Abstract
Background
High‐intensity ultrasound (HIUS) has been studied for the past two decades as a new therapeutic option for solid tumor direct treatment and a method for better chemotherapy delivery and perfusion. This treatment approach has not been tested to our knowledge in peritoneal metastatic therapy, where limited tissue penetration of intraperitoneal chemotherapy has been a main problem. Both liquid instillations and pressurized aerosols are affected by this limitation. This study was performed to evaluate whether HIUS improves chemotherapy penetration rates.
Methods
High-intensity ultrasound (HIUS) was applied for 0, 5, 30, 60, 120, and 300 seconds on the peritoneal tissue samples from fresh postmortem swine. Samples were then treated with doxorubicin via pressurized intraperitoneal aerosol chemotherapy (PIPAC) under 12 mmHg and 37°C temperature. Tissue penetration of doxorubicin was measured using fluorescence microscopy on frozen thin sections.
Results
Macroscopic structural changes, identified by swelling of the superficial layer of the peritoneal surface, were observed after 120 seconds of HIUS. Maximum doxorubicin penetration was significantly higher in peritoneum treated with HIUS for 300 seconds, with a depth of 962.88 ± 161.4 μm (p < 0.05). Samples without HIUS had a penetration depth of 252.25 ± 60.41. Tissue penetration was significantly increased with longer HIUS duration, with up to 3.8-fold increased penetration after 300 sec of HIUS treatment.
Conclusion
Our data indicate that HIUS may be used as a method to prepare the peritoneal tissue for intraperitoneal chemotherapy. Higher tissue penetration rates can be achieved without increasing chemotherapy concentrations and preventing structural damage to tissue using short time intervals. More studies need to be performed to analyze the effect of HIUS in combination with intraperitoneal chemotherapy.
1. Introduction
Peritoneal metastasis (PM) is a commonly seen manifestation of advanced gastrointestinal and gynecological cancers. It is known that the antitumor effect of intraperitoneal chemotherapy (IPC) is strongly limited by penetration of chemotherapy drugs well below 1 mm into peritoneal nodules [1, 2]. Various approaches have been made to improve the availability of chemotherapy in these tumor nodules.
For example, it has been shown that hyperthermia [3] and intraperitoneal pressure [4] increase drug penetration and efficiency. These concepts have already led to new therapies like hyperthermic intraperitoneal chemotherapy (HIPEC) combined with cytoreductive surgery [5]. The application of pressure has been proposed and was ultimately applied through pressurized intraperitoneal aerosol chemotherapy (PIPAC) in the treatment of more advanced peritoneal metastasis [6, 7]. Clinical as well as experimental studies have also tested irradiation [8–10] and new drug formulas [11–13] as alternate methods of increasing chemotherapy penetration. Despite several attempts to improve penetration rates, these studies have had only limited success. For example, application parameters in PIPAC that may affect chemotherapeutic penetration depth, such as the micropump position and dose of doxorubicin, have been tested [14]. Although many of these aforementioned efforts have been made, penetration levels still were mostly described to be less than 500 μm [15–17].
Therefore, further methods for improved drug delivery and increasing depth penetration are needed to be developed. In this regard, high intensity ultrasound (HIUS) has been a very promising method to potentially achieve this goal. HIUS has been investigated for over two decades in solid tumor therapy with promising results in particular cases [18–20]. It is already known that HIUS can improve the perfusion of chemotherapy agents in liver tumors and glioblastoma [21, 22]. HIUS systems provide unique advantages of low invasiveness and absence of radiation.
However, to our knowledge, its interaction in combination with any form of intraperitoneal chemotherapy on the peritoneum has not been thoroughly investigated. It is known that HIUS enhances the delivery of doxorubicin in a preclinical model of solid pancreatic cancer [23]. We aimed to evaluate its effect on the penetration depth of doxorubicin in a well-established model of fresh postmortem peritoneal tissue samples [17, 24].
