Abstract
Objectives
To investigate whether the presence of peroxisome proliferator-activated receptor gamma (PPARG) gene polymorphisms is associated with unexplained mild visual impairment (UMVI) in patients with type 2 diabetes mellitus (T2DM).
Methods
A total of 135 T2DM residents with UMVI and 133 with normal vision (NV; best-corrected visual acuity ≥ 20/25 in both eyes) were enrolled. UMVI was defined as best-corrected visual acuity (BCVA) < 20/25 and ≥ 20/63 in both eyes, with no visual impairment-causing diseases found. Four PPARG gene single-nucleotide polymorphisms (SNPs) (rs3856806, rs1801282, rs709158, and rs10865710) were assessed with the HAPLOVIEW 4.0 software to examine the statistical association of PPARG polymorphisms and UMVI in patients with T2DM.
Results
Four SNPs qualified the Hardy–Weinberg equilibrium (p > 0.05). The frequency of genotype GC at SNP rs10865710 was significantly higher in the UMVI group than in the NV group (p < 0.001; GG + GC versus CC) (OR = 8.94, 95% CI: 4.90–16.31), whereas genotype CC decreased the risk (OR = 0.07, 95% CI: 0.03–0.14). Genotype TT at SNP rs3856806 was strongly associated with UMVI (p < 0.0001, TT + TC versus CC) (OR = 4.74, 95% CI: 2.68–8.54), whereas genotype CC appeared to be protective for UMVI (OR = 0.55, 95% CI: 0.37–0.82).
Conclusions
Susceptibilities of PPARG variants may lead to differences in PPARG transcription, result in early function loss of retinal photoreceptor cells, and eventually cause UMVI.
1. Introduction
Diabetes is a group of metabolic disorders characterized by hyperglycemia resulting from defects in insulin secretion, insulin action, or both [1, 2]. The World Health Organization (WHO) estimated that by 2025, there would be 300 million people worldwide with diabetes mellitus [3], and Type 2 diabetes mellitus (T2DM) accounted for 90%–95% of those with diabetes [1]. Over the last three decades, there had been a major rise in the prevalence of T2DM globally [4]. T2DM is associated with many complications, among which ocular complications are common and usually emerging earlier than other complications [5]. Owing to complications such as cataracts, diabetic retinopathy (DR), and glaucoma, the prevalence of visual impairment is much higher in the T2DM population than the nondiabetic populations. Over the past decades, measures for prevention of visual impairment focused on moderate to severe visual impairment and blindness (best-corrected visual acuity (BCVA) < 20/63) [6]. Accordingly, few health administration members or ophthalmologists paid attention to mild visual impairment (BCVA < 20/25 and ≥20/63), [6] which also reduced the activities of daily living and life quality of patients with T2DM [7, 8].
From October 2014 to January 2015, we conducted a cross-sectional, epidemiological study of eye disease among 2,216 adults with T2DM in the Xinjing community, Shanghai [9]. Mild visual impairment was found in 1,891 eyes—42.7% of all eyes examined (4,432 eyes in 2,216 patients). The primary causes of mild visual impairment in patients with T2DM were cataract and DR [10]. In addition, we also identified 420 eyes of 210 patients with unexplained mild visual impairment (UMVI) in both eyes—21.3% of cases and 9.1% of all 2,216 participants. We believe that UMVI occurred because of the early function loss of the macular photoreceptor cells when no morphological changes could be detected in the population-based epidemiological studies in which fundus photography and optical coherence tomography (OCT) were the major detective techniques. Given that such a high proportion of patients with UMVI and the global increase in T2DM [11], the number of diabetic patients with UMVI is about to increase rapidly [5]. To date, no published study had addressed the pathogenesis of UMVI, while patients with UMVI continued to seek an explanation, because the characterization of the etiology as “unknown” would indicate a possible rapid progression to moderate or severe visual loss and imply that there is no effective prevention or treatment.
The mechanism of the ocular complications of T2DM is complex and still not well-demonstrated. Genetic susceptibility, inflammation, oxidative stress, and environmental influences were all reported to be involved [12–15]. Peroxisome proliferator-activated receptor gamma (PPARG) is a ligand-activated transcription factor that plays an important role in the control of a variety of physiological processes such as metabolism, angiogenesis, fibrosis, inflammation, and oxidative stress in various blind-causing diseases, such as DR, age-related macular degeneration, and optic neuropathy [16–19]. Genetic susceptibility determines the different responses to factors like inflammation. For example, the incidence of DR differed in different individuals with the same blood glucose level. Therefore, we speculated that the susceptibility of certain genes in the diabetic population may result in UMVI. In the present study, peroxisome proliferator-activated receptor gamma (PPARG) was chosen as a candidate gene, and we investigated whether the presence of PPARG gene polymorphisms was associated with UMVI in a Chinese Han T2DM population to provide novel insight into the pathogenesis of UMVI.
