Abstract
Background
The traditional anti-inflammation disease-modifying anti-rheumatic drugs (DMARDs) have limited therapeutic effects in rheumatoid arthritis (RA) patients. We previously reported the safety and efficacy of umbilical cord mesenchymal stem cell (UC-MSC) treatment in RA patients that were observed for up to 8 months after UC-MSC infusion. The aim of this study is to assess the long-term efficacy and safety of UC-MSC along with DMARDs for the treatment of RA.
Methods
64 RA patients aged 18–64 years were recruited in the study. During the treatment, patients were treated with 40 mL UC-MSC suspension product (2 × 107 cells/20 mL) via intravenous injection immediately after the infusion of 100 mL saline. The serological markers tests were used to assess safety and the 28-joint disease activity score (DAS28) and the Health Assessment Questionnaire (HAQ) to assess efficacy.
Results
1 year and 3 years after UC-MSC cells treatment, the blood routine, liver and kidney function and immunoglobulin examination showed no abnormalities, which were all in the normal range. The ESR, CRP, RF of 1 year and 3 years after treatment and anti-CCP of 3 years after treatment were detected to be lower than that of pretreatment, which showed significant change (P < 0.05). Health index (HAQ) and joint function index (DAS28) decreased 1 year and 3 years after treatment than before treatment (P < 0.05).
Conclusion
UC-MSC cells plus DMARDs therapy can be a safe, effective and feasible therapeutic option for RA patients.
Keywords: rheumatoid arthritis, umbilical cord mesenchymal stem cell, cell therapy
Introduction
Rheumatoid arthritis (RA) is a chronic, autoimmune disease affecting multiple joints symmetrically.1 The main symptoms at the early stage of the disease are joint pain and swelling; at late stage, arthritis leads to joint stiffness, malformation, loss of function, and even disability.2 Currently, available medication for RA treatment has limited efficacy, including non-steroidal anti-inflammatory drugs (NSAIDs), disease-modifying anti-rheumatic drugs (DMARDs) and immunosuppressants.3 The target cytokine therapy has some therapeutic effects but without significant repair of injured joints, which restricts its widespread clinical use.
Mesenchymal stem cells (MSCs) are characterized by their regenerative property to repair parenchymal tissue and organs through differentiating into lineages of mesenchymal tissues, as well as immunomodulation.4 Injected MSCs can migrate to injured tissues and facilitate the recovery of damaged cells.5 Clinical studies have demonstrated the clinical benefits of MSCs in RA therapy.6 However, the therapeutic benefits of umbilical cord tissue-derived MSCs (UC-MSCs) for the treatment of RA have not been well studied clinically. As is known that the key factor in the pathogenesis of RA is the elevated cytokines arising from numerous synovial cells.7 MSCs can express various receptors for pro-inflammatory cytokines8 to reduce inflammation in RA patients. We have previously reported the safety and efficacy of UC-MSC for the treatment of RA patients for up to 8 months after UC-MSC infusion.9 The aim of this 3-year cohort study is to assess the long-term efficacy and safety of UC-MSC plus DMARDs therapy for RA. The data demonstrated that UC-MSC treatment was safe and able to benefit RA patients in long term when combined with DMARDs. Continuous UC-MSC treatment can resume patients’ physical activity function to achieve excellent clinical benefits.
Materials and Methods
Patients
According to the diagnostic criteria of RA from American Rheumatism Association,10 64 RA patients from 986 hospital of People’s Liberation Army Air Force were recruited in this study between January 2000 and January 2017. The inclusion criteria: swelling is present in at least one joint, and three or more joint swelling; morning stiffness for 3 hrs on average which did not have another cause; results from at least one blood test indicate the presence of RA; symptoms have been present for at least 6 weeks. Exclusion criteria: Patients were excluded from the study if they are; pregnant, aged under 12 or over 70 years, had received any RA treatment before. The study was registered in ClinicalTrials.gov (NCT01547091) and approved by the ethics committee of 986 hospital of People’s Liberation Army Air Force. The authors intend to share all individual deidentified participant data which will be accessible on ClinicalTrials.gov (NCT01547091) for 1 year.
The disease activity for all the patients could not be well controlled by the previous DMARDs or NSAIDs. Data regarding the clinical characteristic were collected and retrospectively analyzed (Table 1). All patients had morning stiffness for 3 h on average and three or more joint swelling. There were 8 males and 56 females, aged 16–64 years, with an average age of 42 years. The course of the disease was from 6 months to 35 years. In addition, 3 Juvenile Rheumatoid Arthritis (JRA) patients and 4 ankylosing spondylitis (AS) patients were included in this study. All patients’ written informed consent was obtained in accordance with the Declaration of Helsinki and the ethics committee of 986 hospital of People’s Liberation Army Air Force, and the written informed consent for any patient under the age of 18 years was provided by a parent or legal guardian.
Table 1.
