronchoscopy, the need for endoscope angulation is eliminated; the bronchoscope can remain stationary and the robotic manipulators can access the entirety of the tracheal cross section. This enables the patient’s neck to maintain a neutral position for the duration of the procedure, and the endoscope is able to adopt a configuration that minimizes lateral force exertion on oral and glottic structures. The added distal dexterity of the robot has the additional benefit of ensuring a constant and unchanging field of view during tool manipulations within the trachea, since the tool motion is decoupled from the motion of the bronchoscope.
This study has several limitations worthy of mention. The use of cadaveric tissue as opposed to live tissue invariably affects the absolute results of the force study. However, given that we compared relative performance between robotic and conventional bronchoscopy (rather than absolute forces), the cadaver presents a suitable analog that captures the complex airway anatomy which is the primary factor contributing to complications associated with bronchoscope tilting. The use of a single cadaver prevents our force results from capturing any gender- or age-specific biases. The force and resection experiments were both performed by an experienced interventional pulmonologist (Dr. Maldonado), motivating our desire to conduct a future multi-user follow up study to capture the effects of training and experience on intraoperative loading and resection efficiency. Finally, we emphasize that the robotic system as described is still in the proof-of-concept phase, and development is currently underway to address issues regarding sterilizability, designing for manufacturability and assembly, and obtaining regulatory approval.
5. Conclusion
This study is the first of its kind to demonstrate the use of robotic rigid bronchoscopy as a viable and potentially superior alternative to conventional rigid bronchoscopy in the management of central airway obstructions. The additional distal dexterity and two-handed capability offered by the robotic system enables effective resection of obstructions while reducing lateral forces on the patient due to bronchoscope angulation. These results, while preliminary, indicate significant promise in terms of reducing intraoperative forces applied to the patient, and thereby reducing the incidence of corresponding complications. Current work is focused on the preparation and execution of a statistically-powered performance evaluation of the robotic system in human cadavers, where we aim to quantitatively and statistically demonstrate that physicians can perform CAO surgeries more safely, effectively, and efficiently with our system than they can with conventional bronchoscopy.
While CAO resection serves as an excellent proving ground for this system, we also envision many potential applications in endoscopic thoracic surgery where distal maneuverability is limited and endoluminal space is at a premium. In particular, this platform could serve many bronchoscopic approaches to airway diseases, such as treatment of dehiscence, stenosis24, tracheomalacia via bronchoscopic tracheoplasty33, and perhaps even thoracic natural orifice transluminal endoscopic surgery (NOTES)23. There are also potential applications in the ear, nose and throat (ENT) space; in particular, endoscopic treatment of benign subglottic stenosis36, and resection of post-tracheostomy airway stenosis. Thus, we foresee the new platform we have described in this paper as having many future applications beyond the initial proof-of-concept experiments described in this paper, with the ultimate goal of helping physicians treat many patients more safely and less invasively.
Acknowledgments
Financial Support: The authors thank the National Institutes of Health (NIH) Small Business Technology Transfer (STTR) for Grant R41 HL140709 which supported the work described in this paper. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Institutes of Health.
Role of Sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Footnotes
Conflict of Interest
The authors have reported to Annals of Biomedical Engineering the following conflicts of interest: The robot concept described in this paper originated in R.W.’s laboratory at Vanderbilt University, and is in the early stages of technology transfer to a startup company, Virtuoso Surgical, Inc., created for purposes of bringing this technology to market, under a Phase I STTR grant from the National Institutes of Health. S.W., N.D., E.B., R.H., D.H. and R.W. are equity holders in Virtuoso. S.W., N.D., E.B. and R.H. are employed by Virtuoso. R.W. and R.H. are founders and board members of Virtuoso, with R.W. serving as president and R.H. serving as Chief Operating Officer. The robot described in this paper is an early-stage prototype, and has not yet begun to go through the FDA approval process. It is not cleared for human use or available for purchase. It will undergo a number of extensive design revisions before becoming a commercial product.