2. Materials and Methods
This was a population-based case-control study. The patients in the UMVI group and NV group were mainly diabetic residents in the Shanghai Xinjing community [9]. The study was approved by the Ethics Committee of the Shanghai general hospital, Shanghai Jiaotong University (2013KY023). All the procedures were conducted according to the tenets of the Declaration of Helsinki. Informed consent was obtained from all subjects after a full explanation of the study protocol.
3. Patient Selection
The inclusion criteria were (1) provision of the written informed consent, (2) diagnosis of T2DM based on the WHO diagnostic criteria, [20] (3) ability to comply with all the required examinations, (4) BCVA < 20/25 and ≥20/63 in both eyes, with no visual impairment-causing ophthalmic diseases, and (5) age- and gender-matched patients with BCVA ≥ 20/25 in both eyes [6].
The exclusion criteria were (1) eyelid diseases, strabismus, corneal diseases, lens diseases, and other eye diseases that may affect the results of OCTA or fundus photograph examinations; (2) eye diseases, such as glaucoma and macular degeneration, which may cause other fundus retinal microvasculopathy; (3) primary systemic diseases, including those involving the respiratory system, circulatory system, and urinary system in addition to DM; and (4) a history of cancer or major surgery.
The research team consisted of the same fully trained and experienced routine members as introduced before [9, 21]. First, the baseline characteristics were surveyed using a questionnaire. Patients met the above terms and went through a thorough eye examination; 1 ml of fasting whole peripheral blood was collected from each participant, and DNA extraction was performed according to the kit instructions (QIAN amp Blood kit Hilden, Germany).
4. SNPs Selection
Four PPARG single-nucleotide polymorphisms (SNPs) (rs1801282, rs3856806, rs709158, and rs10865710) in previous studies associated with metabolic disorders captured in the locus were selected [22–27]. Among them, rs1801282 is a confirmed type 2 diabetes susceptibility locus of PPARG [28]. rs3856806, rs709158, and rs10865710 are all associated with loci of lipoprotein metabolism and obesity in the Chinese Han population [25, 29, 30]. Probe sequences of four SNPs were shown in Table 1; positions and functional consequences were also listed.
Table 1.
Probe sequence for four SNPs used for Fluidigm sequencing analysis.
| Rs number | SNP | Ch | Functional consequence | Position | SNP_SEQ |
|---|---|---|---|---|---|
| rs10865710 | Intron C>G | 3 | Upstream transcript variant | 12,311,699 | AGTTTCATGTAGGTAAGACTGTGTAGAATGTCGGGTCTCGATGTTGGCGCTATTCAAGCCCTGATGATAAGGCTTTTGGCATTAGATGCTGTTTTGTCTT[C/G]ATGGAAAATACAGCTATTCTAGGATCCTTGAGCCTTTCATAAGAGATAAGGTTGTGAATCCTAAGACCCTAGGACCRTTTACTTAGATGATCTGCTCTCT |
|
| |||||
| rs1801282 | Intron C>G | 3 | Missense variant/coding sequence variant | 12,351,626 | TTGATCTTTTGCTAGATAGAGACAAAATATCAGTGTGAATTACAGCAAACCCCTATTCCATGCTGTTATGGGTGAAACTCTGGGAGATTCTCCTATTGAC[C/G]CAGAAAGCGATTCCTTCACTGATACACTGTCTGCAAACATATCACAAGGTAAAGTTCCTTCCAGATACGGCTATTGGGGACGTGGGGGCATTTATGTAAG |
|
| |||||
| rs709158 | Intron A>G | 3 | Genic downstream transcript variant | 12,403,176 | CTCTGCAGCAGGCAAAAGCTCTTTTTGTTAATTCAAAACAGTTTGGAATCCATTTCAGTTCTTCCTAAACCTCCAAGATACGGGGGAGGAAATTCACTGG[A/G]TTTTACAATATATTTTTCAAGGCAAATTGCCATCGCCGTCCTAATGACAGAGAAGCTGCCGATATCACTACAACGGCTGCAGATGGCAAGTCATCCAGCC |
|
| |||||
| rs3856806 | C1341T | 3 | 3 prime UTR variant/synonymous variant | 12,415,557 | CCCTGGAGCTCCAGCTGAAGCTGAACCACCCTGAGTCCTCACAGCTGTTTGCCAAGCTGCTCCAGAAAATGACAGACCTCAGACAGATTGTCACGGAACA[C/T]GTGCAGCTACTGCAGGTGATCAAGAAGACGGAGACAGACATGAGTCTTCACCYGCTCCTGCAGGAGATCTACAAGGACTTGTACTAGCAGAGAGTCCTGA |
Ch: chromosome.
