Abstract
Aims
Recent evidence suggests that the opioid system is implicated in the pathophysiology of alcohol use disorder (AUD). We aimed to examine the genetic influence of opioid receptors on susceptibility to AUD and its clinical and psychological characteristics including harmful drinking behavior and various aspects of impulsivity in AUD patients.
Methods
Three μ‐opioid receptor gene (OPRM1) variants and two κ‐opioid receptor gene (OPRK1) variants were examined in 314 male patients with AUD and 324 male controls. We applied the Alcohol Use Disorders Identification Test (AUDIT), Obsessive Compulsive Drinking Scale (OCDS), and Alcohol Dependence Scale. AUD patients also completed the stop‐signal task, delay discounting task, balloon analogue risk task, and the Barratt Impulsiveness Scale version 11 (BIS‐11).
Results
No significant differences in genotype distributions or haplotype frequencies were found between AUD patients and controls. However, OPRK1 SNP rs6473797 was significantly related to the severity of alcohol‐related symptoms as measured by AUDIT and OCDS and a haplotype containing rs6473797 was also related to OCDS scores in AUD patients. For other psychological traits, OPRM1 SNP rs495491 was significantly associated with scores on the motor subfactor of the BIS‐11.
Conclusion
Genetic variations in opioid receptors may contribute to symptom severity and impulsivity in AUD patients.
Keywords: alcohol use disorder, drinking severity, impulsivity, opioid receptor gene
1. INTRODUCTION
Alcohol use disorder (AUD) is a complex illness involving multiple genetic and environmental factors. Based on studies of twins and adoption, the hereditary component of alcohol dependence has been estimated at 50%‐60%.1 The endogenous opioid system has been implicated in the development of alcohol dependence due to its prominent role in the central reward mechanism.2 Existing studies suggest that the level of alcohol‐dependent activation in endogenous opioid transmission might be in part genetically determined.3 Among opioid receptor genes, OPRM1 encoding the μ‐opioid receptor is the most intensively studied in relation to drug dependence and alcoholism. Several studies on the effect of single nucleotide polymorphisms (SNPs) in OPRM1, such as rs1799971, on alcohol dependence report contradictory results.4, 5 Furthermore, the role of the κ‐opioid receptor in the regulation of reward stimuli via the modulation of dopaminergic tone8 has prompted research on the association of OPRK1 —which encodes the κ‐opioid receptor—with alcoholism.9 However, the role of OPRK1 in AUD is unclear due to conflicting results.10, 11
Apart from the influence of genetic factors on the development of AUD, researchers have also sought to identify markers associated with the severity of AUD symptoms.12 However, few studies have explored the genetic effects of opioid receptor genes on symptom severity in AUD. In a study investigating the association of the OPRM1 variant rs1799971 with alcoholism severity, no significant results emerged from questionnaire scores.13 The μ‐opioid receptor is known to play a crucial role in modulating the reinforcement effects of substances by reward circuits14; it is therefore valuable to investigate the link between OPRM1 and AUD severity. The OPRK1 gene is known to be associated with levels of alcohol use in patients with heroin dependence undergoing methadone maintenance treatment.15 Given the absence of studies investigating the direct genetic influence of OPRK1 on alcohol severity in the AUD, additional research is necessary. A number of questionnaires have been designed to measure symptom severity in AUD; the Alcohol Use Disorders Identification Test (AUDIT) is considered a useful tool to identify harmful drinking.16 The Alcohol Dependence Scale (ADS) is one of the most broadly used instruments to assess the severity of alcohol dependence,17 and the Obsessive Compulsive Drinking Scale (OCDS) has been widely used to measure the severity of cravings.18
Previous studies in humans have established that impulsivity is closely related to alcohol problems.19 Impulsivity as measured in prospective studies has been shown to predict the development of AUD20 and to mediate the relationship between parental substance‐use disorders and the eventual development of substance‐use disorders in offspring.21 There is growing consensus that impulsivity is heterogeneous, involving a variety of behaviors and processes. The Barratt Impulsiveness Scale (BIS) is a well‐validated self‐report questionnaire that measures multidimensional impulsivity consisting of motor, attentional (cognitive), and nonplanning impulsivity.22 Several behavioral tasks have also been developed and used for performance‐based measurement of impulsivity. In terms of behavioral disinhibition, the stop‐signal task (SST) is commonly used; in a previous study using SST, the patients with alcohol dependence showed impaired inhibitory control.23 The delay discounting task (DDT) is a well‐known modality to assess the tendency to discount future rewards in relation to impulsive decision making.24 In relatively recent years, poor outcomes resulting from risky behavior have been considered an important factor in impulsivity; the Balloon Analogue Risk Task (BART) is being used to measure the dimension of risk‐taking propensity.25 It is important to understand the role of impulsivity in AUD by comprehensively considering the various dimensions of impulsivity using self‐rating measures and various performance results. From the perspective of linkage in impulsivity and opioid receptor genes, the OPRM1 variant rs1799971 has shown association with impulsivity26; moreover, OPRM1 ‐knockout mice exhibited a marked reduction in motor impulsivity.27 In addition, the occurrence of impulsivity in patients with Parkinson's disease receiving dopamine replacement therapy is known to be related to OPRK1 genotypes28; however, evidence of a relationship between opioid receptor genes and impulsivity is still lacking.
The aim of our study was to investigate the potential role of OPRM1 and OPRK1 in susceptibility to AUD, as well as in its symptom severity characteristics using AUDIT, OCDS, and ADS in a Korean population. In addition, we explored the association between multidimensional impulsivity and OPRM1 and OPRK1 using questionnaires, BIS‐11, and behavioral tasks including SST, DDT, and BART in patients with AUD. We hypothesize that opioid receptor gene polymorphisms are associated with symptom severity and impulsivity in AUD patients.