Statement of Ethical Approval: All experiments were performed by a single interventional pulmonologist (F.M.). We did not recruit a multiple-user cohort for these proof-of-concept experiments. Based on this consideration, and the fact that no patients or live animals were involved in our experiments, prior approval from an ethics committee (IRB or IACUC) was not required.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Breatnach E, Abbott G, and Fraser R Dimensions of the Normal Human Trachea. American Journal of Roentgenology 142:903–906, 1984. [DOI] [PubMed] [Google Scholar]
- 2.Brichet A, Verkindre C, Dupont J, Carlier M, Darras J, Wurtz A, Ramon P, and Marquette C Multidisciplinary approach to management of postintubation tracheal stenoses. European Respiratory Journal 13:888–893, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Brodie A and Vasdev N The future of robotic surgery. Robotics: Annals of the Royal College of Surgeons of England pp. 4–13, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casal RF, Iribarren J, Eapen G, Ost D, Morice R, Lan C, Cornwell L, Almeida FA, Grosu H, and Jimenez CA Safety and effectiveness of microdebrider bronchoscopy for the management of central airway obstruction. Respirology 18:1011–1015, 2013. [DOI] [PubMed] [Google Scholar]
- 5.Chan E Malignant airway obstruction: treating central airway obstruction in the oncologic setting. UWOMJ 80:7–9, 2011. [Google Scholar]
- 6.Chan JYK, Wong EWY, Tsang RK, Holsinger FC, Tong MCF, Chiu PWY, and Ng SSM Early results of a safety and feasibility clinical trial of a novel single - port flexible robot for transoral robotic surgery. European Archives of Oto-Rhino-Laryngology 274:3993–3996, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen C, Bent JP, and Parikh SR Powered debridement of suprastomal granulation tissue to facilitate pediatric tracheotomy decannulation. International journal of pediatric otorhinolaryngology 75:1558–1561, 2011. [DOI] [PubMed] [Google Scholar]
- 8.Chen K, Joseph Varon M, Wenker OC et al. Malignant airway obstruction: recognition and management. The Journal of emergency medicine 16:83–92, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Chhajed PN, Baty F, Pless M, Somandin S, Tamm M, and Brutsche MH Outcome of treated advanced non-small cell lung cancer with and without central airway obstruction. Chest 130:1803–1807, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Ernst A, Feller-Kopman D, Becker HD, and Mehta AC Central airway obstruction. American journal of respiratory and critical care medicine 169:1278–1297, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin J-C, Pujol S, Bauer C, Jennings D, Fennessy F, Sonka M, Buatti J, Aylward S, Miller JV, Pieper S, and Kikinis R 3d slicer as an image computing platform for the quantitative imaging network. Magnetic Resonance Imaging 30:1323–1341, 2012. Quantitative Imaging in Cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feins RH, Burkhart HM, Conte JV, Coore DN, Fann JI, Hicks GL Jr, Nesbitt JC, Ramphal PS, Schiro SE, Shen KR et al. Simulation-based training in cardiac surgery. The Annals of thoracic surgery 103:312–321, 2017. [DOI] [PubMed] [Google Scholar]
- 13.Gilbert HB, Rucker DC, and Webster RJ III. Concentric Tube Robots: The State of the Art and Future Directions. In: Robotics Research, volume 114, pp. 253–269. 2016. [Google Scholar]
- 14.Ginsberg R, Vokes E, and Raben A Non-small cell lung cancer. In: Cancer: Principles and Practice of Oncology:, pp. 858–910. 1997. [Google Scholar]
- 15.Gompelmann D, Eberhardt R, and Herth F Novel endoscopic approaches to treating chronic obstructive pulmonary disease and emphysema In: Seminars in respiratory and critical care medicine, volume 36, pp. 609–615, Thieme Medical Publishers2015. [DOI] [PubMed] [Google Scholar]
- 16.Hans S, Delas B, Gorphe P, Ménard M, and Brasnu D Transoral robotic surgery in head and neck cancer. European Annals of Otorhinolaryngology, Head and Neck diseases 129:32–37, 2012. [DOI] [PubMed] [Google Scholar]
- 17.Hendrick RJ, Mitchell CR, Herrell SD, and Iii RJW Hand-held transendoscopic robotic manipulators : A transurethral laser prostate surgery case study. International Journal of Robotics Research 34:1559–1572, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hohenforst-Schmidt W, Zarogoulidis P, Pitsiou G, Linsmeier B, Tsavlis D, Kioumis I, Papadaki E, Freitag L, Tsiouda T, Turner JF et al. Drug eluting stents for malignant airway obstruction: a critical review of the literature. Journal of Cancer 7:377, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ingenito EP, Wood DE, and Utz JP Bronchoscopic lung volume reduction in severe emphysema. Proceedings of the American Thoracic Society 5:454–460, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar A and Asaf BB Robotic thoracic surgery: the state of the art. Journal of minimal access surgery 11:60, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahmood K and Wahidi MM Ablative Therapies for Central Airway Obstruction. Semin Respir Crit Care Med pp. 681–692, 2014. [DOI] [PubMed] [Google Scholar]
- 22.Mahoney AW, Gilbert HB, and Webster RJ Chapter 7: A Review of Concentric Tube Robots: Modeling, Control, Design, Planning, and Sensing. In: The Encyclopedia of Medical Robotics, volume 1, pp. 181–202. 2018. [Google Scholar]