5. Sequencing Methods
The sequences, which included both upstream and downstream regions of the target SNPs (), were sent to Fluidigm (http://Assay_Design_Group@fluidigm.com), and the Fluidigm SNP genotyping markers which was composed of a specific target amplification (STA) primer, a locus-specific (LS) primer, and two allele-specific primers were designed. Genotyping was performed following the Fluidigm SNP genotyping instructions by the IRRI genotyping service laboratory (http://gsl@irri.org) as introduced by Kim et al. [31]. Briefly, the target region was amplified with the STA and LS primers under a thermal cycler. The diluted PCR products from the 268 samples, four Fluidigm SNP markers, and PCR reagents were simultaneously mated in a FR192.24 Dynamic Array by the IFC Controller. Then, PCR was performed in the FC1™ Cycler, and the fluorescence signals from the end PCR products were finally read under the EP1TM Reader.
6. Statistical Analysis
Student's t-test and the χ2 test were used to compare continuous clinical data and categorical variables, respectively. Allelic and genotypic frequencies between the UMVI and NV groups were compared by the χ2 test or Fisher's exact test. Hardy–Weinberg equilibrium (HWE) for genotype frequencies of the SNPs was tested by the χ2 test. The correction for multiple testing in the haplotype analysis was performed by permutation testing. Pairwise linkage disequilibrium (LD, D′) analyses between the polymorphisms and EM-based haplotype association analysis were performed by HAPLOVIEW (ver. 4.0) and SPSS 22.0 software (IBM Corporation, US). Odds ratios (OR) and 95% confidence intervals (CI) were also calculated. A p < 0.05 was considered statistically significant.
7. Results
A total of 135 T2DM residents with UMVI were admitted in the case group of this study. Another 133 normal vision (NV; BCVA ≥ 20/25 in both eyes) residents with T2DM were enrolled in the control group of this study.
Basic information for the subjects in the two groups is shown in Table 2. Except for the difference in the waistline and hipline, the two groups showed no statistically significant intergroup differences in gender, age, age at onset of T2DM, duration of diabetes, hemoglobin A1c levels, fasting blood glucose levels, and systolic and diastolic blood pressure. The UMVI group had a slightly shorter waistline and hipline than the NV group, but the well-acknowledged indicator of obesity degree, BMI, did not differ significantly between the two groups. Logistic regression analysis also did not reveal any correlation between the waistline, hipline, and target SNPs.
Table 2.
Demographic and clinical characteristics of 135 residents with unexplained mild visual impairment (UMVI) and 133 residents with normal vision (NV).
| UMVI residents | NV residents | p value∗ | |
|---|---|---|---|
| Gender (male) | 53 | 60 | 0.39 |
| Age (year) | 65.34 ± 5.41 | 64.82 ± 8.45 | 0.59 |
| Age at diabetes onset (years) | 58.48 ± 10.76 | 57.51 ± 10.15 | 0.39 |
| Duration of diabetes (years) | 6.86 ± 5.21 | 7.30 ± 5.86 | 0.52 |
| Hemoglobin A1c (%) | 7.24 ± 1.46 | 7.09 ± 1.34 | 0.36 |
| Fasting blood glucose (mmol/l) | 7.21 ± 2.08 | 7.17 ± 1.88 | 0.87 |
| Body mass index (kg/m2) | 25.00 ± 3.62 | 25.75 ± 3.51 | 0.09 |
| Waistline (cm) | 85.68 ± 9.93 | 88.27 ± 9.19 | 0.03 |
| Hipline (cm) | 94.98 ± 9.84 | 97.03 ± 6.77 | 0.04 |
| Systolic blood pressure (mmHg) | 140.02 ± 20.84 | 143.77 ± 19.69 | 0.14 |
| Diastolic blood pressure (mmHg) | 81.03 ± 11.97 | 80.74 ± 11.35 | 0.84 |
∗Student's t-test and χ2 test.
Out of the 4 SNPs selected, rs709158 had a low genotyping call rate (= 51%), while the remaining three had a full call rate. 4 SNPs tested in the UMVI and NV groups all qualified the HWE (p > 0.05). The allelic frequencies for each of the four sequence variants analyzed (rs3856806, rs1801282, rs709158, and rs10865710) in all the UMVI, and control cases are shown in Table 1.
