Abstract
Older people living with HIV (PLWH) experience multimorbidity that can negatively impact quality of life (QoL). Exercise can improve physical function, but effects on QoL are not well understood. 32 PLWH and 37 controls aged 50-75 completed 12-weeks of moderate-intensity exercise, then were randomized to moderate or high-intensity for 12 additional weeks. Depressive symptoms (CES-D scores) were significantly greater and QOL (SF-36 mental and physical summary scores) significantly lower among PLWH at baseline (all p<0.05). PLWH had significantly greater worsening in CES-D scores compared to controls (3.4 [0.7, 6.0]; p=0.01) between 13-24 weeks. Mental QoL changed minimally, with no significant difference in changes by serostatus between weeks 0-12 or weeks 13-24 (p≤0.22). Changes in physical function summary scores were similar by serostatus between 0-12 weeks (1.5 [−1.6, 4.6], p=0.35), but declined significantly more among PLWH between 13-24 weeks (−4.1 [−7.2,−1], p=0.01). Exercise intensity had no significant effect on changes in CES-D or SF-36 summary scores; high-intensity exercise was associated with greater improvements in vitality/fatigue (4.1 [0.8, 7.3], p=0.02), compared to moderate-intensity. Exercise initiation failed to improve depressive symptoms or QoL among PLWH. Additional interventions may be needed to maximize these patient-reported outcomes among older PLWH initiating an exercise program.
Keywords: HIV, Older Adults, Quality of Life, Exercise, Depression
Background
In the United States, the prevalence of people living with HIV (PLWH) over the age of 50 was approximately 45% in 2014 and projected to increase to 75% by 2030 (Siegler and Brennan-Ing, 2017) due to advancements in the efficacy and availability of effective antiretroviral therapy (ART) (Costagliola, 2014). As HIV infection is now considered a chronic disease, this expanding population presents with synergistic risks for multi-morbidity (Chambers et al., 2014). PLWH may experience aging-related conditions earlier than their uninfected peers, due in part to toxicity of some ART regimens, polypharmacy, and prolonged exposure to the virus (Hawkins, Brown, Margolick, & Erlandson, 2017; Hodes et al., 2016; Siegler and Brennan-Ing, 2017). Furthermore, the added burden of depression, anxiety (Monteiro, Canavarro, & Pereira, 2016; Nosrat, Whitworth, & Ciccolo, 2017), HIV- and age-related stigma (Emlet et al., 2015; Slater et al., 2015), and social isolation (Grov, Golub, Parsons, Brennan, & Karpiak, 2010) contributes to declines in cognitive (Moore et al., 2014; Vance, 2013) and physical function (Grov, et al., 2010) in PLWH.
As important, or perhaps more important, than the burden of comorbid conditions on cognitive and physical function, is the impact on quality of life (QoL). For example, depression is the most commonly reported comorbid condition for PLWH (Balderson et al., 2013) and is linked to decreased ART adherence (Halkitis et al., 2014; Nosrat, et al., 2017; Sin and DiMatteo, 2014), increased mortality rates (Nosrat, et al., 2017), and diminished QoL (Millar, Starks, Gurung, & Parsons, 2017). Improved QoL for all older adults is a goal for Healthy People 2020, particularly among populations such as older PLWH who experience additional health disparities that may negatively impact QoL (Balderson, et al., 2013). Thus, identification and incorporation of interventions that improve QoL is a high priority area in the management of older PLWH.
Physical activity is recommended by the Centers for Disease Control and Prevention (CDC) to alleviate chronic conditions and provide health benefits for older adults, but about a quarter of the older adult population are sedentary, and this prevalence only increases with age (Diaz et al., 2016). We have previously shown that higher self-reported physical activity is associated with greater physical and mental QoL in PLWH (Erlandson et al., 2014). To our knowledge, however, few studies have examined the benefits of exercise on the QoL of older PLWH, especially in comparison to adults without HIV (Shah et al., 2016). Further research is needed to determine the efficacy of specific exercise strategies to improve QoL among older PLWH (Kamitani, Sipe, Higa, Mullins, & Soares, 2017), and whether these responses differ from age-matched, uninfected controls. The Exercise for Healthy Aging study compared the effects of 24 weeks of moderate- or high-intensity cardiovascular and resistance exercise on physiological outcomes among older PLWH and uninfected controls. The purpose of this planned secondary analysis was to determine the effects of exercise on depressive symptoms and QoL, and whether these changes differed among older adults with or without HIV.
Methods
Study Design & Population
The Exercise for Healthy Aging study was a randomized clinical trial that examined the effects of a 24-week exercise intervention to improve physical function in sedentary PLWH (n=32) and uninfected controls (n=37), aged 50-75 years ( NCT02404792). Briefly, PLWH were on an ART regimen for a minimum of two years with a HIV-1 RNA <200 copies/mL and a CD4 count ≥200 cells/μL. PLWH were recruited primarily from the University of Colorado Infectious Diseases Clinic, in addition to community groups, and referrals by other HIV providers around the Denver Metropolitan area. Uninfected controls were recruited through referrals from partners and friends of participants with HIV, advertisements through the University of Colorado clinical trials website, advertisements around the University of Colorado Anschutz Medical Campus and local businesses, and Craigslist. All participants were sedentary by self-report (<60 minutes of physical activity each week for 6 months preceding) and had no comorbid conditions that would interfere with the ability to participate in an exercise program.
