Abstract
Background
We assessed the utility of the systemic immune-inflammatory index (SII) in estimating the in-hospital and long-term prognosis of elderly patients with acute myocardial infarction (AMI) who received percutaneous coronary intervention (PCI).
Material/Methods
Our study evaluated 711 consecutive elderly patients (age 65–85 years) from January 2015 to December 2017. The correlation between clinical outcomes and SII was analyzed through the stepwise Cox regression analysis and the Kaplan-Meier approach. The clinical endpoints were all-cause mortality and major adverse cardiovascular and cerebrovascular events (MACCE) in-hospital and during 3-year follow-up.
Results
The study enrolled 711 elderly patients with AMI (66.95% male, 71.99±0.19 years). Kaplan-Meier analysis showed a lower survival rate in patients with higher SII scores, which also predicted in-hospital and long-term (≤3 years) outcomes. In multivariate analyses, SII showed an independent predictive value for in-hospital mortality (hazard ratio (HR), 3.32; 95% confidence interval (CI), 1.55–7.10; p<0.01), in-hospital MACCE (HR, 1.43; 95%CI, 1.02–2.00; p=0.04), long-term mortality (HR, 1.95; 95%CI, 1.23–3.09; p<0.01), along with long-term MACCE (HR, 1.72; 95%CI, 1.23–2.40; p<0.01). Moreover, SII showed a weak but significant positive relationship with the Gensini score among patients developing non-ST-segment elevation myocardial infarction (r=0.18; p<0.01).
Conclusions
SII, a readily available laboratory marker, is a potential indicator to predict the clinical endpoints for elderly patients with AMI undergoing PCI.
MeSH Keywords: Inflammation, Myocardial Infarction, Prognosis, Systemic Immune-Inflammatory Index
Background
An epidemiological survey showed that a subtype of severe coronary artery disease (CAD) manifestation – acute myocardial infarction (AMI) – causes more than 1/3 of all deaths in developed nations annually [1]. AMI is a complex, multifactorial disease that has been reported to be associated with acute inflammation and stress response [2].
Clinically, inflammatory and immune circulatory cells, including neutrophils, platelets, and lymphocytes, have pivotal roles in development of heart disease [3–5]. As suggested in cross-sectional research, neutrophilic granulocytosis in the early stage of AMI can increase the risk of subsequent congestive heart failure [6]. Higher platelet counts reflect increased thrombocyte activation, aggravate release of inflammatory mediators, and the undesirable inflammatory process [7]. Conversely, lower lymphocyte counts associated with poor prognosis have been shown in AMI [8,9]. Accordingly, growing attention has focused on the clinical application of various inflammatory ratios, such as lymphocyte-to-monocyte ratio (LMR), atherogenic index of plasma (AIP), and neutrophil-to-lymphocyte ratio (NLR) [10–13]. These measurable biochemical markers only represent local immune response and inflammation in the progression of AMI, but inflammation can also be systemic [14].
Recently, based on an ambispective cohort study, Hu et al. developed an innovative predictable marker called the systemic immune-inflammatory index (SII) [15]. SII is an inflammation-related indicator that integrates neutrophil, platelet, and lymphocyte counts and can reflect the comprehensive immune and inflammation situation in the body [16,17]. Since then, SII was shown to be a useful indicator to predict clinical outcomes for tumors and other inflammatory diseases, and has attracted much research attention [18–20]. However, to date, the predictive ability of SII in all-cause mortality and major adverse cardiovascular and cerebrovascular events (MACCE) has not been reported in AMI. The present study investigated the relationship of SII with clinical endpoints in elderly AMI patients and explored the relationship between SII and the severity of coronary lesions.
Material and Methods
Study population
Between January 2015 and December 2017, 711 consecutive elderly patients (age 65–85 years) were retrospectively enrolled at Tong Ji Hospital in Wuhan, China. All enrolled patients were diagnosed with AMI and underwent percutaneous coronary intervention (PCI). They were classified into 2 groups: an ST-segment elevation myocardial infarction (STEMI) group and a non-ST-segment elevation myocardial infarction (NSTEMI) group. STEMI was defined as typical chest pain, an increase in myocardial necrosis biomarkers, and electrocardiogram characteristics of sustained ST elevation in no less than 2 continuous leads or new left branch bundle block pattern [21]. NSTEMI was defined as typical chest pain, a rise in myocardial necrosis biomarkers, with no elevation at ST segment on electrocardiograph [22].
Exclusion criteria were: (1) age under 65 years or over 85 years, (2) changes in inflammatory or immune markers other than AMI (e.g., autoimmune diseases, sepsis, trauma, recent major surgery, active malignancy, severe liver, and renal failure), and (3) receiving inhibitors of glycoprotein IIb/IIIa or thrombolytic therapy.
This study was by the Ethics Committee of Tong Ji Hospital of Huazhong University of Science and Technology (TJ-C20141112) and complied with the Helsinki Declaration.
Clinical data collection
Data on study population baseline characteristics, including demographic information and the history of diagnosed cardiovascular and cerebrovascular diseases (e.g., diabetes, CAD, and hypertension), were collected from hospital records, as were data on smoking/drinking status (including previous history and current tobacco/alcohol use). In addition, the Killip class and data on vital signs (e.g., diastolic and systolic blood pressures and heart rate) were recorded on admission. We also collected data on history of antiplatelet therapy (aspirin, clopidogrel/ticagrelor), beta-blockers therapy, lipids-modulating therapy (statins) and the inhibitors of angiotensin-converting enzymes/blockers of angiotensin II receptor during hospitalization.
Laboratory analysis included the results of routine blood tests, creatinine, aminopherase (aspartate aminotransferase, alanine transaminase), serum lipids (low- and high-density lipoprotein cholesterols, total triglyceride, and total cholesterol), cardiac troponin I (CTnI) and N-terminal pro-brain natriuretic peptide (NT-proBNP). Echocardiographic parameters included left ventricular ejection fraction (LVEF). Total measurements were obtained from first examination in hospital.
Coronary angiography
All enrolled patients were given aspirin (300 mg) and clopidogrel/ticagrelor (300–600 mg) as adjuvant antiplatelet therapy before coronary intervention on the basis of the established guideline [23]. If necessary, the heparin/low molecular heparin or tirofiban therapy was administered in the perioperative period. The results of coronary angiography were determined by 2 cardiovascular physicians. According to coronary angiography results and clinical findings, doctors selected different treatment strategies according to the current practice guidelines [24].
The blood flow grade in thrombolysis in myocardial infarction (TIMI) and Gensini score were obtained from angiographic data. TIMI is a semi-quantitative index of blood flow in infarct-related vessels. Scores ranged from 0 to 3, based on vessels with significant stenosis [25]. Gensini score is a widely use angiographic scoring system in which the vascular score of the lesion is determined by multiplying the basic score of the stenosis of each coronary artery by the coefficient of the lesion site. The total score of all diseased vessels is the final score of the stenosis degree of the coronary artery [26].
