Abstract
Background and Objectives:
Previously, we have shown that 9-cis retinoic acid (9-cis RA) stimulates lymphangiogenesis and limits postsurgical lymphedema in animal models when administered intraperitoneally. In this study, we investigate whether 9-cis RA contained within a single-use depot drug delivery system (DDS) can mitigate development of lymphedema in a clinically relevant mouse limb model.
Methods:
Hind limb lymphedema was induced via surgical lymphadenectomy and irradiation. Animals were divided into 2 treatment groups: 1) 9-cis RA DDS, 2) placebo DDS. Outcomes measured included paw thickness, lymphatic clearance and density, epidermal thickness, and collagen deposition.
Results:
Compared to control animals, 9-cis RA-treated animals had significantly less paw swelling from postoperative week 3 (P=0.04) until the final timepoint at week 6 (P=0.0007). Moreover, 9-cis RA-treated animals had significantly faster lymphatic clearance (P<0.05), increased lymphatic density (P=0.04), reduced lymphatic vessel size (P=0.02), reduced epidermal hyperplasia (P=0.04), and reduced collagen staining (P=0.10).
Conclusions:
Animals receiving 9-cis RA sustained-release implants at the time of surgery had improved lymphatic function and structure, indicating reduced lymphedema progression. Thus, we demonstrate that 9-cis RA contained within a single-use depot DDS has favorable properties in limiting pathologic responses to lymphatic injury and may be an effective strategy against secondary lymphedema.
Keywords: 9-cis retinoic acid, retinoic acid, depot, sustained release, drug delivery system, drug pellet, lymphedema, lymphangiogenesis, postsurgical lymphedema, secondary lymphedema
Introduction
Lymphedema is a disfiguring disease resulting from injury, infection or congenital malformation of the lymphatic system affecting over 250 million individuals worldwide [1]. The pathogenesis of lymphedema originates from impaired lymphatic transport capacity resulting in the buildup of fluid, protein and fibroadipose tissue in the interstitial space, causing progressive enlargement of the affected body part. Depending on the location of injury, lymphedema can affect the upper extremity, lower extremity, genitalia, abdomen, or head and neck. Primary lymphedema is rare, affecting 1:6,000–10,000 live births due to inborn defects of lymphangiogenesis [2]. Secondary lymphedema is more common and results in a significant disease burden, particularly for the cancer patient population. In the US, secondary lymphedema affects over 5 million cancer survivors and is most often caused by direct lymphatic injury during the resection or irradiation of solid organ tumors, including breast, gynecologic and lower extremity malignancies [1].
Unfortunately, no curative therapy exists for lymphedema. Symptoms of this progressive disease are variable but can include pain, heaviness, disfigurement, impaired ambulation, challenges with activities of daily living, increased risk for infection and wound breakdown, and risk of malignant transformation. The mainstay of lymphedema therapy consists of conservative management in the form of combined decongestive therapy (CDT). CDT includes four elements: manual lymphatic drainage, compression bandaging, exercise and skin care. Since conservative management results only in symptomatic relief, is time-consuming, and is associated with high healthcare costs, patient adherence is poor in the long-term [3]. For this reason, advances in the surgical and pharmacologic management of lymphedema have recently garnered scientific interest. New microsurgical procedures, such as vascularized lymph node transfer (VLNT) and lymphaticovenous bypass (LVB), aim to redirect obstructed lymphatic flow into the venous circulation to decongest the affected body part. More extreme debulking procedures, which resect subcutaneous and fibroadipose tissue and cover the resulting defect with skin grafts, are used for patients with extensive tissue fibrosis characteristic of end-stage lymphedema [4].
In an effort to explore novel options for lymphedema management, current research efforts include the investigation of pharmacologic agents with pro-lymphangiogenic properties. Several growth factors have already been shown to stimulate lymphangiogenesis in animal models, such as VEGF-C and VEGF-D [5–8]. However, these drugs have been linked to the promotion of tumor metastasis, making their use relatively contraindicated in patients with a history of cancer, which comprise a significant proportion of lymphedema patients in the US [9–11]. A retinoic acid derivative, 9-cis retinoic acid (9-cis RA) has recently been shown to stimulate lymphangiogenesis without promoting tumor metastasis. Landmark studies have revealed significantly increased lymphatic endothelial cell proliferation, migration and vessel formation when treated with 9-cis RA in vitro. Animal studies have corroborated these findings by demonstrating increased lymphatic vessel density and function in vivo [12,13].
Importantly, unlike the pro-lymphangiogenic growth factors VEGF-C and VEGF-D, 9-cis RA demonstrates a favorable risk profile in patients with a history of cancer. Moreover, it has already been FDA-approved for Kaposi sarcoma of the skin and chronic hand eczema in the US. Its lack of known associated teratogenicity, unlike other retinoic acid compounds such as isotretinoin, further makes it a favorable candidate for studies on lymphedema therapy. Previous animal studies have tested 9-cis RA given through daily intraperitoneal injections, a method of drug administration that has poor translatability to future clinical trials due to the painful nature of the injection and the need for daily follow-up. In this study, we aim to investigate the pro-lymphangiogenic effects of 9-cis RA contained within an implantable, single-use pellet for sustained drug delivery in a clinically relevant mouse lymphedema model. Data from this study may set the stage for the use of a 9-cis RA drug delivery system (DDS) in future human trials and aid in the development of this promising pharmacologic agent for the prevention of postsurgical lymphedema.
