Abstract
In previous work, we applied the rotation-limiting strategy and introduced a substituent at the 3-position of the pyrazolo [3,4-d]pyrimidin-4-amine as the affinity element to interact with the deeper hydrophobic pocket, discovered a series of novel quinazolinones as potent PI3Kδ inhibitors. Among them, the indole derivative 3 is one of the most selective PI3Kδ inhibitors and the 3,4-dimethoxyphenyl derivative 4 is a potent and selective dual PI3Kδ/γ inhibitor. In this study, we replaced the carbonyl group in the quinazolinone core with a sulfonyl group, designed a series of novel 2H-benzo[e][1,2,4]thiadiazine 1,1-dioxide derivatives as PI3Kδ inhibitors. After the reduction of nitro group in N-(2,6-dimethylphenyl)-2-nitrobenzenesulfonamide 5 and N-(2,6-dimethylphenyl)-2-nitro-5-fluorobenzenesulfonamide 6, the resulting 2-aminobenzenesulfonamides were reacted with trimethyl orthoacetate to give the 3-methyl-2H-benzo[e][1,2,4]thiadiazine 1,1-dioxide derivatives. After bromination of the 3-methyl group, the nucleophilic substitution with the 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine provided the respective iodide derivatives, which were further reacted with a series of arylboronic acids via Suzuki coupling to furnish the 2H-benzo[e][1,2,4]thiadiazine 1,1-dioxide derivatives 15a–J and 16a–d. In agreement with the quinazolinone derivatives, the introduction of a 5-indolyl or 3,4-dimethoxyphenyl at the affinity pocket generated the most potent analogues 15a and 15b with the IC50 values of 217 to 266 nM, respectively. In comparison with the quinazolinone lead compounds 3 and 4, these 2H-benzo[e][1,2,4]thiadiazine 1,1-dioxide derivatives exhibited much decreased PI3Kδ inhibitory potency, but maintained the high selectivity over other PI3K isoforms. Unlike the quinazolinone lead compound 4 that was a dual PI3Kδ/γ inhibitor, the benzthiadiazine 1,1-dioxide 15b with the same 3,4-dimethoxyphenyl moiety was more than 21-fold selective over PI3Kγ. Moreover, the introducing of a fluorine atom at the 7-position of the 2H-benzo[e][1,2,4]thiadiazine 1,1-dioxide core, in general, was not favored for the PI3Kδ inhibitory activity. In agreement with their high PI3Kδ selectivity, 15a and 15b significantly inhibited the SU-DHL-6 cell proliferation.
Keywords: PI3Ks; PI3Kδ inhibitors; 2H-benzo[e][1,2,4]thiadiazine 1,1-dioxide; anticancer; anticancer agents
1. Introduction
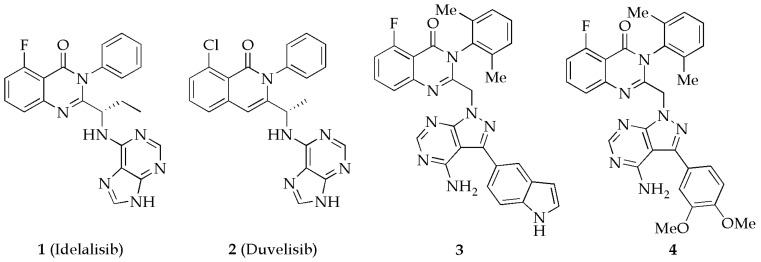
Phosphoinositide 3-kinases (PI3Ks) are a family of lipid kinases that regulate numerous biological functions, including cell growth, proliferation, differentiation, motility, and intracellular trafficking, through the phosphorylation of the phosphatidylinositol 4,5-bisphosphate (PIP2) to generate the lipid second messenger phosphatidylinositol 3,4,5-trisphosphate (PIP3) [1,2,3]. There are three classes of the PI3K enzyme, of which class I PI3Ks are the mostly studied and further divided into subgroups IA (PI3Kα, PI3Kβ, and PI3Kδ) and IB (PI3Kγ) based on the signaling pathways and the regulatory proteins to which they bind [4,5]. The class IA PI3K isoforms mediate the signal transduction from receptor tyrosine kinases [6], while the IB isoform PI3Kγ is principally activated by G-protein coupled receptors [7]. PI3Kα and PI3Kβ are ubiquitously expressed, while PI3Kδ and PI3Kγ are dominantly expressed in leukocytes [8,9,10]. All the class I PI3K isoforms are implicated in cancer [11,12,13,14,15,16]. There are also mounting evidences that support a therapeutic role for inhibition of PI3Kα in diabetes [17,18], PI3Kβ in thrombosis [19,20], PI3Kδ and PI3Kγ in both rheumatoid arthritis and asthma [21,22,23,24], PI3Kδ in activated PI3Kδ syndrome (APDS) [25,26,27], and PI3Kγ in idiopathic pulmonary fibrosis [28]. Therefore, the development of PI3K isoform selective inhibitors is a promising therapeutic strategy for the treatment of these PI3Ks-related diseases. The selective PI3Kδ inhibitor idelalisib (Figure 1) is approved by FDA for follicular lymphoma (FL) and small lymphocytic lymphoma (SLL) and for chronic lymphocytic leukemia (CLL) in combination with rituximab [29,30]. The dual PI3Kδ/γ inhibitor duvelisib (Figure 1) is approved for adult patients with relapsed or refractory CLL or SLL, and relapsed or refractory FL after at least two prior systemic therapies [31]. However, in the clinical application of idelalisib, infectious and autoimmune toxicities were observed, and the unique toxicities are associated with inhibition of different isoforms of the PI3K enzyme [32]. To improve the isoform selectivity of the quinazolinone-based PI3Kδ inhibitors, in previous work, we introduced a pyrazolo [3,4-d]pyrimidin-4-amine moiety as the hinge region binding group, a substituent at the 3-position of the pyrazolo[3,4-d]pyrimidine core as the affinity element to interact with the deeper hydrophobic pocket, and a 2,6-dimethylphenyl to limit the free rotation of the 3-phenyl in idelalisib, discovered the indole derivative 3 as one of the most selective PI3Kδ inhibitors (IC50 = 8.6 nM) with more than 3630-fold, 390-fold and 40-fold selective for PI3Kδ over PI3Kα, β and γ, and the 3,4-dimethoxyphenyl derivative 4 as a potent and selective dual PI3Kδ/γ inhibitor (IC50 = 8.4 nM for PI3Kδ, IC50 = 62 nM for PI3Kγ) with more than 1400-fold, 820-fold selective for PI3Kδ over PI3Kα and PI3Kβ [33]. Considering the importance of sulfonamides in drug discovery [34,35,36], we replaced the carbonyl group in the quinazolinone core, and reported here the synthesis and preliminary evaluation of 2H-benzo[e][1,2,4]thiadiazine 1,1-dioxide derivatives as PI3Kδ inhibitors.
Figure 1.
Selective PI3Kδ and dual PI3Kδ/γ inhibitors.
2. Results and Discussion
2.1. Chemistry
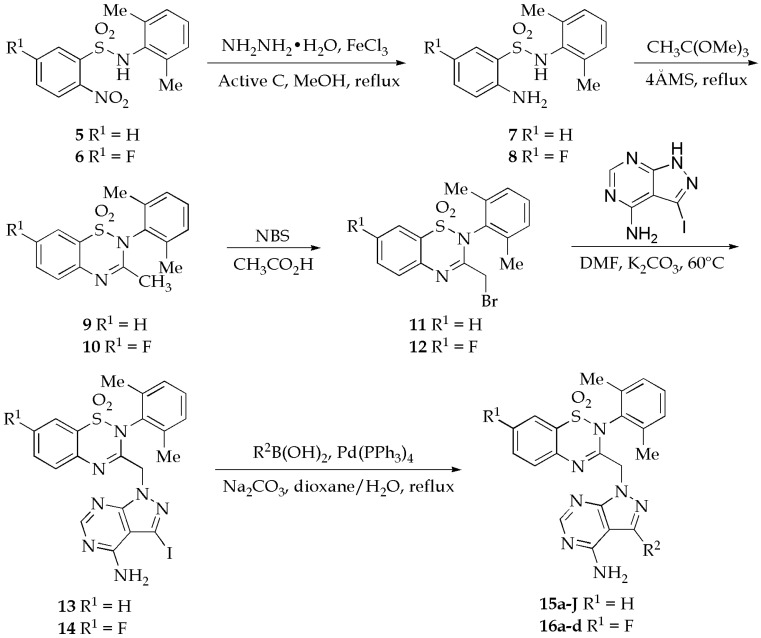
All the new 2H-benzo[e][1,2,4]thiadiazine 1,1-dioxide derivatives were prepared following a general synthetic route shown in Scheme 1. The 2-nitrobenzene-1-sulfonamides 5 and 6 were readily prepared according to the reported method by the reaction of 2-nitrobenzene-1-sulfonyl chloride or 5-fluoro-2-nitrobenzene-1-sulfonyl chloride with 2,6-dimethylbenzenamine in methanol and water solution in the presence of CH3COONa under refluxing conditions [37]. Reduction of the nitro group to amine was carried out using hydrazine monohydrate in the presence of ferric chloride and activated charcoal in methanol under reflux conditions in excellent yields (95% and 99%). The resulting 2-aminobenzenesulfonamides 7 and 8 were reacted with trimethyl orthoacetate to give the corresponding 3-methyl-2H-benzo[e][1,2,4]thiadiazine 1,1-dioxide derivatives 9 and 10 in 51% and 40%, respectively. In the bromination of the allyl methyl group using N-bromosuccinimide (NBS), the main compounds were found to be the dibrominated products. Therefore, compounds 9 and 10 were reacted with only 0.5 equivalent of NBS in glacial acetic acid to give the monobrominated derivatives 11 and 12 in moderate yields (79% and 72% based on NBS). Nucleophilic substitution of 11 and 12 with 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine, which was readily prepared from 5-amino-1H-pyrazole-4-carbonitrile in two steps by the known procedures [38], resulted in the iodides 13 and 14 in 86 and 58% yields, respectively. Finally, the incorporation of the affinity elements was achieved through the Suzuki coupling of 13 and 14 with the appropriate boronic acid in dioxane and water catalyzed by Pd(PPh3)4 under refluxing conditions, and the target 2H-benzo[e][1,2,4]thiadiazine 1,1-dioxide derivatives 15a–J and 16a–d were obtained in 34–91% and 51–84% yields. The structures of these 2H-benzo[e][1,2,4]thiadiazine 1,1-dioxide derivatives were characterized by 1H-nuclear magnetic resonance (NMR) and 13C-NMR (please refer to the Supplementary Materials).