Follow-up and study endpoints
Clinical endpoints in our study included all-cause mortality and MACCE. We assessed in-hospital mortality (during the hospital stay) and long-term mortality (up to 3-year follow-up). In-hospital and long-term MACCE were defined as in-hospital cardiovascular and cerebrovascular accident and up to 3-year follow-up, respectively. In-hospital data were obtained from the in-patient management system and follow-up data were collected from telephone interviews with patients or their relatives (regular re-examination and telephone follow-up).
Statistical analysis
SII was calculated as (neutrophil counts)×(platelet counts)/(lymphocyte counts) [15]. Continuous parameters were expressed as mean±standard error, and the Kolmogorov-Smirnov test was conducted to assess normality of distribution. Comparisons among continuous variables were performed using the Mann-Whitney U test or the independent-sample t test, depending on whether the variables were parametric values or nonparametric values. Categorical parameters were compared using the chi-square test. Correlation analysis was performed to assess the overall correlation between 2 indicators. When comparing 2 parametric variables, Pearson correlation coefficient was used; if they were nonparametric variables, Spearman correlation coefficient was used. The threshold for predicting clinical outcomes was determined by receiver operating characteristic (ROC) curve analysis. Survival curve was obtained using Kaplan-Meier analysis and log-rank test. Factors related to clinical endpoints were identified through univariate analysis. The possible predictive factors, including parameters that had p value <0.1 in univariate analysis or several established AMI risk factors (including sex, age, smoking status, and histories of diabetes, CAD and hypertension) [27,28], were incorporated into the stepwise multivariate Cox regression model. P value <0.05 denoted a statistically significant difference. All data analyses were performed using SPSS 22.0.
Results
Patients characteristics
Table 1 shows demographic, laboratory, and angiographic data. All 711 participants (age 65–85 years) were enrolled and were divided into a STEMI group (n=405, 272 males) and a NSTEMI group (n=306, 204 males). The mean age was 71.99±0.19 years and 66.95% of subjects were males. There were no significant differences in medical history or in-hospital medications between the 2 groups. Patients with STEMI were more likely to have worse vital signs at admission, elevated CTnI and aspartate aminotransferase levels, and worse LVEF, as well as higher WBC counts and neutrophil counts. However, lymphocyte counts were higher in the NSTEMI group.
Table 1.
Demographic, laboratory, and angiographic characteristics of study population.
| Characteristics | All patients (n=711) | STEMI (n=405) | NSTEMI (n=306) | p |
|---|---|---|---|---|
| Age, year | 71.99±0.19 | 71.73±0.25 | 72.32±0.30 | 0.13 |
| Sex (Male), n (%) | 476 (66.95) | 272 (67.16) | 204 (66.67) | 0.89 |
| Smoking, n (%) | 210 (29.54) | 130 (32.10) | 80 (26.14) | 0.09 |
| Drinking, n (%) | 150 (21.10) | 86 (21.23) | 64 (20.92) | 0.94 |
| Hypertension, n (%) | 423 (59.49) | 237 (58.52) | 186 (60.78) | 0.54 |
| Prior CAD, n (%) | 78 (10.97) | 42 (10.37) | 36 (11.76) | 0.56 |
| Diabetes, n (%) | 179 (25.18) | 97 (23.95) | 82 (26.80) | 0.39 |
| Stroke, n (%) | 96 (13.50) | 53 (13.09) | 43 (14.05) | 0.71 |
| SBP on admission (mmHg) | 129.72±0.93 | 127.95±1.28 | 132.06±1.33 | 0.03 |
| DBP on admission (mmHg) | 75.62±0.55 | 75.82±0.77 | 75.36±0.75 | 0.67 |
| HR on admission (beats/min) | 77.11±0.60 | 78.94±0.75 | 74.69±0.86 | <0.01 |
| hospitalization day | 8.69±0.26 | 8.40±0.28 | 9.07±0.47 | 0.20 |
| Killip class, III–IV, n (%) | 101 (14.21) | 63 (15.56) | 38 (12.42) | 0.24 |
| LVEF, % | 53.04±0.46 | 50.93±0.47 | 55.82±0.68 | <0.01 |
| Creatinine (μmol/L) | 91.93±0.33 | 93.96±1.84 | 89.24±1.96 | 0.08 |
| AST (u/L) | 91.27±4.07 | 123.77±6.15 | 77.05±4.41 | <0.01 |
| ALT (u/L) | 35.83±1.45 | 38.19±1.91 | 32.71±2.22 | 0.06 |
| NT-proBNP, pg/mL | 4635.84±292.81 | 4823.00±367.95 | 4388.14±475.45 | 0.46 |
| CTnI, pg/mL | 15015.62±699.78 | 18398.24±1001.14 | 10538.61±881.01 | <0.01 |
| HDL, mmol/L | 1.09±0.02 | 1.08±0.02 | 1.10±0.03 | 0.72 |
| LDL, mmol/L | 2.58±0.04 | 2.62±0.05 | 2.54±0.05 | 0.26 |
| Total triglyceride, mmol/L | 1.36±0.04 | 1.30±0.05 | 1.42±0.06 | 0.14 |
| Total cholesterol, mmol/L | 4.12±0.04 | 4.17±0.06 | 4.05±0.06 | 0.17 |
| WBC count (109/L) | 9.18±0.14 | 9.84±0.20 | 8.30±0.19 | <0.01 |
| Platelet count (109/L) | 202.78±2.53 | 206.37±3.43 | 198.03±3.71 | 0.10 |
| Neutrophil count (109/L) | 7.14±0.14 | 7.86±0.20 | 6.17±0.19 | <0.01 |