The observed genotype frequencies of the 4 PPARG SNPs met the HWE (p > 0.05) in both the UMVI and NV groups (as shown in Table 3). Statistically significant differences were observed between the UMVI subjects and controls when the genotypic frequencies for each of the 3 SNPs with significantly increased allelic frequency (rs10865710, rs709158, and rs3856806) were compared. The frequency of genotype GC at SNP rs10865710 was significantly higher in the UMVI group (p < 0.001; GG + GC versus CC), conferring an approximately 8.94-fold increased risk for UMVI (OR = 8.94, 95% CI: 4.90–16.31), whereas genotype CC decreased the risks (OR = 0.07, 95% CI: 0.03–0.14). Genotype TT at SNP rs3856806 was strongly associated with UMVI (p < 0.0001, TT + TC versus CC) conferring a more than 3-fold increased risk (OR = 4.74, 95% CI: 2.68–8.54), whereas genotype CC appeared to be protective for UMVI (OR = 0.55, 95% CI: 0.37–0.82).
Table 3.
PPARG allele frequencies in the 135 residents with unexplained mild visual impairment (UMVI) and 133 residents with normal vision (NV).
| SNP | Alleles | UMVI residents | NV residents | p value∗ | MAF | OR (95%CI) |
|---|---|---|---|---|---|---|
| number (%) | number (%) | |||||
| rs10865710 | G | 163 (60.4) | 14 (5.3) | 6.57E − 42 | 0.33 | 27.42 (15.18–49.51) |
| C | 107 (39.6) | 252 (94.7) | ||||
| rs1801282 | G | 22 (8.1) | 0 (0) | 1.99E − 06 | 0.04 | NA |
| C | 248 (91.9) | 266 (100) | ||||
| rs709158 | G | 95 (58.6) | 24 (21.4) | 1.00E − 09 | 0.43 | 5.20 (3.00–9.00) |
| A | 67 (41.4) | 88 (78.6) | ||||
| rs3856806 | T | 88 (32.6) | 21 (7.9) | 1.22E − 12 | 0.20 | 5.64 (3.38–9.42) |
| C | 182 (67.4) | 245 (92.1) |
MAF = minor allele frequency; OR = odds ratio; CI = confidence interval; NA, the odds ratio was not available where the number of individuals with two copies of the risk allele was zero. ∗χ2 test.
The pairwise LD analysis identified one block (39 kb) (Figure 1), which included 4 SNPs in strong LD, as observed by the D0 value. The SNP rs10865710 was in complete LD with rs1801282 (coefficient of LD [D0] = 1.00). The frequency of these haplotypes and their associations with UMVI is shown in Table 4.
Figure 1.
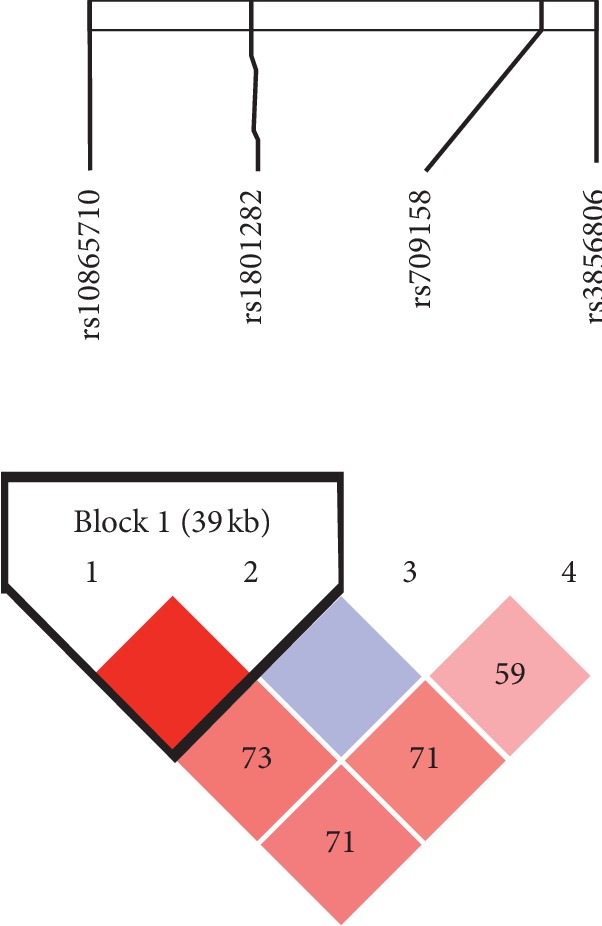
PPARG linkage disequilibrium plot of the PPARG single-nucleotide polymorphisms rs3856806, rs1801282, rs709158, and rs10865710. The number in the diamond refers to D0 (100 9 D0). The linkage disequilibrium block was defined according to the standard confidence intervals. The strength of linkage disequilibrium is depicted by the intensity of red coloring, which moves from white to light red as D0 progresses from 0 to 100.
Table 4.