Scheme 1.
Synthesis of 2H-benzo[e][1,2,4]thiadiazine 1,1-dioxide derivatives.
2.2. PI3Kδ Inhibitory Activity and Isoform Selectivity
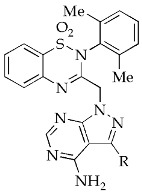
Compounds 15a–J were first tested for their inhibitory activity against PI3Kδ using the ADP-Glo luminescent assay [39], using a pan-PI3K inhibitor PI-103 as a positive control [40]. As shown in Table 1, the substitution at the 3-position of the pyrazolo[3,4-d]pyrimidine with 5-indolyl or 3,4-dimethoxyphenyl led to the relative potent analogues 15a and 15b with IC50 values of 217 to 266 nM, respectively. The 6-methoxypyridin-3-yl derivative 15d exhibited moderate PI3Kδ inhibitory activity (IC50 = 498 nM), whereas the 3-fluoro-4-methoxyphenyl analogue 15c only had marginal activity. In comparison with 15b, the substitution of the 3,4-dimethoxypheny group for 2,3-dihydrobenzo[b][1,4]dioxin-6-yl (15e), benzo[d][1,3]dioxol-5-yl (15f), 4-methoxyphenyl (15g), 3-methoxyphenyl (15h), 4-(trifluoromethoxy)phenyl (15i), and phenyl (15j) was not tolerated, indicating the subtle requirements at the affinity pocket of PI3Kδ.
Table 1.
PI3Kδ inhibitory activity of 15a–j and 16a–d.
| Compd. | Structure | R | IC50 (nM) a |
|---|---|---|---|
| 15a |
|
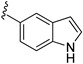
|
217 ± 28 |
| 15b |
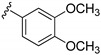
|
266 ± 31 | |
| 15c |
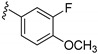
|
980 ± 45 | |
| 15d |
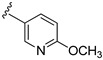
|
498 ± 33 | |
| 15e |
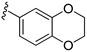
|
>1000 | |
| 15f |
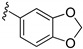
|
>1000 | |
| 15g |
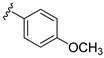
|
>1000 | |
| 15h |
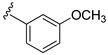
|
>1000 | |
| 15i |
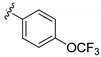
|
>1000 | |
| 15j |
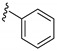
|
>1000 | |
| 16a |
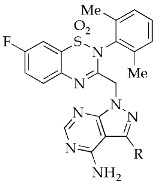
|
|
>1000 |
| 16b |
|
518 ± 62 | |
| 16c |
|
824 ± 76 | |
| 16d |
|
823 ± 69 | |
| PI-103 | 1.6 ± 0.1 | ||
a The IC50 values are shown as the mean ± SD from two separate experiments.
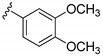
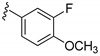
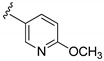
In order to increase the inhibitory activity of compound 15a–d, a fluorine atom was introduced at the 7-position of the 2H-benzo[e][1,2,4]thiadiazine 1,1-dioxide, and compounds 16a–d were prepared and evaluated for their PI3Kδ inhibitory activity. In comparison with 15a, the fluorinated compound 16a lost its activity (Table 1), and compounds 16b and 16d showed almost 2-fold decrease in potency. In contrast, compound 16c with a 3-fluoro-4-methoxyphenyl moiety at the affinity pocket showed a slight increase in potency.
Compared with the leading quinazolinone derivatives 3 and 4, these 2H-benzo[e][1,2,4]-thiadiazine 1,1-dioxide derivatives 15a–j and 16a–d showed much decreased PI3Kδ inhibitory activity. However, the most potent derivatives 15a and 15b proved to be selective PI3Kδ inhibitors (Table 2). The indole derivative 15a showed significantly lower potency against other three isoforms of class I PI3K and was more than 140-fold selective for PI3Kδ over PI3Kα, β and γ. The 3,4-dimethoxyphenyl derivative 15b was more than 60-fold, 90-fold and 20-fold selective for PI3Kδ over PI3Kα, PI3Kβ, and PI3Kγ, respectively. In comparison with the lead 4, 15b was more selective over PI3Kγ (21-fold vs. 7-fold).
Table 2.
The isoform selectivity and SU-DHL-6 cell growth inhibitory activity of 15a and 15b.
| Compound | IC50 (nM) a | GI50 (μM) a | |||
|---|---|---|---|---|---|
| PI3Kα | PI3Kβ | PI3Kδ | PI3Kγ | SU-DHL-6 | |
| 15a | >50,000 | 30596 ± 875 | 217 ± 28 | >50,000 | 2.13 ± 0.09 |
| 15b | 16364 ± 768 | 24189 ± 495 | 266 ± 31 | 5838 ± 135 | 2.50 ± 0.11 |
| PI-103 | 6.5 ± 0.7 | 23 ± 1.6 | 1.6 ± 0.1 | 78 ± 4.3 | 0.039 ± 0.011 b |
a The IC50 or GI50 values are shown as the mean ± SD from two separate experiments; b CAL-101 was the positive control.
2.3. SU-DHL-6 Cell Growth Inhibitory Activity
The selective PI3Kδ inhibitors 15a and 15b were further evaluated for their antiproliferative activity against human B-cell SU-DHL-6. 15a and 15b significantly inhibited SU-DHL-6 cell proliferation with the GI50 of 2.13 and 2.50 μM, respectively (Table 2), which were in consistent with their PI3Kδ inhibitory potency.
2.4. Molecular Modeling Study
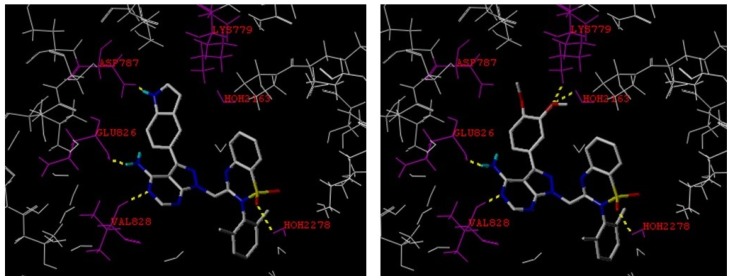
Molecular docking studies were conducted on the new discovered selective PI3Kδ inhibitors 15a and 15b. As shown in Figure 2, the pyrazolo[3,4-d]pyrimidine portion in both compounds 15a and 15b forms hydrogen bonds with Glu826 and Val828 in the hinge region.
Figure 2.
Molecular docking studies of 15a (left) and 15b (right).
Both inhibitors bind to the PI3Kδ isoform in an ‘induced fit’ conformation in which the 2H-benzo[e][1,2,4]thiadiazine 1,1-dioxide moiety is sandwiched between Trp760 and Met752 as these residues move apart to create the specificity pocket. The indol-5-yl (15a) in the specificity pocket forms an additional hydrogen bond with Asp787. Like the carbonyl oxygen in 3 and 4, one sulfonyl oxygen in both 15a and 15b acts as hydrogen bond acceptor from the H2O2278. In compound 15b, only the 3-methoxy group forms hydrogen bonding with Lys779, while in lead 4, the 3-methoxy interacts with Tyr813 and Asp911, the 4-methoxy interacts with Lys779 [33]. These differences may contribute its less potency against PI3Kδ than 4. In comparison with 3 and 4, the lack of a 8-fluorine at the 2H-benzo[e][1,2,4]thiadiazine 1,1-dioxide core in 15a and 15b may also be related to their lower PI3Kδ inhibitory activity.
