Abstract
Type 2 diabetes mellitus (DM2) is strongly associated with other comorbidities such as obesity, atherosclerosis, and hypertension. Obesity is associated with sustained low-grade inflammatory response due to the production of proinflammatory cytokines. This inflammatory process promotes the differentiation of some myeloid cells, including myeloid-derived suppressor cells (MDSCs). In this study, two groups of individuals were included: DM2 patients and non-DM2 individuals with similar characteristics. Immunolabeling of CD15+ CD14- and CD33+ HLA-DR-/low was performed from whole peripheral blood, and samples were analyzed by flow cytometry, and frequencies of MDSCs and the relationship of these with clinical variables, cytokine profile (measured by cytometric bead array), and anthropometric variables were analyzed. The frequency of CD33+ HLA-DR-/low MDSCs (that produce IL-10 and TGF-β, according to an intracellular detection) is higher in patients with DM2 (P < 0.05), and there is a positive correlation between the frequency of CD15+ CD14- and CD33+ HLA-DR-/low MDSC phenotypes. DM2 patients have an increased concentration of serum IL-5 (P < 0.05). Also, a negative correlation between the frequency of CD15+ CD14- MDSCs and LDL cholesterol was found. Our group of DM2 patients have an increased frequency of mononuclear MDSC CD33+ HLA-DR-/low that produce TGF-β and IL-10. These cytokines have been associated with immune modulation and reduced T cell responses. DM2 and non-DM2 subjects show a similar cytokine profile, but the DM2 patients have an increased concentration of IL-5.
1. Introduction
According to the American Diabetes Association (ADA), diabetes mellitus is a metabolic disease characterized by severe hyperglycemia due to defects in insulin secretion or the lack of proper action of this hormone in the target tissues. Type 2 diabetes mellitus (DM2) is the one that has the greatest impact worldwide, accounting for 90-95% of all reported cases of diabetes worldwide as reported by the ADA [1, 2]. The World Health Organization (WHO) estimated that 347 million cases of DM2 exist worldwide as of 2014 [3], and also, recent estimations suggest that DM2 will be the 7th cause of mortality by 2030 [4]. Data from the International Diabetes Federation suggest 4.9 million deaths associated with diabetes and its related complications [5]. In Mexico, the National Institute for Statistics and Geography (INEGI, acronym in Spanish) has reported that 70 out 1000 deaths were caused by diabetes and its complications causing a great economic burden to the national health institutions [6].
It has been described that the main factors associated with an increased risk of developing DM2 are obesity, unhealthy eating habits, sedentarism, advanced age, family history of diabetes, ethnicity, etc. [7–9]. The relationship between diabetes and obesity has been widely documented, and around 90% of diabetics are overweight or obese [10]. Obesity has also been associated with low-grade chronic inflammatory processes, and several cytokines such as tumor necrosis factor alpha (TNF-α) have been shown to be elevated in obese individuals due to an increased activity of adipose tissue-derived cytokine production and insulin resistance [11, 12]. Other bioactive molecules such as leptin, IL- (interleukin-) 6, resistin, and monocyte chemoattractant protein 1 (MCP-1) have been associated with insulin resistance [13–17].
Chronic inflammatory processes have been associated with alterations in leucocyte composition. In the seventies, a subset of myeloid cells with suppressor properties was described in patients with several types of cancer. These cells were at the moment described as “natural suppressor cells,” but the functional properties and markers of such cells have been recently described and named as myeloid-derived suppressor cells (MDSCs) [18–20]. These cells are a heterogeneous group of myeloid cells such as myeloid progenitors, immature myeloid cells, immature granulocytes, and immature dendritic cells. In humans, the main MDSCs have been described as immature monocytic and granulocytic cells, and several reports suggest an elevation in the frequency of MDSCs in some pathologic conditions, including cancer, sepsis, and acute and chronic inflammatory processes [19, 20]. Several phenotypes for these cells have been described such as CD15+ CD14- and also CD33+ HLA-DR-/low [19] to mention some [21]. It has been described that these cells have immune suppressive functions [22], and they are producers of interferon gamma (IFN-γ), IL-10, and transforming growth factor beta (TGF-β) [23].
As described previously, obesity is the biggest risk factor for developing DM2. This has also been associated with low-grade chronic inflammation with overproduction of TNF-α, IL-6, and IL-1β and other bioactive molecules in the adipose tissue such as granulocyte-macrophage colony-stimulating factor (GM-CSF), granulocyte colony-stimulating factor (G-CSF), macrophage colony-stimulating factor (M-CSF), vascular endothelial growth factor (VEGF), or IFN-γ [24–27]. Taking all the previously described data and the fact that diabetic individuals with DM2 have a higher predisposition to infection due to diminished immune responses [28–30], it becomes really important to understand if several cell populations that suppress the immune response such as the MDSCs are major conditioning factors that promote deficiencies in the development of several immune-mediated mechanisms in DM2 individuals [19]. The aim of the present paper was to compare the frequency of myeloid cells with the phenotypes CD15+ CD14- and CD33+ HLA-DR-/low producers of IL-10 and TGF-β in peripheral blood of patients with DM2 and non-DM2 subjects. The correlation between the MDSC immunophenotypes with common comorbidities to diabetics, laboratory, cytokine levels, cell suppressive function, and anthropometric data was also analyzed.
2. Material and Methods
2.1. Participants' Inclusion Criteria
DM2 patients of the study were recruited at the medical family care unit # 4 (Zacatecas, Mexico) according to approved protocols (R-2011-785-063); the visits were made between March 19 and May 19 in 2015. DM2 subjects (n = 23, age range of 35-62 years old) were invited to participate. DM2 subjects complied with the ADA criteria for DM2 diagnosis as follows: random glucose measurement of >120 mg/dl and/or glucose tolerance test > 200 mg/dl (for newly diagnosed individuals) and/or glycated hemoglobin (HbA1c) > 6.5%. Nondiabetic subjects (non-DM2) (n = 21) were negative for diabetes according to ADA criteria and were recruited at the same clinic with that from a similar age group. Clinical and laboratory data from all participants was collected from the routine follow-up at their clinic. Common comorbidities (reported by the treating physician) were also recovered from the medical records.