Patients Characteristics and Demographics
| Characteristics | N (%) | |
|---|---|---|
| Patients | 64 | |
| Gender | ||
| Male | 8(12.5%) | |
| Female | 56(87.5%) | |
| Age(years) | Median | 48 |
| Mean (Range) | 42(16–64) | |
| Duration of the disease (years) | Median | 15 |
| Mean (Range) | 10(0.5–35) | |
| Joints pain number | 4 (3–10) | |
| Morning stiffness (hours) | 3.4 (2–5) | |
| Disease status | Mean (Range) | |
| HAQ | 5.20(2–12) | |
| DAS 28 | 4.25(1.63–7.21) | |
| Along with the symptoms(n) | ||
| JRA | 3(4.7%) | |
| AS | 4(6.25%) |
Abbreviations: HAQ, the Health Assessment Questionnaire; DAS 28, the 28-joint disease activity score; AS, ankylosing spondylitis; JRA, juvenile rheumatoid arthritis.
Treatment Process
The study design is illustrated in Figure 1. To prevent allergy during and after UC-MSC treatment, 2–5 mg of dexamethasone diluted in 100 mL of saline was given to patients intravenously right before the infusion of UC-MSC. For patients with hyperglycemia (Fasting glucose >7.0 mmol/L) and hypertension, 12.5–25 mg of promethazine (Phenergan) was given intramuscularly instead of dexamethasone. UC-MSCs were obtained from Stem Cell Biology and Regenerative Medicine Institution, Yi-Chuang Institute of Bio-Industry Cells met the eligible criteria for clinical use.11 During treatment, each patient received 40mL of UC-MSC product (2 × 107 cells/20 mL) intravenously immediately after 100mL normal saline infusion, at the infusion rate of 1 mL/minute.
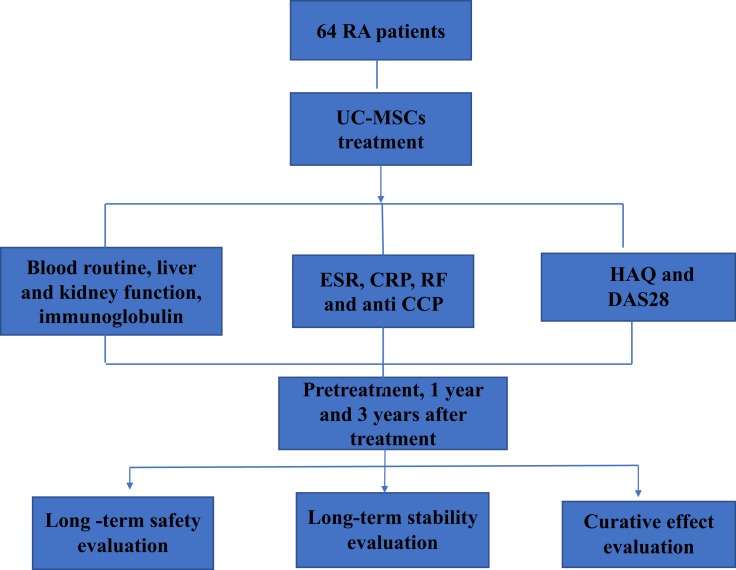
Figure 1.
Schematic of clinical evaluation for enrolled RA patients treatment by UC-MSC cells.
All patients maintained individualized long-term application of small dose DMARDs: leflunomide 10 mg/day, hydroxychloroquine sulfate 0.2 g/day or methotrexate 7.5 mg/week. ECG monitoring was performed in the whole process. Adverse events and the clinical information were recorded. Tests for serological markers to assess safety and the 28-joint disease activity score, and the Health Assessment Questionnaire to assess efficacy.
Clinical Evaluation
All patients’ blood samples were collected before treatment, 1 year, and 3 years after UC-MSC treatment. Safety assessment was performed by testing blood routine, liver and kidney function and immunoglobulin, stability assessment by HAQ and DAS28 and serological markers included ESR, CRP, RF, and anti-CCP antibody.
According to the clinical relief of American Rheumatism Association’s standard clinical relief of RA10 should meet at least 4 of the 5 following criteria: morning stiffness time is less than 15 mins; no fatigue sensation; no joint pain and tenderness; no joint swelling; ESR was less than 30 mm/h in women and 20 mm/h in men. Obvious relief means that in addition to meet 4 of the above 5 criteria, ESR and CRP each improvement of 50%, and RF decreased by more than 30%. Effective criteria are to meet half of the above obvious effect criteria. Invalid effect criteria means that patients who fail to reach 3 of the 6 criteria for obvious effect or RA condition worsens. The DAS28 was used to assess the disease status, with examining the number of tenderness (T28) and swelling joints (SW28). The pain score (0–10) was used to assess the patients' pain status during the follow-up. Before treatment,1 patient had no pain (score 0), 16 patients had minor pain (score 1–3), 28 patients had moderate pain (score 4–6), 19 patients had severe pain (score 7–10). After treatment, 13 patients had no pain (score 0), 16 patients had minor pain (score 1–3),23 patients had moderate pain (score 4–6), 12 patients had severe pain (score 7–10).
