Abstract
Background
Randomized trials have shown favorable clinical outcomes for coronary CT angiography (CTA) in patients with suspected acute coronary syndrome (ACS). Our goal was to estimate the cost-effectiveness of coronary CTA as compared to alternative management strategies for ACP patients over lifetime.
Methods
Markov microsimulation model was developed to compare cost-effectiveness of competitive strategies for ACP patients: 1) coronary CTA, 2) standard of care (SOC), 3) AHA/ACC Guidelines, and 4) expedited emergency department (ED) discharge protocol with outpatient testing. ROMICAT-II trial was used to populate the model with low to intermediate risk of ACS patient data, whereas diagnostic test-, treatment effect-, morbidity/mortality-, quality of life- and cost data were obtained from the literature. We predicted test utilization, costs, 1-, 3-, 10-year and over lifetime cardiovascular morbidity/mortality for each strategy. We determined quality adjusted life years (QALY) and incremental cost-effectiveness ratio. Observed outcomes in ROMICAT-II were used to validate the short-term model.
Results
Estimated short-term outcomes accurately reflected observed outcomes in ROMICAT-II as coronary CTA was associated with higher costs ($4,490 vs. $2,513-$4,144) and revascularization rates (5.2% vs. 2.6%−3.7%) compared to alternative strategies. Over lifetime, coronary CTA dominated SOC and ACC/AHA Guidelines and was cost-effective compared to expedited ED protocol ($49,428/QALY). This was driven by lower CV mortality (coronary CTA vs. expedited discharge: 3-year: 1.04% vs. 1.10–1.17; 10-year: 5.06% vs. 5.21–5.36%; respectively).
Conclusion
Coronary CTA in patients with suspected ACS renders affordable long-term health benefits as compared to alternative strategies.
Keywords: Coronary CTA, Acute Chest Pain, Acute Coronary Syndrome, Cost-Effectiveness Analysis, Markov Microsimulation Model
1. INTRODUCTION
Several randomized comparative effectiveness trials have demonstrated that early coronary computed tomographic angiography (CTA) improves the efficiency of emergency department (ED) triage in acute chest pain (ACP) patients with suspected acute coronary syndrome (ACS) by significantly reducing hospital admissions and decreasing length of hospital stay as compared to standard of care.1–3 This improvement is driven by an increased diagnostic certainty rendered through CTA by establishing the absence of coronary artery disease (CAD). Absence of CAD, which is the most important finding in 50% of patients, carries a very high negative predictive value for major adverse cardiac events (MACE) during index hospitalization and moreover, a warranty period for at least two more years.4 On the other hand, the detection of obstructive CAD by coronary CTA in 10% of patients leads to increased rates of invasive coronary angiography (ICA) and percutaneous coronary intervention (PCI) and – at least in systems without gate keeper or closed referral system – 5 subsequently higher cost of care in coronary CTA-based strategies.1 Not surprisingly, studies of short follow-up from 30 days to one year were not able to demonstrate improvement in health outcomes.6 Thus, concerns have been voiced as to whether coronary CTA is indeed beneficial to these patients, especially considering national efforts such as the “less is more” initiative and evidence from non-US studies showing that expedited ED protocols without inpatient diagnostic testing may lower costs while remaining equally safe in the short-term.7,8 Besides absence of CAD or obstructive CAD, coronary CTA detects non-obstructive CAD in about 40% of patients, a finding that has significant prognostic implications, with some studies suggesting that aggressive lipid lowering therapy based on obstructive and non-obstructive CAD, independent of statin eligibility based on ASCVD score, results in improved outcomes.9
Strategies that are alternatives to early coronary CTA include functional testing, expedited ED protocols with the intent to perform diagnostic testing in an outpatient setting6, 8, and a strategy based on the American Heart Association/ American College of Cardiology (AHA)/ACC) guidelines.10 Despite the fact that ROMICAT-II has been conducted several years ago and there have been several other more recent publications in ACP population, there are still no long-term data on resource utilization and outcomes available with the longest reported follow-up of 19 months.11 To determine whether the availability of information on the presence and extent of CAD by coronary CTA will offset higher initial costs by a significant improvement in health outcomes in the long term, we developed a Markov microsimulation model to determine lifetime health outcomes and cost-effectiveness of available ED management strategies for patients with suspected ACS.
2. METHODS
2.1. Simulation Model Overview
We developed a Markov microsimulation model to simulate four management strategies for individual patients who present to the ED with suspected ACS. These strategies are: 1) early coronary CTA as observed in ROMICAT-II, 2) standard of care (functional testing) as observed in ROMICAT-II, 3) Expert Consensus strategy based on current ACC/AHA guidelines and 4) an expedited ED protocol strategy with early discharge and the intent to perform diagnostic testing in an outpatient setting. 2,8,10,12
2.2. Patient Population
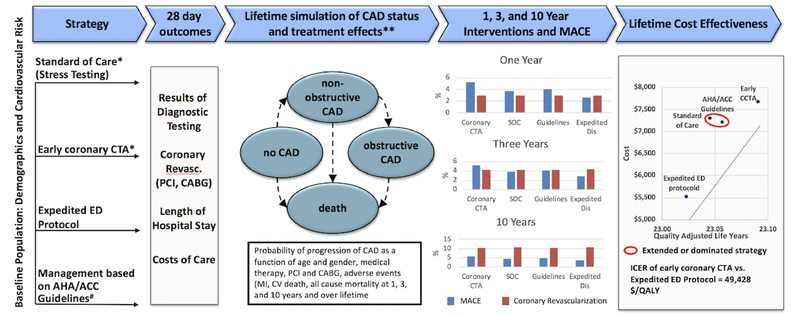
The Markov model was populated using individual data on demographics and cardiovascular risk factors from the 1,000 patients enrolled in the “Rule Out Myocardial Infarction/Ischemia Using Computer Assisted Tomography” (ROMICAT) II trial (Table 1).2 In brief, the ROMICAT-II trial randomized 1000 patients at low-intermediate risk of ACS who presented to the ED with suspicion of ACS and in whom ED caregivers ordered diagnostic testing to rule out ACS between April 2010 and January 2012 at nine US hospitals to either an early coronary CTA strategy or standard of care (SOC). Model input parameters beyond the 28-day follow up were estimated using published sources (Appendix, Supplemental Tables 3–5). Thus, the simulation model had two distinctive components: a short-term model (ED presentation and the first month afterwards) and a long-term Markov microsimulation model (second month until end of life) (Fig. 1).
Table 1.
Baseline Population characteristics for the Markov Model.
| Population# (n=1,000) | |
|---|---|
| Age (years) | 54.2 ± 8.1 |
| Men, n (%) | 532 (53.2) |
| Cardiovascular risk factors, n (%) | |
| Hypertension | 541 (54.1) |
| Diabetes mellitus | 173 (17.3) |
| Dyslipidemia | 454 (45.4) |
| Former or current smoker | 492 (49.2) |
| Family history of premature CAD | 271 (27.1) |
| Number of cardiovascular risk factors, n (%) | |
| 0 or 1 | 373 (37.3) |
| 2 or 3 | 528 (52.8) |
| ≥ 4 | 99 (9.9) |
| TIMI score, n (%) | |
| 0 | 614 (61.4) |
| 1 | 288 (28.8) |
| 2 | 85 (8.5) |
| 3 | 13 (1.3) |
| Acute coronary syndrome | |
| Myocardial Infarction | 23 (2.3) |
| Unstable Angina | 52 (5.2) |
| Coronary artery disease (CAD)* | |
| No CAD | 507 (50.7) |
| Non-obstructive CAD (<50% stenosis) | 430 (43.0) |
| Obstructive CAD (>/=50% stenosis) | 63 (6.3) |
ROMICAT II patient level data
CAD status was determined using invasive cardiac catheterization, coronary CTA, and functional test results.
Fig. 1.
Markov microsimulation model with data sources for short and long term health and economic outcomes of four competing ED management strategies. Baseline population characteristics as observed in the ROMICAT-II Trial. Short term model validation based on 28 day management and outcomes observed in the ROMICAT-II. MACE (dark grey bars) includes myocardial infarction and cardiovascular death, wherease Coronary Revascularization (light grey bars) represents PCI and CABG. Major model inputs for long term simulation: ROMICAT-II, Ottawa cohort of stable chest pain and literature. CABG = Coronary artery bypass graft; CAD = Coronary artery disease; CTA = Computed tomography angiography; ED = Emergency Department; MACE = Major adverse cardiovascular events; PCI = Percutan coronary intervention.