2.2. Ethics Statement
The present study was approved by the national commission on scientific investigation (CNIC) at the Mexican Institute for Social Security (IMSS) as well as the national ethics commission with registration number R-2011-785-063. All protocols were based on the International Declaration of Helsinki and in the principles of not malevolence, justice, and equality. All participants that agreed to participate signed an informed consent, and blood samples were drawn from only these individuals.
2.3. MDSC Phenotype and Flow Cytometry Analysis
Immunophenotypes were identified according to Ko et al. [31]. Briefly, blood samples were obtained from the participants by means of venipuncture. 4 ml of blood was collected. 100 μl of whole blood was used with a lyse/wash protocol for staining. For the identification of the MDSC phenotypes, a combination of the following antibodies was used: CD14 PECy7 and CD15 PE-Cy5 (BD Biosciences, USA); CD33 APC and HLA-DR PerCP-Cy5.5 (BD Biosciences, USA). All antibodies were titrated before use. Immunostained samples were analyzed by flow cytometry in a FACSCanto II (Becton Dickinson, USA) with 488 and 633 laser lines and a standard configuration of 4-2 detectors. Data acquisition was performed by FACSDiva software v. 6.1 (BD Biosciences, USA), and 50000 events were acquired for each sample. The time parameter was used as quality control, observing stability between the performed analyses. Analysis of flow cytometry data was carried out in the FCS 3.0 files after export in FlowJo Software v. 8.7 (FlowJo LLC, USA).
2.4. Intracellular Staining of IL-10 and TGF-β
Blood samples were obtained from 4 DM2 patients and 4 nondiabetic participants by venipuncture. 100 μl of whole blood was used with a lyse/wash protocol, and for cellular permeabilization, we used the BD Cytofix/Cytoperm™ Kit (BD Biosciences, USA) according to the manufacturer's instructions. Intracellular staining was performed immediately afterwards with the antibodies for TGF-β PE and IL-10 PE in separate tubes (BD, Biosciences, USA). Fluorescence minus one (FMO) was used as the control. Immunostained samples were analyzed by flow cytometry in a FACSCanto II (Becton Dickinson, USA), and analysis of flow cytometry data was carried out in the FCS 3.0 files after export in FlowJo Software v. 8.7 (FlowJo LLC, USA).
2.5. Cytokine Quantification by Cytometric Bead Array
The concentration of TNF-α, IFN-γ, IL-1α, IL-5, IL-10, IL-12p70, IL-17, and eotaxin was analyzed by cytometric bead array using the manufacturer's specifications (BD Biosciences, USA). Briefly, starting with cytokine standard preparation and cytokine capture bead mixture, 50 μl serum samples from each individual and dilutions for the standard curve were placed in a multiscreen 1.2 μm, 96-well filter plate (Merck Millipore, Germany), and the mixed capture beads and phycoerythrin detection reagent were added. After 3-hour incubation and repeated wash steps, the data was acquired in a FACSCanto II flow cytometer (Becton Dickinson, USA) and analyzed using FCAP Array v3.0 (BD Biosciences, USA) to convert fluorescent intensity values to concentrations using a standard curve.
2.6. Statistical Analysis
Normality of data was verified by means of a Kolmogorov-Smirnov test or a D'Agostino-Pearson normality test. Group comparisons for continuous quantitative variables were made with a Mann-Whitney U test for the nonparametric data or a Welch corrected t-test for normally distributed data. Categorical variables were analyzed with a Fisher exact test. A correlation analysis was made to explore the correlation of MDSC, laboratory, and anthropometric data by using a Spearman correlation (nonnormal distribution was verified). The two-tailed level of significance used for all analysis was α = 0.05. All statistical analysis was carried out in the GraphPad Prism software v6.0 (GraphPad Software, USA).
3. Results
3.1. Clinical Features of Patients with DM2 and Nondiabetic Controls
Clinical features, laboratory data, and anthropometric data were analyzed in order to determine whether differences were present due to heterogeneity of the population or whether differences in MDSC cells were associated with other parameters such as metabolic markers of disease or cardiovascular risk factors. As shown in Table 1, no major differences were identified in the groups in the variables such as age, sex, body mass index, waist to hip ratio, low-density lipoprotein cholesterol (cLDL), high-density lipoprotein cholesterol (cHDL), and total cholesterol. As expected, there were significant differences between DM2 subjects for both Hb1Ac% and glucose levels due to the inclusion criteria. No differences were identified either for markers associated with alterations in lipid metabolism or cardiovascular risk.
Table 1.