Statistical Analysis
All continuous variables were showed as descriptive statistics, which were recorded as a mean ± standard error. The change between baseline and end point was compared by the one-way ANOVA on Graph Pad Prism 7.0. Data for evaluation before and after the treatment were compared by Tukey’s test and Bonferroni multiple comparison correction tests. P<0.05 was considered as statistical significance.
Results
Long-Term Safety
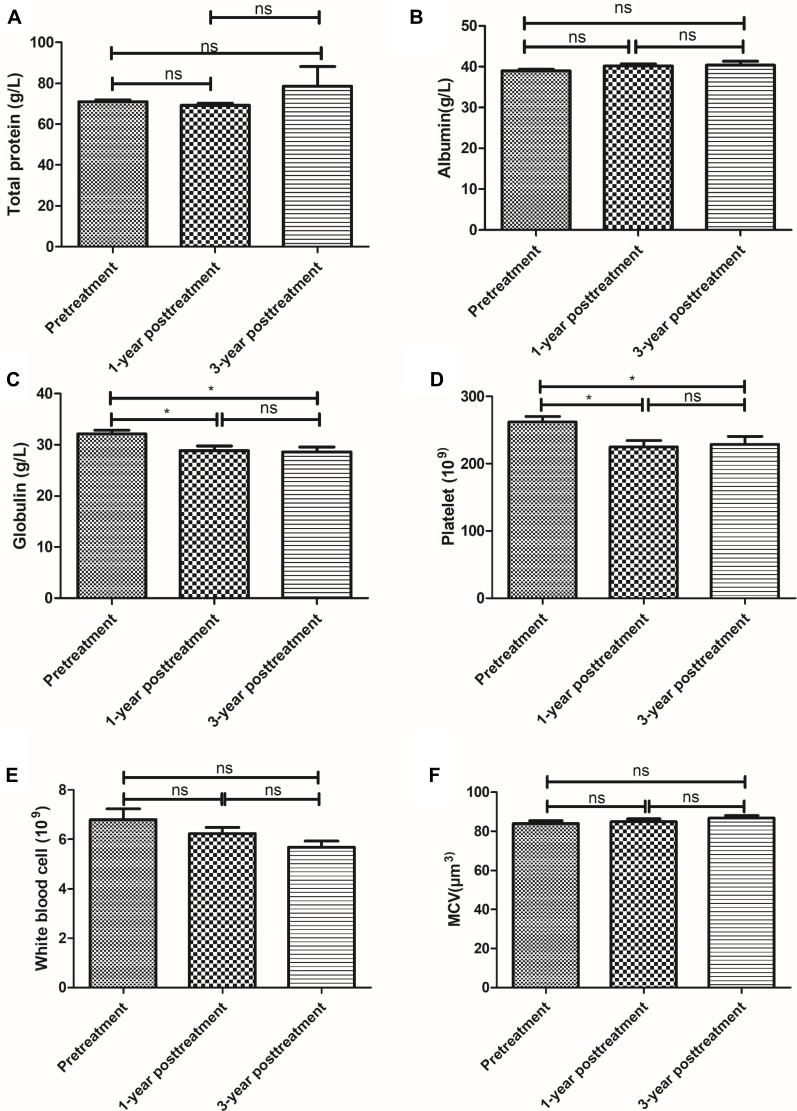
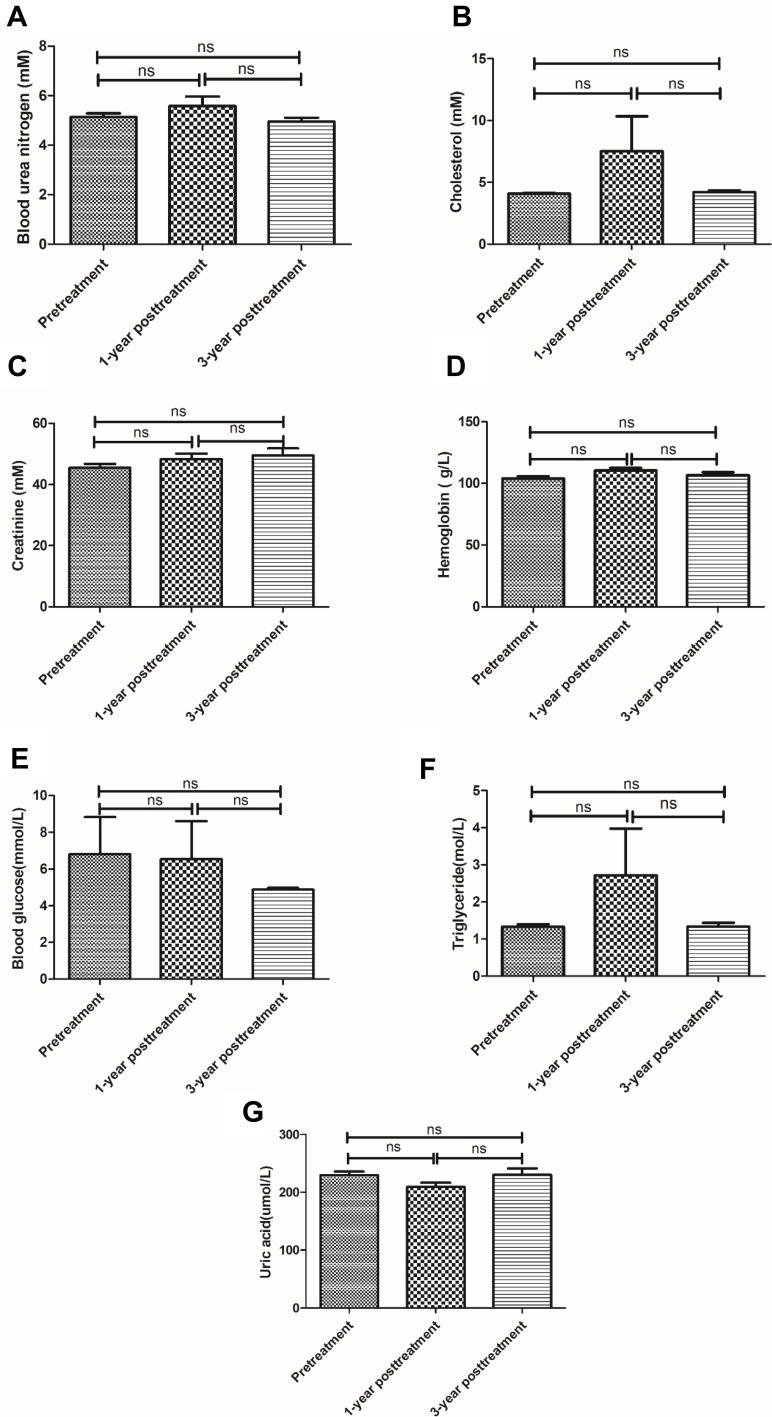
All patients showed no abnormality in the blood routine examination. Routine blood marker, TP (Figure 2A), ALB (Figure 2B), WB (Figure 2E) and MCV (Figure 2F) had no significant change at 1-year or 3-year posttreatment with UC-MSC plus DMARDs as compared to pretreatment (P>0.05). Globulin (Figure 2C) and Platelet (Figure 2D) had significant difference between pretreatment and 1 year or 3 years after treatment (P<0.05). Liver, kidney function and immunoglobulins levels from each patient were all within normal range before, 1 year and 3 years after UC-MSC treatment (Figure 3).
Figure 2.
Routine blood marker changes during different times. TP (A), ALB (B), globulin (C), platelet (D), WB (E) and MCV (F) before treatment and 1-year posttreatment and 3-year posttreatment with umbilical cord mesenchymal stem cells (UC-MSCs) plus disease-modifying anti-rheumatic drugs (DMARDs). Pre-treatment versus after the first or second treatment; * represents P < 0.05, ns represents no significance difference (n = 64).