2.3. Short-term model
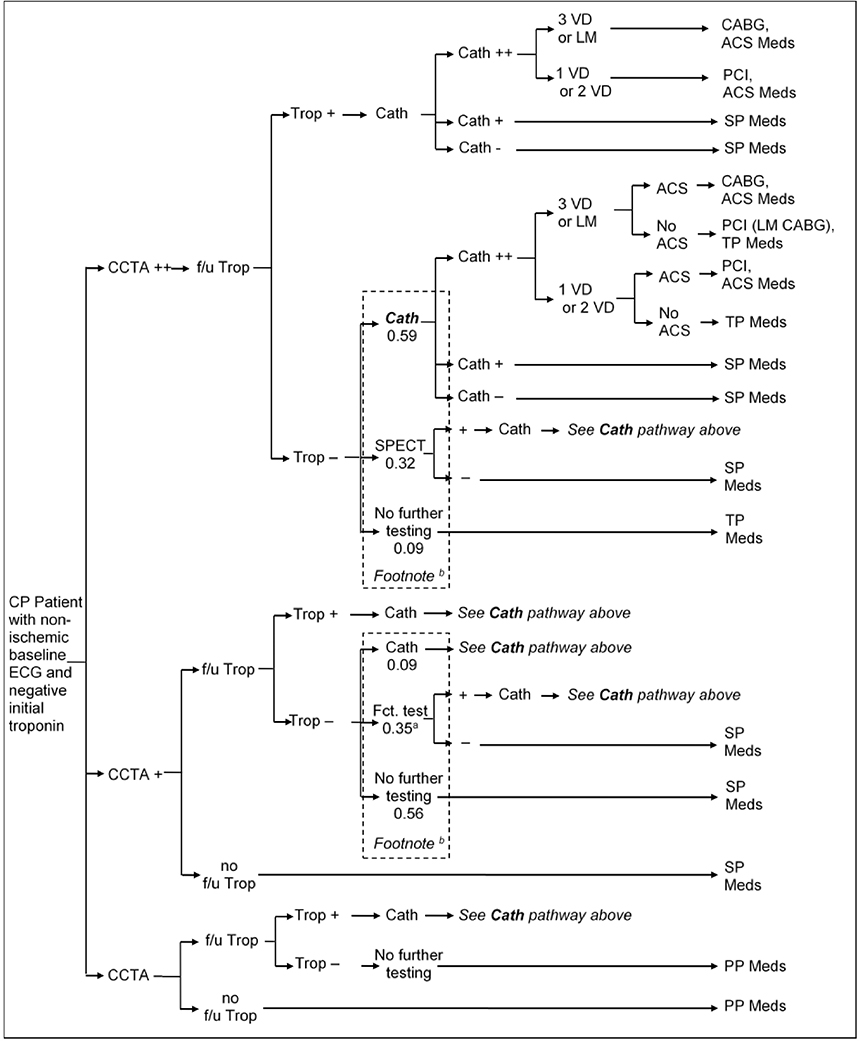
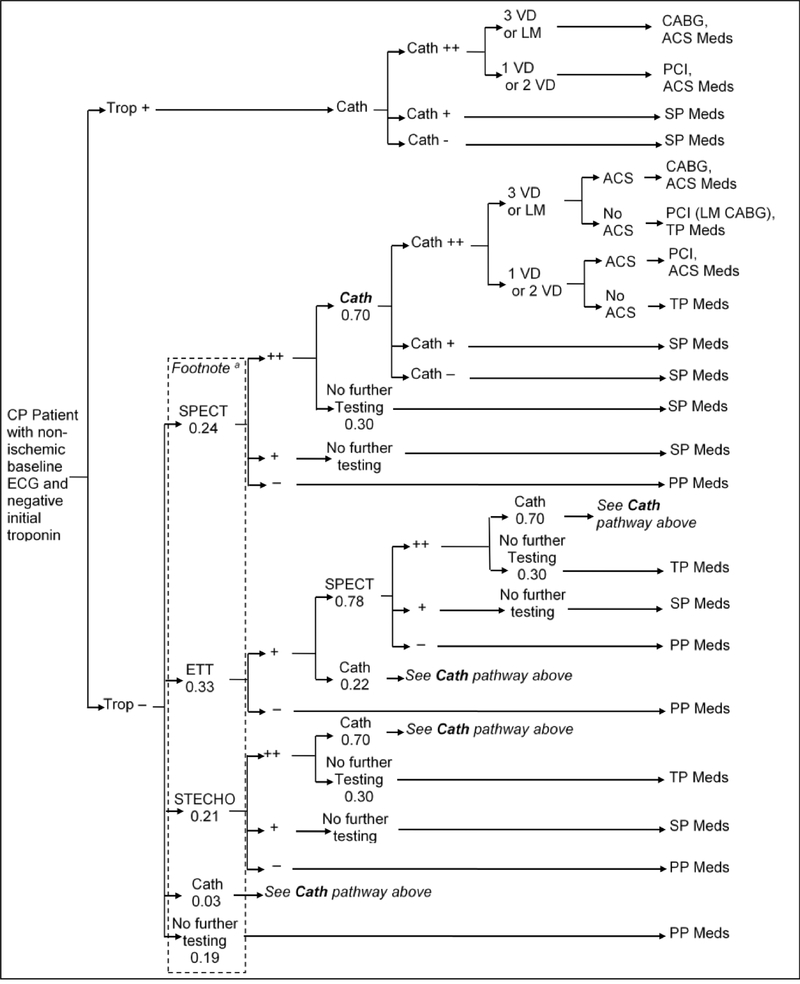
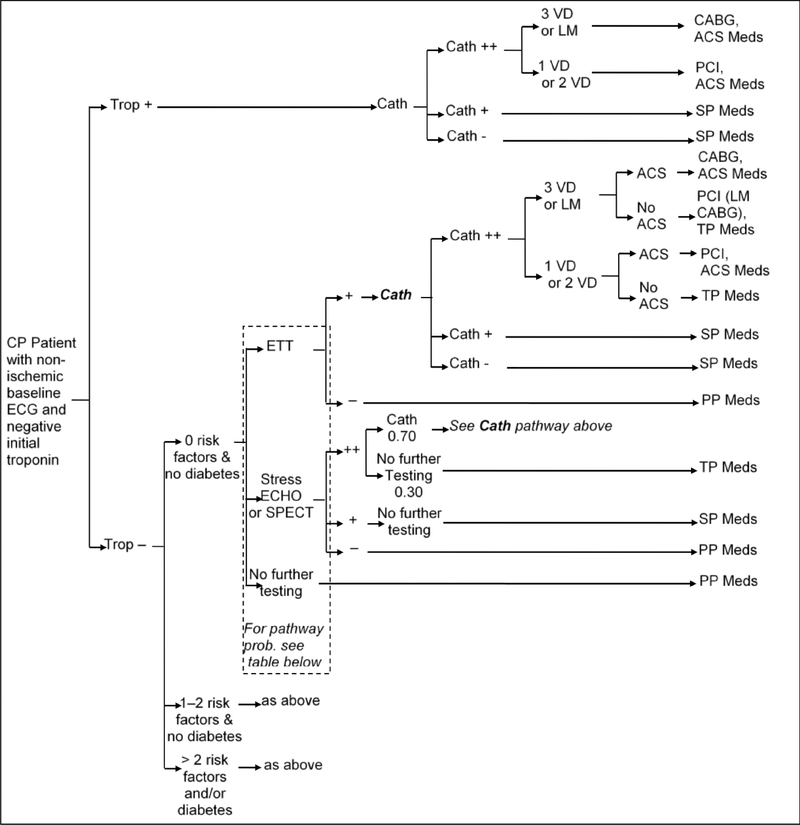
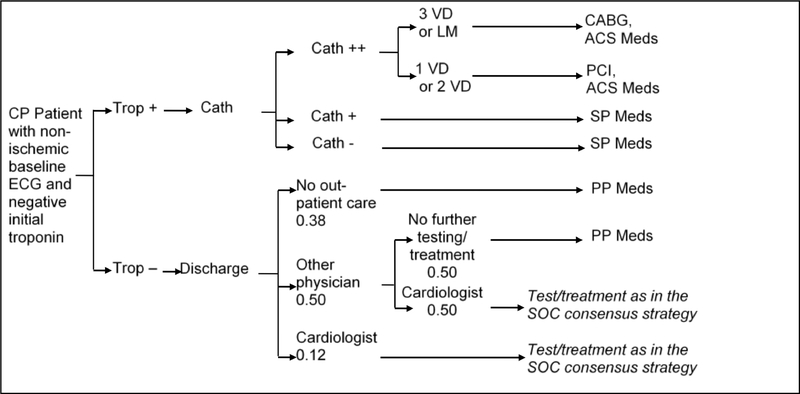
In the short-term model, we estimated the probability of each strategy to accurately detect underlying CAD and ACS, as well as test and treatment utilization and costs within the first month after ED presentation. The management strategies are outlined in detail in Figures 2a–d. Briefly, the early coronary CTA and SOC strategy reflect the actual patient management as observed in ROMICAT-II.2 The hallmark of the early coronary CTA strategy was the early discharge of more than 50% of patients after a single troponin test, while the SOC performed functional testing (75% myocardial perfusion imaging) in nearly 80% of patients after serial troponin testing (Figures 2a and 2b). The expert consensus strategies were based on an abstraction of the current ACC/AHA guidelines for patients with ACP (Figure 2c).10 In this strategy, patients in whom ACS was ruled out were managed based on their cardiovascular risk profile and diagnostic test results. The strategy of expedited ED discharge is based on the study by Than et al.8 (Figure 2d) and assumes that advanced diagnostic testing is performed on an outpatient basis. An important limitation of this strategy is the reportedly limited compliance with recommendations for outpatient testing.13, 14 Based on available data, we assumed in this strategy that 12% of patients with negative troponin results will follow-up with a cardiologist and 50% with a primary care physician (of which 50% will be referred to a cardiologist), leaving 38% of patients discharged without any physician follow-up.
