Abstract
Objectives
To estimate birth prevalence of congenital cytomegalovirus (cCMV) in HIV-exposed uninfected children born in the current era of combination antiretroviral therapy and describe cCMV-related neurodevelopmental and hearing outcomes.
Study design
The Surveillance Monitoring for ART Toxicities cohort study follows HIV-exposed uninfected children at 22 sites in the US and Puerto Rico. Birth cCMV prevalence was estimated in a subset of participants who had blood pellets collected within three weeks of birth and underwent ≥1 of 6 assessments evaluating cognitive and language development including an audiologic examination between 1 and 5 years of age. Detection of CMV DNA by polymerase chain reaction testing of peripheral blood mononuclear cells was used to diagnose cCMV. Proportions of suboptimal assessment scores were compared by cCMV status using Fisher exact test.
Results
Mothers of 895 eligible HIV-exposed uninfected children delivered between 2007 and 2015. Most (90%) were on combination antiretroviral therapy, 88% had an HIV viral load of ≤400 copies/mL, and 93% had CD4 cell counts of ≥200 cells/μL. Eight infants were diagnosed with cCMV, yielding an estimated prevalence of 0.89% (95% CI, 0.39%−1.75%). After adjusting for a sensitivity of 70%−75% for the testing method, projected prevalence was 1.2%−1.3%. No differences were observed in cognitive, language and hearing assessments by cCMV status.
Conclusions
Although birth cCMV prevalence in HIV-exposed uninfected children born to women with well-controlled HIV is trending down compared with earlier combination antiretroviral therapy-era estimates, it is above the 0.4% reported for the general US population. HIV-exposed uninfected children remain at increased risk for cCMV.
Cytomegalovirus (CMV) is ubiquitous with world-wide distribution and is the leading cause of congenital infections and nongenetic hearing loss in children. Birth prevalence of congenital CMV (cCMV) infection varies widely, is population dependent, and parallels maternal seroprevalence.1 The cCMV and hearing multicenter screening study conducted at seven centers within the US reported a birth prevalence of 0.4%.2 Lower socioeconomic status and young maternal age are other important demographic characteristics associated with higher birth prevalence.1 Cohort studies in high-income countries before the implementation of combination antiretroviral (ARV) therapy (cART) have shown rates of in utero transmission of CMV in infants born to women living with HIV-1 (WLHIV) infection ranging from 2.5% to 9.2%, identifying this subpopulation of women as very high risk.3–7 Of these studies, those that specifically examined the birth prevalence of cCMV in HIV-exposed uninfected infants reported estimates that ranged from 2.8% to 4.5%.3–5 The Pediatric Pulmonary and Cardiovascular Complications Study of infants born to WLHIV from 1990 to 1994 reported a prevalence of 4.3% in HIV-exposed uninfected infants, well above the general population.4 Studies that addressed cCMV prevalence following implementation of cART showed inconsistent conclusions. The French Perinatal Cohort Study demonstrated a decline of cCMV from 3.0% in 1993–1996 to 1.5% in 2001–2004, as did a Spanish study by Marin Gabriel et al, from 9.2% in 1987–1996 to 1.3% in 1997–2003.5,6 Other investigators have reported an overall high cCMV prevalence occurring in the era of cART: 4.8% from 1997–2002, 3% from 1997–2005, and 2.2% from 1997–2013.5,8,9 We are now on the verge of entering the third decade in the era of cART, with mother-to-child transmission rates of HIV well below 1%, and approximately 8700 infants born annually in the US who are HIV-exposed and uninfected.10
We therefore undertook this study to estimate the birth prevalence of cCMV in HIV-exposed uninfected infants and children followed in the Surveillance Monitoring for ART Toxicities (SMARTT) cohort who were born to WLHIV over the last 10 years at participating clinical sites in the US and Puerto Rico.11 Most of these women were closely monitored and treated with cART during their pregnancy; their HIV infection was well-controlled. Their children underwent longitudinal neurodevelopmental (ND) testing from 1 through 5 years of age, just before entrance into kindergarten, and so we also sought to compare hearing and ND outcomes in HIV-exposed uninfected children with and without cCMV.
Methods
SMARTT is an ongoing observational cohort study conducted at 22 sites within the US, including Puerto Rico, that evaluates the growth and development of HIV-exposed uninfected children. The dynamic cohort of SMARTT commenced enrolling HIV-exposed uninfected infants at birth in 2007 and as of February 1, 2017, had accrued 2579 infants and children. Medical history, physical examinations, and a battery of ND assessments were performed at protocoldefined intervals as previously described; this included a peripheral blood mononuclear cell (PBMC) pellet collected from infants at birth.11,12 Medical diagnoses were obtained from the medical record and indexed using the MedDRA coding system.13 The study was approved by the Institutional Review Boards of the Harvard T. H. Chan School of Public Health and all participating sites. Written informed consent was obtained from legal guardians.