Materials and Methods
Animals
All experiments were performed in accordance with the statutes from The Guide for the Care and Use of Laboratory Animals (National Research Council, 8th ed, 2011), the Animal Welfare Act, and other Federal regulations. Our protocol was approved by the University of Southern California Institutional Animal Care and Use Committee. Male and female Prox-1 green fluorescent protein (GFP) transgenic mice, 12–16 weeks old, weighing 20–30 g were bred within the University of Southern California animal facility. This GFP-expressing lymphatic reporter mouse was used because it conveniently allows for the visualization of lymphatic vessels and other Prox1-expressing tissue under fluorescence microscopy [13,14]. Animals were housed in a light- and temperature-controlled environment with access to food and water ad libitum.
Hind Limb Lymphedema Model
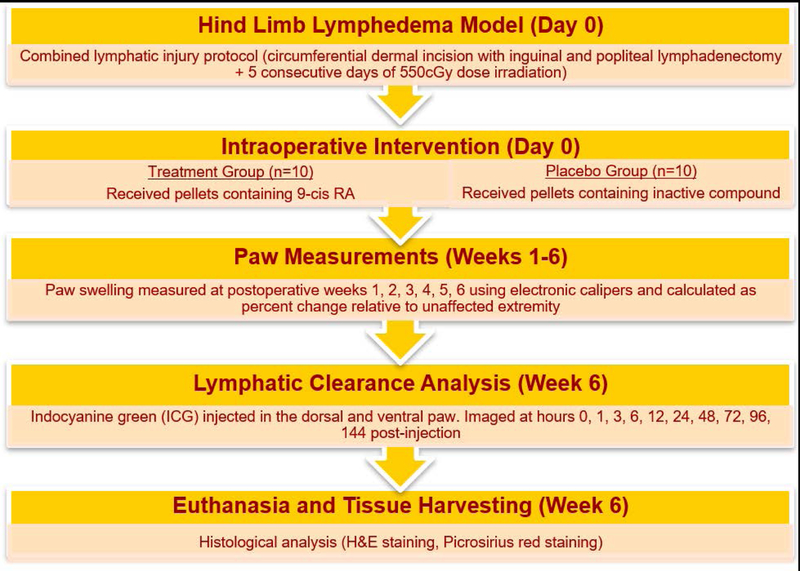
A timeline of the experimental methodology is depicted in Figure 1. Under isoflurane-inhaled anesthesia, the lymphatic injury protocol was used on all experimental mice to induce hind limb lymphedema. This consisted of hind limb lymph node dissection followed by adjuvant radiation therapy. Using a dissecting microscope, a circumferential intradermal incision was made around the thigh, after which the ipsilateral inguinal fat pad (containing the inguinal lymph nodes) and the ipsilateral popliteal lymph node were removed, as previously described [13]. Visualization of the inguinal and popliteal lymphatics was facilitated by the injection of methylene blue dye on the dorsum of the paw and by the use of a green fluorescent microscope. Skin sutures were placed in 7–8 mm gaps to reinforce discontinuity of dermal lymphatics. The surgical area was then irradiated using a clinically relevant dose of 27.5 Gy radiation delivered by an XRad 320 device over a period of 5 days (550 cGy per day), which is the human equivalent dose for mouse.
Figure 1. Timeline of the experimental methodology.
Starting from day 0 (day of surgery), paw measurements were obtained weekly for a total of 6 weeks. At the final timepoint, animals underwent lymphatic clearance analysis before being euthanized for tissue harvesting.
Experimental Design
Using the Prox-1 GFP-expressing lymphatic reporter mouse hind limb lymphedema model, twenty (20) mice were divided into two groups to test the effect of 9-cis RA on postsurgical lymphedema: (1) the treatment group received implantable DDS pellets containing 9-cis RA, 2) the control group received implantable placebo pellets containing inactive compound. DDS pellets were placed within the surgical wound intraoperatively and sutured in place to the adductor muscles of the thigh. DDS treatment pellets were custom-manufactured by Innovative Research of America (Sarasota, FL) to contain 100 μg of 9-cis RA, released over a period of 30 days using a matrix-driven delivery system. The carrier-binder excipients of the pellet matrix were synthetic and non-toxic, and included cholesterol, lactose, celluloses, phosphates, and stearates. This resulted in a biodegradable matrix that effectively and continuously released the active product in the animal. Animals that developed tissue necrosis or surgical site infection were sacrificed and excluded from the analysis (in total, 2 out of 20 animals met exclusion criteria: 1 from the treatment group and 1 from the control group).
