Abstract
Background:
Treatment of autoimmune diseases has relied on broad immunosuppression. Knowledge of specific interactions between human leukocyte antigen (HLA), the autoantigen, and effector immune cells, provides the foundation for antigen-specific therapies. These studies investigated the role of HLA, specific myeloperoxidase (MPO) epitopes, CD4+ T cells, and ANCA specificity in shaping the immune response in patients with anti-neutrophil cytoplasmic autoantibody (ANCA) vasculitis.
Methods:
HLA sequence-based typing identified enriched alleles in our patient population (HLA-DPB1*04:01 and HLA-DRB4*01:01), while in silico and in vitro binding studies confirmed binding between HLA and specific MPO epitopes. Class II tetramers with MPO peptides were utilized to detect autoreactive CD4+ T cells. TCR sequencing was performed to determine the clonality of T cell populations. Longitudinal peptide ELISAs assessed the temporal nature of anti-MPO447–461 antibodies. Solvent accessibility combined with chemical modification determined the buried regions of MPO.
Results:
We identified a restricted region of MPO that was recognized by both CD4+ T cells and ANCA. The autoreactive T cell population contained CD4+CD25intermediateCD45RO+ memory T cells and secreted IL-17A. T cell receptor (TCR) sequencing demonstrated that autoreactive CD4+ T cells had significantly less TCR diversity when compared to naïve and memory T cells, indicating clonal expansion. The anti-MPO447–461 autoantibody response was detectable at onset of disease in some patients and correlated with disease activity in others. This region of MPO that is targeted by both T cells and antibodies is not accessible to solvent or chemical modification, indicating these epitopes are buried.
Conclusions:
These observations reveal interactions between restricted MPO epitopes and the adaptive immune system within ANCA vasculitis that may inform new antigen-specific therapies in autoimmune disease while providing insight into immunopathogenesis.
Keywords: ANCA vasculitis, autoreactive T cells, epitope specificity, immunodominant epitopes, ANCA specificity
1.1. INTRODUCTION
Initiation of the autoimmune response centers around the specific interactions of the major histocompatibility complex (MHC), peptides from the self-antigen, and recognition by T cell receptors (TCRs) on T cells. Autoreactive T cells then help B cells produce high-affinity, class-switched autoantibodies. These interactions are key to understanding the immunopathogenesis of autoimmune disease and must be fully understood before clinical attempts to initiate antigen-specific therapies.
Numerous autoimmune diseases have benefited from GWAS by identification of human leukocyte antigens (HLAs) that are highly associated with disease. This holds true in ANCA vasculitis as well (1–3). However, the autoantigen for most autoimmune diseases remains poorly understood, thereby hindering investigation into immunopathogenesis. ANCA vasculitis studies benefit from the knowledge that proteinase-3 (PR3) and MPO are the targeted autoantigens in disease. Furthermore, studies of MPO antigenicity have repeatedly demonstrated the pathogenic potential of anti-MPO ANCA both in vitro and in vivo (4).
While the presence of autoantibodies is a classic hallmark of numerous autoimmune diseases (anti-type IV collagen antibodies in anti-glomerular basement membrane disease (5, 6), anti-mitochondrial antibodies in primary biliary cirrhosis (7–9), anti-PLA2R antibodies in membranous nephropathy (10, 11)), these antibody responses are normally polyclonal and how the epitope specificity of autoantibodies impact disease is relatively unknown. Fortunately, several studies have mapped epitopes within MPO that are targeted by ANCA (12–18). Our group previously utilized epitope excision mass spectrometry and identified MPO epitopes targeted during active disease, remission, and natural autoantibody epitopes targeted by healthy individuals (12). One of these epitopes was linear (MPO447–459) and could be utilized for ELISA-based studies to determine that reactivity to this epitope was associated with disease activity in a subset of patients. Additional studies in a mouse model of MPO-ANCA glomerulonephritis (GN) mapped MPO epitopes targeted by CD4+ and CD8+ T cells (19, 20). Collectively, these mouse studies have bolstered the findings in patients, and demonstrated the ability to reintroduce immune tolerance using nasal insufflation of an immunodominant MPO epitope (mouse MPO409–428 (21). Treatment with this singular epitope induced tolerance to the specific region of MPO, but downregulated the immune response to the entire MPO molecule. This underscores the immunoregulatory potential of this MPO region.
Previous studies from our group reported an expanded population of memory effector CD25intermediate T cells that secrete pro-inflammatory cytokines (22), determined epitope specificity of MPO-ANCA, and identified potentially pathogenic regions of interest within MPO (12). The region identified by our epitope excision studies of MPO-ANCA (MPO447–459) overlaps significantly with an immunodominant CD4+ T cell epitope identified in a mouse model of MPO-induced GN (20). Identification of these epitopes set the stage to investigate the specific interactions between HLA alleles, previously reported epitopes, and CD4+ T cells in patients. The ANCA vasculitis field is poised to answer questions about disease immunopathogenesis and provide a foundation for future, antigen-specific therapies.
The studies presented herein are aimed to decipher the epitope specificity of the adaptive immune response in the periphery of ANCA vasculitis patients. Furthermore, these studies elucidate the specific MPO epitopes these CD4+ T cells react with, the phenotype of the autoreactive cells, and their clonal restriction. Additionally, with a large longitudinal cohort of MPO-ANCA vasculitis patients, we examined the temporal nature of the anti-MPO447–461 ANCA response and how it relates to disease status. Further investigation into the biochemical structure of the targeted MPO region provide insight into the initiation of the MPO-ANCA response. Collectively, these new findings elucidate the possibility that environmental alterations to the MPO molecule may unveil an otherwise hidden epitope to which both CD4+ T cells and B cells are targeting within MPO-ANCA vasculitis patients.
1.2. METHODS
1.2.1. Study Cohort for HLA and Tetramer Studies
Patients with ANCA vasculitis were diagnosed according to the Chapel Hill Consensus Conference (23, 24), were enrolled at University of North Carolina at Chapel Hill clinics and followed in the Glomerular Disease Collaborative Network (25, 26). Patients and healthy volunteers were recruited according to the guidelines of the Institutional Review Board (IRB) (study no. 97–0523) by the University of North Carolina Office of Human Research Ethics. Study subjects gave informed, written consent and participated according to University IRB guidelines.
Patient charts were reviewed by clinicians to confirm disease activity status, periods of relapse, periods of remission, and serotype, prior to analysis. Disease activity was determined by the 2003 Birmingham Vasculitis Activity Score (BVAS) in conjunction with clinical signs of activity. Patients with a BVAS of 0 and no clinical or laboratory evidence of active disease were considered to be in remission. Active disease was defined as a BVAS >0 with clinical and/or laboratory evidence of disease (27). ANCA serotypes were assessed by indirect immunofluorescence and antigen-specific PR3 and MPO ELISAs. Patients were excluded from the study if they were ANCA negative by ELISA, had suspected or confirmed drug-induced forms of ANCA vasculitis, or had overlapping diseases. Demographic features of the study subjects can be found in Supplementary Table 3.
1.2.2. Patient Cohort for ELISAs
A total of 979 serum samples were tested by ELISA for reactivity to native MPO and MPO447–459 (Inova, Biomatik). Control samples included serum from 93 healthy controls, 24 patients with systemic lupus erythematosus, and 48 patients with PR3-ANCA, of which half were in remission (Supplemental Tables 4 and 5). The remaining serum samples were acquired from 51 patients with MPO-ANCA at multiple time points spanning disease course (>4 samples n=45, >3 samples n=50) as determined by clinical manifestations of disease and conventional assays. We also compared patients who were ever positive for anti-MPO447–461 to those who were negative for reactivity to MPO447–461 and PR3 controls to identify any potentially confounding differences in cohort characteristics (Supplemental Table 5).
1.2.3. HLA Sequence-Based Typing
High-resolution HLA typing was performed at the Institute for Immunology and Infectious Diseases (IIID) in Perth Western Australia. All steps in the pipeline, from sample preparation to the reporting, were quality assessed and tracked using an accredited Laboratory Information and Management System (ELab). Automated liquid handlers and data analysis was performed using the accredited proprietary software applications HLA Allele Caller and HLA Analysis Suite (Murdoch University, Western Australia). HLA loci were PCR amplified for Class I (A, B, C Exons 2 and 3) and Class II (DQB1, Exons 2 and 3; DRB,DQA1 and DPB1, Exon 2) MHC genes using MID tagged primers optimised to minimize allele dropouts and primer bias. Amplified DNA products from unique MID tagged products were pooled in equimolar ratios and prepared for Illumina sequencing. Libraries were quantified by qPCR and quality assessed on the Agilent Bio analyser or Tape Station. Normalized libraries were then sequenced using Illumina MiSeq platform (MiSeq V3 600-cycle kit, 2X300bp paired end reads). The quality filtered data was demultiplexed by MID tags, merged and the alleles called using the HLA Allele Caller software that minimises the influence of sequencing errors. Alleles were called using the latest IMGT HLA allele database as the allele reference library. The HLA Analysis Suite reporting software performed comprehensive allele balance checks, and assigned putative allele calls for ambiguous results and contamination checks on the final dataset.
