Abstract
Background
This study was to investigate the correlation between osteoporosis and serum uric acid in ankylosing spondylitis (AS) patients, and to further identify potential factors that might be associated with osteoporosis in AS patients.
Material/Methods
We included 182 AS patients, consisted of 143 male patients and 39 female patients, who visited our hospital from January 1, 2014 to December 31, 2018. We used dual-energy x-ray absorptiometry to measure bone mineral density (BMD) of orthotopic lumbar vertebrae in patients with AS. The gender, age, disease duration, BMD, T-score, Z-score, uric acid, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), blood platelet (PLT), and status of treatment with biologics of the patients were collected. Then, the Spearman correlation coefficient and multivariate liner regression analysis were applied to identify the relationship between the factors and BMD, T-score, and Z-score in AS patients.
Results
Male AS patients between the ages of 16 and 30 years old had a higher risk of osteoporosis (P<0.05). AS patients with uric acid value between 300–360 μmol/L had the highest BMD, T-score, and Z-score. The BMD had a positive correlation with age and disease duration (P<0.01) while had a negative correlation with PLT (P<0.05). BMD in AS patients with elevated ESR was significantly (P<0.05) lower than in AS patients with normal ESR. There were no significant differences in BMD between AS patients with elevated CRP and the patients with normal CRP and PLT. Treatment with TNFi (tumor necrosis factor alpha inhibitor) did not improve BMD in AS patients.
Conclusions
The relationship between uric acid and BMD in AS patients was observed as inverted “U”-type. Keeping uric acid within 300–360 μmol/L might be helpful in preventing AS patients from developing osteoporosis.
MeSH Keywords: Osteoporosis; Spondylitis, Ankylosing; Uric Acid
Background
Ankylosing spondylitis (AS) is a disease characterized by inflammation of the spine and ligaments. The occurrence of AS is mainly related to factors such as heredity, chronic infection, and imbalance of autoimmune function. About 96% of patients with AS have been detected with human leukocyte antigen B27 (HLA-B27), probably because the HLA-B27 gene population suffers from some acquired stimulation leading to specific pathological changes.
The prevalence of osteoporotic fractures in AS patients is increasing [1]. Osteopenia was found in the early stage of AS [2]. Osteoporosis is characterized by decreased bone mass and micro-structure damage, and increased bone fragility is more prone to fractures [3]. Due to differences in measurement method and measurement site of bone mineral density (BMD), the incidence of osteoporosis in AS patients significantly differs from report to report. A Chinese study, which included 1051 AS patients with BMD evaluated using dual-energy x-ray method, found that the rate of osteoporosis (at significance T-score ≤–2.5) was 26.83% at the lumbar spine, 7.33% at the femoral neck, and 11.23% at the femoral trochanter [4]. For vertebrae, the progression of AS is associated with osteitis and trabecular bone loss, leading to an increased risk of osteoporosis and fracture in AS patients [5].
Oxidative stress is one of the pathological mechanisms in osteoporosis. The anti-oxidation function of serum uric acid (UA) might be the reason why UA can resist osteoporosis [6,7]. A large-sample cross-sectional study had found that serum UA over moderate levels was associated with an elevated BMD and a reduction in the incidence of osteoporosis [8]. Another large-scale cross-sectional clinical in vitro study showed that serum UA prevented osteoporosis by reducing reactive oxygen species production in osteoclast precursors, and by inhibiting osteoclastogenesis [9].
The relationship between UA and osteoporosis are being investigated. A study [10] from South Korea which included 150 AS patients showed that UA levels were positively correlated with BMD in AS patients. As UA level increased, the BMD level increased, and the T-score and Z-score were highly consistent.
As a natural antioxidant, UA is correlated with osteoporosis. However, current studies have not proven that whether the higher the UA concentration is, the more benefit to the patient. The main purpose of this study was to investigate the correlation between osteoporosis and serum UA in Chinese AS patients, and to further identify potential factors that might be associated with osteoporosis in Chinese AS patients.