Clinical features of patients.
| Variable | Group | P value | |
|---|---|---|---|
| Non-DM2 (n = 21) | DM2 (n = 23) | ||
| Gender (female/male) | 11/10 | 12/11 | 1.000a |
| Age (years) | 43 (q1 = 42, q3 = 50) | 50 (q1 = 40, q3 = 62) | 0.264b |
| Years with diabetes | 6.0 (q1 = 2, q3 = 14) | ||
| BMI (kg/m2) | 27.55 (q1 = 26.07, q3 = 30.22) | 27.4 (q1 = 24.2, q3 = 28.7) | 0.275b |
| Waist to hip ratio | 0.94 (q1 = 0.91, q3 = 0.96) | 0.93 (q1 = 0.89, q3 = 0.98) | 0.913b |
| Glucose (mg/dl) | 112 (q1 = 93, q3 = 117) | 185.4 (q1 = 111, q3 = 233) | 0.001∗b |
| Hb1Ac (%) | 5.9 (q1 = 5.8, q3 = 6.2) | 8.5 (q1 = 6.3, q3 = 10) | 0.000∗b |
| Total cholesterol (mg/dl) | 207 (q1 = 260, q3 = 231) | 209.4 (q1 = 170, q3 = 230) | 1.000b |
| cHDL (mg/dl) | 48.3 (q1 = 40.9, q3 = 52.6) | 49 (q1 = 38.2, q3 = 55.8) | 0.869b |
| cLDL (mg/dl) | 119.8 (q1 = 99.7, q3 = 156.3) | 130.6 (q1 = 81.1, q3 = 160.9) | 0.860b |
aFisher's exact test. bMann-Whitney U test. ∗P < 0.05.
3.2. MDSCs with CD15+ CD14- and CD33+ HLA-DR-/Low Phenotypes Are Present in Both DM2 and Non-DM2 Subjects
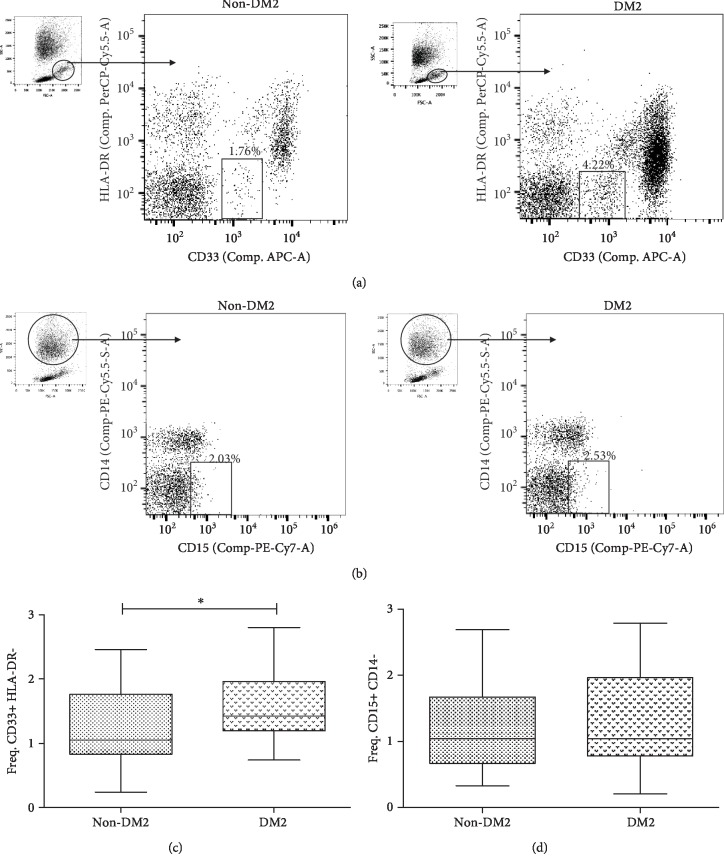
As mentioned above, several phenotypes of MDSCs have been identified and thoroughly characterized. The CD33+ HLA-DR-/low MDSCs are a subtype of myeloid cells reported by Ko et al. to have suppressive functions in renal cell carcinoma [31]. As shown in Figure 1(a), we identified such population with a similar gating strategy, and differences in both diabetics and nondiabetic subjects were also identified. For the CD15+ CD14- MDSC population (which has been described as another important subpopulation regulating in a negative manner the immune responses in some cancer patients) [31], samples of diabetics and nondiabetics show the presence of this population in low frequency (Figure 1(b)).
Figure 1.
Gating strategy and frequencies of MDSCs in DM2. (a) Dotplot APC vs. PerCP-Cy5.5 shows PBMCs and the gate mark of the CD33+ HLA-DR-/low MDSCs. (b) Dotplot PE-Cy7 vs. PE-Cy5 shows PBMCs and PMNs and the gate mark of the CD15+ CD14- MDSCs. (c) Frequency of CD33+ HLA-DR-/low MDSCs, comparing the group of DM2 (n = 22) with the non-DM2 group (n = 21). The graph shows the median with interquartile ranges. (d) Frequency of CD15+ CD14- MDSCs, comparing the group of DM2 (n = 20) with the non-DM2 group (n = 21). The graph shows the mean and standard deviation. A P value < 0.05 was considered as statistically significant which was calculated using the statistical test of Mann-Whitney. Statistical analysis was performed with the GraphPad Prism® v5.0 software. Data were obtained in a BD FACSCanto II® Flow Cytometer and were analyzed with the software FlowJo® v10.0.
3.3. Patients with DM2 Have Different Frequencies of MDSCs
Given the suppressive role of MDSCs over other immune cells, one of the main goals of this study was to evaluate the frequency of MDSCs in patients with DM2 compared to non-DM2 subjects. For this purpose, the frequency of total cells was evaluated for both the CD33+ HLA-DR-/low and the CD15+ CD14- phenotypes. Significant differences were identified for the CD33+ HLA-DR-/low mononuclear MDSCs in patients with DM2 as compared to non-DM2 (P < 0.05, Figure 1(c)). For the CD15+ CD14- MDSC cell population, no differences between groups were found (P > 0.05, Figure 1(d)).