| Lymphocyte count (109/L) | 1.40±0.02 | 1.32±0.03 | 1.50±0.04 | <0.01 |
| Monocyte count (109/L) | 0.57±0.01 | 0.58±0.02 | 0.55±0.01 | 0.21 |
| Hemoglobin (mg/dL) | 127.03±0.71 | 126.94±0.92 | 127.15±1.10 | 0.88 |
| SII | 1392.69±56.85 | 1645.60±81.60 | 1057.95±71.86 | <0.01 |
| Medications in-hospital, n (%) | ||||
| Antiplatelet therapy | 666 (93.67) | 385 (95.06) | 281 (91.83) | 0.08 |
| Beta-blocker | 507 (71.31) | 281 (69.38) | 226 (73.86) | 0.19 |
| ACEI/ARB | 500 (70.32) | 276 (68.15) | 224 (73.20) | 0.14 |
| Statin | 694 (97.61) | 393 (97.04) | 301 (98.37) | 0.25 |
| Angiographic and procedural characteristics | ||||
| Culprit vessel, n (%) | ||||
| LAD | 622 (87.48) | 362 (89.38) | 260 (84.97) | 0.08 |
| LCX | 451 (63.43) | 243 (60.00) | 208 (67.97) | 0.03 |
| RCA | 458 (64.42) | 260 (64.20) | 198 (64.71) | 0.89 |
| No. of diseased vessels, n (%) | 0.56 | |||
| 0 | 1 (0.14) | 0 (0.00) | 1 (0.33) | |
| 1 | 196 (27.57) | 115 (28.40) | 81 (26.47) | |
| 2 | 208 (29.25) | 121 (29.88) | 87 (28.43) | |
| 3 | 306 (43.04) | 169 (41.73) | 137 (44.77) | |
| Onset to reperfusion time, h | 14.29±0.33 | 13.37±0.43 | 15.51±0.50 | <0.01 |
| Tirofiban use, n (%) | 591 (83.12) | 348 (85.93) | 243 (79.41) | 0.03 |
| Stent use, n (%) | 533 (74.96) | 318 (78.52) | 215 (70.26) | 0.01 |
| Use of thrombus aspiration, n (%) | 72 (10.13) | 60 (14.81) | 12 (3.92) | <0.01 |
| Stent diameter, mm | 2.27±0.06 | 2.40±0.08 | 2.08±0.08 | <0.01 |
| Stent length, mm | 20.45±0.51 | 21.84±0.69 | 18.60±0.74 | <0.01 |
| Preprocedural TIMI grade, n (%) | 0.01 | |||
| 0 | 439 (61.74) | 270 (66.67) | 169 (55.23) | |
| 1 | 245 (34.46) | 124 (30.62) | 121 (39.54) | |
| 2 | 24 (3.38) | 11 (2.72) | 13 (4.25) | |
| 3 | 3 (0.42) | 0 (0.00) | 3 (0.98) | |
| Postprocedural TIMI grade, n (%) | 0.11 | |||
| 0 | 90 (12.66) | 45 (11.11) | 45 (14.71) | |
| 1 | 40 (5.63) | 18 (4.44) | 22 (7.19) | |
| 2 | 28 (3.94) | 19 (4.69) | 9 (2.94) | |
| 3 | 553 (77.78) | 323 (79.75) | 230 (75.16) | |
| Gensini score | 93.76±1.99 | 94.59±2.39 | 92.67±3.36 | 0.64 |
Mean±SEM and n (%) are reported for continuous and categorical variables, respectively. SEM – standard error of the mean.
STEMI – ST-segment elevation myocardial infarction; NSTEMI – non-ST-elevation myocardial infarction; CAD – coronary artery disease; SBP – systolic blood pressure; DBP – diastolic blood pressure; HR – heart rate; LVEF – left ventricular ejection fraction; AST – aspartate aminotransferase; ALT – alanine aminotransferase; NT-proBNP – N-terminal pro-brain natriuretic peptide; CTnI – cardiac troponin I; HDL – high-density lipoprotein cholesterol; LDL – low-density lipoprotein cholesterol; WBC – white blood cell; SII – systemic immune-inflammatory index; ACEI/ARB – angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker; LAD – left coronary artery; LCX – left circumflex; RCA – right coronary artery; TIMI – thrombolysis in myocardial infarction.
Additionally, as shown in Table 1, the onset to reperfusion time was shorter in the STEMI group. Thrombus aspiration use (p<0.01), stent use (p=0.01), and tirofiban use (p=0.03) were remarkably higher in the STEMI group. Differences in number of culprit vessels and Gensini score were not significantly different between the 2 groups. Longer stents (p<0.01) and wider stents (p<0.01) were needed in patients with STEMI. The flow grade of postprocedural TIMI in the STEMI group was significantly different from that in the NSTEMI group (p=0.01). Supplementary Table 1 lists data on clinical results.
Predictive value of SII
When predicting the long-term outcomes, the area under the ROC curve (AUC) was 0.64 (95% confidence interval (CI), 0.58–0.71; p<0.01). The sensitivity and specificity for SII in predicting the long-term clinical events were 50.00% and 74.10%, respectively, at the threshold of 1423.12×109/L (Figure 1).
Figure 1.
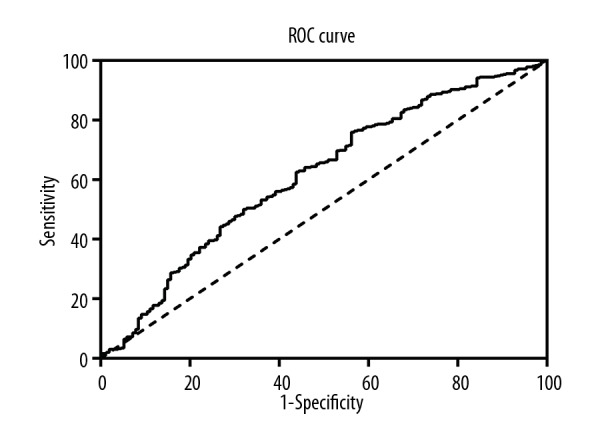
ROC curves analysis showing the predictive cutoff value of SII in AMI patients according to long-term clinical outcomes (AUC=0.64; 95%CI, 0.58–0.71; p<0.01).ROC – receiver operator characteristic; SII – systemic immune-inflammatory index; AMI – acute myocardial infarction; AUC – area under the curve; CI – confidence interval.
Survival analysis
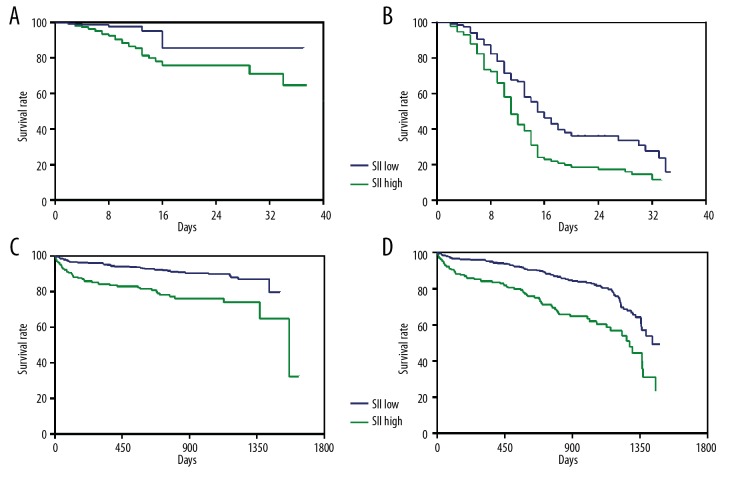
The hospital stay was 8.69±6.90 days and follow-up time was 737.45±16.74 days. In our study population, 52 (7.31%) patients died during hospitalization and 80 (11.25%) died during follow-up. Kaplan-Meier survival curves (Figure 2A–2D) show that patients with higher SII scores had significantly worse in-hospital and long-term outcomes than those with lower SII scores (log-rank test, all p values are <0.01).