As previously described (Erlandson et al., 2018), each participant attended supervised exercise sessions 3 times/week for 24 weeks at the University of Colorado-Anschutz Medical Campus Exercise Research Laboratory. Participants began with a 2-week supervised, low-intensity exercise acclimation for machine familiarization consisting of 20-30 minutes of treadmill walking at 30-40% of VO2 max (measured by heart rate monitor during exercise) and 3 sets of 8 repetitions of 4 weight-assisted machine exercises (bench press, leg press, lateral pulldown, and a rotating 4th exercise) at low-intensity (40-50% of the 1-RM). After 2 weeks, participants increased cardiovascular endurance exercise intensity to 40-50% VO2 max and time by 5 minutes every week to achieve a goal of 50 minutes/session by the end of 12 weeks. Resistance exercise was increased to 60-70% of 1-RM; 1-RM was reassessed every 3 weeks and target weight loads adjusted as needed. At week 12, VO2 max measurements were repeated and participants were randomized to either continue moderate-intensity exercise or advance to high-intensity (60-70% of week 13 VO2 max and >80% 1-RM) for the remaining 12 weeks. The randomization was balanced by HIV serostatus, gender, and age.
Depression and QoL Measurement Surveys
Depressive symptoms were measured using the Center for Epidemiologic Studies Depression Scales (CES-D)(Micheal Irwin, 1999). Scores ranged from 0 - 60 with ≥16 indicative of clinical depression. QoL was assessed with the 36-Item Short Form Health Survey (SF-36), a valid and reliable method for measuring physical and mental QoL (Ping-Chuan Hsiung, 2005). The SF-36 has eight QoL subscales that measure physical functioning, role limitations due to physical health (role physical), bodily pain, general health, emotional functioning, role limitations due to emotional well-being (role emotional), social functioning, and vitality-fatigue. These subscales can be weighted and aggregated to create a physical health summary score and mental health summary score. The scores were normalized to a population mean of 50 and ranged from 0 – 100 with higher scores indicating better subjective QoL. Participants completed SF-36 and CES-D surveys at 0 (baseline), 12, and 24 weeks.
Statistical Analysis
Demographic differences between serostatus groups were examined using independent t-tests for continuous variables (age, BMI), and Chi-Squared or Fisher’s Exact Tests for categorical variables. Baseline CES-D scores, SF-36 physical and mental summary scores, and SF-36 subscales were compared using independent t-tests with unequal variances.
Multiple linear regression models first adjusted for baseline CES-D or SF-36 scores only, and then for additional covariates. Covariates related to HIV, QoL, and depression were considered based on literature and clinical judgement including: marijuana use, body mass index (BMI), education, employment status, smoking status, age, number of comorbidities, and anxiety, depression, or bipolar disorde. Backwards selection was performed and covariates were retained in the model if there was a greater than 10- 20% change in SF-36 or CES-D scores. Baseline (0-12 week change) or midpoint (13-24 week change) scores were included in all adjusted models, centered to the mean of the respective measurement tool. Partial f-tests were used to determine the final model from the full model with all covariates. Similar model approaches were used to assess the effect of high- versus moderate-intensity exercise. Because outcomes were exploratory, we did not adjust for multiple comparisons. The alpha level for significance was set to p<0.05 to allow for the investigation of direction of relationships.
Results