2. Materials and Methods
2.1. High-Intensity Ultrasound
The experiments were performed on commercially available tissue samples; hence, no approval of the Local Board on Animal Care was required. Fresh postmortem swine peritoneum was purchased (local pork supplier, Zerniki Wielkie) and cut into proportional sections. Samples were then placed into Petri dishes. and NaCl 0.9% was added until the peritoneal surface was covered with 5 mm of liquid. High-intensity ultrasound was applied with a metal pen to the center of the peritoneal tissue using a sonicator (Bandelin Sonoplus, UW 2070). The tip of the pen was held 3 mm from the tissue. Samples were divided into six groups which were treated for 0 seconds, 5 seconds, 30 seconds, 60 seconds, 120 seconds, and 300 seconds, respectively. Each treatment contained 0.3 seconds of active and 0.7 seconds of passive interval, with 20 kHz frequency, output power of 70 W, and 50% of amplitude.
2.2. Ex Vivo PIPAC Model
Samples and untreated controls were placed in a well-described ex vivo model and treated with PIPAC with doxorubicin (PFS®, 2 mg/ml, Pfizer Europe, Sandwich, United Kingdom, purity ≥98%). A commercially available hermetic sealable plastic box with a total volume of 3.5 liters, representing the abdominal cavity, was used. In the center of the top cover of the plastic box, a 5 mm trocar (Kii® Balloon Blunt Tip System, Applied Medical, Rancho Santa Margarita, CA, USA) was placed. The nozzle of the microcatheter (MC, Olympus, PW-205V Olympus Surgical Technologies Europe, Hamburg, Germany) was introduced into the trocar. The plastic box was kept at a constant temperature of 27°C during the whole procedure. Fresh tissue specimens of peritoneum (German landrace pigs), each measuring 4.0 × 4.0 × 0.5 cm, were placed at the center of the plastic box. The distance between the nozzle of the MC and the bottom of the plastic box was 10 cm. The plastic box was then tightly sealed, and a constant CO2 capnoperitoneum of 12 mmHg (Olympus UHI-3, Olympus medical life science and industrial divisions, Olympus Australia, Notting Hill, Australia) was maintained during the entire PIPAC procedure. 3 mg of doxorubicin were dissolved in 50 ml NaCl 0.9% at 27°C and aerosolized.
2.3. Microscopic Analysis
After treatments, all tissue samples were rinsed with a sterile NaCl 0.9% solution in order to eliminate superficial chemotherapy and immediately frozen in liquid nitrogen. Cryosections (10 μm) were prepared from different areas of each specimen. Sections were mounted with a ProLong™ Gold Antifade Mountant (Thermo Fisher Scientific) containing 1.5 μg/ml 4',6-diamidino-2-phenylindole (DAPI) to stain nuclei. Penetration depth of doxorubicin was monitored using a Nikon Eclipse 80i fluorescence microscope (Nikon Instruments Europe B.V. Amsterdam, Netherlands). The distance between the luminal surface and the innermost positive staining for doxorubicin accumulation was measured and reported in micrometers.
2.4. Statistical Analyses
Experiments were independently performed three times. A total of eight tissue sections per tissue sample were subject to doxorubicin penetration measurement. Prism 7.0 software (GraphPad, La Jolla, CA, USA) was utilized to analyze the data. One-way ANOVA with a multiple comparison test was used for analyses of independent groups. A significant p value was considered at p < 0.05.