Fig. 2.
A. Diagnostic Pathways in coronary CTA Strategy.
a: Functional tests: 0.23 SPECT, 0.06 stress ECHO, 0.06 ETT.
b: Average pathway probabilities; Patients undergo pathways according to a split based on risk factors.
ACS = Acute coronary syndrome; CABG = Coronary artery bypass graft; Cath = Coronary catheterization (++ = significant stenosis (> 70%), + = mild stenosis); CCTA=Coronary computed tomographic angiography (++ = significant stenosis, + = mild stenosis) f/u = follow up; ECHO = Echocardiography; ETT = Exercise tolerance test; LM = Left-main disease; PCI = Percutaneous coronary intervention; SPECT = Single-photon emission computed tomography; Trop = Troponin test; VD=Vessel disease. PP Meds = Primary prevention medication: Aspirin and Statin (for patients with hypertension, diabetes, or dyslipidemia), SP Meds = Secondary prevention medication: Aspirin and Statin, TP Meds = Tertiary prevention medication: Aspirin, Statin, and Betablocker (non-diabetic patient) or ACE (diabetic patient), ACS Meds = ACS medication: Aspirin, high-dose Statin, Clopidogrel (one year only), Beta blocker and ACE.
B. Diagnostic Pathways in the Standard of Care Strategy.
a: Average pathway probabilities; Patients undergo pathways according to their propensity score.
ACS = Acute coronary syndrome; CABG = Coronary artery bypass graft; Cath = Coronary catheterization (++ = significant stenosis (> 50%), + = mild stenosis); CP = Chest pain; ECG = Electrocardiogram; ETT = Exercise tolerance test; LM = Left-main disease; PCI = Percutaneous coronary intervention; SPECT = Singlephoton emission computed tomography; STECHO = Stress echocardiography; Trop = Troponin test; VD = Vessel disease. PP Meds = Primary prevention medication: Aspirin and Statin (for patients with hypertension, diabetes, or dyslipidemia), SP Meds = Secondary prevention medication: Aspirin and Statin, TP Meds = Tertiary prevention medication: Aspirin, Statin, and Betablocker (non-diabetic patient) or ACE (diabetic patient), ACS Meds = ACS medication: Aspirin, high-dose Statin, Clopidogrel (one year only), Beta blocker, and ACE.
C. Diagnostic Pathways per AHA/ACC Guidelines.
Per AHA/ACC guidelines patient management after stress testing is guided by the presence of traditional risk factors and diabetes. The table at the bottom of the figure demonstrates the probabilities to receive type of functional testing.
ACS = Acute coronary syndrome; CABG = Coronary artery bypass graft; Cath = Coronary catheterization (++ = significant stenosis (> 50%), + = mild stenosis); CP = Chest pain; ECG = Electrocardiogram; ECHO = Echocardiography; ETT = Exercise tolerance test; LM = Left-main disease; PCI = Percutaneous coronary intervention; SPECT = Single-photon emission computed tomography; Trop = Troponin test; Txt = Treatment; VD = Vessel disease. PP Meds = Primary prevention medication: Aspirin and Statin (for patients with hypertension, diabetes, or dyslipidemia), SP Meds = Secondary prevention medication: Aspirin and Statin, TP Meds = Tertiary prevention medication: Aspirin, Statin, and Betablocker (non-diabetic patient) or ACE (diabetic patient), ACS Meds = ACS medication: Aspirin, high-dose Statin, Clopidogrel (one year only), Beta blocker, and ACE.
D2. Diagnostic Pathways in the Standard of Care Expedited ED Protocol Strategy.
ACS=Acute coronary syndrome; CABG=Coronary artery bypass graft; Cath=Coronary catheterization (++ = significant stenosis (> 50%), + = mild stenosis); CP=Chest pain; ECG=Electrocardiogram; LM=Left-main disease; PCI=Percutaneous coronary intervention; Trop=Troponin test; VD=Vessel disease.
PP Meds=Primary prevention medication: Aspirin and Statin (for patients with hypertension, diabetes, or dyslipidemia), SP Meds=Secondary prevention medication: Aspirin and Statin, ACS Meds=ACS medication: Aspirin, high-dose Statin, Clopidogrel (one year only), Beta blocker, and ACE.
Some aspects of management were similar for all strategies. For example, patients with a positive troponin were always referred to ICA. While the diagnostic testing sequence varied between the strategies, the model assumes conditional independence and uses the same accuracies for individual tests regarding the detection of CAD and ACS across all strategies based on the most recent literature (Appendix, Supplemental Table 1). Guideline-recommended treatment (Appendix, Supplemental Table 2) was based on the estimated CAD and ACS status.
2.4. Long-term model
The long-term model was used to estimate quality adjusted life years (QALYs) and lifetime costs of care. As a basis we estimated the probability of future coronary revascularization procedures including PCI and CABG, adverse cardiovascular events, including myocardial infarctions and CV death, as well as overall mortality rates at 1, 3, and 10 years and over a lifetime.
Progression of CAD, an important determinant for future coronary revascularization procedures including PCI and CABG, and adverse cardiovascular events, including myocardial infarctions and CV death, was modeled as a function of age, gender, disease status and National Cholesterol Education Program (NCEP) risk score from a cohort of stable chest pain patients using a simulated annealing approach.15,16 Cardiovascular mortality and morbidity rates were modeled based on the presence and extent of CAD in the CONFIRM registry.17,18 Mortality rates for non-ST segment elevation myocardial infarction (NSTEMI) and unstable angina (UA) depended on the underlying CAD status, and mortality due to causes other than CAD was based on US life tables (Appendix, Supplemental Table 3).19–22 We assumed that patients newly diagnosed with significant CAD were started on medical therapy per AHA/ACC guidelines (Appendix, Supplemental Table 2). We assumed that appropriate treatment reduced CAD mortality (proportionally) by 23% (Preventive fraction: one minus odds ratio of 0.77).23 A second assumption was that missed ACS increased 30 day mortality by 70–90% (hazard ratios between 1.70–1.90).24
Across a lifetime, transition to different health states is modeled in monthly cycles at which time the CAD status of each patient is determined. The CAD status could remain the same or progress, and patients could suffer from myocardial infarctions and die from either cardiovascular disease or other causes (Figure 1).
2.5. Health-related quality of life, healthcare costs data, and incremental cost-effectiveness ratio
Quality of life was determined by the presence and extent of CAD, the treatment method (medical treatment or intervention), and whether obstructive CAD was treated or not. In our approach we follow Ladapo et al..25 As such, we estimated the probability of having typical angina versus nonspecific or atypical chest pain based on the underlying CAD status according to the CASS registry, and whether a patient was treated for CAD. We used utility values for various chest discomfort classifications that were derived from Lalonde et al. (Appendix, Supplemental Table 4).26, 27 We combined the data to calculate the impact of CABG and PCI on chest pain. Quality adjusted life years (QALYs) were calculated by weighting life years with quality of life values.
Costs were derived from three different sources: 1) The costs of diagnostic tests, initial and recurrent cardiac interventions, and hospitalizations were directly derived from the micro-costing at each ROMICAT-II clinical site. Thus, actual health care costs such as cost for diagnostic testing (CTA, ETT, SPECT, Stress Echo, ICA) and interventions (PCI, CABG) during the index care episode were assessed from reports from hospital cost-accounting systems and physician billing records;2, 12 2) costs of outpatient office visits were based on Medicare reimbursements, and 3) costs of long-term medical therapy were derived from the RedBook (Appendix, Supplemental Table 5).
The incremental cost-effectiveness ratio (ICER) expresses the costs per additional QALY, i.e. the costs to live an additional year in perfect health. The ICER was calculated as the difference in costs between two strategies and divided by the difference in quality adjusted life years. To estimate the ICER, costs and QALYs were discounted at 3% annually.
2.6. Sensitivity analyses
2.6.1. Risk of ACS
To assess the impact of the risk profile of the incoming cohort, we performed a sensitivity analysis in which we replaced the ROMICAT-II cohort with a cohort whose demographic and clinical characteristics reflected the CT-STAT study population.28 Although we did not have access to patient level data for this trial, we were able to simulate a cohort that met its described characteristics well (Appendix, Supplemental Table 6).
2.6.2. Diagnostic Accuracy of coronary CTA to detect obstructive CAD
As diagnostic accuracy for obstructive CAD may determine rates of referral to invasive coronary angiography and may vary between CT scanners and readers, we conducted a bivariate sensitivity analysis to determine the ICER across a range of sensitivities (85% to 100%) and specificities (50% to 100%).
