Abstract
Background.
Inappropriate sinus tachycardia (IST) is a rare clinical disorder characterized by an elevated resting heart rate and an exaggerated rate response to exercise or autonomic stress. Pharmacologic therapy and catheter ablation are considered first-line treatments for IST but can yield suboptimal relief of symptoms. The results of surgical ablation at our center were reviewed for patients with refractory IST.
Methods.
Between 1987 and 2018, 18 patients underwent surgical sinoatrial (SA) node isolation for treatment-refractory IST. All 18 patients had previously failed pharmacologic therapy, and 15 patients had failed catheter ablation of the SA node.
Results.
Ten patients underwent a median sternotomy, and 8 patients underwent a minimally invasive right thoracotomy. The SA node was isolated with the use of surgical incisions, cryoablation, or bipolar radiofrequency ablations. Sinus tachycardia was eliminated in 100% of patients in the immediate postoperative period. Long-term follow-up data were available for 17 patients, with a mean follow-up of 11.4 ± 7.9 years. At last follow-up, 100% of patients were free from recurrent symptomatic IST. More than 80% of patients were completely asymptomatic, whereas 3 patients reported occasional palpitations. Four patients were on b-blockers, and 5 patients required subsequent pacemaker implantation. All 8 patients who underwent minimally invasive isolation were in normal sinus rhythm at last follow-up, and only 1 patient complained of palpitations.
Conclusions.
Surgical isolation of the SA node is a feasible treatment for IST refractory to pharmacologic therapy and catheter ablation. A minimally invasive surgical approach offers a less morbid alternative to traditional median sternotomy.
Inappropriate sinus tachycardia (IST) is a rare clinical condition characterized by a mean 24-hour heart rate more than 90 beats/min, a resting heart rate more than 100 beats/min, and an exaggerated rate response to exercise or autonomic stress.1–4 No set criteria exist for diagnosing IST, and it is, therefore, a diagnosis of exclusion, once other possible systemic causes of tachycardia have been ruled out, including hyperthyroidism, pheochromocytoma, and physical deconditioning.3–6 Before consideration for any interventional procedure, all patients should be carefully evaluated to rule out any medical or psychiatric cause of their tachycardia. Although the precise pathophysiology of IST remains unknown, proposed mechanisms include sympathetic receptor hypersensitivity, blunted parasympathetic tone, or enhanced automaticity of the sinoatrial (SA) node.2–4,7 Although IST was previously considered a disorder limited to women between the ages of 15 and 50 years, recent studies have shown that IST also affects middle-aged men and that its incidence has been underestimated.3,8
The first-line treatment for IST is pharmacologic therapy with b-blockers for heart rate control and the addition of antiarrhythmic drugs, calcium channel blockers, or both as required.3,4,6 More recently, the sinus node If channel blocker, ivabradine, has been reported in a randomized clinical trial to safely and effectively control IST-related symptoms and to lower patient heart rate at rest, while standing, and during exertion.9,10 However, the treatment of IST with ivabradine is currently an off-label therapy and not all patients respond similarly. Patients for whom medical therapy does not provide relief often undergo catheter ablation of the SA node. However, the difficulty of creating transmural lesions that fully isolate the SA node from the atrium with catheter ablation is well known.11,12 Symptomatic control and long-term outcomes after catheter ablation have been variable; a 2015 report found that long-term freedom from IST at 6 months ranged from 23% to 85% between different studies.13–15
Surgical ablation treatment for IST was first used in the 1980s for patients refractory to pharmacologic treatment and catheter ablation, but current clinical guidelines list IST surgical ablation as a class III recommendation.3,4,6 The purpose of this study was to examine our operative results and the late outcomes of surgical treatment for IST. With greater awareness and recognition of the role of surgery in selected patients, we would hope to increase referrals and provide a more robust evidence base to allow for the future addition in the guideline recommendations for surgical ablation.