3. Results
3.1. Ex Vivo Experiment
PIPAC and HIUS were applied without complications. After applying HIUS, a visual control of the sample was performed. No macroscopic damage of the peritoneal surface was observed with shorter HIUS duration. However, after 120 seconds, some whitening and swelling of the peritoneum were noted. Doxorubicin was detected in fluorescence microscopy in both groups. Microscopic analysis of the different tissue specimens showed a substantial difference in the penetration depth of doxorubicin. Tissue penetration levels after HIUS were 361 μm ± 34.5 μm at 5 seconds, 409 μm ± 69.7 μm at 30 seconds, 598 μm ± 136.9 μm at 60 seconds, 725 μm ± 126.4 μm at 120 seconds, and 962 μm ± 161.4 μm at 300 seconds. Controls without HIUS showed penetration levels with (A) 252 μm ± 60.4 μm. Penetration increased significantly with longer HIUS duration (A-F vs. controls, p < 0.05) and reached a maximum in the sample (F). The penetration reached the 1 mm level (F) and increased up to 3.8 folds to the control without HIUS (control vs. F, p < 0.0001).
The differences between the penetration depths observed in this study summarized in Figures 1 and 2 display representative photos showing doxorubicin fluorescence in the analyzed tissue samples.
Figure 1.
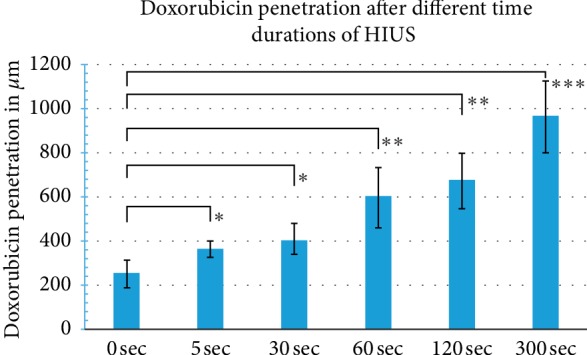
Tissue penetration depth of doxorubicin in μm after HIUS treatment for 0, 5, 30, 60, 120, and 300 sec (∗p < 0.01; ∗∗p < 0.001; ∗∗∗p < 0.0001).
Figure 2.
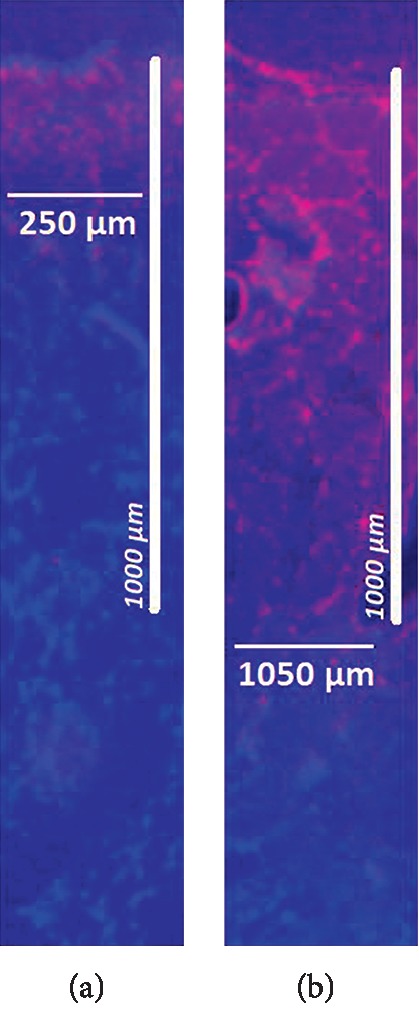
Microscopic analysis of the penetration depth of doxorubicin into fresh peritoneal samples of German landrace pigs. Nuclei (blue) were stained with 4',6-diamindino-2-phenylidole (DAPI). (a) In-tissue penetration of doxorubicin without HIUS. (b) In-tissue penetration of doxorubicin after 300 seconds HIUS.
4. Discussion
In spite of progress in chemotherapeutic regimens and new drug compositions, poor response to systemic and local treatment is observed in a considerable part of patients, mainly due to molecular mechanisms and limited drug distribution in the tumor [1, 25].