Material and Methods
The patients
This was a retrospective analysis of medical records obtained from the First Affiliated Hospital of Guangzhou University of Chinese Medicine (Guangzhou, China). We enrolled 519 consecutive patients with AS evaluated from January 1, 2014 to December 31, 2018. There were 182 patients (143 male patients and 39 female patients) with AS who met our inclusion criteria. Patients with AS between the ages of 15 and 50 years old fulfilled the modified New York criteria for the classification of AS [11]. The study exclusion criteria were as follows: 1) patients with AS whose age was not within 15 to 50 years old (to avoid the potential interference due to the increased incidence of osteoporosis post-menopause in female patients). 2) Patients with AS who were unable to perform BMD measurements. 3) Patients who had used certain drugs: uric acid-lowering drugs such as allopurinol, fenofibrate, benzbromarone, and other medicines including diuretics, β-blocker; patients who had undergone long-term treatment with glucocorticoids; and patients who had used bisphosphonates in the past 1 year. 4) Patients with certain diseases: psoriasis, inflammatory bowel disease, reactive arthritis, thyroid or parathyroid disorders, chronic liver disease, and chronic kidney disease, diabetes. 5) Patients with vertebral fractures. 6) Patients who underwent joint replacement. Figure 1 shows our process of screening AS patients. This study was approved by the ethics committees of the First Affiliated Hospital of Guangzhou University of Chinese Medicine (No. ZYYECK [2018]173).
Figure 1.

Flowchart of inclusion and exclusion.
Baseline data
Gender, age, disease duration, and BMD were accurately recorded for all participants.
BMD test
BMD was tested in the lumbar vertebrae L1–L4 region of the AS patients by dual-energy x-ray absorptiometry analyzer. The results were calculated using the BMD of the lumbar spine. BMD tests were all taken at the same parameters and expressed as the number of grams of bone mineral per square centimeter (g/cm2). The T-score was the number of standard deviation (SD) of BMD above or below the mean of a healthy 30-year-old adult of the same sex and ethnicity as the patient. The Z-score was the number of SD compared to the age-matched mean BMD. The BMD coefficient of variation measured by the instrument is <1%. BMD, T-score, and Z-score are the indicators to diagnose osteoporosis.
Laboratory measurements
Tests included UA, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and blood platelet (PLT). Although ESR and CRP were not specific diagnostic indicators for patients with AS, they were significant for the auxiliary diagnosis of AS and therefore we incorporated them in our analysis.
UA was measured by enzymatic colorimetric method, with a normal reference value of 150~360 μmol/L in women and 150–420 μmol/L in men. ESR was measured via the Westergren method with a normal reference value of 0~15 mm/hour. CRP was detected via the nephelometry method with a normal reference value of 0~8 mg/L. PLT was calculated via flow cytometry with a normal reference value of 100~300×109/L.
Statistical analysis
SPSS 23.0 was utilized for statistical analysis and Prism 7.0 software was used for graphics generation. The categorical outcome can be described by frequency or percentage, and the continuous outcomes can be expressed by the mean and SD. The comparison between groups was performed by 2 independent sample t-test or 2 independent sample rank-sum test; Differences between groups were analyzed via the chi-square test. P<0.05 was considered statistically significant. Correlation factors were analyzed using Spearman correlation factor analysis and multivariate analysis using multivariate liner regression analysis.
Results
Characteristics of the AS patients
The mean age of the 182 AS patients was 30.07±9.61 years. The gender ratio of male and female patients was 3.7: 1. The mean disease duration was 83.73±72.21 months. The mean BMD was 0.87±0.18 g/cm2. HLA-B27 test was positive in 96.8% of the AS patients. 42.3% of the AS patients had treated with biologic disease-modifying antirheumatic drugs (bDMARDs) only. Celecoxib was the most frequently used drug. Over 90% of the patients had taken nonsteroidal anti-inflammatory drugs (NSAIDs). Close 40% of the patients had taken sulfasalazine which was the most used conventional synthetic DMARDs (csDMARDs) (Table 1, Figure 2).
Table 1.