Patients and Methods
Eighteen consecutive patients undergoing surgical SA node isolation for IST at our center between 1987 and 2018 were included for analysis. Rate and rhythm follow-up data were concurrently collected in our institutional arrhythmia outcomes database that has dated back to 1987. Our institutional Society of Thoracic Surgeons (STS) database was used to obtain preoperative patient demographic characteristics, previous treatments for IST, duration of symptoms, operative approach and concomitant procedures, and perioperative results. Missing data were ascertained through chart review and contact with patients and referring physicians when needed. In our series of 18 patients, only 1 patient (patient 2) was lost to follow-up, and the medical records could not be found.
The primary end points were postoperative arrhythmia recurrence as well as heart rhythm and symptoms at the last available follow-up. Postoperative and follow-up heart rhythm was obtained by 12-lead electrocardiogram or prolonged monitoring by either a 24-hour or longer Holter monitor or pacemaker interrogation.
This study was approved by the Washington University School of Medicine Institutional Review Board. Informed consent and permission for release of information were obtained from all patients.
Results
Preoperative Patient Characteristics
Preoperative patient characteristics are shown in Table 1. The mean age was 34 ± 11 years. Of the 18 patients, 16 patients were women. All patients had symptomatic IST. The mean duration of symptoms before surgery was 5.3 ± 4.7 years. Before surgical intervention, all patients had failed medical therapy, including b-blockers, antiarrhythmic drugs, calcium channel blockers, ivabradine, or a combination. Fifteen patients underwent at least one failed catheter ablation procedure for sinus node modification. The mean number of catheter ablations before surgery was 2 ± 1.5 (median 1.5, interquartile range: 1 to 3.25). Before surgical intervention, additional catheter-based electrophysiological procedures were performed before surgery in 7 patients, including atrioventricular node ablation (n = 3) or ablation for AV nodal reentrant tachycardia (n = 4). Four patients had pacemaker implantations before surgery.
Table 1.
Preoperative Patient Characteristics
| Patient | Age y | Sex | Duration, y | Medical Treatment | Catheter Ablations, n | Additional Prior EP Procedures |
|---|---|---|---|---|---|---|
| 1 | 36 | F | 3 | BB + AA | 4 | None |
| 2 | 33 | F | 7 | BB | 1 | None |
| 3 | 29 | F | 12 | BB | 2 | AVN ablation/pacemaker |
| 4 | 42 | F | 10 | BB | 2 | None |
| 5 | 29 | F | 6 | BB + AA | 2 | AVN ablation/pacemaker |
| 6 | 25 | F | 1 | BB | 4 | AVNRT ablation |
| 7 | 42 | F | 16 | BB | 3 | AVNRT ablation/pacemaker |
| 8 | 25 | F | 1 | BB | 1 | None |
| 9 | 34 | F | 6 | BB | 1 | AVNRT ablation |
| 10 | 52 | M | 2 | BB + AA | 2 | None |
| 11 | 42 | F | 2 | BB + AA | 0 | None |
| 12 | 26 | M | 6 | BB + AA | 4 | AVNRT ablation |
| 13 | 21 | F | 2 | AA | 5 | ablation/pacemaker |
| 14 | 27 | F | 15 | BB + Iv | 0 | None |
| 15 | 18 | F | 2 | BB | 0 | None |
| 16 | 22 | F | 2 | BB + CCB + Iv | 1 | None |
| 17 | 62 | F | 2 | BB + CCB + Iv | 1 | None |
| 18 | 48 | F | 1 | BB + CCB | 1 | None |
| Mean ± SD | 34 ± 11 | … | 5.3 ± 4.7 | … | 2 ± 1.5 | … |
AA, antiarrhythmic medication; AVN, atrioventricular node; AVNRT, atrioventricular nodal reentrant tachycardia; BB, b-blocker medication; CCB, calcium channel blocker; EP, electrophysiological; F, female; Iv, ivabradine; M, male.
Surgical Details
The details of each procedure are shown in Table 2, with patients listed in chronologic order. Surgical isolation of the anatomic SA node was performed in all patients. Ten patients underwent a median sternotomy, with 8 patients requiring cardiopulmonary bypass. The remaining 8 patients underwent a minimally invasive operation by right mini-thoracotomy as previously described at our institution16 with only 1 patient (patient 12) requiring cardiopulmonary bypass due to a need for concomitant right atrial Cox-Maze procedure. We have previously described the right atrial portion of the Cox-Maze lesion set.17
Table 2.