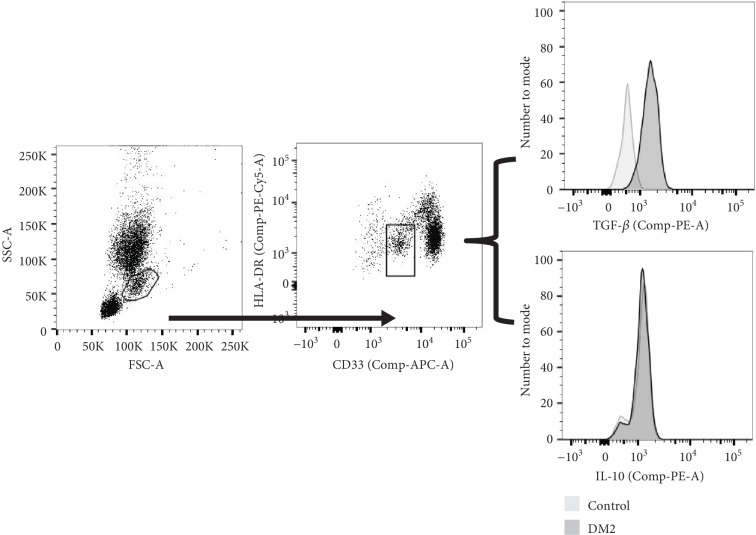
3.4. CD33+ HLA-DR-/Low MDSCs Produce TGF-β and IL-10
One of the major mechanisms by which MDSCs regulate the T cell-mediated immune response is by the production of IL-10 and TGF-β. Therefore, we evaluated the production of these cytokines in DM2 patients and controls. To demonstrate that the CD33+ HLA-DR-/low MDSCs are an immunoregulatory subset of cells, we did an intracellular staining of IL-10 and TGF-β and we found that this subpopulation actually produces these cytokines (Figure 2), suggesting that the immunosuppressive function of the MDSCs is also increased in the DM2 patients.
Figure 2.
CD33+ HLA-DR-/low MDSCs produce IL10 and TGF-β. Representative data of the intracellular staining of IL-10 and TGF-β on CD33+ HLA-DR-/low MDSCs. Data were obtained in a BD FACSCanto II® Flow Cytometer and were analyzed with the software FlowJo® v10.0.
3.5. DM2 and Non-DM2 Subjects Show a Similar Cytokine Profile
Several reports suggest an association of increased frequencies of MDSCs and overproduction of inflammatory cytokines. Due to the elevation of the frequencies in the CD33+ HLA-DR-/low MDSCs in the diabetic group, we wondered whether this increased frequency of CD33+ HLA-DR-/low MDSCs could be related to cytokine overproduction. Therefore, several proinflammatory cytokines such as IL-1α, TNF-α, IFN-γ, IL-5, IL-12p70, and IL-17 and one anti-inflammatory IL-10 were quantified by cytometric bead array and analyzed through FCAP Array. The concentrations were classified for groups and compared between groups. Only significant differences were found in the comparison of IL-5 (P = 0.02); the other cytokines did not show any significant differences (Table 2). To corroborate, a correlation analysis was made between the concentrations of the cytokines and the frequencies of CD33+ HLA-DR-/low MDSCs. No correlation between these parameters was found (P > 0.05) (Supplementary ). Finally, the same correlation analysis was made; considering only the DM2 group, no significant correlations were identified either (Supplementary ).
Table 2.
Comparison of serum cytokine concentrations among study groups#.
| Variable | Non-DM2 | DM2 | P value |
|---|---|---|---|
| Eotaxin () | 4475 pg/dl ± 1987 | 5338 pg/dl ± 2409 | 0.2127a |
|
| |||
| IL-1α (m ± IQR) | 482.1 pg/dl q1 = 365.8, q3 = 665 |
415.1 pg/dl q1 = 323.1, q3 = 487.8 |
0.1741b |
|
| |||
| TNF-α (m ± IQR) | 69.64 pg/dl q1 = 14.16, q3 = 149.4 |
42.75 pg/dl q1 = 34.94, q3 = 133.5 |
0.8591b |
|
| |||
| IFN-γ (m ± IQR) | 642.8 pg/dl q1 = 397, q3 = 1534 |
400.1 pg/dl q1 = 83.98, q3 = 1252 |
0.4202b |
|
| |||
| IL-5 (m ± IQR) | 2.850 pg/dl q1 = 0.4175, q3 = 15.89 |
38.4 pg/dl q1 = 4.42, q3 = 74.72 |
0.0200∗b |
|
| |||
| IL-10 (m ± IQR) | 16.65 pg/dl q1 = 13.03, q3 = 32.66 |
26.68 pg/dl q1 = 11.49, q3 = 46.71 |
0.5652b |
|
| |||
| IL-12p70 (m ± IQR) | 22.18 pg/dl q1 = 2.40, q3 = 60.23 |
22.65 pg/dl q1 = 16.11, q3 = 34.25 |
0.9004b |
|
| |||
| IL-17 (m ± IQR) | 117.9 pg/dl q1 = 10.76, q3 = 298.4 |
93.97 pg/dl q1 = 65.10, q3 = 434.9 |
0.9999b |
#Only the concentrations obtained above detection limits for each cytokine were considered for analysis. aStudent's t-test. bMann-Whitney U test. ∗P < 0.05.