The baseline characteristics of study participants.
| Variable | Mean±SD (N=182) |
|---|---|
| Age, mean (years)±SD | 30.07±9.61 |
| Gender, n (%) | |
| Male | 143 (78.6%) |
| Disease duration (month), mean±SD | 83.73±72.21 |
| Body mass index (kg/m2), mean±SD | 0.87±0.18 |
| T-score, mean±SD | −1.94±2.0 |
| Z-score, mean±SD | −1.80±1.9 |
| Positive rate of HLA-B27, n (%) | 176 (96.8%) |
| Medicine n (%) | |
| Celecoxib | 138 (75.82%) |
| bDMARDs | 75 (41.21%) |
| D-cal | 66 (36.26%) |
| SSZ | 63 (34.07%) |
| Alfacalcitriol | 32 (17.58%) |
| Calcitriol | 30 (16.48%) |
| Meloxicam | 21 (11.54%) |
| Caltrate | 13 (7.14%) |
| Etoricoxib | 10 (5.49%) |
| Thalidomide | 9 (4.95%) |
| MTX | 7 (3.85%) |
| Predinisone | 3 (1.65%) |
| Aclasta | 2 (1.10%) |
Figure 2.
Medications used in ankylosing spondylitis patients.
Correlation analysis between the factors and BMD in AS patients
By using Spearman correlation factor analysis, we found that BMD was positively correlated with disease duration and age (P<0.01), negatively correlated with PLT (P<0.05), and no correlation with ESR, CRP, and UA (Figure 3, Table 2). The results of T-score and Z-score were consistent with BMD results (not shown). Subsequently, we performed a multivariate liner regression analysis of variables including age, duration, and PLT. The results showed that the correlation was significant between BMD and age (r=0.347, P<0.01), but not disease duration and PLT (Table 3).
Figure 3.
Linear regression analysis between bone mineral density (at the lumbar spine) and uric acid (UA), age, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), blood platelet (PLT), and disease duration of ankylosing spondylitis patients. (A) UA; (B) Age; (C) ESR; (D) CRP; (E) PLT; and (F) Disease duration.
Table 2.
Spearman correlation analysis of BMD with age, duration, uric acid, C-reactive protein, erythrocyte sedimentation rate, and blood platelet count.
| Age | Duration | UA | CRP | ESR | PLT | ||
|---|---|---|---|---|---|---|---|
| BMD | r valve | 0.431 | 0.213 | −0.147 | −0.137 | −0.142 | −0.176 |
| P valve | 0.001 | 0.004 | 0.052 | 0.065 | 0.057 | 0.018 |
Spearman correlations performed for continuous variables. BMD – bone mineral density; UA – uric acid; CRP – C-reactive protein; ESR – erythrocyte sedimentation rate; PLT – blood platelet
Table 3.
Multivariate liner regression model predicting the relationship between BMD and variables.
| Effect | Partial regression coefficient | SE | t | P | 95% CI |
|---|---|---|---|---|---|
| Age | 0.006 | 0.002 | 3.619 | <0.001 | 0.003~0.009 |
| Duration | 0.001 | 0.001 | 1.562 | 0.120 | 0.000~0.001 |
| PLT | 0.001 | 0.001 | −1.325 | 0.187 | – |
| Constant | 0.748 | 0.07 | 10.658 | <0.001 | 0.609~0.886 |
BMD – bone mineral density; SE – standard error.
Serum UA and osteoporosis acted as an inverted “U” type correlation in AS patients especially in male AS patients
The patients were divided into 3 groups: UA ≤300 μmol/L group, UA within 300–360 μmol/L group, and UA ≥360 μmol/L group. Results showed that BMD was significantly higher in the patients with UA within 300–360 μmol/L group than the other 2 groups. The results of T-scores and Z-scores were consistent with BMD. The same result was observed in male patients. These results suggested that AS patients with UA level ranging from 300–360 μmol/L had the best BMD, T-score, and Z-score (Figure 4), especially in male AS patients. This result suggested that UA acted as an inverted “U”-type correlation with osteoporosis.
Figure 4.
(A–C) Bone mineral density (BMD) in the lumbar spine according to serum uric acid (UA) tertiles. (A) BMD; (B) T-score; and (C) Z-score. (D–K) Differences in BMD, T-score, Z-score, UA, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and blood platelet (PLT) between male and female ankylosing spondylitis patients.