2.6.3. Treatment effect of aggressive medical therapy for obstructive CAD
In our main analysis we assume that life time statin therapy reduces CAD mortality by 23% assuming full compliance.23 In order to address uncertainty regarding the treatment effect, we conducted a sensitivity analysis using the upper and lower bounds of the confidence interval, i.e. 18% and 30% relative risk reduction. In addition, we performed sensitivity analyses for variations in compliance, including a scenario with 5 years of full compliance followed by 5 years of declining compliance (in monthly steps with none of the patients on statins after 10 years), and full compliance for 5 years and no treatment effect afterwards.
2.7. Statistical analyses and computer software
All 1,000 ROMICAT-II patients ran through each of the four strategies 1000 times. We first validated the short-term model by comparing model-predicted short-term outcomes in the coronary CTA and SOC strategy with the observed short-term outcomes (e.g. length of stay, number of interventions, cost of care) in ROMICAT-II.
All comparisons are reported without p values because all analyses were run with sample sizes (1,000,000, i.e., each of the 1,000 patients 1000 times) large enough to generate stable estimates of the effect sizes of interest, ensuring that the difference in QALYs and costs between the interventions was >2 times the (larger) standard error of the mean.29 The model was programmed in TreeAge Pro Suite 2009 (TreeAge Software, Williamstown, MA, USA). All data and statistical analyses were performed using Stata 13.0 (StataCorp, College Station, TX, USA).
3. RESULTS
3.1. Population Characteristics
The ROMICAT-II population (n=1000) was defined as middle aged (54.2 ± 8.1 years), equally representing gender (53.2% male), and by a substantial cardiovascular risk factor (RF) burden (52.8%: 2 – 3 RF; 9.9%: >3 RF). Overall, 50.7% of patients had no CAD, 43% non-obstructive CAD and 6.3% obstructive CAD. The incident of ACS during index hospitalization was 7.5% (NSTEMI: 2.3%; UA: 5.2% (Table 1).
3.2. Short-term outcomes – index hospitalization and 28 day outcomes
Overall, the short-term model predicted length of stay, testing, and interventions for both the coronary CTA and SOC strategy very accurately predicted the observations made during the ROMICAT-II trial (Table 2). This data validates the accuracy of the short-term model.
Table 2.
Model validation.
| ROMICAT II Early CCTA | ROMICAT II Standard of Care | |||
|---|---|---|---|---|
| Variables | Trial | Simulation | Trial | Simulation |
| Length of Stay (hours) | 23.2 | 24.7 | 30.8 | 30.1 |
| Functional Testing | ||||
| SPECT (%) | 12 | 9.6 | 27 | 29.4 |
| Stress ECHO (%) | 4 | 1.3 | 20 | 20.2 |
| ETT (%) | 4 | 1.1 | 32 | 32.5 |
| Cath (%) | 12 | 17.5 | 8 | 11.2 |
| Intervention | ||||
| PCI (%) | 5 | 5.0 | 3 | 2.7 |
| CABG (%) | 1 | 1.2 | 1 | 0.6 |
| Radiation exposure - mSv | 14.3 | 13.5 | 5.3 | 5,1 |
| Cost of Care – U.S. $ * | ||||
| Emergency Department | 2,101 | 2,246 | 2,566 | 2,558 |
| Hospital | 1,925 | 2,377 | 1,308 | 1,340 |
| Total (with F/U) | 4,289 | 4,623 | 4,060 | 3,899 |
CABG = coronary artery bypass graft; Cath = coronary catheterization; ECHO = echocardiography; ETT = exercise tolerance test; PCI = percutaneous coronary intervention; SPECT = Single-photon emission computed tomography.
As in ROMICAT-II, early coronary CTA was most accurate in identifying patients with obstructive CAD (98%), followed by Expert Consensus (75%), and SOC (69%). In contrast, the predicted accuracy of the expedited ED discharge strategy to identify patients with obstructive CAD was very low (46%), while CAD status remained unknown to patients and providers in more than 50% of patients with underlying CAD. The higher yield in diagnosis of obstructive CAD correlated with the frequency of subsequent revascularizations. For example, twice as many patients underwent PCI after early coronary CTA as compared to expedited ED discharge (5.2% vs. 2.6%). In contrast, the predicted length of stay was shortest for expedited ED discharge (12.3 hours) followed by early coronary CTA (23.4 hours), SOC (30.6 hours) and Expert Consensus (30.9 hours).
The diagnostic costs during index hospitalization were highest for coronary CTA ($2,692) followed by Expert Consensus ($2,535), SOC ($2,501), and only $1,891 for expedited ED discharge. The higher revascularization rate with early coronary CTA resulted in significantly higher total costs as compared to expedited ED discharge ($4,490 vs. $2,513), while difference to SOC ($4,144) and expert consensus ($4,064) was relatively small (Table 3).
Table 3.
Short-term outcomes – Comparison between four competing management strategies.
| ROMICAT II CCTA* | ROMICATI SOC* | Expert Consensus* | Expedited ED Protocol* | |
|---|---|---|---|---|
| Length of Hospital Stay (hours) | 23.4 | 30.6 | 30.9 | 12.3 |
| Noninvasive Diagnostic testing | ||||
| CCTA (%) | 100.0 | 0.0 | 0.0 | 0.0 |
| SPECT (%) | 8.8 | 29.8 | 22.1 | 8.2 |
| Stress ECHO (%) | 1.3 | 20.6 | 28.4 | 10.5 |
| ETT (%) | 1.2 | 32.5 | 29.0 | 10.8 |
| Invasive Coronary Angiography (%) | 16.1 | 11.3 | 14.1 | 6.6 |
| Accuracy to detect obstructive CAD# | ||||
| True positive (%) | 98.6 | 68.9 | 75.0 | 45.5 |
| False positive (%) | 3.7 | 1.4 | 1.3 | 0.5 |
| Coronary Revascularization | ||||
| PCI (%) | 4.3 | 3.0 | 3.3 | 2.1 |
| CABG (%) | 0.9 | 0.7 | 0.7 | 0.5 |
| Cost of Care ($) | ||||
| Diagnostic costs (incl. angiography) | 2,692 | 2,501 | 2,535 | 1,891 |
| Treatment costs | 1,798 | 1,643 | 1,529 | 622 |
| Total | 4,490 | 4,144 | 4,064 | 2,513 |
CCTA = Coronary Computed Tomographic Angiography; ECHO = Echocardiography; ETT = Exercise Tolerance Test; PCI = Percutaneous Coronary Intervention; SPECT = Single-Photon Emission Computed Tomography.
The outcomes were based on a simulation of each strategy in 1000 patients from ROMICAT II trial, #estimated based on published diagnostic accuracy data for each test
Among all strategies, patients without CAD had the lowest rate of invasive angiographies after early coronary CTA (1.4%) followed by expedited ED discharge (3.8%), SOC (7.1%), and Expert consensus (9.4%). This is accompanied by a reduction in length of stay and cost for the early coronary CTA strategy, which was similar to the expedited ED discharge (13.2 hours vs. 9.9 hours and $2,262 vs. $2,035). In contrast, patients without CAD in the SOC or Expert consensus strategy had a length of stay that was twice as long (27.6 hours and 26.9 hours) and costs that were 50% higher ($3,482 and $3,289).
3.3. Long-term health and economic outcomes (Figure 1)
The major health and economic outcomes one, three, and 10 years after ED presentation and over a lifetime are shown in Table 4. Overall, the differences in rates of MI were relatively small between the strategies, albeit slightly higher rates were observed in the SOC and the expedited ED discharge strategies. MI rates increased from around 2.6% after a year to around 12.2% over a lifetime. However, the relative differences in cardiovascular mortality for early coronary CTA versus expedited ED discharge were noticeable after 10 years (5.06% vs 5.36%) and further increased over a lifetime (45.64% versus 46.10%). In contrast, the cardiovascular mortality benefit of early coronary CTA was smaller when compared to the other two strategies.
Table 4.
Simulated long-term health and economic outcomes – one, three, and ten years after ED presentation and over lifetime.
| 1 Year | 3 Years | 10 Years | Lifetime | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RII CCTA | RII SOC | Exp Con | Exp D/C | RII CCTA | RII SOC | Exp Con | Exp D/C | RII CCTA | RII SOC | Exp Con | Exp D/C | RII CCTA | RII SOC | Exp Con | Exp D/C | |
| MI (%) | 2.57 | 2.59 | 2.57 | 2.58 | 3.15 | 3.17 | 3.15 | 3.16 | 5.33 | 5.37 | 5.34 | 5.38 | 12.17 | 12.29 | 12.22 | 12.26 |
| PCI (%) | 4.34 | 3.03 | 3.30 | 2.18 | 4.39 | 3.11 | 3.39 | 2.30 | 4.75 | 3.59 | 3.84 | 2.85 | 7.00 | 6.03 | 6.23 | 5.37 |
| CABG (%) | 0.88 | 0.71 | 0.75 | 0.47 | 0.89 | 0.74 | 0.77 | 0.50 | 1.05 | 0.91 | 0.94 | 0.68 | 2.04 | 1.93 | 1.94 | 1.72 |
| CV death (%) | 0.35 | 0.38 | 0.38 | 0.42 | 1.04 | 1.11 | 1.10 | 1.17 | 5.06 | 5.23 | 5.21 | 5.36 | 45.64 | 45.91 | 45.89 | 46.10 |
| Overall mortality (%) | 1.05 | 1.07 | 1.07 | 1.08 | 3.11 | 3.16 | 3.16 | 3.21 | 13.46 | 13.52 | 13.55 | 13.64 | 100.00 | 100.00 | 100.00 | 100.00 |
| Cost total ($) | 4,580 | 4,230 | 4,149 | 2,590 | 4,756 | 4,397 | 4,320 | 2,741 | 5,417 | 5,037 | 4,965 | 3,333 | 7,662 | 7,288 | 7,205 | 5,498 |
CABG = coronary artery bypass graft; CCTA = Coronary Computed Tomographic Angiography; CV = Cardiovascular; Exp. Con = Expert Consensus; Exp. D/C = Expedited Discharge (Expedited ED Protocol) MI = Myocardial Infarction; PCI = percutaneous coronary intervention; SOC = Standard of Care.