The birth prevalence of cCMV was estimated in a subset of the SMARTT dynamic component participants meeting 2 specific inclusion criteria outlined elsewhere in this article: first, they must have had ≥1 of 6 assessments (5 ND and one audiology, described elsewhere in this article) performed at ages 1 through 5 years and second, a PBMC pellet collected at ≤3 weeks of life. All cases of cCMV identified were categorized as symptomatic or asymptomatic, and their ND outcomes described.
Diagnosis of Symptomatic and Asymptomatic cCMV
All PBMC pellets in participants meeting inclusion criteria had DNA extracted and tested by a CMV DNA polymerase chain reaction (PCR) assay in a laboratory at the University of California, San Diego, using nested PCR as previously described.14 A diagnosis of cCMV was made based on a positive result. Children with cCMV were categorized as symptomatic if any of the following signs were present at birth: microcephaly, small for gestational age, petechial or purpuric rash, hepatosplenomegaly, neurologic deficits, or seizures.15 If none of these signs were present, cCMV-positive participants were categorized as asymptomatic. Small for gestational age was defined as a birth weight of <10th percentile and microcephaly as a head circumference of <3rd percentile for gestational age.16
For children without a PBMC pellet collected at ≤3 weeks of life, the SMARTT database of MedDRA diagnoses was queried for all cCMV diagnoses. Some participating sites routinely screen all HIV-exposed uninfected newborns for CMV, whereas other sites do not conduct routine screening, but may have identified cCMV based on an evaluation of symptoms. Each identified case was reviewed to ascertain that cases met the study definition of cCMV: positive urine CMV culture, salivary nucleic acid amplification testing, or other diagnostic testing performed within the first 3 weeks of life. To avoid introducing bias, only cases identified by testing an available PBMC pellet were included in the estimate of cCMV prevalence.
ND and Hearing Assessments
Longitudinal examination of developmental outcomes of language, intelligence, and hearing that were part of the SMARTT battery of assessments were evaluated based on data submitted as of August 1, 2018. At 1 year of age, the MacArthur-Bates Communicative Development Inventory and the Bayley Scales of Infant and Toddler Development were performed.17,18 At 2 years of age, the Ages and Stages was performed.19 At 5 years of age, the Wechsler Preschool and Primary Scale of Intelligence, the Test of Language Development, and a comprehensive audiologic evaluation using earphones were performed.20,21 For this study ND scores of ≥1 SD below the normative mean were categorized as lower than normal. Sensorineural hearing loss was defined as pure tone average of worse ear of >25 dB hearing level at 0.5, 1.0, 2.0, and 4.0 kHz, after excluding children with flat tympanograms. The MacArthur-Bates Communicative Development Inventory and Ages and Stages were administered in Spanish or English; the Bayley Scales of Infant and Toddler Development, Wechsler Preschool and Primary Scale of Intelligence, and Test of Language Development was available and administered in English.
Statistical Analyses
The proportion with cCMV infection in the tested sample was determined, along with the Clopper-Pearson exact 95% CI. For developmental outcomes at ages 1, 2, and 5 years, summary statistics were calculated for continuous measures by cCMV infection status and the groups were compared with a Wilcoxon rank-sum test. The continuous outcome measures were also used to define discrete outcome groups (lower or delayed in development, vs normal), and for these and other discrete measures the cross-tabulation frequencies were calculated, and the 2 groups were compared with the Fisher exact test. The same approach was followed for basic demographic, delivery, and newborn characteristics, measures of maternal HIV disease severity and treatment (HIV RNA viral load, CD4 cell count and percent, and ARV exposure during pregnancy), and for measures of caregiver education and income. Due to the low number of cCMV events observed, no models were fit for comparison of ND outcomes by cCMV status adjusting for other covariates.
Results
As of February 1, 2017, there were 2212 HIV-exposed uninfected participants in the dynamic cohort who were ≥1 year of age, of whom 895 met study criteria for estimation of cCMV birth prevalence (Figure 1). The major reason for exclusion from study was lack of a birth PBMC sample. Compared with the 1317 excluded children, the 895 children were less often African American (63% vs 79%), more often Hispanic (40% vs 23%), and had slightly better maternal HIV disease control status (87% vs 82% with suppressed viral load and 93% vs 88% with CD4 ≥200 cells/mL).
Figure 1.
Flow chart describing the identification of cCMV infection in HIV-exposed uninfected children followed in the Surveillance Monitoring of Antiretroviral Therapy and Toxicity Study. aSurveillance Monitoring for Antiretroviral Therapy Toxicities cohort.