Genotypic association analysis in 135 residents with unexplained mild visual impairment (UMVI) and 133 residents with normal vision (NV).
| SNP | Genotype | UMVI residents | NV residents | HWpval | p value∗ | OR (95% CI)∗ | p value# | OR# (95% CI) |
|---|---|---|---|---|---|---|---|---|
| number | number | |||||||
| rs10865710 | GG | 36 | 0 | 0.08 | 7.17E − 42 | NA | ||
| GC | 91 | 14 | 6.40 (3.47–11.80) | 1.86E − 41 | 0.01 (0.00–0.02) | |||
| CC | 8 | 119 | 0.07 (0.03–0.14) | (CC: GG + GC) | ||||
| rs1801282 | GG | 0 | 0 | 1 | NA | NA | ||
| GC | 22 | 0 | NA | 7.46E − 6 | 0.46 (0.40, 0.53) | |||
| CC | 113 | 133 | 0.86 (0.61–1.21) | (CC: GG + GC) | ||||
| rs709158 | GG | 25 | 4 | 0.33 | 3.35E − 09 | 4.43 (1.46–13.43) | 0.41 | 2.22 (0.67, 7.38) |
| GA | 45 | 16 | 1.94 (1.00–3.78) | 6.9E − 7 | 20.46 (5.84, 71.61) | |||
| AA | 11 | 36 | 0.21 (0.10–0.45) | 1.39E − 6 | 0.11 (0.04, 0.26) | |||
| rs3856806 | TT | 16 | 0 | 0.10 | 1.24E − 10 | NA | ||
| TC | 56 | 21 | 2.63 (1.51–4.58) | 8.92E − 10 | 0.16 (0.09–0.29) | |||
| CC | 63 | 112 | 0.55 (0.37–0.82) | (CC: TT + TC) |
p value∗ (chi-square test); OR (95% CI) ∗(chi-square test); OR# (95 % CI); p value# (Bonferroni correction); OR' (Bonferroni correction); HWpval, Hardy–Weinberg equilibrium p value. OR = odds ratio; CI = confidence interval; NA, the odds ratio was not available where the number of individuals with two copies of the risk allele was zero. ∗χ2 test.
8. Discussion
A PubMed search indicated that there is no worldwide study that has investigated the association of PPARG gene polymorphisms with UMVI in a T2DM population. Therefore, the statistically significant relationship between UMVI and the SNPs rs10865710 and rs3856806 found in our study will be important for elucidating the gene susceptibility and possible pathogenesis of UMVI. Haplotype analysis for PPARG SNPs in the groups UMVI and NV is shown in Table 5.
Table 5.
Haplotype analysis for PPARG SNPs in 135 residents with unexplained mild visual impairment (UMVI) and 133 residents with normal vision (NV).
| rs10865710 | rs1801282 | UMVI residents | NV residents | p value∗ | OR (95% CI) |
|---|---|---|---|---|---|
| haplotype frequency | haplotype frequency | ||||
| C | C | 0.34 | 0.95 | 6.57E − 42 | 0.04 (0.02–0.07) |
| G | C | 0.52 | 0.05 | 4.04E − 33 | 19.67 (10.92–35.45) |
| G | G | 0.08 | 0 | 1.99E − 06 | NA |
∗ χ 2 test.
Visual impairment in patients with T2DM is attributed primarily to retinal damage. [19, 32] The outer retina consists of photoreceptor neurons and Müller cells, which are metabolically coupled to support the generation of electrochemical impulses in response to stimulation of light, with nutrients and oxygen diffusing from choroidal vessels through the pigmented epithelium layer. The retina and choroid, as high-energy consumption targets, are highly prone to hyperglycemia-induced molecular damage. Quite a few published papers have confirmed that PPARG plays an important role in reactive oxygen species generation, inflammation, apoptosis, and antiangiogenesis-induced retinal and choroidal dysfunction [33–36]. Suppression of PPARG via activation of nuclear factor kappa B is reported to be involved in the pathogenesis of experimental DR and oxygen-induced retinopathy [37]. As an important constituent of mitochondrial reactive oxygen species imbalance, PPARG was also confirmed to be an initiated and sustained factor in the general pathways of DR after short-term stimulation by hyperglycemia and directly mediated the inhibitory effect of statins on reactive oxygen species, thus reducing early retinal injury in diabetic eyes [38, 39].
Specific variants rs10865710 (introns) and rs3856806 (synonymous mutation) (Table 1) account for UMVI, do not change the sequence of amino acids, and mainly affect the process of PPARG transcription. Therefore, we speculate that the differences in PPARG gene susceptibility lead to different levels of PPARG protein, which further result in differences in the response to oxidative stress in retina/choroid under the stimulus of hyperglycemia, causing early function loss of macular photoreceptor cells and eventually resulting in UMVI.