Operative Details and Postoperative Results
| Patient | Surgical Procedure | Long-term Results | Follow-up, y |
|---|---|---|---|
| 1 | SAN isolation | Asymptomatic; PM | 30.3 |
| 2 | SAN isolation | … | … |
| 3 | SAN isolation | Asymptomatic | 22.9 |
| 4 | SAN isolation (Cryo) | Asymptomatic; BB; PM | 22.6 |
| 5 | SAN isolation | Occasional palpitations | 21.6 |
| 6 | SAN isolation (Cryo) | Occasional palpitations; PM; BB | 5.0 |
| 7 | SAN isolation (Cryo) | Asymptomatic | 15.2 |
| 8 | SAN isolation (BRF)a | Asymptomatic; PM | 14.0 |
| 9 | SAN isolation (BRF)a | Occasional palpitations | 13.9 |
| 10 | SAN isolation (BRF) | Asymptomatic; PM | 11.5 |
| 11 | SAN isolation (BRF)a | Asymptomatic | 9.5 |
| 12 | SAN isolation (BRF + Cryo)a right atrial maze | Asymptomatic; BB | 8.1 |
| 13 | SAN isolation (BRF + Cryo) | Asymptomatic | 7.7 |
| 14 | SAN isolation (BRF) SAN denervation (Cryo) | Asymptomatic | 2.0 |
| 15 | SAN isolation (BRF)a SAN denervation (Cryo) | Asymptomatic | 2.0 |
| 16 | SAN isolation (BRF)a SAN denervation (Cryo) | Asymptomatic | 0.4 |
| 17 | SAN isolation (BRF)a SAN denervation (Cryo) | Asymptomatic; BB | 0.1 |
| 18 | SAN isolation (BRF)a SAN denervation (Cryo) | Asymptomatic; BB | 0.1 |
| Mean ± SD | … | … | 11.4 ± 7.9 |
Mini-thoracotomy approach was used.
SAN ablation was performed using surgical lesions unless noted otherwise.
BB, postoperative b-blocker usage; BRF, bipolar radiofrequency ablation; Cryo, cryoablation; PM, postoperative pacemaker placement; SAN, sinoatrial node.
In the first 5 patients, an intraoperative epicardial mapping system was used to identify the area of early activation in the right atrium. A circumferential incision was performed to isolate this area and the anatomic SA node from the lower atrium. With the advent of technology, bipolar radiofrequency (RF) clamps with or without the use of cryoablation were used to replace surgical incisions. In 9 patients, cryoablation was used in addition to or in place of the circumferential incision. In patient 4, the region of the SA node was directly cryoablated before the circumferential incision was performed. In patient 6, the site of previous catheter ablation was suspected as the origin of the tachycardia and was ablated using cryoablation without an isolating incision. Cryoablation was used in patients 7 and 12 to connect the atrial incision line and to make a complete circumferential lesion around the right atrium. In addition, over the past several years, as our understanding of SA node physiology has improved, we have also added SA node denervation and another lesion isolating a cuff of atrial tissue around the inferior vena cava (IVC).
Intraoperative Management
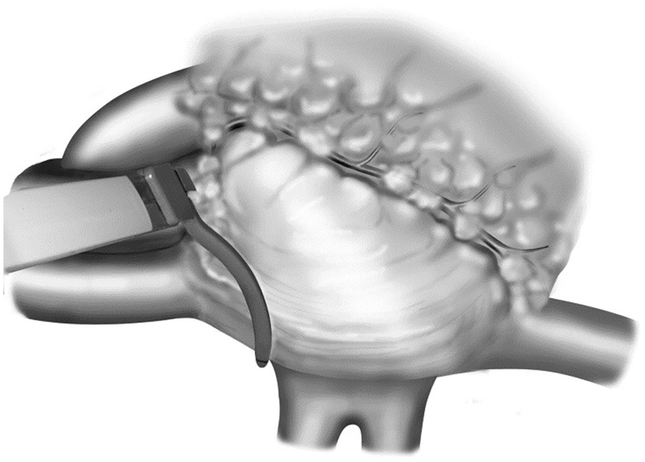
Intraoperative administration of isoproterenol (up to 1 mg/kg) was used to induce sinus tachycardia with rates of 120 to 180 beats/min. In our recent minimally invasive patients, a bipolar RF clamp was used to isolate the SA node and as large a cuff of surrounding right atrium as possible (Figure 1). After successful isolation, isoproterenol was again infused. If there was no blunting of the tachycardia response, further ablations were performed. Circumferential ablations of the surrounding right atrium were performed until both an inversion of the P-wave morphologic structure was observed in the inferior leads of the electrocardiogram and a blunting of the response to isoproterenol was achieved (Figures 2A, 2B). Epicardial cryoablation of the fat pads overlying the right pulmonary veins and surrounding the superior vena cava–right atrial junction was performed to modify the neural input to the SA node region. A 15-mm bell-shaped cryoprobe was used, and ablation was performed for 3 minutes at −60°C (Video). In all patients, bipolar pacing was then used to confirm electrical isolation of the SA node and the surrounding cuff of atrium from the remainder of the right atrium.