Of the 895 patients who met study criteria, 8 had a positive CMV DNA PCR, yielding estimated birth prevalence for cCMV of 0.89% (95% CI, 0.39%−1.75%). Six were asymptomatic and 2 were symptomatic. One of these 8 children already had a known diagnosis of cCMV recorded in the SMARTT database. Of the 1317 participants who did not meet the inclusion criteria, an additional 5 cases of cCMV were reported in the SMARTT database: 4 were identified by routine birth screening performed according to the clinical sites standard practice, and 1 was symptomatic at birth, prompting evaluation and subsequent diagnosis.
Maternal and Birth Characteristics
The mothers delivered in the US and Puerto Rico between 2007 and 2015 and ranged in age from 15.2 to 50.1 years (median, 29.1 years; IQR, 24.6–33.6 years), with 55% selfidentified as African American, 32% as White, and 40% as Latino (Table). Of participants with available data, 31% of mothers reported not receiving a high school degree, and 77% reported annual household income of £$20 000. These caregiver demographic characteristics were not different by child’s cCMV status. Among 895 mothers, 90% were on cART at some point during their pregnancy, 6% on ARV regimens not constituting cART, and 1% who received no ARV treatment (no data available for 3%). Exposures during pregnancy to 5 ARV drug classes are shown and similar by cCMV status. The proportion of mothers whose last (before delivery) available HIV viral load was ≤400 copies/mL and last CD4 cell counts ≥200 cells/μL were 88.0% and 92.9%, respectively. Mothers of children with cCMV were more likely to have a last CD4+ T cell before delivery of <20% compared with mothers of uninfected children (37.5% vs 12.8%; P = .07). Preterm deliveries occurred for 16% of all HIV-exposed uninfected children, but in none of the 8 with cCMV identified by PCR. The mean number of years of follow-up (equivalent to age) for HIV-exposed uninfected children with cCMV was 7.2 years (range, 4.5–9.5 years) compared with 5.9 years (range, 1.610.3 years) for those without (P = .1). There were no differences in newborn head circumference, weight, or length by cCMV status. Six of 8 newborns with cCMV fell within normal percentiles for weight, length, and head circumference for gestational age. Two babies had symptomatic cCMV by study criteria: both were small for gestational age, one at the 8th and the other at the 5th percentile for weight. They were not identified by their sites as cCMV infected at birth, but by PCR testing. One baby failed the newborn hearing screen (but passed on repeat testing).
Table.
Infant and maternal demographic and delivery characteristics, and maternal HIV virologic and immunologic measures during pregnancy, by cCMV status
| Characteristics | cCMV positive (n = 8) | cCMV negative (n = 887) | Total (N = 895) | P value* |
|---|---|---|---|---|
| Infant characteristics | ||||
| Current age (years) | 7.4 (6.2–8.0) | 5.9 (4.0–7.8) | 5.9 (4.0–7.8) | .11 |
| Birth year | ||||
| 2007–2009 | 4 (50%) | 251 (28%) | 255 | |
| 2010–2012 | 3 (38%) | 339 (38%) | 342 | |
| 2013–2015 | 1 (13%) | 297 (33%) | 298 | |
| Male sex | 4 (50%) | 473 (53%) | 477 (53%) | >.99 |
| Race | ||||
| Black | 6 (75%) | 482 (54%) | 488 (55%) | .54 |
| White | 1 (13%) | 284 (32%) | 285 (32%) | |
| Other/unknown | 1 (13%) | 121 (14%) | 122 (14%) | |