1.2.4. In Silico and In Vitro Binding Studies
In silico predictions were made by the Immune Epitope Database (www.iedb.org) to identify antigen candidates that were then tested by in vitro binding studies as previously described (28). Reported predicted percentile rank is for the peptide and the allele most frequent in positives, and is calculated based on how the predicted affinity of each epitope ranks compared to a very large set of epitopes randomly extracted from UniProt.
Assays to quantitatively measure peptide binding to HLA-DRB4*01:01 and HLADPB1*0401 (class II) MHC molecules were based on the inhibition of binding of a high affinity radiolabeled peptide to purified MHC molecules, and were performed essentially as described elsewhere (29–31).
1.2.5. Tetramer Staining and Flow Cytometry
Tetramers were generated (Benaroya Institute, CA) for HLA-DPB1*04:01, HLADRB4*01:01, and HLA-DRB1*04:01 as previously described (32) with the following MPO epitopes: MPO447–461(RKIVGAMVQIITYRD), MPO435–454(PRWDGERLYQEARKIVGAMV), MPO488–502(PRIANVFTNAFRYGH), MPO545–559(PILRGLMATPAKLNR), and MPO409–423(MPELTSMHTLLLREH). Tetramers created with MPO447–461 scramble (IKAVRIYIGDVMRQT) were used as a control for all HLA alleles.
Patient peripheral blood mononuclear cells (PBMCs) were isolated from cell preparation tubes (CPT, BD Biosciences), washed with phosphate buffered saline (PBS, Life Technologies) and stored in RPMI with 10% FBS at 4° C overnight. Cells were counted and resuspended at 5×106/mL in RPMI with 10% FBS, blocked (Human TruStain FcX, Biolegend) for 10 minutes at 37° C. 5×105 cells per condition were incubated with either no tetramer, or 2 uL of tetramer for 1 hour at 37°C. Cells were then washed, stained with a panel including CD4 (BioLegend, clone RPA-T4), CD8 (BioLegend, clone HIT8a), CD25 (BioLegend, clone BC96), and CD127 (BioLegend, clone A019D5) or a panel including CD4 (BioLegend, clone RPA-T4), CD8 (BioLegend, clone HIT8a), and CD45RO (BioLegend, clone UCHL1). Cells were acquired on an LSRII flow cytometer (BD), and analyzed using FlowJo software (Tree Star).
Cytokine capture for IL-17A production was performed on patient PBMCs in addition to tetramer staining. Staining was performed following the kit protocol (IL-17A secretion assay PE, Miltenyi Biotec). Cells were stained with tetramer, incubated with CytoStim (human, Miltenyi Biotec) for 4 hours at 37° C, and then stained with capture and detection antibodies, CD4, CD8, CD25, CD127 before fixation and analysis on a LSRII flow cytometer (BD), and analyzed using FlowJo software (Tree Star).
For sorting experiments, PBMCs were isolated and prepared as previously mentioned. PBMCs were then incubated with 3 uL of DRB4*01:01-MPO435–454 per 30×106 cells for 1 hour at 37° C. Cells were then washed, stained with a panel including CD4 (BioLegend, clone RPA-T4), CD8 (BioLegend, clone HIT8a), CD14 (BioLegend, clone 63D3), CD19 (Life Technologies, clone SJ25-C1), CD25 (BioLegend, clone BC96), and CD127 (BioLegend, clone A019D5). Cells were sorted into PBS using a Becton Dickinson FACSAria II (BSL-2) flow cytometer with a 70 μm nozzle.
1.2.6. RNA Isolation
RNA was isolated from sorted T cells using RNAqueous Micro Total RNA Isolation Kit (TermoFisher Scientific), and frozen at −80° C until library preparation.
1.2.7. TCR Sequencing
T cell receptor (TCR) beta repertoire profiling was done with repertoire diversity measures adjusted for differential read coverage as previously described (33, 34). Briefly, RNA quantity was assessed by bioanalyzer before processing with the SMARTer ® Human TCR a/b Profiling Kit (kit lot # 1512025A). Post library prep, library concentration was measured by Qubit 2.0 Fluorometer using the dsDNA HS Assay Kit (lot# 1910791). Qubit was calibrated using the dsDNA HS standards #1 and #2 (0 ng/uL and 10 ng/uL respectively), then sample was diluted 1uL in 199 uL of the assay buffer and run on Qubit after the standards. Samples were then run on an Agilent Technologies 2200 Tapestation to determine library size in base pairs using the High Sensitivity D1000 screentape and associated buffer (2 uL buffer:2 uL sample). Samples were then diluted to 10 nM with SMARTer ® Human TCR a/b Profiling Kit elution buffer, based on Qubit concentrations and pooled in equal volumes. Qubit and tapestation were repeated on the final pool. The pool was loaded onto a MiSeq at the UNC High Throughput Sequencing Facility at 13.5 pM.
1.2.8. Myeloperoxidase ELISA
The MPO ANCA serum samples were screened for MPO titers regardless of disease activity or previously reported titers. The MPO ANCA kits were purchased from INOVA and all the samples were run in duplicate following the recommended protocol. Absorbance values were measured by a Tecan Infinite M200 Pro plate reader.
1.2.9. Peptide ELISA and Alanine Scanning
ELISA studies utilized a peptide with the addition of two amino acids (MPO447–461 instead of MPO447–459) to increase solubility of the peptide. Solid white ELISA plates (Grenier) were coated with 0.01mM peptide coating solution made in 0.5M carbonate-bicarbonate buffer at pH 9.3 and incubated overnight at 4° C. Plates were blocked in Dulbecco’s PBS (Gibco) with 1% goat serum (Gibco) and 0.5% Tween20 (Fisher Scientific) for 2 hours at 37° C. The serum samples were diluted in blocking buffer (1:100), applied to the plate and incubated at 37° C for 1 hour. Goat anti-human with alkaline phosphatase conjugate secondary antibody specific to Ig (H+L) (Millipore) (1:10,000) was diluted in blocking buffer and then incubated on the plate for 1 hour at room temperature and detected using CDP-Star substrate in Roche Detection Buffer with enhancer in a Tecan Infinite M200 Pro plate reader programmed for luminescence. To determine the residues required for binding, each amino acid in MPO447–461 (RKIVGAMVQIITYRD) was replaced with an alanine. All peptides were run in triplicate with positive (known positive patient against MPO447–461) and negative (healthy serum and secondary alone) controls consistent across plates, with one patient per plate. Chemiluminescence was measured using a Tecan Infinite M200 Pro plate reader (Life Sciences).
1.2.10. Circular Dichroism (CD) Spectroscopy
The spectra were the average of four scans obtained by collecting data at 0.2 nm intervals from 260 to 190 nm, with a response time of 2s and a bandwidth of 1 nm. The raw CD data were adjusted by subtracting a buffer blank. Peptide concentration in all samples was 100 uM in 10 mM phosphate buffer, pH 7.4 containing 150 mM NaCl.
1.2.11. Solvent Predictions
Solvent accessibility was calculated using the ‘rolling ball’ algorithm and the GETAREA software (35). The atomic coordinates were obtained from the Protein Data Bank (entry 1CXP). A sphere (of solvent) with the radius of 1.4 Å was used to ‘probe’ the surface of MPO. Residues were considered to be solvent exposed if the ratio value exceeded 50% and to be buried if the ratio was less than 20%.
1.2.12. Isotope Labeling with Succinic Anhydride
Succinylation of lysine-ε-amino and N-terminal amino groups were performed at pH 7.8 for 30 min at 37°C in 100 mM NaHCO3 coupling buffer (succinic-d4 anhydride, CDN isotopes; succinic-d0 anhydride, Sigma). A two-step d0-succinylation/d4-succinylation protocol was employed for the quantitative estimation of relative reactivities of each amino group. First a 0.5–225-fold molar excess of succinic-d0 anhydride relative to the protein (MPO, Lee Biosolutions) was used for its partial modification at the reaction condition described above. The reaction was stopped by adding 100 mM Tris quenching buffer, pH 8.0, the disulfide bridges were reduced by 5 mM DTT, they were alkylated by 15 mM iodoacetamide, and the quenching buffer was removed by performing buffer exchange with the coupling buffer on a PD 10 column. Complete modification was subsequently obtained by the reaction of the protein with a 2250-fold excess of succinic-d4 anhydride. The excess of reagent was removed from the succinylated proteins by performing an additional buffer exchange with a 50 mM NH4HCO3 digestion buffer, pH 7.8, the resulting protein mixture was digested by trypsin, and the tryptic peptides were analyzed by liquid chromatography electrospray mass spectrometry. Relative reactivities were calculated from the ratio of the intensities of the modified and isotopically labeled peptides.
1.2.13. Protein Modeling
Human MPO structure was obtained using Protein Data Bank code 1CXP and visualized using PyMOL by Schrödinger.
1.2.14. Statistical Analysis
Cohort HLA allele prevalence was provided by the percentage. GraphPad Prism was used to generate figures (Version 7, GraphPad Software, La Jolla, CA) and analyses were conducted using SAS software (Version 9.4 SAS Institute, Cary, NC). Paired differences were evaluated between CD4+ T cell tetramers among MPO patients (Figure 1 C and D) and tested for differences from zero using a paired sign rank test. A paired sign rank test was also used to evaluate anti-MPO447–461 reactivity for patients with measures at onset with active disease and during remission (Figure 5 B). For non-paired comparisons, Wilcoxon two sample tests were used for continuous measures and Fisher Exact test for categorical variables. Comparisons of anti-MPO447–461 reactivity between healthy controls and patient groups including those with PR3-ANCA, SLE and MPO-ANCA (Figure 5 A) were done using Wilcoxon two sample test with a Boferreoni correction for multiple comparisons applied (critical value = 0.0083).