Figure 3.
Live, kidney function and immunoglobulin character of test. BUN (A), cholesterol (B), creatinine (C), hemoglobin (D), blood glucose (E), triglyceride (F) and uric acid (G) before treatment and 1-year posttreatment and 3-year posttreatment with umbilical cord mesenchymal stem cells (UC-MSCs) plus disease-modifying anti-rheumatic drugs (DMARDs). Pre-treatment versus after the first or second treatment, ns represents no significance difference (n = 64).
Long-Term Changes of Inflammatory and Serological Markers
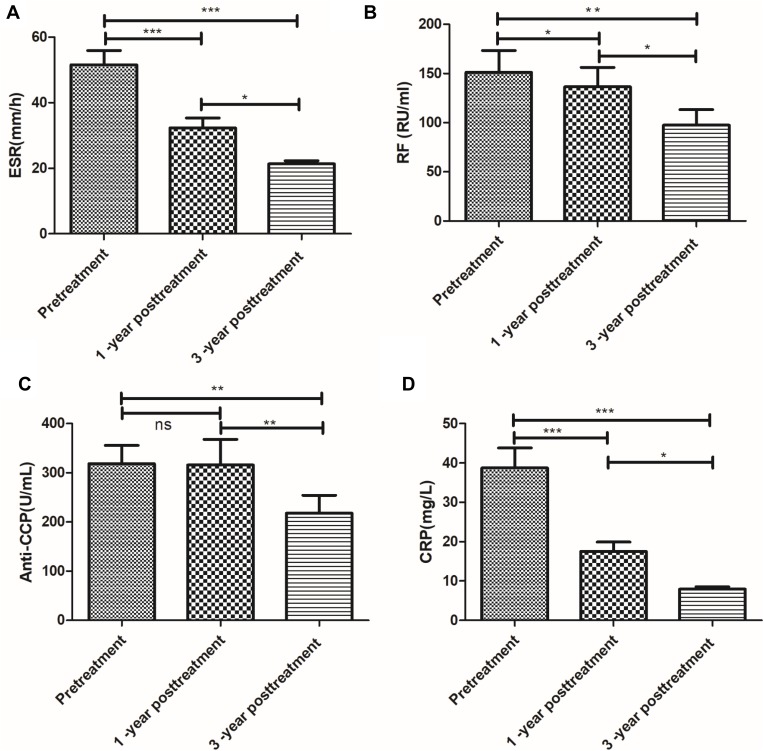
There are significant decreases for the Inflammatory and/or RA Serological Makers including ESR, CRP, RF and anti-CCP at 1 year and 3 years after treatment. Compared with the pretreatment level from patients with RA, they showed decreased levels of ESR, CRP, RF, and anti-CCP after 1 year and 3-year posttreatment. The ESR, CRP decreased significant difference (P<0.01) (Figure 4A and D). Another marker, RF, also showed a decreased trend, particularly at 3 years after the treatment (P<0.05) (Figure 4B). The anti-CCP levels between pretreatment and 1-year posttreatment show no significant difference (Figure 4C). The value of ESR, CRP, RF, and anti-CCP remained stable between 1-year posttreatment and 3 years posttreatment, which showed no significance (P>0.05) (Figure 4).