Pressurized intraperitoneal and pressurized intraluminal aerosol chemotherapies have been introduced to improve the treatment of advanced, multiresistant surface malignancies by overcoming limitations in drug penetration through the use of pressure and microaerosol [26, 27]. However, attempts to further improve were only partially successful, as changes of treatment parameters have only modestly improved penetration rates [4, 14]. Adding irradiation and modifying application modes [10] did not improve performance either, as penetration levels were mostly limited to the first few hundred microns. However, we know that increasing tissue penetration enhances the antitumor effect with a higher local drug disposition [1]. In our study, we demonstrate the previously unrecognized potential of HIUS to enhance drug penetration to many folds in the peritoneal tissue.
In the clinical setting, HIUS is being increasingly used as noninvasive treatment of both primary and metastatic tumors. Besides its effects described here, it has additional antitumor effects including ablation and mechanical disruption of cancer tissue [28, 29]. HIUS has already been shown to be useful in the treatment of uterine fibroids [30], various solid tumors of pancreas, liver, renal system, and prostate, and breast cancer [31–34]. So far, there have been no or few studies for potential use in peritoneal metastases (PM). By improving tissue penetration, higher drug concentrations in the tumor tissue could be reached without increasing the drug dose, which is important to limit systemic side effects of the chemotherapy.
Other attempts to improve current PIPAC and IPC have been studied recently. One such attempt to improve overall results is synchronous intravenous chemotherapy. Feasibility for this kind of bidirectional approach has been demonstrated, and results on tumor regression and survival have been promising [35]. However, it is unclear whether this effect is predominantly that of PIPAC or rather one of the intravenous chemotherapies. Studies indicate that this might be an effect of PIPAC [36], while the effect of the additional intravenous chemotherapy is unknown.
Another attempt to improve PIPAC was the introduction of electrostatic precipitation as an additional feature to the procedure. A recent study from Giger-Pabst et al. [37] analyzing the effect of electrostatic PIPAC (ePIPAC) versus PIPAC alone did not show any tissue increase or any other change demonstrating the efficancy of PIPAC by adding an electrostatic device. Additionally, clinical studies could not detect any differences between these two approaches in terms of biological effect [38]. Data on electrostatic augmentation is scarce, and the potential of electrostatic PIPAC is unknown. Analyzing the effects of electrostatic precipitation combined with the applied aerosol itself is quite a challenge, and while ongoing studies present new locations and various applications for chemoaerosol [39, 40], there is an ongoing effort to understand the applied chemoaerosol itself [39, 41].
The application of heat in IPC is well studied. Heat has shown to increase cytotoxicity and has therefore been an integral part in HIPEC [42]. However, the application of heat in PIPAC is a technical challenge because heat would have to be distributed through the applied gaseous capnoperitoneum. Therefore, it remains unclear if heat has a role in PIPAC. Despite these limitations, concepts based on basic physical principles like heat, electrostatic effects, changing physical properties of applied substances [39], or mechanic alteration [43] of the biological surface have gained more interest recently as they seem to have more potential than initially expected.
Our data indicate that HIUS plus PIPAC can overcome the 1 mm barrier on the peritoneum, which is a very promising result. HIUS resulted in better penetration of doxorubicin into swine peritoneum samples from 1.4 to 3.8 folds depending on the duration of HIUS application (5 sec to 300 sec). These findings require further studies in this field and ideas for a possible clinical approach to the application of HIUS in PM via PIPAC or via any other intraperitoneal chemotherapy.
5. Conclusions
Our data indicate that pretreatment of tissue samples with HIUS enhances doxorubicin penetration after the PIPAC procedure. Depth of penetration increases with longer duration of HIUS. This can be a new promising approach in IPC for better outcomes. Further research needs to be conducted for translation of this ex vivo method into clinical practice.
Acknowledgments
This study was funded by Institutional Funds.