For TRB analysis, amplicon read quality was assessed using FastQC, indicating read counts and Phred scores were comparable for all samples with all samples having over 250K reads. TRB clones were aligned and assembled using MiXCR 2.1.9 (36) with imgt library version 201802–5 (37). Diveristy metrics were calculated on the nucleotide sequence of Complementarity-Determining Region 3 (CDR3) of productive clones. The following normalized diversity metrics were calculated using VDJTools (38) to bootstrap counts 100x to the lowest number of aligned reads for all samples: Observed Diversity, Shannon-Wiener, Normalized Shannon-Wiener and d50. Diversity metrics were compared using a T-test for two-group comparison and an ANOVA for more than two groups. Patient Identity was taken into account for these comparisons. False Discovery Rate corrections were done using the Benjamini-Hochberg method (39) using R ((40); stats::p.adjust). Morisita Horn Similarity Indices were performed on the amino acid CDR3 sequence of productive clones using the ‘horn’ method (41) of vegan::vegdist (42). The Morisita-Horn Dissimilarity Indices were subtracted from one to get the Similarity Indices presented. T-tests were used to compare differences in these indices between groups. Graphs were made using ggplot2 (43).
1.3. RESULTS
1.3.1. In Silico and In Vitro Binding Studies Confirm the Ability of MPO Epitopes to Bind HLA
Previous GWAS identified an association between HLA and ANCA vasculitis (1–3) and recently identified HLA-DPB1 as being associated with PR3-ANCA vasculitis, and HLA-DQB1 with MPO-ANCA vasculitis (3). Expanding on the GWAS results, Hilhorst et al. demonstrated increased risk of relapse in patients carrying HLA-DPB1*04:01 regardless of disease serotype (44). These studies demonstrate the importance of specific HLA alleles and their contribution to ANCA vasculitis. To identify specific HLA variants through an unambiguous method, HLA typing was performed on our cohort of MPO-ANCA vasculitis patients (n=167) and a combined total of 104 local healthy controls (n=44 submitted to IIID for sequencing, n=60 from HLA organ donor typing at UNC). In our cohort, the two most prevalent class II alleles in MPO-ANCA vasculitis were HLA-DPB1*04:01 (38%) and HLA-DRB4*01:01 (39%) (Supplementary Table 1). These alleles are similar in prevalence to the local healthy control population: HLA-DPB1*04:01 (32%) and HLA-DRB4*01:01 (16%) (Supplementary Table 2), though HLA-DRB4*01:01 may be more prevalent in our cohort of MPO-ANCA vasculitis patients. Allele prevalence accounts for the fact that each patient contains two alleles per HLA locus. However, when examining HLA frequency (homozygous and heterozygous patients are considered equally), 52% percent of our MPO-ANCA vasculitis patients had HLA-DPB1*04:01 and 51% had HLA-DRB4*01:01 (Supplementary Table 1).
To determine which MPO epitopes could bind these two HLAs, we utilized the Immune Epitope Database, which provides unbiased in silico binding predictions. From these predictions, five putative MPO epitopes were identified (15 amino acids in length based on algorithm limitations) with their respective HLA binding partner(s) (Table 1). Additionally, HLA-DRB4*01:01 was predicted to bind our previously identified B cell epitope MPO447–461, and a 15 amino acid long section of the mouse immunodominant MPO epitope (5) was included in this analysis (mouse MPO413–427), and predicted to bind HLA-DRB5*01:01 (Table 1).
Table 1.
In silico binding predictions using the Immune Epitope Database. Predicted percentile rank reports affinity of peptide compared to other randomly selected peptides with a lower percentile rank correlating to higher affinity.
| Protein | Peptide | Predicted Percentile Rank | Associated HLA Allele |
|---|---|---|---|
| Mouse MPO | MPO413–427 (GEKLYQEARKIVGAM) |
12.19 | HLA-DRB5*01:01 |
| Human MPO | MPO447–461 (RKIVGAMVQIITYRD) |
2.83 | HLA-DRB4*01:01 |
| Human MPO | MPO105–119 (PVAATRTAVRAADYL) |
10.88 | HLA-DQA1*05:01/ DQB1*02:01 |
| Human MPO | MPO488–502 (PRIANVFTNAFRYGH) |
7.82 | HLA-DRB5*01:01 |
| Human MPO | MPO245–259 (DQERSLMFMQWGQLL) |
1.84 | HLA-DPA1 *01:03/ DPB1*04:02 |
| Human MPO | MPO545–559 (PILRGLMATPAKLNR) |
2.48 | HLA-DRB 1*04:04 |
| Human MPO | MPO409–423 (MPELTSMHTLLLREH) |
3.05 | HLA-DPA1*01:01/ DPB1*04:01 |
Further in vitro competitive binding studies were used to validate seven HLA-epitope pairs in addition to the previously identified T cell epitope MPO435–454 and scrambled control epitope MPO447–461scramble. These HLA-epitope pairs were chosen for in vitro validation based on HLA frequency within our patient population as a technical measure and based on predicted strength of interaction (lower predicted percentile rank equals higher predicted binding capacity). The HLAs used for the in vitro validation are a pre-set panel which encompasses multiple alleles of 7 HLAs. Binding values (IC50 values) under 100 indicate high affinity binding of peptide to HLA, values between 100 and 1000 represent moderate affinity, and those values exceeding 1000 indicate low or no binding to HLA.
Several HLA/MPO peptide pairings demonstrated moderate to high affinity binding (Table 2). Focusing on HLA-DPB1*04:01 due to patient carrier frequency, we proceeded with three MPO peptides (MPO409–423, MPO435–454, and MPO447–461). With HLA-DRB4*01:01, the following MPO peptides were chosen based on affinity binding and prior epitope mapping studies (MPO409–423, MPO435–454, MPO447–461, and MPO545–559). Additionally, the control epitope MPO447–461scramble bound both HLA-DPB1*04:01 and HLA-DRB4*01:01 with moderate to high affinity (Table 2). Given the frequency of these two alleles in our patient cohort (52% and 51% respectively) and the in vitro binding results, we proceeded with MHC class II tetramer studies utilizing the moderate to high affinity MPO epitopes and the scrambled control epitope MPO447–461scramble to assess CD4+ T cell autoreactivity in patients as a proof of concept study. The in vitro binding studies revealed several additional HLA epitope partners, however the low allele prevalence of these HLA prevent their extensive investigation.
Table 2.
In vitro binding studies. IC50 values under 100 are indicative of high affinity binding to HLA, values between 100 and 1000 represent moderate binding pairs, and values greater than 1000 indicate low or no binding to HLA.
| Protein | Peptide | HLA-DPA1*01:03/ HLA-DPB1*04:01 | HLA-DPA1*01:03/ HLA-DPB1*04:02 | HLA-DQB1*02:01/ *02:02 | HLA-DRB1*03:04/ *03:07 | HLA-DRB1*04:01-*04:13 | HLA-DRB4*01:01/ *01:03 | HLA-DRB5*01:01/ *01:02/ *02:02 |
|---|---|---|---|---|---|---|---|---|
| Mouse MPO |
MPO413–427 (GEKLYQEARKIVGAM) |
53700 | 19400 | 56400 | 31100 | 933 | 9350 | 1440 |
| Human MPO |
MPO447–461 (RKIVGAMVQIITYRD) |
98 | 74 | 615 | 13900 | 46 | 240 | 1210 |
| Human MPO |
MPO105–119 (PVAATRTAVRAADYL) |
7120 | 6165 | 285 | 14500 | 433 | 876 | 321 |
| Human MPO |
MPO488–502 (PRIANVFTNAFRYGH) |
5620 | 1345 | 1000 | 2970 | 2 | 725 | 5 |
| Human MPO |
MPO245–259 (DQERSLMFMQWGQLL) |
1090 | 910 | 7050 | 70000 | 4140 | 381 | 1180 |
| Human MPO |
MPO545–559 (PILRGLMATPAKLNR) |
12000 | 2475 | 2550 | 32800 | 8 | 68 | 29 |
| Human MPO |
MPO409–423 (MPELTSMHTLLLREH) |
409 | 826 | 1210 | 11000 | 16 | 217 | 81 |
| Human MPO |
MPO435–454 (PRWDGERLYQEAYRKIVGAMV) |
1050 | N.D. | N.D. | N.D. | N.D. | 253 | N.D. |
| Human MPO |
MPO447–461scramble (IKAVRIYIGDVMRQT) |
38 | N.D. | N.D. | N.D. | N.D. | 17 | N.D. |
1.3.2. Patients with HLA-DPB1*04:01 and/or HLA-DRB4*01:01 Have Autoreactive CD4+ T Cells that Bind Specific MPO Epitopes
To identify autoreactive CD4+ cells, we utilized class II tetramers (HLA-DPB1*04:01 or HLA-DRB4*01:01) loaded with the MPO409–423, MPO435–454, MPO447–461, and MPO545–559, or a scrambled MPO447–461 peptide as a negative control. For HLA-DPB1*04:01, the associated alpha chain in the tetramer was DPA1*01:03 and for HLA-DRB4*01:01, the associated alpha chain was DRA1*01:01. To assess specificity of one peptide (MPO435–454), a tetramer with HLA-DPB1*04:01 and corresponding mouse MPO409–428 was also used as a negative control in select experiments. Two of these epitopes (MPO435–454 and MPO447–461) within human MPO overlap by eight amino acids, and only two amino acids differ between the human epitope MPO435–454 and mouse MPO409–428 (Figure 1A). Patient and healthy control PBMCs were stained with class II tetramers to assess the frequency of autoreactive CD4+ T cells directly ex vivo without exogenous stimulation. Tetramer-positive cells were detected after exclusion of doublets, followed by gating on lymphocytes and CD4+/CD8- cells. (Figure 1B). When comparing reactivity of patient CD4+ T cells to human MPO435–454 and mouse MPO409–428 using HLA-DPB1*04:01 tetramers, we found consistently higher reactivity to the human epitope (Supplemental Figure 1).