Postoperative Measurements
Prior to surgery, all animals were sex-, age-, and weight-matched to eliminate confounding variables. Bilateral paw thickness, the primary outcome to assess lymphedema development, was measured weekly using electronic calipers for a total of 6 weeks. Hind limbs were photographed using a Nikon D5000 digital SLR flash camera using fixed distance, focal length, background, and positioning. To account for age-appropriate growth and differences in animal size, paw thickness was normalized for each animal by calculating percent change relative to the unoperated paw using the formula (To–Tu)/Tu x 100, where To is the thickness of the operated paw and Tu is the thickness of the unoperated paw. All measurements were performed in triplicate and statistically analyzed as mean values.
Indocyanine Green Lymphography
At postoperative week 6, lymphatic clearance was measured using indocyanine green (ICG) lymphography to compare lymphatic function between the treatment and placebo groups. ICG is a fluorescent dye that, upon activation by a laser light source, displays a signal that can be imaged using near-infrared systems. It has been shown to be superior to lymphoscintigraphy in a clinical setting, with both a sensitivity and specificity of 100% [15]. After inducing anesthesia via isoflurane inhalation, a sub-microliter injection system syringe was used to intradermally inject 2 μL of ICG on the plantar aspect of the second and fifth paw digits. ICG lymphography images were recorded using SPY-Q technology (Novadaq Technologies, BC, Canada) at 1, 3, 6, 12, 24, 48, 72, 96, and 144 hours post-injection. For each post-injection time point, the fluorescence intensity of the lymphography images was quantified in 3 regions of interest in the operated hind limb using ImageJ pixel intensity analysis (National Institutes of Health, Bethesda, MD).
Histological Assessment
Hind limb tissue harvested at euthanasia was fixed in 10% neutral buffered formalin for 24 hours, decalcified using Surgipath Decalcifier II solution (Leica Biosystems Inc., Buffalo Grove, IL), embedded in paraffin, and sectioned using 3–5 μm tissue thickness. Hematoxylin and Eosin (H&E) staining of operated mouse paws was performed to document differences in histological architecture of the soft tissues of the paw between treatment and control groups. Thickness of the Malpighian layer, consisting of the distance from the bottom of the stratum basale to the top of the stratum spinosum of the epidermis, was measured with ImageJ using at least 3 images per cross-section taken at 20x magnification using a BZ-X800E microscope (Keyence Corporation of America, Itasca, IL). Picrosirius red staining of operated mouse paws was performed to detect differences in fibrotic response between the two groups. Staining was performed by bathing cross-sections in picrosirius red solution (Sigma-Aldrich, St. Louis, MO) for 40 minutes, followed by dehydration and mounting. Collagen deposition was quantified using at least 3 images per cross-section taken at 20x magnification by measuring percent collagen area (in red) divided by total tissue area within the dermis.
Immunohistochemical Assessment
Formalin fixed and paraffin-embedded samples of mouse paws were immunostained for LYVE-1 (catalog # NB600–1008; Novus Biologicals, Centennial, CO) according to manufacturer recommendations. Briefly, sections were deparaffinized, heated in citrate buffer for antigen retrieval, and incubated in primary antibody for LYVE-1 (1:200) at 4°C in 5% bovine serum albumin blocking buffer overnight. Sections were then incubated in biotinylated secondary antibody (1:200) for 2 hours, followed by incubation with alkaline phosphatase-conjugated streptavidin and diaminobenzidine. In order to document differences in lymphatic density, the number and diameter of LYVE-1 positive lymphatic vessels was quantified on ImageJ using at least 3 images per cross-section taken at 40x magnification.
Statistical Analysis
Statistical analysis was conducted using GraphPad Prism 8.0 software (GraphPad Software, Inc., San Diego, CA) using a two-tailed Student’s t-test to compare the means of the treatment and placebo groups. All outcomes were analyzed using mean values and standard error. Statistical significance was determined by P-values less than 0.05.
Results
Sustained Release 9-cis RA Implanted at the Lymphedenectomy Defect Prevents Postsurgical Hind Limb Lymphedema
Previously, we have shown that daily intraperitoneal injections of 9-cis RA (0.08 mg/kg) prevent postsurgical lymphedema in mice [13]. To test a more clinically feasible route of administration, 9-cis RA was incorporated into an implantable, 30-day sustained-release pellet and placed within the surgical site intraoperatively, at the same time that hind limb lymphedema was induced in Prox1-GFP mice. A schematic of the hind limb lymphedenectomy model used to establish lymphedema, along with intraoperative images of the lymph node dissection and DDS implantation, is depicted in Figure 2A–F. The progression of lymphedema in control and treatment animals was assessed by measuring paw thickness over a period of 6 weeks. As shown in Figure 3, animals treated with 9-cis RA implants consistently experienced less paw swelling over time, indicating reduced lymphedema progression. When change in operated paw size (relative to the unoperated paw) was plotted over time, animals treated with 9-cis RA implants showed significantly reduced paw sizes than control animals, beginning on postoperative week 3 and then every week thereafter for the duration of the study (Figure 4). The mean difference in “change in paw size” between the two groups was approximately 10% (7% at week 3, 12% at week 4, 9% at week 5, and 11% at week 6). Moreover, change in paw size over time was analyzed within each group (as opposed to between groups) by comparing each postsurgical time point to day 0. This way, we could assess the degree of lymphedema progression over time for each animal group. While the placebo group demonstrated significant increases in paw thickness over time, no significant difference in paw thickness was observed within the treatment group over time. Thus, animals treated with 9-cis RA sustained release DDS did not develop a degree of paw swelling that was consistent with lymphedema development.