2. METHODS
2.1. Subjects
The present study included only Korean males. The patient group consisted of 314 patients with AUD who were hospitalized at the 16 psychiatric hospitals throughout Korea. They were all diagnosed as having alcohol dependence by trained psychiatrists according to the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) criteria and had been abstinent for at least one week. All subjects scored above the cutoff score of 8 on the AUDIT, which is indicative of hazardous drinking.29 Exclusion criteria were as follows: (a) physical or mental illness that would interfere with task performance; (b) history of major psychiatric disorder including schizophrenia and other psychotic disorders, mood disorders, or anxiety disorders; (c) history of other substance dependence in the last 6 months; and (d) a score of less than 26 on the MMSE‐K (Mini‐Mental State Examination—Korean version). For the control group, a total of 324 nonalcoholic healthy males were enrolled from the Cardiovascular Genome Center at Yonsei University College of Medicine in the Republic of Korea between November 2000 and March 2011. They visited Severance Hospital, Yonsei University Health System, for health check‐ups. They did not have any specific medical conditions. Participants provided written informed consent according to the procedures approved by the Severance Hospital Institutional Review Board, and all methods were carried out in accordance with the approved guidelines.
2.2. Measurements
2.2.1. Questionnaires
The severity of symptoms in patients with AUD was assessed using AUDIT,16 OCDS,18 and ADS.17 The AUDIT consists of questions regarding alcohol consumption and the resulting consequences of drinking, with higher scores designating more harmful drinking behavior. The OCDS assesses the efforts and ability to resist thoughts of alcohol and the impulse to drink, with higher scores indicating higher craving intensities. ADS evaluates self‐administered compulsive drinking, problematic drinking behavior, and alcohol withdrawal symptoms. The severity of depressive and anxiety symptoms was assessed using the Beck Depression Inventory (BDI)30 and Beck Anxiety Inventory (BAI).31 All questionnaires were translated into Korean versions. Impulsivity was measured using the Korean version of the BIS‐11 for which we analyzed total scores as well as three subfactors including motor, cognitive, and nonplanning scores.32
2.2.2. Behavior tasks
We conducted the SST to assess the response inhibition and stop‐signal reaction time (SSRT). A longer SSRT value indicates a slower inhibitory response. To assess the impulsivity in a choice situation, the DDT was performed by participants. We calculated the k value, with higher k values designating higher sensitivity to delayed rewards. Lastly, the BART was conducted to measure risk‐taking propensity (a value of BART). A larger adjusted value of BART represents a higher risk‐taking propensity. (See the Appendix S1 for details of the behavior tasks.).
2.3. SNP selection and genotyping
We selected a total of six SNPs including four OPRM1 gene SNPs (rs1799971, rs495491, rs609148, and rs648893) and two OPRK1 gene SNPs (rs6473797 and rs702764). In selecting the SNPs, we reviewed previous genetic studies on alcohol dependence and verified significant association with alcohol use based on SNP or haplotype analyses4, 6, 11, 33, 34 (Table S1). The minor allele frequency for each selected SNP was confirmed to be >0.05 in East Asian populations, based on the 1000 genomes project database (https://www.ncbi.nlm.nih.gov/variation/tools/1000genomes/; GRCh37.p13, Phase 3). Genomic DNA was extracted from blood. Genotyping was conducted using a single‐base primer extension assay (ABI PRISM® SNaPshot™ Multiplex kit; ABI/Life Technologies/Thermo Fisher Scientific) according to the manufacturer's instructions.
2.4. Statistical analyses
Statistical analyses were performed using descriptive statistics for the demographic variables. Genotypes for rs609148 and rs648893 were perfectly linked; therefore, we dropped rs648893 from the analyses and the allele frequencies and association of traits for rs609148 were extended to rs648893. Differences in the allelic distribution of the five SNPs were examined using chi‐square tests. Associations between each SNP genotype and alcohol dependence status were examined using age‐adjusted multivariate logistic regression analysis. Linear regression models were used to evaluate associations between genotypes and various clinical measures of alcohol severity (AUDIT, OCDS, ADS) and impulsivity (BIS, SST, DDT, BART) in AUD subjects. Single‐marker analysis was conducted using the R package SNPassoc.36 We set the statistical significance level at P < 0.01 with Bonferroni correction for multiple comparisons (five SNPs). For the haplotype analysis, the pairwise linkage disequilibrium (LD) pattern of the OPRM1 and OPRK1 SNPs was estimated using Haploview v4.0 (http://www.broadinstitute.org/haploview/haploview)37 and haplotype blocks were determined from the solid spine of LD. The regression analysis of the haplotypes was conducted using the “haplo.score” function of the program “haplo.stats” (http://cran.r-project.org/src/contrib/Descriptions/haplo.stats.html).38 The linear regression analysis of SNPs and haplotypes was conducted with adjustments for age, total BDI score, and total BAI score. The haplotypes with frequencies <0.5% were excluded. For haplotype analyses, permutation adjustments were performed (n = 10 000) and simulated P < 0.05 was regarded statistically significant.
3. RESULTS
3.1. Sociodemographic and clinical characteristics
The sociodemographic and clinical characteristics as well as the behavioral task results of AUD patients and controls are presented in Table 1. All subjects were Korean males. Patients with AUD were significantly younger than the controls (t = 19.5, P < 0.001); therefore, we adjusted for age in subsequent analyses. All participants including AUD patients and controls were male.
Table 1.