Male AS patients between the ages of 16 and 30 had a higher risk of osteoporosis
The patients were divided into 3 groups: T-score <2.5 group, T-score within −1~2.5 group, and T-score >−1 group. We employed the T-score to determine if the BMD was normal, and when T <−2.5, it indicated that there was osteoporosis in AS patients. Stratified by gender, chi-square test showed that age was significantly associated with osteoporosis in male patients with AS (χ2=26.543, P<0.001), while in the female patients was not (χ2=6.611, P>0.1). It was observed that T-score in male AS patients was significantly lower than that in female AS patients, which suggested that the male AS patients were more likely to develop osteoporosis than female AS patients, especially between the ages of 16 and 30 (Figure 5). We further investigated the differences in all the factors between male and female patients. It showed that BMD in female AS patients was significantly higher than that in male AS patients. The results of T-score and Z-score were consistent with BMD. There was a statistically significant difference in UA and CRP between male and female AS patients. There were no significant differences in age, disease duration, PLT, and ESR between genders (Figure 4). This suggested that the male AS patients were more likely to develop osteoporosis than female AS patients.
Figure 5.
The T-score in ankylosing spondylitis patients with different gender and age ranges.
Relationship between inflammatory indicators and osteoporosis
ESR, CRP, and PLT are the most commonly used laboratory tests in assessing the level of inflammation in AS patients in our daily practice.
The patients were divided as either above or below the reference values of ESR of 15 mm/hour, CRP of 8 mg/L and PLT of 300×109/L to assess the correlation between ESR, CRP, PLT, and BMD. It was found that AS patients with ESR ≤15 mm/hour had better BMD than the patients with ESR >15 mm/hour. The results of T-scores and Z-scores were consistent with BMD. But this only happened in male patients not female patients. There were no statistically significant differences in BMD, T-score, and Z-score between the patients with normal CRP, PLT, and elevated CRP, PLT.
This suggested that elevated ESR might be a useful indicator in predicting osteoporosis in male AS patients (Figure 6).
Figure 6.
Bone mineral density (BMD), T-score, and Z-score according to different levels of erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and blood platelet (PLT) in total AS patients. (A) BMD; (B) T-score; (C) Z-score; (D) BMD in male and female patients; (E) T-score in male and female patients; and (F) Z-score in male and female patients.
Besides, it also showed that female patients had higher BMD, T-score, and Z-score when the AS patients had elevated ESR, CRP, or PLT, which also indicated that the male patients were more likely to develop osteoporosis.
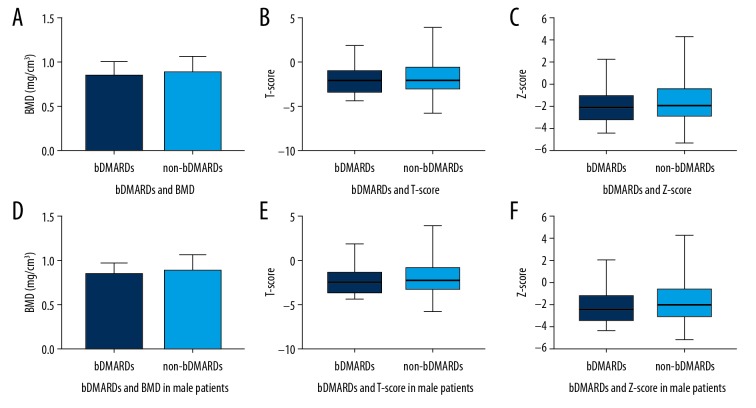
Treatment with bDMARDs and osteoporosis
The patients were divided into 2 groups: the bDMARD group, the AS patients who had previously used or were currently using bDMARDs (Such as Enbrel Humira, Remicaid, Yisaipu (a kind of Enbrel-biomimic) for at least 3 months, and the non-bDMARDs group, AS patients who had never used any bDMARDs or had used some bDMARDs for no more than 3 months. It showed that there was no significant difference between the 2 groups in BMD, T-score, and Z-score.
This might indicate that treating AS with bDMARDs is not helpful in preventing AS patients from developing osteoporosis (Figure 7).
Figure 7.