In that case, PPARG agonists, such as pioglitazone, may help control UMVI and relieve patient anxiety. Pioglitazone has been proven to protect retinal and/or choroidal cells from hyperglycemia-induced injuries in a PPARG-dependent pathway. It can normalize insulin signaling in the diabetic rat retina through reduction in the levels of tumor necrosis factor and suppressor of cytokine signaling 3, [35] modulate the retinal pigmented epithelium survival responses to oxidative stress, inhibit activation of the glial cells, prevent cell apoptosis, and protect the retina from subsequent cellular damage caused by retinal ischemia/reperfusion [33, 40]. In previously clinical studies, pioglitazone has been used to prevent vascular complications of T2DM, such as stroke and atherosclerosis [41, 42]. Therefore, we suppose that pioglitazone may be used to prevent visual impairment progression in patients with T2DM.
In summary, the present study confirmed an independent association between UMVI and PPARG polymorphisms in a T2DM population. The limitations of this study should not be neglected. First, the study was a single-center study based on the Chinese Han population and contained a small number of subjects. Second, more SNPs of PPARG should be sequenced. Further studies are also necessary.
Acknowledgments
This study was funded by the Chinese National Nature Science Foundation (Project nos. 81670898 and 81600778), Chronic Diseases Prevention and Treatment Project of Shanghai Shen Kang Hospital Development Centre (Project nos. SHDC12015315 and SHDC2015644), Shanghai Three Year Public Health Action Program (Project no. GWIV-3.3), Shanghai High-level Oversea Training Team Program on Eye Public Health (Project no. GWTD2015S08), Shanghai Outstanding Academic Leader Program (Project no. 16XD1402300), Science and Technology Commission of Shanghai Municipality (Project no. 17511107901), and Shanghai Municipal Education Commission—Gaofeng Clinical Medicine Grant Support (Project no. 20172022).
Data Availability
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Authors' Contributions
Haidong Zou and Tao Li conceived and designed the study. Haidong Zou, Tao Li, Xian Xu, and Yi Xu analysed and interpreted the data. Tao Li, Xian Xu, Yi Xu, Peiyao Jin, Jianhua Chen, and Yongyong Shi collected data. Haidong Zou was incharge of overall direction and planning.
Supplementary Materials
Fluidigm SNP genotyping markers.
References
- 1.American Diabetes Association, Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(1):S81–S90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 2.Pendse A. A., Johnson L. A., Tsai Y.-S., Maeda N. Pparg-P465L mutation worsens hyperglycemia in Ins2-Akita female mice via adipose-specific insulin resistance and storage dysfunction. Diabetes. 2010;59(11):2890–2897. doi: 10.2337/db10-0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.King H., Aubert R. E., Herman W. H. Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21(9):1414–1431. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 4.Chen L., Magliano D. J., Zimmet P. Z. The worldwide epidemiology of type 2 diabetes mellitus-present and future perspectives. Nature Reviews Endocrinology. 2012;8(4):228–236. doi: 10.1038/nrendo.2011.183. [DOI] [PubMed] [Google Scholar]
- 5.Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703–713. [PMC free article] [PubMed] [Google Scholar]
- 6.Intenational Council of Ophthalmology. The 29th International Congress of Ophthalmology. Sydney, Australia: 2002. Visual standard-aspects and ranges of vision loss with emphasis on population surveys[C/OL] http://www.icoph.org/downloads/visualstandardsreport.pdf. [Google Scholar]
- 7.Vitale S., Cotch M. F., Sperduto R. D. Prevalence of visual impairment in the United States. JAMA. 2006;295(18):2158–2163. doi: 10.1001/jama.295.18.2158. [DOI] [PubMed] [Google Scholar]
- 8.Sharma S., Brown G. C., Brown M. M., et al. Converting visual acuity to utilities. Canadian Journal of Ophthalmology. 2000;35(5):267–272. doi: 10.1016/s0008-4182(00)80077-0. [DOI] [PubMed] [Google Scholar]
- 9.Zou H., Xu X., He J., et al. Prevalence and risk factors of mild vision loss in patients with type 2 diabetes in Beixinjing Community of Shanghai. Zhonghua Yi Xue Za Zhi. 2016;96:210–215. doi: 10.3760/cma.j.issn.0376-2491.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Xu X., He J., Xu X., et al. Cataract was the principle cause of visual impairment and blindness in Shanghai residents with type 2 diabetes. Acta Ophthalmologica. 2016;94(3):e246–e247. doi: 10.1111/aos.12930. [DOI] [PubMed] [Google Scholar]