Figure 4.
Long-term stability evaluation of RA patients. ESR (A), RF (B), Anti-CCP (C) and CRP (D), before treatment and 1-year posttreatment and 3-year posttreatment with disease-modifying anti-rheumatic drugs (DMARDs) plus umbilical cord mesenchymal stem cells (UC-MSCs). Pre-treatment versus after the first or second treatment, *** represents P < 0.001, ** represents P < 0.01;1-year posttreatment versus 3-year posttreatment, * represents P < 0.05; ns represents no significance (n = 64).
Long-Term Efficacy
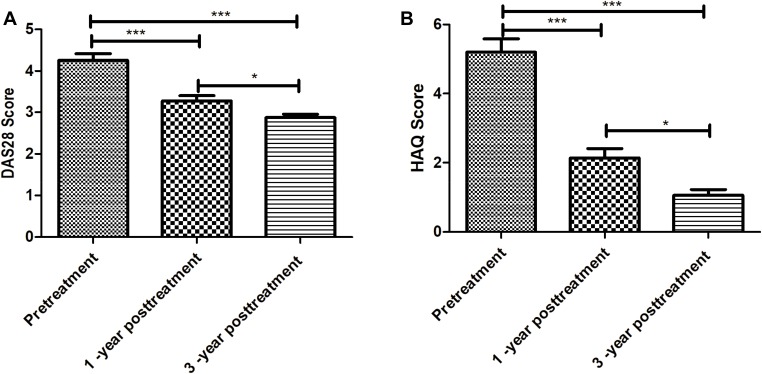
The scores DAS28 decreased significantly at 1 year after treatment compared with pretreatment (P<0.05), and it continued to decrease at 3 years after treatment (Figure 5A). Consistently, the health assessment questionnaire (HAQ) scores also decreased significantly at 1 year and 3 years after treatment compared with pretreatment (P<0.05), and there is a significant difference of HAQ score between 1-year and 3-year posttreatment (P<0.05) (Figure 5B). These data suggest the long-term efficacy of UC-MSC treatment.
Figure 5.
Scores of DAS28 and HAQ were evaluated after twice of UC-MSCs treatment. (A) DAS28 score was evaluated; (B) HAQ score was evaluated. Pre-treatment versus after the first or second treatment; *** represents P < 0.001, 1-year posttreatment versus 3-year posttreatment; * represents P < 0.05 (n = 64).
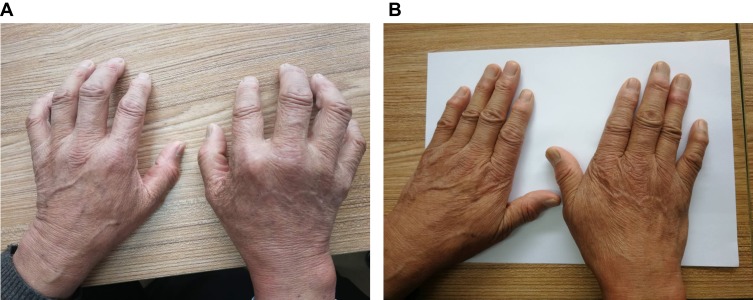
The pictures of two representative patients that received UC-MSC are provided in Figures 6 and 7. The first patient is a 68-year-old man who had serious joint pain, swelling and deformity before treatment had achieved remission since 6 months after UC-MSC treatment and maintained till 3 years after treatment (Figure 6A). Three-year after UC-MSCs treatment, the joint deformity recovered (Figure 6B) and the pain disappeared. What is more, he can move freely and do some physical exercise.
Figure 6.
A 68 year-male was diagnosed with RA in 1998. In 2010, he was admitted to our hospital for the first time. (A) Shows that his hands could not be kept straight. (B) After 3 years posttreatment, he has stopped using anti-rheumatism medicine for 5 years, and his hands stretch freely and the rheumatic nodules around the joints gradually become soft and fade.