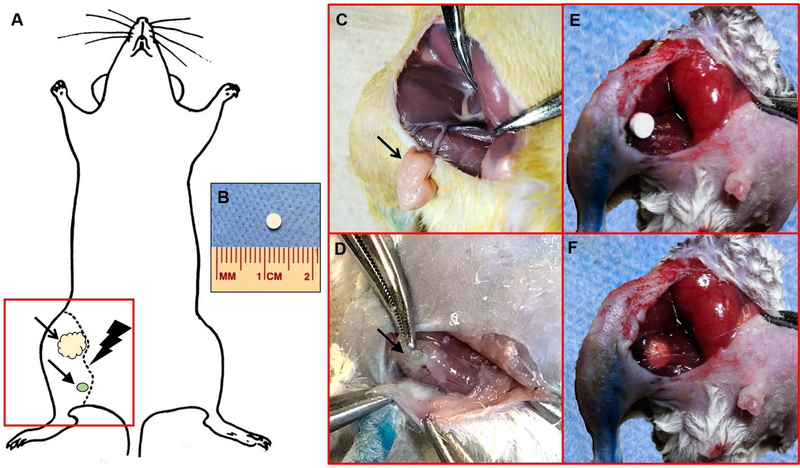
Figure 2. Schematic of the hind limb lymphadenectomy model.
(A) To induce hind limb lymphedema in mice, the inguinal fat pad (in yellow, indicated by the open arrow) and the popliteal lymph node (in green, indicated by the closed arrow) were surgically dissected. A circumferential skin incision was then made around the thigh along the dotted line, followed by hind limb irradiation. The red square indicates the field of view for images C–F. (B) Implantable pellets measured 3 mm x 3 mm. (C) Intraoperative image identifying the inguinal fat pad containing inguinal lymphatics (indicated by the open arrow), supplied by the superficial epigastric vessels. (D) Intraoperative image identifying the popliteal lymph node located within the inferior pole of the adductor thigh muscles (indicated by the closed arrow). (E) Intraoperative image of the implantable DDS placed in situ within the surgical wound. (F) Intraoperative image of the implantable DDS sutured to the fibers of the adductor thigh muscles.
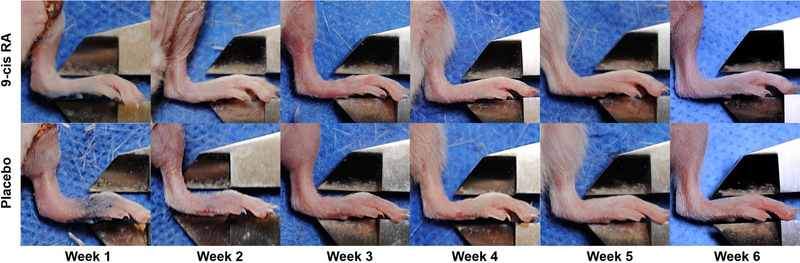
Figure 3. Representative paw images after inducing hind limb lymphedema in treatment and placebo groups.
Compared to control animals receiving placebo, the 9-cis RA group showed consistently less paw swelling as measured by electronic calipers. Subset of data used to plot Figure 4.
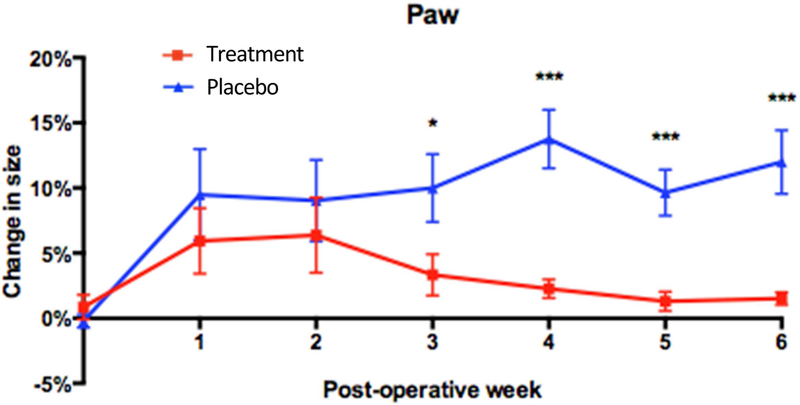
Figure 4. Sustained release 9-cis RA decreases paw swelling over time.