Bone mineral density (BMD), T-score, and Z-score according to whether treatment with biologic disease-modifying antirheumatic drugs (bDMARDs). (A) BMD in total patients; (B). T-score in total patients; (C) Z-score in total patients. (D) BMD in male patients. (E) T-score in male patients. (F) Z-score in male patients.
Discussion
In our study, 78.6% of patients were male, aged between 16 and 30, which was consistent with a previous study in China [12]. We also found that female AS patients had lower risk in developing osteoporosis than AS patients, which is consistent with other studies [12]. It is believed that androgen is involved in the differentiation of osteoblasts, and estrogen might be involved in the regulation of bone resorption [6]. A previous study showed that decreased concentrations of bioactive estradiol in men leads to accelerated bone turnover and decreased BMD, while testosterone deficiency and estrogen were important contributors to osteoporosis [13]. Another study has found that follicle-stimulating hormone can increase bone formation [14]. Unfortunately, it is unknown if the results are related to sex hormones. Further research should be taken in the next step.
In this study, we found that AS patients were more likely to have a better BMD when the serum UA concentration was within 300–360 μmol/L, especially in male AS patients, which was not consistent with the conclusion from a South Korea study [10]. In our study, an inverted “U”-type correlation was observed between osteoporosis and serum UA.
A retrospective study [15] of 2150 samples from Liuzhou, China, found that UA concentration was closely related to BMD. Maintaining a certain level of UA can prevent bone loss, however, patients with UA above that threshold will have a decreased BMD. The conclusion of the aforementioned study is consistent with our findings. So, keeping the UA concentration within 300–360 μmol/L might be helpful in preventing AS patients from osteoporosis.
Oxidative stress has an effect on bone metabolism and is one of the independent risk factors for osteoporosis [16]. Increased oxidative stress or decreased antioxidant capacity in humans might lead to osteoporosis. UA acts as an important antioxidant in the body, which participates in redox reactions, and has anti-oxidation and anti-DNA damage effects [17].
Relatively high levels of UA might be beneficial for BMD maintenance, but abnormally high UA levels would result in crystals form at the joints and increasing bone destruction [18], which was detrimental to the patient’s quality of life. It’s necessary to control the UA levels within a proper range, since studies have shown that hyperuricemia was an independent risk factor for type 2 diabetes, hypertension, and cardiovascular disease.
In AS, there are a variety of inflammatory chemokines and cytokines involved in. Chronic inflammation can inhibit the formation of osteoblast cells and stimulate osteoclast differentiation, and finally lead to systemic bone loss and secondary osteoporosis. In this study we found that elevated ESR was associated with low BMD level in AS patients, but not CRP or PLT. Elevated ESR might be an effective indicator in predicting osteoporosis in AS patients.
In our study, we also found that age and disease duration were positively correlated with BMD. Generally speaking, BMD should gradually decrease with age, but due to the particularity of AS patients, osteoblasts were active during the late stages of disease progression, leading to calcification of ligaments and tendons, and BMD increased. Besides, in this study, the Image result for dual-energy X-ray absorptiometry (DXA) method was used to measure the BMD of lumbar vertebrae, which is widely used in our clinical practice. The data obtained via DXA in the measurement is the area BMD, which cannot distinguish between cortical bone and cancellous bone, also cannot distinguish between pathological osteogenesis, such as ligament callus, and articular surface fusion caused by inflammation in the spine.
In AS, as the osteoporosis developing, accompanied with new bone formation, such as syndesmophytes and facet joint fusion, especially in vertebrae. Osteitis is associated with loss of trabecular bone, leading to increased risk of osteoporosis and fracture, while new bone formation leads to ligament callus formation and ultimately to rigidity. The changes can occur simultaneously in a single AS patient. A study [19] of 204 AS patients with BMD in different sites demonstrated that, the BMD was quite different among the lumbar spine, total hip region, femoral neck and total tibia by DXA method. Another 10-year follow-up study [20] of 15 AS patients found that, BMD decreased significantly by quantitative computed tomography (CT) measurement, however, the BMD increased by 0.15 g/cm2 at anteroposterior lumbar spine by DXA measurement. This also can explain the effect of pathological osteogenesis in AS on lumbar spine BMD.