Sociodemographic and clinical characteristics and behavioral results of the study samples
| Variables | AUD (n = 314)a | Controls (n = 324)a | P‐value |
|---|---|---|---|
| Age, y | 48.5 ± 7.79 | 62.3 ± 10.0 | <0.001 |
| Education, y | 13.5 ± 3.96 | ||
| Age of first drinking | 19.4 ± 6.77 | ||
| Duration of AUD, y | 17.5 ± 10.4 | ||
| AUDIT | 26.7 ± 7.70 | ||
| OCDS | 19.2 ± 7.29 | ||
| ADS | 21.3 ± 10.5 | ||
| BIS | |||
| Total | 51.1 ± 11.0 | ||
| Cognitive | 14.5 ± 3.27 | ||
| Motor | 15.8 ± 4.47 | ||
| Nonplanning | 20.7 ± 4.64 | ||
| BDI | 18.9 ± 12.5 | ||
| BAI | 13.5 ± 11.3 | ||
| Behavioral task | |||
| SSRT (n = 286) | 184 ± 125 | ||
| DDT (k value) (n = 285) | −3.41 ± 3.14 | ||
| BART (n = 279) | 9.16 ± 34.4 | ||
ADS, Alcohol‐Dependent Scale; AUD, alcohol use disorder; AUDIT, Alcohol Use Disorders Identification Test; BAI, Beck Anxiety Inventory; BART, Balloon Analogue Risk Task; BDI, Beck Depression Inventory; BIS, Barratt Impulsiveness Scale; DDT, delayed discount test; OCDS, Obsessive Compulsive Drinking Scale; SSRT, stop‐signal reaction time.
Mean ± standard deviation.
3.2. Association between OPRM1/OPRK1 SNPs and AUD status
There was no significant deviation from Hardy‐Weinberg equilibrium in the SNP data for controls, and the minor allele frequencies were >0.05 for all SNPs (Table S2). Comparison of allele and genotype distributions did not reveal significant differences between AUD cases and normal controls for all SNPs (Table 2). Two haplotype blocks were each revealed for OPRM1 (rs1799971, rs495491, rs609148) and OPRK1 (rs702764, rs6473797; Figure 1). The results of haplotype analysis revealed no significant differences in haplotype frequencies between patients with AUD and controls (Table S3).
Table 2.
Distribution of allelic and genotypic frequencies of target SNPs between AUD and controls
| Gene | rs number | Allele | Genotype | ||||||
|---|---|---|---|---|---|---|---|---|---|
| D/da | AUDb | Controlb | P‐valuec | AUDd | Controld | OR add (95% CI) | P‐valuee | ||
| OPRM1 | rs1799971 | A/G | 394/230 | 396/250 | 0.499 | 125/144/43 | 127/142/54 | 0.86 (0.64‐1.14) | 0.288 |
| OPRM1 | rs495491 | A/G | 532/92 | 559/89 | 0.607 | 226/80/6 | 245/69/10 | 1.20 (0.80‐1.79) | 0.372 |
| OPRM1 | rs609148 | G/A | 582/40 | 595/49 | 0.413 | 272/38/1 | 273/49/0 | 0.89 (0.50‐1.56) | 0.675 |
| OPRK1 | rs6473797 | A/G | 385/239 | 418/226 | 0.236 | 114/157/41 | 128/162/32 | 1.05 (0.78‐1.43) | 0.746 |
| OPRK1 | rs702764 | A/G | 582/40 | 605/41 | 0.951 | 274/34/3 | 283/39/1 | 1.03 (0.59‐1.79) | 0.931 |
AUD, alcohol use disorder; Cl, confidence interval; OR, odds ratio; add, additive; SNP, single nucleotide polymorphism.
Lowercase d denotes the less frequent allele.
Number of major and minor alleles in individuals with AUD and controls.
P‐values by Pearson's chi‐square test for allelic associations.
Number of genotypes in individuals with AUD and controls. Order of genotypes: DD/Dd/dd (d is the minor allele).
P‐values by multivariate logistic regression with adjustment for age.
Figure 1.
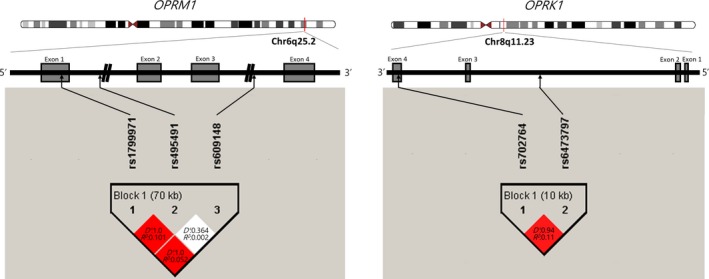
Schematic representation of the μ‐ and κ‐opioid receptor gene with the location of the SNPs analyzed in the present study, and linkage disequilibrium (LD) structure among the SNPs in healthy controls. The grey boxes indicate exons in the OPRM1 and OPRK1 genes. Haplotype blocks and LD structure were derived using Haploview version 4.2. In the haplotype blocks, pairwise D′ values and R‐squared values were presented in squares
3.3. Influences of OPRM1/OPRK1 SNPs on severity of AUD
No OPRM1 SNPs or haplotypes were related to any AUDIT, OCDS, and ADS scores. In contrast, OPRK1 SNP rs6473797 was significantly related to the severity of alcohol‐related symptoms, as measured by the AUDIT (P = 0.0086) and OCDS (P = 0.0005; Table 3). ADS was not significantly related to rs6473797 (P = 0.0189) after adjusting for multiple comparisons. A haplotype containing rs6473797 (block 1, rs6473797‐rs702764) was related to the OCDS scores. The A‐A haplotype was significantly associated with lower OCDS scores (Hap‐score = −3.45, simulated [sim] P = 5e‐4), and the G‐A haplotype was associated with higher OCDS scores (Hap‐score = 2.60, sim P = 0.0088; Table 4). However, rs702764 was not significantly related to any symptom severity scores and haplotype block 1 was not significantly related to AUDIT and ADS scores (Table S4).
Table 3.