Graphical representation of percent change in size of the operated paw relative to the unoperated paw. Compared to control animals, animals treated with 9-cis RA showed significantly reduced paw swelling at postoperative week 3 (7% mean difference, P=0.04), week 4 (12% mean difference, P=0.0002), week 5 (9% mean difference, P=0.0005), and week 6 (11% mean difference, P=0.0007). Within the treatment group, no significant difference in paw swelling was observed between time points.
Effect of Sustained Release 9-cis RA on Lymphatic Clearance After Lymphadenectomy
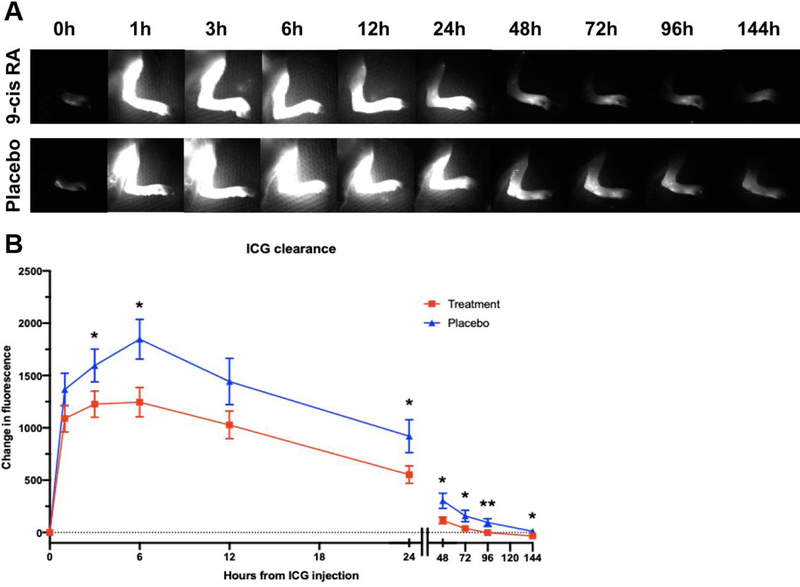
Indocyanine green (ICG) lymphography is a well-established imaging modality used in clinics by healthcare providers to visualize lymphatic vessels in real-time, for the purposes of diagnosis or preoperative planning. When ICG is injected intradermally, it is preferentially picked up and cleared by the lymphatic system. This imaging modality provides rapid visualization of lymphatic vessels and measures lymphatic clearance, acting as a surrogate for lymphatic function. Using the principles of ICG lymphography, we hypothesized that the degree of hind limb lymphedema would correlate with degree of lymphatic blockage (i.e., reduced lymphatic clearance). Thus, at postoperative week 6 and prior to euthanasia, we monitored lymphatic clearance by quantifying the change in dermal fluorescence of operated mouse hind limbs injected with ICG (Figure 5A). Notably, lymphographic analysis of placebo mice revealed that dermal backflow, a characteristic finding of lymphedematous limbs, persisted until 48 hours post-ICG injection, as opposed to the dermal backflow of 9-cis RA-treated mice which began to disappear at 12 hours. When analyzing change in fluorescence intensity over time, animals treated with 9-cis RA revealed significantly faster lymphatic drainage than control animals beginning 3 hours after ICG injection and lasting until hour 144, when lymphographic analysis was terminated (Figure 5B).
Figure 5. Sustained release 9-cis RA results in faster lymphatic clearance.
(A) Representative time-lapse images of ICG lymphography performed on postoperative day 42 (week 6). Subset of data used to plot Figure 5B. The accumulation of fluorescent signal seen at 1 hour post-ICG injection and persisting until hour 24 in the placebo group is consistent with dermal backflow, a characteristic finding of lymphedema on ICG lymphography due to disrupted lymphatic flow within dermal tissue. In 9-cis RA-treated animals, dermal backflow begins to disappear at hour 12, representing increased lymphatic clearance. (B) Change in fluorescence from injection time 0 plotted as a function of time (in arbitrary units). Compared to control animals, animals treated with 9-cis RA showed significantly faster lymphatic drainage as measured by decreased ICG fluorescence at 3, 6, 24, 48, 72, 96 and 144 hours following ICG injection (P<0.05).