| Hispanic ethnicity | 2 (25%) | 357 (40%) | 359 (40%) | .50 |
| Birth weight (g) | 3087 (2623–3374) | 3030 (2710–3365)† | 3030 (2710–3365)† | .96 |
| Head circumference (cm) | 34.6 (32.8–35.3) | 34.0 (32.7–35.0)‡ | 34.0 (32.7–35.0)‡ | .48 |
| Length (cm) | 50.0(48.4–51.1) | 49.0 (47.2–50.8)§ | 49.0 (47.3–50.8)§ | .30 |
| Gestational age (wk) | 39.6 (38.2–39.9) | 38.3 (37.6–39.3)¶ | 38.3 (37.6–39.3)¶ | .11 |
| Gestational age <37 wk | 0 (0%) | 140 (16%)‡ | 140 (16%)‡ | .62 |
| Maternal characteristics | ||||
| Mother’s age at delivery | 29.0(21.4–31.5) | 29.1 (24.6–33.6)‡ | 29.1 (24.6–33.6)‡ | .48 |
| Cesarean delivery | 3 (38%) | 517(58%)† | 520 (58%)† | .40 |
| Caregiver <HS degree | 1 (25%)† | 190(31%)** | 191 (31%) | >.99 |
| Caregiver household income ≤$20 000 | 3 (75%)† | 452 (77%)†† | 455 (77%) | >.99 |
| Maternal immunologic and HIV virologic measurements (first and last values during pregnancy) and maternal HIV ARV drug regimen during pregnancy | ||||
| First CD4% <20 | 3 (37.5%) | 176 (20.2%)‡‡ | 179 (20.3%)‡‡ | .21 |
| First CD4 <200 cells/mm3 | 1 (12.5%) | 95 (10.8%)§§ | 96 (10.9%)§§ | .60 |
| First RNA >400 copies/mL | 5 (62.5%) | 422 (47.9%)¶¶ | 427 (48.0%)¶¶ | .49 |
| Last CD4% <20 | 3 (37.5%) | 112(12.8%)‡‡ | 115(13.0%)‡‡ | .07 |
| Last CD4 <200 cells/mm3 | 1 (12.5%) | 62 (7.1%)§§ | 63 (7.1%)§§ | .45 |
| Last RNA >400 copies/mL | 2 (25.0%) | 105 (11.9%)¶¶ | 107 (12.0%)¶¶ | .25 |
| ARV pregnancy regimen** | ||||
| cART | 7 (88%) | 802 (90%) | 809 (90%) | .11 |
| Other ARVs | 0 (0%) | 53 (6%) | 53 (6%) | |
| No ARVs | 1 (13%) | 7(1%) | 8(1%) | |
| Unknown | 0 (0%) | 25 (3%) | 25 (3%) | |
| NRTI-unexposed | 1 (13%) | 11 (1%) | 12(1%) | .12 |
| NRTI-exposed | 7 (88%) | 853 (96%) | 860 (96%) | |
| NNRTI-unexposed | 6 (75%) | 707 (80%) | 713(80%) | .71 |
| NNRTI-exposed | 2 (25%) | 157 (18%) | 159 (18%) | |
| Protease inhibitor unexposed | 4 (50%) | 191 (22%) | 195(22%) | .14 |
| Protease inhibitor exposed | 4 (50%) | 673 (76%) | 677 (76%) | |
| Integrase inhibitor unexposed | 7 (88%) | 759 (86%) | 766 (86%) | >.99 |
| Integrase inhibitor exposed | 1 (13%) | 105 (12%) | 106 (12%) | |
| Fusion inhibitor unexposed | 8(100%) | 856 (97%) | 864 (97%) | >.99 |
| Fusion inhibitor exposed | 0 (0%) | 8(1%) | 8(1%) |
NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor.
Median with IQRs reported where applicable; numbers with missing values are as follows
4 missing
3 missing
2 missing
8 missing
277 missing
301 missing
14 missing
10 missing
6 missing.
Wilcoxon rank-sum test for continuous variables and Fisher exact test for categorical values.
ARV regimen the mother received during pregnancy. cART was defined as cART consisting of ≥3 ARV drugs from ≥2 different drug classes. Other ARVs were defined as any combination not meeting the definition of cART. ARV drug class exposure at any time during pregnancy is reported for 5 drug classes. ARV drug class data were missing for 23 of the mothers of cCMV-negative participants.
ND Outcomes through 5 Years of Age
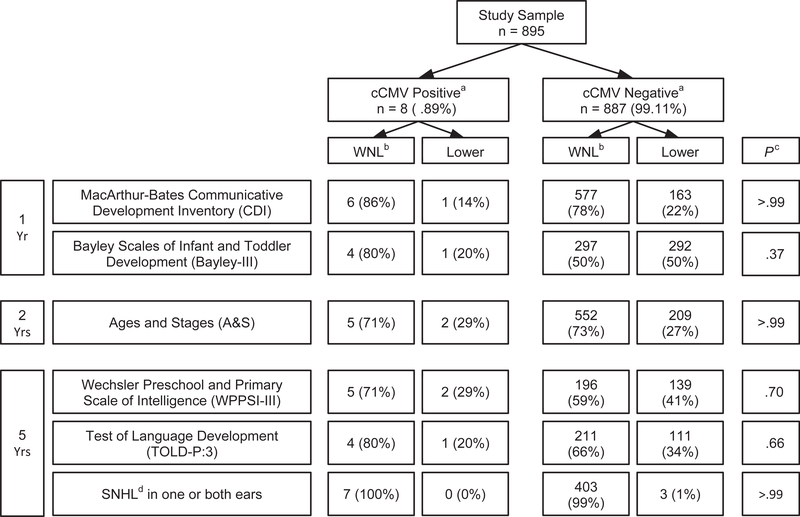
Only the 8 participants identified by PCR testing were included in the comparison with CMV uninfected children. There were no differences in dichotomized outcomes for lower performance by cCMV status (Figure 2; available at www.jpeds.com). Likewise, comparison of continuous measures such as mean and median scores showed no differences between the 2 groups.