Abbreviations
- CO2:
Carbon dioxide
- CRS:
Cytoreductive surgery
- HIPEC:
Hyperthermic intraperitoneal chemotherapy
- HIUS:
High-intensity ultrasounds
- IAP:
Intra-abdominal pressure
- IPC:
Intraperitoneal chemotherapy
- PM:
Peritoneal metastasis
- PIPAC:
Pressurized intraperitoneal aerosol chemotherapy
- ePIPAC:
Electrostatic pressurized intraperitoneal aerosol chemotherapy
- MC:
Microcatheter.
Data Availability
The data used to support the findings of this study are available from the corresponding author on request.
Conflicts of Interest
The authors have no conflicts of interest or financial ties to disclose.
Authors' Contributions
VK was involved in study design, laboratory analysis, and data acquisition. SR was responsible for study design, data analysis, and manuscript drafting. AM was responsible for study design, data acquisition, and manuscript drafting. TK was involved in study design, laboratory analysis, data acquisition, and manuscript drafting. MA was involved in data interpretation and critical revision for important intellectual content of the manuscript. AM was responsible for supervision of the study, drafting, and critical revision for important intellectual content of the manuscript.
References
- 1.Dedrick R. L., Myers C. E., Bungay P. M., DeVita V. T., Jr. Pharmacokinetic rational for the peritoneal drug administration in the treatment of ovarian cancer. Cancer Treatment Reviews. 1978;6:1–11. [PubMed] [Google Scholar]
- 2.Los G., Mutsaers P. H., van der Vijgh W. J., Baldew G. S., de Graaf P. W., McVie J. G. Direct diffusion of cis-diamminedichloroplatinum(II) in intraperitoneal rat tumor after intraperitoneal chemotherapy: a comparison with systemic chemotherapy. Cancer Research. 1989;49:3380–3384. [PubMed] [Google Scholar]
- 3.Morano W. F., Khalili M., Chi D. S., Bowne W. B., Esquivel J. Clinical studies in CRS and HIPEC: trials, tribulations, and future directions-a systematic review. Journal of Surgical Oncology. 2018;117(2):245–259. doi: 10.1002/jso.24813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khosrawipour V., Diaz-Carballo D., Acikelli A. H., et al. Cytotoxic effect of different treatment parameters in pressurized intraperitoneal aerosol chemotherapy (PIPAC) on the in vitro proliferation of human colonic cancer cells. World Journal of Surgical Oncology. 2017;15(1):p. 43. doi: 10.1186/s12957-017-1155-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajeev R., Turaga K. K. Hyperthermic intraperitoneal chemotherapy and cytoreductive surgery in the management of peritoneal carcinomatosis. Cancer Control. 2016;23(1):36–46. doi: 10.1177/107327481602300107. [DOI] [PubMed] [Google Scholar]
- 6.Khosrawipour V., Khosrawipour T., Diaz-Carballo D., Förster E., Zieren J., Giger-Pabst U. Exploring the spatial drug distribution pattern of pressurized intraperitoneal aerosol chemotherapy (PIPAC) Annals of Surgical Oncology. 2016;23(4):1220–1224. doi: 10.1245/s10434-015-4954-9. [DOI] [PubMed] [Google Scholar]
- 7.Bellendorf A., Khosrawipour V., Khosrawipour T., et al. Scintigraphic peritoneography reveals a non-uniform 99mTc-pertechnetat aerosol distribution pattern for pressurized intra-peritoneal aerosol chemotherapy (PIPAC) in a swine model. Surgical Endoscopy. 2018;32(1):166–174. doi: 10.1007/s00464-017-5652-4. [DOI] [PubMed] [Google Scholar]