Figure 1.
Position of the bipolar radiofrequency clamp used to isolate the sinoatrial node and surrounding right atrial tissue. Reproduced from Kreisel and colleagues16 with permission from The Journal of Thoracic and Cardiovascular Surgery, Elsevier. See the Video for more details.
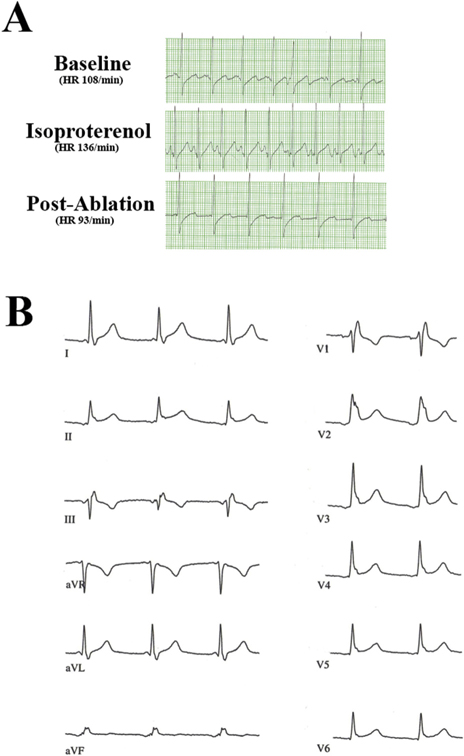
Figure 2.
(A) Inferior lead (II) of intraoperative electrocardiogram in patient 16 undergoing minimally invasive surgical ablation. A baseline electrocardiogram demonstrated an upright P-wave with a change in heart rate (HR) from 108 beats/min to 136 beats/min during the administration of isoproterenol. There was no reaction to isoproterenol after ablation, which led to an atrial rhythm with negative P-wave morphologic characteristics. (B) Twelve-lead electrocardiogram showing stable postoperative atrial escape rhythm. Note negative P-wave morphologic characteristics in inferior leads II, III, and aVF.
Postoperative Outcomes
Sinus tachycardia was eliminated in all patients in the immediate postoperative period. Symptomatic postoperative bradycardia occurred in 5 patients during the follow-up period, all of whom required subsequent pacemaker implantation.
Long-term follow-up data were available for 17 patients. The mean follow-up time was 11.4 ± 7.9 years (Table 2). At last follow-up, no patient had recurrent symptomatic IST. More than 80% of patients (14 of 17) were completely asymptomatic, whereas 3 patients still complained of palpitations. In these patients, there was no evidence of symptomatic tachycardia on pacemaker interrogations or event monitor recordings. Five patients reported b-blocker usage at last follow-up. Postoperative pacemaker implantation was required in 2 patients before discharge, and an additional 3 patients had pacemakers by their last follow-up. Postoperatively, only 2 patients required further catheter ablation procedures: patient 1 underwent ablation for atrial flutter, and patient 3 underwent ablation for ectopic atrial tachycardia arising from the coronary sinus, both of which were confirmed by endocardial mapping. Except for these 2 patients, no other patient experienced a recurrence of symptomatic tachycardia.