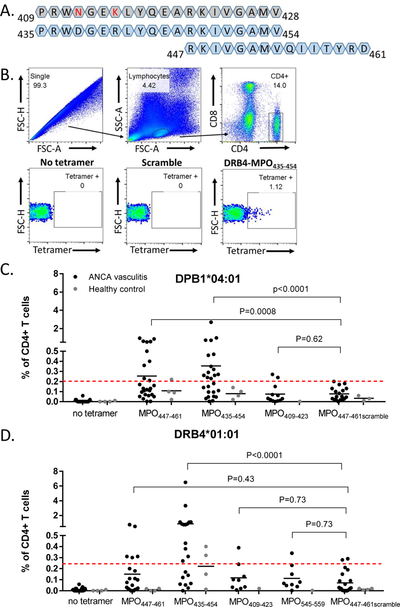
Figure 1.
Patients demonstrate HLA and epitope specific tetramer recognition by CD4+ T cells. (A) Amino acid sequence alignment of mouse MPO409–428 (grey), human MPO435–454, and human MPO447–461. Amino acid differences between mouse and human epitopes in red. (B) Gating strategy for tetramer+CD4+ T cells with no tetramer control, MPO447–461 scramble control, and HLA-DRB4 MPO435–454. Tetramer positivity for each individual was based on the corresponding no-tetramer control. (C-D) CD4+ T cell recognition of tetramers in MPO-ANCA patients (n=27; black dots) and HLA-matched healthy controls (n=6; gray dots). Some patients and healthy controls had both HLAs and were used in both studies while some samples were only used for one HLA. Dashed red line indicates threshold of positivity determined by the average plus two standard deviations of patient and healthy control MPO447–461scramble tetramer positivity. (C) HLA-DPB1*04:01 tetramer carrying MPO peptides (D) HLA-DRB4*01:01 tetramers carrying MPO peptides. P values were calculated by paired signed rank test.
Positive tetramer reactivity was determined by a threshold set by the average plus 2 standard deviations of T cell reactivity to the scrambled peptide (red dashed line Figures 1C & 1D). While some patients had low responses across all tetramers (Figures 1C & 1D), greater reactivity was demonstrated in response to MPO435–454 with both HLAs (Figures 1C & 1D), and a decreased, but still positive, response to MPO447–461 in both HLAs (Figures 1C & 1D) compared to HLA-matched healthy controls. Overall, healthy controls (n=6 HLA-matched healthy controls, some controls were heterozygous and contained both HLAs of interest) had low or undetectable tetramer binding. Patient CD4+ T cell reactivity to MPO409–423 and MPO545–559 was generally below the threshold of positivity determined by the average plus two standard deviations of the reactivity to the scrambled MPO epitope. Additionally, tetramers with HLA-DRB1*04:01 were tested on a small cohort of patients (n=6) and all reactivity was below the threshold of positivity (Supplemental Figure 2).
We retrospectively analyzed our cohort reactivity to determine if those patients who had high T cell reactivity to MPO435–454 were the same patients with high T cell reactivity to MPO447–461. In examining those patients with T cell reactivity >1% of total CD4+ T cells we encountered n=9 patients. Out of this cohort, only one of these patients had strong T cell reactivity to both the MPO435–454 and MPO447–461 tetramers. The other retrospective analysis was to determine if a patient had both HLA-DPB1*04:01 and HLA-DRB4*01:01, could the patient exhibit high T cell reactivity in both HLAs. In this analysis, there were n=7 patients that contained both HLAs of interest and exhibited >1% T cell reactivity to at least one peptide. Within this cohort, only one of these patients had strong reactivity to the same peptide in both HLAs. Collectively, these data suggest that there are both HLA and peptide restricted responses within the patient population.
To further investigate the specificity of tetramer binding, dual tetramer staining was performed with DPB1*04:01-PE-MPO447–461 and DPB1*04:01-PE/Cy5-MPO435–454 loaded in the same HLA. While these 2 peptides have slightly different affinities for HLA-DPB1*04:01 (MPO447–461 with strong affinity with an IC50=98 and MPO435–454 with moderate affinity with an IC50=1050), that should not affect their interaction with the T cell receptor of the cognate T cells as these predictions are solely between peptide and HLA. The majority of tetramer-positive cells were positive for both tetramers (Supplementary Figure 3), suggesting that the overlapping region of these epitopes may be required for binding to HLA. Additionally, while these samples were obtained in a cross-sectional manner, we did retrospectively analyze if disease activity had any bearing on the degree of tetramer positivity. There were no significant trends with disease activity or immunosuppression relative to tetramer positivity. Collectively, these tetramer studies demonstrate that CD4+ T cell reactivity is restricted to the region of MPO encompassed by amino acids 435–461 in a subgroup of patients with ANCA vasculitis.
1.3.3. Patient Tetramer+ T Cells Contain Memory T Cells that Secrete IL-17A
We previously identified an expanded population of CD4+ T cells in ANCA vasculitis that are CD127+, CD25intermediate and antigen-experienced, as evidenced by CD45RO expression (22). Based on these phenotypic markers and functional status, we hypothesized that this population may include autoreactive cells. To test this, tetramer-positive CD4+ T cells were further characterized for CD25, CD127, and CD45RO expression. The gating strategy for T cell populations based on CD25 expression is based on a fluorescence minus one (FMO) control to determine CD25 negativity threshold. A portion of tetramer-positive cells were CD25intermediateCD127+ cells (44.7% average for MPO447–461, 49.6% average for MPO435–454) (Figure 2A) (22). Tetramer-positive cells were also assessed for the presence of the memory marker CD45RO. Consistently greater than 50% of the tetramer-positive population expressed this marker (60% average across all positive tetramers, Figure 2B). Based on prior data from our lab denoting that regulatory T cells (Tregs) are dysfunctional in ANCA vasculitis, we analyzed the Treg population within the tetramer-positive population. In our current cohort, on average, 7.61% of tetramer-positive T cells were CD25high CD127low Tregs, which is not different from the percentage of overall Tregs in the CD4+ population within ANCA vasculitis patients (7.10%).
Figure 2.
Patient CD4+ T cells that bind tetramers are enriched for CD25intermediate cells expressing CD45RO, indicating memory cells memory marker (CD45RO+). (A) Gating strategy for CD25intermediate cells using a representative patient. Consistently half of tetramer positive cells were CD25intermediate T cells (n=14). (B) Representative plot demonstrating that the expression of CD45RO+ was slightly higher in tetramer positive cells than overall CD4+ T cells (n=6).
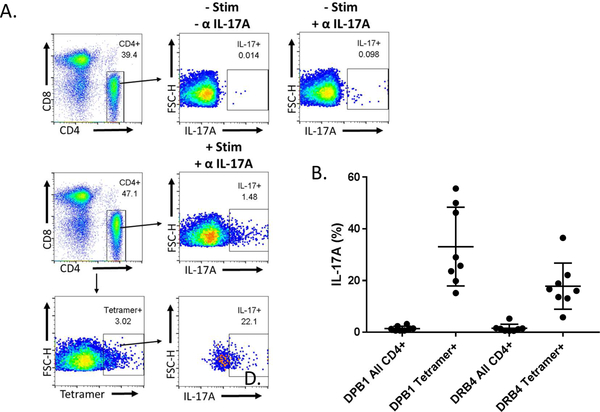
Previous characterization of the CD25intermediate T cell population and its ability to secrete primarily IL-17A led to the investigation of whether tetramer-positive cells were also capable of secreting IL-17A. Therefore, IL-17A secretion from tetramer positive CD4+ T cells was compared to IL-17A secretion by total CD4+ T cells within each patient using both HLA-DPB1 and HLA-DRB4 tetramers carrying the epitope MPO435–454 (Figure 3A). A higher proportion of tetramer-positive cells secreted IL-17A in response to stimulation when compared to total CD4+ T cells for each patient regardless of HLA restriction, confirming the pro-inflammatory activity of these autoreactive CD4+ T cells (Figure 3B).
Figure 3.