Sustained Release 9-cis RA Mitigates Epidermal Hyperplasia and Collagen Deposition that are Hallmarks Characteristic of Chronic Lymphedema
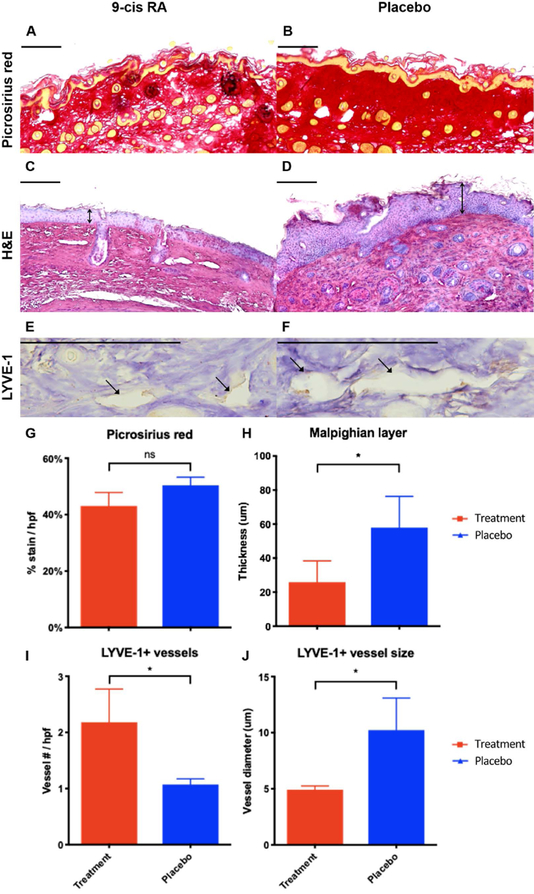
A histological analysis of operated mouse hind limbs was performed to further characterize changes of postsurgical lymphedema. Since longstanding lymphedema results in tissue fibrosis mediated by increased collagen deposition, we chose to quantify collagen density by measuring percent of picrosirius red-stained dermis divided by total tissue area (Figure 6A,B). Picrosirius red staining revealed that the operated paw skin of mice treated with 9-cis RA had a 15% reduction in collagen density compared to animals receiving placebo (P=0.10), indicating a reduced fibrotic response to lymphedema (Figure 6G). Moreover, since epidermal hyperplasia is a common histological finding of skin tissue affected by lymphedema, we chose to quantify epidermal hyperplasia by measuring the thickness of the Malpighian layer in H&E sections of operated mouse paw skin (Figure 6C,D). The Malpighian layer consists of the intrinsic stratum spinosum and stratum basale components of the epidermis. This was specifically measured to exclude the stratum corneum from analysis, as the stratum corneum can be artificially altered by tissue processing and other environmental factors, thus acting as a confounding variable. As shown in Figure 6H, the Malpighian layer was significantly thinner in animals treated with 9-cis RA implants compared to control animals (55% mean difference, P=0.04), suggesting that 9-cis RA released from implantable pellets prevents epidermal hyperplasia and lymphedema progression.
Figure 6. Sustained release 9-cis RA decreases epidermal hyperplasia, collagen deposition, and lymphatic vessel size.
(A) Representative picrosirius red stained cross-section of operated paw skin from a 9-cis RA-treated mouse. Horizontal scale bars represent 100 μm. (B) Representative picrosirius red stained cross-section of operated paw skin from a placebo-treated mouse. Note the decrease in collagen deposition in the operated paw skin of mice treated with 9-cis RA pellets. (C) Representative H&E stained cross-section of operated paw skin from a 9-cis RA-treated mouse. Horizontal scale bars represent 100 μm, vertical arrows represent Malpighian layer thickness. (D) Representative H&E stained cross-section of operated paw skin from a placebo-treated mouse. Note the decrease in hyperkeratosis and acanthosis in the operated paw skin of mice treated with 9-cis RA. (E) Representative LYVE-1 stained cross-section of operated paw skin from a 9-cis RA-treated mouse. Horizontal scale bars represent 100 μm, closed arrows indicate lymphatic vessels. (F) Representative LYVE-1 stained cross-section of operated paw skin from a placebo-treated mouse. Note the smaller lymphatic vessel diameter in the operated paw skin of mice treated with 9-cis RA. (G) Animals receiving 9-cis RA pellets showed a 15% decrease in collagen density per total tissue area compared to animals receiving placebo, indicating a reduced fibrotic response typical of lymphedema (P=0.10). (H) Animals receiving 9-cis RA pellets showed a 55% decrease in Malpighian layer thickness compared to animals receiving placebo (P=0.04), indicating reduced epidermal hyperplasia typical of lymphedema. (I) Animals receiving 9-cis RA pellets showed a 104% increase in number of LYVE-1+ lymphatic vessels compared to animals receiving placebo (P=0.04), indicating increased lymphatic density secondary to lymphangiogenesis. (J) Animals receiving 9-cis RA pellets showed a 52% decrease in LYVE-1+ lymphatic vessel diameter compared to animals receiving placebo (P=0.02), indicating preservation of the normal lymphatic architecture without luminal dilation.
Sustained Release 9-cis RA Maintains Lymphatic Vessel Structure and Density After Lymphadenectomy
Immunostaining for lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1), a lymphatic endothelial cell marker, was performed to visualize lymphatic vessels and characterize their structural response to postsurgical lymphedema. Over time, lymphatic vessels affected by postsurgical lymphedema become dilated due to the buildup of luminal pressure distal to the zone of injury. This was demonstrated by a mean lymphatic vessel diameter of approximately 10 μm in the placebo-treated group, which was twice as large as the size of lymphatic vessels in the 9-cis RA DDS group (P=0.02) (Figure 6J). Moreover, the pro-lymphangiogenic effects of 9-cis RA were specifically observed on lymphatic staining, as animals treated with 9-cis RA DDS had significantly more LYVE-1 positive lymphatic vessels than placebo-treated animals (P=0.04), indicating lymphatic endothelial cell proliferation (Figure 6I). Thus, 9-cis RA implantable pellets have favorable properties in maintaining normal lymphatic architecture and promoting lymphangiogenesis in postsurgical lymphedema.