Figure 7.
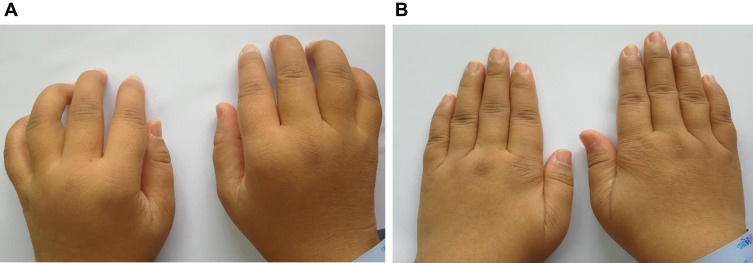
A 33-year female, with 4 years of illness, (A) difficulty in clenching, swelling and pain, morning stiffness, (B) After UC-MSC cells treatment 1 week, the symptoms improved significantly.
Another RA patient, who was a 33-year-old woman with serious joint swelling and deformity before treatment (Figure 7A), also achieved remission. Three-year after UC-MSC treatment, the joint deformity was recovered (Figure 7B) and swelling was well-healed. In addition, she can run sometimes freely and do some physical exercise.
Discussion
In our previous report,9,12 we have shown the UC-MSC treatment was safe and effective in clinical treatment. We observed the patients up to 8 months after UC-MSC treatment in RA patients, which no acute serious side-effects occurred either during or after UC-MSCs infusion, and only few patients (4%) showed mild adverse effects such as flu-like symptoms during the infusion, which disappeared within hours without any treatment. All patients have shown improvements in the diet, sleep, and physical strength after the cell therapy based on patients’ reports. In comparison, there was no such improvement in the control group. In addition, the clinical response to UC-MSCs treatment was rapid (as early as 12 h posttreatment) with the physical evidence after the administration of UC-MSCs. In the placebo group, no significant changes in symptoms were detected throughout the study.
This 3-year cohort study of UC-MSC therapy for RA was aimed to provide further data on the long-term safety and efficacy of UC-MSC treatment. The results demonstrated that DMARD drug combined with UC-MSCs therapy alleviated RA symptoms, reduced HAQ and DAS28 scores, as well as reduced levels of RF, CRP, ESR, and anti-CCP and the effects maintained for 3 years after UC-MSC treatment.
Traditional therapy for RA includes DMARDs, non-steroidal anti-inflammatory drugs (NSAIDs), slow-acting anti-rheumatic drugs (SAARDs) and hormone drugs,13 with high disease recurrence rate and side effects after long-term use.14 Treatments with hormone for a long time can result in reduced immunity and osteoporosis with sodium and water retention. Thus, such treatments have poor patient compliance. Most importantly, they cannot change the progress of the disease. Besides joint injury, RA also affects multiple tissues and organs. Traditional drug therapy could not perform the regeneration and repair of damaged tissues.
In recent years, cytokine targeted therapies have been developed, such as TNF-competitive inhibitor Etanercept and TNF-monoclonal antibody Infliximab,15 which have been widely used to inhibit the proliferation of synovial cells, reduce the release of inflammatory factors and achieve the effect of anti-inflammatory and improvement of RA symptoms.16 However, Bongartz et al17 found that the use of TNF-inhibitors is likely to increase the occurrence of various post-operative infections, and has no obvious ability to repair damaged joints, thus limiting its clinical use. Currently, refractory RA cannot be well controlled by the available clinical treatments. Some patients are intolerant to certain immunosuppressive agents, which limits the scope of drug use. Therefore, new treatments need to be explored.
With the development of bioengineering and cell biology, the research on stem cells continues to spread, and the application of stem cell therapy for RA will become popular. Mesenchymal stem cells have biological characteristics of regenerative repair of parenchymal tissues and organs and immune regulation, providing a new prospect for the treatment of RA.18,19 In our study, the levels of rheumatoid factors and anti-CCP antibodies showed a slow decline, and some of them were even on the high side. The data even reached >500, because the immunomodulation process of different patients has an individual difference and it is a tardiness process, so the rate of decline varies from person to person.