- 11.Chen L., Magliano D. J., Zimmet P. Z. The worldwide epidemiology of type 2 diabetes mellitus-present and future perspectives. Nature Reviews Endocrinology. 2011;8(4):228–236. doi: 10.1038/nrendo.2011.183. [DOI] [PubMed] [Google Scholar]
- 12.Tomic M., Ljubic S., Kastelan S., Gverovic Antunica A., Jazbec A., Poljicanin T. Inflammation, haemostatic disturbance, and obesity: possible link to pathogenesis of diabetic retinopathy in type 2 diabetes. Mediators of Inflammation. 2013;2013 doi: 10.1155/2013/818671.818671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding L., Cheng R., Hu Y., et al. Peroxisome proliferator-activated receptor α protects capillary pericytes in the retina. The American Journal of Pathology. 2014;184(10):2709–2720. doi: 10.1016/j.ajpath.2014.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonne C. Le PPAR gamma: une nouvelle cible pharmacologique contre la néovascularisation rétinienne et choroïdienne. Journal Français d’Ophtalmologie. 2005;28(3):326–330. doi: 10.1016/s0181-5512(05)81062-9. [DOI] [PubMed] [Google Scholar]
- 15.Fletcher B., Gulanick M., Lamendola C. Risk factors for type 2 diabetes mellitus. The Journal of Cardiovascular Nursing. 2002;16(2):17–23. doi: 10.1097/00005082-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Fajas L., Auboeuf D., Raspé E., et al. The organization, promoter analysis, and expression of the human PPARγ gene. Journal of Biological Chemistry. 1997;272(30):18779–18789. doi: 10.1074/jbc.272.30.18779. [DOI] [PubMed] [Google Scholar]
- 17.Yu H., Wark L., Ji H., et al. Dietary wolfberry upregulates carotenoid metabolic genes and enhances mitochondrial biogenesis in the retina of db/db diabetic mice. Molecular Nutrition & Food Research. 2013;57(7):1158–1169. doi: 10.1002/mnfr.201200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.SanGiovanni J. P., Lee P. H., Sapieha P., et al. AMD-associated genes encoding stress-activated MAPK pathway constituents are identified by interval-based enrichment analysis. PloS one. 2013;8(8) doi: 10.1371/journal.pone.0053155.e71239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu J., Zhang J., Ji M., et al. The role of peroxisome proliferator-activated receptor and effects of its agonist, pioglitazone, on a rat model of optic nerve crush: PPARγ in retinal neuroprotection. PLoS One. 2013;8(7) doi: 10.1371/journal.pone.0068935.e68935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hod M., Hadar E., Cabero-Roura L., et al. Prevention of type 2 diabetes among women with prior gestational diabetes mellitus. International Journal of Gynecology & Obstetrics. 2015;131(3):S16–S18. doi: 10.1016/j.ijgo.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Zou X., Lu L., Xu Y., et al. Prevalence and clinical characteristics of dry eye disease in community-based type 2 diabetic patients: the Beixinjing eye study. BMC Ophthalmology. 2018;18:p. 117. doi: 10.1186/s12886-018-0781-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan W., Shen C., Wu M., Zhou Z.-Y., Guo Z.-R. Association and interaction of PPARα, δ, and γ gene polymorphisms with low-density lipoprotein-cholesterol in a Chinese han population. Genetic Testing and Molecular Biomarkers. 2015;19(7):379–386. doi: 10.1089/gtmb.2015.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bego T., Dujic T., Mlinar B., et al. Association of PPARG and LPIN1 gene polymorphisms with metabolic syndrome and type 2 diabetes. Medicinski Glasnik. 2011;8:76–83. [PubMed] [Google Scholar]
- 24.Gouda H. N., Sagoo G. S., Harding A.-H., Yates J., Sandhu M. S., Higgins J. P. T. The association between the peroxisome proliferator-activated receptor-γ2 (PPARG2) Pro12Ala gene variant and type 2 diabetes mellitus: a HuGE review and meta-analysis. American Journal of Epidemiology. 2010;171(6):645–655. doi: 10.1093/aje/kwp450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo W., Guo Z., Wu M., et al. Association of peroxisome proliferator-activated receptor α/δ/γ; with obesity, and Gene–Gene interaction, in the Chinese han population. Journal of Epidemiology. 2013;23(3):187–194. doi: 10.2188/jea.je20120110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gu S.-J., Chen D.-H., Guo Z.-R., Zhou Z.-Y., Hu X.-S., Wu M. Effect of obesity on the association between common variations in the PPAR gene and C-reactive protein level in Chinese Han population. Endocrine. 2015;48(1):195–202. doi: 10.1007/s12020-014-0218-x. [DOI] [PubMed] [Google Scholar]