Figure 2.
Kaplan-Meier cumulative survival curves for in-hospital and long-term mortality and MACCE, according to high SII versus those with low SII. (A) Kaplan-Meier survival curves of in-hospital mortality (log-rank test: p<0.01). (B) Kaplan-Meier survival curves of in-hospital MACCE (log-rank test: p<0.01). (C) Kaplan-Meier survival curves of long-term mortality (log-rank test: p<0.01). (D) Kaplan-Meier survival curves of long-term MACCE (log-rank test: p<0.01). SII – systemic immune-inflammatory index; MACCE – major adverse cardiovascular and cerebrovascular events.
Regression analysis
Upon multivariate regression analysis, history of CAD, Killip class, CTnI, neutrophil counts, hemoglobin, creatinine and SII (harzard ratio (HR), 3.32; 95%CI, 1.55–7.10; p<0.01) were identified to be the factors independently predicting in-hospital mortality (Table 2). Moreover, SII score was the strongest predictor of in-hospital MACCE (HR, 1.43; 95%CI, 1.02–2.00; p=0.04). Additional significant independent predictors were Killip class, LVEF, CTnI, neutrophil counts, and hemoglobin (Table 3).
Table 2.
Predictors of in-hospital mortality in univariate and multivariate Cox regression analysis.
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Age | 1.03 | 0.98–1.09 | 0.24 | NS | ||
| Sex (Male) | 1.55 | 0.89–2.69 | 0.12 | NS | ||
| Gensini score | 1.01 | 1.00–1.01 | 0.01 | NS | ||
| Smoking | 1.55 | 0.85–2.83 | 0.16 | NS | ||
| Hypertension | 1.06 | 0.61–1.85 | 0.85 | NS | ||
| Prior CAD | 2.42 | 1.27–4.62 | 0.01 | 2.77 | 1.38–5.57 | <0.01 |
| Diabetes | 1.06 | 0.59–1.93 | 0.84 | NS | ||
| Killip class | 2.38 | 1.85–3.05 | <0.01 | 1.64 | 1.24–2.18 | <0.01 |
| LVEF | 0.95 | 0.93–0.97 | <0.01 | NS | ||
| CTnI | 2.50 | 1.37–4.57 | <0.01 | 2.62 | 1.31–5.23 | 0.01 |
| WBC | 1.13 | 1.07–1.19 | <0.01 | NS | ||
| Neutrophil counts | 1.15 | 1.09–1.21 | <0.01 | 1.12 | 1.04–1.21 | <0.01 |
| Lymphocyte counts | 0.82 | 0.53–1.28 | 0.38 | |||
| Platelet counts | 1.00 | 0.99–1.00 | 0.26 | |||
| Hemoglobin | 0.96 | 0.95–0.98 | <0.01 | 0.97 | 0.96–0.98 | <0.01 |
| Total cholesterol | 0.83 | 0.64–1.06 | 0.14 | |||
| Total triglyceride | 0.92 | 0.68–1.24 | 0.58 | |||
| LDL | 0.91 | 0.68–1.21 | 0.50 | |||
| HDL | 0.33 | 0.12–0.91 | 0.03 | NS | ||
| creatinine | 1.01 | 1.00–1.02 | <0.01 | 1.01 | 1.00–1.01 | 0.02 |
| SII | 2.95 | 1.27–6.87 | 0.01 | 3.32 | 1.55–7.10 | <0.01 |
HR – hazard ratio; CI – confidence interval; NS – no statistical significance; CAD – coronary artery disease; LVEF – left ventricular ejection fraction; CTnI – cardiac troponin I; WBC – white blood cell; HDL – high-density lipoprotein cholesterol; LDL – low-density lipoprotein cholesterol; SII – systemic immune-inflammation index. NS stands for the factors which have no statistically significance in multivariate analysis. Bolded differences show statistical difference at the p<0.05 for multivariate analysis.
Table 3.
Predictors of in-hospital major adverse cardiovascular and cerebrovascular events in univariate and multivariate Cox regression analysis.
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Age | 1.00 | 0.98–1.03 | 0.85 | NS | ||
| Sex (Male) | 1.31 | 1.01–1.71 | 0.04 | NS | ||
| Gensini score | 1.00 | 0.99–1.00 | 0.57 | |||
| Smoking | 1.05 | 0.81–1.37 | 0.71 | NS | ||
| Hypertension | 1.11 | 0.86–1.45 | 0.42 | NS | ||
| Prior CAD | 1.14 | 0.78–1.67 | 0.51 | NS | ||
| Diabetes | 1.05 | 0.80–1.38 | 0.73 | NS | ||
| Killip class | 1.35 | 1.19–1.52 | <0.01 | 1.17 | 1.01–1.35 | 0.04 |
| LVEF | 0.98 | 0.97–0.99 | <0.01 | 0.98 | 0.97–0.99 | <0.01 |
| CTnI | 1.56 | 1.21–2.02 | <0.01 | 1.35 | 1.03–1.78 | 0.03 |
| WBC | 1.07 | 1.04–1.10 | <0.01 | NS | ||
| Neutrophil counts | 1.08 | 1.05–1.11 | <0.01 | 1.08 | 1.04–1.12 | <0.01 |
| Lymphocyte counts | 0.90 | 0.74–1.09 | 0.28 | |||
| Platelet counts | 1.00 | 0.99–1.00 | 0.31 | |||
| Hemoglobin | 0.99 | 0.99–1.00 | 0.02 | 0.99 | 0.99–1.00 | 0.02 |
| Total cholesterol | 0.98 | 0.88–1.09 | 0.72 | |||
| Total triglyceride | 0.99 | 0.87–1.12 | 0.84 | |||
| LDL | 0.99 | 0.87–1.12 | 0.83 | |||
| HDL | 1.13 | 0.83–1.54 | 0.44 | |||
| creatinine | 1.00 | 1.00–1.01 | 0.05 | NS | ||
| SII | 1.32 | 1.01–1.72 | 0.04 | 1.43 | 1.02–2.00 | 0.04 |
HR – hazard ratio; CI – confidence interval; NS – no statistical significance; CAD – coronary artery disease; LVEF – left ventricular ejection fraction; CTnI – cardiac troponin I; WBC – white blood cell; HDL – high-density lipoprotein cholesterol; LDL – low-density lipoprotein cholesterol; SII – systemic immune-inflammation index. NS stands for the factors which have no statistically significance in multivariate analysis. Bolded differences show statistical difference at the p<0.05 for multivariate analysis.