Coronary revascularization rates remained high over the lifetime in the early coronary CTA strategy, but the difference in revascularization rate between coronary CTA and the other strategies decreased over time. For example, while the PCI rate for early coronary CTA was 1.9 times higher than the rate for expedited ED discharge after 3 years (4.4% versus 2.3%), this decreased to 1.3 times higher over a lifetime (7.0% vs. 5.4%) (Table 4). Additional costs over a lifetime were highest for coronary CTA and approximately $2,000 higher as compared to expedited ED discharge, $370higher as compared to SOC and $460 higher as compared to Expert Consensus.
3.4. Incremental Cost Effectiveness Ratio
In a comparison of all four strategies, performing an early coronary CTA in the ED resulted in a gain of 25 quality adjusted life days (QALD) when compared to expedited ED discharge (QALYs: 23.09 vs. 23.02), 17 QALD compared to SOC (QALYs: 23.09 vs. 23.05) and 12 QALD compared to expert consensus (QALYs: 23.09 vs. 23.06) (Table 5). The coronary CTA strategy extendedly dominated the SOC and the expert consensus strategies, i.e. the ICER of the coronary CTA strategy was lower than the ICER of the SOC strategy and the ICER of the expert consensus strategy (Central Figure 1). Thus, the SOC as well as the expert consensus strategy were inferior to early coronary CTA because they were more expensive for an equal gain in QALYs. Compared to the second most efficient strategy (expedited ED protocol), the coronary CTA strategy rendered a cost per QALY of $ 49,428. In a head to head comparison of coronary CTA to SOC the ICER decreased to $ 13,961.
Table 5.
Incremental Cost-effectiveness ratio of different strategies to manage patients with acute chest pain.
| Cost ($) | Cost difference | QALYs | QALYs difference | ICER ($/QALY) | |
|---|---|---|---|---|---|
| Expedited ED discharge protocol | 5,498 | 23.024 | |||
| Guidelines | 7,205 | 1,707 | 23.058 | 0.034 | Dominated |
| Standard of Care as in ROMICAT II | 7,288 | 83 | 23.046 | −0.012 | Dominated |
| Early coronary CTA as in ROMICAT II | 7,662 | 374 | 23.092 | 0.046 | 49,428 |
Coronary CTA = Coronary computed tomographic angiography; ICER = Incremental cost-effectiveness ratio; QALY = Quality-adjusted life year
Cost and QALYs are reported as undiscounted values; ICER is estimated based on discounted values (3% annual). The ICER shows the costs per additional QALY, i.e. the costs per additional year in perfect health. The Table shows that an early CCTA strategy cost 49,428 per additional QALY compared to expedited ED protocol.
3.5. Sensitivity Analyses
Treatment effect of aggressive medical therapy for obstructive CAD (Figure 3)
Fig. 3.
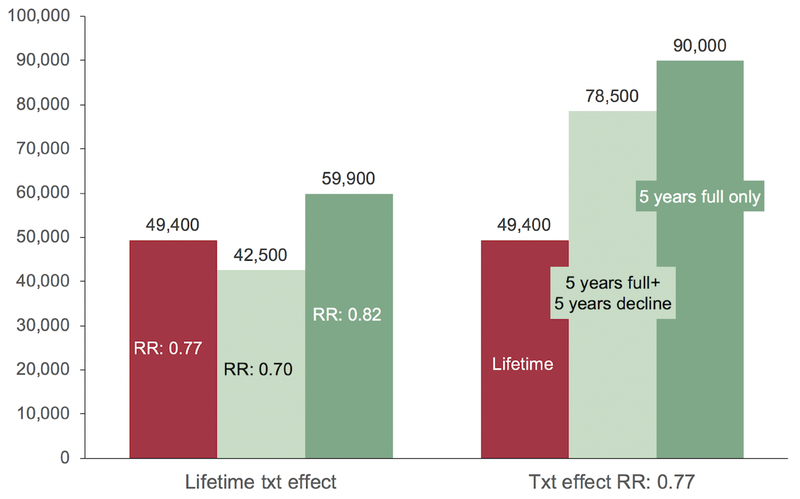
Treatment effect of agressive medical theraphy for obstructive CAD. Left side: Treatment effect of agressive medical theraphy for obstructive CAD (> 50%): light grey column: baseline case assuming that life time statin therapy reduces CAD mortality by 23% (22); mild and dark grey column: sensitivity analysis using the upper (30%) and lower (18%) bounds of the confidence in-terval of relative risk reduction. Right side: Sensitivity analyses for validations in treatment compliance: light grey column: baseline case assuming that life time statin therapy reduces CAD mortality by 23% (22); mild grey column: 5 years of full compliance followed by 5 years of decining compliance (in monthly steps with none of the patients on statins after 10 years); dark grey: full com-pliance for 5 years and no treatment effect afterwards. CAD = Coronary artery disease, RR = Risk reduction.
Assuming a relative risk decrease of only 0.18 representing the lower bound of the 95% CI, the ICER increased from $ 49,000 to $ 60,000.23 Assuming a variation of adherence to medical therapy, we studied two scenarios: 1) 5 years of full compliance followed by 5 years of declining compliance resulted in an increase of the ICER from $ 49,000 to $ 78,500; 2) 5 years of full compliance followed by a complete lack of adherence and no treatment effect resulted in nearly a doubling of the ICER from $ 49,000 to $ 90,000.
Diagnostic Accuracy of coronary CTA to detect obstructive CAD
We determined the ICER across a range of sensitivities (85% to 100%) and specificities (50% to 100%) of coronary CTA to detect obstructive CAD as compared to invasive angiography. Overall, the ICER of coronary CTA versus expedited discharge ranged from $46,000 for a near perfect diagnostic accuracy to $70,000/QALY for a specificity of 50% and a sensitivity of 85%. Notably, changes in specificity resulted in larger changes of ICER (between $17,000 and $20,000 /QALY) than changes in sensitivity (between $2,500 and $5,000/QALY), possibly as a result of unnecessary and ineffective ICA and CABG (Appendix, Supplemental Table 7).
ED Population and Risk of ACS
In populations with a lower prevalence of ACS; i.e.: CT-STAT: 1.8% ACS vs. ROMICAT-II: 7.5% ACS ICER for coronary CTA increased to 73,192$/QALY (Appendix, Supplemental Table 8).28
4. DISCUSSION
This cost-effectiveness analysis based on a Markov microsimulation model compared the lifetime health and economic outcomes of four contemporary management strategies for patients with suspected ACS. The major result is that the data suggest that an early coronary CTA strategy is cost-effective over a lifetime as compared to competing strategies, including early ED discharge. This is due to an early detection of true CAD status by early coronary CTA that despite an initial increase in testing, interventions, and costs results in a reduction in cardiovascular mortality starting to emerge 3 years after the initial ED presentation, primarily through appropriate medical therapy. This effect was not only sustained but expanded throughout life. To the contrary, the initially cost savings through fewer tests resulted in a lack of correct classification of CAD status of many patients. On average, coronary CTA added 12 to 25 days of quality-adjusted life per patient, which is achieved at the cost of ~$50,000 per QALY when compared to a strategy of expedited ED discharge. This was based on the assumption that only 37% of patients expeditiously discharged would have an outpatient cardiologist follow-up, which is consistent with published data. In a sensitivity analysis, assuming a 50% cardiologist follow-up rate, ICER decreased to ~$34,000 per QALY, however, coronary CTA remained more cost effective. In a head-to-head comparison of the two most common strategies of coronary CTA and SOC (functional testing), the ICER was much lower with $14,000 per QALY.