Figure 2.
Comparison of percent with lower and normal language, cognitive and audiologic scores by cCMV status for the cohort of 895 HIV-exposed uninfected children. The cutoff for a classification of “lower” on each assessment was: MacArthur-Bates Communicative Development Inventory: age-adjusted percentile score ≤10th percentile in any of the 4 domains (phrases understood, vocabulary comprehension, word production, or total gestures); Bayley-III: composite score ≤85 in any of the 5 domains (cognitive, language, motor, social-emotional, or adaptive behavior); Ages and Stages: total score ≥1 SD below age specific norms; Wechsler Preschool and Primary Scale of Intelligence: composite score ≤85 in any of the 3 quotients (verbal IQ, performance IQ, full scale IQ); Test of Language Development: Spoken Language Quotient £85; and audiologic evaluation: sensorineural hearing loss with worse ear pure tone average >25 dB hearing level at 0.5, 1.0, 2.0, and 4.0 kHz. aComparison by cCMV status was not significantly different for any of the neurodevelopment, language, and audiology assessments. The highest proportion classified as lower in these assessments was for the Bayley-III where 50% of cCMV negative children met cutoff criteria in ≥1 of 5 domains and are believed to be generally representative of children who are HIV exposed, and uninfected. bWithin normal limits. cFisher exact test. dSensorineural hearing loss.
Discussion
Well into the era of cART, we demonstrate a cCMV birth prevalence of 0.9% in HIV-exposed uninfected children born in the US and Puerto Rico between 2007 and 2015. Compared with the highly sensitive salivary and urine nucleic acid tests used to detect cCMV, blood PBMC DNA PCR is ≥70%–75% sensitive in detecting infants with cCMV.22,23 We therefore project a birth prevalence between 1.2% and 1.3% in HIV-exposed uninfected children in the era of cART as a more realistic estimate, and one that is above what has been reported in the general US population, but below previously discussed estimates of prevalence before the era of cART.
Several reasons exist for the high rate of in utero CMV transmission in WLHIV in the pre-cART era. Both HIV and CMV are more prevalent in women of lower socioeconomic status, where there is almost universal co-infection. There is an established association between maternal immunosuppression with CD4 cell counts of <200 cells/μL and cCMV infection, a finding confirmed in this study as well.5,24 Evidence also suggests an association between high HIV viral load and increased CMV shedding in genital secretions and urine in pregnant WLHIV.25
We hypothesize that higher transmission rates of congenital infection in babies born to WLHIV can occur from either reactivation or reinfection with CMV in these mothers, producing higher CMV viremia and/or more frequent episodes of viremia as a result of their impaired immunity, and that with effective cART the prevalence trends closer to that observed in the general population.26,27
Previous studies on the cCMV prevalence in the era of cART have not been consistent in showing a decrease compared with the pre-cART era. Our cohort’s prevalence was lower than and consistent with the decline in birth prevalence reported by Guibert et al (1.5%) and Marin Gabriel et al (1.3%).5,6 Duryea et al, with a rate of 3% for their HIV-exposed infants, felt it was difficult to place their prevalence in context with the reported literature because their institution served a population of minority women mostly of low socioeconomic status who may have had a higher baseline prevalence of cCMV.8 Frederick et al, with a rate of 4.8% thought their prevalence may have been higher because most mothers were diagnosed with HIV during their pregnancy and referred to their clinic after the first trimester as opposed to pre-pregnancy.7
The SMARTT cohort represents infants born to WLHIV most of whom are receiving cART and have well-controlled HIV infection. Our data for deliveries between 2007 and 2015 reflect this: 90% of these women were receiving cART, 88% had suppressed viral loads, and 93% had CD4 cell counts of >200 cells/μL before delivery. Hence, they did not have many of the risk factors associated with increased transmission rates described elsewhere in this article. Our data support that HIV-exposed uninfected infants born to WLHIV who are well-controlled in antenatal care and without immune suppression in the cART era will likely have lower cCMV prevalence than has been reported previously. Nevertheless, HIV-exposed uninfected children remain at elevated risk for this congenital infection. A possible explanation may be related to the socioeconomic issues that characterize the mothers of HIV-exposed uninfected children followed in SMARTT.