The effects of OPRM1 SNPs and OPRK1 SNPs on severity of alcohol use disorder
| rs number | D/da | DD/Dd/ddb | AUDIT | OCDS | ADS | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DDc | Ddc | ddc | Mean difference (95% CI) | P d | DDc | Ddc | ddc | Mean difference (95% CI) | P d | DDc | Ddc | ddc | Mean difference (95% CI) | P d | |||
| rs1799971 | A/G | 125/144/43 | 26.7 ± 0.71 | 26.4 ± 0.64 | 28.0 ± 1.04 | 0.603 (−0.54, 1.75) | 0.304 | 19.2 ± 0.69 | 18.9 ± 0.59 | 20.4 ± 0.99 | 0.641 (−0.37, 1.65) | 0.215 | 21.3 ± 0.98 | 20.3 ± 0.85 | 24.1 ± 1.45 | 1.01 (−0.49, 2.51) | 0.188 |
| rs495491 | A/G | 226/80/6 | 26.7 ± 0.51 | 26.7 ± 0.90 | 27.2 ± 2.28 | −0.756 (−2.36, 0.84) | 0.355 | 19.0 ± 0.46 | 19.6 ± 0.94 | 22.2 ± 2.17 | −0.236 (−1.65, 1.18) | 0.744 | 21.4 ± 0.69 | 20.9 ± 1.21 | 22.3 ± 4.81 | −1.44 (−3.53, 0.65) | 0.177 |
| rs609148 | G/A | 272/38/1 | 26.7 ± 0.46 | 26.6 ± 1.34 | 36.0 ± 0.00 | −0.695 (−3.00, 1.61) | 0.555 | 19.2 ± 0.43 | 19.2 ± 1.37 | 31.0 ± 0.00 | −0.691 (−2.72, 1.34) | 0.505 | 21.3 ± 0.63 | 21.0 ± 1.81 | 41.0 ± 0.00 | −0.970 (−3.98, 2.04) | 0.528 |
| rs6473797 | A/G | 114/157/41 | 25.2 ± 0.70 | 27.4 ± 0.60 | 28.6 ± 1.31 | 1.58 (0.41, 2.75) | 0.0086 | 17.3 ± 0.64 | 20.1 ± 0.58 | 21.3 ± 1.18 | 1.85 (0.83, 2.87) | 0.0005 | 19.6 ± 0.94 | 21.7 ± 0.83 | 24.4 ± 1.77 | 1.85 (0.31, 3.38) | 0.019 |
| rs702764 | A/G | 274/34/3 | 26.5 ± 0.46 | 28.7 ± 1.28 | 26.3 ± 8.84 | 1.08 (−1.09, 3.24) | 0.331 | 18.9 ± 0.44 | 22.0 ± 1.23 | 20.3 ± 6.69 | 1.84 (−0.07, 3.74) | 0.06 | 21.0 ± 0.63 | 23.6 ± 1.88 | 22.7 ± 7.88 | 1.33 (−1.51, 4.18) | 0.358 |
ADS, Alcohol‐Dependent Scale; AUDIT, Alcohol Use Disorders Identification Test; CI, confidence interval; OCDS, Obsessive Compulsive Drinking Scale; SNP, single nucleotide polymorphism. Bold values indicate statistical significance at the P < 0.01 level.
Lowercase d denotes the less frequent allele.
Number of genotypes.
Mean ± standard error.
P‐values by multivariate linear regression with adjustment for age, Beck Depression Inventory score and Beck Anxiety Inventory score.
Table 4.
The effects of haplotype on OCDS score
| Block | Hap‐Freqa | Hap‐Scoreb | Crude P c | Sim P d | ||
|---|---|---|---|---|---|---|
| OPRK1 (rs6473797‐rs702764)e | ||||||
| A | A | 0.615 | −3.45 | 0.0006 | 5e‐04 | |
| G | G | 0.062 | 1.97 | 0.0493 | 0.0505 | |
| G | A | 0.321 | 2.60 | 0.0093 | 0.0088 | |
| OPRM1 (rs495491‐rs1799971‐rs609148)f | ||||||
| A | A | G | 0.44 | −0.779 | 0.436 | 0.438 |
| G | A | A | 0.0164 | −0.665 | 0.506 | 0.508 |
| A | A | A | 0.0437 | −0.507 | 0.612 | 0.612 |
| G | A | G | 0.131 | −0.137 | 0.891 | 0.890 |
| A | G | G | 0.364 | 1.26 | 0.209 | 0.21 |
OCDS, Obsessive Compulsive Drinking Scale. Bold values indicate statistical significance at the simulated P < 0.05 level.
Hap‐Freq, estimated frequency of the haplotype in the pool of all participants.
Hap‐Score, score for the haplotype.
Asymptotic chi‐square P‐value.
Simulated P‐value.
Global‐stat = 12.5, df = 3, P = 0.0057, global simulated P = 0.0055.
Global‐stat = 1.87, df = 5, P = 0.867, global simulated P = 0.851.
3.4. Association between OPRM1/OPRK1 SNPs and impulsivity in AUD patients
Only rs495491 showed significant association with the motor subfactor score of BIS (Table 5), while other SNPs were not significantly related to either total scores or subfactor scores of BIS. The results of the SNP analysis showed no significant association between OPRM1/OPRK1 SNPs and SSRT calculated from the SST and k values from the DDT and BART representing multidimensional impulsivity (Table 6). No haplotypes were related to BIS total and subfactor scores or behavioral task results (Table S5).
Table 5.
The effects of OPRM1 SNPs and OPRK1 SNPs on motor subfactor score of BIS
| rs number | D/da | DD/Dd/ddb | DDc | Ddc | ddc | Mean difference(95% CI) | P d |
|---|---|---|---|---|---|---|---|
| rs1799971 | A/G | 125/144/43 | 15.9 ± 0.37 | 15.6 ± 0.39 | 16.3 ± 0.74 | 0.17 (−0.48, 0.82) | 0.611 |
| rs495491 | A/G | 226/80/6 | 16.1 ± 0.31 | 15 ± 0.44 | 17.3 ± 1.26 | −1.20 (−2.10,−0.30) | 0.0095 |
| rs609148 | G/A | 272/38/1 | 15.8 ± 0.27 | 16.2 ± 0.82 | 19.0 ± 0.00 | −0.096 (−1.40, 1.22) | 0.886 |
| rs6473797 | A/G | 114/157/41 | 15.8 ± 0.44 | 15.7 ± 0.34 | 16.3 ± 0.70 | −0.055 (−0.73, 0.62) | 0.873 |
| rs702764 | A/G | 274/34/3 | 15.7 ± 0.27 | 16.5 ± 0.83 | 17.7 ± 3.53 | 0.485 (−0.75, 1.72) | 0.44 |
BIS, Barratt Impulsiveness Scale; CI, confidence interval; SNP, single nucleotide polymorphism. The bold value indicates statistical significance at the P < 0.01 level.