In addition, we show that the benefits of coronary CTA vary with adherence to aggressive lipid lowering therapy, although coronary CTA remained cost-effective at higher costs per QALY (up to $90,000). A similar increase in ICER was seen for lower diagnostic accuracy for the detection of stenosis, i.e. using older CT technology and for the assumptions that patients at much lower risk for ACS would undergo early CTA. While there are no long-term studies in patients with suspected ACS who underwent coronary CTA, the recent results from the SCOT-HEART trial in a stable chest pain population validate the assumptions and results of our modeling in that they demonstrate a lower incidence of death from coronary heart disease or myocardial infarction after coronary CTA as compared to stress testing strategy. In addition, the results confirmed our assumptions that an initial increase in testing and revascularization after coronary CTA is followed by less testing over mid-term follow-up, while patients who underwent stress testing experienced an increase in testing and revascularization over time.30
To put these results in perspective, the ACC/AHA Guideline statement on cost and value methodology classifies interventions resulting in gains per QALY costing <50K as a high value, 50–100K as an intermediate value, and >100K as a low value.31 An often-cited value for approval or payment of medical interventions is US $100 000 per QALY.32–34 Hence, our base case scenario suggests that coronary CTA is highly cost-effective ($49,428 per QALY) in patients with suspicion for ACS while sensitivity analyses assuming limitations suggest an intermediate value (70–90K per QALY). It is interesting to note that both scenarios compare favorably with established strategies such as lung cancer screening (130K per QALY 35), and screening for CAD in patients with type 2 diabetes mellitus or HIV.36,37 Even though an addition of 12–25 quality adjusted days on an individual’s lifespan may appear underwhelming to some, from a societal point of view this translates to an additional 0.4 Million QALYs on the life span of the 6 million patients presenting annually to the ED in the US each year. Thus, early coronary CTA in patients with suspected ACS could have a significant public health and economic impact.
It is perhaps not surprising that an expedited ED discharge strategy appears beneficial in the short-term but is inferior in the long-term when compared to a strategy that delivers powerful prognostic information in every patient at the beginning. A major factor driving this difference is that 50% of patients who have obstructive CAD would not be detected with this strategy. We have not considered the cost of repeated ED visits in the expedited discharge strategy, nor the litigation costs for missed detection of CAD. Our model confirms the low risk for major adverse cardiovascular event (MACE) after ED discharge 38,39 in this population consistent with findings of short-term follow-up studies that did not detect any differences in health outcomes between a coronary CTA strategy and SOC.2,40 Moreover, our model highlights that such studies need a minimum follow-up of 3 years in order to detect emerging differences between the strategies. Though there is no such data from randomized trials, several observational studies have uniformly demonstrated that patients with non-obstructive and obstructive CAD detected on coronary CTA are at significantly higher long-term risk for developing MACE compared to those without the disease, even after adjustment for traditional risk factors (HR non-obstructive: 2.5; HR obstructive: 11.2).41 Moreover, the prognostic accuracy of coronary CTA is significantly higher as compared to functional testing.
While the careful use of diagnostic testing is extremely important, our data suggests that a change in how to perceive an ED visit, from a point of care to rule out MI at the lowest costs possible to a unique opportunity to lay the ground work for an optimized medical prevention using coronary CTA to accurately establish CAD status would be cost effective. Moreover, in the future, availability of CT FFR may lead to a lower rate of ICAs and perhaps even subsequent PCI in patients who are positive for anatomicbut not for hemodynamic significant stenosis.
The strengths of our analysis are (1) the use of patient level data for the demographics, clinical presentation, traditional CVD risk factors, and CAD status as observed in the ROMICAT-II trial to populate the baseline model (2) the ability to use real per patient data from ROMICAT-II on testing, interventions, health outcomes and cost during the index hospitalization and over the following 28 days to calibrate and validate the short-term model and (3) the robustness of the model to simulate the natural history of CAD, its associated morbidity and mortality, and the effects of treatment over different time horizons.
It is important to emphasize that our modeling while based on ROMICAT-II includes several important sensitivity analyses; thereby, accommodating difference in populations and outcomes presented in subsequent publications.11 In these, changes in the treatment effect and adherence to aggressive lipid lowering therapy for obstructive CAD resulted in a moderate increase of the ICER between $60,000/QALY and $90,000/QALY which is still below $100,000 per QALY. In addition, we showed that although the strength of coronary CTA is a very high negative predictive value in the absence of CAD, the benefits of coronary CTA are diminished if the incidence of ACS is too low rendering coronary CTA 50% less cost-effective (ICER: 73,200/QALY for ACS incidence of 1.8% as seen in CT-STAT). This increase in costs was primarily related to the lower underlying prevalence of CAD and thus fewer treatments. Similarly, we show that a lower specificity of early coronary CTA for the detection of obstructive CAD leads to an increased ICER (about $10,000 for a decrease in specificity from 90% to 70%), emphasizing the importance of acquiring coronary CTA data sets with diagnostic image quality and having them interpreted by highly skilled readers.
4.1. Limitations
Inherent in this type of research is that the results are based on data simulations and thus represent estimates, which applies to all strategies. As such the model is highly dependent on the quality of the input data. However, as discussed, the main outcomes of our analyses benefitted from the availability of individual patient data from randomized clinical trials. Some of the data we used was derived from studies performed a long time ago, e.g. data regarding the quality of life. It is reassuring, though, that the life expectancy for different trial populations approximates those observed in the CDC vital statistics when accounting for higher disease burden in the trial population compared to the overall population (RII: model: 24.7 years, CDC: 27.0 years, CT-STAT: model 29.0 years, CDC: 31.4 years).42 Moreover, the 2 year outcomes of our model were comparable to the observed 2 year outcomes in clinical trials, i.e. ROMICAT I trial.4
5. CONCLUSION
Considering long-term health outcomes and costs of care, early coronary CTA appears to be the most cost-effective strategy in patients with suspected ACS as compared to alternative strategies including expedited ED discharge. Benefits of early coronary CTA are expected to be seen three years after ED presentation.
Supplementary Material
ACKNOWLEGEMENT
We gratefully acknowledge Nadja Arifovic, BS for her assistance in the preparation and submission of the manuscript.
Funding: This work was supported by grants from the National Heart, Lung, and Blood Institute (U01HL092040 and U01HL092022) and the National Institutes of Health (UL1RR025758, K23HL098370, and L30HL093896, to Dr. Truong). Drs. Goehler and Pursnani received funding from the NIH Ruth Kirschstein National Research Award T32 (5T32HL076136). Dr. Ferencik received support from the American Heart Association (13FTF16450001).
Disclaimer: The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the views of the National Heart, Lung, and Blood Institute, National Institutes of Health, or the United States Department of Health and Human Services.
Disclosure
Dr. G. Scott Gazelle is a consultant to GE Healthcare. Dr. Quynh Truong has received research grants on behalf of the institution from Ziosoft (significant) and is a consultant with HeartFlow (moderate < $5K). Dr. Udo Hoffmann has received research grants on behalf of the institution from Siemens Medical Solutions, HeartFlow, KOWA Ltd., and DCRI. Dr. Benjamin Chow has received grants on behalf of the institution from CV Diagnostix and TeraRecon Inc.. Drs. Alexander Goehler, Thomas Mayrhofer, Amit Pursnani, Maros Ferencik, Heidi S. Lumish, Cordula Barth, Julia Karady, John T. Nagurney, James E. Udelson, Jerome L. Fleg have no disclosures.