CES-D and SF-36 were completed by all participants remaining on study at each time point: 69 (32 PLWH, 37 controls) participants at baseline, 59 (28 PLWH, 31 controls) at week 12, and 56 (27 PLWH, 29 controls) at week 24. The majority of participants were male (91%) and white (74%) (Table 1). Compared to controls, PLWH had a higher prevalence of mood disorders (depression, anxiety, and/or bipolar disorder), lower mean BMI, a higher percentage of high school education or less, and were less likely to be employed, and more likely to use marijuana or other drugs.
Table 1:
Demographics by HIV Serostatus at Baseline Evaluations
| Baseline Characteristic | Overall (n=69) |
PLWH (n=32) |
Controls (n=37) |
p-value |
|---|---|---|---|---|
| Age < 60 years | 42 (61) | 22 (69) | 20 (54) | 0.32 |
| Body mass index | 29 (5) | 27 (4) | 30 (5) | 0.02 |
| Male | 63 (91) | 28 (88) | 35 (95) | 0.54 |
| Race | 0.09 | |||
| White | 51 (74) | 20 (63) | 31 (84) | |
| Black | 12 (17) | 9 (28) | 3 (8) | |
| Other | 6 (9) | 3 (9) | 3 (8) | |
| Hispanic Ethnicity | 8 (12) | 4 (13) | 4 (11) | 1 |
| Educationa | 0.001 | |||
| High School or less | 14 (20) | 10 (31) | 4 (11) | |
| College | 31 (45) | 18 (56) | 13 (35) | |
| Post-Graduate | 23 (34) | 4 (13) | 19 (51) | |
| Employment | <0.001 | |||
| Unemployed, disabled or retired | 35 (51) | 24 (75) | 11 (30) | |
| Part or full-time | 34 (49) | 8 (25) | 26 (70) | |
| Sexual Preference among Males a | <0.001 | |||
| Heterosexual | 28 (44) | 1 (3) | 27 (77) | |
| Prefers sex with men | 31 (49) | 25 (89) | 6 (17) | |
| Bisexual | 2 (3) | 1 (3) | 1 (3) | |
| Sexual Preference among Femalesa | 1 | |||
| Heterosexual | 5 (83) c | 3 (75)c | 2 (100) c | |
| Depression, Anxiety, or Bipolar | 26 (38) | 17 (53) | 9 (24) | 0.03 |
| Use of antidepressant medication | 18 (26) | 8 (25) | 10 (27) | 1 |
| Use of benzodiazepine medication | 9 (13) | 6 (19) | 3 (8) | 0.29 |
| Use of antipsychotic | 7 (10) | 7 (22) | 0 (0) | 0.003 |
| Smoking Status | 0.067 | |||
| Never | 34 (49) | 11 (34) | 23 (62) | |
| Former | 26 (38) | 16 (50) | 10 (27) | |
| Current | 9 (13) | 5 (16) | 4 (11) | |
| Alcohol Use | 0.21 | |||
| No | 16 (23) | 10 (31) | 6 (16) | |
| Weekly | 42 (61) | 19 (59) | 23 (62) | |
| Daily | 11 (16) | 3 (9) | 8 (22) | |
| Drug Use in the Past 2 Years | 6 (9) | 6 (19) | 0 (0) | 0.008 |
| Frequent (daily or weekly) Marijuana Useb | 11 (16) | 11 (34) | 0 (0) | <0.001 |
Reported as mean (standard deviation [SD]) or frequency (%);
not all participants answered
including methamphetamine, cocaine, heroin, poppers;
proportion of the females only
Depressive Symptoms
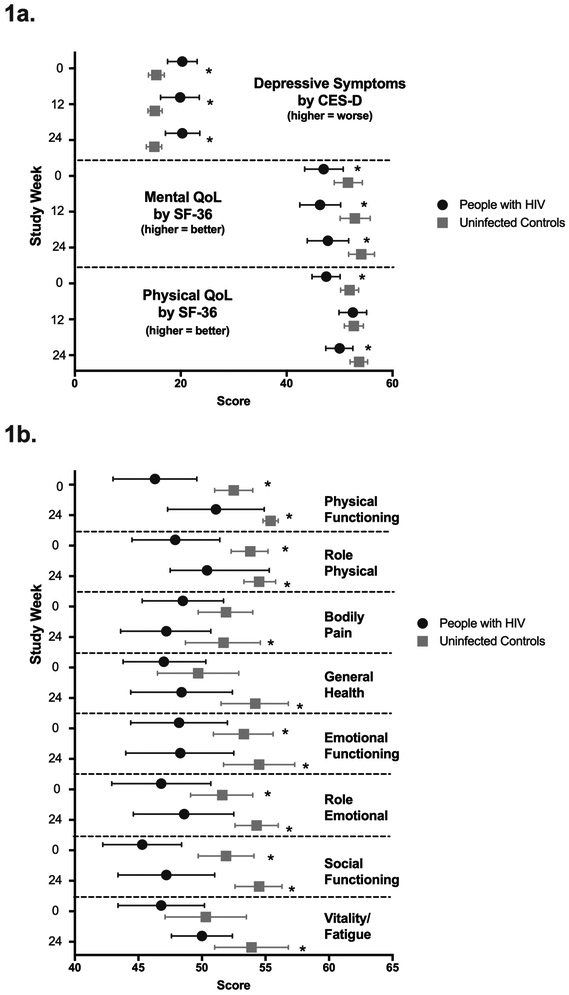
At baseline (Figure 1a), PLWH had significantly higher (worse) CES-D scores than controls (p=0.003), with the mean value among the PLWH (20.3) indicative of clinical depression. CES-D scores remained significantly higher than controls at week 12 (p=0.017) and week 24 (p=0.004). In unadjusted analyses (Table 2), changes in CES-D did not differ signficantly by HIV serostatus between weeks 0-12 (p=0.39 and 0.99, respectively). PLWH had a significantly greater increase in score between weeks 13-24 compared to controls, indicating worsening depressive symptoms (2.4 [0.1, 4.7], p=0.043). Fully adjusted models (Table 3), yielded serostatus difference between weeks 13-24 (3.4 [0.7, 6.0, p=0.01). Change in CES-D scores did not differ significantly by exercise intensity in fully adjusted models (Table 3).
Figure 1a.
Depressive Symptoms by CES-D and Quality of Life by SF-36 Mental and Physical Subscales. Mean scores with 95% confidence intervals are shown at 0, 12, and 24 weeks among people living with HIV (black circles) and uninfected controls (gray squares). Significant between group differences (p<0.05) are indicated by *.
1b. SF-36 Subscale mean scores with 95% confidence intervals are shown at 0 and 24 weeks among people living with HIV (black circles) and uninfected controls (gray squares). Significant between group differences (p<0.05) are indicated by *.
Table 2.