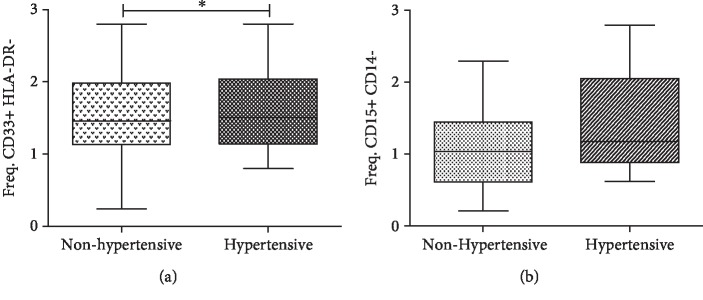
3.6. CD33+ HLA-DR-/Low MDSC Increased Frequency Is Associated with Individuals with Hypertension
It is known that some cell subtypes in the blood are associated with an increase in cardiovascular alterations and comorbidities, such as the case of T helper 17 (Th17) cells that respond to sodium increase in essential hypertension and have been recently linked to the pathogenesis of arterial hypertension [32, 33]. In order to identify if other variables were also associated with the increase in MDSCs, we evaluated if hypertension had any effect on the frequency of MDSCs. For this purpose, all subjects were polled for analysis and categorized according to having or not hypertension (reported in their clinical history) in two groups: hypertensive (which includes diabetic and nondiabetic) and nonhypertensive (which includes diabetic and nondiabetic). When the analysis was performed on these assumptions, we found a difference in the frequencies of CD33+ HLA-DR-/low MDSC phenotype (P < 0.05, Figure 3(a)) between hypertensive (mean = 1.620, SD = 0.602) and nonhypertensive (mean = 1.209, SD = 0.483) groups. These differences are not observed for the frequency of CD15+ CD14- MDSCs, although a slight increase is observed in the group that has hypertension (P > 0.05, Figure 3(b)).
Figure 3.
Frequency of CD33+ HLA-DR-/low and CD15+ CD14- MDSCs comparing if the subjects have hypertension in both groups (DM2 and non-DM2). (a) Frequency of CD33+ HLA-DR-/low MDSCs (hypertensive n = 16, nonhypertensive n = 27). (b) Frequency of CD15+ CD14- MDSCs (hypertensive n = 15, nonhypertensive n = 26). The graphs show the median and interquartile ranges. It was considered as statistically significant if P value < 0.05∗ which was calculated using the statistical t-test for unpaired samples. Statistical analysis was performed with the GraphPad Prism® v5.0 software.
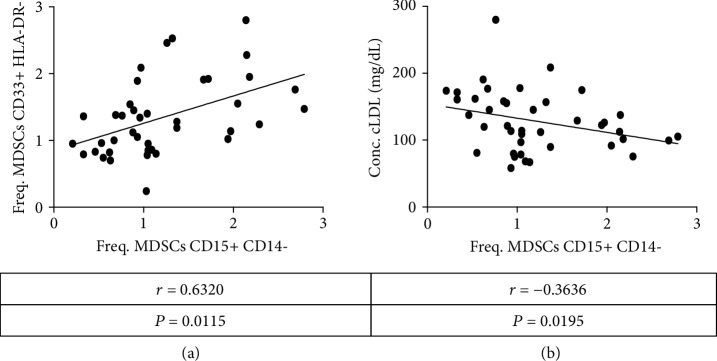
3.7. MDSC Phenotypes Correlate with Cardiovascular Markers of Disease
Given the previously shown results, MDSCs are elevated in the DM2 group and also in hypertensive individuals. To further clarify whether differences are associated with cardiovascular markers or diabetes markers, a correlation analysis was carried out. It was decided to perform the analysis for nonparametric data; of all the variables analyzed (age, BMI, WHR, glucose, Hb1ac%, cholesterol, cHDL, and cLDL), only the LDL cholesterol levels have a negatively statistically significant (P < 0.05) correlation with the frequency of CD15+ CD14- MDSCs (Figure 4(b)). Additionally, the frequency of CD33+ HLA-DR-/low and CD15+ CD14- MDSCs has a statistically significant correlation (P < 0.001, Figure 4(a)), and this correlation is maintained when only the DM2 group is analyzed (P = 0.029, Supplementary ), so this correlation is directly associated with DM2 condition. Data correlations with Spearman's rho value are summarized in Table 3.
Figure 4.
Correlation of MDSC frequency with cardiovascular markers. (a) Correlation between Freq. CD15+ CD14- and CD33+ HLA-DR-/low MDSCs. Data from 44 subjects, including groups in DM2 and nondiabetic controls. (b) Correlation between the concentration of LDL cholesterol and Freq. CD15+ CD14- MDSCs. Shown data are from 44 subjects (DM2 and nondiabetic controls). It was considered as statistically significant if value of P < 0.05. Statistical analysis was performed with the GraphPad Prism ® v5.0 software.
Table 3.
Correlation analysis of variables with the frequency of MDSCs#.
| Freq. MDSCs CD15+ CD14- | Freq. MDSCs CD33+ HLA-DR- | ||
|---|---|---|---|
| Age | Correlation coefficient | -0.083 | -0.117 |
| Significance (two-tailed) | 0.607 | 0.456 | |
| N | 41 | 43 | |
|
| |||
| Time of diagnosis (years) | Correlation coefficient | -0.080 | 0.039 |
| Significance (two-tailed) | 0.744 | 0.869 | |
| N | 19 | 20 | |
|
| |||
| BMI (kg/m2) | Correlation coefficient | 0.180 | 0.054 |
| Significance (two-tailed) | 0.259 | 0.731 | |
| N | 41 | 43 | |
|
| |||
| Waist-hip ratio | Correlation coefficient | -0.259 | -0.139 |
| Significance (two-tailed) | 0.107 | 0.378 | |
| N | 40 | 42 | |
|
| |||
| Fasting glucose | Correlation coefficient | -0.097 | 0.252 |
| Significance (two-tailed) | 0.547 | 0.103 | |
| N | 41 | 43 | |
|
| |||
| HbA1c (%) | Correlation coefficient | -0.040 | 0.255 |
| Significance (two-tailed) | 0.802 | 0.100 | |
| N | 41 | 43 | |
|
| |||
| Total cholesterol (mg/dl) | Correlation coefficient | -0.284 | -0.083 |
| Significance (two-tailed) | 0.071 | 0.597 | |
| N | 41 | 43 | |
|
| |||
| cHDL (mg/dl) | Correlation coefficient | 0.104 | -0.044 |
| Significance (two-tailed) | 0.518 | 0.779 | |
| N | 41 | 43 | |
|
| |||
| cLDL (mg/dl) | Correlation coefficient | -0.364∗ | -0.110 |
| Significance (two-tailed) | 0.019 | 0.482 | |
| N | 41 | 43 | |
|
| |||
| Triglycerides (mg/dl) | Correlation coefficient | -0.068 | -0.068 |
| Significance (two-tailed) | 0.675 | 0.665 | |
| N | 41 | 43 | |
|
| |||
| Freq. MDSCs CD15+ CD14- | Correlation coefficient | 1.000 | 0.511∗∗ |
| Significance (two-tailed) | 0.001 | ||
| N | 41 | 40 | |
|
| |||
| Freq. MDSCs CD33+ HLA-DR- | Correlation coefficient | 0.511∗∗ | 1.000 |
| Significance (two-tailed) | 0.001 | ||
| N | 40 | 43 | |
#Correlations were calculated with Spearman's rho. Differences in the sample size for each correlation may differ depending on the availability of the data for such patients or controls. ∗P < 0.05; ∗∗P < 0.01.