Lowercase d denotes the less frequent allele.
Number of genotypes.
mean ± standard error.
P‐values by multivariate linear regression with adjustment for age, Beck Depression Inventory score and Beck Anxiety Inventory score.
Table 6.
The effects of OPRM1 SNPs and OPRK1 SNPs on impulsivity behavioral task results
| rs number | D/da | SSRT | DDT (k value) | BART | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DD/Dd/ddb | DDc | Ddc | ddc | Mean difference (95% CI) | P d | DD/Dd/ddb | DDc | Ddc | ddc | Mean difference (95% CI) | P d | DD/Dd/ddb | DDc | Ddc | ddc | Mean difference (95% CI) | P d | ||
| rs1799971 | A/G | 111/127/43 | 168 ± 14.2 | 196 ± 6.87 | 178 ± 20.9 | 11.8 (−7.95, 31.6) | 0.242 | 112/128/43 | −3.33 ± 0.32 | −3.45 ± 0.29 | −3.56 ± 0.31 | −0.08 (−0.60, 0.44) | 0.761 | 112/125/40 | 30.2 ± 1.62 | 27.3 ± 1.45 | 33.2 ± 3.04 | 0.351 (−2.57, 3.27) | 0.814 |
| rs495491 | A/G | 204/73/4 | 182 ± 7.19 | 185 ± 18.9 | 153 ± 27.5 | −4.78 (−33.7, 24.1) | 0.746 | 206/73/4 | −3.51 ± 0.21 | −3.24 ± 0.42 | −1.88 ± 0.93 | 0.247 (−0.51, 1.00) | 0.52 | 202/71/4 | 29.9 ± 1.20 | 28.0 ± 2.08 | 21.2 ± 6.24 | −2.32 (−6.56, 1.91) | 0.283 |
| rs609148 | G/A | 245/34/1 | 180 ± 7.87 | 192 ± 17.1 | 213 ± 0.00 | 7.09 (−33.5, 47.7) | 0.732 | 248/33/1 | −3.53 ± 0.19 | −2.50 ± 0.69 | −4.05 ± 0.00 | 0.669 (−0.40, 1.74) | 0.220 | 244/31/1 | 29.1 ± 1.10 | 30.8 ± 3.09 | 21.9 ± 0.00 | 1.44 (−4.65, 7.52) | 0.644 |
| rs6473797 | A/G | 104/141/36 | 195 ± 11.5 | 163 ± 9.95 | 218 ± 20.9 | −2.00 (−23.0, 19.0) | 0.852 | 106/142/35 | −3.39 ± 0.33 | −3.38 ± 0.25 | −3.66 ± 0.50 | −0.16 (−0.71, 0.39) | 0.567 | 105/138/34 | 27.6 ± 1.60 | 30.7 ± 1.53 | 28.7 ± 2.78 | 1.37 (−1.71, 4.44) | 0.384 |
| rs702764 | A/G | 248/30/2 | 182 ± 8.04 | 189 ± 9.25 | 165 ± 1.67 | 0.99 (−39.0, 41.0) | 0.961 | 251/30/1 | −3.50 ± 0.20 | −2.81 ± 0.56 | −2.57 ± 0.00 | 0.509 (−0.59, 1.61) | 0.367 | 245/29/2 | 29.7 ± 1.10 | 26.3 ± 3.14 | 23.7 ± 6.21 | −3.13 (−8.98, 2.73) | 0.296 |
BART, Balloon Analogue Risk Task; CI, confidence interval; DDT, delayed discount test; SNP, single nucleotide polymorphism; SSRT, stop‐signal reaction time.
Lowercase d denotes the less frequent allele.
Number of genotypes.
mean ± standard error.
P‐values by multivariate linear regression with adjustment for age, Beck Depression Inventory score and Beck Anxiety Inventory score.
4. DISCUSSION
This study investigated the associations between five SNPs of the opioid receptor genes OPRM1 and OPRK1 and the affected status of several aspects of alcohol symptom severity and impulsivity related to AUD in Korean male subjects.