Unadjusted Changes in Depression (CES-D) and Quality of Life (SF-36) Scores from 0-12 and 13-24 Weeks by HIV Serostatus and Exercise Intensity
|
Change from 0-12 Weeks |
P- value |
Change from 13-24 Weeks |
P- value |
Change from 13-24 weeks |
P- value |
||||
|---|---|---|---|---|---|---|---|---|---|
| PLWH | Controls | PLWH | Controls | High- Intensity |
Moderate- Intensity |
||||
| CES-D | −1 [−3.3, 1.3] | 0.2 [−1.2, 1.5] | 0.39 | 0.7 [−1.5, 3] | −0.3 [−1.7, 1] | 0.41 | −0.2 [−2.3, 1.8] | 0.7 [−0.8, 2.2] | 0.47 |
| Summary Physical | 5.7 [3.2, 8.3]** | 1.2 [−0.7, 3] | 0.004 | −2.7 [−5.3, −0.1]* | 1 [−0.5, 2.5] | 0.017 | −0.2 [−2.6, 2.3] | −1.4 [−3.1, 0.3] | 0.39 |
| Summary Mental | 1.2 [−1.9, 4.3] | 1 [−0.8, 2.9] | 0.94 | 1.4 [−2.3, 5.1] | 0.7 [−1.4, 2.9] | 0.76 | 2.2 [−0.9, 5.4] | −0.3 [−2.9, 2.4] | 0.21 |
| Physical Functioning | 6.1 [3.2, 9]** | 2.2 [0.4, 4]* | 0.023 | −1.9 [−5.6, 1.7] | 0.7 [−0.5, 1.8] | 0.17 | 0.5 [−1.6, 2.5] | −1.7 [−4.9, 1.5] | 0.25 |
| Role Physical | 5.3 [1.2, 9.4]* | −0.2 [−2.9, 2.4] | 0.026 | −1.9 [−4.9, 1.1] | 1.3 [−1.2, 3.8] | 0.099 | −0.3 [−3.8, 3.3] | −0.3 [−1.8, 1.2] | 0.99 |
| Bodily Pain | 1.5 [−1.9, 4.8] | 0.1 [−2.7, 2.8] | 0.51 | −2.2 [−6, 1.6] | 0 [−2.3, 2.4] | 0.31 | −0.4 [−3, 2.2] | −1.7 [−5.3, 1.9] | 0.55 |
| General Health | 3 [0.4, 5.6]* | 2.2 [0.3, 4]* | 0.59 | −0.4 [−2.4, 1.6] | 1.8 [−0.1, 3.7] | 0.11 | 1.5 [−0.7, 3.7] | −0.1 [−1.7, 1.5] | 0.23 |
| Emotional Function | 1 [−1.4, 3.5] | 0.5 [−1.7, 2.7] | 0.74 | −0.2 [−3.2, 2.7] | 0.3 [−2.4, 3] | 0.78 | 1.3 [−1.7, 4.3] | −1.3 [−3.8, 1.1] | 0.18 |
| Role Emotional | 2.2 [−2.3, 6.6] | 1 [−0.8, 2.7] | 0.61 | 1.1 [−3.5, 5.7] | 1 [−1.1, 3.2] | 0.98 | 1.7 [−1.3, 4.8] | 0.4 [−3.5, 4.3] | 0.58 |
| Social Function | 4.8 [1.8, 7.7]** | 1.3 [−1.4, 3.9] | 0.08 | −2.1 [−4.9, 0.6] | 0.6 [−1.1, 2.2] | 0.09 | 0 [−2, 2] | −1.5 [−4, 1] | 0.35 |
| Vitality Fatigue | 3.7 [0.6, 6.7]* | 2.3 [0.2, 4.4]* | 0.46 | 1.2 [−1.4, 3.9] | 1.4 [−1, 3.8] | 0.93 | 3.9 [1.1, 6.6]** | −1.4 [−3.1, 0.2] | 0.001 |
Data presented as mean (95% confidence interval). P-values indicate the between group change (by HIV serostatus or exercise intensity). The Asterix (*) indicate the within group change, with
indicating a p-value of <0.05 and
indicating a p-value of <0.01.
Table 3.
Changes in Depression (CES-D) and Quality of Life (SF-36) Scores from 0-12 and 13-24 Weeks by HIV Serostatus and Exercise Intensity, Fully-Adjusted Modelsa,b
| Week 0-12 Change in PLWH (reference controls) |
P- value |
Week 13-24 Change in PLWH (reference controls) |
P-Value | Week 13-24 Change in High- Intensity (reference moderate) |
P- Value |
|
|---|---|---|---|---|---|---|
| CES-Da | 0.1 [−3, 3.1] | 0.96 | 3.4 [0.7, 6.0] | 0.01 | −0.1 [−2.6, 2.4] | 0.95 |
| SF-36 Summary and Subscale Scoresb | ||||||
| Summary Physical | 1.5 [−1.6, 4.6] | 0.35 | −4.1 [−7.2, −1.0] | 0.01 | 1.2 [−1.6, 4.1] | 0.38 |
| Summary Mental | −3.2 [−7, 0.6] | 0.10 | −3.7 [−8.2, 0.9] | 0.11 | 0.6 [−3.3, 4.5] | 0.76 |
| Physical Functioning | −0.8 [−3.4, 1.8] | 0.54 | −2.2 [−6.9, 2.5] | 0.36 | 1.6 [−2.3, 5.5] | 0.40 |
| Role Physical | −0.6 [−4.5, 3.3] | 0.75 | −4.6 [−8.3, −1.0] | 0.01 | −0.4 [−3.7, 2.9] | 0.83 |
| Bodily Pain | −0.9 [−5.4, 3.6] | 0.69 | −5.6 [−10.6, −0.7] | 0.03 | 1.6 [−2.7, 5.9] | 0.45 |
| General Health | −1.7 [−5.3, 1.8] | 0.32 | −2.3 [−5.7, 1.1] | 0.18 | 1.7 [−1.2, 4.5] | 0.24 |
| Emotional Functioning | −2.7 [−6.2, 0.8] | 0.12 | −3.8 [−8.4, 0.8] | 0.11 | 1.3 [−2.8, 5.3] | 0.53 |
| Role Emotional | −3 [−7.7, 1.6] | 0.20 | −2.9 [−7.9, 2.1] | 0.24 | −0.1 [−4.3, 4.1] | 0.95 |
| Social Functioning | −2.2 [−6.3, 1.9] | 0.28 | −4.6 [−8.2, −0.9] | 0.01 | 0.5 [−2.8, 3.8] | 0.77 |
| Vitality Fatigue | −2.9 [−6.3, 0.6] | 0.10 | −2.6 [−6.8, 1.7] | 0.23 | 4.1 [0.8, 7.3] | 0.02 |
Data presented as mean (95% confidence interval).
CES-D models adjusted for employment, baseline anxiety, depression or bipolar disorder, baseline score (for 0-12 week change), exercise intensity and week 12 scores (for 13-24 week change).