Tetramer positive T cells are enriched for cells capable of IL-17A secretion. (A) Representative plot shows gating scheme for determining percentage of IL-17A secreting cells for all CD4+ and tetramer positive cells in patients compared to no stimulation and no antibody (α IL-17A) controls. (B) Compared to all CD4+ T cells, a higher proportion of tetramer positive CD4+ T cells secrete IL-17A in patients (n=8)
1.3.4. Autoreactive CD4+ T Cells Have Decreased TCR Diversity
Considering the likelihood that these MPO-specific T cells are clonally expanded in response to autoantigen, we examined the degree of clonality within the tetramer-positive T cells compared to tetramer-negative, CD25−, CD25intermediate, and regulatory T cell populations. The HLA-DRB4*01:01 tetramer carrying MPO435–454 was used exclusively for these experiments based on consistently high patient reactivity (Figure 1D) and patients with previously recorded high frequency of tetramer-positive cells were selected for inclusion. HLA-DRB4*01:01-MPO435–454 specific T cell receptor (TCR) repertoires demonstrated decreased species richness, Shannon entropy, and d50 indices, consistent with diminished TCR repertoire diversity in the autoreactive T cell population (Figure 4). Interestingly, we also observed a decrease in TCR repertoire diversity in the CD25intermediate memory effector cells compared to naïve CD25− T cells but no difference between regulatory T cell diversity and these populations. These findings suggest clonal expansion and TCR restriction of MPO reactive CD4+ T cells in the periphery of ANCA vasculitis patients. Furthermore, these findings stem from cells collected directly ex vivo without exogenous stimulation, indicating that patient cells are chronically exposed to autoantigen in vivo.
Figure 4.

Decreased diversity of MPO435–454 specific T cells (Tet+) relative to tetramer negative CD25−, CD25intermediate, and regulatory T cells. Matched bootstrapped diversity including species richness, Shannon entropy, and d50 diversity indices derived from T cell receptor beta (TRB) profiling are shown (n=5 patients, n=4 for tetramer positive and Treg samples) corrected for false discovery rate (FDR). * p<0.05
1.3.5. Patient Autoantibody Reactivity to MPO447–461 Occurs Primarily at Disease Onset
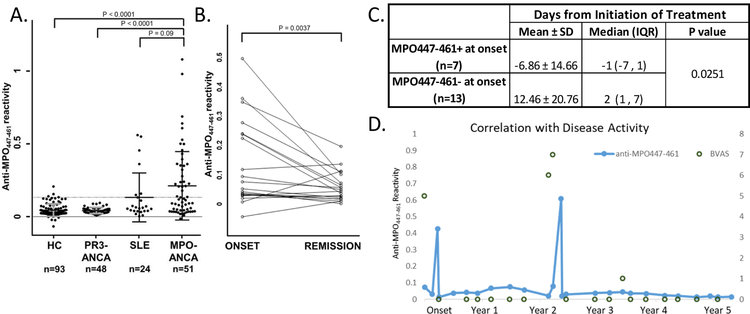
Based on our previous finding that a linear epitope is recognized by patient ANCA during disease activity (12), plus our presented data that CD4+ T cells recognize a restricted portion of MPO in a subgroup of patients, we sought to fully understand the temporal autoantibody response to MPO447–461. In our original study, MPO447–459 was utilized by ELISA, however through follow-up testing and optimization, the slightly longer MPO447–461 had improved solubility and more reproducible ELISA results. Therefore, longitudinal autoantibody responses to MPO447–461 were measured by an in-house ELISA in 51 patients (average follow-up time of cohort was 3.86 years). Fifty-three percent of tested patients (n=27/51) were positive for anti-MPO447–461 autoantibodies at least once during their disease course (Figure 5A) with the positive threshold being set at the average of the healthy control population plus 2 standard deviations. In a subgroup of patients, this specific autoantibody reactivity occurred at disease onset (Figure 5B). To understand any potential impact of therapy on anti-MPO447–461 reactivity, patients who were positive for anti-MPO447–461 were compared to those who were negative for reactivity, and we found that negative samples had a higher incidence of treatment prior to sample collection (Figure 5C). Additionally, patients who had an anti-MPO447–461 response at any point in their course had a longer duration of follow up compared to patients who never exhibited an anti-MPO447–461 response (Supplemental Table 5). Therefore, the true prevalence of anti-MPO447–461 autoantibodies is likely underrepresented in our cohort.
Figure 5.
Anti-MPO447–461 autoantibody is detectable in 53% of patients tested by ELISA (n=27/51), occasionally at disease onset. (A) Measured serum or plasma anti-MPO447–461 ELISA reactivity from healthy controls (HC), patients with PR3-ANCA vasculitis, patients with systemic lupus erythematous (SLE), and patients with MPO-ANCA vasculitis, normalized to a positive control. Threshold of positivity was determined by the average of healthy control samples plus two standard deviations and is indicated by the grey dashed line. P-values calculated using Wilcoxon two sample test with a Bonferroni adjusted critical value of 0.0083. (B) Reactivity of 20 samples collected at onset paired with a remission sample from the same patient, normalized to a positive control. P-value calculated using matched-pairs sign rank test (C) Analysis of onset samples revealed that anti-MPO447–461 negative samples had a higher prevalence of treatment prior to collection. P-values calculated using Wilcoxon two sample test. (D) Representative patient where temporal analysis of patient anti-MPO447–461 reactivity correlates with disease activity (BVAS score).
MPO-ANCA vasculitis patients in this cohort had serial serum samples that were used to assess correlation between anti-MPO447–461 and disease activity. We identified a close correlation between anti-MPO447–461 reactivity and disease activity in a portion of patients (results from illustrative patient shown in Figure 5D), while others demonstrated anti-MPO447–461 reactivity only at disease onset (Figure 5B). In addition, there was no correlation between ANCA reactivity to whole MPO and MPO447–461 (Supplementary Figure 4) suggesting that ANCA reactivity to MPO447–461 is specific, not just a readout for ANCA reactivity to whole MPO.
1.3.6. Autoantibody Binding to MPO447–459 Requires K448, I456, and an α-Helical Structure
With the intriguing finding that anti-MPO447–459 autoantibodies are detected at disease onset in a subset of patients, we sought to understand the dynamics of how the autoantibody interacts with this epitope. The critical amino acids that mediate binding of anti-MPO ANCA to MPO447–461 were elucidated with alanine scanning experiments. Peptides for MPO447–461 were generated using sequential substitutions of alanine for each amino acid in the peptide. Sera from eight anti-MPO447–461 positive patients were used to assess reactivity to each peptide. These experiments revealed that peptides with alanine substitutions for Lysine448 and Isoleucine456 demonstrate significantly decreased serum reactivity to the peptide (Figure 6A). Intriguingly, there was an amino acid substitution at the Glutamine455 that seemed to increase antibody reactivity in some samples, which could represent how natural variant of MPO may be more immunogenic.
Figure 6.
Anti-MPO447–461 antibody binding is dependent on secondary structure. (A) Alanine scanning experiments were performed in triplicate with eight previously positive patients by ELISA. Each letter represents the amino acid switched for alanine in sequential peptides. The positive control was the same highly positive serum included on every plate and the same healthy control was included on every plate. Scramble is the MPO447–461 scrambled control and a secondary antibody only control was also used. (B) Circular dichroism experiments to evaluate the secondary structure of peptides with alanine substitutions. The spectra were the average of four scans obtained by collecting data at 0.2 nm intervals from 260 to 190 nm, with a response time of 2 seconds and a bandwidth of 1 nm. (C) Cartoon model of MPO447–461 highlighted in blue demonstrate alpha-helical structure of the region. All models were generated using Pymol.
Amino acid substitutions often cause changes in the secondary structure; therefore, circular dichroism experiments were performed to examine any resulting structural changes. These experiments revealed that alanine substitutions for Lysine448 or Isoleucine456 resulted in a disrupted alpha helical structure, which could explain the altered autoantibody binding (Figure 6B). This same alpha helix disruption was observed for the MPO447–461scramble epitope (IKAVRIYIGDVMRQT) that was used as a control throughout experiments (Figure 6B). The alpha-helical structure of MPO447–461 can be visualized in Figure 6C as the blue portion of the MPO molecule.
1.3.7. MPO447–459 is Not Accessible to Solvent
From protein modeling investigations (Figure 6C), the MPO447–461 epitope seemed to reside in a potentially buried location within MPO. Therefore, experiments were performed to determine the nature of this MPO region. Solvent accessibility predictions were made based on crystal structure and GETAREA software (Figure 7A). These predictions confirmed that the MPO447–461 epitope is buried inside the MPO molecule and not accessible to the immune system under homeostatic conditions (35). Of interest, a portion of the MPO molecule that does have T cell reactivity (MPO435–454) is exposed to solvent (Figure 7A).
Figure 7.
Solvent accessibility and chemical modification determine the buried nature of MPO447–461. (A) Accessibility to solvent of each amino acid in MPO447–459 determined by predictive algorithms. Residues are considered to be solvent exposed if ratio value > 50%, and buried if the ratio value < 20%. (B) Isotope labeling with succinic anhydride to determine solvent accessibility of MPO epitopes. The top panel examines the percentage of succinylation versus the accessibility of ε-NH2 Lysine and N-terminal α-NH2. MPO447–461 ((R)KIVGAMVQIITYR(D)), represented by the circled data point, had almost undetectable levels of succinylation and solvent accessibility.