We speculate that some ARVs may have anti-CMV activity possibly affecting in utero transmission. Nelfinavir, a protease inhibitor with anti-CMV activity, was not associated with protection against cCMV in HIV-exposed uninfected children.9 Integrase inhibitors demonstrate in vitro anti-CMV activity; however, this property has not yet been demonstrated in vivo.28 We did not find an association with specific ARVs and in utero transmission of CMV in this study, although we acknowledge our sample size may not have been sufficient to detect one.
We were unable to show differences in hearing, language, and ND outcomes in HIV-exposed uninfected children with and without cCMV. Because approximately 1 in 5 children are expected to develop disability following congenital infection with CMV, our small group of 8 children is not large enough to show these differences if they do exist.29 A limitation of this study is that many children did not have PBMCs available for testing. We noted differences in race/ethnicity distribution and a slightly better average HIV disease control status in mothers of children who had blood pellets available and ND test results than mothers of children who did not. If a missing PBMC sample or ND test was associated with cCMV infection, then the estimate of prevalence would be biased. A strength is that our cohort, drawn from 22 sites across the US and Puerto Rico, provides a broad representation of the population of HIV-exposed uninfected children, which permits for greater generalizability of our finding.
Although birth prevalence of cCMV in HIV-exposed uninfected children in the cART era is lower than previous estimates, these children are still at increased risk for this congenital infection. With approximately 8700 HIV-exposed uninfected infants born annually in the US, large cohort studies such as SMARTT may help shed light on the longterm outcomes of cCMV in this population.
Acknowledgments
We thank the children and families for their participation in PHACS, and the individuals and institutions involved in the conduct of PHACS. Data management services were provided by Frontier Science and Technology Research Foundation (PI: Suzanne Siminski, MS, MBA), and regulatory services and logistical support were provided by Westat, Inc (PI: Julie Davidson, MSN).
Glossary
- ARV
Antiretroviral
- cART
Combination antiretroviral therapy
- cCMV
Congenital CMV
- CMV
Cytomegalovirus
- ND
Neurodevelopmental
- PBMC
Peripheral blood mononuclear cell
- PCR
Polymerase chain reaction
- SMARTT
Surveillance Monitoring for ART Toxicities
- WLHIV
Women living with HIV-1
Appendix
Additional members of the Pediatric HIV/AIDS Cohort Study
The following institutions, clinical site investigators and staff participated in conducting PHACS SMARTT in 2018, in alphabetical order: Ann & Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL: Ellen Chadwick, MD, Margaret Ann Sanders, MPH, Scott Hunter, PhD; Baylor College of Medicine, Houston, TX: William Shearer, MD, Mary Paul, MD, Chivon McMullen-Jackson, BSN, Ruth Eser-Jose, MSN, Lynnette Harris, PhD; Bronx Lebanon Hospital Center, Bronx, NY: Mahoobullah Mirza Baig, MD, Alma Villegas, PhD; Children’s Diagnostic & Treatment Center, Fort Lauderdale, FL: Lisa Gaye-Robinson, MD, Jawara Dia Cooley, MEd, James Blood, MSW Patricia Garvie, PhD; New York University School of Medicine, New York, NY: William Borkowsky, MD, Sandra Deygoo, MS, CCRP, Jennifer Lewis; Rutgers–New Jersey Medical School, Newark, NJ: Arry Dieudonne, MD, Linda Bettica, BSN, Juliette Johnson, Karen Surowiec, PsyD; St. Jude Children’s Research Hospital, Memphis, TN: Katherine Knapp, MD, Kim Allison, RN, Megan Wilkins, PhD, Jamie Russell-Bell, MA, BSN; San Juan Hospital/Department of Pediatrics, San Juan, PR: Nicolas Rosario, MD, MSc, Lourdes Angeli-Nieves, MPH, Vivian Olivera, PhD; SUNY Downstate Medical Center, Brooklyn, NY: Stephan Kohlhoff, MD, Ava Dennie, PA, Jean Kaye, RN; Tulane University School of Medicine, New Orleans, LA: Russell Van Dyke, MD, Karen Craig, RN, MSN, CCRC, Patricia Sirois, PhD; University of Alabama, Birmingham, AL: Cecelia Hutto, MD, Paige Hickman, NP, Dan Marullo, PhD; University of California, San Diego, CA: Veronica Figueroa, MA, Megan Loughran, BA, Sharon Nichols, PhD; University of Colorado, Denver, CO: Elizabeth McFarland, MD, Emily Barr, MSN, CPNP, CNM, Christine Kwon, BS, Carrie Glenny, RN, BSN, MA; University of Florida, Center for HIV/AIDS Research, Education and Service, Gainsville, FL: Mobeen Rathore, MD, Kristi Stowers, MPH, Saniyyah Mahmoudi, ARNP, Nizar Maraqa, MD, Rosita Almira, MPH; University of Illinois, Chicago, IL: Karen Hayani, MD, Lourdes Richardson, MSN, FNP, Renee Smith, PhD, Alina Miller, BS; University of Miami, Miami, FL: Gwendolyn Scott, MD, Maria Mogollon, Gabriel Fernandez, BSc, BA, Anai Cuadra, PhD; Keck Medicine of the University of Southern California, Los Angeles, CA: Mariam Davtyan, PhD, Jennifer Vinas, RN, Guadalupe Morales-Avendano, PsyD, PhD; University of Puerto Rico School of Medicine, Medical Science Campus, San Juan, PR: Zoe M. Rodriguez, MD, Lizmarie Torres, RN, BSN, Nydia Scalley, MA.