4. Discussion
It is known that subjects with diabetes have an increased susceptibility to infectious diseases. For active tuberculosis infection, a risk of more than 5-fold has been reported in diabetics compared to nondiabetic subjects [34, 35]. Recent reports suggest that there are alterations in the immune cells such as neutrophil chemotactic and phagocytic functions that contribute to this increased susceptibility to infection in diabetics [36]; however, other cell populations might be associated with this phenomenon. MDSCs were linked in the last few years with increased susceptibility to infectious diseases; particularly, it was recently described that frequencies of these cells are elevated in patients with tuberculosis [37]. Recently, it has been shown that low-grade inflammation induced by obesity and infiltrating cytokine-producing adipose tissue immune cells is associated with several complications of diabetics such as insulin resistance [38]. Given that chronic inflammation is present in these individuals and due to the previously described reports that suggest that this might be associated with MDSC expansion, the present report evaluated whether or not diabetes-associated low-grade inflammatory processes might be associated with increased frequencies of MDSCs.
It has been described that there is a basal frequency of MDSCs in healthy individuals, although very small (around 1%) for both monocytic and polymorphonuclear MDSCs [31]; consistent with these findings, similar frequencies in our groups are reported. The physiological role of these cells is thought to be associated with immune regulation by several mechanisms, such as arginine depletion which causes a reduced activity of T cells and a decrease in CD62L in lymphocytes caused by ADAM metallopeptidase domain 17 (ADAM17), and through the expansion of regulatory T (Treg) cells by TGF-β secretion and IL-10 [20], we confirm that our population of CD33+ HLA-DR- mononuclear MDSCs produces IL-10 and TGF-β in both DM2 and nondiabetic donors.
A statistically significant rise in the frequency of CD33+ HLA-DR-/low MDSCs was found in the DM2 group; these findings are similar to those reported by Wang et al. [39] where they find an increased frequency of MDSC CD11b+ CD33+ and probe how these MDSCs inhibit T cell proliferation; although the phenotype is a little different (CD11b+ CD33+ vs. CDD33+ HLA-DR-/low), both correspond to a mononuclear immunophenotype; our findings reaffirm the importance that MDSCs could play in the pathophysiology of DM2 suppressing T cell response making patients susceptible to some infections and also provide a link to cardiovascular alterations in DM2 patients. The causes behind such increase could be associated with the production of TGF-β and IL-5. Several reports have described that the production of such cytokines in diabetics and obese people is increased and linked to insulin resistance [26]. Also, in diabetics, an increase in signaling pathways such as JNK and IκB kinase beta (IKKβ) has been described to be associated with insulin resistance, and these same signaling pathways are responsible of MDSC expansion in bone marrow [40–43]. Therefore, the low-grade inflammation process characteristic of diabetics could be associated with such observed expansion. Surprisingly, our results did not show differences in the cytokine profile, perhaps because all the patients were already under medical oversight and treatment. In our sample, there were no differences in the BMI (which is one of the variables associated with low-grade inflammation). Interestingly, the only cytokine that is elevated in our sample of DM2 was IL-5. This cytokine that has been reported to be increased in those patients with DM2 and tuberculosis [44], common comorbidity associated with immunodeficiency in DM2 patients; nevertheless, further investigation is needed to confirm the role of IL-5 in susceptibility to infections in DM2.
Systemic arterial hypertension (SAH) is a common comorbidity of DM2 patients, according to the National Health and Nutrition Survey (ENSANUT, acronym in Spanish) in 2016; in Mexico, patients with a previous diagnosis of diabetes have a prevalence of 57.6% with hypertension [45]. Therefore, when we analyzed according to SAH, a significant increase in MDSC frequency was found. Recent reports have documented that hypertension has an important inflammatory component particularly associated with Th17 cells that have a high response to salt concentrations [46]. Given that these cells are able to produce IL-6 and other cytokines [47] that are able to induce proliferation of MDSCs, our hypothesis is that such phenomena are intertwined, meaning that MDSC increased frequencies are probably a consequence of inflammatory processes from diabetes, obesity, and hypertension. This is further supported by our own results given that increased frequency of MDSC cells correlates between the two phenotypes; high levels of LDL cholesterol correlated negatively with CD15+ CD14- MDSCs, and this could be related to the generation of atheromatous plaques in blood vessels given that an increase of such inflammatory cells with phagocytic functions such as these is able to adhere to such plaques. These hypotheses need to be confirmed by experimental observations.
5. Conclusion
Our group of DM2 patients have an increased frequency of mononuclear MDSCs CD33+ HLA-DR-/low that produce TGF-β and IL-10. DM2 and non-DM2 subjects show a similar cytokine profile, but the DM2 patients have an increased concentration of IL-5. CD33+HLA-DR-/low MDSCs are associated with other complications of diabetics such as hypertension and cardiovascular markers of the disease (cLDL).