- 27.Ma J., Li Y., Zhou F., Xu X., Guo G., Qu Y. Meta-analysis of association between the Pro12Ala polymorphism of the peroxisome proliferator-activated receptor-gamma2 gene and diabetic retinopathy in Caucasians and Asians. Molecular Vision. 2012;18:2352–2360. [PMC free article] [PubMed] [Google Scholar]
- 28.Altshuler D., Hirschhorn J. N., Klannemark M., et al. The common PPARγ Pro12Ala polymorphism is associated with decreased risk of type 2 diabetes. Nature Genetics. 2000;26(1):76–80. doi: 10.1038/79216. [DOI] [PubMed] [Google Scholar]
- 29.Lv X., Zhang L., Sun J., et al. Interaction between peroxisome proliferator-activated receptor gamma polymorphism and obesity on type 2 diabetes in a Chinese Han population. Diabetology & Metabolic Syndrome. 2017;9:p. 7. doi: 10.1186/s13098-017-0205-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y., Meng N., Lv Z., Li H., Qu Y. The gene polymorphisms of UCP 1 but not PPAR γ and TCF 7L2 are associated with diabetic retinopathy in Chinese type 2 diabetes mellitus cases. Acta Ophthalmologica. 2015;93(3):e223–e229. doi: 10.1111/aos.12542. [DOI] [PubMed] [Google Scholar]
- 31.Kim S. R., Ramos J., Ashikari M., et al. Development and Validation of Allele-Specific SNP/Indel Markers for Eight Yield-Enhancing Genes using Whole-Genome Sequencing Strategy to Increase Yield Potential of Rice. New York, NY, USA: Oryza sativa L. Rice; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolter J. R. Diabetic retinopathy. American Journal of Ophthalmology. 1961;51:1123–1141. doi: 10.1016/0002-9394(61)91802-5. [DOI] [PubMed] [Google Scholar]
- 33.Rodrigues G. A., Maurier-Mahé F., Shurland D.-L., et al. Differential effects of PPARγ ligands on oxidative stress-induced death of retinal pigmented epithelial cells. Investigative Opthalmology & Visual Science. 2011;52(2):890–903. doi: 10.1167/iovs.10-5715. [DOI] [PubMed] [Google Scholar]
- 34.Malchiodi-Albedi F., Matteucci A., Bernardo A., Minghetti L. PPARγ, microglial cells, and ocular inflammation: new venues for potential therapeutic approaches. PPAR Research. 2008;2008:p. 295784. doi: 10.1155/2008/295784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang Y., Thakran S., Bheemreddy R., et al. Pioglitazone normalizes insulin signaling in the diabetic rat retina through reduction in tumor necrosis factor α and suppressor of cytokine signaling 3. Journal of Biological Chemistry. 2014;289(38):26395–26405. doi: 10.1074/jbc.m114.583880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Z., He T., Du K., et al. Inhibition of oxygen-induced ischemic retinal neovascularization with adenoviral 15-lipoxygenase-1 gene transfer via up-regulation of PPAR-γ and down-regulation of VEGFR-2 expression. PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0085824.e85824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tawfik A., Sanders T., Kahook K., Akeel S., Elmarakby A., Al-Shabrawey M. Suppression of retinal peroxisome proliferator-activated receptor γ in experimental diabetes and oxygen-induced retinopathy: role of NADPH oxidase. Investigative Opthalmology & Visual Science. 2009;50(2):878–884. doi: 10.1167/iovs.08-2005. [DOI] [PubMed] [Google Scholar]
- 38.Ceriello A. The emerging challenge in diabetes: the “metabolic memory”. Vascular Pharmacology. 2012;57(5-6):133–138. doi: 10.1016/j.vph.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 39.Zheng Z., Chen H., Wang H., et al. Improvement of retinal vascular injury in diabetic rats by statins is associated with the inhibition of mitochondrial reactive oxygen species pathway mediated by peroxisome proliferator-activated receptor coactivator 1. Diabetes. 2010;59(9):2315–2325. doi: 10.2337/db10-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang X.-Y., Xiao Y.-Q., Zhang Y., Ye W. Protective effect of pioglitazone on retinal ischemia/reperfusion injury in rats. Investigative Opthalmology & Visual Science. 2013;54(6):3912–3921. doi: 10.1167/iovs.13-11614. [DOI] [PubMed] [Google Scholar]
- 41.Yoshii H., Onuma T., Yamazaki T., et al. Effects of pioglitazone on macrovascular events in patients with type 2 diabetes mellitus at high risk of stroke: the PROFIT-J study. Journal of Atherosclerosis and Thrombosis. 2014;21:563–573. [PubMed] [Google Scholar]
- 42.Xiao C. C., Ren A., Yang J., et al. Effects of pioglitazone and glipizide on platelet function in patients with type 2 diabetes. European Review for Medical and Pharmacological Sciences. 2015;19:963–970. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fluidigm SNP genotyping markers.
Data Availability Statement
The data used to support the findings of this study are included within the article.