SII (HR, 1.95; 95%CI, 1.23–3.09; p<0.01) can independently predict long-term mortality, along with age, Killip class, and LVEF (Table 4). Multivariate regression analysis showed that age, LVEF, Killip class, and SII score (HR, 1.72; 95%CI, 1.23–2.40; p<0.01) were independent predictors for long-term MACCE (Table 5).
Table 4.
Predictors of long-term mortality in univariate and multivariate Cox regression analysis.
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Age | 1.08 | 1.04–1.13 | <0.01 | 1.06 | 1.01–1.11 | 0.01 |
| Sex (Male) | 1.49 | 0.95–2.32 | 0.08 | NS | ||
| Gensini score | 1.00 | 1.00–1.01 | 0.23 | |||
| Smoking | 1.11 | 0.71–1.75 | 0.65 | NS | ||
| Hypertension | 1.26 | 0.80–2.00 | 0.32 | NS | ||
| Prior CAD | 1.01 | 0.49–2.10 | 0.98 | NS | ||
| Diabetes | 1.37 | 0.85–2.22 | 0.20 | NS | ||
| Killip class | 2.01 | 1.61–2.51 | <0.01 | 1.49 | 1.16–1.91 | <0.01 |
| LVEF | 0.96 | 0.94–0.98 | <0.01 | 0.97 | 0.96–0.99 | <0.01 |
| CTnI | 1.50 | 0.95–2.35 | 0.08 | NS | ||
| WBC | 1.11 | 1.06–1.17 | <0.01 | NS | ||
| Neutrophil counts | 1.12 | 1.07–1.17 | <0.01 | NS | ||
| Lymphocyte counts | 0.56 | 0.38–0.85 | 0.01 | NS | ||
| Platelet counts | 1.00 | 0.99–1.00 | 0.55 | |||
| Hemoglobin | 0.98 | 0.97–0.99 | <0.01 | NS | ||
| Total cholesterol | 1.04 | 0.85–1.26 | 0.73 | |||
| Total triglyceride | 0.99 | 0.78–1.26 | 0.95 | |||
| LDL | 1.04 | 0.83–1.30 | 0.76 | |||
| HDL | 1.34 | 0.87–2.06 | 0.19 | |||
| creatinine | 1.01 | 1.00–1.01 | <0.01 | NS | ||
| SII | 2.66 | 1.71–4.13 | <0.01 | 1.95 | 1.23–3.09 | <0.01 |
HR – hazard ratio; CI – confidence interval; NS – no statistical significance; CAD – coronary artery disease; LVEF – left ventricular ejection fraction; CTnI – cardiac troponin I; WBC – white blood cell; HDL – high-density lipoprotein cholesterol; LDL – low-density lipoprotein cholesterol; SII – systemic immune-inflammation index. NS stands for the factors that had no statistical significance in multivariate analysis. Bolded differences show statistically significant difference at p<0.05 in multivariate analysis.
Table 5.
Predictors of long-term major adverse cardiovascular and cerebrovascular events in univariate and multivariate Cox regression analysis.
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Age | 1.06 | 1.03–1.09 | <0.01 | 1.05 | 1.02–1.09 | <0.01 |
| Sex (male) | 1.36 | 0.98–1.88 | 0.06 | NS | ||
| Gensini score | 1.00 | 0.99–1.00 | 0.43 | |||
| Smoking | 1.04 | 0.75–1.44 | 0.83 | NS | ||
| Hypertension | 1.22 | 0.87–1.69 | 0.25 | NS | ||
| Prior CAD | 1.10 | 0.67–1.83 | 0.70 | NS | ||
| Diabetes | 1.27 | 0.89–1.83 | 0.19 | NS | ||
| Killip class | 1.91 | 1.60–2.27 | <0.01 | 1.57 | 1.29–1.90 | <0.01 |
| LVEF | 0.97 | 0.96–0.99 | <0.01 | 0.98 | 0.97–1.00 | 0.02 |
| CTnI | 1.50 | 1.09–2.08 | 0.01 | NS | ||
| WBC | 1.10 | 1.06–1.14 | <0.01 | NS | ||
| Neutrophil counts | 1.10 | 1.06–1.14 | <0.01 | NS | ||
| Lymphocyte counts | 0.66 | 0.50–0.88 | 0.01 | NS | ||
| Platelet counts | 1.00 | 0.99–1.00 | 0.80 | |||
| Hemoglobin | 0.99 | 0.98–1.00 | <0.01 | NS | ||
| Total cholesterol | 0.95 | 0.82–1.11 | 0.52 | |||
| Total triglyceride | 1.08 | 0.92–1.27 | 0.35 | |||
| LDL | 0.92 | 0.77–1.10 | 0.35 | |||
| HDL | 1.11 | 0.75–1.64 | 0.60 | |||
| creatinine | 1.01 | 1.00–1.01 | <0.01 | NS | ||
| SII | 2.22 | 1.61–3.07 | <0.01 | 1.72 | 1.23–2.40 | <0.01 |
HR – hazard ratio; CI – confidence interval; NS – no statistical significance; CAD – coronary artery disease; LVEF – left ventricular ejection fraction; CTnI – cardiac troponin I; WBC – white blood cell; HDL – high-density lipoprotein cholesterol; LDL – low-density lipoprotein cholesterol; SII – systemic immune-inflammation index. NS stands for the factors which have no statistically significance in multivariate analysis. Bolded differences show statistical difference at the p<0.05 for multivariate analysis.
Correlation analysis
Supplementary Table 2 shows that SII score has a weak but significantly positive relationship with Gensini score only in the NSTEMI group (r=0.18; p<0.01). Furthermore, SII showed a positive correlation with Killip class in both groups, as did CTnI and WBC. Also, SII scores were negatively correlated with LVEF among STEMI cases. However, SII was not correlated with TIMI flow grade in all patients.
Discussion
In the present study of 711 AMI patients, we found that SII was a potential indicator for predicting all-cause mortality/MACCE. we also found a significant correlation between SII and cardiovascular-related variables such as Gensini score. To the best of our knowledge, this is the first analysis of the utility of SII in predicting clinical outcomes of elderly AMI patients. The results are mainly applicable to elderly patients age 65 to 85 years.
It was recently shown that inflammatory cells (e.g., WBC and subtypes) can be used to estimate prognosis in AMI patients [29], but inflammatory predictors based on 1 or 2 components are relatively poor predictors of prognosis in AMI [14]. Therefore, SII, an inflammatory index of 3 inflammatory cell types (neutrophils, platelets, and lymphocytes), might more comprehensively represent the balanced status of immune and inflammatory conditions in the host [30,31]. In the present study, the prediction abilities of SII and each parameter were compared through univariate and multivariate Cox regression analyses. All confounding factors were adjusted, and we found that SII had the best prediction ability in all clinical endpoints.