3. Materials and Methods
3.1. General Chemical Experimental Procedures
1H- and 13C-NMR spectra were recorded on a Bruker-600 NMR spectrometer (Brucker Co., Ltd., Zurich, Switzerland). All spectra were recorded at room temperature for DMSO or CDCl3 solutions. High resolution mass spectra (HRMS) were obtained on a 6520 QTOF instrument (Agilent Technologies Inc., Santa Clara, CA, USA by electrospray ionization (ESI). Melting points were determined on an X-6 micromelting point apparatus (Beijing Tech. Co., Ltd., Beijing, China) without corrections. Column chromatography was performed on silica gel (200–300 mesh). All reactions involving oxygen- or moisture sensitive compounds were carried out under a dry N2 atmosphere using anhydrous solvents. Unless otherwise noted, reagents were added by syringe.
2-Amino-N-(2,6-dimethylphenyl)benzenesulfonamide (7)
To a stirred solution of N-(2,6-dimethylphenyl)-2-nitrobenzenesulfonamide (5, 19.4 g, 63.3 mmol) in methanol (200 mL), ferric chloride (5.1 g, 19 mmol) and activated charcoal (6.5 g) was added and refluxed for 30 min. 80% Hydrazine monohydrate (31.7 g, 633 mmol) was then added dropwise and refluxed for 5 h. After filtration, the filtrate was concentrated and the residue was dissolved in EtOAc (200 mL), washed with brine, dried over anhydrous Na2SO4. After filtration and evaporation, the residue was purified by silica gel chromatography (EtOAc/hexane = 1:3) to give 7 (16.5 g, 95%) as a white solid, m.p. 144–146 °C; lit. [41] m.p. 144–145 °C.
2-Amino-N-(2,6-dimethylphenyl)-5-fluorobenzenesulfonamide (8)
According to the procedures described for the synthesis of 7, compound 8 were obtained as a colorless solid (16 g) in 99% yield, m.p. 185–186 °C; 1H-NMR (DMSO-d6) δ 9.44 (s, 1H, SO2NH), 7.21 (td, J = 9.0, 3.0 Hz, 1H, Ar-H), 7.07 (td, J = 8.4, 1.5 Hz, 1H, Ar-H), 7.01 (d, J = 7.8 Hz, 2H, Ar-H), 6.97 (dd, J = 8.4, 3.0 Hz, 1H, Ar-H), 6.85 (dd, J = 9.0, 4.8 Hz, 1H, Ar-H), 5.85 (s, 2H, NH2), 2.04 (s, 6H, 2,6-(CH3)2); MS (ESI) calcd. for C14H14FN2O2S [M − H]−: 293.1, found: 293.3.
2-(2,6-Dimethylphenyl)-3-methy-2H-benzo[e][1,2,4]thiadiazine 1,1-dioxide (9)
A mixture of 7 (5 g, 18.1 mmol), trimethyl orthoacetate (50 mL) and 4Ă molecular sieve (10 g) was refluxed for 10 h. After cooling to room temp., the mixture was concentrated and the residue was dissolved in EtOAc (200 mL), washed with brine, dried over anhydrous Na2SO4. After filtration and evaporation, the residue was purified by silica gel chromatography (EtOAc/hexane = 1:5) to give 9 (2.8 g, 51%) as a white solid, m.p. 162–163 °C; 1H-NMR (CDCl3) δ 7.89 (d, J = 7.8 Hz, 1H, Ar-H), 7.70 (td, J = 8.4, 1.2 Hz, 1H, Ar-H), 7.60 (d, J = 8.4 Hz, 1H, Ar-H), 7.47 (td, J = 7.8, 1.2 Hz, 1H, Ar-H), 7.21 (t, J = 7.2 Hz, 1H, Ar-H), 7.18 (d, J = 7.8 Hz, 2H, Ar-H), 2.22 (s, 6H, 2,6-(CH3)2), 2.11 (s, 3H, 3-CH3); 13C-NMR (CDCl3) δ 154.34, 142.55, 138.58, 133.51, 132.59, 129.99, 129.27, 127.65, 127.23, 126.90, 121.01, 23.38, 18.62; MS (ESI) m/z calcd. for C16H17N2O2S [M + H]+ 301.1, found 301.0.
2-(2,6-Dimethylphenyl)-7-fluoro-3-methy-2H-benzo[e][1,2,4]thiadiazine1,1-dioxide (10)
According to the procedures described for the synthesis of 9, compound 10 were obtained as a colorless solid (6.9 g) in 40% yield, m.p. 150–151 °C; 1H-NMR (DMSO-d6) δ 7.89 (dt, J = 7.2, 1.5 Hz, 1H, Ar-H), 7.73 (dd, J = 7.2, 1.2 Hz, 2H, Ar-H), 7.36 (t, J = 7.2 Hz, 1H, Ar-H), 7.29 (d, J = 7.8 Hz, 2H, Ar-H), 2.13 (s, 6H, 2,6-(CH3)2), 2.06 (s, 3H, 3-CH3); 13C-NMR (DMSO-d6) δ 160.28 (d, JC–F = 249.2 Hz), 154.02, 139.26 (d, JC–F = 3.0 Hz), 138.59, 132.62, 130.88 (d, JC–F = 7.6 Hz), 130.66, 129.79, 127.96 (d, JC–F = 9.1 Hz), 122.58 (d, JC–F = 24.2 Hz), 107.95 (d, JC–F = 27.2 Hz), 23.25, 18.47; MS (ESI) m/z calcd. for C16H16FN2O2S [M + H]+ 319.1, found 319.0.
3-Bromomethyl-2-(2,6-dimethylphenyl)-2H-benzo[e][1,2,4]thiadiazine1,1-dioxide (11)
Compound 9 (1.0 g, 3.3 mmol) was dissolved in glacial acetic acid (10 mL), and then NBS (0.3 g, 1.65 mmol) was added. After the mixture was stirred at room temperature for 0.5 h, distilled water (50 mL) was added. The mixture was extracted by dichloromethane, washed with brine, dried over anhydrous Na2SO4. After filtration and evaporation, the residue was purified by silica gel chromatography (EtOAc/hexane = 1:10) to give 11 (0.5 g) with a conversion yield of 79% as a white solid, m.p. 150–151°C; 1H-NMR (CDCl3) δ 7.90 (dd, J = 7.8, 1.2 Hz, 1H, Ar-H), 7.75 (td, J = 8.4, 1.2 Hz, 1H, Ar-H), 7.69 (dd, J = 7.8, 0.6 Hz, 1H, Ar-H), 7.55 (td, J = 8.4, 1.2 Hz, 1H, Ar-H), 7.29 (t, J = 7.8 Hz, 1H, Ar-H), 7.19 (d, J = 7.8 Hz, 2H, Ar-H), 3.97 (s, 2H, CH2Br), 2.24 (s, 6H, 2,6-(CH3)2); 13C-NMR (CDCl3) δ 151.86, 142.16, 138.89, 133.69, 132.14, 130.36, 129.51, 128.37, 128.25, 127.79, 121.00, 28.91, 18.95; MS (ESI) m/z calcd. for C16H16BrN2O2S [M + H]+ 379.0 and 381.0, found 381.3 and 383.4.
3-Bromomethyl-2-(2,6-dimethylphenyl)-7-fluoro-2H-benzo[e][1,2,4]thiadiazine 1,1-dioxide (12)
According to the procedures described for the synthesis of 11, compound 12 were obtained as a colorless solid (0.65 g) in 72% conversion yield, m.p. 185–186 °C; 1H-NMR (DMSO-d6) δ 7.95 (dd, J = 8.4, 3.0 Hz, 1H, Ar-H), 7.84 (dd, J = 10.8, 5.4 Hz, 1H, Ar-H), 7.79 (td, J = 10.8, 3.0 Hz, 1H, Ar-H), 7.37 (t, J = 8.4 Hz, 1H, Ar-H), 7.29 (d, J = 9.0 Hz, 2H, Ar-H), 4.10 (s, 2H, CH2Br), 2.14 (s, 6H, 2,6-(CH3)2); 13C-NMR (DMSO-d6) δ 161.21 (d, JC–F = 250.6 Hz), 151.77, 138.82, 132.20, 131.54 (d, JC–F = 9.1 Hz), 130.94, 129.97, 129.28, 128.60 (d, JC–F = 9.1 Hz), 122.87 (d, JC–F = 24.2 Hz), 108.32 (d, JC–F = 25.7 Hz), 30.02, 18.72; MS (ESI) m/z calcd.for C16H15BrFN2O2S [M + H]+ 397.0 and 399.0, found 397.2 and 399.1.