Acknowledgments
The authors would like to thank the participants of the study and the clinical and laboratory staff at “medical family care unit # 4 in Zacatecas, Mexico.” MHGH acknowledges project number R-2011-785-063.
Data Availability
The data used in this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflict of interest.
Authors' Contributions
JECD conceived and designed the experiments; JCFR, JCGA, and MAVA performed the experiments; JCFR and JCGA analyzed the data; JECD, MLMF, IGV, ARCV, CJSE, MHGH, and JAEM contributed reagents/materials/analysis tools; JCFR and JECD wrote the paper; JCFR, JCGA, MLMF, IGV, ARCV, MAVA, CJSE, MHGH, JAEM, and JECD critically reviewed the manuscript.
Supplementary Materials
Supplementary Table 1: correlation analysis of cytokine concentrations and MDSC frequency (CD33+ HLA-DR- phenotype). Supplementary Table 2: correlation analysis of cytokine concentrations and frequency of MDSCs CD15+ CD14- in the DM2 group. Supplementary Table 3: correlation analysis of variables with the frequency of MDSCs (only DM2 group).
References
- 1.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Supplement 1):S81–S90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association. Standards of medical care in diabetes--2015: summary of revisions. Diabetes Care. 2015;38, article S4(Supplement 1) doi: 10.2337/dc15-s003. [DOI] [Google Scholar]
- 3.Danaei G., Finucane M. M., Lu Y., et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. The Lancet. 2011;378(9785):31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 4.Mathers C. D., Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Medicine. 2006;3(11, article e442) doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Key Massages for Diabetes (2014) 2014, https://www.idf.org/cvd.
- 6.da Rocha Fernandes J., Ogurtsova K., Linnenkamp U., et al. IDF Diabetes Atlas estimates of 2014 global health expenditures on diabetes. Diabetes Research and Clinical Practice. 2016;117:48–54. doi: 10.1016/j.diabres.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 7.Federación Internacional de la Diabetes. ATLAS de la DIABETES de la FID. International Diabetes Federation; 2013. https://www.diabetesatlas.org/ [Google Scholar]
- 8.Obesity and Overweight. WHO; 2015. https://www.who.int/mediacentre/factsheets/fs311/en/ [Google Scholar]
- 9.Beulens J. W. J., Rutters F., Rydén L., et al. Risk and management of pre-diabetes. European Journal of Preventive Cardiology. 2019;26(2_Supplement):47–54. doi: 10.1177/2047487319880041. [DOI] [PubMed] [Google Scholar]
- 10.Pan B., Wu Y., Yang Q., et al. The impact of major dietary patterns on glycemic control, cardiovascular risk factors, and weight loss in patients with type 2 diabetes: a network meta-analysis. Journal of Evidence-Based Medicine. 2019;12(1):29–39. doi: 10.1111/jebm.12312. [DOI] [PubMed] [Google Scholar]
- 11.Hotamisligil G. S., Shargill N. S., Spiegelman B. M. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 12.Feinstein R., Kanety H., Papa M. Z., Lunenfeld B., Karasik A. Tumor necrosis factor-alpha suppresses insulin-induced tyrosine phosphorylation of insulin receptor and its substrates. Journal of Biological Chemistry. 1993;268(35):26055–26058. [PubMed] [Google Scholar]
- 13.Zhang Y., Proenca R., Maffei M., Barone M., Leopold L., Friedman J. M. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 14.Fried S. K., Bunkin D. A., Greenberg A. S. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. The Journal of Clinical Endocrinology & Metabolism. 1998;83(3):847–850. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- 15.Steppan C. M., Bailey S. T., Bhat S., et al. The hormone resistin links obesity to diabetes. Nature. 2001;409(6818):307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 16.Shimomura I., Funahashi T., Takahashi M., et al. Enhanced expression of PAI–1 in visceral fat: possible contributor to vascular disease in obesity. Nature Medicine. 1996;2(7):800–803. doi: 10.1038/nm0796-800. [DOI] [PubMed] [Google Scholar]
- 17.Fukuhara A., Matsuda M., Nishizawa M., et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307(5708):426–430. doi: 10.1126/science.1097243. [DOI] [PubMed] [Google Scholar]
- 18.Youn J. I., Gabrilovich D. I. The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. European Journal of Immunology. 2010;40(11):2969–2975. doi: 10.1002/eji.201040895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gabrilovich D. I., Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nature Reviews Immunology. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gabrilovich D. I., Ostrand-Rosenberg S., Bronte V. Coordinated regulation of myeloid cells by tumours. Nature Reviews Immunology. 2012;12(4):253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greten T. F., Manns M. P., Korangy F. Myeloid derived suppressor cells in human diseases. International Immunopharmacology. 2011;11(7):802–807. doi: 10.1016/j.intimp.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Facciabene A., Motz G. T., Coukos G. T-regulatory cells: key players in tumor immune escape and angiogenesis. Cancer Research. 2012;72(9):2162–2171. doi: 10.1158/0008-5472.CAN-11-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang B., Pan P. Y., Li Q., et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Research. 2006;66(2):1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 24.Kern P. A., Saghizadeh M., Ong J. M., Bosch R. J., Deem R., Simsolo R. B. The expression of tumor necrosis factor in human adipose tissue. Regulation by obesity, weight loss, and relationship to lipoprotein lipase. The Journal of Clinical Investigation. 1995;95(5):2111–2119. doi: 10.1172/JCI117899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hotamisligil G. S., Arner P., Caro J. F., Atkinson R. L., Spiegelman B. M. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. The Journal of Clinical Investigation. 1995;95(5):2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wellen K. E., Hotamisligil G. S. Inflammation, stress, and diabetes. The Journal of Clinical Investigation. 2005;115(5):1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McArdle M. A., Finucane O. M., Connaughton R. M., McMorrow A. M., Roche H. M. Mechanisms of obesity-induced inflammation and insulin resistance: insights into the emerging role of nutritional strategies. Frontiers in Endocrinology. 2013;4:p. 52. doi: 10.3389/fendo.2013.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peleg A. Y., Weerarathna T., McCarthy J. S., Davis T. M. Common infections in diabetes: pathogenesis, management and relationship to glycaemic control. Diabetes/Metabolism Research and Reviews. 2007;23(1):3–13. doi: 10.1002/dmrr.682. [DOI] [PubMed] [Google Scholar]