Based on the extensive and in-depth research on UC-MSC, UC-MSC should function in different aspects in the treatment of RA. Firstly, basic studies have confirmed that MSCs can differentiate into osteoblasts, cartilage, fat, tendon, muscle and other cells in vitro and in vivo,20 which may participate in the regeneration and repair of damaged tissues. Secondly, joint lesions can promote the aggregation of MSCs to the lesion synovium to repair the damaged joint tissue.21 Chen et al22 reported the feasibility of using MSCs to construct bone and cartilage combined grafts, and confirmed that adding MSCs into solid supports can promote the repair of cartilage defects. In a mice model of arthritis, single intraperitoneal injection of primary mouse MSCs prevented severe damage to the affected bone and cartilage.23 Thirdly, MSCs secrete a variety of cytokines with nutritional effects and can promote the proliferation and differentiation of stem cells.24 Fourthly, MSCs have unique immunomodulatory effects. When added into the mitogen-stimulated peripheral blood lymphocyte culture system, both autogenous and allogeneic MSCs can significantly inhibit the proliferation and activation of T lymphocytes, which plays an important role in the regulation of the immune network and is expected to fundamentally adjust the pathological immune system of patients.25 In addition, when MSCs differentiate into other types of cells, it still retains its immunomodulatory effect;26 Fifthly, MSCs have a chemotaxis effect, which can prevent the release of inflammatory mediators, reduce the inflammatory reaction and tissue damage, and disease progression.27,28
In this study, after the treatment of UC-MSC for RA patients, personalized low dose of DMARDs therapy was maintained for a long time. The serological indexes of the patients were stable for a long time, reaching the low activity level of RA, which was consistent with the improved clinical symptoms. Therefore, UC-MSC therapy is safe for RA treatment. UC-MSC cell combined with DMARDs treatment can maintain low disease activity and improve the quality of life for RA patients for a long time. The underlying mechanism may be related to UC-MSC’s ability to regulate patients’ autoimmune tolerance and improve the tolerance of anti-rheumatism drugs for RA patients.29–31
This study supplements the data of our previous studies and provides the supportive evidence of long-term safety and efficiency in the use of UC-MSC therapy for RA. The limitation of the current study is that all patients were enrolled and treated from a single center, and there is no placebo control for the long-term observation. Therefore, a larger multiple-center, controlled study needs to be confirmed to confirm the current findings. However, the result of this study supported the use of UC-MSC infusion for RA treatment, as all patients failed traditional drug treatment and obtained symptom improvements and evaluation index decrease after UC-MSC treatment.
Overall, our study demonstrated the long-term safety and efficacy of UC-MSC therapy in RA patients. The therapeutic effects of UC-MSC can be maintained for 3 years, with stable clinical outcomes, which significantly improved RA patients’ quality of life.
Acknowledgments
This study was supported by the Shaanxi Province Social Development Public Relations Project (2012K13-02-35), and the Military Medicine and Public Health Plan (CLZ120GA23).
Disclosure
The authors have declared no competing interest.
References
- 1.Hoogduijn MJ. Are mesenchymal stromal cells immune cells? Arthritis Res Ther. 2015;17(1):88. doi: 10.1186/s13075-015-0596-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun Y, Deng W, Geng L, et al. Mesenchymal stem cells from patients with rheumatoid arthritis display impaired function in inhibiting Th17 cells. J Immunol Res. 2015;2015:284215. doi: 10.1155/2015/284215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rasch EK, Hirsch R, Paulose-Ram R, Hochberg MC. Prevalence of rheumatoid arthritis in persons 60 years of age and older in the United States: effect of different methods of case classification. Arthritis Rheum. 2003;48(4):917–926. doi: 10.1002/art.10897 [DOI] [PubMed] [Google Scholar]
- 4.Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98(8):2396–2402. doi: 10.1182/blood.V98.8.2396 [DOI] [PubMed] [Google Scholar]
- 5.Alvaro-Gracia JM, Jover JA, Garcia-Vicuna R, et al. Intravenous administration of expanded allogeneic adipose-derived mesenchymal stem cells in refractory rheumatoid arthritis (Cx611): results of a multicentre, dose escalation, randomised, single-blind, placebo-controlled phase Ib/IIa clinical trial. Ann Rheum Dis. 2017;76(1):196–202. doi: 10.1136/annrheumdis-2015-208918 [DOI] [PubMed] [Google Scholar]
- 6.Munir H, McGettrick HM. Mesenchymal stem cell therapy for autoimmune disease: risks and rewards. Stem Cells Dev. 2015;24(18):2091–2100. doi: 10.1089/scd.2015.0008 [DOI] [PubMed] [Google Scholar]
- 7.Li Z, Jiang CM, An S, et al. Immunomodulatory properties of dental tissue-derived mesenchymal stem cells. Oral Dis. 2014;20(1):25–34. doi: 10.1111/odi.12086 [DOI] [PubMed] [Google Scholar]