SF-36 scores were adjusted for baseline BMI, education, baseline score (for 0-12 week change), exercise intensity and week 12 scores for 13-24 week change)
Quality of Life Summary Scores
At baseline, PLWH had a lower SF-36 physical summary score than controls (Figure 1a, p=0.007). Between weeks 0-12, the physical summary score increased significantly among the PLWH but not the controls (Table 2), resulting in a significant between group difference (p=0.004, Figure 1a). From weeks 13-24, PLWH had a significant decrease while controls had a slight increase, resulting in significantly lower SF-36 physical summary scores among PLWH at week 24 (p=0.015), Figure 1a. In models adjusted for baseline SF-36 score only, changes between weeks 0-12 were not significantly different by HIV serostatus, while changes between weeks 13-24 indicated significantly greater declines among PLWH (−4.1 [−7.2, −1], p=0.01). Exercise intensity was not associated with changes in SF-36 physical summary scores (Table 3).
SF-36 mental summary scores were also significantly lower among PLWH compared to controls at baseline (p=0.042), week 12 (p=0.007), and week 24 (p=0.006), Figure 1a. No significant within group changes were seen among PLWH or controls between weeks 13 or 24 (Table 2). In fully-adjusted models, neither HIV serostatus nor exercise intensity were significantly associated with change in SF-36 mental summary scores (Table 3).
Quality of Life Subscales
At baseline, PLWH had significantly lower scores on the physical functioning, role physical, emotional functioning, role emotional, and social functioning subscales of the SF-36 (all p<0.05; Figure 1b). Significantly lower scores were also seen among PLWH on emotional functioning, role emotional, and social functioning at week 12, and on all subscales by week 24 (Figure 1b). From weeks 0-12, both PLWH and controls had significant increases in physical functioning, general health, and vitality/fatigue, and PLWH additionally on role physical and social function. Compared to controls, these improvements were significantly greater among PLWH on the physical functioning and role physical subscales (Table 2). In the fully adjusted analyses (Table 3), HIV serostatus was associated with significantly lower scores on role physical, social functioning, and bodily pain between weeks 13-24. Higher intensity exercise was associated with significantly improved vitality/fatigue scores (4.1 [0.8, 7.3], p=0.02, Table 3).
Discussion
Among older adults living with and without HIV who completed a supervised exercise intervention, both groups had initial improvements in physical QoL (summary scale). The self-reported improvements in physical health correspond to the improvements in objective physical function that we have previously reported (Erlandson, et al. 2018), with the greatest improvements seen within the first 12 weeks of the intervention. Similarly, although we had limited power, we found significant changes across many SF-36 subscales during the first 12 weeks, supporting the initial beneficial effects of exercise. Many of these improvements were not sustained in the second 12 weeks, however scores among both PLWH and controls were generally improved from pre- intervention (Figure 1). In contrast to several studies that have found supervised exercise interventions increase QoL and/or mental health in adults with HIV (Maharaj and Chetty, 2011; O’Brien, Tynan, Nixon, & Glazier, 2016; Ogalha et al., 2011) or without HIV (Bridle, Spanjers, Patel, Atherton, & Lamb, 2012; Knapen, Vancampfort, Morien, & Marchal, 2015; Loprinzi, 2013; Park, Han, & Kang, 2014), we failed to show significant improvements in most mental health outcomes over the 24 weeks of the study. These findings lead one to wonder what was different with our participants or our intervention?
First, both of our groups had a significant burden of mental illness, as highlighted by high CES-D scores among both PLWH and controls, psychiatric medication burden, and prior diagnoses of depression, anxiety or bipolar illness in >50% in PLWH and nearly 25% of controls. Participants may have experienced fluctuations in their mental health beyond what might be expected in a population without mental health disorders or captured in research-based survey tools. Although the SF-36 and CES-D have been validated among both populations with and without HIV, discrepancies with clinician-based diagnosis of severe depression or mood disorders have been reported (Marando et al., 2016). Indeed, in focus groups conducted among the first 19 PLWH from the exercise intevention, improvements in physical health, mental health, and mood were commonly reported beneficial outcomes (Neff et al., 2018).
Second, the assigned (rather than self-selected) exercise intensity during the second 12 weeks of the study might have attenuated improvements in mood. However, many studies have suggested similar if not improved mood with assigned rather than self-selected intensity (Kellogg et al., 2018; Meyer et al., 2016; Smith, Eston, Tempest, Norton, & Parfitt, 2015). The goals of participating in the intervention and thus impact of outcomes on QoL may have differed. As has been previously shown in other populations, participants who joined the study to improve overall health may have experienced a greater sense of achievement, reflected by improved physical QoL, whereas those who joined the study with a goal of weight loss may have experienced poorer QoL at week 24 if the goal was not achieved (Craft, Carroll, & Lustyk, 2014).
The duration of our study was longer (24 vs 12 weeks) and enrolled an older age group than many other reported studies of supervised exercise interventions in PLWH (Dudgeon, Phillips, Bopp, & Hand, 2004; O'Brien, et al., 2016), which may have influenced the changes in mood and QoL. Many participants expressed study visit and intervention fatigue after 24 weeks of supervised visits, which may have been reflected in our final QoL and mood scores. As with many behavior change interventions, motivation to participate and perceived benefits are high in the first several weeks, but wear off with time (Ory, Lee Smith, Mier, & Wernicke, 2010). Older adults, and PLWH in particular, may need additional interventions to maintain motivation, limit fatigue, and enhance physical QoL improvements, such as greater exercise variety, motivational interviewing and goal setting, incentive programs, or adjuvant therapies (massage, yoga, etc.) (Saajanaho et al., 2014) (Bethancourt, Rosenberg, Beatty, & Arterburn, 2014) (Shah, et al., 2016; Yuri, Takabatake, Nishikawa, Oka, & Fujiwara, 2016). Furthermore, among older adults with a high mental health burden, the exercise regimen alone may not boost depressive symptoms and mental QoL as much as more behavioral-focused interventions, group activities, or social events (Gallegos, Hoerger, Talbot, Moynihan, & Duberstein, 2013) (Azizan and Justine, 2016; Bonura and Tenenbaum, 2014; Lenze et al., 2014; Williams and Lord, 1997), and the addition of supplemental therapy (counseling, mindfulness training, yoga) should be considered to maximize mental health QoL among older adults with or without HIV.