Results from previous experimental and clinical research suggested that patients with elevated SII had greater risk of poor clinical outcomes [32]. It is known that myocardial ischemia is closely associated with inflammation [33]. Sezer et al. reported that neutrophilia in patients with AMI was strongly associated with the incidence of microvascular reperfusion injury after performing coronary artery revascularization [34]. Neutrophil extracellular traps (NETs) are a type of activated neutrophil found in patients with AMI, which is released at the site of pathology in the coronary artery [35]. NETs are highly proinflammatory and prothrombotic, and are positively correlated with infarct area [36]. Neutrophil-driven proinflammatory processes appear to play a role in the adverse prognosis. We found that neutrophils were strongly associated with in-hospital mortality and MACCE, which is consistent with previous research [37].
Growing evidence shows that platelets play a role in initial CAD progression and development [38,39]. Persistent inflammation causes a prothrombotic state due to thrombocytosis [40]. Aggregating platelets adhere to endothelial cell surfaces and recruit monocytes into inflammatory sites. Additionally, platelets release various inflammatory mediators, which can further activate platelets and form a vicious cycle [41–44].
Lymphocytes, as a part of the adaptive immune response, play an important part in the immune regulatory pathway. It was reported that lower lymphocyte concentration was related to the progression of atherosclerosis progression and adverse clinical outcomes in patients with AMI [45–47]. Triggered by aggravated inflammation, the increased apoptosis of lymphocyte can reduce lymphocyte counts [48,49]. In consequence, because of the high levels of neutrophils and platelets and the reduced lymphocyte concentration, an elevated SII might be linked to the increased inflammatory activity and thus lead to poor clinical outcomes.
The present study used the Gensini scoring system, which is objective and credible, to define the severity of coronary artery disease [50]. Our correlation analysis showed a positive association between SII and Gensini score in patients with NSTEMI. This suggests that SII could be useful in clinical risk stratification and optimal management of NSTEMI. The NSTEMI management guidelines emphasize the importance of risk assessment and risk-oriented treatment decision [51–53]. SII values are easy to calculate using counts of WBC subtypes, and these widely available tests are routinely performed at hospital admission. SII is suited for clinical use because it is easy to use and is cost-effective.
Our study has limitations that need to be considered. This was a retrospective study at a single center. Moreover, because some markers (e.g., C-reactive protein, fibrinogen, and myeloperoxidase) are not routinely assessed, we did not compare SII with the conventional inflammatory indicators. Finally, we did not perform in-depth analysis of the relationship between SII and AMI. Therefore, prospective studies in larger populations are needed to validate our conclusions.
Conclusions
We found that a higher value of SII was independently associated with poor clinical prognosis. SII is a potential indicator to predict the in-hospital and long-term clinical results for elderly AMI patients undergoing PCI.
Supplementary Data
Supplementary Table 1.
Clinical outcomes of the study population during hospitalization and long-term follow-up.
| Clinical outcomes | In-hospital | Long-term follow-up |
|---|---|---|
| Death, n (%) | 52 (7.31) | 80 (11.25) |
| Reinfarction, n (%) | 4 (0.56) | 3 (0.42) |
| Revascularization, n (%) | 1 (0.14) | 4 (0.56) |
| Arrhythmia, n (%) | 119 (16.74) | 61 (8.58) |
| Congestive heart failure, n (%) | 179 (25.18) | 116 (16.32) |
| Cerebrovascular accident, n (%) | 13 (1.83) | 7 (0.98) |
Supplementary Table 2.
Correlation between SII and other laboratory measurements.
| STEMI | NSTEMI | |||
|---|---|---|---|---|
| r | p | r | p | |
| Gensini score | −0.05 | 0.29 | 0.18 | <0.01 |
| Preprocedural TIMI grade | −0.06 | 0.23 | −0.08 | 0.15 |
| Postprocedural TIMI grade | −0.06 | 0.20 | −0.05 | 0.38 |
| Killip class | 0.25 | <0.01 | 0.35 | <0.01 |
| CTnI | 0.31 | <0.01 | 0.30 | <0.01 |
| LVEF | −0.21 | <0.01 | −0.04 | 0.45 |
| WBC | 0.63 | <0.01 | 0.57 | <0.01 |
SII – systemic immune-inflammation index; STEMI – ST elevation myocardial infarction; NSTEMI – non-ST elevation myocardial infarction; TIMI – thrombolysis in myocardial infarction; CTnI – cardiac troponin I; WBC –white blood cell; LVEF – left ventricular ejection fraction.
Acknowledgements
We acknowledge the volunteers from Tong Ji Hospital who participated in our study, as well as those that took part in designing the research, collecting and analyzing data, and writing and reviewing the manuscript.
Footnotes
Source of support: Departmental sources
Conflicts of interest
None.