Notably, the 8 patients who underwent minimally invasive surgery reported no palpitations after surgery. At last follow-up, 7 of these 8 patients (88%) were asymptomatic, and no patient had recurrent IST.
Comment
IST is a rare clinical condition in which patients experience an elevated resting heart rate more than 100 beats/min and an exaggerated response to exercise or autonomic stress despite having no identifiable structural abnormalities of the heart.3–6 Studies have shown that the incidence of IST may actually be underestimated and a more diligent work-up is required to identify these patients correctly.3,8 Despite it being a rare clinical condition, we strongly believe that both electrophysiologists and cardiac surgeons performing ablation should be familiar with this disease and its management. This can be a debilitating condition, and medical management is often unsuccessful. This includes b-blockers as the first line, antiarrhythmic drugs, calcium channel blockers, and ivabradine.
Catheter ablation of the SA node is often used when medical management fails.13–15 Numerous studies have examined the effectiveness of catheter ablation for IST but have reported widely variable success rates. A 2015 review of nine studies found that short-term success rates after catheter ablation ranged from 76% to 100%, whereas long-term success rates at 6 months after ablation were between 23% and 85%. The definition of short-term success varied among studies and included a greater than 10% increase in resting sinus cycle length, reduction of resting sinus rate less than 90 beats/min, reduction in maximum sinus rate during isoproterenol infusion, and a caudal shift in sinus node activation.15
The variability in outcomes after catheter ablation may arise because of the difficulty of consistently producing transmural lesions with transvenous catheters in the right atrium to isolate or modify the SA node complex.11 This is likely because of the numerous trabeculae and the widely variable anatomy and wall thickness in this area.18 In particular, the crista terminalis extending from the superior vena cava to the IVC is particularly thick, making transmural ablation difficult and inconsistent. In addition, several studies have found that the anatomic SA node does not necessarily correlate with the functional SA node. An optical mapping study in canines noted the existence of discrete exit pathways of SA node activation that varied by up to 17 mm from the anatomic SA node within the atrial myocardium.12 In addition, high-density mapping studies of human hearts have found the existence of multiple activation pathways within the atrium that vary from beat to beat and may arise from a significantly larger area than the anatomically defined SA node.19,20 Because catheter ablation procedures often target the anatomic SA node and the area of earliest atrial activation, these lesion sets may fail to ablate or isolate all the pathways that comprise the functional SA node.
The surgical treatment of IST has the advantage of isolating the SA node with a wide cuff of surrounding right atrium completely from the right atrium. In 1984, Yee and colleagues21 first described a technique in which a circumferential incision was used to divide the atrium into superior and inferior segments in a patient with disabling paroxysmal sinus tachycardia. This lesion resolved the patient’s tachycardia and replaced it with a stable junctional escape rhythm.21 Similar techniques used to completely isolate the SA node were reported in case reports22,23 and a case series by Hendry and colleagues24 Both case reports noted symptomatic relief and freedom from medications up to 1 year postoperatively,22,23 whereas Hendry and colleagues24 reported that 1 of 3 patients in their series with sinus tachycardia was in sinus rhythm several years postoperatively. With the advent of ablation technology, cryoablation and bipolar RF energy are used to avoid surgical incisions, shorten the operative time, and avoid cardiopulmonary bypass.25–28 The bipolar RF clamps are an attractive technology for this application. They have been shown in numerous long-term animal studies to create transmural lesions on the beating heart, even through the thick and trabeculated myocardium.25,29
The present case series expands on previous findings and, to our knowledge, represents the largest case series to date of patients undergoing surgical SA node isolation at a single institution. Moreover, long-term follow-up was available. In this series, surgical incisions were used in 4 patients, lone cryoablation was performed in 3 patients, lone bipolar RF ablation was performed in 4 patients, and 7 patients underwent a combination of cryoablation and bipolar ablation. A minimally invasive surgical approach by a small right mini-thoracotomy was used in 8 patients.
Although the present report represents one of the largest case series to date of patients undergoing surgical therapy for IST, several limitations must be noted. First, the small sample size (n = 18), long study duration, and multiple surgical approaches make this patient population relatively heterogeneous. However, such heterogeneity is difficult to avoid because of the rarity of patient referral for surgical treatment.