There was no significant difference between single‐marker or haplotype distributions of OPRM1 or OPRK1 and AUD status. The rs1799971 variant of OPRM1 has been the most frequently studied SNP for its association with alcohol dependence, though results have been inconsistent. Certain studies reported a significant association between rs1799971 and alcohol dependence,4, 6, 33 while others reported no genetic effect for this SNP.5, 7, 39 The most recent meta‐analysis reviewing 17 case‐control studies including nine Caucasian studies (8026 patients in total) and eight Asian studies (1587 patients in total) concluded that rs1799971 exhibited no association with alcohol dependence in either ethnicity.40 For the other OPRM1 SNPs we investigated, rs495491 and rs609148, a study among 382 European American patients with alcohol dependency showed the genetic risk effect of the rs495491‐C minor allele and the protective effect of the rs609148‐T minor allele toward alcohol dependence.35 However, a subsequent family‐based study with 1923 European American samples did not find any association with OPRM1 SNPs including rs495491 and rs609148.41 The results of our study demonstrating a lack of association correspond with these previous studies. Although few studies exist on the association between OPRK1 and AUD, those that have been conducted report inconsistent findings. A multicenter study with a family‐based design suggested a significant association of OPRK1 SNP rs6473797 with alcohol dependence.11 However, other studies showed negative results for the genetic effect of OPRK1 in alcoholism, contrary to ours.10, 34, 39
Although we were unable to find any association between OPRM1 or OPRK1 and the development of AUD, there were several significant associations of OPRK1 genetic variants with AUDIT and OCDS scores. The minor G allele of rs6473797 was associated with higher AUDIT scores, signifying that the AUD patients with the G allele showed higher hazardous drinking tendencies. In addition, the G allele of rs6473797 was also related to higher OCDS scores, suggesting more severe craving symptoms. Furthermore, in haplotype block 1 of the OPRK1 gene, the G‐A haplotype of rs6473797‐rs702764 showed higher OCDS scores, while the A‐A haplotype presented significantly lower scores. This result suggests that the rs6473797 SNP and haplotype rs6473797‐rs702764 of OPRK1 play a role in regulating the severity of craving related to obsessive‐compulsive drinking in AUD. Although it did not reach statistical significance after correction for multiple comparisons, rs6473797 showed nominally significant association (P = 0.0189) with ADS. The G allele was linked to increasing ADS scores, indicating heavier drinking and severe withdrawal symptoms.42 This tendency for the G allele of rs6473797 to be associated with higher symptom severity in ADS is consistent with the results of the AUDIT and OCDS. In summary, our results suggest that the AUD patients carrying the G allele of OPRK1 SNP rs6473797 show more harmful drinking behavior, cravings, and withdrawal symptoms, corresponding to the experience of more painful symptoms in AUD patients. To date, there have been few studies on the OPRK1 gene and alcohol severity. However, several animal and human studies indirectly suggest the association between the OPRK1 gene and alcohol severity. In animal studies, OPRK1 ‐knockout mice showed decreased oral alcohol consumption.43 In an alcohol‐dependent rat model, increased κ‐opioid receptor signaling caused excessive alcohol consumption during withdrawal.44 In humans, the T‐T‐C‐T haplotype (rs7832417‐rs16918853‐rs702764‐rs7817710) of the OPRK1 gene showed a relationship with elevated levels of alcohol use in heroin‐dependent patients.15 Regarding OPRM1, there were no associations between any of the SNPs or haplotypes and AUDIT, OCDS, and ADS scores in our study. Previous studies on the influences of OPRM1 SNPs on AUDIT or ADS also reported no associations.13, 45 Considering these results in conjunction with ours, it seems that OPRM1 is not related to the severity in drinking levels or frequency that lead to harmful consequences in AUD.
With respect to impulsivity, we initially hypothesized that μ‐ and κ‐opioid receptor genes influence multidimensional impulsivity as measured by SST, DDT, BART, and self‐report questionnaire BIS‐11 in AUD patients. In the μ‐opioid receptor gene, we found that the G allele of rs495491 in the OPRM1 gene was significantly associated with higher motor impulsivity subfactor scores of BIS‐11. A previous animal study suggested that OPRM1 ‐knockout mice showed significantly decreased motor impulsivity as measured by premature responses on the signaled nose poke task.27 In a study involving PET imaging of μ‐opioid receptors, impulsivity as measured by the NEO PI‐R (NEO Personality Inventory, Revised) showed a significant positive relationship with μ‐receptor binding potential in the right anterior cingulate and adjacent medial frontal cortex, right ventral basal ganglia, and basolateral area of the right amygdala, all of which are areas involved in the pathophysiology of substance abuse.46 However, the instrumental measures of impulsivity such as SST, DDT, and BART were not influenced by OPRM1 or OPRK1 variants in this study.46 Courtney et al conducted a study utilizing functional magnetic resonance imaging while performing SST and explored the functional polymorphism of OPRM1 SNP rs1799971 in alcohol dependence.47 The author demonstrated the difference in functional connectivity within fronto‐striatal networks according to the genotype of OPRM1 SNP rs1799971; nonetheless, there was no significant genotypic effect on behavioral performance results for the SST, as indicated by our results. Regarding DDT and BART, most genetic studies focused on dopamine genes48, 49; there have been no reports on the relationship between OPRM1 or OPRK1 and DDT or BART. A study of the association between the DRD2 Taql A polymorphism and BIS in alcohol‐dependent patients showed a significant dopamine gene effect on impulsiveness; another study showed that DRD2 Taql A and DRD4 VNTR polymorphisms were linked to DDT results.48 Despite the fact that we were unable to determine the association between opioid receptor gene variants and SST, DDT, and BART, the dopamine and opioid systems interact with the reward system and impulsivity in the brain 50; therefore, it is likely that the opioid‐related genes OPRM1 or OPRK1 are involved in impulsivity. Further studies are necessary to determine their influence on various measures of impulsivity.
There are several limitations in the present study. First, the sample size was relatively small, potentially obscuring significant genetic associations. Second, we were unable to select all tagging SNPs covering the entire OPRM1 and OPRK1 genes. Studies with larger sample sizes and greater numbers of SNPs will be needed to clearly confirm the function and impact of opioid receptor genes in AUD. Third, all participants in the present study were exclusively male. To understand the effects of genetic differences in alcohol use and impulsivity comprehensively, it would be necessary to study both male and female subjects. Lastly, impulsivity using the self‐rating scale, BIS‐11, and behavior tasks involving SST, DDT, and BART was assessed only in AUD patients, not in healthy controls. We were therefore unable to determine the regression model containing the control group to define the genetic influence of OPRM1/OPRK1 on impulsivity.
In conclusion, we found no associations between selected SNPs or haplotypes of OPRM1 or OPRK1 genes and the development of AUD in Korean males. However, certain SNPs and haplotypes of OPRK1 were associated with hazardous drinking tendency (AUDIT scores) and craving symptoms (OCDS scores). The OPRM1 SNP rs495491 was related to motor impulsivity as measured by the BIS‐11 in patients with AUD. These findings suggest that the opioid receptor genes may have a modulating influence on several aspects of symptom severity related to AUD and impulsivity in Korean males with AUD.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF‐2018R1A2B2007714). The funding source did not give any influences on the study design, data collection, analysis and interpretation of data, the writing of the report, and the decision to submit the article for publication.