Further studies were performed to confirm the buried nature of this epitope. A two-step amino acid-specific covalent labeling protocol was employed for the quantitative estimation of relative reactivity of each lysine-ε-amino and N-terminal amino groups for several MPO peptides. MPO was first treated with a low molar excess of the lysine modifiers succinic anhydride (succinic-d0 anhydride), followed by reaction with a high molecular excess of an isotopically labeled form of the modification reagent (succinic-d4 anhydride). Relative reactivities were calculated from the ratio of the intensities of the modified and isotopically labeled peptides. Several MPO epitopes were determined to have high degrees of succinylation and/or high solvent accessibility (Figure 7B). However, MPO447–461 ((R)KIVGAMVQIITYR(D)) had only 2.24% succinylation and 7.15 ε-NH2 Lysine and N-terminal α-NH2 solvent accessibility, indicating that this portion of MPO, which is targeted by both arms of the adaptive immune system, contains a buried epitope (Figure 7B).
1.4. DISCUSSION
Collectively, these data pinpoint a restricted region within MPO that is targeted by both CD4+ T cells and ANCA in human MPO-ANCA vasculitis. Through high-resolution HLA typing, HLA-DPB1*04:01 and HLA-DRB4*01:01 were pursued based on prevalence in this cohort. These HLA bound several MPO epitopes with moderate to high affinity. The combination of these initial studies provided the foundation to utilize class II tetramers loaded with MPO epitopes to identify autoreactive CD4+ T cells. Not surprisingly, these autoreactive T cells contain pro-inflammatory, effector memory T cells. In parallel, we confirmed that 53% of our MPO-ANCA vasculitis cohort has autoantibody reactivity to MPO447–461. This reactivity was detectable at disease onset in some patients and correlated with disease activity in others.
Identification of enriched HLA alleles in our cohort of MPO-ANCA vasculitis patients provided the groundwork for downstream analyses and applications. The Immune Epitope Database predicted regions of MPO and their HLA binding partners. A subset of epitopes identified by in silico predictions bound to HLA with high affinity during in vitro binding experiments. Critically, both MPO447–461 and MPO435–454 had moderate to strong binding affinity for HLA-DPB1*04:01 and HLA-DRB4*01:01. These two epitopes were of particular interest based on prior reports from both human and mouse studies. Mouse MPO409–428, which corresponds to human MPO435–454, was reported as an immunodominant CD4+ T cell epitope, and mouse MPO431–439 (human MPO457–465) was reported as a pathogenic CD8+ T cell epitope (19–21). These two epitopes overlap with our previously published B cell epitope (MPO447–459), discovered by epitope excision, by eight and three amino acids respectively (or eight and five amino acids with MPO447–461 used for the studies reported herein) (Figure 8). Additionally, the dual positivity of the HLA-DPB1*04:01 tetramers loaded with the overlapping peptides (Supplementary Figure 3) suggests an eight amino acid region between these two epitopes may contain the most important residues for immune recognition in patients with HLA-DPB1*04:01. While it is possible that this dual staining is explained by non-specific binding of these tetramers, this is unlikely due to the difference in T cell binding to tetramers with the mouse and human versions of the T cell epitope (MPO409–428 and MPO435–454 respectively) (Supplemental Figure 1).
Figure 8.
Human MPO crystal structure reveals overlap between previously reported MPO epitopes. (PDBID:5FIW) Highlighted regions are as follows; dark blue - MPO435–454 (Ooi et al.2012), purple- MPO447–459 (Roth et al. 2013), light blue - MPO457–465 (Chang et al. 2017), gold - overlap between highlighted regions. The active site heme of myeloperoxidase is colored red.
Building on the knowledge of certain HLA-peptide interactions, we utilized class II tetramers with HLA-DPB1*04:01 and HLA-DRB4*01:01 to demonstrate that patients have CD4+ T cells that recognize and bind specific regions of MPO (specifically MPO447–461 and/or MPO435–454). Additional MPO epitopes (MPO409–423, MPO488–502, and MPO545–559) and HLA (DRB1*04:01) combinations were tested based on high affinity HLA binding, but had low CD4+ T cell reactivity. This highlights the assertion that the region of MPO encompassing the amino acids 435–461 is the critical region for CD4+ T cell reactivity in a subgroup MPO-ANCA vasculitis. Unexpectedly, detection of tetramer positive CD4+ T cells was possible when stained directly ex vivo without stimulation, indicating chronic antigen exposure in vivo. The majority of class II tetramer studies in autoimmune disease have relied on in vitro expansion protocols to detect autoreactive CD4+ T cells. Half of the tetramer positive CD4+ T cells are CD25intermediate, CD127+, CD45RO+ cells which suggests that these are activated effector memory cells. We also assessed IL-17A secretion of patient T cells based on results from previous work that demonstrated increased IL-17A, increased IL-4, and unchanged IFN-γ secretion from CD25intermediate cells compared to control CD4+ T cells in patients (22). When compared to overall CD4+ T cells, a higher proportion of tetramer-positive cells secreted IL-17A upon stimulation, which suggests these autoreactive cells are poised to respond in a pro-inflammatory manner. Owing to the expanded memory T cell population in the periphery of patients, we hypothesized that clonal expansion has occurred. Therefore, we employed TCR sequencing to determine the TCR diversity within several T cell populations. These studies revealed significantly decreased diversity of the autoreactive CD4+ TCR repertoire compared to CD25−, CD25intermediate, and regulatory T cells. Additionally, these studies revealed a stepwise decrease in diversity from CD25− naïve cells, to CD25intermediate memory cells, to the MPO435–454 reactive cells, with regulatory T cells showing similar diversity to both the CD25- and CD25intermediate cells. This finding supports chronic antigen exposure leading to clonal expansion of the autoreactive cells in patients and highlights the specificity of this CD4+ T cell response to MPO. Together, these results suggest that tetramer-positive CD25intermediate T cells are clonally expanded autoreactive cells that play a role in the immunopathogenesis of MPO-ANCA vasculitis.
Our studies suggest that CD4+ T cell reactivity is not only restricted to a critical region of MPO, but is HLA restricted as well. CD4+ T cells were only found to react to MPO peptides in the context of DRB4*01:01 and DBP1*04:01. Despite the fact that well-powered GWAS in ANCA vasculitis have pointed to the HLA-DQ allele as associated with MPO-ANCA (1, 3), our in vitro binding data did not find any MPO peptides that bound HLA-DQ with high affinity. The previously discovered variants implicating HLA-DQ are either intergenic or synonymous and are not predicted to alter the coding sequence of HLA-DQ. Their association with ANCA vasculitis could be explained by altering expression or mRNA stability of HLA-DQ, but not binding of MPO peptides. Therefore, other HLAs, such as DRB and DPB, may be biologically important for presentation of autoantigens.
Numerous studies have demonstrated the polyclonal nature of the anti-MPO antibody response in ANCA vasculitis (12, 13, 17, 18). However, not all autoantibodies are pathogenic and some autoantibody-epitope pairings may better predict disease onset or disease relapse. Our previous work discovered a particular autoantibody epitope (MPO447–461) that had an association with active disease in patients with MPO-ANCA vasculitis (12). In our current study, we sought to understand the relevance of reactivity to this epitope in a large, longitudinal cohort of patients. While anti-MPO447–461 autoantibodies do correlate with disease activity in some patients, this is not the case in all patients. Further studies may decipher the clinical differences between these patients and those who only have anti-MPO447–461 autoantibodies at onset disease versus those patients who never have detectable anti-MPO447–461 autoantibodies. Statistically, there is a trend that those patients with anti-MPO447–461 autoantibodies are more likely to have upper respiratory disease.
Additionally, we discovered that antibody binding to this region of MPO is dependent upon two amino acids: Lysine448 and Isoleucine456. Through circular dichroism studies, it is apparent that substitution at these two amino acids disrupts the alpha helix that spans this region of MPO. Therefore, autoantibody binding is likely dependent on secondary structure of this region. Perhaps the most intriguing results from these studies elucidate the hidden nature of this region of MPO. Through solvent exposure and chemical modification studies, it is apparent that MPO447–461 is buried within the MPO molecule and not exposed to the outside environment under homeostatic conditions. How then are anti-MPO447–461 autoantibodies formed and how might they contribute to disease onset and progression?
There are several limitations that are beyond the scope of this study, but should be considered in future investigations. We pursued the HLA-epitope combinations in this manuscript for practical reasons as a proof-of-concept study. However, studies that previously identified epitopes in this region of MPO have also demonstrated autoantibody reactivity to other regions of MPO on both the light (14) and heavy chains (12, 13, 15, 16, 18) and these regions should be further investigated. One study, Fujii et al., used recombinant deletion mutations within specific regions of MPO to identify B and T cell epitopes. Those studies identified reactivity to the same region investigated in our study (Hc; MPO409–474), but noted higher reactivity to other regions of the heavy chain of MPO (Ha; MPO279–341, Hb; MPO341–409, and Hg; MPO598–745)(17). To this same end, the studies presented herein reveal other potential HLA-peptide combinations of interest that should be investigated. While it is clear that the region of MPO we chose to assess is involved in disease immunopathogenesis for some patients, there may be a combinatorial effect with other HLA-peptide combinations, and some patients may have reactivity to other regions of MPO exclusively. It is also possible that patients have T cell autoreactivity to conformational (non-continuous) epitopes, though identification and confirmation of these epitopes is technically challenging. These studies begin to explore the clonality of the adaptive immune response, as single cell T cell receptor (TCR) sequencing may reveal more about whether autoreactivity is due to a diverse T cell repertoire or a clonal expansion of one or few autoreactive cells. As our T cell studies were cross-sectional, we were unable to address the relationship between T cell autoreactivity to this region of MPO and disease activity. This may be addressed in future studies using paired samples from patients at times of active disease and remission. Finally, these studies raise the question of how immunosuppressive medication alters the presence of autoreactive cells. While some of the patients in our cohort were in remission and untreated for a year or more, every patient used for the tetramer studies had a history of some immunosuppressive therapy. Although performing these studies in new-onset ANCA vasculitis patients prior to therapy is ideal, the need for high-resolution HLA typing information makes tetramer experiments technically unattainable unless patients have been HLA typed prior to disease onset.