Note that, although our approach has varied over 3 decades, evolving from an on-bypass, sternotomy approach to an off-pump, thoracoscopic one, our operative strategy and lesion set has remained constant. This has involved isolating the entire superior right atrium and SA node complex from the remainder of the atrium. Because recent experimental work from our group and others has increased our understanding of the SA node complex and its ability to migrate with changes in autonomic tone,19,20 we have expanded our lesion set to also isolate a cuff of atrial tissue around the IVC and to include SA node denervation with cryoablation. In addition, our ablation goal has also remained the same. Inversion of the P-wave and blunting of the heart rate response to isoproterenol were observed in all patients.
Our long-term outcomes after surgical ablation have been excellent in patients who have failed medical therapy or catheter ablation. In our series, all patients had failed medical management, and 15 of 18 (83%) had failed at least one catheter-based ablation. At the last follow-up, no patient had recurrent symptomatic IST. This disease may represent another good indication for surgical ablation, but the evidence base needs to be developed to increase referrals.
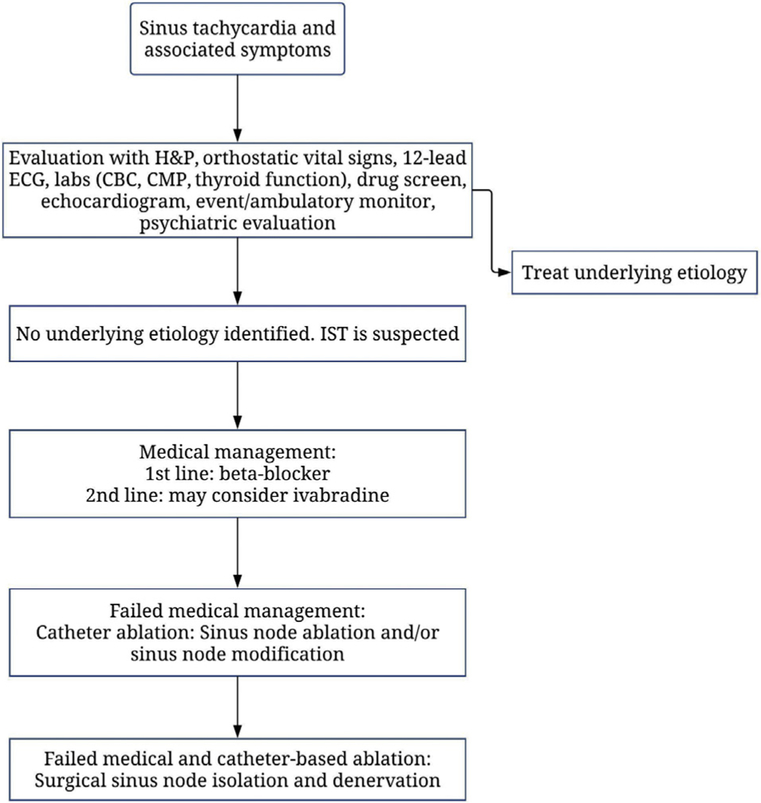
At our center, most patients have been referred from outside institutions familiar with our work. Our general approach to patients with symptomatic sinus tachycardia and presumed diagnoses of IST is outlined in Figure 3. Patients are typically referred to us because of their symptoms of tachycardia and palpitations and were only taken to the operating room when unresponsive to medical therapy with or without prior failed catheter ablation.
Figure 3.
Our general approach to patients with symptomatic sinus tachycardia. (CBC, complete blood count; CMP, comprehensive metabolic panel; ECG, electrocardiogram; H&P, history and physical; IST, inappropriate sinus tachycardia.)
With the advent of bipolar RF clamps and minimally invasive cardiac surgical techniques (eg, right mini-thoracotomy, thoracoscopic guidance), we have now been able to perform this operation minimally invasively, using a single small incision and an off-pump approach with thoracoscopic guidance. This offers a less morbid alternative to sternotomy and may be more attractive to patients and referring cardiologists. Cardiac surgeons should know that surgical ablation is a potential option for a select subset of patients with IST with excellent long-term outcomes.