- 8.Mattar P, Bieback K. Comparing the immunomodulatory properties of bone marrow, adipose tissue, and birth-associated tissue mesenchymal stromal cells. Front Immunol. 2015;6:560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L, Wang L, Cong X, et al. Human umbilical cord mesenchymal stem cell therapy for patients with active rheumatoid arthritis: safety and efficacy. Stem Cells Dev. 2013;22(24):3192–3202. doi: 10.1089/scd.2013.0023 [DOI] [PubMed] [Google Scholar]
- 10.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–324. doi: 10.1002/(ISSN)1529-0131 [DOI] [PubMed] [Google Scholar]
- 11.Horwitz EM, Le Blanc K, Dominici M, et al. Clarification of the nomenclature for MSC: the International Society for Cellular Therapy position statement. Cytotherapy. 2005;7(5):393–395. doi: 10.1080/14653240500319234 [DOI] [PubMed] [Google Scholar]
- 12.Wang L, Huang S, Dang Y, et al. Cord blood-derived cytokine-induced killer cellular therapy plus radiation therapy for esophageal cancer: a case report. Medicine (Baltimore). 2014;93(28):e340–e340. doi: 10.1097/MD.0000000000000340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smolen JS, Aletaha D, Koeller M, Weisman MH, Emery P. New therapies for treatment of rheumatoid arthritis. Lancet (London, England). 2007;370(9602):1861–1874. doi: 10.1016/S0140-6736(07)60784-3 [DOI] [PubMed] [Google Scholar]
- 14.Singh JA, Saag KG, Bridges SL Jr, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheum. 2016;68(1):1–26. [DOI] [PubMed] [Google Scholar]
- 15.Anderson PJ. Tumor necrosis factor inhibitors: clinical implications of their different immunogenicity profiles. In: Seminars in arthritis and rheumatism; Elsevier; 2005:19–22. [DOI] [PubMed] [Google Scholar]
- 16.McCain M, Quinet R, Davis WJ. Etanercept and infliximab associated with cutaneous vasculitis. Rheumatology (Oxford). 2002;41(1):116–117. doi: 10.1093/rheumatology/41.1.116 [DOI] [PubMed] [Google Scholar]
- 17.Bongartz T, AJ S, MJ S, Buchan I, EL M, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295(19):2275–2285. doi: 10.1001/jama.295.19.2275 [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez-Rey E, Gonzalez MA, Varela N, et al. Human adipose-derived mesenchymal stem cells reduce inflammatory and T cell responses and induce regulatory T cells in vitro in rheumatoid arthritis. Ann Rheum Dis. 2010;69(01):241–248. [DOI] [PubMed] [Google Scholar]
- 19.Chen FH, RS T. Mesenchymal stem cells in arthritic diseases. JAr Ther. 2008;10(5):223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singer NG, Caplan AI. Mesenchymal stem cells: mechanisms of inflammation. Annu Rev Pathol Mech Dis. 2011;6:457–478. doi: 10.1146/annurev-pathol-011110-130230 [DOI] [PubMed] [Google Scholar]
- 21.Rosset P, Deschaseaux F, Layrolle P. Cell therapy for bone repair. Orthop Traumatol Surg Res. 2014;100(1 Suppl):S107–S112. doi: 10.1016/j.otsr.2013.11.010 [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Chen H, Li P, et al. Simultaneous regeneration of articular cartilage and subchondral bone in vivo using MSCs induced by a spatially controlled gene delivery system in bilayered integrated scaffolds. Biomaterials. 2011;32(21):4793–4805. doi: 10.1016/j.biomaterials.2011.03.041 [DOI] [PubMed] [Google Scholar]
- 23.Augello A, Tasso R, Negrini SM, Cancedda R, Pennesi G. Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum. 2007;56(4):1175–1186. doi: 10.1002/(ISSN)1529-0131 [DOI] [PubMed] [Google Scholar]
- 24.Bartholomew A, Sturgeon C, Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30(1):42–48. doi: 10.1016/S0301-472X(01)00769-X [DOI] [PubMed] [Google Scholar]
- 25.Le Blanc K. Immunomodulatory effects of fetal and adult mesenchymal stem cells. Cytotherapy. 2003;5(6):485–489. doi: 10.1080/14653240310003611 [DOI] [PubMed] [Google Scholar]
- 26.Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringden O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31(10):890–896. doi: 10.1016/S0301-472X(03)00110-3 [DOI] [PubMed] [Google Scholar]
- 27.Gao F, Chiu SM, Motan DA, et al. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis. 2016;7:e2062. doi: 10.1038/cddis.2015.327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakuma R, Takahashi A, Nakano-Doi A, et al. Comparative characterization of ischemia-induced brain multipotent stem cells with mesenchymal stem cells: similarities and differences. Stem Cells Dev. 2018;27(19):1322–1338. doi: 10.1089/scd.2018.0075 [DOI] [PubMed] [Google Scholar]
- 29.Guan LX, Guan H, Li HB, et al. Therapeutic efficacy of umbilical cord-derived mesenchymal stem cells in patients with type 2 diabetes. Exp Ther Med. 2015;9(5):1623–1630. doi: 10.3892/etm.2015.2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bunpetch V, Wu H, Zhang S, Ouyang H. From “bench to bedside”: current advancement on large-scale production of mesenchymal stem cells. Stem Cells Dev. 2017;26(22):1662–1673. doi: 10.1089/scd.2017.0104 [DOI] [PubMed] [Google Scholar]
- 31.Hamidian Jahromi S, Estrada C, Li Y, Cheng E, Davies JE. Human umbilical cord perivascular cells and human bone marrow mesenchymal stromal cells transplanted intramuscularly respond to a distant source of inflammation. Stem Cells Dev. 2018;27(6):415–429. doi: 10.1089/scd.2017.0248 [DOI] [PubMed] [Google Scholar]