- 8.Khosrawipour V., Giger-Pabst U., Khosrawipour T., et al. Effect of irradiation on tissue penetration depth of doxorubicin after pressurized intra-peritoneal aerosol chemotherapy (PIPAC) in a novel ex-vivo model. Journal of Cancer. 2016;7(8):910–914. doi: 10.7150/jca.14714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khosrawipour V., Bellendorf A., Khosrawipour C., et al. Irradiation does not increase the penetration depth of doxorubicin in normal tissue after pressurized intra-peritoneal aerosol chemotherapy (PIPAC) in an ex vivo model. In Vivo. 2016;30(5):593–597. [PubMed] [Google Scholar]
- 10.Khosrawipour V., Khosrawipour T., Hedayat-Pour Y., et al. Effect of whole-abdominal irradiation on penetration depth of doxorubicin in normal tissue after pressurized intraperitoneal aerosol chemotherapy (PIPAC) in a post-mortem swine model. Anticancer Research. 2017;37(4):1677–1680. doi: 10.21873/anticanres.11498. [DOI] [PubMed] [Google Scholar]
- 11.Robella M., Vaira M., Argenziano M., et al. Exploring the use of pegylated liposomal doxorubicin (Caelyx®) as pressurized intraperitoneal aerosol chemotherapy (PIPAC) Frontiers in Pharmacology. 2019;25(10):p. 669. doi: 10.3389/fphar.2019.00669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mikolajczyk A., Khosrawipour V., Schubert J., et al. Effect of liposomal doxorubicin in pressurized intra-peritoneal aerosol chemotherapy (PIPAC) Journal of Cancer. 2018;9(23):4301–4305. doi: 10.7150/jca.26860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schubert J., Khosrawipour V., Chaudry H., et al. Comparing the cytotoxicity of taurolidine, mitomycin C and oxaliplatin on the proliferation of in-vitro colon carzinoma cells following Pressurized Intra-peritoneal Aerosol Chemotherapy. World Journal of Surgical Oncology. 2019;17(1):p. 93. doi: 10.1186/s12957-019-1633-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khosrawipour V., Khosrawipour T., Falkenstein T. A., et al. Evaluating the effect of micropump position, internal pressure and doxorubicin dosage on efficacy of pressurized intra-peritoneal aerosol chemotherapy (PIPAC) in an ex vivo model. Anticancer Research. 2016;36(9):4595–4600. doi: 10.21873/anticanres.11008. [DOI] [PubMed] [Google Scholar]
- 15.Khosrawipour V., Khosrawipour T., Kern A. J. P., et al. Distribution pattern and penetration depth of doxorubicin after pressurized intraperitoneal aerosol chemotherapy (PIPAC) in a postmortem swine model. Journal of Cancer Research and Clinical Oncology. 2016;142(11):2275–2280. doi: 10.1007/s00432-016-2234-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khosrawipour T., Wu D., Bellendorf A., et al. Feasibility of single tumorspot treatment in peritoneal carcinomatosis via close range doxorubicin impaction in pressurized intra-peritoneal aerosol chemotherapy (PIPAC) Journal of Clinical & Experimental Oncology. 2017;6(3) doi: 10.4172/2324-9110.1000187. [DOI] [Google Scholar]
- 17.Khosrawipour V., Mikolajczyk A., Schubert J., Khosrawipour T. Pressurized intra-peritoneal aerosol chemotherapy (PIPAC)viaEndoscopical microcatheter system. Anticancer Research. 2018;38(6):3447–3452. doi: 10.21873/anticanres.12613. [DOI] [PubMed] [Google Scholar]
- 18.Giles S. L., Imseeh G., Rivens I., ter Haar G. R., Taylor A., deSouza N. M. MR guided high intensity focused ultrasound (MRgHIFU) for treating recurrent gynaecological tumours: a pilot feasibility study. The British Journal of Radiology. 2019;92(1098) doi: 10.1259/bjr.20181037.20181037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.An C., Li X., Liang P., et al. Local tumor control of thoracoabdominal wall seeding tumor from hepatocellular carcinoma with ultrasound-guided interventional treatment: a summarized study. Journal of Cancer Research and Therapeutics. 2019;15(2):404–414. doi: 10.4103/jcrt.JCRT_784_18. [DOI] [PubMed] [Google Scholar]