- 29.Gan Y.-H. Host susceptibility factors to bacterial infections in type 2 diabetes. PLoS Pathogens. 2013;9(12, article e1003794) doi: 10.1371/journal.ppat.1003794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joshi N., Caputo G. M., Weitekamp M. R., Karchmer A. W. Infections in patients with diabetes mellitus. The New England Journal of Medicine. 1999;341(25):1906–1912. doi: 10.1056/NEJM199912163412507. [DOI] [PubMed] [Google Scholar]
- 31.Ko J. S., Zea A. H., Rini B. I., et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clinical Cancer Research. 2009;15(6):2148–2157. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 32.Chen S., Agrawal D. K. Dysregulation of T cell subsets in the pathogenesis of hypertension. Current Hypertension Reports. 2015;17(2):p. 8. doi: 10.1007/s11906-014-0521-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang J., Crowley S. D. Role of T lymphocytes in hypertension. Current Opinion in Pharmacology. 2015;21:14–19. doi: 10.1016/j.coph.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ponce-de-Leon A., Garcia-Garcia M. . L., Garcia-Sancho M. C., et al. Tuberculosis and diabetes in southern Mexico. Diabetes Care. 2004;27(7):1584–1590. doi: 10.2337/diacare.27.7.1584. [DOI] [PubMed] [Google Scholar]
- 35.Delgado-Sánchez G., García-García L., Castellanos-Joya M., et al. Association of pulmonary tuberculosis and diabetes in Mexico: analysis of the National Tuberculosis Registry 2000–2012. PLoS One. 2015;10(6, article e0129312) doi: 10.1371/journal.pone.0129312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delamaire M., Maugendre D., Moreno M., Le Goff M. C., Allannic H., Genetet B. Impaired leucocyte functions in diabetic patients. Diabetic Medicine. 1997;14(1):29–34. doi: 10.1002/(SICI)1096-9136(199701)14:1<29::AID-DIA300>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 37.du Plessis N., Loebenberg L., Kriel M., et al. Increased frequency of myeloid-derived suppressor cells during active tuberculosis and after recent Mycobacterium tuberculosis infection suppresses T-cell function. American Journal of Respiratory and Critical Care Medicine. 2013;188(6):724–732. doi: 10.1164/rccm.201302-0249OC. [DOI] [PubMed] [Google Scholar]
- 38.Herder C., Dalmas E., Boni-Schnetzler M., Donath M. Y. The IL-1 pathway in type 2 diabetes and cardiovascular complications. Trends in Endocrinology & Metabolism. 2015;26(10):551–563. doi: 10.1016/j.tem.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 39.Wang T., Wen Y., Fan X. Myeloid-derived suppressor cells suppress CD4+ T cell activity and prevent the development of type 2 diabetes. Acta Biochimica et Biophysica Sinica. 2018;50(4):362–369. doi: 10.1093/abbs/gmy014. [DOI] [PubMed] [Google Scholar]
- 40.Yuan M., Konstantopoulos N., Lee J., et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkβ. Science. 2001;293(5535):1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 41.Aguirre V., Uchida T., Yenush L., Davis R., White M. F. The c-Jun NH2-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser307. Journal of Biological Chemistry. 2000;275(12):9047–9054. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]
- 42.Hirosumi J., Tuncman G., Chang L., et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420(6913):333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 43.Cai D., Yuan M., Frantz D. F., et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. Nature Medicine. 2005;11(2):183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar N. P., Sridhar R., Banurekha V. V., et al. Type 2 diabetes mellitus coincident with pulmonary tuberculosis is associated with heightened systemic type 1, type 17, and other proinflammatory cytokines. Annals of the American Thoracic Society. 2013;10(5):441–449. doi: 10.1513/AnnalsATS.201305-112OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Campos-Nonato I., Hernandez-Barrera L., Pedroza-Tobias A., Medina C., Barquera S. Hypertension in Mexican adults: prevalence, diagnosis and type of treatment. Ensanut MC 2016. Salud Pública de México. 2018;60(3):233–243. doi: 10.21149/8813. [DOI] [PubMed] [Google Scholar]
- 46.Amador C. A., Barrientos V., Peña J., et al. Spironolactone decreases DOCA–salt–induced organ damage by blocking the activation of T helper 17 and the downregulation of regulatory T lymphocytes. Hypertension. 2014;63(4):797–803. doi: 10.1161/HYPERTENSIONAHA.113.02883. [DOI] [PubMed] [Google Scholar]
- 47.Ahmed M., Gaffen S. L. IL-17 in obesity and adipogenesis. Cytokine & Growth Factor Reviews. 2010;21(6):449–453. doi: 10.1016/j.cytogfr.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: correlation analysis of cytokine concentrations and MDSC frequency (CD33+ HLA-DR- phenotype). Supplementary Table 2: correlation analysis of cytokine concentrations and frequency of MDSCs CD15+ CD14- in the DM2 group. Supplementary Table 3: correlation analysis of variables with the frequency of MDSCs (only DM2 group).
Data Availability Statement
The data used in this study are available from the corresponding author upon request.