Our studies examining the structure of this targeted region within the MPO molecule raise additional questions regarding initiation of the anti-MPO immune response. A large portion of this region of MPO is hidden and would only be accessible to the immune system if the structure of MPO was significantly altered. As such, we can hypothesize that since the MPO447–461 epitope is buried within MPO, the structure of MPO must be substantially disrupted to facilitate an immune response to this epitope in patients. A critical question in autoimmune disease is: what is the inciting event in immunopathogenesis? Therefore, the events that lead to the disruption of MPO and reveal buried epitopes to the immune system may be equally as important to generate the autoimmune process as the presence of autoreactive cells. This is an intriguing possibility because the epitope for anti-glomerular basement membrane (GBM) antibodies is also buried and requires conformational changes of the antigen to be revealed to the immune system (6). Further understanding of potential conformational changes within MPO in ANCA vasculitis will facilitate pinpointing the immunopathogenesis of disease.
Autoimmune disease research is rapidly moving toward the use of disease relevant epitopes to create sustained nonresponsiveness to autoantigen and/or antigen-specific therapies to reduce autoreactive cell burden. Embarking on this quest requires a thorough understanding of the interaction between HLA, autoantigen peptides, and autoreactive T cells. With regard to tolerance, mouse MPO409–428 has been previously used to reintroduce tolerance by nasal insufflation in a mouse model of focal necrotizing glomerulonephritis. Insufflation of this peptide afforded protection before onset of disease and partially ameliorated established disease (21). Additionally, insufflation with this peptide managed to downregulate antibody responses not only to the specific epitope, but downregulated the entire polyclonal response to MPO. Regarding use of antigen-specific therapies, a study by Clemente-Cesares et al. utilized disease-relevant, peptide-coated nanoparticles to ameliorate disease severity in several mouse models of autoimmunity (45). In type 1 diabetes, a study used nanoparticles to induce tolerance in mice (46), while in patients, nasal administration of insulin conferred minor improvements at the level of cellular function, but did not slow the loss of beta cell function (47). Additionally, CAR T cell technologies are being utilized to specifically target autoreactive B cells (48) and develop antigen specific regulatory T cells (49). Collectively, our work provides new insight into the interactions between HLA, antigenic peptides, and the CD4+ T cell immune response—key factors necessary to move forward towards antigen-specific therapies. These findings not only inform the development of these new therapies for patients with MPO-ANCA vasculitis but also provide a foundation for studies in other autoimmune diseases.
Supplementary Material
Highlights.
Autoreactive CD4+ T cells are detectable in the periphery of ANCA vasculitis
T cells target a restricted, buried region of MPO
B cell autoantibodies target a linear epitope associated with disease
Epitope-mapping of adaptive immunity provide foundation for immunotherapy
Acknowledgements:
Thank you to Caroline Poulton, Candace Henderson, Kristin Kennedy, and Lauren Blazek for their help procuring patient samples and managing and retrieving patient demographic information. We would like to thank Drs. John Schmitz and Eric Weimer for providing HLA information from UNC’s healthy organ donor population. We also thank Robert Petrovich from the Protein Expression Core Facility of the National Institute of Environmental Health Sciences (NIEHS) for help with acquisition of CD spectra. Finally, thank you the staff of the UNC Flow Cytometry Core Facility for all of their guidance and troubleshooting on the many variations of these experiments.
This work was supported by federal grant P01 DK058335–06 from the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases. KGS was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Award Number TL1TR001110 and by an NIH, National Institute of Diabetes and Digestive and Kidney Diseases Ruth L. Kirschstein National Research Service Award (NRSA) Institutional Research Training Grant (T32DK007750; PI RJ Falk). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The UNC Flow Cytometry Core Facility is supported in part by P30 CA016086 Cancer Center Core Support Grant to the UNC Lineberger Comprehensive Cancer Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have declared that no conflict of interest exists.
References
- 1.Lyons PA, Rayner TF, Trivedi S, Holle JU, Watts RA, Jayne DR, Baslund B, Brenchley P, Bruchfeld A, Chaudhry AN, et al. 2012. Genetically distinct subsets within ANCA-associated vasculitis. N Engl J Med 367:214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xie G, Roshandel D, Sherva R, Monach PA, Lu EY, Kung T, Carrington K, Zhang SS, Pulit SL, Ripke S, et al. 2013. Association of granulomatosis with polyangiitis (Wegener’s) with HLA-DPB1*04 and SEMA6A gene variants: evidence from genome-wide analysis. Arthritis Rheum 65:2457–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merkel PA, Xie G, Monach PA, Ji X, Ciavatta DJ, Byun J, Pinder BD, Zhao A, Zhang J, Tadesse Y, et al. 2016. Identification of functional and expression polymorphisms associated with risk for anti-neutrophil cytoplasmic autoantibody-associated vasculitis. Arthritis Rheumatol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falk RJ, and Jennette JC 2002. ANCA are pathogenic--oh yes they are! J Am Soc Nephrol 13:1977–1979. [DOI] [PubMed] [Google Scholar]
- 5.Ooi JD, Chang J, O’Sullivan KM, Pedchenko V, Hudson BG, Vandenbark AA, Fugger L, Holdsworth SR, and Kitching AR 2013. The HLA-DRB1*15:01-restricted Goodpasture’s T cell epitope induces GN. J Am Soc Nephrol 24:419–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pedchenko V, Bondar O, Fogo AB, Vanacore R, Voziyan P, Kitching AR, Wieslander J, Kashtan C, Borza DB, Neilson EG, et al. 2010. Molecular architecture of the Goodpasture autoantigen in anti-GBM nephritis. N Engl J Med 363:343–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kita H, He XS, and Gershwin ME 2003. Application of tetramer technology in studies on autoimmune diseases. Autoimmun Rev 2:43–49. [DOI] [PubMed] [Google Scholar]
- 8.Maverakis E, Kim K, Shimoda M, Gershwin ME, Patel F, Wilken R, Raychaudhuri S, Ruhaak LR, and Lebrilla CB 2015. Glycans in the immune system and The Altered Glycan Theory of Autoimmunity: a critical review. J Autoimmun 57:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L, Wang FS, and Gershwin ME 2015. Human autoimmune diseases: a comprehensive update. J Intern Med 278:369–395. [DOI] [PubMed] [Google Scholar]
- 10.Cui Z, Xie LJ, Chen FJ, Pei ZY, Zhang LJ, Qu Z, Huang J, Gu QH, Zhang YM, Wang X, et al. 2016. MHC Class II Risk Alleles and Amino Acid Residues in Idiopathic Membranous Nephropathy. J Am Soc Nephrol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seitz-Polski B, Dolla G, Payre C, Girard CA, Polidori J, Zorzi K, Birgy-Barelli E, Jullien P, Courivaud C, Krummel T, et al. 2016. Epitope Spreading of Autoantibody Response to PLA2R Associates with Poor Prognosis in Membranous Nephropathy. J Am Soc Nephrol 27:1517–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roth AJ, Ooi JD, Hess JJ, van Timmeren MM, Berg EA, Poulton CE, McGregor J, Burkart M, Hogan SL, Hu Y, et al. 2013. Epitope specificity determines pathogenicity and detectability in ANCA-associated vasculitis. J Clin Invest 123:1773–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erdbrugger U, Hellmark T, Bunch DO, Alcorta DA, Jennette JC, Falk RJ, and Nachman PH 2006. Mapping of myeloperoxidase epitopes recognized by MPO-ANCA using human-mouse MPO chimers. Kidney Int 69:1799–1805. [DOI] [PubMed] [Google Scholar]
- 14.Pedrollo E, Bleil L, Bautz FA, Kalden JR, and Bautz EK 1993. Antineutrophil cytoplasmic autoantibodies (ANCA) recognizing a recombinant myeloperoxidase subunit. Adv Exp Med Biol 336:87–92. [DOI] [PubMed] [Google Scholar]
- 15.Tomizawa K, Mine E, Fujii A, Ohashi YY, Yamagoe S, Hashimoto Y, Ishida-Okawara A, Ito M, Tanokura M, Yamamoto T, et al. 1998. A panel set for epitope analysis of myeloperoxidase (MPO)-specific antineutrophil cytoplasmic antibody MPO-ANCA using recombinant hexamer histidine-tagged MPO deletion mutants. J Clin Immunol 18:142–152. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki K, Kobayashi S, Yamazaki K, Gondo M, Tomizawa K, Arimura Y, Nakabayashi K, Ozaki S, Yoshida M, Yoshida T, et al. 2007. Analysis of risk epitopes of anti-neutrophil antibody MPO-ANCA in vasculitis in Japanese population. Microbiol Immunol 51:1215–1220. [DOI] [PubMed] [Google Scholar]