- 20.Mikolajczyk A., Khosrawipour V., Kulas J., et al. Release of doxorubicin from its liposomal coating via high intensity ultrasound. Molecular and Clinical Oncology. 2019;11(5):483–487. doi: 10.3892/mco.2019.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lyon P. C., Griffiths L. F., Lee J., et al. Clinical trial protocol for TARDOX: a phase I study to investigate the feasibility of targeted release of lyso-thermosensitive liposomal doxorubicin (ThermoDox®) using focused ultrasound in patients with liver tumours. Journal of Therapeutic Ultrasound. 2017;2(5):p. 28. doi: 10.1186/s40349-017-0104-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo Z., Jin K., Pang Q., et al. On-demand drug release from dual-targeting small nanoparticles triggered by high-intensity focused ultrasound enhanced glioblastoma-targeting therapy. ACS Applied Materials & Interfaces. 2017;9(37):31612–31625. doi: 10.1021/acsami.7b10866. [DOI] [PubMed] [Google Scholar]
- 23.Li T., Wang Y.-N., Khokhlova T. D., et al. Pulsed high-intensity focused ultrasound enhances delivery of doxorubicin in a preclinical model of pancreatic cancer. Cancer Research. 2015;75(18):3738–3746. doi: 10.1158/0008-5472.can-15-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mikolajczyk A., Khosrawipour V., Schubert J., Chaudhry H., Pigazzi A., Khosrawipour T. Particle stability during pressurized intra-peritoneal aerosol chemotherapy (PIPAC) Anticancer Research. 2018;38(8):4645–4649. doi: 10.21873/anticanres.12769. [DOI] [PubMed] [Google Scholar]
- 25.Jacquet P., Sugarbaker P. H. Peritoneal-plasma barrier. Cancer Treatment and Research. 1996;82:53–63. doi: 10.1007/978-1-4613-1247-5_4. [DOI] [PubMed] [Google Scholar]
- 26.Mikolajczyk A., Khosrawipour V., Schubert J., et al. Feasibility and characteristics of pressurized aerosol chemotherapy (PAC) in the bladder as a therapeutical option in early-stage urinary bladder cancer. In Vivo. 2018;32(6):1369–1372. doi: 10.21873/invivo.11388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Göhler D., Khosrawipour V., Khosrawipour T., et al. Technical description of the microinjection pump (MIP) and granulometric characterization of the aerosol applied for pressurized intraperitoneal aerosol chemotherapy (PIPAC) Surgical Endoscopy. 2017;31(4):1778–1784. doi: 10.1007/s00464-016-5174-5. [DOI] [PubMed] [Google Scholar]
- 28.Alkins R. D., Mainprize T. G. High-intensity focused ultrasound ablation therapy of gliomas. Progress in Neurological Surgery. 2018;32:39–47. doi: 10.1159/000469678. [DOI] [PubMed] [Google Scholar]
- 29.Maloney E., Hwang J. H. Emerging HIFU applications in cancer therapy. International Journal of Hyperthermia. 2015;31(3):302–309. doi: 10.3109/02656736.2014.969789. [DOI] [PubMed] [Google Scholar]
- 30.Zhang L., Zhang W., Orsi F., Chen W., Wang Z. Ultrasound-guided high intensity focused ultrasound for the treatment of gynaecological diseases: a review of safety and efficacy. International Journal of Hyperthermia. 2015;31(3):280–284. doi: 10.3109/02656736.2014.996790. [DOI] [PubMed] [Google Scholar]
- 31.De Senneville B. D., Moonen C., Ries M. MRI-guided HIFU methods for the ablation of liver and renal cancers. Advances in Experimental Medicine and Biology. 2016;880:43–63. doi: 10.1007/978-3-319-22536-4_3. [DOI] [PubMed] [Google Scholar]