Lastly, participants randomized to high-intensity exercise experienced greater gains in vitality than those who stayed in the moderate regimen. While conceptually, a higher-intensity of exercise might lead to greater fatigue, particularly among PLWH with high levels of baseline fatigue, our finding instead suggests that older sedentary adults may benefit from a stronger exercise “dose” to maximize improvements in function. Further, we found no evidence that this greater exercise intensity was associated with detrimental effects on other outcomes. Importantly, bodily pain did not worsen with greater exercise intensity.
Several limitations of this study should be acknowledged: first, the small sample size and large standard deviations limited our ability to detect differences between groups on some outcomes, in particular, the SF-36 subscales. The majority of the cohort were white males willing to initiate exercise, thus, our cohort may not be representative of the general population. Ethnic minorities and women may have differing barriers to initiating exercise and mood responses to exercise (Brian C. Martinson, 2010; Craft, et al., 2014). Determining and reducing barriers to exercise in diverse, older PLWH is a critical element in the prevention of QoL disparities.
In summary, older, PLWH initiating an exercise intervention report lower QoL and more depressive symptoms when compared to uninfected peers. Although objective physical function improvements were readily experienced by both groups (Erlandson, et al., 2018), exercise alone may not be enough to result in consistent improvements in depressive symptoms or QoL in an older population with a high burden of mental illness. Additional research on the impact of group exercise, exercise variety including more mindfulness-based therapy (i.e., yoga or tai chi), or adjunct therapies are needed to optimize mental health and QoL among older adults with HIV.
Acknowledgments
Funding
This work was supported by the National Institute of Aging of the National Institutes of Health [K23AG050260] and NCATS Colorado CTSA Grant Number UL1TR002535 (to KME). The funding sources had no a role in data collection, analysis, or interpretation; trial design; or patient recruitment. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Azizan A, & Justine M (2016). Effects of a Behavioral and Exercise Program on Depression and Quality of Life in Community-Dwelling Older Adults: A Controlled, Quasi-Experimental Study. Journal of Gerontological Nursing, 42(2), pp. 45–54. doi: 10.3928/00989134-20151124-01 [DOI] [PubMed] [Google Scholar]
- Balderson BH, Grothaus L, Harrison RG, McCoy K, Mahoney C, & Catz S (2013). Chronic illness burden and quality of life in an aging HIV population. AIDS care, 25(4), pp. 451–458. doi: 10.1080/09540121.2012.712669 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/22894702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethancourt HJ, Rosenberg DE, Beatty T, & Arterburn DE (2014). Barriers to and facilitators of physical activity program use among older adults. Clin Med Res, 12(1-2), pp. 10–20. doi: 10.3121/cmr.2013.1171 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/24415748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonura KB, & Tenenbaum G (2014). Effects of yoga on psychological health in older adults. J Phys Act Health, 11(7), pp. 1334–1341. doi: 10.1123/jpah.2012-0365 [DOI] [PubMed] [Google Scholar]
- Martinson Brian C., L. C., Sherwood Nancy E., Hayes Marcia, Pronk Nico P., O’Connor Patrick J. (2010). Population Reach and Recruitment Bias in a Maintenance RCT in Physically Active Older Adults. Physical Active Health, 7(1), pp. 127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridle C, Spanjers K, Patel S, Atherton NM, & Lamb SE (2012). Effect of exercise on depression severity in older people: systematic review and meta-analysis of randomised controlled trials. British Journal of Psychiatry, 201(3), pp. 180–185. doi: 10.1192/bjp.bp.111.095174 [DOI] [PubMed] [Google Scholar]
- Chambers LA, Wilson MG, Rueda S, Gogolishvili D, Shi MQ, Rourke SB, & Positive Aging Review, T. (2014). Evidence informing the intersection of HIV, aging and health: a scoping review. AIDS and Behavior, 18(4), pp. 661–675. doi: 10.1007/s10461-013-0627-5 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24185708 [DOI] [PubMed] [Google Scholar]
- Costagliola D (2014). Demographics of HIV and aging. Current Opinion in HIV and AIDS, 9(4), pp. 294–301. doi: 10.1097/COH.0000000000000076 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24824889 [DOI] [PubMed] [Google Scholar]
- Craft BB, Carroll HA, & Lustyk MK (2014). Gender Differences in Exercise Habits and Quality of Life Reports: Assessing the Moderating Effects of Reasons for Exercise. Int J Lib Arts Soc Sci, 2(5), pp. 65–76. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27668243 [PMC free article] [PubMed] [Google Scholar]
- Diaz KM, Howard VJ, Hutto B, Colabianchi N, Vena JE, Blair SN, & Hooker SP (2016). Patterns of Sedentary Behavior in US Middle-Age and Older Adults: The REGARDS Study. Medicine and science in sports and exercise, 48(3), pp. 430–438. doi: 10.1249/MSS.0000000000000792 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/26460633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudgeon WD, Phillips KD, Bopp CM, & Hand GA (2004). Physiological and psychological effects of exercise interventions in HIV disease. AIDS patient care and STDs, 18(2), pp. 81–98. doi: 10.1089/108729104322802515 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/15006183 [DOI] [PubMed] [Google Scholar]
- Emlet CA, Brennan DJ, Brennenstuhl S, Rueda S, Hart TA, & Rourke SB (2015). The impact of HIV-related stigma on older and younger adults living with HIV disease: does age matter? AIDS Care, 27(4), pp. 520–528. doi: 10.1080/09540121.2014.978734 [DOI] [PubMed] [Google Scholar]