- 23.Makris KI, Rieder E, and Swanstrom LL Natural Orifice Trans-Luminal Endoscopic Surgery (NOTES) in Thoracic Surgery. Seminars in Thoracic and Cardiovascular Surgery 22:302–309, 2010. [DOI] [PubMed] [Google Scholar]
- 24.Maloney JD, Weigel TL, and Love RB Endoscopic repair of bronchial dehiscence after lung transplantation. Annals of Thoracic Surgery 72:2109–2111, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Mattheis S, Hasskamp P, Holtmann L, Sch C, Geisthoff U, Dominas N, and Lang S Flex Robotic System in transoral robotic surgery : The first 40 patients. Head and Neck pp. 471–475, 2017. [DOI] [PubMed] [Google Scholar]
- 26.McDougall J and Cortese D Neodymium-yag laser therapy of malignant airway obstruction. a preliminary report. In: Mayo Clinic proceedings, volume 58, pp. 35–39. 1983. [PubMed] [Google Scholar]
- 27.Mineshita M and Slebos D-J Bronchoscopic interventions for chronic obstructive pulmonary disease. Respirology 19:1126–1137, 2014. [DOI] [PubMed] [Google Scholar]
- 28.Mokadam NA, Fann JI, Hicks GL, Nesbitt JC, Burkhart HM, Conte JV, Coore DN, Ramphal PS, Shen KR, Walker JD et al. Experience with the cardiac surgery simulation curriculum: results of the resident and faculty survey. The Annals of thoracic surgery 103:322–328, 2017. [DOI] [PubMed] [Google Scholar]
- 29.Mudambi L, Miller R, and Eapen GA Malignant central airway obstruction. Journal of thoracic disease 9:S1087, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murgu S and Colt H Subjective assessment using still bronchoscopic images misclassifies airway narrowing in laryngotracheal stenosis. Interactive Cardiovascular and Thoracic Surgery 16:655–660, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murgu S and Colt HG Morphometric bronchoscopy in adults with central airway obstruction: Case illustrations and review of the literature. Laryngoscope 119:1318–1324, 2009. [DOI] [PubMed] [Google Scholar]
- 32.Nicastri DG and Weiser TS Rigid Bronchoscopy : Indications and Techniques. YOTCT 17:44–51, 2012. [Google Scholar]
- 33.Nouraei SA, Kapoor KV, Nouraei SM, Ghufoor K, Howard DJ, and Sandhu GS Results of endoscopic tracheoplasty for treating tracheostomy-related airway stenosis. Clinical Otolaryngology 32:471–475, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Ost DE, Ernst A, Grosu HB, Lei X, Diaz-Mendoza J, Slade M, Gildea TR, Machuzak MS, Jimenez CA, Toth J et al. Therapeutic bronchoscopy for malignant central airway obstruction. Chest 147:1282–1298, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pathak V, Welsby I, Mahmood K, Wahidi M, Macintyre N, and Shofer S Ventilation and Anesthetic Approaches for Rigid Bronchoscopy. Ann Am Thorac Soc 11:628–634, 2014. [DOI] [PubMed] [Google Scholar]
- 36.Raman T, Chatterjee K, Alzghoul BN, Innabi AA, Tulunay O, Bartter T, and Meena NK A bronchoscopic approach to benign subglottic stenosis. SAGE Open Medical Case Reports 5:2050313X1771315, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Remacle M, Prasad V, Lawson G, Plisson L, Bachy V, and Vorst SVD Transoral robotic surgery ( TORS ) with the Medrobotics Flex System : first surgical application on humans. European Archives of Oto-Rhino-Laryngology 272:1451–1455, 2015. [DOI] [PubMed] [Google Scholar]
- 38.Schuler PJ, Hoffmann TK, Veit JA, Friedrich DT, and Scheithauer MO Hybrid procedure for total laryngectomy with a flexible robot-assisted surgical system. Int J Med Robotics Comput Assist Surg 13:1–7, 2017. [DOI] [PubMed] [Google Scholar]
- 39.Stahl D, Richard K, and Papadimos T Complications of bronchoscopy: A concise synopsis. International Journal of Critical Illness and Injury Science 5:189–95, 2015–07-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stratakos G, Gerovasili V, Dimitropoulos C, Giozos I, Filippidis FT, Gennimata S, Zarogoulidis P, Zissimopoulos A, Pataka A, Koufos N et al. Survival and quality of life benefit after endoscopic management of malignant central airway obstruction. Journal of Cancer 7:794, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Toma TP, Hopkinson NS, Hillier J, Hansell DM, Morgan C, Goldstraw PG, Polkey MI, and Geddes DM Bronchoscopic volume reduction with valve implants in patients with severe emphysema. The Lancet 361:931–933, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Vishwanath G, Madan K, Bal A, Aggarwal AN, Gupta D, and Agarwal R Rigid bronchoscopy and mechanical debulking in the management of central airway tumors: an indian experience. Journal of bronchology & interventional pulmonology 20:127–133, 2013. [DOI] [PubMed] [Google Scholar]
- 43.Williamson J, Phillips M, Hillman D, and Eastwood P Managing obstruction of the central airways. Internal medicine journal 40:399–410, 2010. [DOI] [PubMed] [Google Scholar]
- 44.Yang B, Zhao F, Zong Z, Yuan J, Song X, Ren M, Meng Q, Dai G, Kong F, Xie S et al. Preferences for treatment of lobectomy in chinese lung cancer patients: video-assisted thoracoscopic surgery or open thoracotomy? Patient preference and adherence 8:1393, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang L and Gao S Robot-assisted thoracic surgery versus open thoracic surgery for lung cancer: a system review and meta-analysis. International journal of clinical and experimental medicine 8:17804, 2015. [PMC free article] [PubMed] [Google Scholar]