3-((4-Amino-3-iodo-1H-pyrazolo[3,4-d]pyrimidin-1-yl)methyl)-2-(2,6-dimethylphenyl)-2H-benzo[e]-[1,2,4]thiadiazine 1,1-dioxide (13)
To a solution of 11 (1.1 g, 2.9 mmol) and 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (1.1 g, 4.4 mmol) in DMF (8 mL), K2CO3 (0.8 g, 5.8 mmol) was added. After stirring at 60 °C for 5 h, the mixture was poured into water (100 mL), extracted by EtOAc. The organic layer was washed with brine, dried over anhydrous Na2SO4. After filtration and evaporation, the residue was purified by silica gel chromatography (EtOAc/hexane = 1:10) to give 13 (1.4 g, 86%) as a white solid, m.p. 242–243 °C; 1H-NMR (DMSO-d6) δ 8.07 (s, 1H, Ar-H), 7.94 (dd, J = 7.8, 1.2 Hz, 1H, Ar-H), 7.80 (td, J = 7.8, 1.2 Hz, 1H, Ar-H), 7.65 (td, J = 7.8, 1.2 Hz, 1H, Ar-H), 7.43 (d, J = 7.8 Hz, 1H, Ar-H), 7.25 (t, J = 7.2 Hz, 1H, Ar-H), 7.17 (d, J= 7.2 Hz, 2H, Ar-H), 5.12 (s, 2H, NCH2), 2.07 (s, 6H, 2,6-(CH3)2); 13C-NMR (CDCl3) δ 162.77, 161.35, 159.35, 156.17, 146.35, 143.45, 139.66, 136.44, 135.32, 134.48, 133.91, 133.26, 132.44, 108.30, 95.82, 60.13, 54.33, 23.17; MS (ESI) m/z calcd. for C21H19IN7O2S [M + H]+ 560.0, found 560.2.
3-((4-Amino-3-iodo-1H-pyrazolo[3,4-d]pyrimidin-1-yl)methyl)-2-(2,6-dimethylphenyl)-7-fluoro-2H-benzo-[e][1,2,4]thiadiazine 1,1-dioxide (14)
Following the procedures described for the synthesis of 13, compound 14 were obtained as a white solid (1.6 g) in 58% yield, m.p. 243–244 °C; 1H-NMR (DMSO-d6) δ 8.08 (s, 1H, Ar-H), 7.92 (dd, J = 11.4, 4.2 Hz, 1H, Ar-H), 7.68 (td, J = 13.2, 4.2 Hz, 1H, Ar-H), 7.54 (dd, J = 13.2, 7.2 Hz, 1H, Ar-H), 7.26 (dd, J = 13.2, 10.2 Hz, 1H, Ar-H), 7.17 (d, J = 11.4 Hz, 2H, Ar-H), 5.13 (s, 2H, NCH2), 2.06 (s, 6H, 2,6-(CH3)2); 13C-NMR (DMSO-d6) δ 160.89 (d, JC–F = 250.7 Hz), 158.02, 156.61, 154.57, 150.89, 138.69, 131.55, 131.46 (d, JC–F = 7.6 Hz), 130.64, 129.76, 128.54 (d, JC–F = 7.6 Hz), 122.71 (d, JC–F = 22.7 Hz), 108.28 (d, JC–F = 25.7 Hz), 103.55, 91.13, 79.59, 49.55, 18.40; MS (ESI) m/z calcd. for C21H18FIN7O2S [M + H]+ 578.0, found 578.0.
3-((4-Amino-3-(1H-indol-5-yl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl)methyl)-2-(2,6-dimethylphenyl)-2H-benzo[e][1,2,4]thiadiazine 1,1-dioxide (15a)
To a solution of 13 (180 mg, 0.30 mmol) in dioxane (4 mL) and distilled water (1.5 mL) was added 1H-indol-5-ylboronic acid (88 mg, 0.55 mmol), sodium carbonate anhydrous (103 mg, 0.97 mmol) and Pd(PPh3)4 (12 mg, 0.03 mmol). The mixture was degassed with N2, and refluxed for 4 h. After cooling to room temperature, EtOAc (50 mL) and distilled water (10 mL) were added, and the organic layer was washed brine, dried over Na2SO4. After filtration and evaporation, the residue was purified by silica gel chromatography (EtOAc/hexane = 1:1) to give 15a (161 mg, 91%) as a white solid, m.p. 216–217 °C; 1H-NMR (CDCl3) δ 11.32 (s, 1H, NH), 8.09 (s, 1H, Ar-H), 7.95 (dd, J = 7.8, 1.2 Hz, 1H, Ar-H), 7.81 (td, J = 7.8, 1.2 Hz, 2H, Ar-H), 7.65 (td, J = 7.8, 0.6 Hz, 1H, Ar-H), 7.57 (d, J = 8.4 Hz, 1H, Ar-H), 7.52 (d, J = 7.8 Hz, 1H, Ar-H), 7.45 (t, J = 3.0 Hz, 1H, Ar-H), 7.38 (dd, J = 8.4, 1.8 Hz, 1H, Ar-H), 7.25 (t, J = 7.8 Hz, 1H, Ar-H), 7.15 (d, J = 7.8 Hz, 2H, Ar-H), 6.55 (t, J = 8.4, 1.8 Hz, 1H, Ar-H), 5.20 (s, 2H, NCH2), 2.04 (s, 6H, 2,6-(CH3)2); 13C-NMR (CDCl3) δ 158.53, 156.22, 155.43, 152.04, 146.79, 141.79, 138.70, 136.53, 134.86, 131.96, 130.48, 129.70, 129.09, 128.51, 128.47, 127.83, 127.04, 123.90, 121.76, 121.20, 120.52, 112.68, 102.22, 97.86, 49.61, 18.35; HRMS (ESI) m/z calcd. for C29H25N8O2S [M + H]+ 549.1816, found 549.1829.
3-((4-Amino-3-(3,4-dimethoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl)methyl)-2-(2,6-dimethylphenyl)-2H-benzo[e][1,2,4]thiadiazine 1,1-dioxide (15b)
According to the procedures described for the synthesis of 15a, compound 15b were obtained as a white solid (61 mg) in 75% yield, m.p. 220–222 °C; 1H-NMR (DMSO-d6) δ 8.31 (s, 1H, NH), 8.09 (s, 1H, Ar-H), 7.94 (dd, J = 7.8, 1.2 Hz, 1H, Ar-H), 7.81 (td, J = 7.8, 1.2 Hz, 1H, Ar-H), 7.65 (td, J = 7.2, 1.2 Hz, 1H, Ar-H), 7.50 (d, J = 8.4 Hz, 1H, Ar-H), 7.26 (t, J = 7.8 Hz, 1H, Ar-H), 7.15–7.19 (m, 4H, Ar-H), 7.12 (d, J = 8.4 Hz, 1H, Ar-H), 5.18 (s, 2H, NCH2), 3.81 (s, 3H, OCH3), 3.80 (s, 3H, OCH3), 2.02 (s, 6H, 2,6-(CH3)2); 13C-NMR (DMSO-d6) δ 158.50, 156.26, 155.50, 151.98, 149.83, 149.51, 145.20, 141.74, 138.71, 134.86, 131.98, 130.50, 129.70, 129.13, 128.47, 127.84, 125.53, 121.19, 121.03, 112.71, 111.99, 97.70, 79.63, 56.04, 55.89, 49.64, 18.32; HRMS (ESI) m/z calcd. for C29H28N7O4S [M + H]+ 570.1918, found 570.1921.
3-((4-Amino-3-(3-fluoro-4-methoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl)methyl)-2-(2,6-dimethyl-phenyl)-2H-benzo[e][1,2,4]thiadiazine 1,1-dioxide (15c)
According to the procedures described for the synthesis of 15a, compound 15c were obtained as a white solid (59 mg) in 66% yield, m.p. 226–227 °C; 1H-NMR (DMSO-d6) δ 8.31 (s, 1H, NH), 8.10 (s, 1H, Ar-H), 7.94 (d, J = 7.8 Hz, 1H, Ar-H), 7.81 (t, J = 7.8 Hz, 1H, Ar-H), 7.65 (t, J = 7.8 Hz, 1H, Ar-H), 7.49 (d, J = 8.4 Hz, 1H, Ar-H), 7.43–7.38 (m, 2H, Ar-H), 7.33 (t, J = 8.4 Hz, 1H, Ar-H), 7.25 (t, J = 7.8 Hz, 1H, Ar-H), 7.15 (d, J = 7.8 Hz, 2H, Ar-H), 5.18 (s, 2H, NCH2), 3.90 (s, 3H, OCH3), 2.02 (s, 6H, 2,6-(CH3)2); 13C-NMR (DMSO-d6) δ 158.50, 156.33, 155.58, 152.84, 152.03 (d, JC–F = 244.6 Hz), 151.85, 148.13 (d, JC–F = 9.1 Hz), 144.02, 141.72, 138.69, 134.86, 131.93, 130.51, 129.71, 128.82 (d, JC–F = 95.1 Hz), 127.82, 125.78 (d, JC–F = 6.0 Hz), 125.21, 121.20, 116.11 (d, JC–F = 19.6 Hz), 114.91, 97.67, 79.65, 56.57, 49.64, 18.32; HRMS (ESI) m/z calcd. for C28H25FN7O3S [M + H]+ 558.1718, found 558.1733.