Future studies should seek to expand the population size and to define optimal patient selection. Furthermore, differences in outcomes after different ablation techniques warrant further investigation. Although a difference in efficacy between ablation technologies and techniques may exist, the small sample size of our case series was inadequate to address this issue.
In conclusion, surgical ablation for IST is a reasonable treatment option for patients refractory to pharmacologic therapy and catheter ablation. Minimally invasive surgical techniques that use bipolar RF and cryoablation have been successful and may provide patients and clinicians with a more attractive option for treatment of refractory IST.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health grants R01-HL032257 (to R.J.D. and R.B.S.) and T32-HL007776 (to R.J.D. and A.J.K.).
Footnotes
The Video can be viewed in the online version of this article [https://doi.org/10.1016/j.athoracsur.2019.03.10] on http://www.annalsthoracicsurgery.org.
References
- 1.Krahn AD, Yee R, Klein GJ, Morillo C. Inappropriate sinus tachycardia: evaluation and therapy. J Cardiovasc Electrophysiol. 1995;6:1124–1128. [DOI] [PubMed] [Google Scholar]
- 2.Bauernfeind RA, Amat-Y-Leon F, Dhingra RC, Kehoe R, Wyndham C, Rosen KM. Chronic nonparoxysmal sinus tachycardia in otherwise healthy persons. Ann Intern Med. 1979;91:702–710. [DOI] [PubMed] [Google Scholar]
- 3.Shen WK. How to manage patients with inappropriate sinus tachycardia. Heart Rhythm. 2005;2:1015–1019. [DOI] [PubMed] [Google Scholar]
- 4.Sheldon RS, Grubb II BP, Olshansky B, et al. 2015 Heart Rhythm Society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm. 2015;12:e41–e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olshansky B, Sullivan RM. Inappropriate sinus tachycardia. J Am Coll Cardiol. 2013;61:793–801. [DOI] [PubMed] [Google Scholar]
- 6.Blomstrom-Lundqvist C, Scheinman MM, Aliot EM, et al. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias–executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Supraventricular Arrhythmias). Circulation. 2003;108:1871–1909. [DOI] [PubMed] [Google Scholar]
- 7.Leon H, Guzman JC, Kuusela T, Dillenburg R, Kamath M, Morillo CA. Impaired baroreflex gain in patients with inappropriate sinus tachycardia. J Cardiovasc Electrophysiol. 2005;16:64–68. [DOI] [PubMed] [Google Scholar]
- 8.Still AM, Raatikainen P, Ylitalo A, et al. Prevalence, characteristics and natural course of inappropriate sinus tachycardia. Europace. 2005;7:104–112. [DOI] [PubMed] [Google Scholar]
- 9.Cappato R, Castelvecchio S, Ricci C, et al. Clinical efficacy of ivabradine in patients with inappropriate sinus tachycardia: a prospective, randomized, placebo-controlled, double-blind, crossover evaluation. J Am Coll Cardiol. 2012;60: 1323–1329. [DOI] [PubMed] [Google Scholar]
- 10.Annamaria M, Lupo PP, Foresti S, et al. Treatment of inappropriate sinus tachycardia with ivabradine. J Interv Card Electrophysiol. 2016;46:47–53. [DOI] [PubMed] [Google Scholar]
- 11.Mantovan R, Thiene G, Calzolari V, Basso C. Sinus node ablation for inappropriate sinus tachycardia. J Cardiovasc Electrophysiol. 2005;16:804–806. [DOI] [PubMed] [Google Scholar]
- 12.Fedorov VV, Schuessler RB, Hemphill M, et al. Structural and functional evidence for discrete exit pathways that connect the canine sinoatrial node and atria. Circ Res. 2009;104: 915–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Man KC, Knight B, Tse HF, et al. Radiofrequency catheter ablation of inappropriate sinus tachycardia guided by activation mapping. J Am Coll Cardiol. 2000;35:451–457. [DOI] [PubMed] [Google Scholar]