References
- 1.Yeh RW, Sidney S, Chandra M, et al. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362(23):2155–65. doi: 10.1056/NEJMoa0908610. [DOI] [PubMed] [Google Scholar]
- 2.Nichols M, Townsend N, Scarborough P, Rayner M. Cardiovascular disease in Europe 2014: Epidemiological update. Eur Heart J. 2014;35(42):2950–59. doi: 10.1093/eurheartj/ehu299. [DOI] [PubMed] [Google Scholar]
- 3.Falk E. Pathogenesis of atherosclerosis. J Am Coll Cardiol. 2006;47(8 Suppl):C7–12. doi: 10.1016/j.jacc.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 4.Davi G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357(24):2482–94. doi: 10.1056/NEJMra071014. [DOI] [PubMed] [Google Scholar]
- 5.Xu L, Su Y, Zhao Y, et al. Melatonin differentially regulates pathological and physiological cardiac hypertrophy: Crucial role of circadian nuclear receptor RORα signaling. J Pineal Res. 2019;67(2):e12579. doi: 10.1111/jpi.12579. [DOI] [PubMed] [Google Scholar]
- 6.Rashidi F, Rashidi A, Golmohamadi A, et al. Does absolute neutrophilia predict early congestive heart failure after acute myocardial infarction? A cross-sectional study. South Med J. 2008;101(1):19–23. doi: 10.1097/SMJ.0b013e31815d3e11. [DOI] [PubMed] [Google Scholar]
- 7.Amraotkar AR, Song DD, Otero D, et al. Platelet count and mean platelet volume at the time of and after acute myocardial infarction. Clin Appl Thromb Hemost. 2017;23(8):1052–59. doi: 10.1177/1076029616683804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hofmann U, Frantz S. Role of T-cells in myocardial infarction. Eur Heart J. 2016;37(11):873–79. doi: 10.1093/eurheartj/ehv639. [DOI] [PubMed] [Google Scholar]
- 9.Kounis NG, Soufras GD, Tsigkas G, et al. White blood cell counts, leukocyte ratios, and eosinophils as inflammatory markers in patients with coronary artery disease. Clin Appl Thromb Hemost. 2015;21(2):139–43. doi: 10.1177/1076029614531449. [DOI] [PubMed] [Google Scholar]
- 10.Ding S, Lin N, Sheng X, et al. Melatonin stabilizes rupture-prone vulnerable plaques via regulating macrophage polarization in a nuclear circadian receptor RORα-dependent manner. J Pineal Res. 2019;67(2):e12581. doi: 10.1111/jpi.12581. [DOI] [PubMed] [Google Scholar]
- 11.Kiris T, Çelik A, Variş E, et al. Association of lymphocyte-to-monocyte ratio with the mortality in patients with ST-elevation myocardial infarction who underwent primary percutaneous coronary intervention. Angiology. 2017;68(8):707–15. doi: 10.1177/0003319716685480. [DOI] [PubMed] [Google Scholar]
- 12.Mazidi M, Katsiki N, Mikhailidis DP, Banach M. Association of ideal cardiovascular health metrics with serum uric acid, inflammation and atherogenic index of plasma: A population-based survey. Atherosclerosis. 2019;284:44–49. doi: 10.1016/j.atherosclerosis.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 13.Guo TM, Cheng B, Ke L, et al. Prognostic value of neutrophil to lymphocyte ratio for in-hospital mortality in elderly patients with acute myocardial infarction. Curr Med Sci. 2018;38(2):354–59. doi: 10.1007/s11596-018-1887-0. [DOI] [PubMed] [Google Scholar]
- 14.Budzianowski J, Pieszko K, Burchardt P, et al. The role of hematological indices in patients with acute coronary syndrome. Dis Markers. 2017;2017 doi: 10.1155/2017/3041565. 3041565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20(23):6212–22. doi: 10.1158/1078-0432.CCR-14-0442. [DOI] [PubMed] [Google Scholar]
- 16.Montecucco F, Liberale L, Bonaventura A, et al. The role of inflammation in cardiovascular outcome. Curr Atheroscler Rep. 2017;19(3):11. doi: 10.1007/s11883-017-0646-1. [DOI] [PubMed] [Google Scholar]
- 17.Ma M, Yu N, Wu B. High systemic immune-inflammation index represents an unfavorable prognosis of malignant pleural mesothelioma. Cancer Manag Res. 2019;11:3973–79. doi: 10.2147/CMAR.S201269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jomrich G, Gruber ES, Winkler D, et al. Systemic Immune-Inflammation Index (SII) predicts poor survival in pancreatic cancer patients undergoing resection. J Gastrointest Surg. :2019. doi: 10.1007/s11605-019-04187-z. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furuncuoğlu Y, Tulgar S, Dogan AN, et al. How obesity affects the neutrophil/lymphocyte and platelet/lymphocyte ratio, systemic immune-inflammatory index and platelet indices: A retrospective study. Eur Rev Med Pharmacol Sci. 2016;20(7):1300–6. [PubMed] [Google Scholar]
- 20.Zhou ZQ, Pang S, Yu XC, et al. Predictive values of postoperative and dynamic changes of inflammation indexes in survival of patients with resected colorectal cancer. Curr Med Sci. 2018;38(5):798–808. doi: 10.1007/s11596-018-1946-6. [DOI] [PubMed] [Google Scholar]
- 21.O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127(4):e362–425. doi: 10.1161/CIR.0b013e3182742cf6. [DOI] [PubMed] [Google Scholar]
- 22.Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64(24):e139–228. doi: 10.1016/j.jacc.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 23.Bittl JA, Baber U, Bradley SM, et al. Duration of dual antiplatelet therapy: A systematic review for the 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. J Am Coll Cardiol. 2016;68(10):1116–39. doi: 10.1016/j.jacc.2016.03.512. [DOI] [PubMed] [Google Scholar]
- 24.Windecker S, Kolh P, Alfonso F, et al. 2014 ESC/EACTS guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Eur Heart J. 2014;35(37):2541–619. doi: 10.1093/eurheartj/ehu278. [DOI] [PubMed] [Google Scholar]
- 25.Sarkar A, Lee JJ. TIMI grade flow. Cardiovasc Res. 2018;114(Suppl 1):S118. [Google Scholar]
- 26.Rampidis GP, Benetos G, Benz DC, et al. A guide for Gensini Score calculation. Atherosclerosis. 2019;28(7):181–83. doi: 10.1016/j.atherosclerosis.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 27.Canto JG, Rogers WJ, Goldberg RJ, et al. Association of age and sex with myocardial infarction symptom presentation and in-hospital mortality. JAMA. 2012;307(8):813–22. doi: 10.1001/jama.2012.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gale CP, Manda SO, Batin PD, et al. Predictors of in-hospital mortality for patients admitted with ST-elevation myocardial infarction: A real-world study using the Myocardial Infarction National Audit Project (MINAP) database. Heart. 2008;94(11):1407–12. doi: 10.1136/hrt.2007.127068. [DOI] [PubMed] [Google Scholar]