ABBREVIATIONS
- ACP
Acute chest pain
- ACS
Acute coronary syndrome
- AHA/ACC
American Heart Association/American College of Cardiology
- ASCVD
Atherosclerotic cardiovascular disease
- CABG
Coronary artery bypass graft
- CAD
Coronary artery disease
- CTA
Computed tomography angiography
- ED
Emergency department
- ICA
Invasive coronary angiography
- ICER
Incremental cost-effectiveness ratio
- MACE
Major adverse cardiovascular event
- NSTEMI
Non-ST segment elevation myocardial infarction
- PCI
Percutan coronary intervention
- QALY
Quality adjusted life years
- RF
Risk Factor
- ROMICAT-II
Rule Out Myocardial Infarction/Ischemia Using Computer Assisted Tomography
- SOC
Standard of care
- UA
Unstable angina
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Litt HI, Gatsonis C, Snyder B, Singh H, Miller CD, Entrikin DW, Leaming JM, Gavin LJ, Pacella CB and Hollander JE. CT angiography for safe discharge of patients with possible acute coronary syndromes. N Engl J Med. 2012;366:1393–403. [DOI] [PubMed] [Google Scholar]
- 2.Hoffmann U, Truong QA, Schoenfeld DA, Chou ET, Woodard PK, Nagurney JT, Pope JH, Hauser TH, White CS, Weiner SG, Kalanjian S, Mullins ME, Mikati I, Peacock WF, Zakroysky P, Hayden D, Goehler A, Lee H, Gazelle GS, Wiviott SD, Fleg JL, Udelson JE and Investigators R-I. Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med. 2012;367:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldstein JA, Chinnaiyan KM, Abidov A, Achenbach S, Berman DS, Hayes SW, Hoffmann U, Lesser JR, Mikati IA, O’Neil BJ, Shaw LJ, Shen MY, Valeti US and Raff GL. The CT-STAT (Coronary Computed Tomographic Angiography for Systematic Triage of Acute Chest Pain Patients to Treatment) trial. J Am Coll Cardiol. 2011;58:1414–22. [DOI] [PubMed] [Google Scholar]
- 4.Schlett CL, Banerji D, Siegel E, Bamberg F, Lehman SJ, Ferencik M, Brady TJ, Nagurney JT, Hoffmann U and Truong QA. Prognostic value of CT angiography for major adverse cardiac events in patients with acute chest pain from the emergency department: 2-year outcomes of the ROMICAT trial. JACC Cardiovasc Imaging. 2011;4:481–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones RL, Thomas DM, Barnwell ML, Fentanes E, Young AN, Barnwell R, Foley AT, Hilliard M, Hulten EA, Villines TC, Cury RC and Slim AM. Safe and rapid disposition of low-to-intermediate risk patients presenting to the emergency department with chest pain: a 1-year high-volume single-center experience. J Cardiovasc Comput Tomogr. 2014;8:375–83. [DOI] [PubMed] [Google Scholar]
- 6.Redberg RF. Coronary CT angiography for acute chest pain. N Engl J Med. 2012;367:375–6. [DOI] [PubMed] [Google Scholar]
- 7.Redberg R, Katz M and Grady D. Diagnostic tests: another frontier for less is more: or why talking to your patient is a safe and effective method of reassurance. Archives of internal medicine. 2011;171:619. [DOI] [PubMed] [Google Scholar]
- 8.Than M, Cullen L, Reid CM, Lim SH, Aldous S, Ardagh MW, Peacock WF, Parsonage WA, Ho HF, Ko HF, Kasliwal RR, Bansal M, Soerianata S, Hu D, Ding R, Hua Q, Seok-Min K, Sritara P, Sae-Lee R, Chiu TF, Tsai KC, Chu FY, Chen WK, Chang WH, Flaws DF, George PM and Richards AM. A 2-h diagnostic protocol to assess patients with chest pain symptoms in the Asia-Pacific region (ASPECT): a prospective observational validation study. Lancet. 2011;377:1077–84. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann U, Ferencik M, Udelson JE, Picard MH, Truong QA, Patel MR, Huang M, Pencina M, Mark DB, Heitner JF, Fordyce CB, Pellikka PA, Tardif JC, Budoff M, Nahhas G, Chow B, Kosinski AS, Lee KL, Douglas PS and Investigators P. Prognostic Value of Noninvasive Cardiovascular Testing in Patients With Stable Chest Pain: Insights From the PROMISE Trial (Prospective Multicenter Imaging Study for Evaluation of Chest Pain). Circulation. 2017;135:2320–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amsterdam EA, Kirk JD, Bluemke DA, Diercks D, Farkouh ME, Garvey JL, Kontos MC, McCord J, Miller TD, Morise A, Newby LK, Ruberg FL, Scordo KA and Thompson PD. Testing of low-risk patients presenting to the emergency department with chest pain: a scientific statement from the American Heart Association. Circulation. 2010;122:1756–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gongora CA, Bavishi C, Uretsky S and Argulian E. Acute chest pain evaluation using coronary computed tomography angiography compared with standard of care: a meta-analysis of randomised clinical trials. Heart. 2018;104:215–221. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann U, Truong QA, Fleg JL, Goehler A, Gazelle S, Wiviott S, Lee H, Udelson JE and Schoenfeld D. Design of the Rule Out Myocardial Ischemia/Infarction Using Computer Assisted Tomography: a multicenter randomized comparative effectiveness trial of cardiac computed tomography versus alternative triage strategies in patients with acute chest pain in the emergency department. Am Heart J. 2012;163:330–8, 338 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Czarnecki A, Chong A, Lee DS, Schull MJ, Tu JV, Lau C, Farkouh ME and Ko DT. Association Between Physician Follow-Up and Outcomes of Care After Chest Pain Assessment in High-Risk Patients. Circulation. 2013;127:1386–1394. [DOI] [PubMed] [Google Scholar]
- 14.Czarnecki A, Wang JT, Tu JV, Lee DS, Schull MJ, Lau C, Farkouh ME, Wijeysundera HC and Ko DT. The role of primary care physician and cardiologist follow-up for low-risk patients with chest pain after emergency department assessment. Am Heart J. 2014;168:289–95. [DOI] [PubMed] [Google Scholar]
- 15.Chow BJ, Small G, Yam Y, Chen L, Achenbach S, Al-Mallah M, Berman DS, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Cheng V, Chinnaiyan KM, Delago A, Dunning A, Hadamitzky M, Hausleiter J, Kaufmann P, Lin F, Maffei E, Raff GL, Shaw LJ, Villines TC, Min JK and Investigators C. Incremental prognostic value of cardiac computed tomography in coronary artery disease using CONFIRM: COroNary computed tomography angiography evaluation for clinical outcomes: an InteRnational Multicenter registry. Circ Cardiovasc Imaging. 2011;4:463–72. [DOI] [PubMed] [Google Scholar]
- 16.Kong CY, McMahon PM and Gazelle GS. Calibration of disease simulation model using an engineering approach. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2009;12:521–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Min JK, Dunning A, Lin FY, Achenbach S, Al-Mallah M, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Cheng V, Chinnaiyan K, Chow BJ, Delago A, Hadamitzky M, Hausleiter J, Kaufmann P, Maffei E, Raff G, Shaw LJ, Villines T and Berman DS. Age- and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings results from the International Multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) of 23,854 patients without known coronary artery disease. J Am Coll Cardiol. 2011;58:849–60. [DOI] [PubMed] [Google Scholar]
- 18.Hulten E, Villines TC, Cheezum MK, Berman DS, Dunning A, Achenbach S, Al-Mallah M, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Cheng VY, Chinnaiyan K, Chow BJ, Cury RC, Delago A, Feuchtner G, Hadamitzky M, Hausleiter J, Kaufmann PA, Karlsberg RP, Kim YJ, Leipsic J, Lin FY, Maffei E, Plank F, Raff GL, Labounty TM, Shaw LJ and Min JK. Usefulness of coronary computed tomography angiography to predict mortality and myocardial infarction among Caucasian, African and East Asian ethnicities (from the CONFIRM [Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter] Registry). Am J Cardiol. 2013;111:479–85. [DOI] [PubMed] [Google Scholar]
- 19.Stone GW, Ware JH, Bertrand ME, Lincoff AM, Moses JW, Ohman EM, White HD, Feit F, Colombo A, McLaurin BT, Cox DA, Manoukian SV, Fahy M, Clayton TC, Mehran R and Pocock SJ. Antithrombotic strategies in patients with acute coronary syndromes undergoing early invasive management: one-year results from the ACUITY trial. JAMA. 2007;298:2497–506. [DOI] [PubMed] [Google Scholar]
- 20.Armstrong PW, Fu Y, Chang WC, Topol EJ, Granger CB, Betriu A, Van de Werf F, Lee KL and Califf RM. Acute coronary syndromes in the GUSTO-IIb trial: prognostic insights and impact of recurrent ischemia. The GUSTO-IIb Investigators. Circulation. 1998;98:1860–8. [DOI] [PubMed] [Google Scholar]
- 21.Roe MT, Harrington RA, Prosper DM, Pieper KS, Bhatt DL, Lincoff AM, Simoons ML, Akkerhuis M, Ohman EM, Kitt MM, Vahanian A, Ruzyllo W, Karsch K, Califf RM and Topol EJ. Clinical and therapeutic profile of patients presenting with acute coronary syndromes who do not have significant coronary artery disease.The Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin Therapy (PURSUIT) Trial Investigators. Circulation. 2000;102:1101–6. [DOI] [PubMed] [Google Scholar]
- 22.Arias E United States Life Tables, 2009. National Vital Statistics Report. 2014;62. [PubMed] [Google Scholar]
- 23.LaRosa JC, He J and Vupputuri S. Effect of statins on risk of coronary disease: a meta-analysis of randomized controlled trials. JAMA. 1999;282:2340–6. [DOI] [PubMed] [Google Scholar]
- 24.Pope JH, Aufderheide TP, Ruthazer R, Woolard RH, Feldman JA, Beshansky JR, Griffith JL and Selker HP. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med. 2000;342:1163–70. [DOI] [PubMed] [Google Scholar]