Funding and Conflicts of Interest Disclosure
The Pediatric HIV/AIDS Cohort Study (PHACS) was supported by the Eunice Kennedy Shriver National Institute Of Child Health & Human Development (NICHD) with cofunding from the National Institute of Dental & Craniofacial Research (NIDCR), the National Institute of Allergy and Infectious Diseases (NIAID), the National Institute of Neurological Disorders and Stroke (NINDS), the National Institute on Deafness and Other Communication Disorders (NIDCD), Office of AIDS Research (OAR), the National Institute of Mental Health (NIMH), the National Institute on Drug Abuse (NIDA), and the National Institute on Alcohol Abuse and Alcoholism (NIAAA), through cooperative agreements with the Harvard T.H. Chan School of Public Health (HD052102) and the Tulane University School of Medicine (HD052104). The conclusions and opinions expressed in this article are those of the authors and do not necessarily reflect those of the National Institutes of Health or U.S. Department of Health and Human Services. The funders did not have any role in the study design, collection, analysis and interpretation of data, writing of the report, or the decision to submit for publication. The authors declare no conflicts of interest.
Footnotes
Additional funding and disclosure information is available at www.jpeds.com.
Data Statement
Data sharing statement available at www.jpeds.com.
References
- 1.Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol 2007;17: 253–76. [DOI] [PubMed] [Google Scholar]
- 2.Fowler KB, McCollister FP, Sabo DL, Shoup AG, Owen KE, Woodruff JL, et al. A Targeted approach for congenital cytomegalovirus screening within newborn hearing screening. Pediatrics 2017;139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doyle M, Atkins JT, Rivera-Matos IR. Congenital cytomegalovirus infection in infants infected with human immunodeficiency virus type 1. Pediatr Infect Dis J 1996;15:1102–6. [DOI] [PubMed] [Google Scholar]
- 4.Kovacs A, Schluchter M, Easley K, Demmler G, Shearer W, La Russa P, et al. Cytomegalovirus infection and HIV-1 disease progression in infants born to HIV-1-infected women. Pediatric Pulmonary and Cardiovascular Complications of Vertically Transmitted HIV Infection Study Group. N Engl J Med 1999;341:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guibert G, Warszawski J, Le Chenadec J, Blanche S, Benmebarek Y, Mandelbrot L, et al. Decreased risk of congenital cytomegalovirus infection in children born to HIV-1-infected mothers in the era of highly active antiretroviral therapy. Clin Infect Dis 2009;48: 1516–25. [DOI] [PubMed] [Google Scholar]
- 6.Marin Gabriel MA, Fernandez Ibieta M, Gonzalez Tome MI, Saavedra Lozano J, Barajas Sanchez V, Rojo Conejo P, et al. [Congenital cytomegalovirus infection in the infants of HIV-infected mothers]. An Pediatr (Barc) 2005;62:38–42. [DOI] [PubMed] [Google Scholar]
- 7.Frederick T, Homans J, Spencer L, Kramer F, Stek A, Operskalski E, et al. The effect of prenatal highly active antiretroviral therapy on the transmission of congenital and perinatal/early postnatal cytomegalovirus among HIV-infected and HIV-exposed infants. Clin Infect Dis 2012;55:877–84. [DOI] [PubMed] [Google Scholar]
- 8.Duryea EL, Sanchez PJ, Sheffield JS, Jackson GL, Wendel GD, McElwee BS, et al. Maternal human immunodeficiency virus infection and congenital transmission of cytomegalovirus. Pediatr Infect Dis J 2010;29:915–8. [DOI] [PubMed] [Google Scholar]
- 9.Gantt S, Leister E, Jacobsen DL, Boucoiran I, Huang ML, Jerome KR, et al. Risk of congenital cytomegalovirus infection among HIV-exposed uninfected infants is not decreased by maternal nelfinavir use during pregnancy. J Med Virol 2016;88:1051–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitmore SK, Zhang X, Taylor AW, Blair JM. Estimated number of infants born to HIV-infected women in the United States and five dependent areas, 2006. J Acquir Immune Defic Syndr 2011;57:218–22. [DOI] [PubMed] [Google Scholar]