3-((4-Amino-3-(6-methoxypyridin-3-yl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl)methyl)-2-(2,6-dimethylphenyl)-2H-benzo[e][1,2,4]thiadiazine 1,1-dioxide (15d)
According to the procedures described for the synthesis of 15a, compound 15d were obtained as a white solid (41 mg) in 47% yield, m.p. 248–249 °C; 1H-NMR (DMSO-d6) δ 8.39 (d, J = 1.8 Hz, 1H, Ar-H), 8.31 (s, 1H, NH), 8.11 (s, 1H, Ar-H), 7.94 (dd, J = 7.8, 1.2 Hz, 1H, Ar-H), 7.92 (dd, J = 8.4, 2.4 Hz, 1H, Ar-H), 7.81 (td, J = 8.4, 1.2 Hz, 1H, Ar-H), 7.65 (td, J =7.8, 1.2 Hz, 1H, Ar-H), 7.49 (d, J = 7.8 Hz, 1H, Ar-H), 7.26 (t, J = 7.8 Hz, 1H, Ar-H), 7.16 (d, J = 7.8 Hz, 2H, Ar-H), 6.98 (d, J = 8.4 Hz, 1H, Ar-H), 5.20 (s, 2H, NCH2), 3.92 (s, 3H, OCH3), 2.03 (s, 6H, 2,6-(CH3)2); 13C-NMR (DMSO-d6) δ 164.14, 158.60, 156.38, 155.61, 151.81, 146.69, 142.36, 141.71, 139.29, 138.69, 134.87, 131.91, 130.51, 129.70, 129.13, 128.50, 127.80, 122.70, 121.19, 111.48, 97.92, 79.64, 53.89, 49.66, 18.33; HRMS (ESI) m/z calcd. for C27H25N8O4S [M + H]+ 541.1765, found 541.1781.
3-((4-Amino-3-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl)methyl)-2-(2,6-dimethylphenyl)-2H-benzo[e][1,2,4]thiadiazine 1,1-dioxide (15e)
According to the procedures described for the synthesis of 15a, compound 15e were obtained as a white solid (71 mg) in 64% yield, m.p. 225–226 °C; 1H-NMR (DMSO-d6) δ 8.31 (s, 1H, NH), 8.08 (s, 1H, Ar-H), 7.94 (dd, J = 7.8, 1.2 Hz, 1H, Ar-H), 7.82 (td, J = 7.8, 1.2 Hz, 1H, Ar-H), 7.65 (td, J = 7.8, 1.2 Hz, 1H, Ar-H), 7.51 (d, J = 8.4 Hz, 1H, Ar-H), 7.24 (t, J = 8.4 Hz, 1H, Ar-H), 7.14 (d, J = 7.8 Hz, 2H, Ar-H), 7.11–7.09 (m, 2H, Ar-H), 7.02 (d, J = 7.8 Hz, 1H, Ar-H), 5.18 (s, 2H, NCH2), 4.30 (s, 4H, OCH2CH2O), 2.01 (s, 6H, 2,6-(CH3)2); 13C-NMR (DMSO-d6) δ 158.45, 156.25, 155.47, 151.92, 144.79, 144.60, 144.23, 141.74, 138.66, 134.86, 131.94, 130.47, 129.68, 129.12, 128.48, 127.83, 126.18, 121.59, 121.19, 118.30, 117.23, 97.63, 79.64, 64.67, 64.59, 49.62, 18.30; HRMS (ESI) m/z calcd. for C29H26N7O4S [M + H]+ 568.1761, found 568.1775.
3-((4-Amino-3-(benzo[d][1,3]dioxol-5-yl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl)methyl)-2-(2,6-dimethyl-phenyl)-2H-benzo[e][1,2,4]thiadiazine 1,1-dioxide (15f)
According to the procedures described for the synthesis of 15a, compound 15f were obtained as a white solid (29 mg) in 34% yield, m.p. > 250 °C; 1H-NMR (DMSO-d6) δ 8.31 (s, 1H, NH), 8.08 (s, 1H, Ar-H), 7.94 (dd, J = 7.8, 1.2 Hz, 1H, Ar-H), 7.82 (td, J = 7.8, 1.2 Hz, 1H, Ar-H), 7.65 (td, J = 7.8, 1.2 Hz, 1H, Ar-H), 7.50 (d, J = 7.8 Hz, 1H, Ar-H), 7.25 (t, J = 7.8 Hz, 1H, Ar-H), 7.15 (d, J = 7.2 Hz, 2H, Ar-H), 7.12–7.09 (m, 2H, Ar-H), 7.07 (d, J = 8.4, 1H, Ar-H), 6.10 (s, 2H, OCH2O), 5.17 (s, 2H, NCH2), 2.01 (s, 6H, 2,6-(CH3)2); 13C-NMR (DMSO-d6) δ 158.44, 156.28, 155.47, 151.89, 148.36, 144.98, 141.73, 138.67, 134.87, 131.93, 130.49, 129.70, 129.14, 128.50, 127.82, 126.85, 122.65, 121.19, 109.44, 108.80, 101.89, 97.65, 79.64, 49.62, 18.30; HRMS (ESI) m/z calcd. for C28H24N7O4S [M + H]+ 554.1605, found 554.1614.
3-((4-Amino-3-(4-methoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl)methyl)-2-(2,6-dimethylphenyl)-2H-benzo[e][1,2,4]thiadiazine 1,1-dioxide (15g)
According to the procedures described for the synthesis of 15a, compound 15g were obtained as a white solid (45 mg) in 74% yield, m.p. 248–249 °C; 1H-NMR (DMSO-d6) δ 8.32 (s, 1H, NH), 8.09 (s, 1H, Ar-H), 7.94 (dd, J = 7.8, 1.2 Hz, 1H, Ar-H), 7.82 (td, J = 7.8, 1.2 Hz, 1H, Ar-H), 7.65 (td, J = 7.8, 1.2 Hz, 1H, Ar-H), 7.57 (d, J = 8.4 Hz, 2H, Ar-H), 7.50 (d, J = 8.4 Hz, 1H, Ar-H), 7.25 (t, J = 7.8 Hz, 1H, Ar-H), 7.15 (d, J = 7.8 Hz, 2H, Ar-H), 7.11 (d, J = 8.4 Hz, 2H, Ar-H), 5.19 (s, 2H, NCH2), 3.82 (s, 3H, OCH3), 2.02 (s, 6H, 2,6-(CH3)2); 13C-NMR (DMSO-d6) δ160.18, 158.51, 156.26, 155.51, 151.92, 145.03, 141.75, 138.69, 134.85, 131.93, 130.48, 129.95, 129.69, 129.10, 128.49, 127.82, 125.43, 121.19, 115.12, 97.70, 79.63, 55.72, 49.60, 18.34; HRMS (ESI) m/z calcd. for C28H26N7O3S [M + H]+ 540.1812, found 540.1829.
3-((4-Amino-3-(3-methoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl)methyl)-2-(2,6-dimethylphenyl)-2H-benzo[e][1,2,4]thiadiazine 1,1-dioxide (15h)
According to the procedures described for the synthesis of 15a, compound 15h were obtained as a white solid (46 mg) in 59% yield, m.p. 221–222 °C; 1H-NMR (DMSO-d6) δ 8.31 (s, 1H), 8.10 (s, 1H, Ar-H), 7.94 (dd, J = 7.8, 1.2 Hz, 1H, Ar-H), 7.82 (td, J = 7.8, 1.2 Hz, 1H, Ar-H), 7.63 (td, J = 7.8, 1.2 Hz, 1H, Ar-H), 7.50 (d, J = 7.8 Hz, 1H, Ar-H), 7.47 (t, J = 7.8 Hz, 1H, Ar-H), 7.26 (t, J = 7.8 Hz, 1H, Ar-H), 7.23 (d, J = 7.8 Hz, 1H, Ar-H), 7.20–7.11 (m, 3H, Ar-H), 7.06 (dd, J = 8.4, 2.4 Hz, 1H, Ar-H), 5.21 (s, 2H, NCH2), 3.82 (s, 3H, OCH3), 2.02 (s, 6H, 2,6-(CH3)2); 13C-NMR (DMSO-d6) δ 160.11, 158.43, 156.31, 155.56, 151.88, 145.02, 141.72, 138.70, 134.87, 134.36, 131.95, 130.86, 130.51, 129.71, 129.14, 128.48, 127.83, 121.20, 120.76, 115.19, 113.85, 97.74, 79.64, 55.63, 49.68, 18.32; HRMS (ESI) m/z calcd. for C28H26N7O3S [M + H]+ 540.1812, found 540.1825.
3-((4-Amino-3-(4-(trifluoromethoxy)phenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl)methyl)-2-(2,6-dimethyl-phenyl)-2H-benzo[e][1,2,4]thiadiazine 1,1-dioxide (15i)
According to the procedures described for the synthesis of 15a, compound 15i were obtained as a white solid (61 mg) in 61% yield, m.p. 206–207 °C; 1H-NMR (DMSO-d6) δ 8.12 (s, 1H, Ar-H), 7.94 (dd, J = 8.4, 1.2 Hz, 1H, Ar-H), 7.81 (dt, J = 8.4, 1.2 Hz, 1H, Ar-H), 7.76 (d, J =8.4 Hz, 2H, Ar-H), 7.65 (dt, J = 8.4, 0.6 Hz, 1H, Ar-H), 7.52 (d, J = 7.8 Hz, 2H, Ar-H), 7.48 (d, J = 7.8 Hz, 1H, Ar-H), 7.26 (t, J = 7.8 Hz, 1H, Ar-H), 7.16 (d, J = 7.8 Hz, 2 H, Ar-H), 5.21 (s, 2H), 2.03 (s, 6H); 13C-NMR (DMSO-d6) δ 158.52, 156.38, 155.72, 151.77, 149.08, 143.91, 141.70, 138.70, 134.87, 132.28, 131.90, 130.59, 130.53, 129.72, 129.14, 128.50, 127.80, 122.10, 121.21, 120.57 (q, JC–F = 256.70 Hz), 97.73, 49.68, 18.34; HRMS (ESI) m/z calcd. for C28H23F3N7O3S [M + H]+ 594.1530, found 594.1547.