Park CI, Hwang SS, Kim HW, Kang JI, Lee SH, Kim SJ. Association of opioid receptor gene polymorphisms with drinking severity and impulsivity related to alcohol use disorder in a Korean population. CNS Neurosci Ther. 2020;26:30–38. 10.1111/cns.13138
REFERENCES
- 1. Heath AC, Bucholz KK, Madden P, et al. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: consistency of findings in women and men. Psychol Med. 1997;27(6):1381‐1396. [DOI] [PubMed] [Google Scholar]
- 2. Di Chiara G, Acquas E, Tanda G. Ethanol as a neurochemical surrogate of conventional reinforcers: the dopamine‐opioid link. Alcohol. 1996;13(1):13‐17. [DOI] [PubMed] [Google Scholar]
- 3. Heilig M, Goldman D, Berrettini W, O'Brien CP. Pharmacogenetic approaches to the treatment of alcohol addiction. Nat Rev Neurosci. 2011;12(11):670‐684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Deb I, Chakraborty J, Gangopadhyay PK, Choudhury SR, Das S. Single‐nucleotide polymorphism (A118G) in exon 1 of OPRM1 gene causes alteration in downstream signaling by mu‐opioid receptor and may contribute to the genetic risk for addiction. J Neurochem. 2010;112(2):486‐496. [DOI] [PubMed] [Google Scholar]
- 5. Kim S‐G, Kim C‐M, Kang D‐H, et al. Association of functional opioid receptor genotypes with alcohol dependence in Koreans. Alcohol Clin Exp Res. 2004;28(7):986‐990. [DOI] [PubMed] [Google Scholar]
- 6. Koller G, Zill P, Rujescu D, et al. Possible association between OPRM1 genetic variance at the 118 locus and alcohol dependence in a large treatment sample: relationship to alcohol dependence symptoms. Alcohol Clin Exp Res. 2012;36(7):1230‐1236. [DOI] [PubMed] [Google Scholar]
- 7. Rouvinen‐Lagerstrom N, Lahti J, Alho H, et al. mu‐Opioid receptor gene (OPRM1) polymorphism A118G: lack of association in Finnish populations with alcohol dependence or alcohol consumption. Alcohol Alcohol. 2013;48(5):519‐525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kreek MJ, LaForge KS, Butelman E. Pharmacotherapy of addictions. Nat Rev Drug Discov. 2002;1(9):710‐726. [DOI] [PubMed] [Google Scholar]
- 9. Levran O, Yuferov V, Kreek MJ. The genetics of the opioid system and specific drug addictions. Hum Genet. 2012;131(6):823‐842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. el Loh W, Fann CS, Chang YT, Chang CJ, Cheng AT. Endogenous opioid receptor genes and alcohol dependence among Taiwanese Han. Alcohol Clin Exp Res. 2004;28(1):15‐19. [DOI] [PubMed] [Google Scholar]
- 11. Xuei X, Dick D, Flury‐Wetherill L, et al. Association of the kappa‐opioid system with alcohol dependence. Mol Psychiatry. 2006;11(11):1016‐1024. [DOI] [PubMed] [Google Scholar]
- 12. Jung MH, Park BL, Lee B‐C, et al. Association of CHRM2 polymorphisms with severity of alcohol dependence. Genes Brain Behav. 2011;10(2):253‐256. [DOI] [PubMed] [Google Scholar]
- 13. Ray LA, Bujarski S, MacKillop J, Courtney KE, Monti PM, Miotto K. Subjective response to alcohol among alcohol‐dependent individuals: effects of the mu‐opioid receptor (OPRM1) gene and alcoholism severity. Alcohol Clin Exp Res. 2013;37(suppl 1):E116‐E124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Contet C, Kieffer BL, Befort K. Mu opioid receptor: a gateway to drug addiction. Curr Opin Neurobiol. 2004;14(3):370‐378. [DOI] [PubMed] [Google Scholar]
- 15. Wang SC, Tsou HH, Chung RH, et al. The association of genetic polymorphisms in the kappa‐opioid receptor 1 gene with body weight, alcohol use, and withdrawal symptoms in patients with methadone maintenance. J Clin Psychopharmacol. 2014;34(2):205‐211. [DOI] [PubMed] [Google Scholar]
- 16. Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on early detection of persons with harmful alcohol consumption–II. Addiction. 1993;88(6):791‐804. [DOI] [PubMed] [Google Scholar]
- 17. Skinner HA, Allen BA. Alcohol dependence syndrome: measurement and validation. J Abnorm Psychol. 1982;91(3):199. [DOI] [PubMed] [Google Scholar]
- 18. Anton RF, Moak DH, Latham P. The Obsessive Compulsive Drinking Scale: a self‐rated instrument for the quantification of thoughts about alcohol and drinking behavior. Alcohol Clin Exp Res. 1995;19(1):92‐99. [DOI] [PubMed] [Google Scholar]
- 19. Verdejo‐Garcia A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance‐use disorders: review of findings from high‐risk research, problem gamblers and genetic association studies. Neurosci Biobehav Rev. 2008;32(4):777‐810. [DOI] [PubMed] [Google Scholar]
- 20. Dawes MA, Tarter RE, Kirisci L. Behavioral self‐regulation: correlates and 2 year follow‐ups for boys at risk for substance abuse. Drug Alcohol Depend. 1997;45(3):165‐176. [DOI] [PubMed] [Google Scholar]
- 21. Tarter RE, Kirisci L, Reynolds M, Mezzich A. Neurobehavior disinhibition in childhood predicts suicide potential and substance use disorder by young adulthood. Drug Alcohol Depend. 2004;76(Suppl):S45‐52. [DOI] [PubMed] [Google Scholar]
- 22. Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51(6):768‐774. [DOI] [PubMed] [Google Scholar]
- 23. Lawrence AJ, Luty J, Bogdan NA, Sahakian BJ, Clark L. Impulsivity and response inhibition in alcohol dependence and problem gambling. Psychopharmacology. 2009;207(1):163‐172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kirby KN, Maraković NN. Modeling myopic decisions: Evidence for hyperbolic delay‐discounting within subjects and amounts. Organ Behav Hum Decis Process. 1995;64(1):22‐30. [Google Scholar]