The result from the lumbar spine might be falsely higher than the real value due to the interference of calcification and rigidity of the late spinal pathogenesis. It would be necessary and helpful for predicting the risk of osteoporosis and fracture in AS patient by taking BMD test at both the lumbar spine and femoral neck.
In this study, we found that bDMARD treatment might not be beneficial in preventing osteoporosis in patients with AS. It concluded that the bDMARD treatment of AS patients did not increase the incidence of osteoporosis, considering the lower dose of bDMARD used in the treatment of AS. A series of studies suggested that different kind of bDMARDs have different effects on bone metabolism [21–23], such as leflunomide and hydroxychloroquine inhibiting the differentiation and maturation of osteoclasts and reducing bone loss, cyclophosphamide might aggravate the symptoms of osteoporosis because it inhibits bone formation and affects cellular calcium absorption, low doses of methotrexate generally have no effect on BMD in patients with AS, SSZ (sulfasalazine) inhibits bone formation mainly through Inhibition of cysteine or glutamate transporters. MMF (mycophenolate mofetil), cyclosporine, and azathioprine have relatively limited effects on bone metabolism and might require more study.
Conclusions
The relationship between UA and BMD in AS patients was observed as an inverted “U”-type correlation, especially in male patients. Keeping UA within 300–360 μmol/L might be helpful in preventing AS patients from developing osteoporosis. Male AS patients between the ages of 16 and 30 years old were more likely to develop osteoporosis. Low BMD level was correlated with elevated ESR, but not CRP or PLT.
Limitations
Unfortunately, the BASDAI (Bath Ankylosing Spondylitis Disease Activity Index) scores, imaging grading, and BMI data of the AS patients were not collected and analyzed, which these measures might likely be correlated to BMD.
Besides, this is a retrospective cross-sectional study, and we are unable to determine if these potential factors are causally correlated to the BMD in AS. Further longitudinal follow-up studies would be necessary and helpful.
Footnotes
Source of support: This study was supported by the 2019 Science Fund from Traditional Chinese Medicine Bureau of Guangdong Province (No. 20191110), and Guangzhou Science Technology and Innovation Commission (No. 201904010177)
Conflicts of interests
None.
References
- 1.Cooper C, Carbone L, Michet CJ, et al. Fracture risk in patients with ankylosing spondylitis: a population-based study. J Rheumatol. 1994;21(10):1877–82. [PubMed] [Google Scholar]
- 2.Lee YS, Schlotzhauer T, Ott SM, et al. Skeletal status of men with early and late ankylosing spondylitis. Am J Med. 1997;103(3):233–41. doi: 10.1016/s0002-9343(97)00143-5. [DOI] [PubMed] [Google Scholar]
- 3.Gao M, Li SL. [Research progress on the relationship between serum uric acid and osteoporosis]. Chinese Journal of Osteoporosis. 2016;22:641–46. [in Chinese] [Google Scholar]
- 4.Kong WP, Zhang W, Tao QW, et al. [Analysis of bone mineral density in 1051 patients with ankylosing spondylitis]. Chinese Journal of Osteoporosis. 2012;18(11):1036–41. [in Chinese] [Google Scholar]
- 5.Vosse D, Landew é R, van der Hejide HD, et al. Ankylosing spondylitis and the risk of fracture: Results from a large primary care-based nested case-control study. Ann Rheum Dis. 2009;68(12):1839–42. doi: 10.1136/ard.2008.100503. [DOI] [PubMed] [Google Scholar]
- 6.Manolagas SC. From estrogen-centric to aging and oxidative stress: Revised perspective of the pathogenesis of osteoporosis. Endocr Rev. 2010;31(3):266–300. doi: 10.1210/er.2009-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Almedia M, O’Brien CA. Basic biology of skeletal aging: Role of stress response pathways. J Gerontol A Biol Sci Med Sci. 2013;68(10):1197–208. doi: 10.1093/gerona/glt079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nabipour I, Sambrook PN, Blyth FM, et al. Serum uric acid is associated with bone health in older men: A cross-sectional population-based study. J Bone Miner Res. 2011;26(5):955–64. doi: 10.1002/jbmr.286. [DOI] [PubMed] [Google Scholar]