3-((4-Amino-3-phenyl-1H-pyrazolo[3,4-d]pyrimidin-1-yl)methyl)-2-(2,6-dimethylphenyl)-2H-benzo[e]-[1,2,4]thiadiazine 1,1-dioxide (15j)
According to the procedures described for the synthesis of 15a, compound 15j were obtained as a white solid (40 mg) in 73% yield, m.p. 193–194 °C; 1H-NMR (DMSO-d6) δ 8.30 (s, 1H, NH), 8.09 (s, 1H, Ar-H), 7.92 (dd, J = 7.8, 1.2 Hz, 1H, Ar-H), 7.80 (td, J = 7.8, 1.2 Hz, 1H, Ar-H), 7.66–7.62 (m, 3H, Ar-H), 7.54 (t, J = 7.8 Hz, 2H, Ar-H), 7.52–7.32 (m, 2H, Ar-H), 7.24 (t, J = 7.8 Hz, 2H, Ar-H), 7.14 (d, J = 7.8 Hz, 2H, Ar-H), 5.19 (s, 2H, NCH2), 2.01 (s, 6H, 2,6-(CH3)2); 13C-NMR (DMSO-d6) δ 1158.46, 156.31, 155.60, 151.86, 145.16, 141.72, 138.69, 134.54, 133.53, 132.51, 131.99, 131.92, 130.51, 130.47, 129.71, 129.67, 129.27, 129.20, 128.60, 127.81, 121.20, 97.73, 79.65, 49.65, 18.34; HRMS (ESI) m/z calcd. for C27H24N7O2S [M + H]+ 510.1707, found 510.1722.
3-((4-Amino-3-(1H-indol-5-yl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl)methyl)-2-(2,6-dimethylphenyl)-7-fluoro-2H-benzo[e][1,2,4]thiadiazine 1,1-dioxide (16a)
According to the procedures described for the synthesis of 15a, compound 16a were obtained as a white solid (65 mg) in 73% yield, m.p. 132–133 °C; 1H-NMR (DMSO-d6) δ 8.30 (s, 1H, NH), 8.08 (s, 1H, Ar-H), 7.91 (dd, J = 7.2, 2.4 Hz, 1H, Ar-H), 7.81 (brs, 1H, Ar-H), 7.69 (td, J = 9.6, 3.0 Hz, 1H, Ar-H), 7.61 (dd, J = 9.0, 4.8 Hz, 1H, Ar-H), 7.56 (d, J = 8.4 Hz, 1H, Ar-H), 7.44 (t, J = 3.0 Hz, 1H, Ar-H), 7.36 (dd, J = 8.4, 1.8 Hz, 1H, Ar-H), 7.25 (t, J = 7.28Hz, 1H, Ar-H), 7.16 (t, J = 7.8 Hz, 2H, Ar-H), 6.54 (s, 1H, 3-CH-indolyl), 5.20 (s, 2H, NCH2), 2.01 (s, 6H, 2,6-(CH3)2); 13C-NMR (DMSO-d6) δ 160.08 (d, JC–F = 250.7 Hz), 158.52, 156.23, 155.41, 151.51, 146.84, 138.68, 138.61 (d, JC–F = 3.0 Hz), 136.53, 131.81, 131.44 (d, JC–F = 9.1 Hz), 130.55, 129.74, 128.66 (d, JC–F = 9.1 Hz), 128.46, 127.05, 123.87, 122.67 (d, JC–F = 24.2 Hz), 121.74, 120.52, 112.67, 108.19 (d, JC–F = 27.2 Hz), 102.21, 97.85, 79.65, 49.57, 18.32; HRMS (ESI) m/z calcd. for C29H24FN8O2S [M + H]+ 567.1721, found 567.1739.
3-((A-amino-3-(3,4-dimethoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl)methyl)-2-(2,6-dimethylphenyl)-7-fluoro-2H-benzo[e][1,2,4]thiadiazine 1,1-dioxide (16b)
According to the procedures described for the synthesis of 15a, compound 16b were obtained as a white solid (47 mg) in 51% yield, m.p. 229–230 °C; 1H-NMR (DMSO-d6) δ 8.31 (s, 1H, NH), 8.09 (s, 1H, Ar-H), 7.91 (dd, J = 7.2, 2.4 Hz, 1H, Ar-H), 7.69 (td, J = 9.0, 2.4 Hz, 1H, Ar-H), 7.59 (dd, J = 9.0, 4.8 Hz, 1H, Ar-H), 7.26 (t, J = 7.8 Hz, 1H, Ar-H), 7.21–7.10 (m, 5H, Ar-H), 5.19 (s, 2H, NCH2), 3.82 (s, 3H, OCH3), 3.81 (s, 3H, OCH3), 2.01 (s, 6H, 2,6-(CH3)2); 13C-NMR (DMSO-d6) δ 160.78 (d, JC–F = 250.7 Hz), 158.51, 156.27, 155.49, 151.46, 149.84, 149.52, 145.24, 138.70, 138.57 (d, JC–F = 3.0 Hz), 131.84, 131.41 (d, JC–F = 7.6 Hz), 130.57, 129.74, 128.69 (d, JC–F = 7.6 Hz), 125.52, 122.66 (d, JC–F = 24.2 Hz), 121.03, 112.72, 112.02, 108.20 (d, JC–F = 27.2 Hz), 97.71, 79.65, 56.04, 55.89, 49.60, 18.31; HRMS (ESI) m/z calcd. for C29H27FN7O4S [M + H]+ 588.1824, found 588.1838.
3-((4-Amino-3-(3-fluoro-4-methoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl)methyl)-2-(2,6-dimethyl-phenyl)-7-fluoro-2H-benzo[e][1,2,4]thiadiazine 1,1-dioxide (16c)
According to the procedures described for the synthesis of 15a, compound 16c were obtained as a white solid (76 mg) in 84% yield, m.p. 201–202 °C; 1H-NMR (DMSO-d6) δ 8.31 (s, 1H, NH), 8.09 (s, 1H, Ar-H), 7.91 (dd, J = 7.2, 3.0 Hz, 1H, Ar-H), 7.69 (td, J = 7.4, 2.4 Hz, 1H, Ar-H), 7.59 (dd, J = 9.0, 4.2 Hz, 1H, Ar-H), 7.44–7.38 (m, 2H, Ar-H), 7.33 (t, J = 9.0 Hz, 1H, Ar-H), 7.26 (t, J = 7.2 Hz, 1H, Ar-H), 7.15 (d, J = 7.8 Hz, 2H, Ar-H), 5.19 (s, 2H, NCH2), 3.90 (s, 3H, OCH3), 2.01 (s, 6H, 2,6-(CH3)2); 13C-NMR (DMSO-d6) δ 160.89 (d, JC–F = 250.7 Hz), 158.50, 156.34, 155.56, 152.02 (d, JC–F = 246.1 Hz), 151.32, 148.14 (d, JC–F = 10.6 Hz), 144.06, 138.68, 138.62 (d, JC–F = 19.6 Hz), 131.78, 131.44 (d, JC–F = 7.6 Hz), 130.59, 129.74, 128.66 (d, JC–F = 7.6 Hz), 125.76 (d, JC–F = 7.6 Hz), 125.21, 122.68 (d, JC–F = 22.7 Hz), 116.11 (d, JC–F = 19.6 Hz), 114.92, 108.21 (d, JC–F = 25.7 Hz), 97.67, 79.63, 56.59, 49.61, 18.30; HRMS (ESI) m/z calcd. for C28H24F2N7O3S [M + H]+ 576.1624, found 576.1637.
3-((4-Amino-3-(6-methoxypyridin-3-yl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl)methyl)-2-(2,6-dimethylphenyl)-7-fluoro-2H-benzo[e][1,2,4]thiadiazine 1,1-dioxide (16d)
According to the procedures described for the synthesis of 15a, compound 16d were obtained as a white solid (51 mg) in 58% yield, m.p. 225–226 °C; 1H-NMR (DMSO-d6) δ 8.38 (s, 1H, NH), 8.31 (s, 1H, Ar-H), 8.10 (s, 1H, Ar-H), 7.90 (d, J = 8.4 Hz, 2H, Ar-H), 7.69 (t, J = 7.2 Hz, 1H, Ar-H), 7.59 (dd, J = 9.0, 4.2 Hz, 1H, Ar-H), 7.25 (t, J = 7.2 Hz, 1H, Ar-H), 7.15 (d, J = 8.4 Hz, 2H, Ar-H), 6.97 (d, J = 8.4 Hz, 1H, Ar-H), 5.20 (s, 2H, NCH2), 3.92 (s, 3H, OCH3), 2.01 (s, 6H, 2,6-(CH3)2); 13C-NMR (DMSO-d6) δ 164.15, 160.89 (d, JC–F = 250.7 Hz), 158.60, 156.39, 155.59, 151.29, 146.69, 142.41, 139.29, 138.69, 138.54 (d, JC–F = 3.0 Hz), 131.77, 131.45 (d, JC–F = 7.6 Hz), 130.59, 129.74, 128.66 (d, JC–F = 9.1 Hz), 122.75, 122.68, 122.67 (d, JC–F = 22.7 Hz), 111.48, 108.20 (d, JC–F = 27.2 Hz), 97.93, 79.64, 53.89, 49.63, 18.32; HRMS (ESI) m/z calcd. for C27H24FN8O3S [M + H]+ 559.1671, found 559.1678.