- 25. Fernie G, Cole JC, Goudie AJ, Field M. Risk‐taking but not response inhibition or delay discounting predict alcohol consumption in social drinkers. Drug Alcohol Depend. 2010;112(1–2):54‐61. [DOI] [PubMed] [Google Scholar]
- 26. Pfeifer P, Sariyar M, Eggermann T, et al. Alcohol consumption in healthy OPRM1 G allele carriers and its association with impulsive behavior. Alcohol Alcohol. 2015;50(4):379‐384. [DOI] [PubMed] [Google Scholar]
- 27. Olmstead MC, Ouagazzal AM, Kieffer BL. Mu and delta opioid receptors oppositely regulate motor impulsivity in the signaled nose poke task. PLoS ONE. 2009;4(2):e4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kraemmer J, Smith K, Weintraub D, et al. Clinical‐genetic model predicts incident impulse control disorders in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2016;87(10):1106‐1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Babor TF, de la Fuente JR, Saunders J, Grant M. The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Health Care. Geneva, Switzerland: World Health Organization; 2011. WHO Publication No. 92.4. [Google Scholar]
- 30. Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: twenty‐five years of evaluation. Clin Psychol Rev. 1988;8(1):77‐100. [Google Scholar]
- 31. Beck AT, Steer RA. Manual for the Beck Anxiety Inventory. San Antonio, TX: Psychological Corporation; 1993. [Google Scholar]
- 32. Chung YO, Lee CW. A study of factor structures of the Barratt Impulsiveness Scale in Korean university students. Korean J Clin Psychol. 1997;16(1):117–129. [Google Scholar]
- 33. Miranda R, Ray L, Justus A, et al. Initial evidence of an association between OPRM1 and adolescent alcohol misuse. Alcohol Clin Exp Res. 2010;34(1):112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang H, Kranzler HR, Yang BZ, Luo X, Gelernter J. The OPRD1 and OPRK1 loci in alcohol or drug dependence: OPRD1 variation modulates substance dependence risk. Mol Psychiatry. 2008;13(5):531–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang H, Luo X, Kranzler HR, et al. Association between two mu‐opioid receptor gene (OPRM1) haplotype blocks and drug or alcohol dependence. Hum Mol Genet. 2006;15(6):807–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gonzalez JR, Armengol L, Sole X, et al. SNPassoc: an R package to perform whole genome association studies. Bioinformatics. 2007;23(5):654–655. [DOI] [PubMed] [Google Scholar]
- 37. Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2004;21(2):263–265. [DOI] [PubMed] [Google Scholar]
- 38. Schaid DJ, Rowland CM, Tines DE, Jacobson RM, Poland GA. Score tests for association between traits and haplotypes when linkage phase is ambiguous. Am J Hum Genet. 2002;70(2):425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cupic B, Stefulj J, Zapletal E, et al. Opioid system genes in alcoholism: a case‐control study in Croatian population. Neuropeptides. 2013;47(5):315–319. [DOI] [PubMed] [Google Scholar]
- 40. Kong X, Deng H, Gong S, Alston T, Kong Y, Wang J. Lack of associations of the opioid receptor mu 1 (OPRM1) A118G polymorphism (rs1799971) with alcohol dependence: review and meta‐analysis of retrospective controlled studies. BMC Med Genet. 2017;18(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xuei X, Flury‐Wetherill L, Bierut L, et al. The opioid system in alcohol and drug dependence: family‐based association study. Am J Med Genet B Neuropsychiatr Genet. 2007;144b(7):877–884. [DOI] [PubMed] [Google Scholar]
- 42. Kahler CW, Strong DR, Stuart GL, Moore TM, Ramsey SE. Item functioning of the alcohol dependence scale in a high‐risk sample. Drug Alcohol Depend. 2003;72(2):183–192. [DOI] [PubMed] [Google Scholar]
- 43. Kovacs KM, Szakall I, O'Brien D, et al. Decreased oral self‐administration of alcohol in kappa‐opioid receptor knock‐out mice. Alcohol Clin Exp Res. 2005;29(5):730–738. [DOI] [PubMed] [Google Scholar]
- 44. Kissler JL, Sirohi S, Reis DJ, et al. The one‐two punch of alcoholism: role of central amygdala dynorphins/kappa‐opioid receptors. Biol Psychiatry. 2014;75(10):774–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hendershot CS, Wardell JD, McPhee MD, Ramchandani VA. A prospective study of genetic factors, human laboratory phenotypes, and heavy drinking in late adolescence. Addict Biol. 2017;22(5):1343–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Love TM, Stohler CS, Zubieta JK. Positron emission tomography measures of endogenous opioid neurotransmission and impulsiveness traits in humans. Arch Gen Psychiatry. 2009;66(10):1124–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Courtney KE, Ghahremani DG, Ray LA. Fronto‐striatal functional connectivity during response inhibition in alcohol dependence. Addict Biol. 2013;18(3):593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Eisenberg DT, Mackillop J, Modi M, et al. Examining impulsivity as an endophenotype using a behavioral approach: a DRD2 TaqI A and DRD4 48‐bp VNTR association study. Behav Brain Funct. 2007;3:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mata R, Hau R, Papassotiropoulos A, Hertwig R. DAT1 polymorphism is associated with risk taking in the Balloon Analogue Risk Task (BART). PLoS ONE. 2012;7(6):e39135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Comings DE, Blum K. Reward deficiency syndrome: genetic aspects of behavioral disorders. Prog Brain Res. 2000;126:325–341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