- 11.Van Dyke RB, Chadwick EG, Hazra R, Williams PL, Seage GR 3rd. The PHACS SMARTT Study: assessment of the Safety of In Utero Exposure to Antiretroviral Drugs. Front Immunol 2016;7:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griner R, Williams PL, Read JS, Seage GR 3rd, Crain M, Yogev R, et al. In utero and postnatal exposure to antiretrovirals among HIV-exposed but uninfected children in the United States. AIDS Patient Care STDS 2011;25:385–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown EG, Wood L, Wood S. The medical dictionary for regulatory activities (MedDRA). Drug Saf 1999;20:109–17. [DOI] [PubMed] [Google Scholar]
- 14.Torre PR, Zeldow B, Yao TJ, Hoffman HJ, Siberry GK, Purswani MU, et al. Newborn hearing screenings in human immunodeficiency virusexposed uninfected infants. J AIDS Immune Res 2016;1. [PMC free article] [PubMed] [Google Scholar]
- 15.Kimberlin DW, Jester PM, Sanchez PJ, Ahmed A, Arav-Boger R, Michaels MG, et al. Valganciclovir for symptomatic congenital cytomegalovirus disease. N Engl J Med 2015;372:933–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeSilva M, Munoz FM, Sell E, Marshall H, Tse Kawai A, Kachikis A, et al. Congenital microcephaly: case definition & guidelines for data collection, analysis, and presentation of safety data after maternal immunisation. Vaccine 2017;35:6472–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fenson L, Marchman VA, Thai DJ, Dale PS, Reznick JS, Bates E. MacArthur-Bates communicative development inventories. Baltimore (MD): Paul H. Brookes Publishing Company; 2007. [Google Scholar]
- 18.Bayley N Bayley Scales of Infant and Toddler Development. 3rd ed. San Antonio (TX): Harcourt Assessment; 2006. [Google Scholar]
- 19.Squires J, Bricker D. Ages & Stages Questionnaires. Baltimore (MD): Paul H. Brookes Publishing Co; 2002. [Google Scholar]
- 20.Wechsler D Wechsler Preschool and Primary Scale of Intelligence. 3rd ed. San Antonio (TX): Psychological Corporation; 2002. [Google Scholar]
- 21.Newcomer PL, Hammill DD. Test of Language Development-Primary. 3rd ed. Austin (TX): Pro-Ed; 1997. [Google Scholar]
- 22.Atkinson C, Walter S, Sharland M, Tookey P, Luck S, Peckham C, et al. Use of stored dried blood spots for retrospective diagnosis of congenital CMV. J Med Virol 2009;81:1394–8. [DOI] [PubMed] [Google Scholar]
- 23.Ross SA, Novak Z, Fowler KB, Arora N, Britt WJ, Boppana SB. Cytomegalovirus blood viral load and hearing loss in young children with congenital infection. Pediatr Infect Dis J 2009;28:588–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manicklal S, van Niekerk AM, Kroon SM, Hutto C, Novak Z, Pati SK, et al. Birth prevalence of congenital cytomegalovirus among infants of HIV-infected women on prenatal antiretroviral prophylaxis in South Africa. Clin Infect Dis 2014;58:1467–72. [DOI] [PubMed] [Google Scholar]
- 25.Adachi K, Xu J, Ank B, Watts DH, Mofenson LM, Pilotto JH, et al. Cytomegalovirus urinary shedding in HIV-infected pregnant women and congenital cytomegalovirus infection. Clin Infect Dis 2017;65:405–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boucoiran I, Mayer BT, Krantz EM, Marchant A, Pati S, Boppana S, et al. Nonprimary maternal cytomegalovirus infection after viral shedding in infants. Pediatr Infect Dis J 2018;37:627–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pokalyuk C, Renzette N, Irwin KK, Pfeifer SP, Gibson L, Britt WJ, et al. Characterizing human cytomegalovirus reinfection in congenitally infected infants: an evolutionary perspective. Mol Ecol 2017;26:1980–90. [DOI] [PubMed] [Google Scholar]
- 28.Yan Z, Bryant KF, Gregory SM, Angelova M, Dreyfus DH, Zhao XZ, et al. HIV integrase inhibitors block replication of alpha-, beta-, and gammaherpesviruses. MBio 2014;5:e01318–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Townsend CL, Forsgren M, Ahlfors K, Ivarsson SA, Tookey PA, Peckham CS. Long-term outcomes of congenital cytomegalovirus infection in Sweden and the United Kingdom. Clin Infect Dis 2013;56:1232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]