- 9.Ahn SH, Lee SH, Kim BJ, et al. Higher serum uric acid is associated with higher bone mass, lower bone turnover, and lower prevalence of vertebral fracture in healthy postmenopausal women. Osteoporos Int. 2013;24(12):2961–70. doi: 10.1007/s00198-013-2377-7. [DOI] [PubMed] [Google Scholar]
- 10.Kang KY, Hong YS, Park SH, et al. Low levels of serum uric acid increase the risk of low bone mineral density in young male patients with ankylosing spondylitis. J Rheumatol. 2015;42(6):968–74. doi: 10.3899/jrheum.140850. [DOI] [PubMed] [Google Scholar]
- 11.Linden SM, Valkenburg HA, Cats A. Evaluation of the diagnostic criteria for ankylosing spondylitis: A proposal for modification of the New York Criteria. Arthritis Rheum. 1984;27:361–68. doi: 10.1002/art.1780270401. [DOI] [PubMed] [Google Scholar]
- 12.Jin Y, Yang S, Yan ZR, et al. [Analysis of bone mineral density in men and women with ankylosing spondylitis]. Chinese Journal of Osteoporosis. 2015:612–17. [in Chinese] [Google Scholar]
- 13.Fink HA, Ewing SK, Ensrud KE, et al. Association of testosterone and estradiol deficiency with osteoporosis and rapid bone loss in older men. J Clin Endocrinol Metab. 2006;91(10):3908–15. doi: 10.1210/jc.2006-0173. [DOI] [PubMed] [Google Scholar]
- 14.Seitz S, Keller J, Schilling AF, et al. Pharmacological estrogen administration causes a FSH-independent osteo-anabolic effect requiring ER alpha in osteoblasts. PLoS One. 2012;7:e50301. doi: 10.1371/journal.pone.0050301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li RS, Li WJ, Wang Y, et al. The relationship between blood uric acid levels and bone mineral density in people of Liuzhou City. Medical Innovation of China. 2016;13(15):138–41. [Google Scholar]
- 16.Glantzounis GK, Tsimoyiannis EC, Kappas AM, Galaris DA. Uric acid and oxidative stress. Curr Pharm Des. 2005;11(32):4145–51. doi: 10.2174/138161205774913255. [DOI] [PubMed] [Google Scholar]
- 17.Strazzullo P, Puig J. Uric acid and oxidative stress: Relative impact on cardiovascular risk. Nutr Metab Cardiovasc Dis. 2007;17:409–14. doi: 10.1016/j.numecd.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Ashika C, Callon KE, Bregina P, et al. Monosodium urate monohydrate crystals inhibit osteoblast viability and function: Implications for development of bone erosion in gout. Ann Rheum Dis. 2011;70:1684–91. doi: 10.1136/ard.2010.144774. [DOI] [PubMed] [Google Scholar]
- 19.Klingberg E, Lorentzon M, Mellström D, et al. Osteoporosis in ankylosing spondylitis – prevalence, risk factors and methods of assessment. Arthritis Res Ther. 2012;14(3):R108. doi: 10.1186/ar3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korkosz M, Gasowski J, Grzanka P, et al. Baseline new bone formation does not predict bone loss in ankylosing spondylitis as assessed by quantitative computed tomography (QCT): 10-year follow-up. BMC Musculoskelet Disord. 2011;12(1):121. doi: 10.1186/1471-2474-12-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin C, Zhang P, Zhang M, et al. Inhibition of SLC7A11 by sulfasalazine enhances osteogenic differentiation of mesenchymal stem cells by modulating BMP2/4 expression and suppresses bone loss in ovariectomized mice. J Bone Miner Res. 2017;32:508–21. doi: 10.1002/jbmr.3009. [DOI] [PubMed] [Google Scholar]
- 22.Rexhepi S, Rexhepi M, Sahatçiu-Meka V, et al. The impact of low-dose disease-modifying anti-rheumatics drugs (DMARDs) on bone mineral density of premenopausal women in early rheumatoid arthritis. Med Arch. 2016;70:101–3. doi: 10.5455/medarh.2016.70.101-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang Z, Feng F, Li T. [Effect of cDMARD on bone metabolism]. New Medicine. 2018;49(3):155–58. [in Chinese] [Google Scholar]