Discussion
Postsurgical lymphedema commonly occurs in the setting of lymphadenectomy and radiation in the treatment of solid cancers. The incidence of postsurgical lymphedema is high in the population of cancer survivors; it develops in 16–39% of breast cancer patients following axillary lymph node dissection, 20–30% of lower extremity sarcoma and melanoma patients, and 20–49% of patients with gynecological malignancies following ilioinguinal lymph node dissection [16,17]. The extremities are the area most commonly affected by lymphedema. Of the patients with postsurgical lymphedema, approximately 90% have lower extremity lymphedema and 10% have upper extremity lymphedema [18]. The mainstay of lymphedema therapy is achieved through conservative management with variable rates of success, significant healthcare costs and poor patient adherence. Moreover, no curative therapy exists for established disease, which results in significant fibroadipose tissue deposition causing physical deformity and chronic functional deficits. For this reason, early interventions aimed at improving lymphatic function have the greatest potential at limiting disease progression.
Alternative therapies for lymphedema, such as surgical procedures that restore lymphatic flow and pharmacologic agents that promote lymphatic vessel formation, are currently being investigated. Of the more promising pharmacologic agents for lymphedema are members of the VEGF family (VEGF-C and VEGF-D) and 9-cis retinoic acid. The VEGF molecules are contraindicated in the cancer population, as they have been associated with the promotion of tumor metastasis. On the other hand, 9-cis RA is already FDA-approved in the US for other conditions and has been shown to stimulate lymphangiogenesis and lymphatic vessel formation both in vitro and in vivo. To date, 9-cis RA has been tested in animal studies via daily intraperitoneal administration, which is cumbersome and impractical in clinical settings, as it requires daily follow-up over a period of multiple weeks. To facilitate the potential development of 9-cis RA into a form suitable for human use and testing in clinical trials, we developed a more optimal protocol for 9-cis RA administration. Through the intraoperative single-use implantation of a biodegradable pellet containing 100 μg 9-cis RA, we were able to provide sustained drug release over a period of 30 days and mitigate the development of postsurgical lymphedema in our mouse hind limb lymphedema model.
In this study, we assessed paw size, lymphatic clearance, lymphatic density, collagen deposition, and epidermal thickness in order to characterize the development of lymphedema in the treatment group of animals receiving 9-cis RA implantable pellets and in the control group of animals receiving placebo pellets. Paw swelling served as clinical evidence of distal hind limb lymphedema. Lymphatic clearance analysis was used to assess lymphatic vessel function, while lymphatic size and density were quantified to assess structural changes of the lymphatic system. Collagen deposition and epidermal hyperplasia were used as histological evidence of lymphedema. Animals treated with 9-cis RA had significantly reduced paw swelling, increased lymphatic clearance and density, reduced lymphatic vessel diameter, and reduced epidermal hyperplasia compared to placebo-treated animals. A reduction in collagen deposition was also noted on picrosirius red-stained sections of 9-cis RA-treated mouse paws; however, this was statistically not significant. Together, these findings indicate that 9-cis RA contained within a single-use depot pellet DDS has favorable properties in limiting postsurgical lymphedema.
Importantly, the maximal difference in paw swelling between the treatment and placebo groups was seen at postoperative week 4 (12% mean difference, P=0.0002). This coincides with the time at which postsurgical changes caused by local cellular mediators of inflammation and edema have begun to subside. By eliminating postoperative swelling as a confounding factor, the observed decrease in paw swelling in the treatment group is more likely to represent a true reduction in lymphedema. Postoperative week 4 also coincides with the end of the 30-day release period of 9-cis RA from treatment pellets. The fact that a significant difference in paw swelling between the two groups was still observed until postoperative week 6 suggests that the effect of 9-cis RA in limiting postsurgical lymphedema lasts beyond the period of known drug release. Thus, 9-cis RA released from our custom-made implantable pellets might have lasting effects in limiting the progression of symptoms of postsurgical lymphedema.
Conclusions
In summary, we present a clinically feasible method of drug delivery for the management of postsurgical lymphedema. The clinical applicability of an implantable pellet DDS that results in sustained local release of the pro-lymphangiogenic drug, 9-cis RA, has significant implications to the field of lymphedema. It allows for the convenient administration of 9-cis RA, which, from a patient and provider perspective, would result in 100% compliance (i.e. no missed drug doses), fewer follow-up appointments and decreased treatment costs than the previously-investigated daily intraperitoneal administration of 9-cis RA. Moreover, it would facilitate early disease intervention, thus preventing establishment of late-stage lymphedema, which is a true clinical challenge to revert and manage. While further work is necessary to determine the safety and efficacy of this route of administration in large animals and eventually humans, the data presented here further support 9-cis RA as a safe and effective pharmacologic agent for the treatment of secondary postsurgical lymphedema in humans.