- 29.Shiyovich A, Gilutz H, Plakht Y. White blood cell subtypes are associated with a greater long-term risk of death after acute myocardial infarction. Tex Heart Inst J. 2017;44(3):176–88. doi: 10.14503/THIJ-16-5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fest J, Ruiter R, Ikram MA. Reference values for white blood-cell-based inflammatory markers in the Rotterdam Study: A population-based prospective cohort study. Sci Rep. 2018;8(1):10566. doi: 10.1038/s41598-018-28646-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fest J, Ruiter R, Mulder M, et al. The systemic immune-inflammation index is associated with an increased risk of incident cancer-A population-based cohort study. Int J Cancer. 2020;146(3):692–98. doi: 10.1002/ijc.32303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li B, Li W, Li X, Zhou H. Inflammation: A novel therapeutic target/direction in atherosclerosis. Curr Pharm Des. 2017;23(8):1216–27. doi: 10.2174/1381612822666161230142931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaplan O, Demircan G. Relationship of autophagy and apoptosis with total occlusion of coronary arteries. Med Sci Monit. 2018;24:6984–88. doi: 10.12659/MSM.910763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sezer M, Okcular I, Goren T, et al. Association of haematological indices with the degree of microvascular injury in patients with acute anterior wall myocardial infarction treated with primary percutaneous coronary intervention. Heart. 2007;93(3):313–18. doi: 10.1136/hrt.2006.094763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mangold A, Alias S, Scherz T, et al. Coronary neutrophil extracellular trap burden and deoxyribonuclease activity in ST-elevation acute coronary syndrome are predictors of ST-segment resolution and infarct size. Circ Res. 2015;116(7):1182–92. doi: 10.1161/CIRCRESAHA.116.304944. [DOI] [PubMed] [Google Scholar]
- 36.Suarez-Cuenca JA, Ruiz-Hernandez AS, Mendoza-Castaneda AA, et al. Neutrophil-to-lymphocyte ratio and its relation with pro-inflammatory mediators, visceral adiposity and carotid intima-media thickness in population with obesity. Eur J Clin Invest. 2019;49(5):e13085. doi: 10.1111/eci.13085. [DOI] [PubMed] [Google Scholar]
- 37.Dragu R, Khoury S, Zuckerman R, et al. Predictive value of white blood cell subtypes for long-term outcome following myocardial infarction. Atherosclerosis. 2008;196(1):405–12. doi: 10.1016/j.atherosclerosis.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 38.Ibrahim H, Schutt RC, Hannawi B, et al. Association of immature platelets with adverse cardiovascular outcomes. J Am Coll Cardiol. 2014;64(20):2122–29. doi: 10.1016/j.jacc.2014.06.1210. [DOI] [PubMed] [Google Scholar]
- 39.Zhang H, Qiu B, Zhang Y, et al. The value of pre-infarction angina and plasma D-dimer in predicting no-reflow after primary percutaneous coronary intervention in ST-segment elevation acute myocardial infarction patients. Med Sci Monit. 2018;24:4528–35. doi: 10.12659/MSM.909360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Velders MA, Wallentin L, Becker RC, et al. Biomarkers for risk stratification of patients with ST-elevation myocardial infarction treated with primary percutaneous coronary intervention: Insights from the Platelet Inhibition and Patient Outcomes trial. Am Heart J. 2015;169(6):879–89. doi: 10.1016/j.ahj.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 41.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352(16):1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 42.Ferroni P, Basili S, Davi G. Platelet activation, inflammatory mediators and hypercholesterolemia. Curr Vasc Pharmacol. 2003;1(2):157–69. doi: 10.2174/1570161033476772. [DOI] [PubMed] [Google Scholar]
- 43.Balta S, Demirkol S, Kucuk U. The platelet lymphocyte ratio may be useful inflammatory indicator in clinical practice. Hemodial Int. 2013;17(4):668–69. doi: 10.1111/hdi.12058. [DOI] [PubMed] [Google Scholar]
- 44.Balta S, Ozturk C. The platelet-lymphocyte ratio: A simple, inexpensive and rapid prognostic marker for cardiovascular events. Platelets. 2015;26(7):680–81. doi: 10.3109/09537104.2014.979340. [DOI] [PubMed] [Google Scholar]
- 45.Marçula M, de Souza Buto MF, Madaloso BA, et al. Lymphocyte count and prognosis in patients with heart failure. Int J Cardiol. 2015;188:60–62. doi: 10.1016/j.ijcard.2015.04.043. [DOI] [PubMed] [Google Scholar]
- 46.Vaduganathan M, Ambrosy AP, Greene SJ, et al. Predictive value of low relative lymphocyte count in patients hospitalized for heart failure with reduced ejection fraction: insights from the EVEREST trial. Circ Heart Fail. 2012;5(6):750–58. doi: 10.1161/CIRCHEARTFAILURE.112.970525. [DOI] [PubMed] [Google Scholar]
- 47.Li J, Ley K. Lymphocyte migration into atherosclerotic plaque. Arterioscler Thromb Vasc Biol. 2015;35(1):40–49. doi: 10.1161/ATVBAHA.114.303227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bian C, Wu Y, Shi Y, et al. Predictive value of the relative lymphocyte count in coronary heart disease. Heart Vessels. 2010;25(6):469–73. doi: 10.1007/s00380-010-0010-7. [DOI] [PubMed] [Google Scholar]
- 49.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348(2):138–50. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 50.Iscanli MD, Metin Aksu N, Evranos B, et al. Comparison of TIMI and Gensini score in patients admitted to the emergency department with chest pain, who underwent coronary angiography. Med Sci Monit. 2014;20:343–49. doi: 10.12659/MSM.889600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Damman P, van’t Hof AW, Ten Berg JM, et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Comments from the Dutch ACS working group. Neth Heart J. 2017;25(3):181–85. doi: 10.1007/s12471-016-0939-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang M, Hao J, Jian Z, et al. Effects of preoperative risk stratification on direct in-hospital costs for Chinese patients with coronary artery bypass graft: A single center analysis. Curr Med Sci. 2018;38(6):1075–80. doi: 10.1007/s11596-018-1986-y. [DOI] [PubMed] [Google Scholar]
- 53.Bohula EA, Bonaca MP, Braunwald E, et al. Atherothrombotic risk stratification and the efficacy and safety of vorapaxar in patients with stable ischemic heart disease and previous myocardial infarction. Circulation. 2016;134(4):304–13. doi: 10.1161/CIRCULATIONAHA.115.019861. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.
Clinical outcomes of the study population during hospitalization and long-term follow-up.
| Clinical outcomes | In-hospital | Long-term follow-up |
|---|---|---|
| Death, n (%) | 52 (7.31) | 80 (11.25) |
| Reinfarction, n (%) | 4 (0.56) | 3 (0.42) |
| Revascularization, n (%) | 1 (0.14) | 4 (0.56) |
| Arrhythmia, n (%) | 119 (16.74) | 61 (8.58) |
| Congestive heart failure, n (%) | 179 (25.18) | 116 (16.32) |
| Cerebrovascular accident, n (%) | 13 (1.83) | 7 (0.98) |
Supplementary Table 2.
Correlation between SII and other laboratory measurements.
| STEMI | NSTEMI | |||
|---|---|---|---|---|
| r | p | r | p | |
| Gensini score | −0.05 | 0.29 | 0.18 | <0.01 |
| Preprocedural TIMI grade | −0.06 | 0.23 | −0.08 | 0.15 |
| Postprocedural TIMI grade | −0.06 | 0.20 | −0.05 | 0.38 |
| Killip class | 0.25 | <0.01 | 0.35 | <0.01 |
| CTnI | 0.31 | <0.01 | 0.30 | <0.01 |
| LVEF | −0.21 | <0.01 | −0.04 | 0.45 |
| WBC | 0.63 | <0.01 | 0.57 | <0.01 |
SII – systemic immune-inflammation index; STEMI – ST elevation myocardial infarction; NSTEMI – non-ST elevation myocardial infarction; TIMI – thrombolysis in myocardial infarction; CTnI – cardiac troponin I; WBC –white blood cell; LVEF – left ventricular ejection fraction.