- 17.Fujii A, Tomizawa K, Arimura Y, Nagasawa T, Ohashi YY, Hiyama T, Mizuno S, and Suzuki K 2000. Epitope analysis of myeloperoxidase (MPO) specific anti-neutrophil cytoplasmic autoantibodies (ANCA) in MPO-ANCA-associated glomerulonephritis. Clin Nephrol 53:242–252. [PubMed] [Google Scholar]
- 18.Bruner BF, Vista ES, Wynn DM, and James JA 2011. Epitope specificity of myeloperoxidase antibodies: identification of candidate human immunodominant epitopes. Clin Exp Immunol 164:330–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang J, Eggenhuizen P, O’Sullivan KM, Alikhan MA, Holdsworth SR, Ooi JD, and Kitching AR 2017. CD8+ T Cells Effect Glomerular Injury in Experimental Anti-Myeloperoxidase GN. J Am Soc Nephrol 28:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ooi JD, Chang J, Hickey MJ, Borza DB, Fugger L, Holdsworth SR, and Kitching AR 2012. The immunodominant myeloperoxidase T-cell epitope induces local cell-mediated injury in antimyeloperoxidase glomerulonephritis. Proc Natl Acad Sci U S A 109:E2615–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gan PY, Tan DS, Ooi JD, Alikhan MA, Kitching AR, and Holdsworth SR 2016. Myeloperoxidase Peptide-Based Nasal Tolerance in Experimental ANCA-Associated GN. J Am Soc Nephrol 27:385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Free ME, Bunch DO, McGregor JA, Jones BE, Berg EA, Hogan SL, Hu Y, Preston GA, Jennette JC, Falk RJ, et al. 2013. Patients with antineutrophil cytoplasmic antibody-associated vasculitis have defective Treg cell function exacerbated by the presence of a suppression-resistant effector cell population. Arthritis Rheum 65:1922–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jennette J, Falk R, Bacon P, Basu N, Cid M, Ferrario F, Flores-Suarez L, Gross W, Guillevin L, and Hagen E 2013. 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis & Rheumatism 65:1–11. [DOI] [PubMed] [Google Scholar]
- 24.Falk RJ, Gross WL, Guillevin L, Hoffman G, Jayne DR, Jennette JC, Kallenberg CG, Luqmani R, Mahr AD, and Matteson EL 2011. Granulomatosis with polyangiitis (Wegener’s): an alternative name for Wegener’s granulomatosis. Annals of the Rheumatic Diseases 70:704–704. [DOI] [PubMed] [Google Scholar]
- 25.Hogan SL, Falk RJ, Chin H, Cai J, Jennette CE, Jennette JC, and Nachman PH 2005. Predictors of relapse and treatment resistance in antineutrophil cytoplasmic antibody-associated small-vessel vasculitis. Ann Intern Med 143:621–631. [DOI] [PubMed] [Google Scholar]
- 26.Pagnoux C, Hogan SL, Chin H, Jennette JC, Falk RJ, Guillevin L, and Nachman PH 2008. Predictors of treatment resistance and relapse in antineutrophil cytoplasmic antibody-associated small-vessel vasculitis: comparison of two independent cohorts. Arthritis Rheum 58:2908–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luqmani R, Bacon P, Moots R, Janssen B, Pall A, Emery P, Savage C, and Adu D 1994. Birmingham vasculitis activity score (BVAS) in system necrotizinig vasculitis. QJM 87:671–678. [PubMed] [Google Scholar]
- 28.Sette A, Paul S, Vaughan K, and Peters B 2015. The Use of the Immune Epitope Database to Study Autoimmune Epitope Data Related to Alopecia Areata. J Investig Dermatol Symp Proc 17:36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sidney J, Assarsson E, Moore C, Ngo S, Pinilla C, Sette A, and Peters B 2008. Quantitative peptide binding motifs for 19 human and mouse MHC class I molecules derived using positional scanning combinatorial peptide libraries. Immunome Res 4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sidney J, Southwood S, Mann DL, Fernandez-Vina MA, Newman MJ, and Sette A 2001. Majority of peptides binding HLA-A*0201 with high affinity crossreact with other A2-supertype molecules. Hum Immunol 62:1200–1216. [DOI] [PubMed] [Google Scholar]
- 31.Sidney J, Southwood S, Moore C, Oseroff C, Pinilla C, Grey HM, and Sette A 2013. Measurement of MHC/peptide interactions by gel filtration or monoclonal antibody capture. Current protocols in immunology / edited by John E. Coligan … [et al.] Chapter 18:Unit 18 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Novak EJ, Liu AW, Nepom GT, and Kwok WW 1999. MHC class II tetramers identify peptide-specific human CD4(+) T cells proliferating in response to influenza A antigen. J Clin Invest 104:R63–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Venturi V, Kedzierska K, Turner SJ, Doherty PC, and Davenport MP 2007. Methods for comparing the diversity of samples of the T cell receptor repertoire. J Immunol Methods 321:182–195. [DOI] [PubMed] [Google Scholar]
- 34.Vincent B, Buntzman A, Hopson B, McEwen C, Cowell L, Akoglu A, Zhang H, and Frelinger J 2016. iWAS--A novel approach to analyzing Next Generation Sequence data for immunology. Cell Immunol 299:6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shrake A, and Rupley JA 1973. Environment and exposure to solvent of protein atoms. Lysozyme and insulin. J Mol Biol 79:351–371. [DOI] [PubMed] [Google Scholar]
- 36.Bolotin DA, Poslavsky S, Mitrophanov I, Shugay M, Mamedov IZ, Putintseva EV, and Chudakov DM 2015. MiXCR: software for comprehensive adaptive immunity profiling. Nat Methods 12:380–381. [DOI] [PubMed] [Google Scholar]
- 37.Lefranc MP, Giudicelli V, Ginestoux C, Bosc N, Folch G, Guiraudou D, Jabado-Michaloud J, Magris S, Scaviner D, Thouvenin V, et al. 2004. IMGT-ONTOLOGY for immunogenetics and immunoinformatics. In Silico Biol 4:17–29. [PubMed] [Google Scholar]
- 38.Shugay M, Bagaev DV, Turchaninova MA, Bolotin DA, Britanova OV, Putintseva EV, Pogorelyy MV, Nazarov VI, Zvyagin IV, Kirgizova VI, et al. 2015. VDJtools: Unifying Post-analysis of T Cell Receptor Repertoires. PLoS Comput Biol 11:e1004503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klipper-Aurbach Y, Wasserman M, Braunspiegel-Weintrob N, Borstein D, Peleg S, Assa S, Karp M, Benjamini Y, Hochberg Y, and Laron Z 1995. Mathematical formulae for the prediction of the residual beta cell function during the first two years of disease in children and adolescents with insulin-dependent diabetes mellitus. Med Hypotheses 45:486–490. [DOI] [PubMed] [Google Scholar]
- 40.Team, R.C. 2017. R: A language and environment for statistical computing. . Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 41.Horn HS 1966. Measurement of “Overlap” in Comparative Ecological Studies. The American Naturalist 100:419–424. [Google Scholar]
- 42.Jari Oksanen, F.G. B., Michael Friendly, Roeland Kindt, Pierre Legendre, Dan McGlinn, Peter R. Minchin, R. B. O’Hara, Gavin L. Simpson, Peter Solymos, M. Henry H. Stevens, Eduard Szoecs and Helene Wagner. 2017. vegan: Community Ecology Package. R package version 2.4–4.
- 43.Wickham H 2009. ggplot2: Elegant Graphics for Data Analysis. New York: Springer-Verlag. [Google Scholar]
- 44.Hilhorst M, Arndt F, Joseph Kemna M, Wieczorek S, Donner Y, Wilde B, Thomas Epplen J, van Paassen P, and Cohen Tervaert JW 2016. HLA-DPB1 as a Risk Factor for Relapse in Antineutrophil Cytoplasmic Antibody-Associated Vasculitis: A Cohort Study. Arthritis Rheumatol 68:1721–1730. [DOI] [PubMed] [Google Scholar]
- 45.Clemente-Casares X, Blanco J, Ambalavanan P, Yamanouchi J, Singha S, Fandos C, Tsai S, Wang J, Garabatos N, Izquierdo C, et al. 2016. Expanding antigen-specific regulatory networks to treat autoimmunity. Nature 530:434–440. [DOI] [PubMed] [Google Scholar]
- 46.Prasad S, Neef T, Xu D, Podojil JR, Getts DR, Shea LD, and Miller SD 2017. Tolerogenic Ag-PLG nanoparticles induce tregs to suppress activated diabetogenic CD4 and CD8 T cells. J Autoimmun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fourlanos S, Perry C, Gellert SA, Martinuzzi E, Mallone R, Butler J, Colman PG, and Harrison LC 2011. Evidence that nasal insulin induces immune tolerance to insulin in adults with autoimmune diabetes. Diabetes 60:1237–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ellebrecht CT, Bhoj VG, Nace A, Choi EJ, Mao X, Cho MJ, Di Zenzo G, Lanzavecchia A, Seykora JT, Cotsarelis G, et al. 2016. Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science 353:179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adair PR, Kim YC, Zhang AH, Yoon J, and Scott DW 2017. Human Tregs Made Antigen Specific by Gene Modification: The Power to Treat Autoimmunity and Antidrug Antibodies with Precision. Front Immunol 8:1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.