Synopsis.
A depot drug delivery system containing 9-cis retinoic acid implanted immediately at the site of the lymphadenectomy defect reduces the clinical, histological, and physiological manifestations of postsurgical lymphedema in a murine animal model.
Acknowledgments
The authors would like to extend their gratitude to Alex Trana and to the laboratory of Dr. Yang Chai for their support and technical advice.
Grant Support
NIH 1K08HL132110-01A1 to A.K.W.
Footnotes
Disclosures
The authors have no conflicts of interest to disclose in relation to the contents of this manuscript.
Data Availability Statement
The data that support the findings of the studies referenced in this article are openly available in PubMed and/or PubMed Central.
References
- 1.Schulze H, Nacke M, Gutenbrunner C, Hadamitzky C (2018) Worldwide assessment of healthcare personnel dealing with lymphoedema. Health Econ Rev 8: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rockson SG, Rivera KK (2008) Estimating the population burden of lymphedema. Ann N Y Acad Sci 1131: 147–154. [DOI] [PubMed] [Google Scholar]
- 3.Shih YC, Xu Y, Cormier JN, Giordano S, Ridner SH, et al. (2009) Incidence, treatment costs, and complications of lymphedema after breast cancer among women of working age: a 2-year follow-up study. J Clin Oncol 27: 2007–2014. [DOI] [PubMed] [Google Scholar]
- 4.Dellon AL, Hoopes JE (1977) The Charles procedure for primary lymphedema. Long-term clinical results. Plast Reconstr Surg 60: 589–595. [DOI] [PubMed] [Google Scholar]
- 5.Szuba A, Skobe M, Karkkainen MJ, Shin WS, Beynet DP, et al. (2002) Therapeutic lymphangiogenesis with human recombinant VEGF-C. FASEB J 16: 1985–1987. [DOI] [PubMed] [Google Scholar]
- 6.Honkonen KM, Visuri MT, Tervala TV, Halonen PJ, Koivisto M, et al. (2013) Lymph node transfer and perinodal lymphatic growth factor treatment for lymphedema. Ann Surg 257: 961–967. [DOI] [PubMed] [Google Scholar]
- 7.Tammela T, Saaristo A, Holopainen T, Lyytikka J, Kotronen A, et al. (2007) Therapeutic differentiation and maturation of lymphatic vessels after lymph node dissection and transplantation. Nat Med 13: 1458–1466. [DOI] [PubMed] [Google Scholar]
- 8.Jin DP, An A, Liu J, Nakamura K, Rockson SG (2009) Therapeutic responses to exogenous VEGF-C administration in experimental lymphedema: immunohistochemical and molecular characterization. Lymphat Res Biol 7: 47–57. [DOI] [PubMed] [Google Scholar]
- 9.Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, et al. (2001) Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med 7: 192–198. [DOI] [PubMed] [Google Scholar]
- 10.Cao R, Ji H, Feng N, Zhang Y, Yang X, et al. (2012) Collaborative interplay between FGF-2 and VEGF-C promotes lymphangiogenesis and metastasis. Proc Natl Acad Sci U S A 109: 15894–15899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peppicelli S, Bianchini F, Calorini L (2014) Inflammatory cytokines induce vascular endothelial growth factor-C expression in melanoma-associated macrophages and stimulate melanoma lymph node metastasis. Oncol Lett 8: 1133–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi I, Lee S, Kyoung Chung H, Suk Lee Y, Eui Kim K, et al. (2012) 9-cis retinoic acid promotes lymphangiogenesis and enhances lymphatic vessel regeneration: therapeutic implications of 9-cis retinoic acid for secondary lymphedema. Circulation 125: 872–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bramos A, Perrault D, Yang S, Jung E, Hong YK, et al. (2016) Prevention of Postsurgical Lymphedema by 9-cis Retinoic Acid. Ann Surg 264: 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi I, Chung HK, Ramu S, Lee HN, Kim KE, et al. (2011) Visualization of lymphatic vessels by Prox1-promoter directed GFP reporter in a bacterial artificial chromosome-based transgenic mouse. Blood 117: 362–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mihara M, Hara H, Araki J, Kikuchi K, Narushima M, et al. (2012) Indocyanine green (ICG) lymphography is superior to lymphoscintigraphy for diagnostic imaging of early lymphedema of the upper limbs. PLoS One 7: e38182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams AF, Franks PJ, Moffatt CJ (2005) Lymphoedema: estimating the size of the problem. Palliat Med 19: 300–313. [DOI] [PubMed] [Google Scholar]
- 17.Moffatt CJ, Franks PJ, Doherty DC, Williams AF, Badger C, et al. (2003) Lymphoedema: an underestimated health problem. QJM 96: 731–738. [DOI] [PubMed] [Google Scholar]
- 18.Greene AK, Borud LJ, Slavin SA (2010) Lymphedema. Plastic Surgery Secrets Plus. Second ed: Mosby pp. 630–635.