3.2. PI3K Kinase Assay
The ADP-Glo luminescent assay was used for PI3Kδ, PI3Kβ and PI3Kγ isoforms and the kinase-Glo luminescent assay was used for PI3Kα according to the standard protocols of Promega [40]. PI-103 was used as a positive control. The compounds were tested from 1 μM or 10 μM, 3-fold dilution, in duplicate for 10 concentrations. The kinase reaction was done in 384-well plate (Corning, Los Altos, MA, USA). Each well was loaded with test compounds (in 100% DMSO) and reaction buffer containing PI substrate. After the PI3K proteins were then added, the reaction was started by the addition of PIP2 and ATP prepared in the reaction buffer and ran for either 60 (for PI3Kα, PI3Kβ, and PI3Kγ) or 120 min (for PI3Kδ). ADP-Glo reagent was then added to terminate the reavtion. The plates were then read in a Synergy 2 reader (BioTek, Shanghai, China) for luminescence detection.
3.3. Cell Proliferation Assay
Cell proliferation was evaluated by The CellTiter-Glo luminescent cell viability assay (Promega, Shanghai, China) was used to evaluate the inhibitory activity of compounds 15a and 15b following the manufacturer’s protocol. In brief, SU-DHL-6 (ATCC) cells were seeded in 96-well plates (Corning, Los Altos, MA, USA) at density of 1 × 104 cells per well, and incubated with medium alone or with the tested compounds at the indicated concentrations (50 μM in DMSO, 3-fold dilution, in duplicate for 10 concentrations). 50 μL CellTiter-Glo (Promega, Shanghai, China) reagent was added to each well to induce cell lysis, and the plate was incubated at room temperature for 10 min to stabilize luminescent signal. After 100 μL of the mixture from each well was transferred to a new 96-well black plate, the fluorescence signal was read on EnVision (Shanghai, China) and the data were analysized by XLFit 4 software (IDBS, Berlin, Germany).
3.4. Molecular Docking
X-ray cocrystal structure of PI3Kδ enzyme was downloaded from RCSB Protein Date Bank (PDB ID: 2WXH) [42]. The molecular docking of 15a and 15b was carried out following the same procedures as reported for compounds 3 and 4 [33].
4. Conclusions
In a continuous study to find more potent and selective PI3Kδ inhibitors based on the rotation-limiting strategy, we substituted the carbonyl in the quinazolinone core for the sulfonyl group, designed and synthesized a series of novel 2H-benzo[e][1,2,4]thiadiazine 1,1-dioxide derivatives 15a–J and 16a–d. In agreement with the quinazolinone derivatives, the introduction of a 5-indolyl or 3,4-dimethoxyphenyl at the affinity pocket generated the most potent analogues 15a and 15b with the IC50 values of 217 to 266 nM, respectively. In comparison with the quinazolinone lead compounds 3 and 4, the 2H-benzo[e][1,2,4]thiadiazine 1,1-dioxide derivatives exhibited much reduced PI3Kδ inhibitory activity, but maintained high selectivity over other PI3K isoforms. Unlike the quinazolinone lead compound 4 that was a dual PI3Kδ/γ inhibitor, the 2H-benzo[e][1,2,4]thiadiazine 1,1-dioxide 15b was more than 21-fold selective over PI3Kγ. This may provide a structural base for the further design of more potent and selective PI3Kδ inhibitors. In agreement with their high PI3Kδ inhibitory activity, 15a and 15b exhibited high antiproliferative potency against B-cell leukemia SU-DHL-6 cells.
Abbreviations
| PI3Ks | Phosphoinositide 3-kinases |
| PIP2 | Phosphatidylinositol 4,5-bisphosphate |
| NBS | N-Bromosuccinimide |
| ADP | Adenosine diphosphate |
| ATP | Adenosine triphosphate |
| DMSO | Dimethyl sulfoxide |
| APDS | PI3Kδ syndrome |
| FL | Follicular lymphoma |
| SLL | Small lymphocytic lymphoma |
| CLL | Chronic lymphocytic leukemia |
Supplementary Materials
Supplementary materials are available online.
Author Contributions
Conceptualization, Y.-P.G. and Z.-P.L.; methodology, Y.-P.G., L.-Q.T. and T.-S.L.; validation, Y.-P.G., L.-Q.T. and T.-S.L.; formal analysis, L.-Q.T. and Y.-P.G.; investigation, Y.-P.G., L.-Q.T. and T.-S.L.; resources, Z.-P.L.; data curation, L.-Q.T. and Y.-P.G.; writing—original draft preparation, L.-Q.T.; writing—review and editing, Z.-P.L.; visualization, Y.-P.G. and L.-Q.T.; supervision, Z.-P.L.; project administration, Z.-P.L.; funding acquisition, Z.-P.L.
Funding
This work was partially supported by the key research and development program of Shandong province (2017CXGC1401).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 15a–j and 16a–d are available from the authors.
References
- 1.Toker A., Cantley L.C. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature. 1997;387:673–676. doi: 10.1038/42648. [DOI] [PubMed] [Google Scholar]
- 2.Cantley L.C. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 3.Brachmann S.M., Yballe C.M., Innocenti M., Deane J.A., Fruman D.A., Thomas S.M., Cantley L.C. Role of phosphoinositide 3-kinase regulatory isoforms in development and actin rearrangement. Mol. Cell. Biol. 2005;25:2593–2606. doi: 10.1128/MCB.25.7.2593-2606.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vanhaesebroeck B., Guillermet-Guibert J., Graupera M., Bilanges B. The emerging mechanisms of isoform specific PI3K signaling. Nat. Rev. Mol. Cell Biol. 2010;11:329–341. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- 5.Engelman J.A., Luo J., Cantley L.C. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 2006;7:606−619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 6.Jimenez C., Hernandez C., Pimentel B., Carrera A.C. The p85 regulatory subunit controls sequential activation of phosphoinositide 3-kinase by tyr kinases and ras. J. Biol. Chem. 2002;277:41556–41562. doi: 10.1074/jbc.M205893200. [DOI] [PubMed] [Google Scholar]
- 7.Brock C., Schaefer M., Reusch H.P., Czupalla C., Michalke M., Spicher K., Schultz G., Nurnberg B. Roles of Gβγ in membrane recruitment and activation of p110γ/p101 phosphoinositide 3-kinase γ. J. Cell Biol. 2003;160:89–99. doi: 10.1083/jcb.200210115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okkenhaug K., Vanhaesebroeck B. PI3K in lymphocyte development, differentiation and activation. Nat. Rev. Immunol. 2003;3:317–330. doi: 10.1038/nri1056. [DOI] [PubMed] [Google Scholar]
- 9.Liu L., Puri K.D., Penninger J.M., Kubes P. Leukocyte PI3Kγ and PI3Kδ have temporally distinct roles for leukocyte recruitment in vivo. Blood. 2007;110:1191–1198. doi: 10.1182/blood-2006-11-060103. [DOI] [PubMed] [Google Scholar]
- 10.Thomas M.J., Smith A., Head D.H., Milne L., Nicholls A., Pearce W., Vanhaesebroeck B., Wymann M.P., Hirsch E., Trifilieff A., et al. Airway inflammation: Chemokine-induced neutrophilia and the class I phosphoinositide 3-kinases. Eur. J. Immunol. 2005;35:1283–1291. doi: 10.1002/eji.200425634. [DOI] [PubMed] [Google Scholar]
- 11.Fruman D.A., Rommel C. PI3K and cancer: Lessons, challenges and opportunities. Nat. Rev. Drug Discov. 2014;13:140–156. doi: 10.1038/nrd4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thorpe L.M., Yuzugullu H., Zhao J.J. PI3K in cancer: Divergent roles of isoforms, modes of activation and therapeutic targeting. Nat. Rev. Cancer. 2015;15:7–24. doi: 10.1038/nrc3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janku F. Phosphoinositide 3-kinase (PI3K) pathway inhibitors in solid tumors: From laboratory to patients. Cancer Treat. Rev. 2017;59:93–101. doi: 10.1016/j.ctrv.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Fruman D.A., Chiu H., Hopkins B.D., Bagrodia S., Cantley L.C., Abraham R.T. The PI3K pathway in human disease. Cell. 2017;170:605–635. doi: 10.1016/j.cell.2017.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao W., Qiu Y., Kong D. Class I phosphatidylinositol 3-kinase inhibitors for cancer therapy. Acta Pharm. Sin. B. 2017;7:27–37. doi: 10.1016/j.apsb.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garces A.E., Stocks M.J. Class 1 PI3K clinical candidates and recent inhibitor design strategies: A medicinal chemistry perspective. J. Med. Chem. 2019;62:4815–4850. doi: 10.1021/acs.jmedchem.8b01492. [DOI] [PubMed] [Google Scholar]