- Erlandson KM, Allshouse AA, Jankowski CM, Mawhinney S, Kohrt WM, & Campbell TB (2014). Relationship of physical function and quality of life among persons aging with HIV infection. AIDS, 28(13), pp. 1939–1943. doi: 10.1097/qad.0000000000000384 Retrieved from http://ovidsp.tx.ovid.com/ovftpdfs/FPDDNCIBAELGDB00/fs047/ovft/live/gv024/00002030/00002030-201408240-00011.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlandson KM, MaWhinney S, Wilson M, Gross L, McCandless SA, Campbell TB, … Jankowski CM (2018). Physical function improvements with moderate or high-intensity exercise among older adults with or without HIV infection. AIDS, 32(16), pp. 2317–2326. doi: 10.1097/QAD.0000000000001984 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/30134299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallegos AM, Hoerger M, Talbot NL, Moynihan JA, & Duberstein PR (2013). Emotional benefits of mindfulness-based stress reduction in older adults: the moderating roles of age and depressive symptom severity. Aging & Mental Health, 17(7), pp. 823–829. doi: 10.1080/13607863.2013.799118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grov C, Golub SA, Parsons JT, Brennan M, & Karpiak SE (2010). Loneliness and HIV-related stigma explain depression among older HIV-positive adults. AIDS Care, 22(5), pp. 630–639. doi: 10.1080/09540120903280901 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/20401765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halkitis PN, Perez-Figueroa RE, Carreiro T, Kingdon MJ, Kupprat SA, & Eddy J (2014). Psychosocial burdens negatively impact HIV antiretroviral adherence in gay, bisexual, and other men who have sex with men aged 50 and older. AIDS Care, 26(11), pp. 1426–1434. doi: 10.1080/09540121.2014.921276 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24865599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins KL, Brown TT, Margolick JB, & Erlandson KM (2017). Geriatric syndromes: new frontiers in HIV and sarcopenia. AIDS, 31 Suppl 2, pp. S137–S146. doi: 10.1097/QAD.0000000000001444 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28471944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes RJ, Sierra F, Austad SN, Epel E, Neigh GN, Erlandson KM, … Hunt PW (2016). Disease drivers of aging. Ann N Y Acad Sci, 1386(1), pp. 45–68. doi: 10.1111/nyas.13299 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27943360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamitani E, Sipe TA, Higa DH, Mullins MM, & Soares J (2017). Evaluating the Effectiveness of Physical Exercise Interventions in Persons Living With HIV: Overview of Systematic Reviews. AIDS Education and Prevention, 29(4), pp. 347–363. doi: 10.1521/aeap.2017.29.4.347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg E, Cantacessi C, McNamer O, Holmes H, von Bargen R, Ramirez R, … Astorino TA (2018). Comparison of Psychological and Physiological Responses to Imposed vs. Self-selected High-Intensity Interval Training. J Strength Cond Res doi: 10.1519/JSC.0000000000002528 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29742746 [DOI] [PubMed] [Google Scholar]
- Knapen J, Vancampfort D, Morien Y, & Marchal Y (2015). Exercise therapy improves both mental and physical health in patients with major depression. Disability and Rehabilitation, 37(16), pp. 1490–1495. doi: 10.3109/09638288.2014.972579 [DOI] [PubMed] [Google Scholar]
- Lenze EJ, Hickman S, Hershey T, Wendleton L, Ly K, Dixon D, … Wetherell JL (2014). Mindfulness-based stress reduction for older adults with worry symptoms and co-occurring cognitive dysfunction. International Journal of Geriatric Psychiatry, 29(10), pp. 991–1000. doi: 10.1002/gps.4086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loprinzi PD (2013). Objectively measured light and moderate-to-vigorous physical activity is associated with lower depression levels among older US adults. Aging & Mental Health, 17(7), pp. 801–805. doi: 10.1080/13607863.2013.801066 [DOI] [PubMed] [Google Scholar]
- Maharaj SS, & Chetty V (2011). Rehabilitation program for the quality of life for individuals on highly active antiretroviral therapy in KwaZulu-Natal, South Africa: a short report. Int J Rehabil Res, 34(4), pp. 360–365. doi: 10.1097/MRR.0b013e32834d2bab Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22002109 [DOI] [PubMed] [Google Scholar]
- Marando F, Gualberti G, Costanzo AM, di Luzio Paparatti U, Franzetti M, Ammassari A, … Galli M (2016). Discrepancies between physician’s perception of depression in HIV patients and self-reported CES-D-20 assessment: the DHIVA study. AIDS care, 28(2), pp. 147–159. doi: 10.1080/09540121.2015.1080794 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/26461177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JD, Ellingson LD, Koltyn KF, Stegner AJ, Kim JS, & Cook DB (2016). Psychobiological Responses to Preferred and Prescribed Intensity Exercise in Major Depressive Disorder. Medicine and science in sports and exercise, 48(11), pp. 2207–2215. doi: 10.1249/MSS.0000000000001022 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27387295 [DOI] [PubMed] [Google Scholar]
- Irwin Micheal, K. H. A., Oxman Michael (1999). Screening for Depression in the Older Adult. Arch Intern Medicine, 159(15), pp. 1701–1704. doi:doi: 10.1001/archinte.159.15.1701 [DOI] [PubMed] [Google Scholar]