- 14.Marrouche NF, Beheiry S, Tomassoni G, et al. Three-dimensional nonfluoroscopic mapping and ablation of inappropriate sinus tachycardia. Procedural strategies and long-term outcome. J Am Coll Cardiol. 2002;39:1046–1054. [DOI] [PubMed] [Google Scholar]
- 15.Gianni C, Di Biase L, Mohanty S, et al. Catheter ablation of inappropriate sinus tachycardia. J Interv Card Electrophysiol. 2016;46:63–69. [DOI] [PubMed] [Google Scholar]
- 16.Kreisel D, Bailey M, Lindsay BD, Damiano RJ Jr. A minimally invasive surgical treatment for inappropriate sinus tachycardia. J Thorac Cardiovasc Surg. 2005;130:598–599. [DOI] [PubMed] [Google Scholar]
- 17.Gaynor SL, Diodato MD, Prasad SM, et al. A prospective, single-center clinical trial of a modified Cox maze procedure with bipolar radiofrequency ablation. J Thorac Cardiovasc Surg. 2004;128:535–542. [DOI] [PubMed] [Google Scholar]
- 18.Ren JF, Marchlinski FE, Callans DJ, Zado ES. Echocardio-graphic lesion characteristics associated with successful ablation of inappropriate sinus tachycardia. J Cardiovasc Electrophysiol. 2001;12:814–818. [DOI] [PubMed] [Google Scholar]
- 19.Stiles MK, Brooks AG, Roberts-Thomson KC, et al. High-density mapping of the sinus node in humans: role of preferential pathways and the effect of remodeling. J Cardiovasc Electrophysiol. 2010;21:532–539. [DOI] [PubMed] [Google Scholar]
- 20.Fedorov VV, Glukhov AV, Chang R, et al. Optical mapping of the isolated coronary-perfused human sinus node. J Am Coll Cardiol. 2010;56:1386–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yee R, Guiraudon GM, Gardner MJ, Gulamhusein SS, Klein GJ. Refractory paroxysmal sinus tachycardia: management by subtotal right atrial exclusion. J Am Coll Cardiol. 1984;3(2 Pt 1):400–404. [DOI] [PubMed] [Google Scholar]
- 22.Esmailzadeh B, Bernat R, Winkler K, Meybehm M, Pfeiffer D, Kirchhoff PG. Surgical excision of the sinus node in a patient with inappropriate sinus tachycardia. J Thorac Cardiovasc Surg. 1997;114:861–864. [DOI] [PubMed] [Google Scholar]
- 23.Selten K, Van Brakel TJ, Van Swieten HA, Smeets JL. Mapping-guided total excision of the sinoatrial node for inappropriate sinus tachycardia. J Thorac Cardiovasc Surg. 2014;147:e56–e58. [DOI] [PubMed] [Google Scholar]
- 24.Hendry PJ, Packer DL, Anstadt MP, Plunkett MD, Lowe JE. Surgical treatment of automatic atrial tachycardias. Ann Thorac Surg. 1990;49:253–259 [discussion 259–260]. [DOI] [PubMed] [Google Scholar]
- 25.Damiano RJ Jr. Alternative energy sources for atrial ablation: judging the new technology. Ann Thorac Surg. 2003;75:329–330. [DOI] [PubMed] [Google Scholar]
- 26.Doll N, Kiaii BB, Fabricius AM, et al. Intraoperative left atrial ablation (for atrial fibrillation) using a new argon cryocatheter: early clinical experience. Ann Thorac Surg. 2003;76: 1711–1715 [discussion 1715]. [DOI] [PubMed] [Google Scholar]
- 27.Taketani T, Wolf RK, Garrett JV. Partial cardiac denervation and sinus node modification for inappropriate sinus tachycardia. Ann Thorac Surg. 2007;84:652–654. [DOI] [PubMed] [Google Scholar]
- 28.Beaver TM, Miles WM, Conti JB, et al. Minimally invasive ablation of a migrating focus of inappropriate sinus tachycardia. J Thorac Cardiovasc Surg. 2010;139:506–507. [DOI] [PubMed] [Google Scholar]
- 29.Prasad SM, Maniar HS, Diodato MD, Schuessler RB, Damiano RJ. Physiological consequences of bipolar radiofrequency energy on the atria and pulmonary veins: a chronic animal study. Ann Thorac Surg. 2003;76:836–841 [discussion 841–842]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.