- 25.Ladapo JA, Hoffmann U, Bamberg F, Nagurney JT, Cutler DM, Weinstein MC and Gazelle GS. Cost-effectiveness of coronary MDCT in the triage of patients with acute chest pain. AJR Am J Roentgenol. 2008;191:455–63. [DOI] [PubMed] [Google Scholar]
- 26.Chaitman BR, Bourassa MG, Davis K, Rogers WJ, Tyras DH, Berger R, Kennedy JW, Fisher L, Judkins MP, Mock MB and Killip T. Angiographic prevalence of high-risk coronary artery disease in patient subsets (CASS). Circulation. 1981;64:360–7. [DOI] [PubMed] [Google Scholar]
- 27.Lalonde L, Clarke AE, Joseph L, Mackenzie T and Grover SA. Comparing the psychometric properties of preference-based and nonpreference-based health-related quality of life in coronary heart disease. Canadian Collaborative Cardiac Assessment Group. Qual Life Res. 1999;8:399–409. [DOI] [PubMed] [Google Scholar]
- 28.Hulten E, Pickett C, Bittencourt MS, Villines TC, Petrillo S, Di Carli MF and Blankstein R. Outcomes after coronary computed tomography angiography in the emergency department: a systematic review and meta-analysis of randomized, controlled trials. J Am Coll Cardiol. 2013;61:880–92. [DOI] [PubMed] [Google Scholar]
- 29.Ritter FE, Schoelles MJ, Quigley KS and Klein LC. Determining the number of simulation runs: Treating simulations as theories by not sampling their behavior. Human-in-the-Loop Simulations: Methods and Practice 2011;ISBN-10: 0857298828:97–116. [Google Scholar]
- 30.Investigators S-H, Newby DE, Adamson PD, Berry C, Boon NA, Dweck MR, Flather M, Forbes J, Hunter A, Lewis S, MacLean S, Mills NL, Norrie J, Roditi G, Shah ASV, Timmis AD, van Beek EJR and Williams MC. Coronary CT Angiography and 5-Year Risk of Myocardial Infarction. N Engl J Med. 2018;379:924–933. [DOI] [PubMed] [Google Scholar]
- 31.Anderson JL, Heidenreich PA, Barnett PG, Creager MA, Fonarow GC, Gibbons RJ, Halperin JL, Hlatky MA, Jacobs AK, Mark DB, Masoudi FA, Peterson ED and Shaw LJ. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2304–22. [DOI] [PubMed] [Google Scholar]
- 32.Culyer A, McCabe C, Briggs A, Claxton K, Buxton M, Akehurst R, Sculpher M and Brazier J. Searching for a threshold, not setting one: the role of the National Institute for Health and Clinical Excellence. Journal of health services research & policy. 2007;12:56–8. [DOI] [PubMed] [Google Scholar]
- 33.Newby LK, Eisenstein EL, Califf RM, Thompson TD, Nelson CL, Peterson ED, Armstrong PW, Van de Werf F, White HD, Topol EJ and Mark DB. Cost effectiveness of early discharge after uncomplicated acute myocardial infarction. N Engl J Med. 2000;342:749–55. [DOI] [PubMed] [Google Scholar]
- 34.Rawlins MD and Culyer AJ. National Institute for Clinical Excellence and its value judgments. BMJ (Clinical research ed). 2004;329:224–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McMahon PM, Kong CY, Bouzan C, Weinstein MC, Cipriano LE, Tramontano AC, Johnson BE, Weeks JC and Gazelle GS. Cost-effectiveness of computed tomography screening for lung cancer in the United States. J Thorac Oncol. 2011;6:1841–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayashino Y, Shimbo T, Tsujii S, Ishii H, Kondo H, Nakamura T, Nagata-Kobayashi S and Fukui T. Cost-effectiveness of coronary artery disease screening in asymptomatic patients with type 2 diabetes and other atherogenic risk factors in Japan: factors influencing on international application of evidence-based guidelines. International journal of cardiology. 2007;118:88–96. [DOI] [PubMed] [Google Scholar]
- 37.Nolte JE, Neumann T, Manne JM, Lo J, Neumann A, Mostardt S, Abbara S, Hoffmann U, Brady TJ, Wasem J, Grinspoon SK, Gazelle GS and Goehler A. Cost-effectiveness analysis of coronary artery disease screening in HIV-infected men. European journal of preventive cardiology. 2013;21:972–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoffmann U, Bamberg F, Chae CU, Nichols JH, Rogers IS, Seneviratne SK, Truong QA, Cury RC, Abbara S, Shapiro MD, Moloo J, Butler J, Ferencik M, Lee H, Jang IK, Parry BA, Brown DF, Udelson JE, Achenbach S, Brady TJ and Nagurney JT. Coronary computed tomography angiography for early triage of patients with acute chest pain: the ROMICAT (Rule Out Myocardial Infarction using Computer Assisted Tomography) trial. J Am Coll Cardiol. 2009;53:1642–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hollander JE, Chang AM, Shofer FS, Collin MJ, Walsh KM, McCusker CM, Baxt WG and Litt HI. One-year outcomes following coronary computerized tomographic angiography for evaluation of emergency department patients with potential acute coronary syndrome. Academic emergency medicine : official journal of the Society for Academic Emergency Medicine. 2009;16:693–8. [DOI] [PubMed] [Google Scholar]
- 40.Foy AJ, Liu G, Davidson WR Jr., Sciamanna C and Leslie DL. Comparative Effectiveness of Diagnostic Testing Strategies in Emergency Department Patients With Chest Pain: An Analysis of Downstream Testing, Interventions, and Outcomes. JAMA Intern Med. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakazato R, Arsanjani R, Achenbach S, Gransar H, Cheng VY, Dunning A, Lin FY, Al-Mallah M, Budoff MJ, Callister TQ, Chang HJ, Cademartiri F, Chinnaiyan K, Chow BJ, Delago A, Hadamitzky M, Hausleiter J, Kaufmann P, Raff G, Shaw LJ, Villines T, Cury RC, Feuchtner G, Kim YJ, Leipsic J, Berman DS and Min JK. Age-related risk of major adverse cardiac event risk and coronary artery disease extent and severity by coronary CT angiography: results from 15 187 patients from the International Multisite CONFIRM Study. Eur Heart J Cardiovasc Imaging. 2014;15:586–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murphy SL, Xu J and Kochanek KD. National Vital Statitics Reports. NVSS. 2013;61:1–118. [PubMed] [Google Scholar]
- 43.Schuetz GM, Schlattmann P and Dewey M. Use of 3×2 tables with an intention to diagnose approach to assess clinical performance of diagnostic tests: meta-analytical evaluation of coronary CT angiography studies. BMJ (Clinical research ed). 2012;345:e6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lau J, Ioannidis JP, Balk EM, Milch C, Terrin N, Chew PW and Salem D. Diagnosing acute cardiac ischemia in the emergency department: a systematic review of the accuracy and clinical effect of current technologies. Ann Emerg Med. 2001;37:453–60. [DOI] [PubMed] [Google Scholar]
- 45.Garber AM and Solomon NA. Cost-effectiveness of alternative test strategies for the diagnosis of coronary artery disease. Ann Intern Med. 1999;130:719–28. [DOI] [PubMed] [Google Scholar]
- 46.Lima RS, Watson DD, Goode AR, Siadaty MS, Ragosta M, Beller GA and Samady H. Incremental value of combined perfusion and function over perfusion alone by gated SPECT myocardial perfusion imaging for detection of severe three-vessel coronary artery disease. J Am Coll Cardiol. 2003;42:64–70. [DOI] [PubMed] [Google Scholar]
- 47.Butler J, Shapiro M, Reiber J, Sheth T, Ferencik M, Kurtz EG, Nichols J, Pena A, Cury RC, Brady TJ and Hoffmann U. Extent and distribution of coronary artery disease: a comparative study of invasive versus noninvasive angiography with computed angiography. Am Heart J. 2007;153:378–84. [DOI] [PubMed] [Google Scholar]
- 48.Weintraub WS, Grau-Sepulveda MV, Weiss JM, O’Brien SM, Peterson ED, Kolm P, Zhang Z, Klein LW, Shaw RE, McKay C, Ritzenthaler LL, Popma JJ, Messenger JC, Shahian DM, Grover FL, Mayer JE, Shewan CM, Garratt KN, Moussa ID, Dangas GD and Edwards FH. Comparative effectiveness of revascularization strategies. N Engl J Med. 2012;366:1467–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuntz KM, Fleischmann KE, Hunink MG and Douglas PS. Cost-effectiveness of diagnostic strategies for patients with chest pain. Ann Intern Med. 1999;130:709–18. [DOI] [PubMed] [Google Scholar]
- 50.Morcos SK. Review article: Acute serious and fatal reactions to contrast media: our current understanding. Br J Radiol. 2005;78:686–93. [DOI] [PubMed] [Google Scholar]
- 51.Weinberg L, Kandasamy K, Evans SJ and Mathew J. Fatal cardiac rupture during stress exercise testing: case series and review of the literature. South Med J. 2003;96:1151–3. [DOI] [PubMed] [Google Scholar]
- 52.Ladapo JA, Hoffmann U, Bamberg F, Nagurney JT, Cutler DM, Weinstein MC and Gazelle GS. Cost-effectiveness of coronary MDCT in the triage of patients with acute chest pain. AJR Am J Roentgenol. 2008;191:455–63. [DOI] [PubMed] [Google Scholar]
- 53.Lalonde L, Clarke AE, Joseph L, Mackenzie T and Grover SA. Comparing the psychometric properties of preference-based and nonpreference-based health-related quality of life in coronary heart disease. Canadian Collaborative Cardiac Assessment Group. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 1999;8:399–409. [DOI] [PubMed] [Google Scholar]
- 54.Boden WE, O’Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, Chaitman BR, Shaw L, Gosselin G, Nawaz S, Title LM, Gau G, Blaustein AS, Booth DC, Bates ER, Spertus JA, Berman DS, Mancini GB, Weintraub WS and Group CTR. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–16. [DOI] [PubMed] [Google Scholar]
- 55.Gandhi SK, Jensen MM, Fox KM, Smolen L, Olsson AG and Paulsson T. Cost-effectiveness of rosuvastatin in comparison with generic atorvastatin and simvastatin in a Swedish population at high risk of cardiovascular events. Clinicoecon Outcomes Res. 2012;4:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.