- 32.Huang L., Chen Q., Yu L., Bai D. Pyropheophorbide-α methyl ester mediated photodynamic therapy induces apoptosis and inhibits LPS-induced inflammation in RAW264.7 macrophages. Photodiagnosis and Photodynamic Therapy. 2019;25:p. 148156. doi: 10.1016/j.pdpdt.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 33.Merckel L. G., Knuttel F. M., Deckers R., et al. First clinical experience with a dedicated MRI-guided high-intensity focused ultrasound system for breast cancer ablation. European Radiology. 2016;26(11):4037–4046. doi: 10.1007/s00330-016-4222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mortezavi A., Krauter J., Gu D. A., et al. Extensive histological sampling following focal therapy of clinically significant prostate cancer with high-intensity focused ultrasound. Journal of Urology. 2019;1:p. 101097. doi: 10.1097/JU.0000000000000298. [DOI] [PubMed] [Google Scholar]
- 35.Khomyakov V., Ryabov A., Ivanov A., et al. Bidirectional chemotherapy in gastric cancer with peritoneal metastasis combining intravenous XELOX with intraperitoneal chemotherapy with low-dose cisplatin and doxorubicin administered as a pressurized aerosol: an open-label, phase-2 study (PIPAC-GA2) Pleura Peritoneum. 2016;1(3):159–166. doi: 10.1515/pp-2016-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Somashekhar S. P., Ashwin K. R., Rauthan C. A., Rohit K. C. Randomized control trial comparing quality of life of patients with end-stage peritoneal metastasis treated with pressurized intraperitoneal aerosol chemotherapy (PIPAC) and intravenous chemotherapy. Pleura Peritoneum. 2018;3(3):p. 20180110. doi: 10.1515/pp-2018-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giger-Pabst U., Bucur P., Roger S., et al. Comparison of tissue and blood concentrations of oxaliplatin administrated by different modalities of intraperitoneal chemotherapy. Annals of Surgical Oncology. 2019;26(13):4445–4451. doi: 10.1245/s10434-019-07695-z. [DOI] [PubMed] [Google Scholar]
- 38.Graversen M., Detlefsen S., Ellebaek S. B., Fristrup C., Pfeiffer P., Mortensen M. B. Pressurized intraperitoneal aerosol chemotherapy with one minute of electrostatic precipitation (ePIPAC) is feasible, but the histological tumor response in peritoneal metastasis is insufficient. European Journal of Surgical Oncology. 2019 doi: 10.1016/j.ejso.2019.08.024. [DOI] [PubMed] [Google Scholar]
- 39.Khosrawipour T., Schubert J., Kulas J., et al. Creating nanocrystallized chemotherapy: the differences in pressurized aerosol chemotherapy (PAC) via intracavitary (IAG) and extracavitary aerosol generation (EAG) regarding particle generation, morphology and structure. Journal of Cancer. 2019 doi: 10.7150/jca.39097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khosrawipour V., Mikolajczyk A., Paslawski R., et al. Intrathoracic aerosol chemotheray via spray-catheter. Molecular and Clinical Oncology. 2019 doi: 10.3892/mco.2020.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khosrawipour T., Schubert J., Khosrawipour V., et al. Particle stability and structure on the peritoneal surface in pressurized intra-peritoneal aerosol chemotherapy (PIPAC) analysed by electron microscopy: first evidence of a new physical concept for PIPAC. Oncology Letters. 2019;17(6):4921–4927. doi: 10.3892/ol.2019.10162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.González-Moreno S., González-Bayón L. A., Ortega-Pérez G. Hyperthermic intraperitoneal chemotherapy: rationale and technique. World Journal of Gastrointestinal Oncology. 2010;2(2):68–75. doi: 10.4251/wjgo.v2.i2.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schubert J., Khosrawipour T., Pigazzi A., et al. Evaluation of cell-detaching effect of EDTA in combination with oxaliplatin for a possible application in HIPEC after cytoreductive surgery: a preliminary in-vitro study. Current Pharmaceutical Design. 2019;25 doi: 10.2174/1381612825666191106153623. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author on request.