- Millar BM, Starks TJ, Gurung S, & Parsons JT (2017). The Impact of Comorbidities, Depression, and Substance Use Problems on Quality of Life Among Older Adults Living With HIV. AIDS and Behavior, 21(6), pp. 1684–1690. doi: 10.1007/s10461-016-1613-5 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27864625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro F, Canavarro MC, & Pereira M (2016). Factors associated with quality of life in middle-aged and older patients living with HIV. AIDS Care, 28 Suppl 1, pp. 92–98. doi: 10.1080/09540121.2016.1146209 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/26881294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RC, Fazeli PL, Jeste DV, Moore DJ, Grant I, Woods SP, & Group, H. I. V. N. R. P. (2014). Successful cognitive aging and health-related quality of life in younger and older adults infected with HIV. AIDS and Behavior, 18(6), pp. 1186–1197. doi: 10.1007/s10461-014-0743-x Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24633788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff HA, Kellar-Guenther Y, Jankowski CM, Worthington C, McCandless SA, Jones J, & Erlandson KM (2018). Turning disability into ability: barriers and facilitators to initiating and maintaining exercise among older men living with HIV. AIDS care, pp. 1–5. doi: 10.1080/09540121.2018.1493186 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29968493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosrat S, Whitworth JW, & Ciccolo JT (2017). Exercise and mental health of people living with HIV: A systematic review. Chronic Illn, 13(4), pp. 299–319. doi: 10.1177/1742395317694224 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29119865 [DOI] [PubMed] [Google Scholar]
- O’Brien KK, Tynan AM, Nixon SA, & Glazier RH (2016). Effectiveness of aerobic exercise for adults living with HIV: systematic review and meta-analysis using the Cochrane Collaboration protocol. BMC Infect Dis, 16, p 182. doi: 10.1186/s12879-016-1478-2 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27112335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogalha C, Luz E, Sampaio E, Souza R, Zarife A, Neto MG, … Brites C (2011). A randomized, clinical trial to evaluate the impact of regular physical activity on the quality of life, body morphology and metabolic parameters of patients with AIDS in Salvador, Brazil. Journal of acquired immune deficiency syndromes, 57 Suppl 3, pp. S179–185. doi: 10.1097/QAI.0b013e31821e9bca Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/21857315 [DOI] [PubMed] [Google Scholar]
- Ory MG, Lee Smith M, Mier N, & Wernicke MM (2010). The science of sustaining health behavior change: the health maintenance consortium. Am J Health Behav, 34(6), pp. 647–659. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/20604691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SH, Han KS, & Kang CB (2014). Effects of exercise programs on depressive symptoms, quality of life, and self-esteem in older people: a systematic review of randomized controlled trials. Applied Nursing Research, 27(4), pp. 219–226. doi: 10.1016/j.apnr.2014.01.004 [DOI] [PubMed] [Google Scholar]
- Hsiung Ping-Chuan, C.-T. F., Change Yu-Yin, Chen Mao-Yen, Wang Jung-Der. (2005). Comparison of WHOQOL-BREF and SF-36 in patients with HIV infection. Quality of Life Research, 14, pp. 141–150. [DOI] [PubMed] [Google Scholar]
- Saajanaho M, Viljanen A, Read S, Rantakokko M, Tsai LT, Kaprio J, … Rantanen T (2014). Older women’s personal goals and exercise activity: an 8-year follow-up. Journal of Aging and Physical Activity, 22(3), pp. 386–392. doi: 10.1123/japa.2012-0339 [DOI] [PubMed] [Google Scholar]
- Shah KN, Majeed Z, Yoruk YB, Yang H, Hilton TN, McMahon JM, … Ryan RM (2016). Enhancing physical function in HIV-infected older adults: A randomized controlled clinical trial. Health Psychol, 35(6), pp. 563–573. doi: 10.1037/hea0000311 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/26867045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegler EL, & Brennan-Ing M (2017). Adapting Systems of Care for People Aging With HIV. J Assoc Nurses AIDS Care, 28(5), pp. 698–707. doi: 10.1016/j.jana.2017.05.006 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28602461 [DOI] [PubMed] [Google Scholar]
- Sin NL, & DiMatteo MR (2014). Depression treatment enhances adherence to antiretroviral therapy: a meta-analysis. Annals of Behavioral Medicine, 47(3), pp. 259–269. doi: 10.1007/s12160-013-9559-6 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24234601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater LZ, Moneyham L, Vance DE, Raper JL, Mugavero MJ, & Childs G (2015). The multiple stigma experience and quality of life in older gay men with HIV. Journal of the Association of Nurses in AIDS Care, 26(1), pp. 24–35. doi: 10.1016/j.jana.2014.06.007 [DOI] [PubMed] [Google Scholar]
- Smith AE, Eston R, Tempest GD, Norton B, & Parfitt G (2015). Patterning of physiological and affective responses in older active adults during a maximal graded exercise test and self-selected exercise. Eur J Appl Physiol, 115(9), pp. 1855–1866. doi: 10.1007/s00421-015-3167-z Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/25876526 [DOI] [PubMed] [Google Scholar]
- Vance D (2013). the cognitive consequences of stigma social withdrawal and depression in adults aging with hiv Psychosocial Nursing, 51(5), pp. 18–20. doi: 10.3928/02793695-20130315-01 [DOI] [PubMed] [Google Scholar]
- Williams P, & Lord SR (1997). Effects of group exercise on cognitive functioning and mood in older women. Australian and New Zealand Journal of Public Health, 21(1), pp. 45–52. [DOI] [PubMed] [Google Scholar]
- Yuri Y, Takabatake S, Nishikawa T, Oka M, & Fujiwara T (2016). The effects of a life goal-setting technique in a preventive care program for frail community-dwelling older people: a cluster nonrandomized controlled trial. BMC Geriatrics, 16, p 101. doi: 10.1186/s12877-016-0277-3 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27175793 [DOI] [PMC free article] [PubMed] [Google Scholar]