- 17.Knight Z.A., Gonzalez B., Feldman M.E., Zunder E.R., Goldenberg D.D., Williams O., Loewith R., Stokoe D., Balla A., Toth B., et al. A pharmacological map of the PI3-K family defines a role for P110α in insulin signaling. Cell. 2006;125:733–747. doi: 10.1016/j.cell.2006.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang X., Liu G., Guo J., Su Z. The PI3K/AKT Pathway in obesity and type 2 diabetes. Int. J. Biol. Sci. 2018;14:1483–1496. doi: 10.7150/ijbs.27173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson S.P., Schoenwaelder S.M., Goncalves I., Nesbitt W.S., Yap C.L., Wright C.E., Kenche V., Anderson K.E., Dopheide S.M., Yuan Y., et al. PI 3-Kinase P110β: A new target for antithrombotic therapy. Nat. Med. 2005;11:507–514. doi: 10.1038/nm1232. [DOI] [PubMed] [Google Scholar]
- 20.Jackson S.P., Schoenwaelder S.M. Antithrombotic phosphoinositide 3-kinase β inhibitors in humans: A ‘shear’ delight! J. Thromb. Haemostasis. 2012;10:2123–2126. doi: 10.1111/j.1538-7836.2012.04912.x. [DOI] [PubMed] [Google Scholar]
- 21.Cushing T.D., Metz D.P., Whittington D.A., McGee L.R. PI3Kδ and PI3Kγ as targets for autoimmune and inflammatory diseases. J. Med. Chem. 2012;55:8559–8581. doi: 10.1021/jm300847w. [DOI] [PubMed] [Google Scholar]
- 22.Rowan W.C., Smith J.L., Affleck K., Amour A. Targeting phosphoinositide 3-kinase δ for allergic asthma. Biochem. Soc. Trans. 2012;40:240–245. doi: 10.1042/BST20110665. [DOI] [PubMed] [Google Scholar]
- 23.Yoo E.J., Ojiaku C.A., Sunder K., Panettieri R.A. Phosphoinositide 3-kinase in asthma: Novel roles and therapeutic approaches. Am. J. Respir. Cell Mol. Biol. 2017;56:700–707. doi: 10.1165/rcmb.2016-0308TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perry M.W.D., Abdulai R., Mogemark M., Petersen J., Thomas M.J., Valastro B., Eriksson A.W. Evolution of PI3Kγ and δ Inhibitors for inflammatory and autoimmune diseases. J. Med. Chem. 2019;62:4783–4814. doi: 10.1021/acs.jmedchem.8b01298. [DOI] [PubMed] [Google Scholar]
- 25.Kracker S., Curtis J., Ibrahim M.A.A., Sediva A., Salisbury J., Campr V., Debré M., Edgar J.D.M., Imai K., Picard C., et al. Occurrence of B-cell lymphomas in patients with activated phosphoinositide 3-kinase δ syndrome. J. Allergy Clin. Immunol. 2014;134:233–236. doi: 10.1016/j.jaci.2014.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elkaim E., Neven B., Bruneau J., Mitsui-Sekinaka K., Stanislas A., Heurtier L., Lucas C.L., Matthews H., Deau M.-C., Sharapova S., et al. Clinical and immunologic phenotype associated with activated phosphoinositide 3-kinase δ syndrome 2: A cohort study. J. Allergy Clin. Immunol. 2016;138:210–218. doi: 10.1016/j.jaci.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 27.Coulter T.I., Chandra A., Bacon C.M., Babar J., Curtis J., Screaton N., Goodlad J.R., Farmer G., Steele C.L., Leahy T.R., et al. Clinical spectrum and features of activated phosphoinositide 3-kinase δ syndrome: A large patient cohort study. J. Allergy Clin. Immunol. 2017;139:597–606. doi: 10.1016/j.jaci.2016.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conte E., Gili E., Fruciano M., Korfei M., Fagone E., Iemmolo M., Lo Furno D., Giuffrida R., Crimi N., Guenther A., et al. PI3K P110γ overexpression in idiopathic pulmonary fibrosis lung tissue and fibroblast cells: In vitro effects of its inhibition. Lab. Investig. 2013;93:566–576. doi: 10.1038/labinvest.2013.6. [DOI] [PubMed] [Google Scholar]
- 29.Furman R.R., Sharman J.P., Coutre S.E., Cheson B.D., Pagel J.M., Hillmen P., Barrientos J.C., Zelenetz A.D., Kipps T.J., Flinn I., et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 2014;370:997–1007. doi: 10.1056/NEJMoa1315226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheah C.Y., Fowler N.H. Idelalisib in the management of lymphoma. Blood. 2016;128:331–336. doi: 10.1182/blood-2016-02-702761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vangapandu H.V., Jain N., Gandhi V. Duvelisib: A phosphoinositide-3 kinase δ,γ inhibitor for chronic lymphocytic leukemia. Expert Opin. Investig. Drugs. 2017;26:625–636. doi: 10.1080/13543784.2017.1312338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greenwell I.B., Flowers C.R., Blumb K.A., Cohen J.B. Clinical use of PI3K inhibitors in B-cell lymphoid malignancies: Today and tomorrow. Expert Rev. Anticancer Ther. 2017;17:271–279. doi: 10.1080/14737140.2017.1285702. [DOI] [PubMed] [Google Scholar]
- 33.Ma C.-C., Zhang C.-M., Tang L.-Q., Liu Z.-P. Discovery of novel quinazolinone derivatives as high potent and selective PI3Kδ and PI3Kδ/γ inhibitors. Eur. J. Med. Chem. 2018;151:9–17. doi: 10.1016/j.ejmech.2018.03.068. [DOI] [PubMed] [Google Scholar]
- 34.Khan F.A., Mushtaq S., Naz S., Farooq U., Zaidi A., Bukhari S.M., Rauf A., Mubarak M.S. Sulfonamides as potential bioactive scaffolds. Curr. Org. Chem. 2018;22:818–830. doi: 10.2174/1385272822666180122153839. [DOI] [Google Scholar]
- 35.Gulcin I., Taslimi P. Sulfonamide inhibitors: A patent review 2013-present. Expert Opin. Ther. Pat. 2018;28:541–549. doi: 10.1080/13543776.2018.1487400. [DOI] [PubMed] [Google Scholar]
- 36.Zhao C., Rakesh K.P., Ravidar L., Fang W.-Y., Qin H.-L. Pharmaceutical and medicinal significance of sulfur (SVI)-Containing motifs for drug discovery: A critical review. Eur. J. Med. Chem. 2019;162:679–734. doi: 10.1016/j.ejmech.2018.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rassadin V.A., Tomashevskiy A.A., Sokolov V.V., Ringe A., Magull J., de Meijere A. Facile access to bicyclic sultams with methyl-1-sulfonylcyclopropane-1-carboxylate moieties. Eur. J. Org. Chem. 2009:2635–2641. doi: 10.1002/ejoc.200900113. [DOI] [Google Scholar]
- 38.Murphy R.C., Ojo K.K., Larson E.T., Castellanos-Gonzalez A., Perera B.G., Keyloun K.R., Kim J.E., Bhandari J.G., Muller N.R., Verlinde C.L., et al. Discovery of potent and selective inhibitors of CDPK1 from C. parvum and T. gondii. ACS Med. Chem. Lett. 2010;1:331–335. doi: 10.1021/ml100096t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koresawa M., Okabe T. High-throughput screening with quantitation of ATP consumption: A universal non-radioisotope, homogeneous assay for protein kinase. Assay Drug Dev. Technol. 2004;2:153–160. doi: 10.1089/154065804323056495. [DOI] [PubMed] [Google Scholar]
- 40.Raynaud F.I., Eccles S.A., Patel S., Alix S., Box G., Chuckowree I., Folkes A., Gowan S., De Haven Brandon A., Di Stefano F., et al. Biological properties of potent inhibitors of class I phosphatidylinositide 3-kinases: From PI-103 through PI-540, PI-620 to the oral agent GDC-0941. Mol. Cancer Ther. 2009;8:1725–1738. doi: 10.1158/1535-7163.MCT-08-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freeman J.H., Wagner E.C. 3,4-Dihydro-1,2,4-benzothiadiazine. J. Org. Chem. 1951;16:815–837. doi: 10.1021/jo01146a001. [DOI] [Google Scholar]
- 42.Berndt A., Miller S., Williams O., Le D.D., Houseman B.T., Pacold J.I., Gorrec F., Hon W.C., Ren P., Liu Y., et al. The p110δ structure: Mechanisms for selectivity and potency of new PI(3)K inhibitors. Nat. Chem. Biol. 2010;6:117–124. doi: 10.1038/nchembio.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.